Characterization of a Magnesium Fluoride Conversion Coating on Mg-2Y-1Mn-1Zn Screws for Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Coating Process
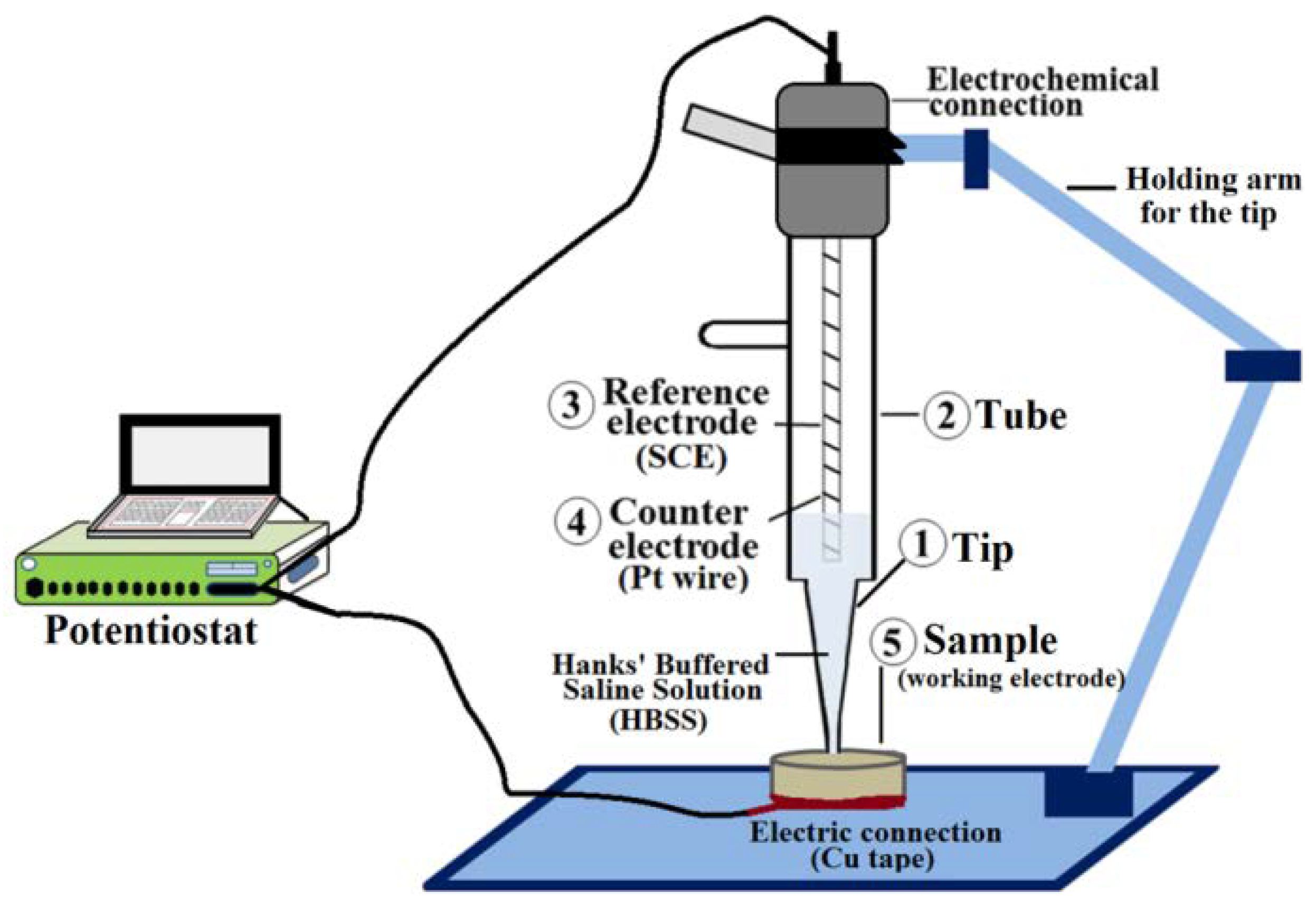
2.2. Samples Characterization
