Chestnut Shell-Activated Carbon Mixed with Pyrolytic Snail Shells for Methylene Blue Adsorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Reagents
2.2. Preparation of the Adsorbent and MB Solution
2.3. Materials Characterization
2.3.1. Dye Adsorptive Experiments
2.3.2. Equilibrium Isotherm Studies
2.3.3. Adsorption Kinetics Studies
2.3.4. Desorption Studies
3. Results and Discussions
3.1. Characterization of the Adsorbent
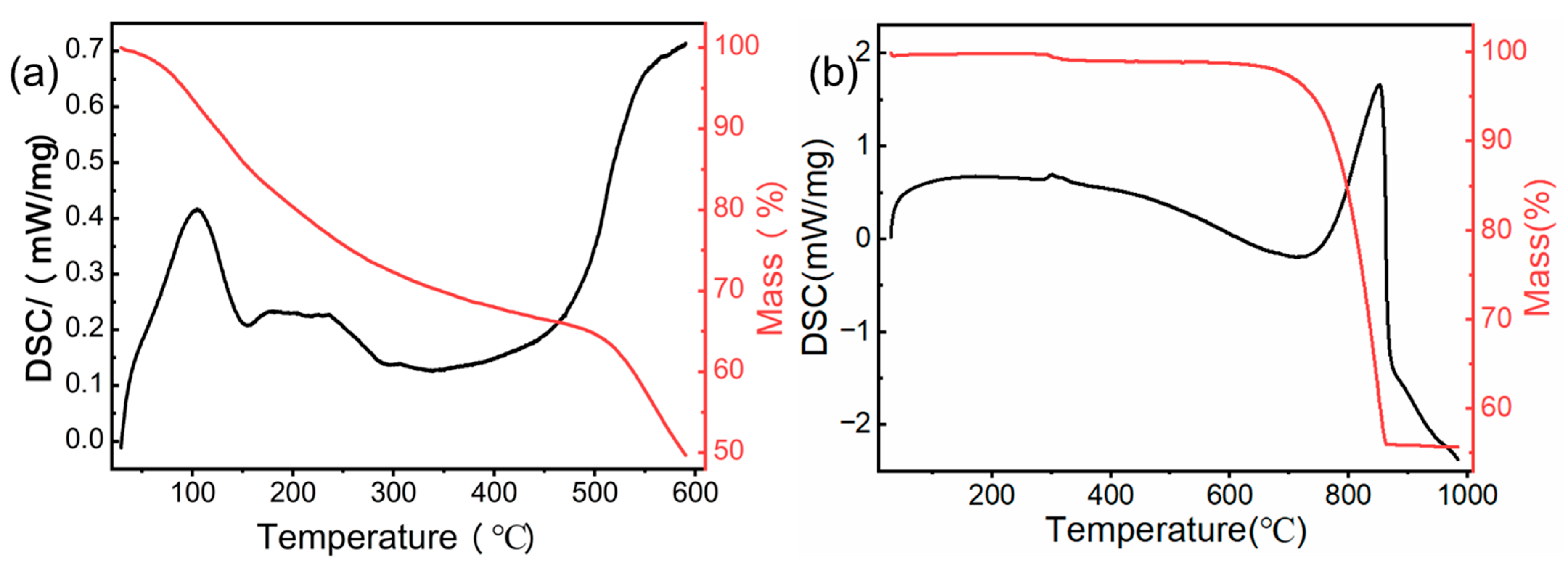
3.1.1. TGA-DSC Analysis
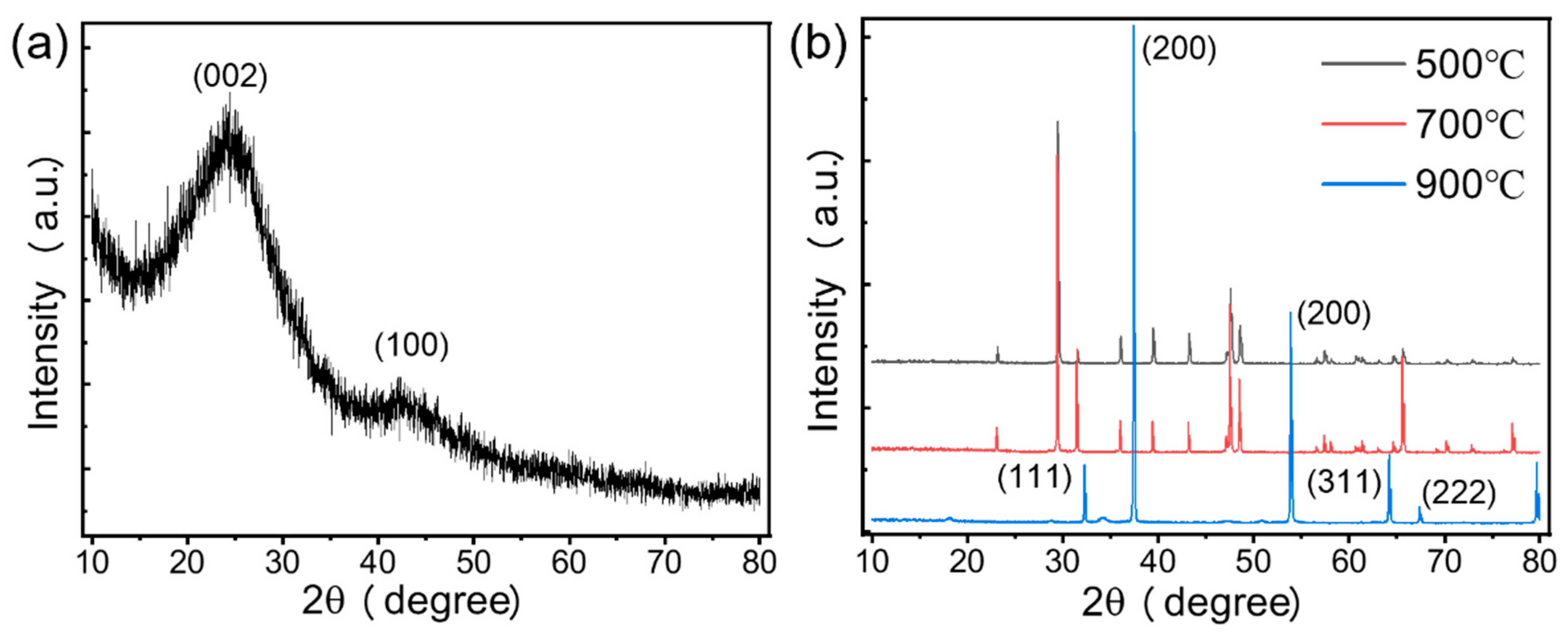
3.1.2. XRD Analysis
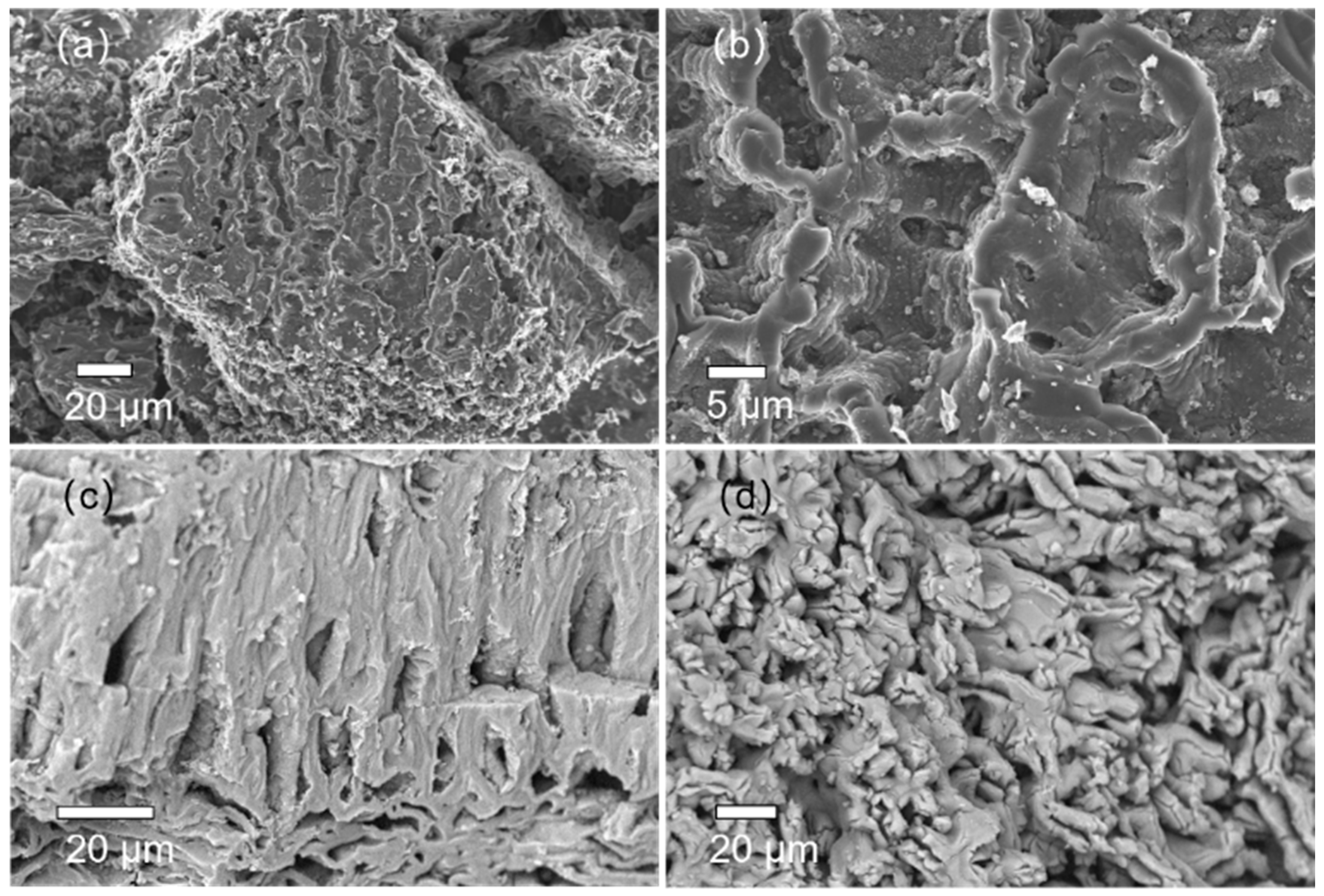
3.1.3. SEM-EDS Analysis
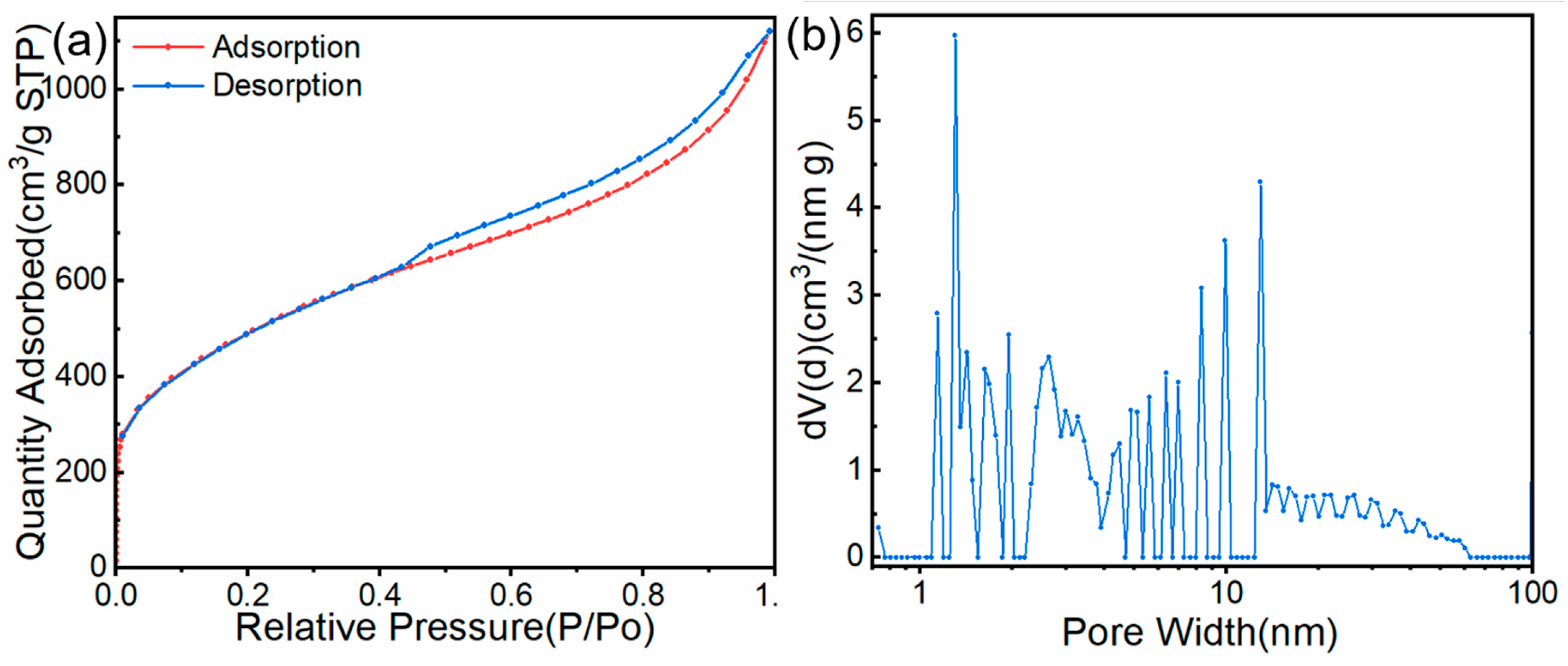
3.1.4. BET Analysis
3.1.5. FTIR Analysis
