Dispersion Mechanism of Styrene–Butadiene Rubber Powder Modified by Itaconic Acid and Its Toughening Effect on Oil Well Cement
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Three Latex Powders
2.3. Characterization of Latex Powder
2.3.1. Absorbance of the Latex Powder
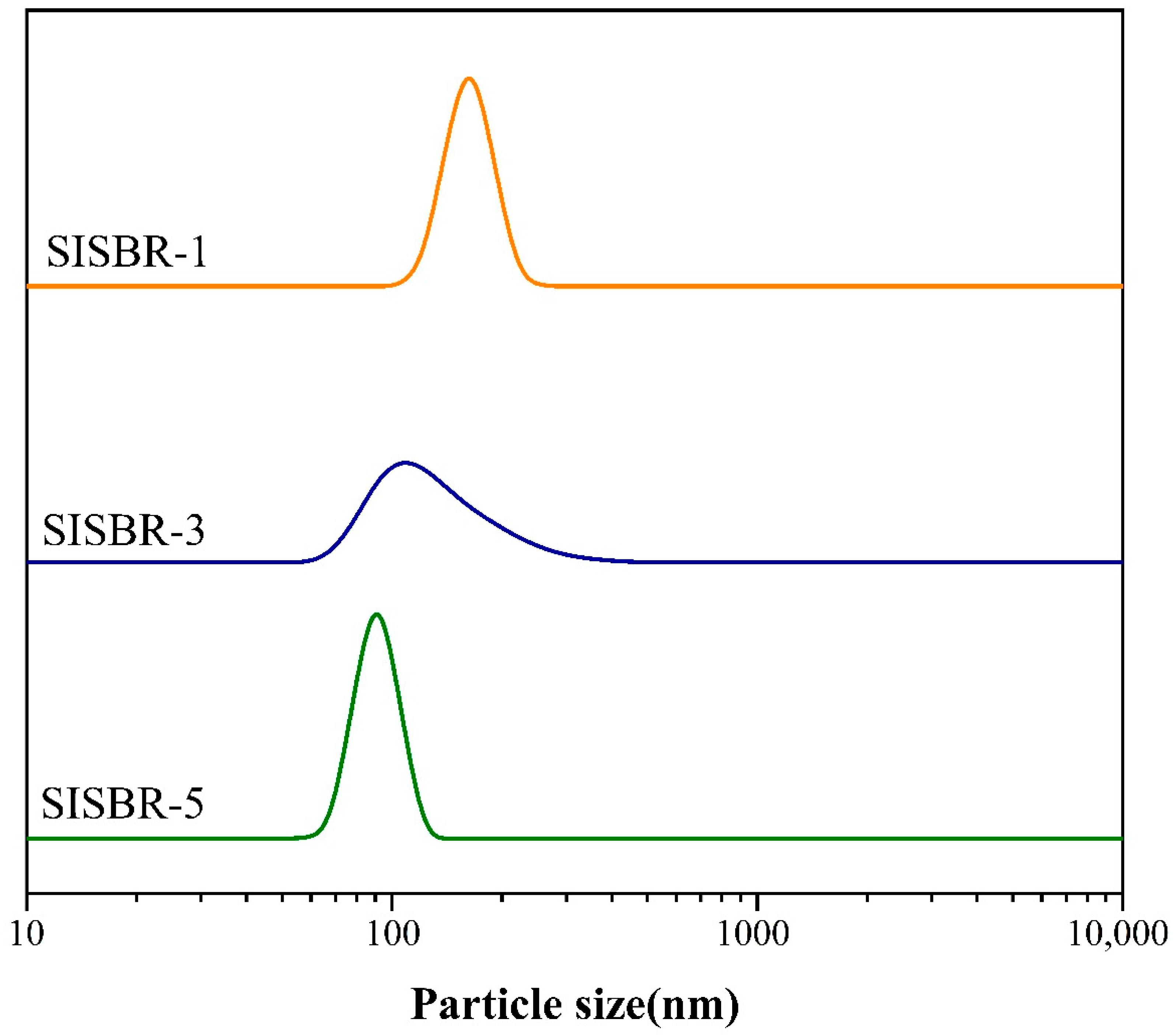
2.3.2. Particle Size of the Latex Powder
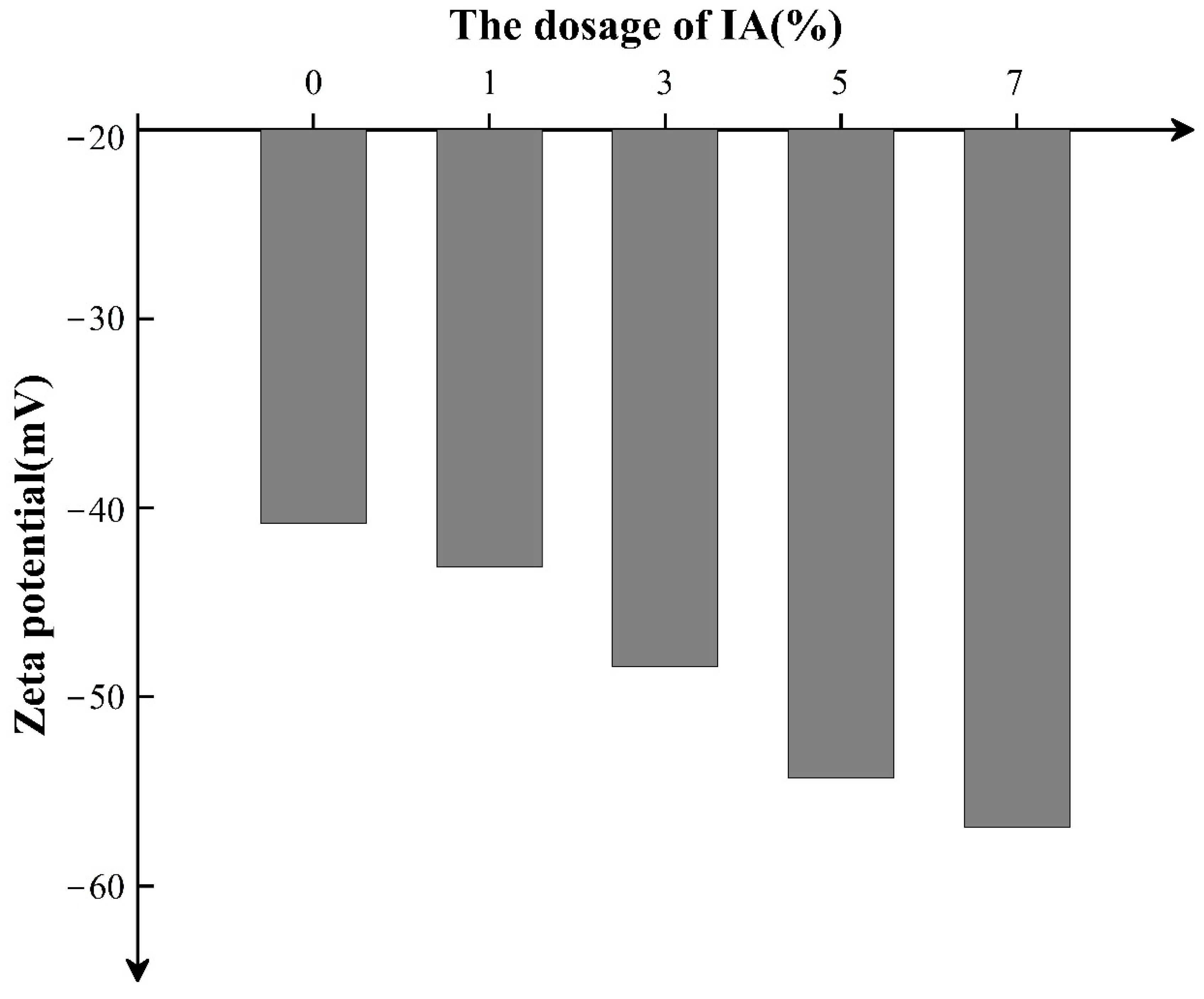
2.3.3. Zeta Potential of the Latex Powder
2.4. Measurements of Cement Paste with Latex
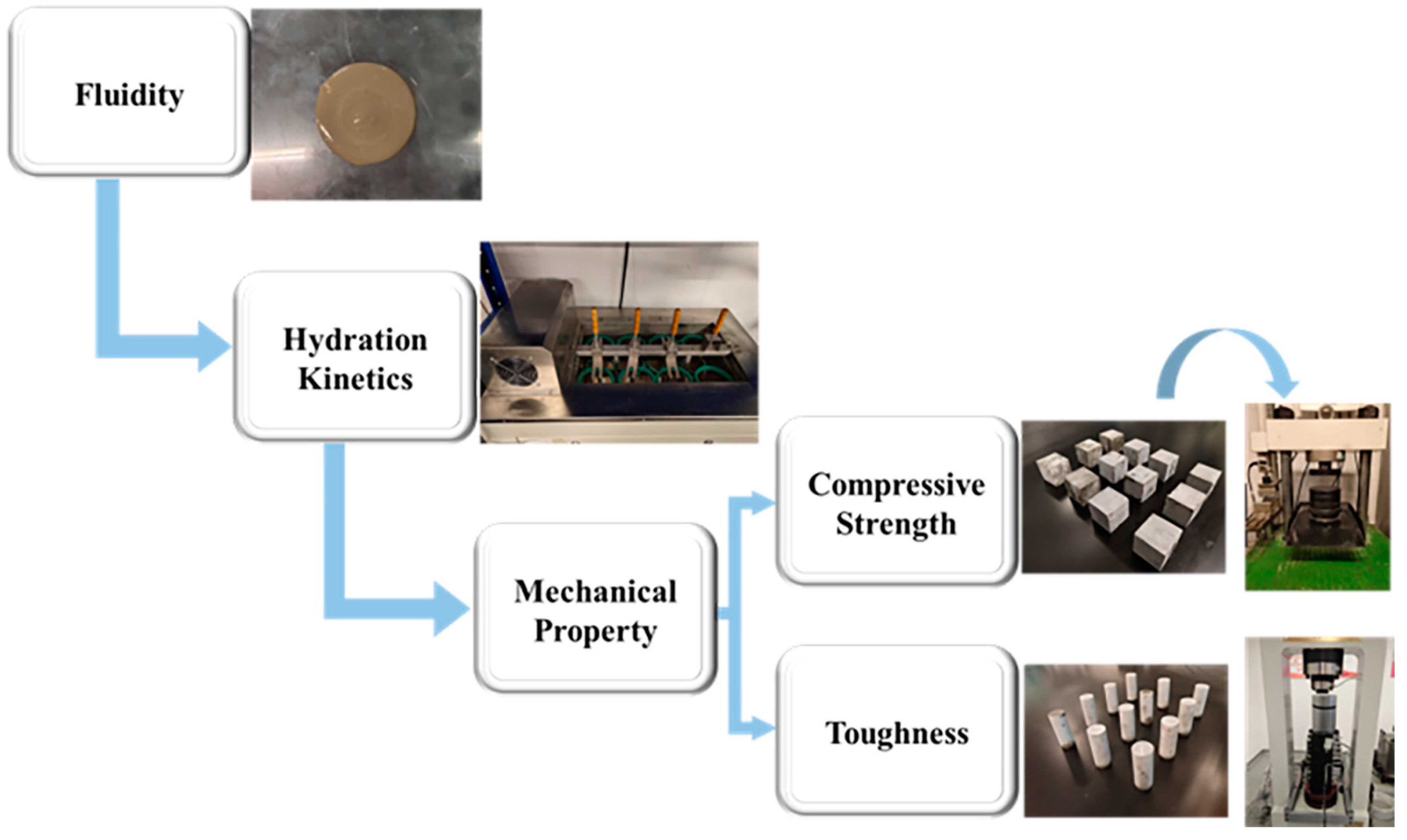
2.4.1. Fluidity of Cement Paste
2.4.2. Isothermal Heat Flow Calorimetry
2.4.3. Triaxial Mechanical Test
2.4.4. Compressive Strength
2.5. Reaction Mechanism of Latex Powder
2.5.1. X-ray Diffraction Tests
2.5.2. Adsorption Measurement
2.5.3. Scanning Electron Microscope (SEM) Tests
3. Results and Discussion
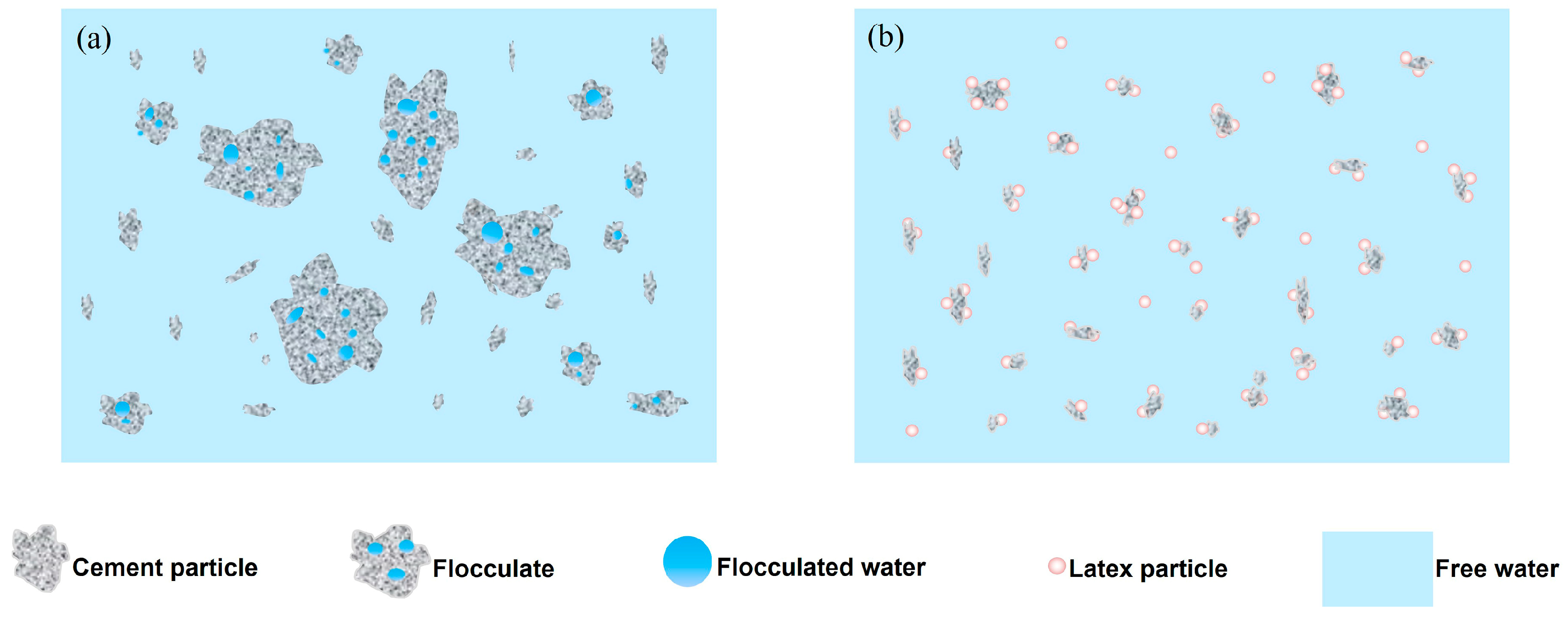
3.1. Dispersion State Analysis of SISBR Latex Powder
3.2. Influence of SISBR Latex on Cement Slurry
3.2.1. Fluidity
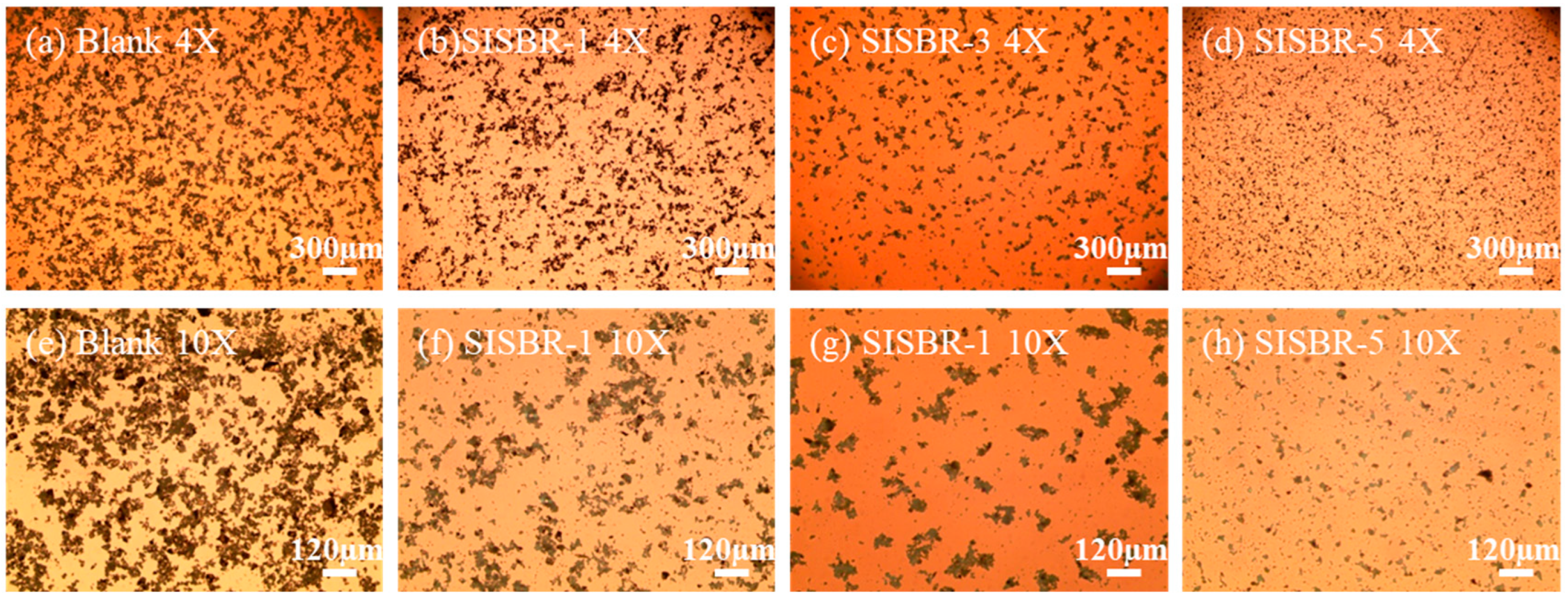
3.2.2. Optical Microstructure of Cement Slurry
3.3. Effects of SISBR Latex Powders on Cement Hydration
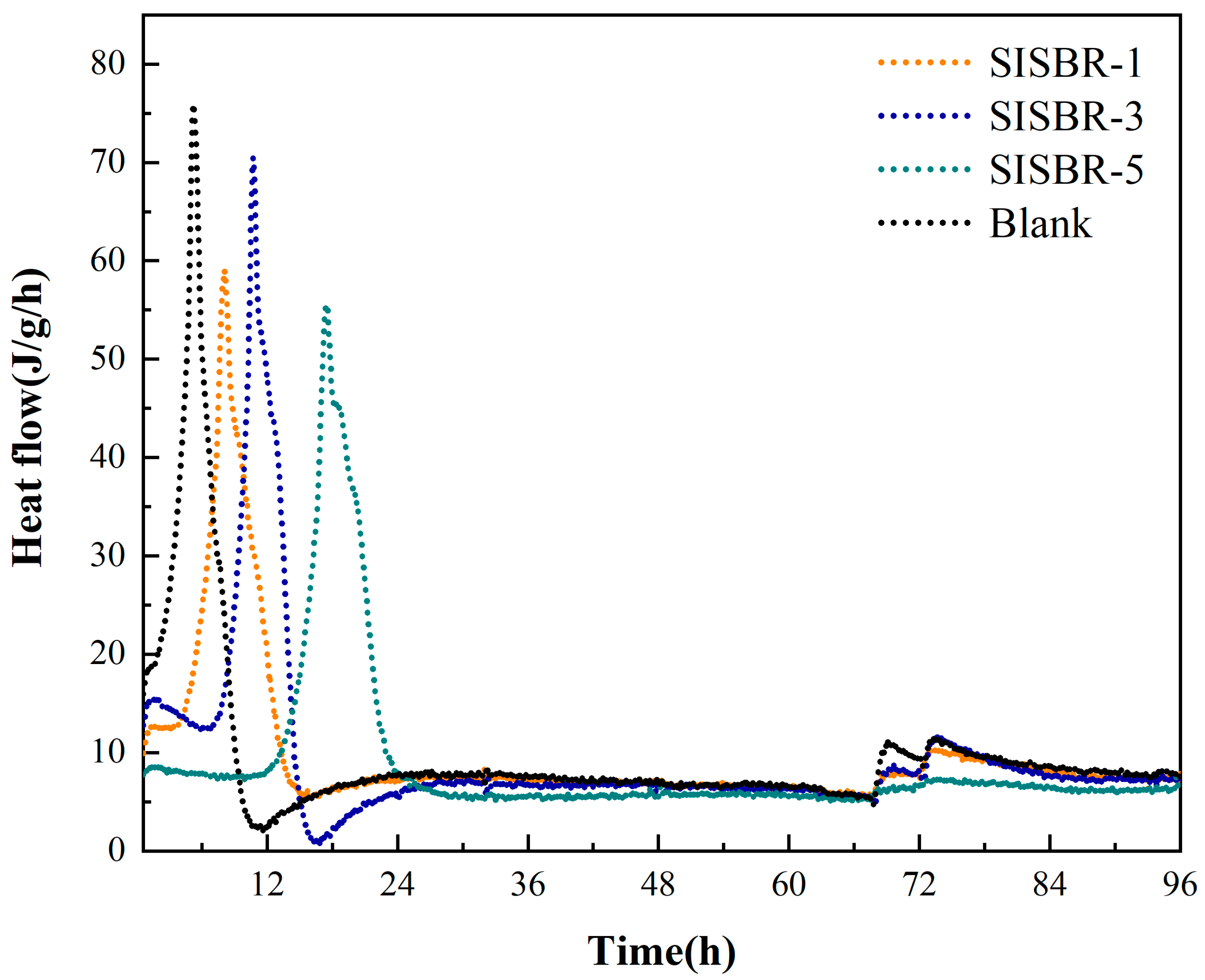
3.3.1. Hydration Kinetics
3.3.2. X-ray Diffraction Analysis
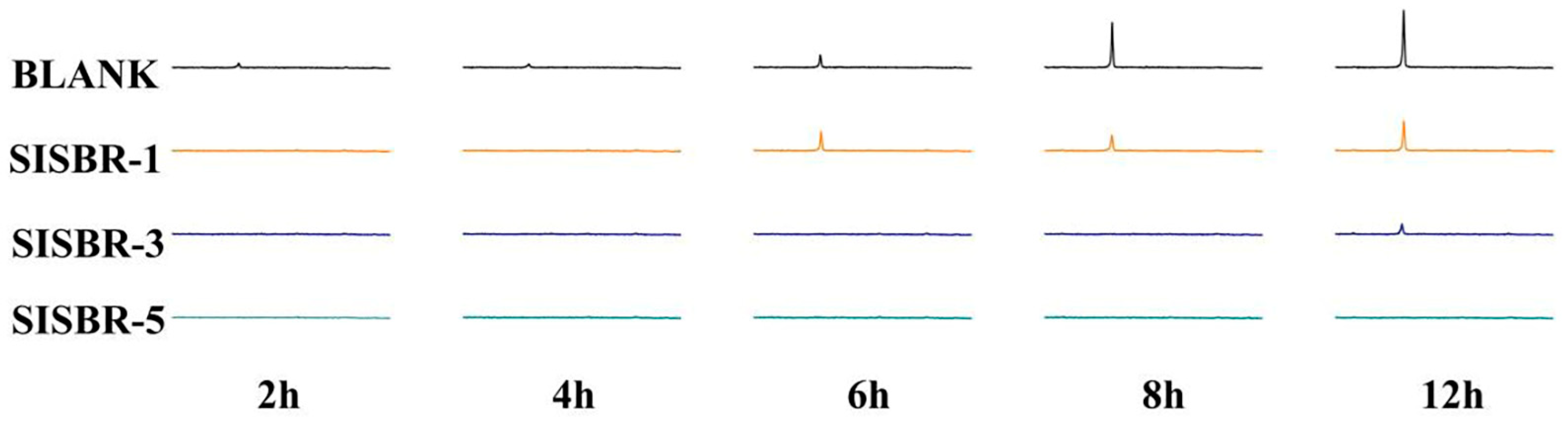
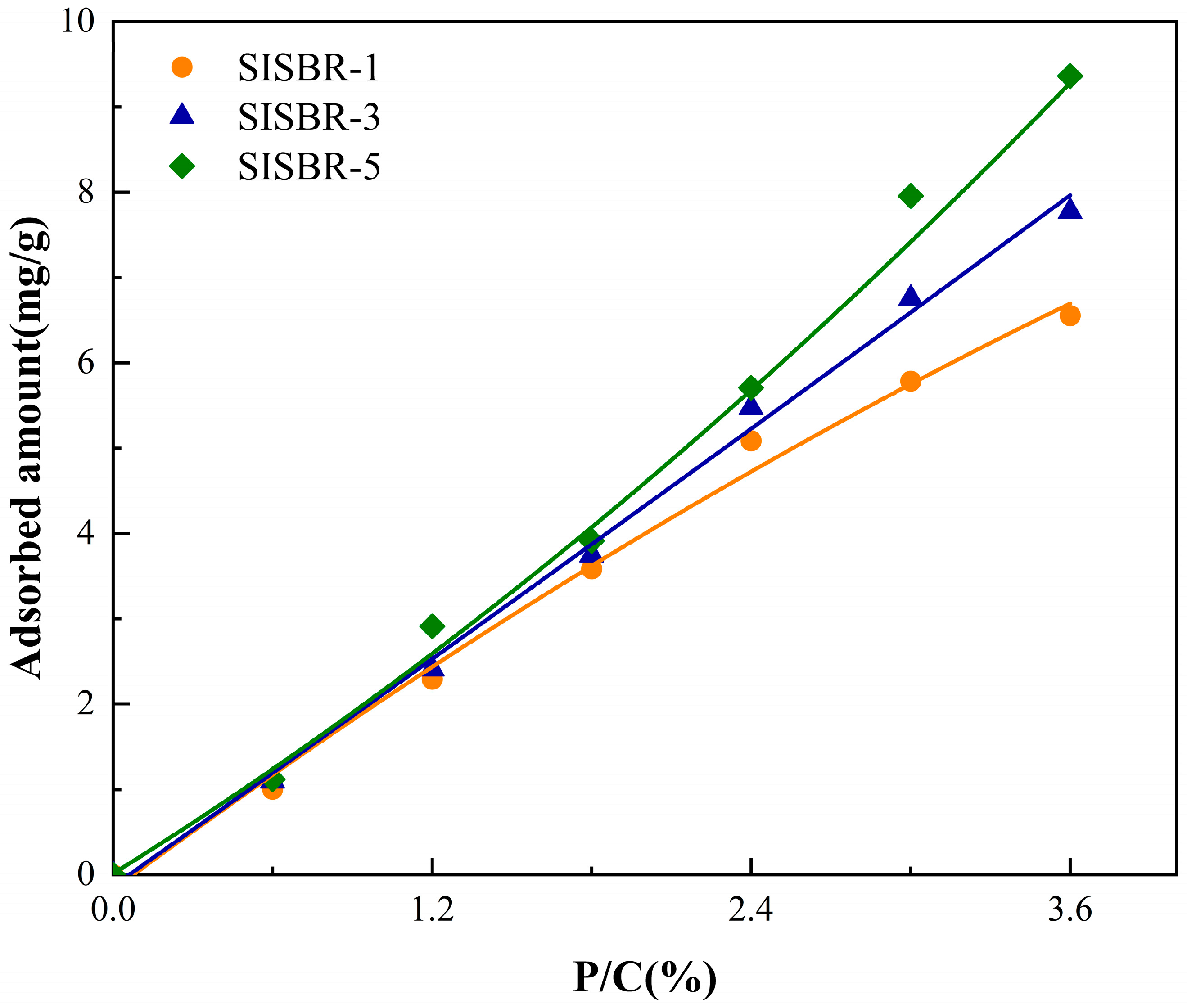
3.3.3. Adsorption Behavior of SISBR Latex Powders on Cement Surface
3.4. Effects of SISBR Latex on Mechanical Property of Cement
3.4.1. Effects of SISBR Latex on Compressive Strength of Cement
3.4.2. Effects of SISBR Latex on Toughness of Cement
3.4.3. Effects of SISBR Latex on Microstructure of Cement Stone