3. Results and Discussion
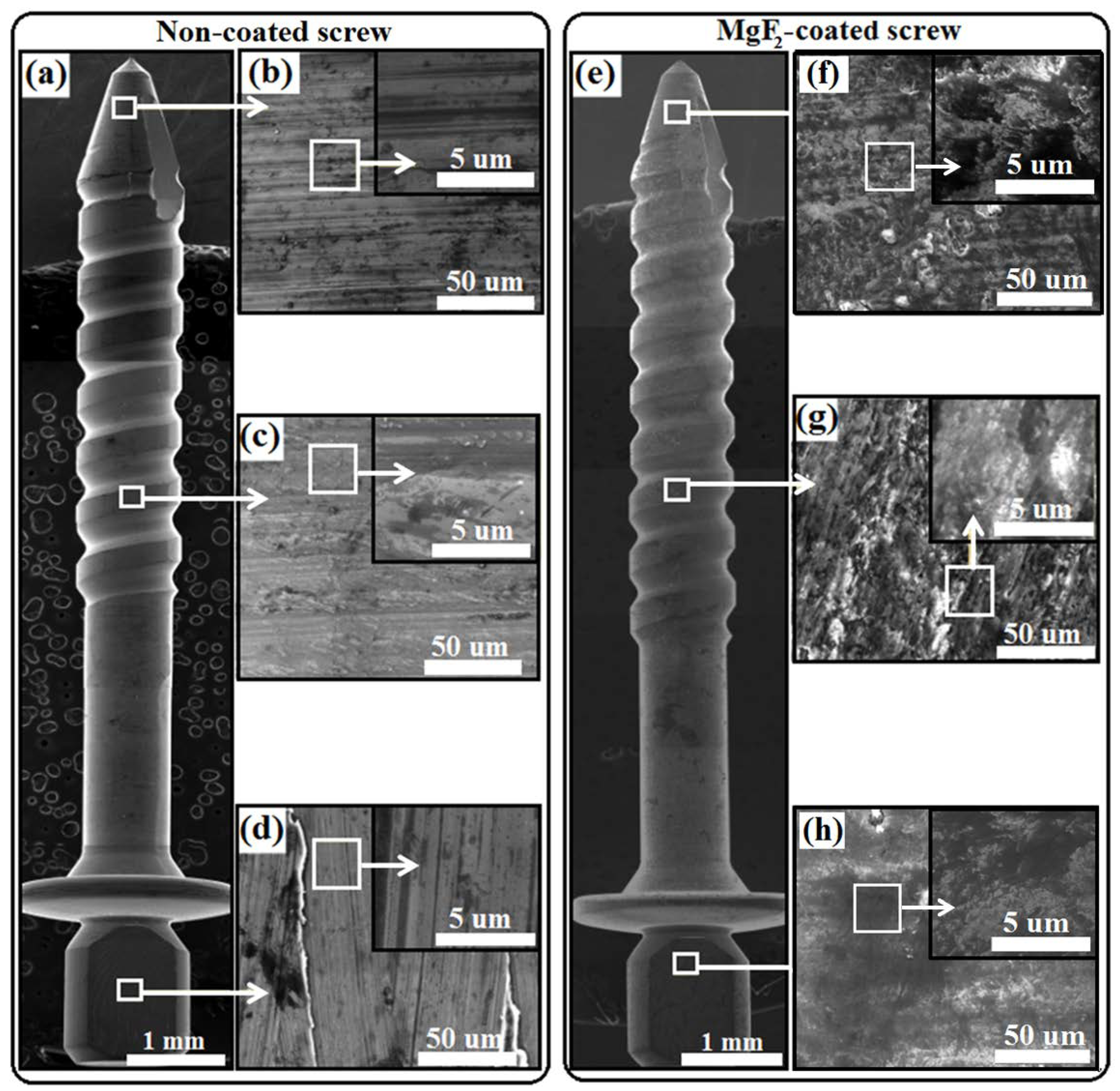
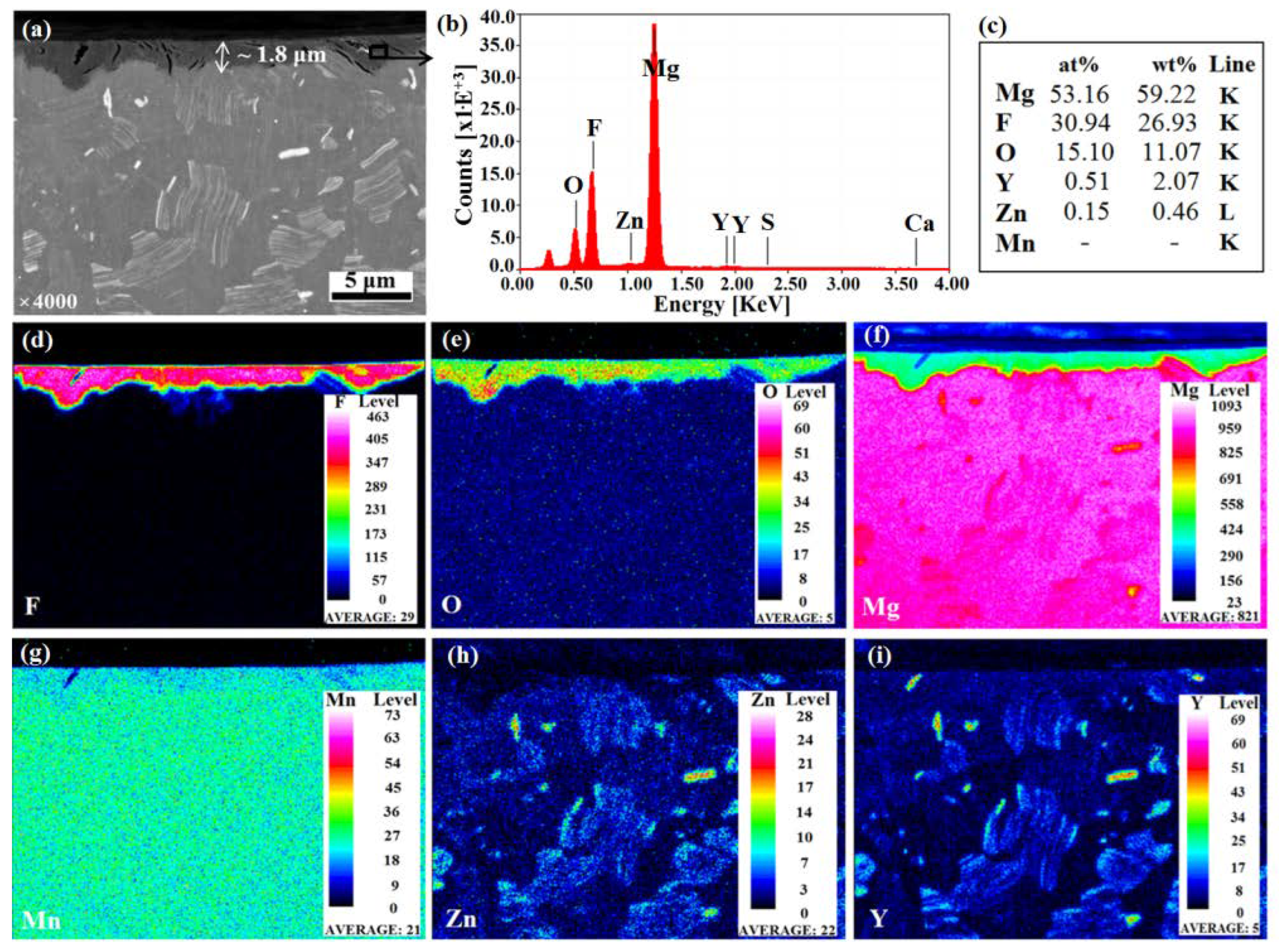
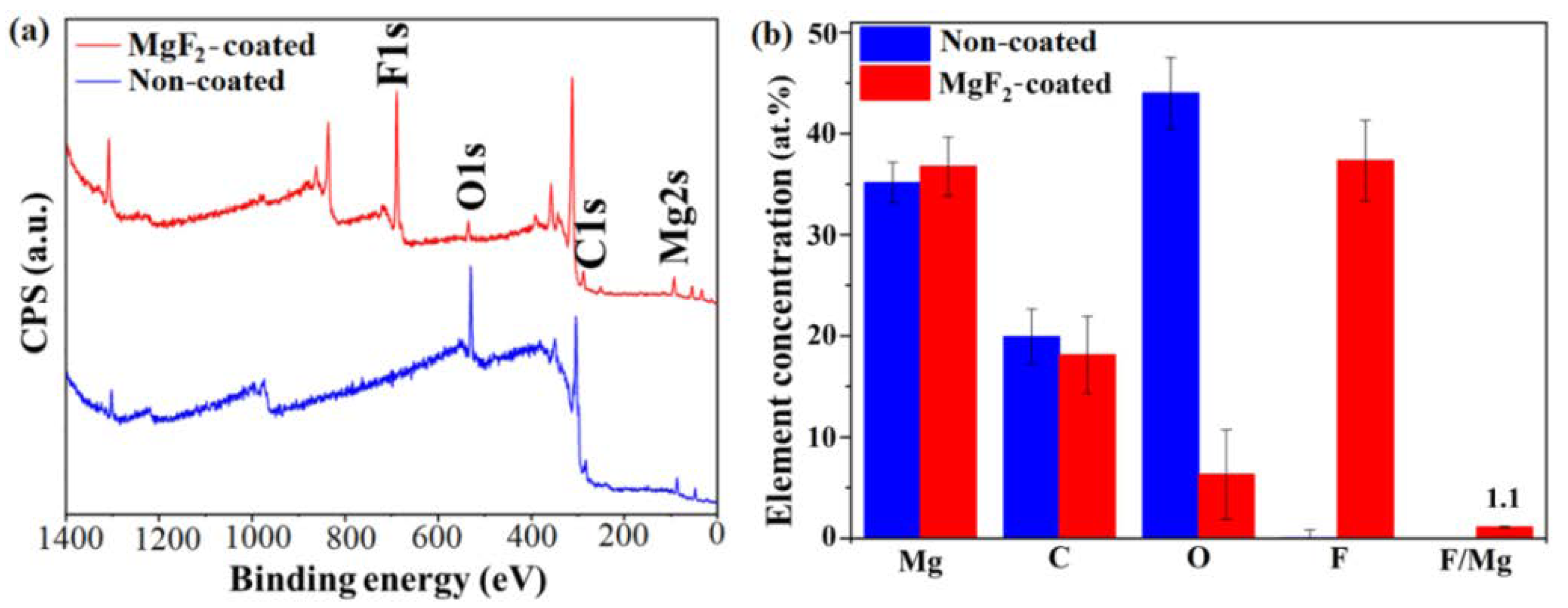
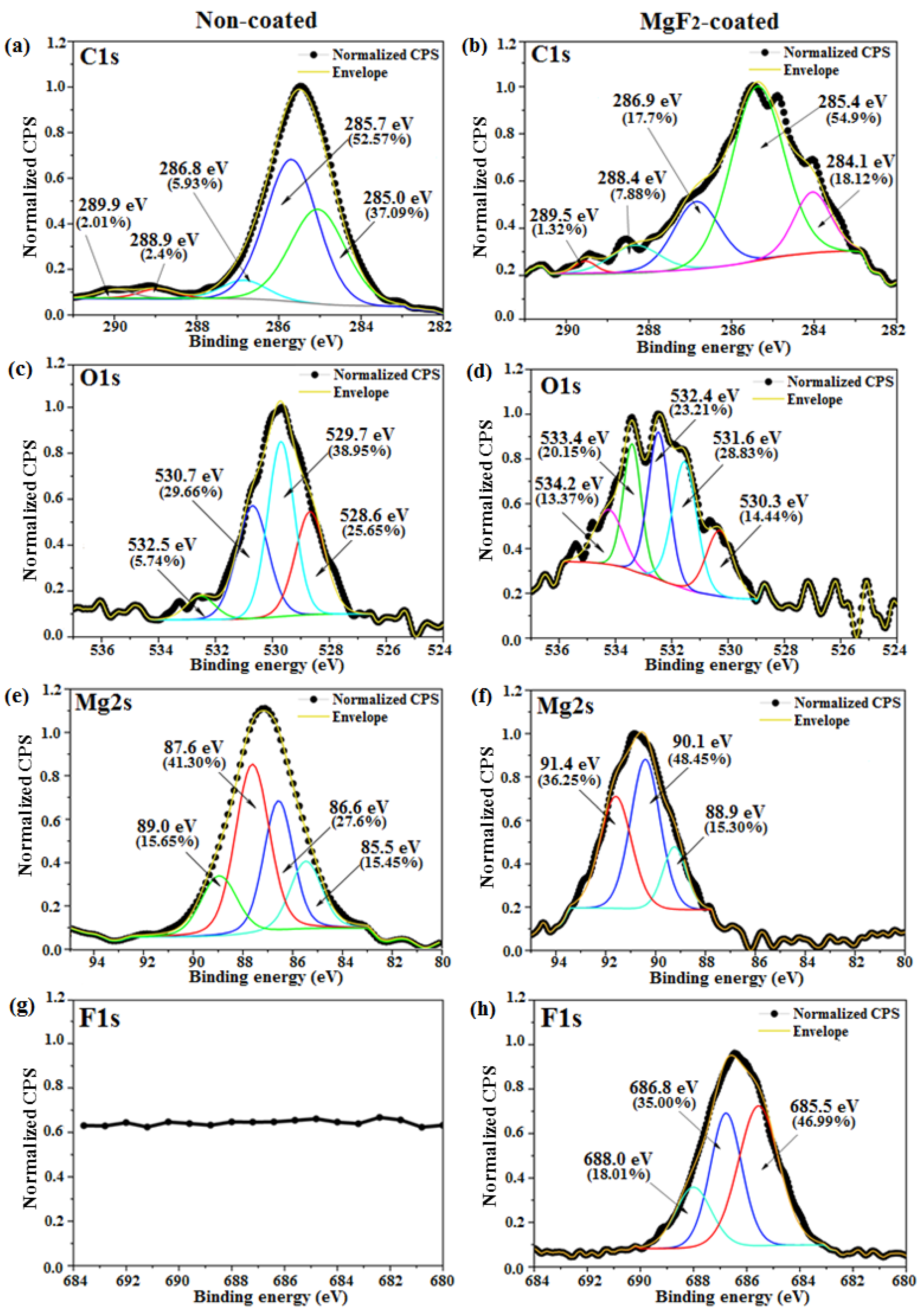
3.1. Coating Morphology and Chemical Composition