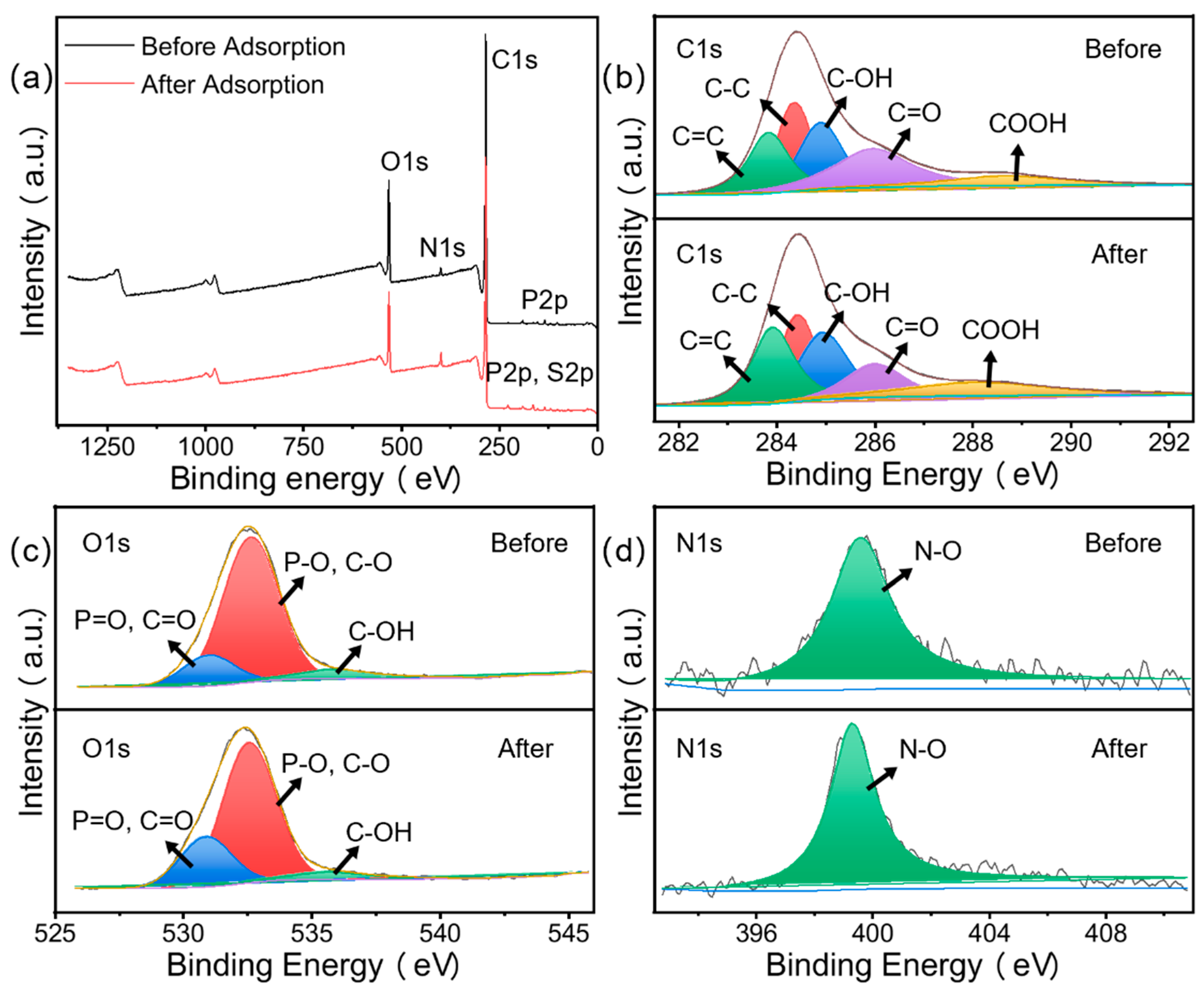
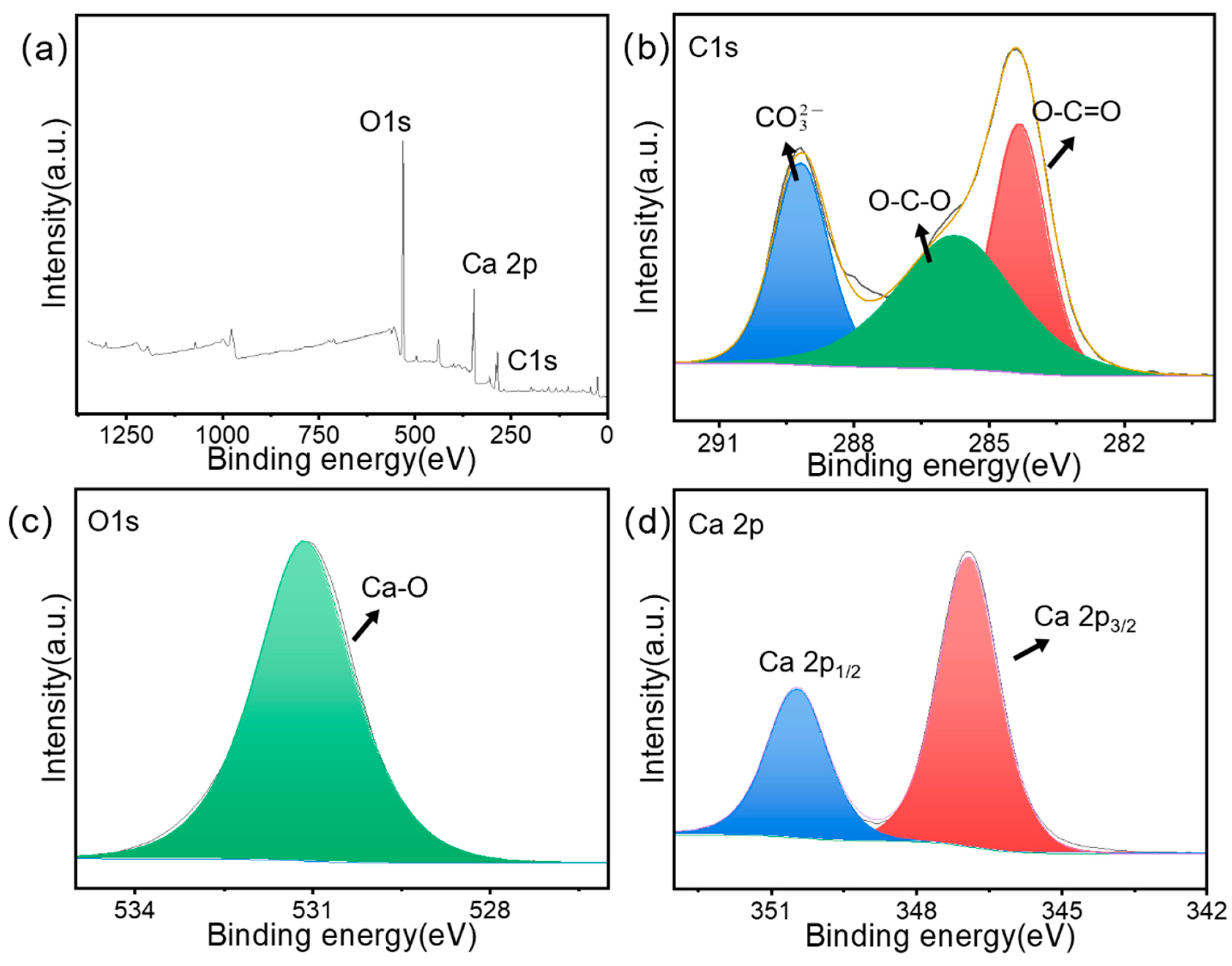
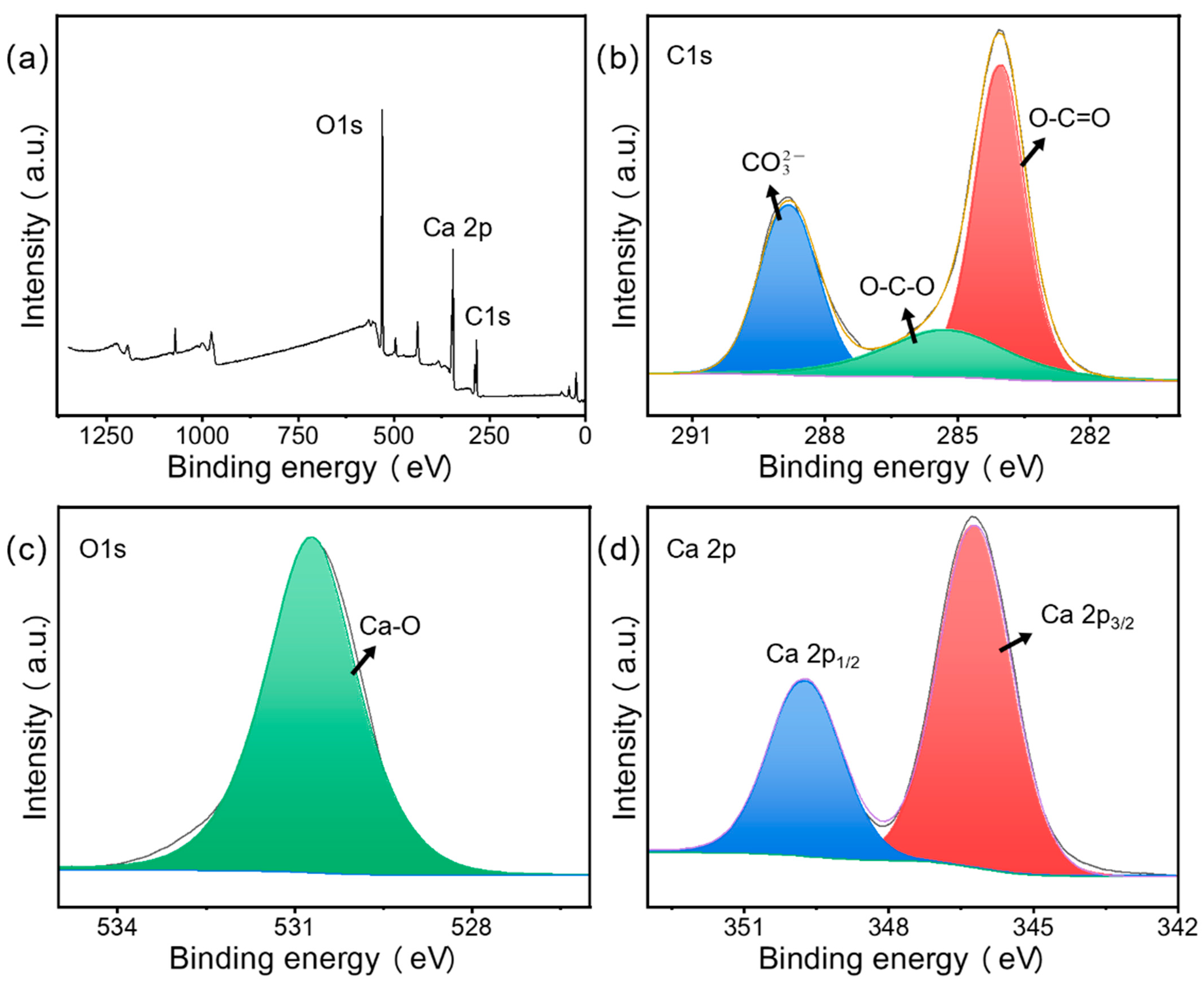
3.1.6. XPS Analysis
3.2. Adsorption Experiments
3.2.1. ZETA Potential and Effect of pH
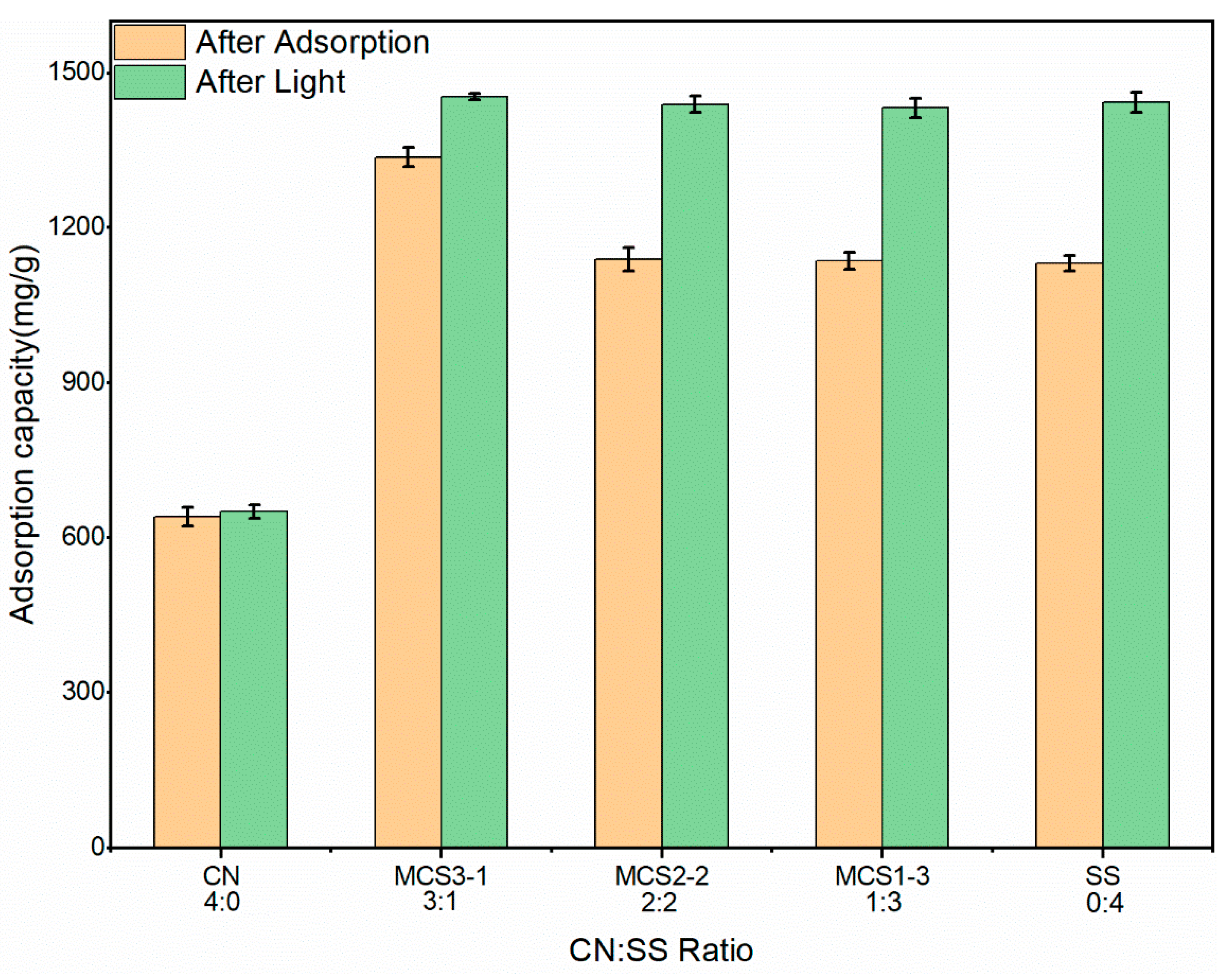
3.2.2. Effect of Adsorbent Ratio
3.2.3. Effect of Initial MB Concentration and Contact Time
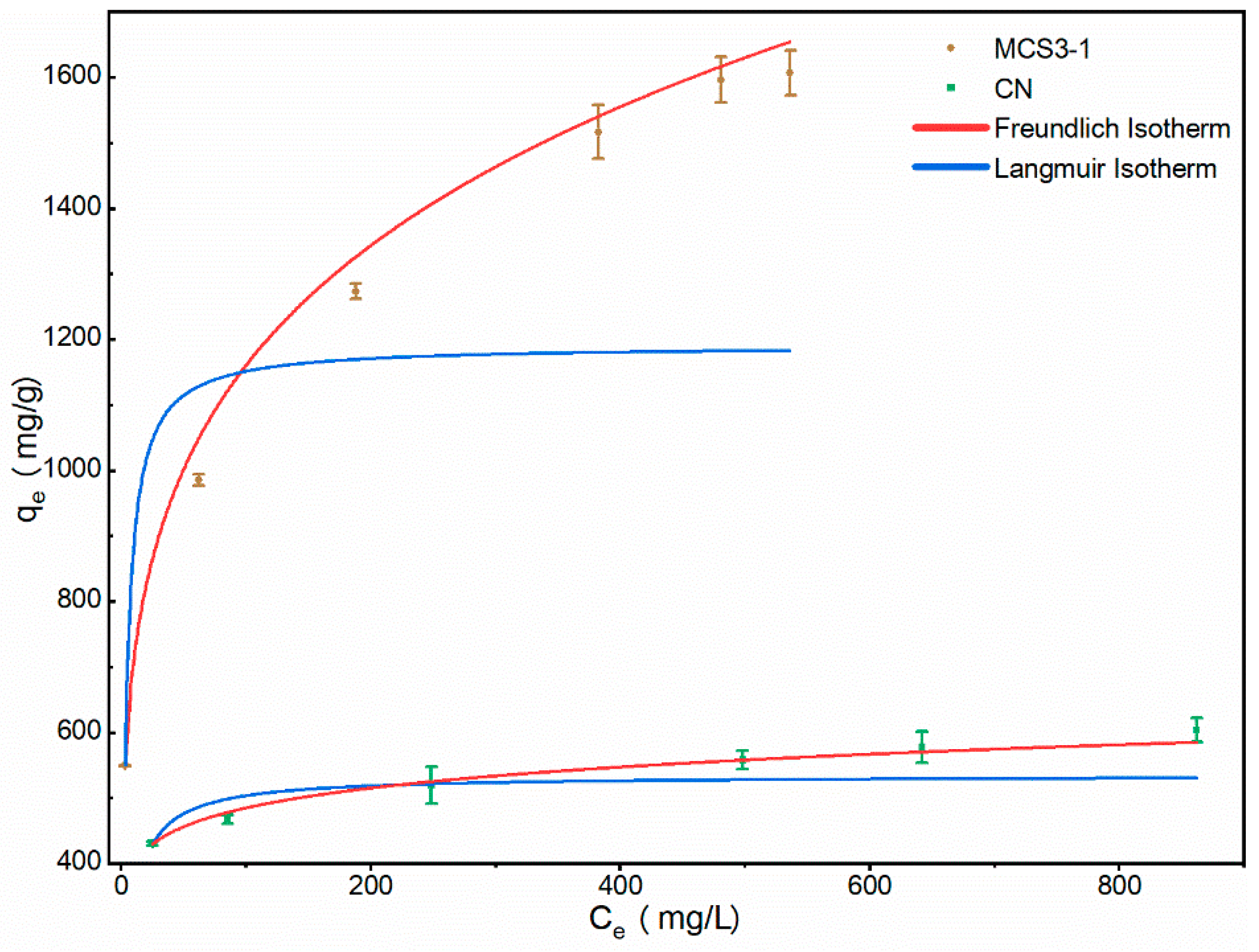