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jackson, R.B. The integrity of oil and gas wells. Proc. Natl. Acad. Sci. USA 2014, 111, 10902–10903. [Google Scholar] [CrossRef]
- Bu, Y.; Ma, R.; Guo, S.; Du, J.; Liu, H.; Cao, X. A theoretical evaluation method for mechanical sealing integrity of cementing sheath. Appl. Math. Model. 2020, 84, 571–589. [Google Scholar] [CrossRef]
- Chu, S.H. Effect of paste volume on fresh and hardened properties of concrete. Constr. Build. Mater. 2019, 218, 284–294. [Google Scholar] [CrossRef]
- Chu, S.H.; Li, L.G.; Kwan, A.K.H. Development of extrudable high strength fiber reinforced concrete incorporating nano calcium carbonate. Addit. Manuf. 2021, 37, 101617. [Google Scholar] [CrossRef]
- Plank, J.; Gretz, M. Study on the interaction between anionic and cationic latex particles and Portland cement. Colloid Surf. A-Physicochem. Eng. Asp. 2008, 330, 227–233. [Google Scholar] [CrossRef]
- Cheng, X.; Chen, Z.; Gu, T.; Zeng, L.; Yao, L.; Chen, Z.; Huang, K.; Zhang, Z.; Zhang, C.; Liu, K.; et al. Study on the dynamic and static mechanical properties of microsphere rubber powder reinforced oil well cement composites. Constr. Build. Mater. 2021, 309, 125145. [Google Scholar] [CrossRef]
- Fan, J.; Guo, J.; Chen, D.; Hu, M.; Cao, L.; Xu, Y.; Wang, M. Effects of submicron core-shell latexes with different functional groups on the adsorption and cement hydration. Constr. Build. Mater. 2018, 183, 127–138. [Google Scholar] [CrossRef]
- Wang, M.; Wang, R.; Yao, H.; Farhan, S.; Zheng, S.; Wang, Z.; Du, C.; Jiang, H. Research on the mechanism of polymer latex modified cement. Constr. Build. Mater. 2016, 111, 710–718. [Google Scholar] [CrossRef]