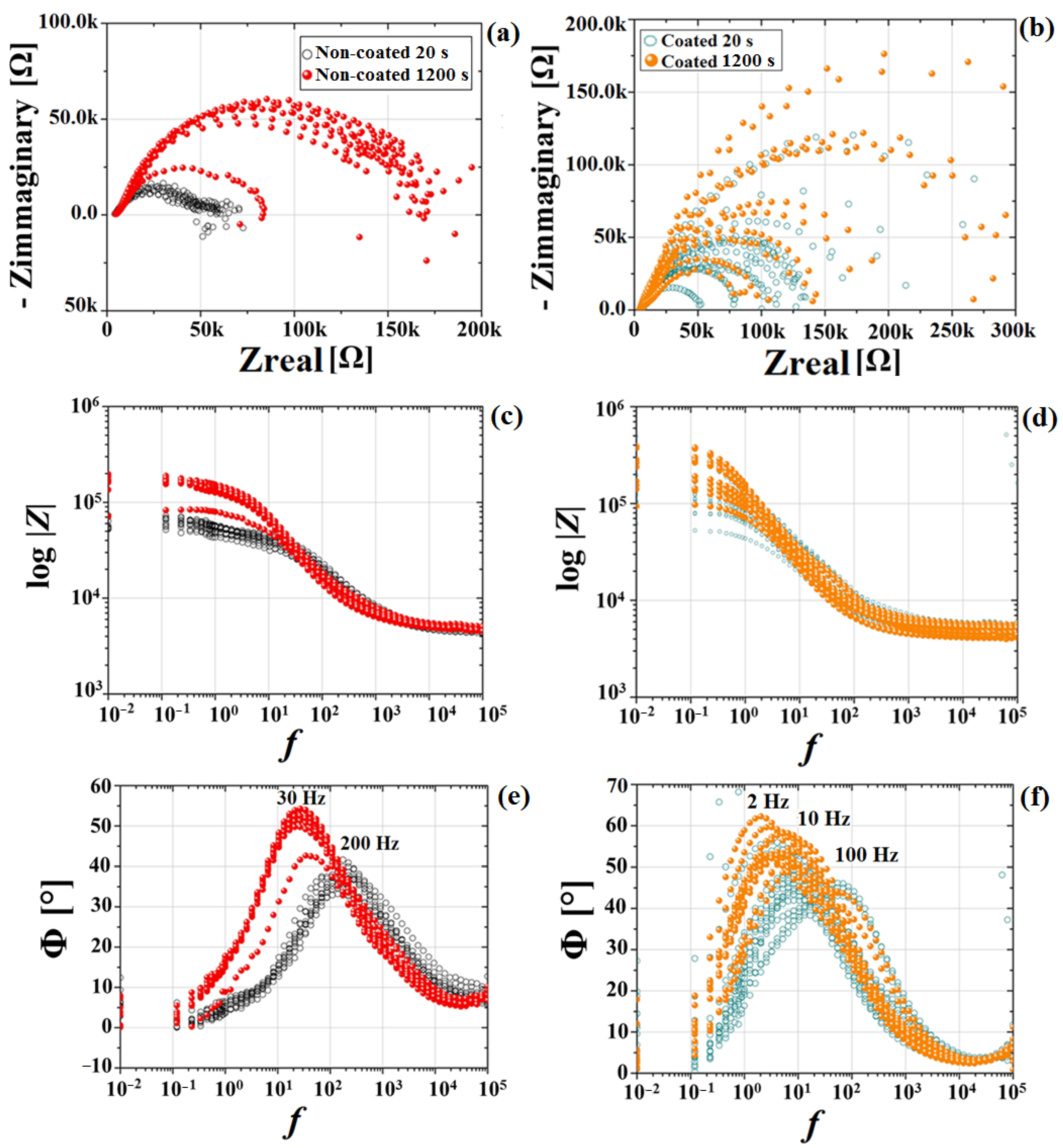
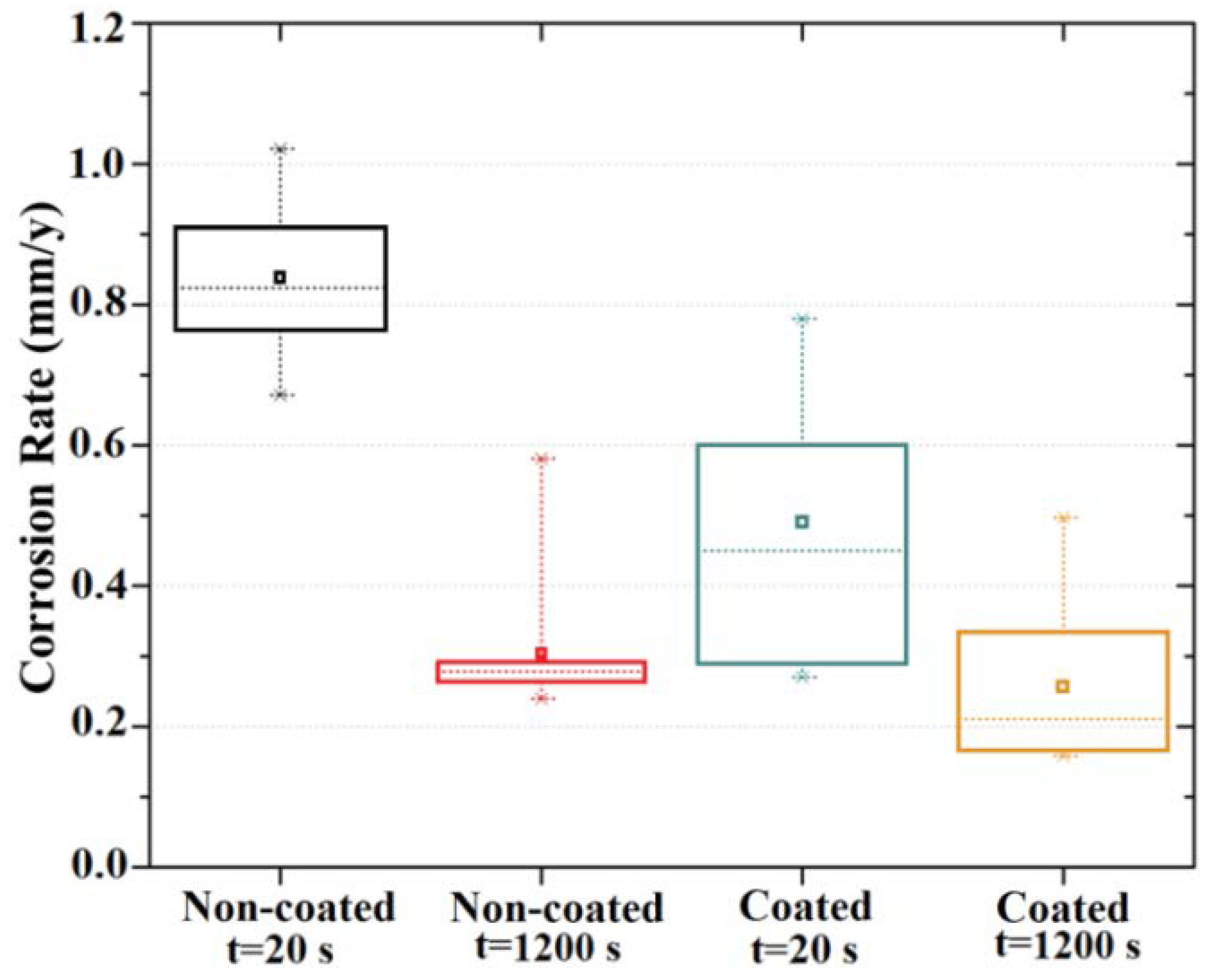
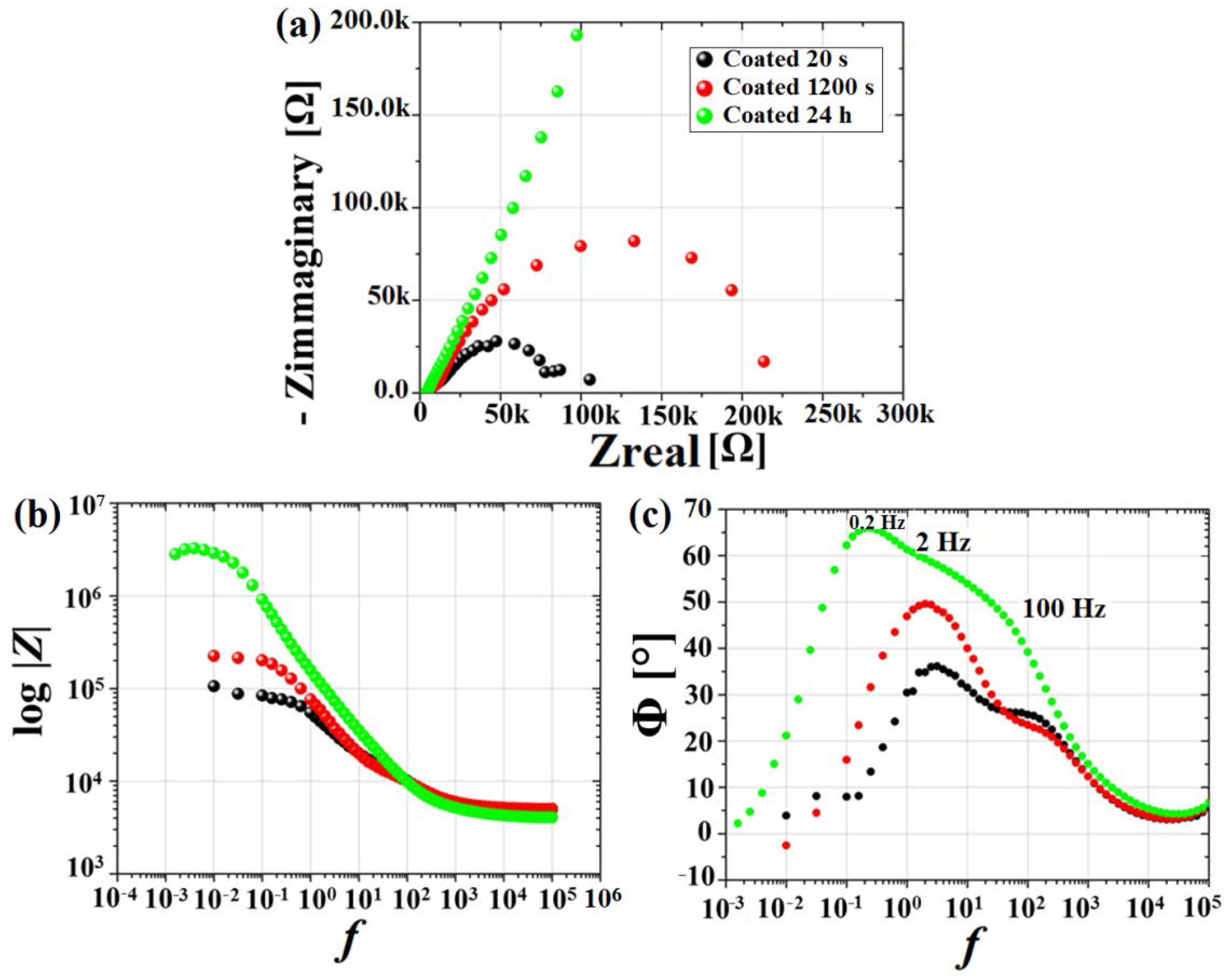
3.2. Electrochemical Results
4. Conclusions
- (1)
- A successful formation of a mixed MgF2/MgO