3.2.4. Adsorption Isotherm
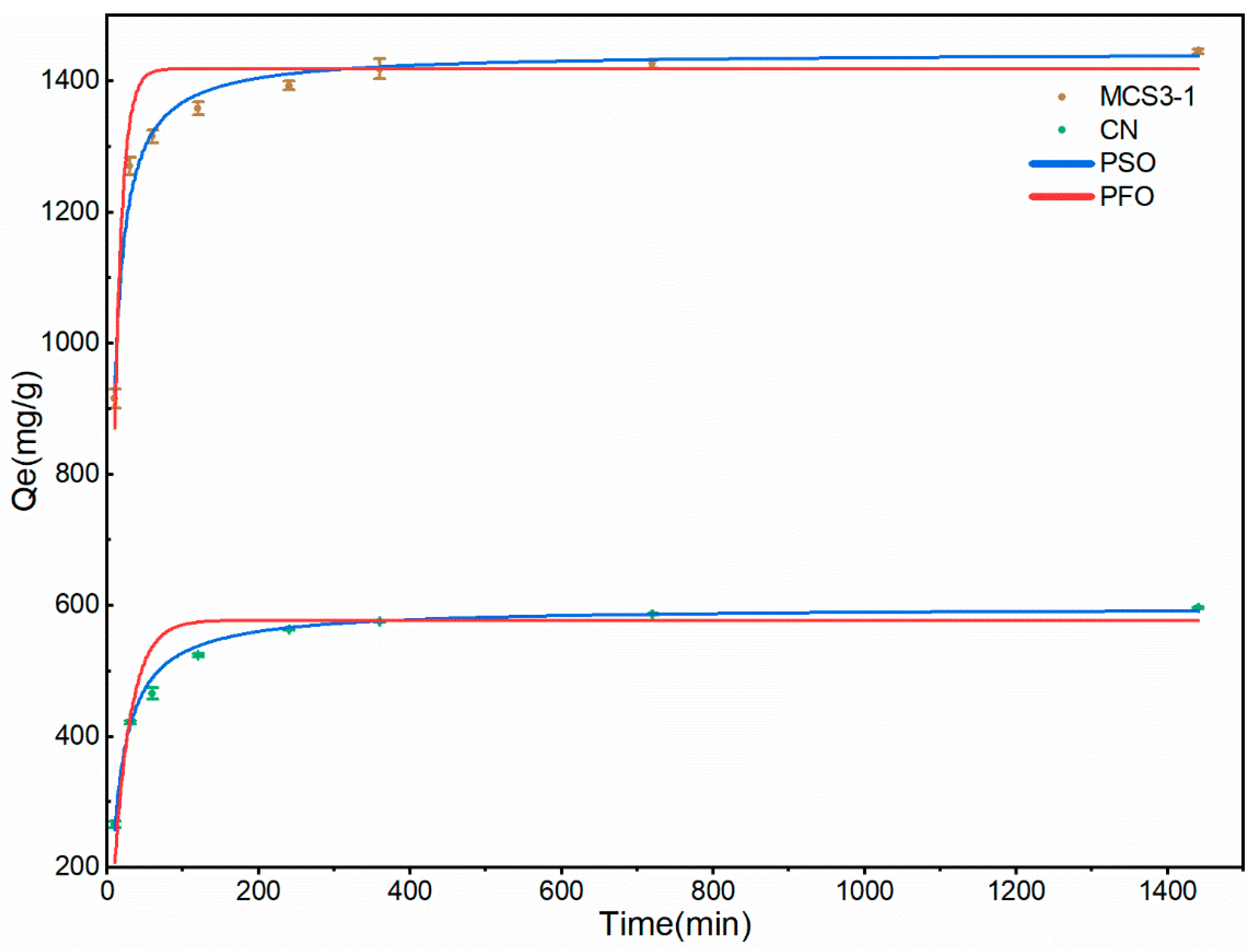
3.2.5. Adsorption Kinetics
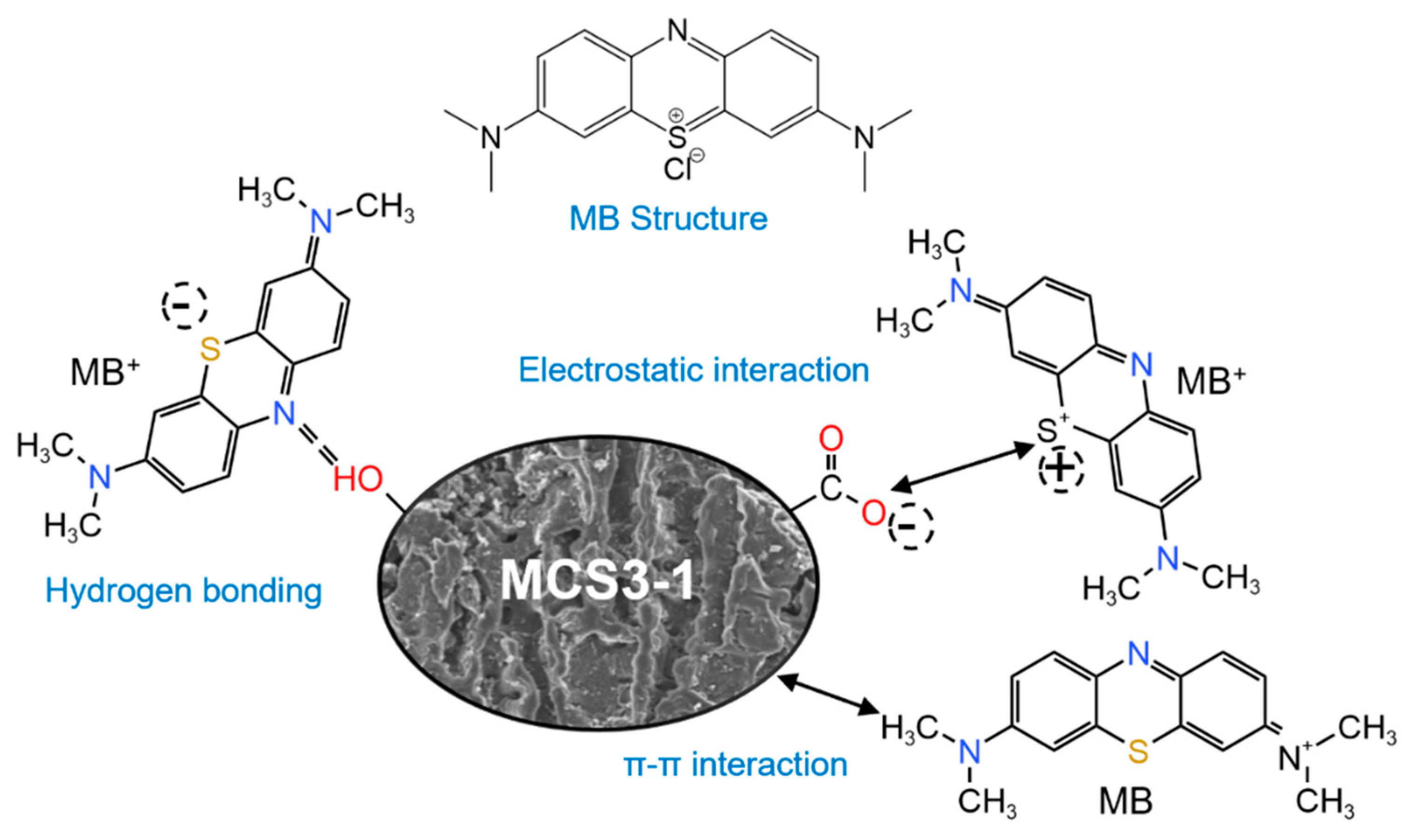
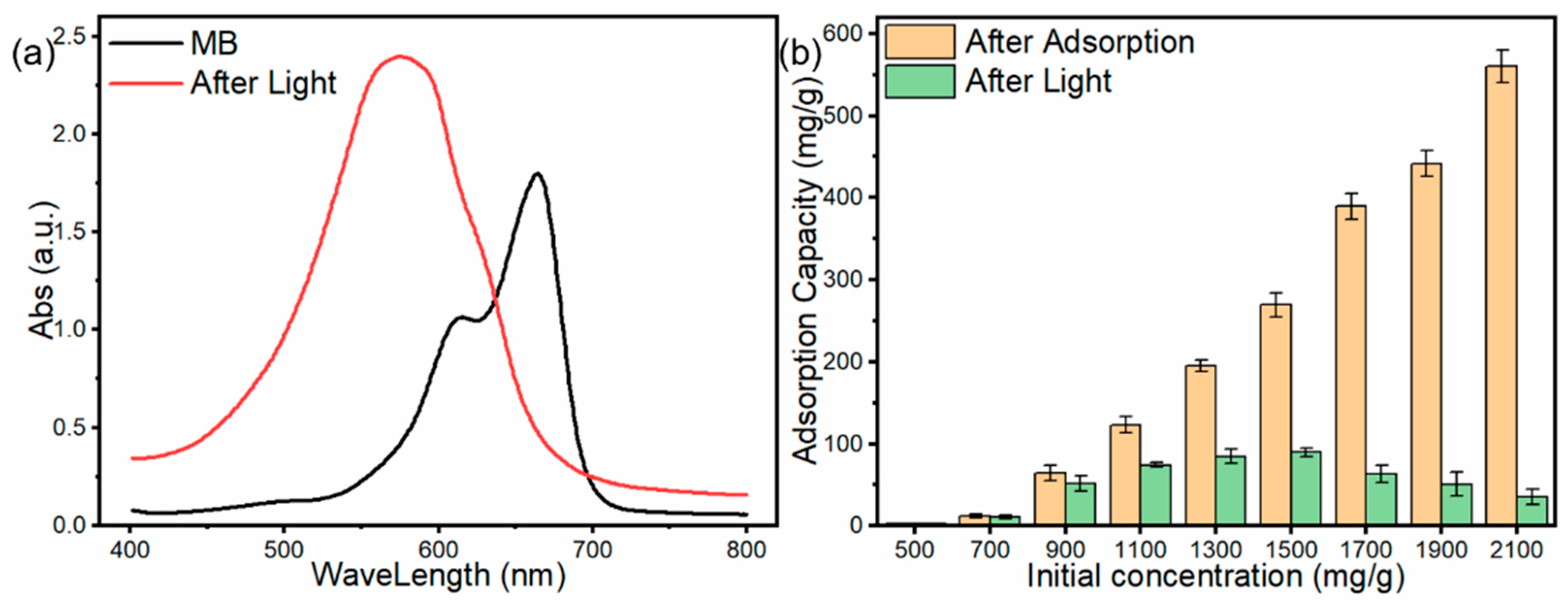
3.2.6. Mechanism of MB Adsorption and Photocatalysis
- MB + hv → MB * (absorption of photons and excitation of MB molecules);
- hv + CaO → e−(CB) + H *(VB) (electron–hole pair formation);
- e−(CB) + O2 → ·O2− (oxygen ion sorption);
- 2O2− + 2H2O → H2O2 + 2OH− + O2 (·O2− neutralization by protons);
- H2O2 + e−(CB) → -OH + ·OH (decomposition of H2O2 and ·OH formation);
- H+ + H2O → H+ + ·OH (water splitting by photo-hole to produce ·OH radicals);
- ·OH/·O2−+ ·MB → MB degradation (electrophilic attack on MB molecules).