- Idrees, M.; Saeed, F.; Amin, A.; Hussain, T. Improvement in compressive strength of Styrene-Butadiene-Rubber (SBR) modified mortars by using powder form and nanoparticles. J. Build. Eng. 2021, 44, 102651. [Google Scholar] [CrossRef]
- Du Chesne, A.; Bojkova, A.; Gapinski, J.; Seip, D.; Fischer, P. Film formation and redispersion of waterborne latex coatings. J. Colloid Interface Sci. 2000, 224, 91–98. [Google Scholar] [CrossRef]
- Kim, J.; Bang, J.; Kim, Y.J.; Kim, J.C.; Hwang, S.W.; Yeo, H.; Choi, I.G.; Kwak, H.W. Eco-friendly alkaline lignin/cellulose nanofiber drying system for efficient redispersion behavior. Carbohydr. Polym. 2022, 282, 119122. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Sun, X.; Bai, Y.; Meng, X.; Li, C. Isolation of dicarboxy cellulose nanocrystal from spent fungi substrate and redispersion with gelatin. J. Mol. Liq. 2022, 367, 120397. [Google Scholar] [CrossRef]
- Keerthika, N.; Jayalakshmi, A.; Ramkumar, S.G. Anchored block-copolymer surfactants for the synthesis of redispersible polystyrene latexes. J. Appl. Polym. Sci. 2019, 137, 48875. [Google Scholar] [CrossRef]
- Bazrkar, H.; Lork, A.; Aminnejad, B. Application of a Synthetic Polymer Nanocomposite Latex in a Wellbore Cement Slurry for Gas Blockage Functions. ACS Omega 2022, 7, 27469–27478. [Google Scholar] [CrossRef]
- Christensen, K.L.; Pedersen, G.P.; Kristensen, H.G. Preparation of redispersible dry emulsions by spray drying. Int. J. Pharm. 2001, 212, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Hou, D.; Meng, L.; Sun, G.; Lu, C.; Li, Z. Mechanism of cement paste reinforced by graphene oxide/carbon nanotubes composites with enhanced mechanical properties. RSC Adv. 2015, 5, 100598–100605. [Google Scholar] [CrossRef]