coating, about 2 µm thick, after immersion in HF, was obtained.
- (2)
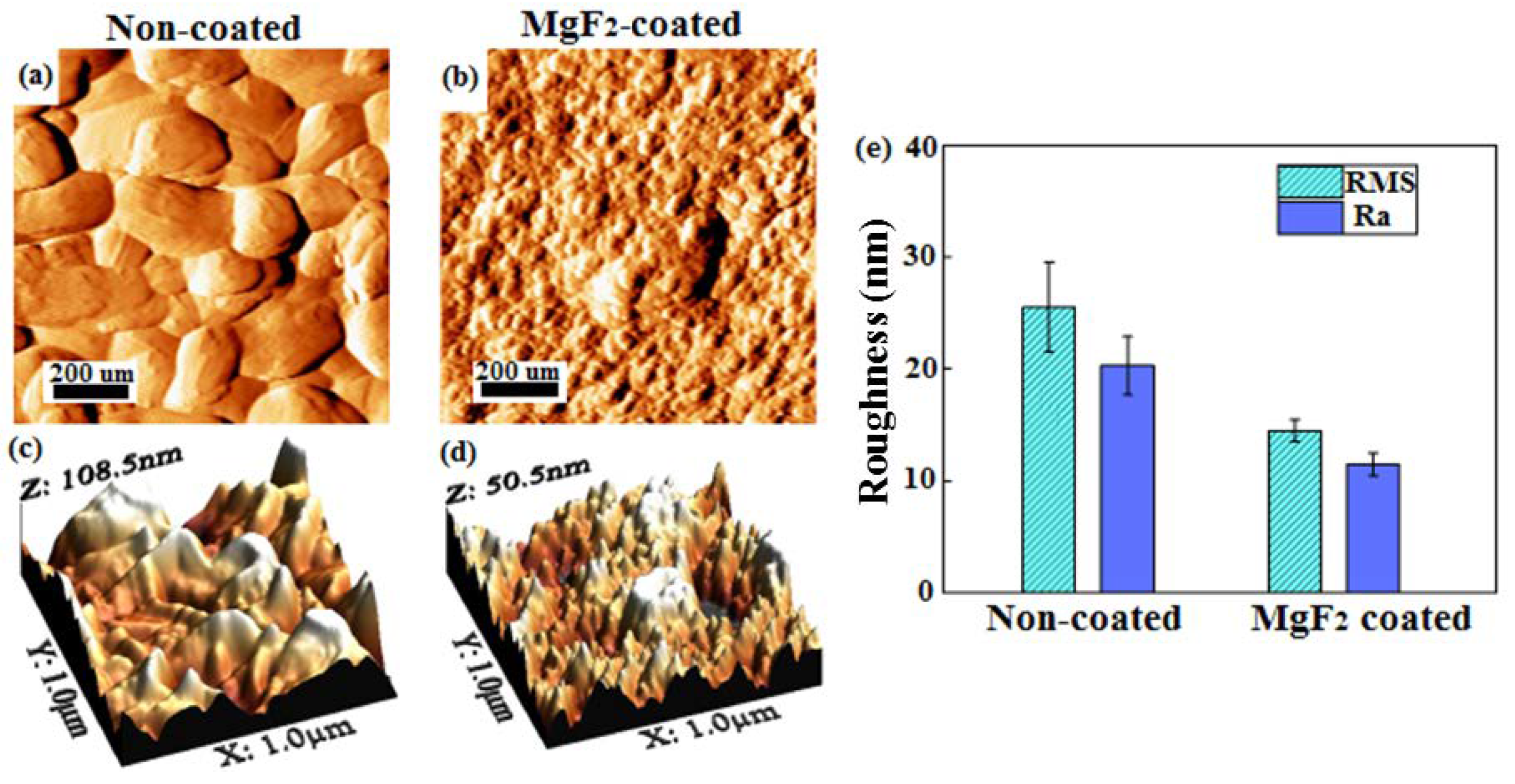
- The topography of the coated screws, being smoother than that of the non-coated screws, showed homogenous features, exhibiting the formation of grooves with a more similar dimension and were evenly distributed.
- (3)
- The presence of both metal-bound-F and metal-bound-O at the uppermost nm of the coated samples surfaces were detected.