3.2.7. Photocatalysis after Adsorption
3.2.8. Desorption Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gleick, P.H. The human right to water. Water Policy 1998, 1, 487–503. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Gupta, V.K. Rice Husk and Its Ash as Low-Cost Adsorbents in Water and Wastewater Treatment. Ind. Eng. Chem. Res. 2011, 50, 13589–13613. [Google Scholar] [CrossRef]
- Fadillah, G.; Saleh, T.A.; Wahyuningsih, S.; Ninda Karlina Putri, E.; Febrianastuti, S. Electrochemical removal of methylene blue using alginate-modified graphene adsorbents. Chem. Eng. J. 2019, 378, 122140. [Google Scholar] [CrossRef]
- Jabar, J.M.; Odusote, Y.A. Removal of cibacron blue 3G-A (CB) dye from aqueous solution using chemo-physically activated biochar from oil palm empty fruit bunch fiber. Arab. J. Chem. 2020, 13, 5417–5429. [Google Scholar] [CrossRef]
- Mu, Y.; Du, H.; He, W.; Ma, H. Functionalized mesoporous magnetic biochar for methylene blue removal: Performance assessment and mechanism exploration. Diam. Relat. Mater. 2022, 121, 108795. [Google Scholar] [CrossRef]
- Zhou, D.; Li, D.; Li, A.; Qi, M.; Cui, D.; Wang, H.; Wei, H. Activated carbons prepared via reflux-microwave-assisted activation approach with high adsorption capability for methylene blue. J. Environ. Chem. Eng. 2021, 9, 104671. [Google Scholar] [CrossRef]
- Jadhav, J.P.; Kalyani, D.C.; Telke, A.A.; Phugare, S.S.; Govindwar, S.P. Evaluation of the efficacy of a bacterial consortium for the removal of color, reduction of heavy metals, and toxicity from textile dye effluent. Bioresour. Technol. 2010, 101, 165–173. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Reghioua, A.; Yaseen, Z.M. Zwitterion composite chitosan-epichlorohydrin/zeolite for adsorption of methylene blue and reactive red 120 dyes. Int. J. Biol. Macromol. 2020, 163, 756–765. [Google Scholar] [CrossRef]
- Jawad, A.H.; Mohammed, I.A.; Abdulhameed, A.S. Tuning of Fly Ash Loading into Chitosan-Ethylene Glycol Diglycidyl Ether Composite for Enhanced Removal of Reactive Red 120 Dye: Optimization Using the Box–Behnken Design. J. Polym. Environ. 2020, 28, 2720–2733. [Google Scholar] [CrossRef]
- Karale-Unde, N.J.; Nikumbh, A.K.; Khanvilkar, M.B.; Nagawade, P.A.; Pawar, R.A.; Nighot, D.V.; Misal, S.B.; Gugale, G.S. Synthesis, structural and electrical conduction of some dual doped semiconductor oxides nanoparticles for photocatalytic degradation of Victoria blue-B and Brilliant yellow under solar light irradiation. J. Mater. Sci. Mater. Electron. 2021, 32, 4998–5034. [Google Scholar] [CrossRef]
- Tong, Y.; McNamara, P.J.; Mayer, B.K. Adsorption of organic micropollutants onto biochar: A review of relevant kinetics, mechanisms and equilibrium. Environ. Sci. Water Res. Technol. 2019, 5, 821–838. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, N.; Giri, B.S.; Chowdhary, P.; Chaturvedi, P. Removal of methylene blue dye using rice husk, cow dung and sludge biochar: Characterization, application, and kinetic studies. Bioresour. Technol. 2020, 306, 123202. [Google Scholar] [CrossRef] [PubMed]
- Konneh, M.; Wandera, S.M.; Murunga, S.I.; Raude, J.M. Adsorption and desorption of nutrients from abattoir wastewater: Modelling and comparison of rice, coconut and coffee husk biochar. Heliyon 2021, 7, e08458. [Google Scholar] [CrossRef] [PubMed]
- Medhat, A.; El-Maghrabi, H.H.; Abdelghany, A.; Abdel Menem, N.M.; Raynaud, P.; Moustafa, Y.M.; Elsayed, M.A.; Nada, A.A. Efficiently activated carbons from corn cob for methylene blue adsorption. Appl. Surf. Sci. Adv. 2021, 3, 100037. [Google Scholar] [CrossRef]
- Vigneshwaran, S.; Sirajudheen, P.; Nikitha, M.; Ramkumar, K.; Meenakshi, S. Facile synthesis of sulfur-doped chitosan/biochar derived from tapioca peel for the removal of organic dyes: Isotherm, kinetics and mechanisms. J. Mol. Liq. 2021, 326, 115303. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Y.; Yang, Y.; Huang, J.; Zimmerman, A.R.; Chen, H.; Hu, X.; Gao, B. Mechanisms and adsorption capacities of hydrogen peroxide modified ball milled biochar for the removal of methylene blue from aqueous solutions. Bioresour. Technol. 2021, 337, 125432. [Google Scholar] [CrossRef]
- Toscano Miranda, N.; Lopes Motta, I.; Maciel Filho, R.; Wolf Maciel, M.R. Sugarcane bagasse pyrolysis: A review of operating conditions and products properties. Renew. Sustain. Energy Rev. 2021, 149, 111394. [Google Scholar] [CrossRef]
- Sud, D.; Mahajan, G.; Kaur, M.P. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions—A review. Bioresour. Technol. 2008, 99, 6017–6027. [Google Scholar] [CrossRef]
- Blasi, C.D.; Galgano, A.; Branca, C. Exothermic Events of Nut Shell and Fruit Stone Pyrolysis. ACS Sustain. Chem. Eng. 2019, 7, 9035–9049. [Google Scholar] [CrossRef]
- Missau, J.; Bertuol, D.A.; Tanabe, E.H. Highly efficient adsorbent for removal of Crystal Violet Dye from Aqueous Solution by CaAl/LDH supported on Biochar. Appl. Clay Sci. 2021, 214, 106297. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Lv, Z.; Xie, R.; Huang, L.; Jiang, J. Impacts of biochar and oyster shells waste on the immobilization of arsenic in highly contaminated soils. J. Environ. Manag. 2018, 217, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Elwakeel, K.Z.; Elgarahy, A.M.; Mohammad, S.H. Use of beach bivalve shells located at Port Said coast (Egypt) as a green approach for methylene blue removal. J. Environ. Chem. Eng. 2017, 5, 578–587. [Google Scholar] [CrossRef]