- Guo, S.; Bu, Y.; Lu, Y. Addition of tartaric acid to prevent delayed setting of oil-well cement containing retarder at high temperatures. J. Pet. Sci. Eng. 2019, 172, 269–279. [Google Scholar] [CrossRef]
- Cheng, X.; Dong, Q.; Ma, Y.; Zhang, C.; Gao, X.; Yu, Y.; Wen, Z.; Zhang, C.; Guo, X. Mechanical and thermal properties of aluminate cement paste with blast furnace slag at high temperatures. Constr. Build. Mater. 2019, 228, 116747. [Google Scholar] [CrossRef]
- Alimardani, M.; Abbassi-Sourki, F.; Bakhshandeh, G.R. An investigation on the dispersibility of carbon nanotube in the latex nanocomposites using rheological properties. Compos. Part B-Eng. 2014, 56, 149–156. [Google Scholar] [CrossRef]
- Baueregger, S.; Perello, M.; Plank, J. Influence of carboxylated styrene–butadiene latex copolymer on Portland cement hydration. Cem. Concr. Compos. 2015, 63, 42–50. [Google Scholar] [CrossRef]
- Pei, Y.; Ren, X.; Xie, D.; Zhang, X. Stabilization mechanism of the reconstituted emulsion of polyacrylate redispersible powder. Chem. Eng. Commun. 2015, 202, 1245–1250. [Google Scholar] [CrossRef]
- Delibaş, A.; Yıldız, U.; Tauer, K. Composite latex production with high solid content. J. Appl. Polym. Sci. 2019, 136, 47423. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, M.; Chen, D.; Liu, Z.; Yu, Y.; Zhang, H.; Guo, J. Performance and working mechanism of amphoteric polycarboxylate-based dispersant and sulfonated acetone formaldehyde polycondensate-based dispersant in oil well cement. Constr. Build. Mater. 