- (4)
- The MgF2 coating demonstrated an efficient role in retarding alloy degradation during the initial stages of exposure to the chloride-containing physiological solution up to 24 h, with the corrosion rate determined using EIS spectra decreasing from 0.49 mm/y to 0.01 mm/y.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Francolini, I.; Hall-Stoodley, L.; Stoodley, P. Biofilms, Biomaterials, and Device-Related Infections. In Biomaterials Science, 4th ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 271–287. ISBN 978-0-12-816137-1. [Google Scholar] [CrossRef]
- Hu, R.-G.; Zhang, S.; Bu, J.-F.; Lin, C.-J.; Song, G.-L. Recent progress in corrosion protection of magnesium alloys by organic coatings. Prog. Org. Coat. 2012, 73, 129–141. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef]
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef]
- Hartwig, A. Role of magnesium in genomic stability. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2001, 475, 113–121. [Google Scholar] [CrossRef]
- Trumbo, P.; Schlicker, S.; Yates, A.; Poos, M. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Xu, Y.-L.; Wang, L.; Huang, M.; Gensch, F.; Kainer, K.U.; Hort, N. The Effect of Solid Solute and Precipitate Phase on Young’s Modulus of Binary Mg-RE Alloys. Adv. Eng. Mater. 2018, 20, 1800271. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Ruan, Y.C.; Yu, M.K.; O’Laughlin, M.; Wise, H.; Chen, D.; Tian, L.; Shi, D.; Wang, J.; et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 2016, 22, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-C.; Chaya, A.; Liu, K.; Verdelis, K.; Sfeir, C. The role of magnesium ions in bone regeneration involves the canonical Wnt signaling pathway. Acta Biomater. 2019, 98, 246–255. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, H.; Sun, J. Long-term in vivo evolution of high-purity Mg screw degradation—Local and systemic effects of Mg degradation products. Acta Biomater. 2018, 71, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Kannan, M.B.; Raman, R.S. In vitro degradation and mechanical integrity of calcium-containing magnesium alloys in modified-simulated body fluid. Biomaterials 2008, 29, 2306–2314. [Google Scholar] [CrossRef]
- Jafari, S.; Raman, R.S.; Davies, C.H. Corrosion fatigue of a magnesium alloy in modified simulated body fluid. Eng. Fract. Mech. 2015, 137, 2–11. [Google Scholar] [CrossRef]
- Gu, X.; Zhou, W.; Zheng, Y.; Cheng, Y.; Wei, S.; Zhong, S.; Xi, T.; Chen, L. Corrosion fatigue behaviors of two biomedical Mg alloys—AZ91D and WE43—In simulated body fluid. Acta Biomater. 2010, 6, 4605–4613. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, L.; Raman, R.S. Magnesium alloys as body implants: Fracture mechanism under dynamic and static loadings in a physiological environment. Acta Biomater. 2012, 8, 916–923. [Google Scholar] [CrossRef]
- Pichler, K.; Fischerauer, S.; Ferlic, P.; Martinelli, E.; Brezinsek, H.-P.; Uggowitzer, P.J.; Löffler, J.F.; Weinberg, A.-M. Immunological Response to Biodegradable Magnesium Implants. JOM 2014, 66, 573–579. [Google Scholar] [CrossRef]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Bamberger, M.; Dehm, G. Trends in the Development of New Mg Alloys. Annu. Rev. Mater. Sci. 2008, 38, 505–533. [Google Scholar] [CrossRef]
- Deng, M.; Wang, L.; Höche, D.; Lamaka, S.V.; Wang, C.; Snihirova, D.; Jin, Y.; Zhang, Y.; Zheludkevich, M.L. Approaching “stainless magnesium” by Ca micro-alloying. Mater. Horiz. 2020, 8, 589–596. [Google Scholar] [CrossRef]
- Jana, A.; Das, M.; Balla, V.K. In vitro and in vivo degradation assessment and preventive measures of biodegradable Mg alloys for biomedical applications. J. Biomed. Mater. Res. Part A 2021, 110, 462–487. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Peng, F.; Zhang, D.; Li, M.; Huang, J.; Liu, X. A tightly bonded reduced graphene oxide coating on magnesium alloy with photothermal effect for tumor therapy. J. Magnes. Alloys 2021. [Google Scholar] [CrossRef]
- Geng, F.; Tan, L.L.; Jin, X.X.; Yang, J.Y.; Yang, K. The preparation, cytocompatibility, and in vitro biodegradation study of pure β-TCP on magnesium. J. Mater. Sci. Mater. Med. 2009, 20, 1149–1157. [Google Scholar] [CrossRef]
- Xin, Y.; Jiang, J.; Huo, K.; Tang, G.; Tian, X.; Chu, P.K. Corrosion resistance and cytocompatibility of biodegradable surgical magnesium alloy coated with hydrogenated amorphous silicon. J. Biomed. Mater. Res. Part A 2008, 89A, 717–726. [Google Scholar] [CrossRef]
- Chowdhury, M.A.; Hossain, N.; Shahid, A.; Alam, J.; Hossain, S.M.; Uddin, I.; Rana, M. Development of SiC–TiO2-Graphene neem extracted antimicrobial nano membrane for enhancement of multiphysical properties and future prospect in dental implant applications. Heliyon 2022, 8, e10603. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.A.; Hossain, H.; Hossain, N.; Hossen, Z.; Kowser, A.; Rana, M. Advances in Coatings on Mg Alloys and Their Anti-Microbial Activity for Implant Applications. Arab. J. Chem. 2022, 15, 104214. [Google Scholar] [CrossRef]
- Rahman, M.; Chowdhury, M.A.; Mia, S.; Ali, R.; Rahman, A.; Ali, O.; Mahmud, S. Fabrication and characterization of hybrid coating on Mg–Zn–Ca Mg alloy for enhanced corrosion and degradation resistance as medical implant. Ceram. Int. 2022, 48, 23314–23324. [Google Scholar] [CrossRef]
- Song, Y.; Shan, D.; Han, E. Electrodeposition of hydroxyapatite coating on AZ91D magnesium alloy for biomaterial application. Mater. Lett. 2008, 62, 3276–3279. [Google Scholar] [CrossRef]
- Xu, L.; Pan, F.; Yu, G.; Yang, L.; Zhang, E.; Yang, K. In vitro and in vivo evaluation of the surface bioactivity of a calcium phosphate coated magnesium alloy. Biomaterials 2009, 30, 1512–1523. [Google Scholar] [CrossRef]
- Chiu, K.Y.; Wong, M.; Cheng, F.; Man, H. Characterization and corrosion studies of fluoride conversion coating on degradable Mg implants. Surf. Coat. Technol. 2007, 202, 590–598. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Zhang, S.; Wang, Z. Comparison of calcium phosphate coatings on AZ31 and fluoride-treated AZ31 alloy prepared by hydrothermal method and their electrochemical corrosion behaviour. Mater. Chem. Phys. 2018, 220, 395–401. [Google Scholar] [CrossRef]