- Sawant, S.A.; Somani, S.P.; Omanwar, S.K.; Somani, P.R. Photocatalytic Degradation of Methylene Blue Dye in Distilled and Sea Water Using Indian Edible Chuna (Calcium Oxide/Hydroxide) as Photocatalyst. Adv. Sci. Eng. Med. 2014, 6, 227–230. [Google Scholar] [CrossRef]
- Jaiswal, K.K.; Dutta, S.; Pohrmen, C.B.; Verma, R.; Kumar, A.; Ramaswamy, A.P. Bio-waste chicken eggshell-derived calcium oxide for photocatalytic application in methylene blue dye degradation under natural sunlight irradiation. Inorg. Nano-Met. Chem. 2020, 51, 995–1004. [Google Scholar] [CrossRef]
- Vanthana Sree, G.; Nagaraaj, P.; Kalanidhi, K.; Aswathy, C.A.; Rajasekaran, P. Calcium oxide a sustainable photocatalyst derived from eggshell for efficient photo-degradation of organic pollutants. J. Clean. Prod. 2020, 270, 122294. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platnum. J. Chem. Phys. 1918, 40, 1361–1403. [Google Scholar]
- Langmuir, I. The Constitution and Fundamental Properties of Solids and Liquids. Part I. Solids. J. Am. Chem. Soc. 1915, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H. Über die Adsorption in Lösungen. Z. Phys. Chem. 1906, 57U, 385–470. [Google Scholar] [CrossRef]
- Jabar, J.M.; Odusote, Y.A. Utilization of prepared activated biochar from water lily (Nymphaea lotus) stem for adsorption of malachite green dye from aqueous solution. Biomass Convers. Biorefin. 2021, 1–12. [Google Scholar] [CrossRef]
- Jabar, J.M.; Odusote, Y.A.; Ayinde, Y.T.; Yılmaz, M. African almond (Terminalia catappa L) leaves biochar prepared through pyrolysis using H3PO4 as chemical activator for sequestration of methylene blue dye. Results Eng. 2022, 14, 100385. [Google Scholar] [CrossRef]
- Yagmur, E.; Tunc, M.S.; Banford, A.; Aktas, Z. Preparation of activated carbon from autohydrolysed mixed southern hardwood. J. Anal. Appl. Pyrolysis 2013, 104, 470–478. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, S.; Zheng, Y.; Luo, Z.; Cen, K. Mechanism study of wood lignin pyrolysis by using TG–FTIR analysis. J. Anal. Appl. Pyrolysis 2008, 82, 170–177. [Google Scholar] [CrossRef]
- Tran, H.N.; Tomul, F.; Thi Hoang Ha, N.; Nguyen, D.T.; Lima, E.C.; Le, G.T.; Chang, C.T.; Masindi, V.; Woo, S.H. Innovative spherical biochar for pharmaceutical removal from water: Insight into adsorption mechanism. J. Hazard. Mater. 2020, 394, 122255. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Ji, Y.; Liu, X.; Mu, L.; Zhu, J. Sorption mechanism of organic dyes on a novel self-nitrogen-doped porous graphite biochar: Coupling DFT calculations with experiments. Chem. Eng. Sci. 2021, 242, 116739. [Google Scholar] [CrossRef]
- Guo, Y.; Hong Nhung, N.T.; Dai, X.; He, C.; Wang, Y.; Wei, Y.; Fujita, T. Strontium Ion Removal from Artificial Seawater Using a Combination of Adsorption with Biochar and Precipitation by Blowing CO2 Nanobubble with Neutralization. Front. Bioeng. Biotechnol. 2022, 10, 819407. [Google Scholar] [CrossRef]
- Dai, X.; Thi Hong Nhung, N.; Hamza, M.F.; Guo, Y.; Chen, L.; He, C.; Ning, S.; Wei, Y.; Dodbiba, G.; Fujita, T. Selective adsorption and recovery of scandium from red mud leachate by using phosphoric acid pre-treated pitaya peel biochar. Sep. Purif. Technol. 2022, 292, 121043. [Google Scholar] [CrossRef]
- Niazi, L.; Lashanizadegan, A.; Sharififard, H. Chestnut oak shells activated carbon: Preparation, characterization and application for Cr (VI) removal from dilute aqueous solutions. J. Clean. Prod. 2018, 185, 554–561. [Google Scholar] [CrossRef]
- Wang, R.-F.; Deng, L.-G.; Li, K.; Fan, X.-J.; Li, W.; Lu, H.-Q. Fabrication and characterization of sugarcane bagasse–calcium carbonate composite for the efficient removal of crystal violet dye from wastewater. Ceram. Int. 2020, 46, 27484–27492. [Google Scholar] [CrossRef]
- Gottipati, R.; Mishra, S. Application of biowaste (waste generated in biodiesel plant) as an adsorbent for the removal of hazardous dye-methylene blue—From aqueous phase. Braz. J. Chem. Eng. 2009, 27, 357–367. [Google Scholar] [CrossRef]
- Postai, D.L.; Demarchi, C.A.; Zanatta, F.; Melo, D.C.C.; Rodrigues, C.A. Adsorption of rhodamine B and methylene blue dyes using waste of seeds of Aleurites Moluccana, a low cost adsorbent. Alex. Eng. J. 2016, 55, 1713–1723. [Google Scholar] [CrossRef]
- Ayesha, M.; Khoja, A.H.; Butt, F.A.; Sikandar, U.; Javed, A.H.; Naqvi, S.R.; Din, I.u.; Mehran, M.T. Sorption enhanced steam reforming of methane over waste-derived CaO promoted MgNiAl hydrotalcite catalyst for sustainable H2 production. J. Environ. Chem. Eng. 2022, 10, 107651. [Google Scholar] [CrossRef]
- Nadeem, F.; Bhatti, I.A.; Ashar, A.; Yousaf, M.; Iqbal, M.; Mohsin, M.; Nisar, J.; Tamam, N.; Alwadai, N. Eco-benign biodiesel production from waste cooking oil using eggshell derived MM-CaO catalyst and condition optimization using RSM approach. Arab. J. Chem. 2021, 14, 103263. [Google Scholar] [CrossRef]