2020, 233, 117147. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, X. Influences of superplasticizer, polymer latexes and asphalt emulsions on the pore structure and impermeability of hardened cementitious materials. Constr. Build. Mater. 2014, 53, 392–402. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, X.; Kong, X.; Wang, F.; Gao, L. Rheological properties and microstructure of fresh cement pastes with varied dispersion media and superplasticizers. Powder Technol. 2018, 330, 219–227. [Google Scholar] [CrossRef]
- Zhang, K.; Pan, L.; Li, J.; Lin, C.; Cao, Y. How does adsorption behavior of polycarboxylate superplasticizer effect rheology and flowability of cement paste with polypropylene fiber. Cem. Concr. Compos. 2019, 95, 228–236. [Google Scholar] [CrossRef]
- Ferrari, L.; Kaufmann, J.; Winnefeld, F.; Plank, J. Interaction of cement model systems with superplasticizers investigated by atomic force microscopy, zeta potential, and adsorption measurements. J. Colloid Interface Sci. 2010, 347, 15–24. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, J.; Han, K.; Wang, J.; Hu, X. Study on the structural build-up of cement-ground limestone pastes and its micro-mechanism. Constr. Build. Mater. 2020, 263, 120656. [Google Scholar] [CrossRef]
- Termkhajornkit, P.; Nawa, T. The fluidity of fly ash–cement paste containing naphthalene sulfonate superplasticizer. Cem. Concr. Res. 2004, 34, 1017–1024. [Google Scholar] [CrossRef]
- Kong, X.; Emmerling, S.; Pakusch, J.; Rueckel, M.; Nieberle, J. Retardation effect of styrene-acrylate copolymer latexes on cement hydration. Cem. Concr. Res. 2015, 75, 23–41. [Google Scholar] [CrossRef]