- Whitford, G. Intake and Metabolism of Fluoride. Adv. Dent. Res. 1994, 8, 5–14. [Google Scholar] [CrossRef]
- Drynda, A.; Hassel, T.; Hoehn, R.; Perz, A.; Peuster, M.; Bach, F.-W. Development and biocompatibility of a novel corrodible fluoride-coated magnesium-calcium alloy with improved degradation kinetics and adequate mechanical properties for cardiovascular applications. J. Biomed. Mater. Res. Part A 2009, 93A, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.M.; Pérez-Maceda, B.T.; Carboneras, M.; Onofre-Bustamante, E.; García-Alonso, M.C.; Escudero, M.L. Response of MC3T3-E1 osteoblasts, L929 fibroblasts, and J774 macrophages to fluoride surface-modified AZ31 magnesium alloy. J. Biomed. Mater. Res. Part A 2013, 101, 2753–2762. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Tan, L.; Xiong, D.; Liu, X.; Zhang, B.; Yang, K. Fluoride treatment and in vitro corrosion behavior of an AZ31B magnesium alloy. Mater. Sci. Eng. C 2010, 30, 740–748. [Google Scholar] [CrossRef]
- Casanova, P.Y.; Jaimes, K.J.; Parada, N.J.; A Hernández-Barrios, C.; Aparicio, M.; Viejo, F.; Coy, A.E. Synthesis and evaluation of MgF2 coatings by chemical conversion on magnesium alloys for producing biodegradable orthopedic implants of temporary use. J. Phys. Conf. Ser. 2013, 466, 012003. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Idris, M.H.; Kadir, M.R.A.; Daroonparvar, M. Effect of fluoride treatment on corrosion behavior of Mg–Ca binary alloy for implant application. Trans. Nonferrous Met. Soc. China 2013, 23, 699–710. [Google Scholar] [CrossRef]
- Witte, F.; Fischer, J.; Nellesen, J.; Vogt, C.; Vogt, J.; Donath, T.; Beckmann, F. In vivo corrosion and corrosion protection of magnesium alloy LAE442. Acta Biomater. 2010, 6, 1792–1799. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, J.; Chen, M.; Liu, D. Biological activity evaluation of magnesium fluoride coated Mg-Zn-Zr alloy in vivo. Mater. Sci. Eng. C 2017, 75, 1068–1074. [Google Scholar] [CrossRef]
- Wang, P.; Liu, J.; Shen, S.; Li, Q.; Luo, X.; Xiong, P.; Gao, S.; Yan, J.; Cheng, Y.; Xi, T. In Vitro and In Vivo Studies on Two-Step Alkali-Fluoride-Treated Mg–Zn–Y–Nd Alloy for Vascular Stent Application: Enhancement in Corrosion Resistance and Biocompatibility. ACS Biomater. Sci. Eng. 2019, 5, 3279–3292. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Dai, C.Y.; Jia, Q.; Zhai, C.; Shi, H.; Yang, Y.; Zhao, B.C.; Cai, H.; Lee, E.-S.; Jiang, H.B. In Vivo Corrosion Behavior of Biodegradable Magnesium Alloy by MAF Treatment. Scanning 2021, 2021, 5530788. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Sun, Y.; Chen, Y.; Zan, R.; Peng, H.; Yang, S.; Kang, X.; Peng, Z.; Wang, W.; Zhang, X. Effects of MgF2 coating on the biodegradation and biological properties of magnesium. Surf. Coat. Technol. 2021, 422, 127552. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, W.; Wang, D.; Li, B.; Zhou, J.; Zhang, D.; Liu, L.; Peng, F.; Liu, X.; Zhang, Y. An in vitro and in vivo comparison of Mg(OH)2-, MgF2- and HA-coated Mg in degradation and osteointegration. Biomater. Sci. 2020, 8, 3320–3333. [Google Scholar] [CrossRef]
- Amberg, R.; Elad, A.; Rothamel, D.; Fienitz, T.; Szakacs, G.; Heilmann, S.; Witte, F. Design of a migration assay for human gingival fibroblasts on biodegradable magnesium surfaces. Acta Biomater. 2018, 79, 158–167. [Google Scholar] [CrossRef]
- Kačarević, P.; Rider, P.; Elad, A.; Tadic, D.; Rothamel, D.; Sauer, G.; Bornert, F.; Windisch, P.; Hangyási, D.B.; Molnar, B.; et al. Biodegradable magnesium fixation screw for barrier membranes used in guided bone regeneration. Bioact. Mater. 2021, 14, 15–30. [Google Scholar] [CrossRef]
- Liu, J.; Kerns, D.G. Mechanisms of Guided Bone Regeneration: A Review. Open Dent. J. 2014, 8, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Tadic, D.; Bielenstein, O. Implant for Covering Maxillary Bone Defects in the Jaw Region and Method for Producing the Same. U.S. Patent US20180036127, 8 February 2018. [Google Scholar]
- Müller, W.D.; Nascimento, M.L.; Zeddies, M.; Córsico, M.; Gassa, L.M.; Mele, M.A.F.L.D. Corrosion Studies Using Potentiodynamic and EIS Electrochemical Techniques. Mater. Res. 2007, 10, 5–10. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Stern, M., Geary, A.L., Eds.; Wiley: New York, NY, USA, 2001; Volume 104, pp. 56–63. [Google Scholar]
- King, A.; Birbilis, N.; Scully, J. Accurate Electrochemical Measurement of Magnesium Corrosion Rates; a Combined Impedance, Mass-Loss and Hydrogen Collection Study. Electrochim. Acta 2014, 121, 394–406. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Mohan, R.; Harshavardhana, N.; Chaudhari, M.; Jeyanthi, S.; Abimannan, G. Analysis on surface finish and chip morphology during dry turning process. Mater. Today Proc. 2021, 46, 999–1002. [Google Scholar] [CrossRef]
- Han, Y.; Liu, M.; Li, K.; Zuo, Y.; Wei, Y.; Xu, S.; Zhang, G.; Song, C.; Zhang, Z.; Guo, X. Facile synthesis of morphology and size-controlled zirconium metal–organic framework UiO-66: The role of hydrofluoric acid in crystallization. CrystEngComm 2015, 17, 6434–6440. [Google Scholar] [CrossRef]
- Aljarrah, M.; Alnahas, J.; Alhartomi, M. Thermodynamic Modeling and Mechanical Properties of Mg-Zn-{Y, Ce} Alloys: Review. Crystals 2021, 11, 1592. [Google Scholar] [CrossRef]
- Makkar, P.; Kang, H.J.; Padalhin, A.R.; Park, I.; Moon, B.-G.; Lee, B.T. Development and properties of duplex MgF2/PCL coatings on biodegradable magnesium alloy for biomedical applications. PLoS ONE 2018, 13, e0193927. [Google Scholar] [CrossRef]
- Wan, H.; Hu, X. One-step solve-thermal process for the construction of anticorrosion bionic superhydrophobic surfaces on magnesium alloy. Mater. Lett. 2016, 174, 209–212. [Google Scholar] [CrossRef]