- Liu, X.-J.; Li, M.-F.; Singh, S.K. Manganese-modified lignin biochar as adsorbent for removal of methylene blue. J. Mater. Res. Technol. 2021, 12, 1434–1445. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, T.; Dang, B.; Wang, H.; Xiong, Y.; Yao, Q.; Wang, C.; Sun, Q.; Jin, C. The properties of fibreboard based on nanolignocelluloses/CaCO3/PMMA composite synthesized through mechano-chemical method. Sci. Rep. 2018, 8, 5121. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Wei, D.; Khuda, F.; Ma, R.; Ismail, M.; Ai, Y. Comparative adsorption capabilities of rubbish tissue paper-derived carbon-doped MgO and CaCO3 for EBT and U(VI), studied by batch, spectroscopy and DFT calculations. Environ. Sci. Pollut. Res. Int. 2020, 27, 13114–13130. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-J.; Li, M.-F.; Ma, J.-F.; Bian, J.; Peng, F. Chitosan crosslinked composite based on corncob lignin biochar to adsorb methylene blue: Kinetics, isotherm, and thermodynamics. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128621. [Google Scholar] [CrossRef]
- Almeida, C.A.; Debacher, N.A.; Downs, A.J.; Cottet, L.; Mello, C.A. Removal of methylene blue from colored effluents by adsorption on montmorillonite clay. J. Colloid Interface Sci. 2009, 332, 46–53. [Google Scholar] [CrossRef]
- Ganguly, P.; Sarkhel, R.; Das, P. Synthesis of pyrolyzed biochar and its application for dye removal: Batch, kinetic and isotherm with linear and non-linear mathematical analysis. Surf. Interfaces 2020, 20, 100616. [Google Scholar] [CrossRef]
- Liu, L.; Lin, Y.; Liu, Y.; Zhu, H.; He, Q. Removal of Methylene Blue from Aqueous Solutions by Sewage Sludge Based Granular Activated Carbon: Adsorption Equilibrium, Kinetics, and Thermodynamics. J. Chem. Eng. Data 2013, 58, 2248–2253. [Google Scholar] [CrossRef]
- Guo, D.; Li, Y.; Cui, B.; Hu, M.; Luo, S.; Ji, B.; Liu, Y. Natural adsorption of methylene blue by waste fallen leaves of Magnoliaceae and its repeated thermal regeneration for reuse. J. Clean. Prod. 2020, 267, 121903. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Chen, X.; Fu, H.; Chen, Y.; Ning, S.; Fujita, T.; Wei, Y.; Wang, X. Layered ammonium vanadate nanobelt as efficient adsorbents for removal of Sr(2+) and Cs(+) from contaminated water. J. Colloid Interface Sci. 2022, 615, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Liu, F.; Huang, Y.; Yang, Q.; Tian, C.; Zhou, W. Preparation of peanut shell-like calcium carbonate from biowaste chicken eggshell and its application for aqueous Victoria Blue B removal. Microporous Mesoporous Mater. 2022, 329, 111549. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J.; Li, X. Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: Adsorption kinetics, equilibrium, thermodynamics and mechanism. J. Environ. Chem. Eng. 2017, 5, 601–611. [Google Scholar] [CrossRef]
- Gupta, K.; Khatri, O.P. Fast and efficient adsorptive removal of organic dyes and active pharmaceutical ingredient by microporous carbon: Effect of molecular size and charge. Chem. Eng. J. 2019, 378, 122218. [Google Scholar] [CrossRef]
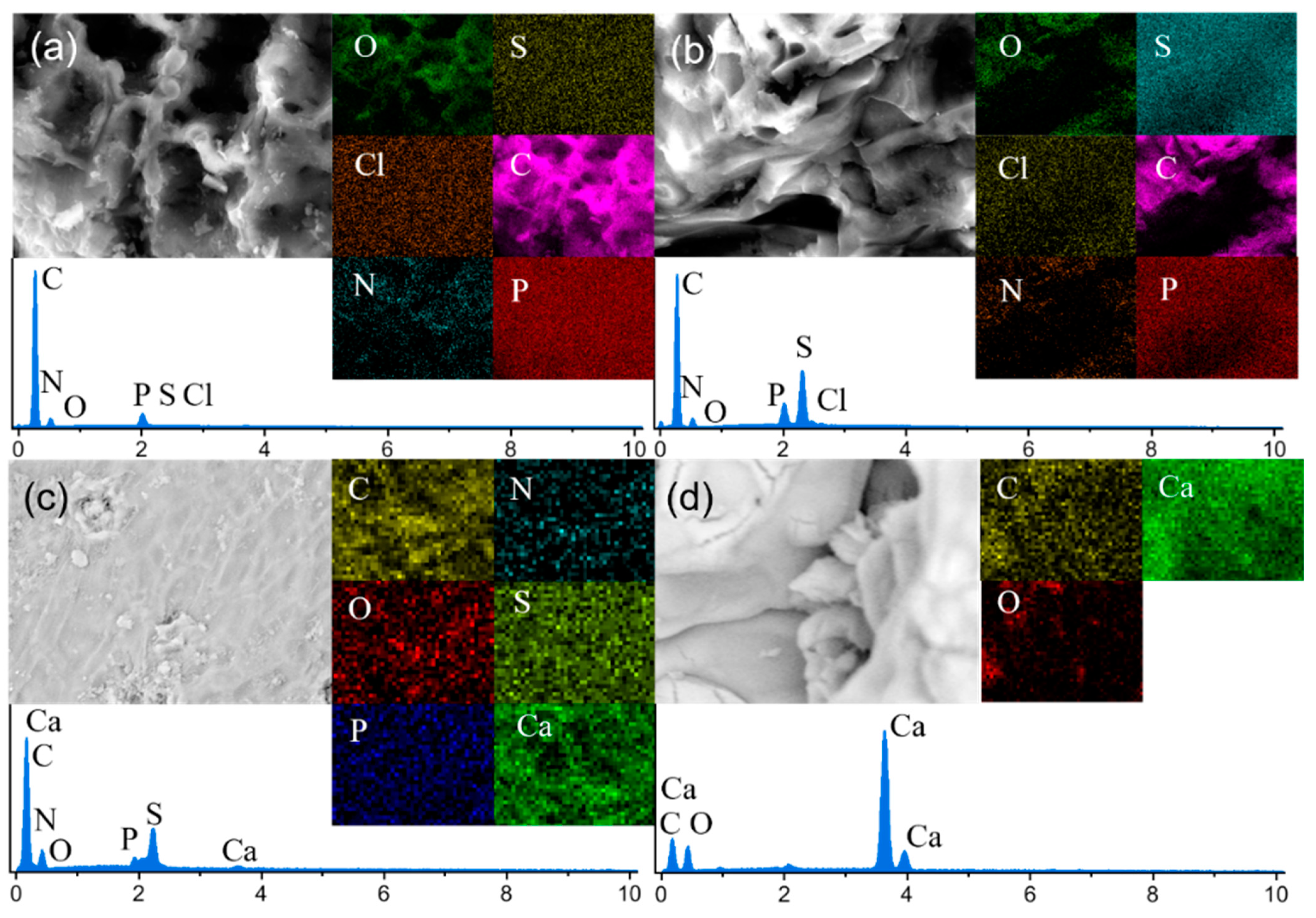





| Element | Atomic % | |||
|---|---|---|---|---|
| CN before Adsorption | CN after Adsorption | SS | MCS3-1 after Adsorption | |
| C | 84.81 | 80.94 | 35.63 | 68.19 |
| N | 0.00 | 3.30 | - | 14.89 |
| O | 12.64 | 7.16 | 42.31 | 14.47 |
| P | 2.48 | 2.18 | - | 0.41 |
| S | 0.05 | 6.20 | - | 1.84 |
| Ca | - | - | 22.05 | 0.19 |
| Isotherms | Parameters | Adsorbents | |
|---|---|---|---|
| MSC3-1 | CN | ||
| Langmuir | KL (L/mg) | 0.29 ± 0.05 | 0.16 ± 0.05 |
| qm (mg/g) | 1191.1 ± 98.5 | 534.7 ± 25.8 | |
| R2 | 0.918 | 0.767 | |
| Freundlich | 1/n | 0.211 ± 0.008 | 0.087 ± 0.006 |
| KF ((mg/g) (L/mg)1/n) | 439.3 ± 4.3 | 325.4 ± 7.5 | |
| R2 | 0.983 | 0.980 | |
| Kinetic Models | Parameters | Adsorbents | |
|---|---|---|---|
| MSC3-1 | CN | ||
| Pseudo-first-order | k1 (min−1) | 0.095 ± 0.016 | 0.045 ± 0.007 |
| qe (mg/g) | 1418.5 ± 16.2 | 557.2 ± 7.4 | |
| R2 | 0.827 | 0.872 | |
| Pseudo-second-order | k1 (×10−4) (g·mg−1·min−1) | 1.25 ± 0.11 | 1.28 ± 0.08 |
| qe (mg/g) | 1443.3 ± 6.6 | 596.9 ± 2.4 | |
| R2 | 0.977 | 0.992 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, J.; Nhung, N.T.H.; Ding, Y.; Chen, H.; He, C.; Wang, X.; Fujita, T. Chestnut Shell-Activated Carbon Mixed with Pyrolytic Snail Shells for Methylene Blue Adsorption. Materials 2022, 15, 8227. https://doi.org/10.3390/ma15228227
An J, Nhung NTH, Ding Y, Chen H, He C, Wang X, Fujita T. Chestnut Shell-Activated Carbon Mixed with Pyrolytic Snail Shells for Methylene Blue Adsorption. Materials. 2022; 15(22):8227. https://doi.org/10.3390/ma15228227
Chicago/Turabian StyleAn, Jiahao, Nguyen Thi Hong Nhung, Yaxuan Ding, Hao Chen, Chunlin He, Xinpeng Wang, and Toyohisa Fujita. 2022. "Chestnut Shell-Activated Carbon Mixed with Pyrolytic Snail Shells for Methylene Blue Adsorption" Materials 15, no. 22: 8227. https://doi.org/10.3390/ma15228227
APA StyleAn, J., Nhung, N. T. H., Ding, Y., Chen, H., He, C., Wang, X., & Fujita, T. (2022). Chestnut Shell-Activated Carbon Mixed with Pyrolytic Snail Shells for Methylene Blue Adsorption. Materials, 15(22), 8227. https://doi.org/10.3390/ma15228227