- Lu, Z.; Kong, X.; Zhang, C.; Xing, F.; Cai, Y.; Jiang, L.; Zhang, Y.; Dong, B. Effect of surface modification of colloidal particles in polymer latexes on cement hydration. Constr. Build. Mater. 2017, 155, 1147–1157. [Google Scholar] [CrossRef]
- Lu, Z.; Kong, X.; Zhang, C.; Cai, Y.; Zhang, Q.; Zhang, Y. Effect of polymer latexes with varied glass transition temperature on cement hydration. J. Appl. Polym. Sci. 2017, 134, 45264. [Google Scholar] [CrossRef]
- Krakowiak, K.J.; Thomas, J.J.; Musso, S.; James, S.; Akono, A.-T.; Ulm, F.-J. Nano-chemo-mechanical signature of conventional oil-well cement systems: Effects of elevated temperature and curing time. Cem. Concr. Res. 2015, 67, 103–121. [Google Scholar] [CrossRef]
- Kong, X.; Pakusch, J.; Jansen, D.; Emmerling, S.; Neubauer, J.; Goetz-Neuhoeffer, F. Effect of polymer latexes with cleaned serum on the phase development of hydrating cement pastes. Cem. Concr. Res. 2016, 84, 30–40. [Google Scholar] [CrossRef]
- Wang, X.; Ran, Q.; Yang, Y.; Shu, X.; Yu, C. Influence of sequence structure of polycarboxylate superplasticizers on early age properties of cement paste. J. Mater. Civ. Eng. 2016, 10, 04016112. [Google Scholar] [CrossRef]
- De Windt, L.; Bertron, A.; Larreur-Cayol, S.; Escadeillas, G. Interactions between hydrated cement paste and organic acids: Thermodynamic data and speciation modeling. Cem. Concr. Res. 2015, 69, 25–36. [Google Scholar] [CrossRef]
- Nalet, C.; Nonat, A. Ionic complexation and adsorption of small organic molecules on calcium silicate hydrate: Relation with their retarding effect on the hydration of C3S. Cem. Concr. Res. 2016, 89, 97–108. [Google Scholar] [CrossRef]