- Yang, C.-W.; Liu, C.; Lin, D.-J.; Yeh, M.-L.; Lee, T.-M. Hydrothermal treatment and butylphosphonic acid derived self-assembled monolayers for improving the surface chemistry and corrosion resistance of AZ61 magnesium alloy. Sci. Rep. 2017, 7, 16910. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, G.; Peng, X.; Li, L.; Feng, H.; Gao, B.; Huo, K.; Chu, P.K. Mitigation of Corrosion on Magnesium Alloy by Predesigned Surface Corrosion. Sci. Rep. 2015, 5, 17399. [Google Scholar] [CrossRef] [PubMed]
- Palchan, I.; Crespin, M.; Estrade-Szwarckopf, H.; Rousseau, B. Graphite fluorides: An XPS study of a new type of C-F bonding. Chem. Phys. Lett. 1989, 157, 321–327. [Google Scholar] [CrossRef]
- He, Z.X.; Pong, W. X-ray photoelectron spectra of MgH2. Phys. Scr. 1990, 41, 930. [Google Scholar] [CrossRef]
- Le Febvrier, A.; Jensen, J.; Eklund, P. Wet-cleaning of MgO(001): Modification of surface chemistry and effects on thin film growth investigated by x-ray photoelectron spectroscopy and time-of-flight secondary ion mass spectroscopy. J. Vac. Sci. Technol. A Vac. Surf. Films 2017, 35, 021407. [Google Scholar] [CrossRef]
- Murch, G.; Thorn, R. Relation between orbital binding energies and ionicities in alkali and alkaline earth flourides. J. Phys. Chem. Solids 1980, 41, 785–791. [Google Scholar] [CrossRef]
- Kouisni, L.; Azzi, M.; Dalard, F.; Maximovitch, S. Phosphate coatings on magnesium alloy AM60: Part 2: Electrochemical behaviour in borate buffer solution. Surf. Coat. Technol. 2005, 192, 239–246. [Google Scholar] [CrossRef]
- Guadarrama-Muñoz, F.; Mendoza-Flores, J.; Duran-Romero, R.; Genesca, J. Electrochemical study on magnesium anodes in NaCl and CaSO4–Mg(OH)2 aqueous solutions. Electrochim. Acta 2006, 51, 1820–1830. [Google Scholar] [CrossRef]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef]
- Jamesh, M.; Kumar, S.; Narayanan, T.S. Corrosion behavior of commercially pure Mg and ZM21 Mg alloy in Ringer’s solution—Long term evaluation by EIS. Corros. Sci. 2011, 53, 645–654. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, R.; Peng, C.; Cai, Z. Microstructure and corrosion behavior of as-extruded Mg-xLi-3Al-2Zn-0.2Zr alloys (x = 5, 8, 11 wt.%). Corros. Sci. 2020, 167, 108487. [Google Scholar] [CrossRef]
- Hou, R.; Victoria-Hernandez, J.; Jiang, P.; Willumeit-Romer, R.; Feyerabend, B.L.; Yi, S.; Letzig, D.; Feyerabend, F. In vitro evaluation of the ZX11 magnesium alloy as potential bone plate. Acta Biomater. 2019, 97, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Snihirova, D.; Deng, M.; Wang, C.; Vaghefinazari, B.; Wiese, G.; Langridge, M.; Höche, D.; Lamaka, S.V.; Zheludkevich, M.L. Insight into physical interpretation of high frequency time constant in electrochemical impedance spectra of Mg. Corros. Sci. 2021, 187, 109501. [Google Scholar] [CrossRef]
- Zaffora, A.; Franco, F.D.; Virtù, D.; Pavia, F.C.; Ghersi, G.; Virtanen, S.; Santamaria, M. Tuning of the Mg alloy AZ31 anodizing process for biodegradable implants. ACS Appl. Mater. Interfaces 2021, 13, 12866–12876. [Google Scholar] [CrossRef]



| Mg-Alloy | Sample Shape | Treatment | Outcomes | Applications | Ref. |
|---|---|---|---|---|---|
| Mg-3Zn-0.5Zr | Cylinders (Ø = 3 mm; h = 10 mm) | HF (20%); 6 h; 37 °C | Decreased corrosion rate; promotion of new bone formation | Orthopedic applications | 2017 [38] |
| Mg-2Zn-0.5Y-0.5Nd | Discs (Ø = 10 mm; h = 2 mm) | HF (40%); 24 h; room temperature | No subcutaneous gas cavities or significant inflammatory cell infiltration | Cardiovascular stents | 2019 [39] |
| AZ31 | Plates (5 mm × 7 mm × 2 mm) | Microfluoruration in saturated NH4HF2 solution at 190 V | Improved corrosion resistance; reduced H2(g) bubbles generation | Medical implant materials | 2021 [40] |
| Mg (99.98%) | Sheets (12 mm × 10 mm × 1 mm) | HF (40%); 24, 48, 96 h; room temperature | Decreased corrosion rate; great cytocompatibility; good attachment and growth of osteoblasts on the surface | Orthopedic applications | 2021 [41] |
| Mg (>99.99 wt.%) | Plates (10 mm × 1 mm × 2 mm) Rodes (Ø = 10 mm; h = 2 mm) | HF (40%); 72 h; room temperature | Beneficial for the osteogenic differentiation of MC3T3-E1 cells and the vascularization of human umbilical vein endothelial cells (HUVECs) | Orthopedic applications | 2020 [42] |
| Mg-2Y-1Mn-1Zn | Screws | HF (40%); room temperature | Decreased corrosion rate; reduced H2(g) bubbles generation | Orthopedic applications; Bone regeneration | This work |
| Y | Zn | Mn | Mg | |
|---|---|---|---|---|
| WZM211 | 2.0 | 1.0 | 1.0 | bal. |
| Non-Coated | Coated | Non-Coated | Coated | |
|---|---|---|---|---|
| Rp [Ω.cm2] | CR [mm/y] | |||
| 20 s | 359 ± 14 | 694 ± 85 | 0.84 ± 0.03 | 0.49 ± 0.06 |
| 1200 s | 1036 ± 198 | 1323 ± 144 | 0.30 ± 0.03 | 0.26 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambaro, S.; Nascimento, M.L.; Shekargoftar, M.; Ravanbakhsh, S.; Sales, V.; Paternoster, C.; Bartosch, M.; Witte, F.; Mantovani, D. Characterization of a Magnesium Fluoride Conversion Coating on Mg-2Y-1Mn-1Zn Screws for Biomedical Applications. Materials 2022, 15, 8245. https://doi.org/10.3390/ma15228245
Gambaro S, Nascimento ML, Shekargoftar M, Ravanbakhsh S, Sales V, Paternoster C, Bartosch M, Witte F, Mantovani D. Characterization of a Magnesium Fluoride Conversion Coating on Mg-2Y-1Mn-1Zn Screws for Biomedical Applications. Materials. 2022; 15(22):8245. https://doi.org/10.3390/ma15228245
Chicago/Turabian StyleGambaro, Sofia, M. Lucia Nascimento, Masoud Shekargoftar, Samira Ravanbakhsh, Vinicius Sales, Carlo Paternoster, Marco Bartosch, Frank Witte, and Diego Mantovani. 2022. "Characterization of a Magnesium Fluoride Conversion Coating on Mg-2Y-1Mn-1Zn Screws for Biomedical Applications" Materials 15, no. 22: 8245. https://doi.org/10.3390/ma15228245
APA StyleGambaro, S., Nascimento, M. L., Shekargoftar, M., Ravanbakhsh, S., Sales, V., Paternoster, C., Bartosch, M., Witte, F., & Mantovani, D. (2022). Characterization of a Magnesium Fluoride Conversion Coating on Mg-2Y-1Mn-1Zn Screws for Biomedical Applications. Materials, 15(22), 8245. https://doi.org/10.3390/ma15228245