- Sowoidnich, T.; Rachowski, T.; Rößler, C.; Völkel, A.; Ludwig, H.-M. Calcium complexation and cluster formation as principal modes of action of polymers used as superplasticizer in cement systems. Cem. Concr. Res. 2015, 73, 42–50. [Google Scholar] [CrossRef]
- Sakai, E.; Kasuga, T.; Sugiyama, T.; Asaga, K.; Daimon, M. Influence of superplasticizers on the hydration of cement and the pore structure of hardened cement. Cem. Concr. Res. 2006, 36, 2049–2053. [Google Scholar] [CrossRef]
- Lu, Z.; Kong, X.; Zhang, C.; Cai, Y. Effect of highly carboxylated colloidal polymers on cement hydration and interactions with calcium ions. Cem. Concr. Res. 2018, 113, 140–153. [Google Scholar] [CrossRef]
- Ma, R.; Chen, Q.; Jiang, Z.; Qian, X.; Song, Y.; Ruan, S. Performances of UHPC bonded cementitious composite systems containing styrene-butadiene rubber latex in a chloride-rich environment. Constr. Build. Mater. 2022, 353, 129126. [Google Scholar] [CrossRef]
- Li, P.; Lu, W.; An, X.; Zhou, L.; Du, S. Effect of epoxy latexes on the mechanical behavior and porosity property of cement mortar with different degrees of hydration and polymerization. Materials 2021, 14, 517. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Ma, Y.; Qiu, C.; Sun, T.; Liu, J.; Zhou, Q. Effect of graphene oxide nanosheets of microstructure and mechanical properties of cement composites. Constr. Build. Mater. 2013, 49, 121–127. [Google Scholar] [CrossRef]
- Lu, Z.; Kong, X.; Zhang, Q.; Cai, Y.; Zhang, Y.; Wang, Z.; Dong, B.; Xing, F. Influences of styrene-acrylate latexes on cement hydration in oil well cement system at different temperatures. Colloid Surf. A-Physicochem. Eng. Asp. 2016, 507, 46–57. [Google Scholar] [CrossRef]






| Chemical Compositions (wt.%) | |||||||
|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Fe2O3 | SO3 | Al2O3 | MgO | K2O | Others |
| 69.68 | 15.13 | 6.45 | 3.39 | 2.34 | 0.92 | 0.79 | 1.3 |
| Latex Samples | IA Dosage (%) in Latex | Elastic Modulus (GPa) |
|---|---|---|
| SBR | 0 | 8.34 |
| SISBR-1 | 1 | 5.9 |
| SISBR-3 | 3 | 3.16 |
| SISBR-5 | 5 | 8.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Y.; Hu, M.; Cao, C.; Yu, J.; Zhao, J.; Zheng, H.; Guo, J. Dispersion Mechanism of Styrene–Butadiene Rubber Powder Modified by Itaconic Acid and Its Toughening Effect on Oil Well Cement. Materials 2022, 15, 8345. https://doi.org/10.3390/ma15238345
Xing Y, Hu M, Cao C, Yu J, Zhao J, Zheng H, Guo J. Dispersion Mechanism of Styrene–Butadiene Rubber Powder Modified by Itaconic Acid and Its Toughening Effect on Oil Well Cement. Materials. 2022; 15(23):8345. https://doi.org/10.3390/ma15238345
Chicago/Turabian StyleXing, Yubing, Miaomiao Hu, Chengzhang Cao, Jiayu Yu, Jiaqi Zhao, Hongbing Zheng, and Jintang Guo. 2022. "Dispersion Mechanism of Styrene–Butadiene Rubber Powder Modified by Itaconic Acid and Its Toughening Effect on Oil Well Cement" Materials 15, no. 23: 8345. https://doi.org/10.3390/ma15238345
APA StyleXing, Y., Hu, M., Cao, C., Yu, J., Zhao, J., Zheng, H., & Guo, J. (2022). Dispersion Mechanism of Styrene–Butadiene Rubber Powder Modified by Itaconic Acid and Its Toughening Effect on Oil Well Cement. Materials, 15(23), 8345. https://doi.org/10.3390/ma15238345







