Structural Phase Transitions and Thermal Degradation Process of MAPbCl3 Single Crystals Studied by Raman and Brillouin Scattering
Abstract
1. Introduction
2. Experimental Section
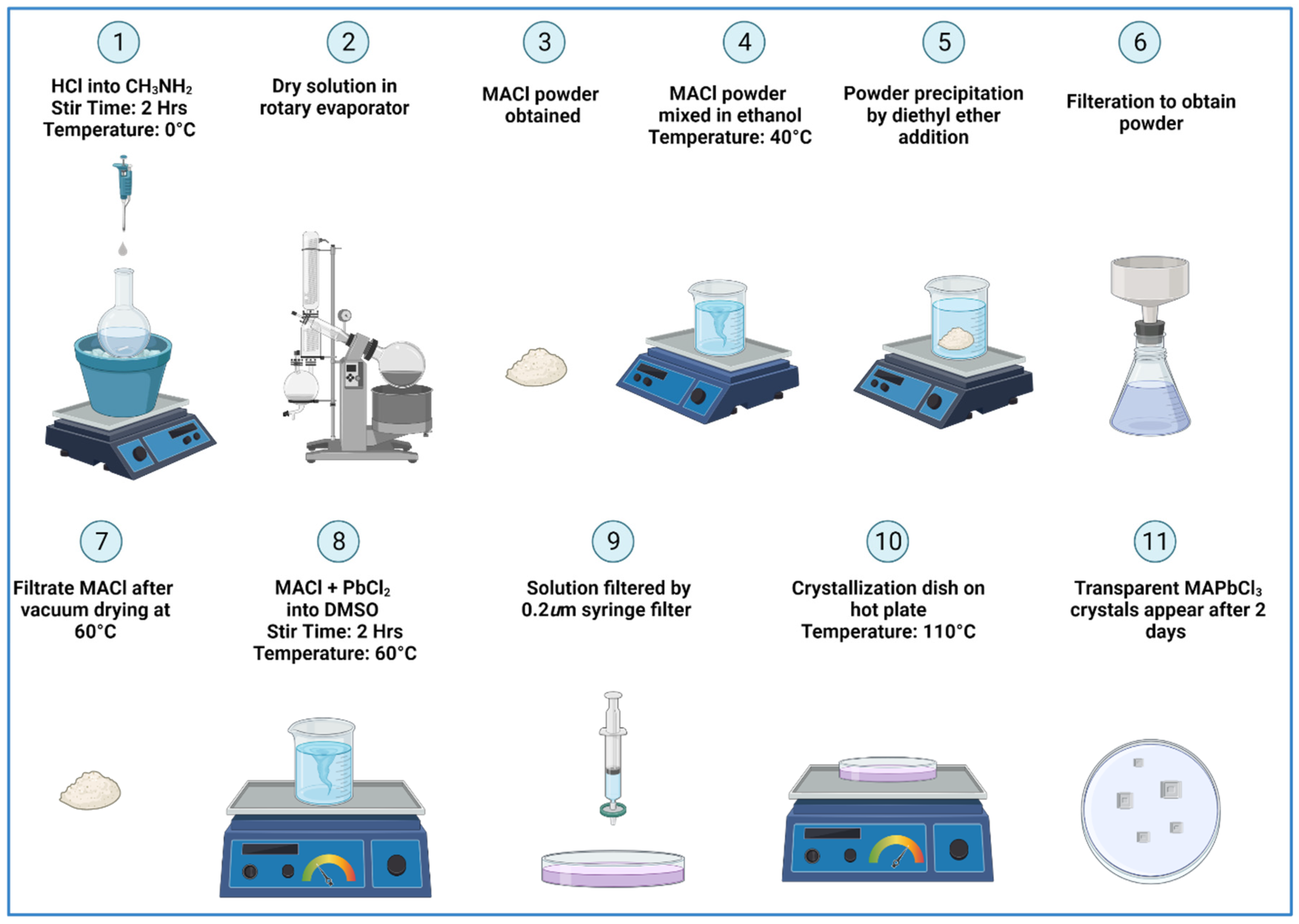
2.1. Precursors
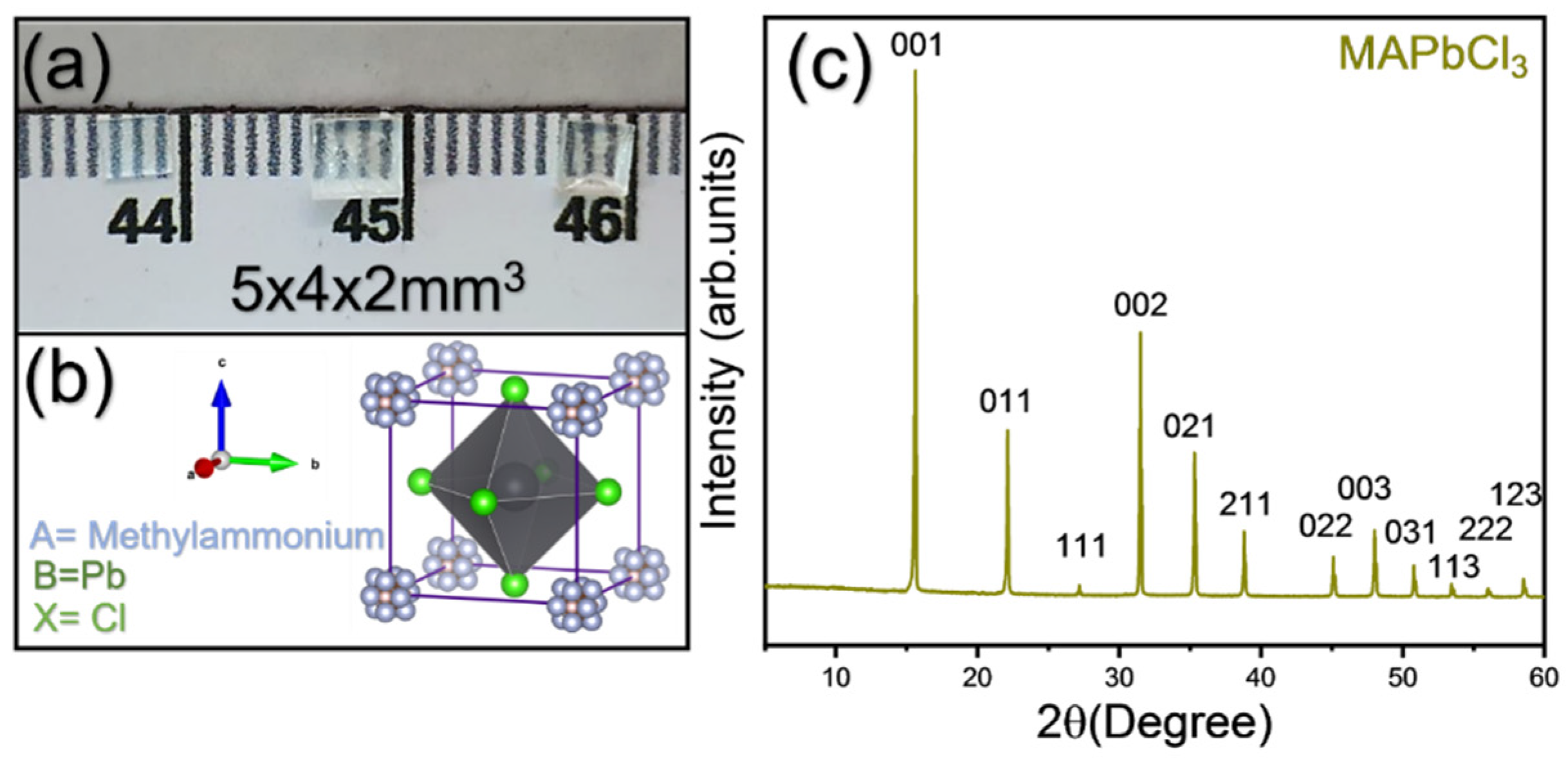
2.2. Single Crystal Synthesis
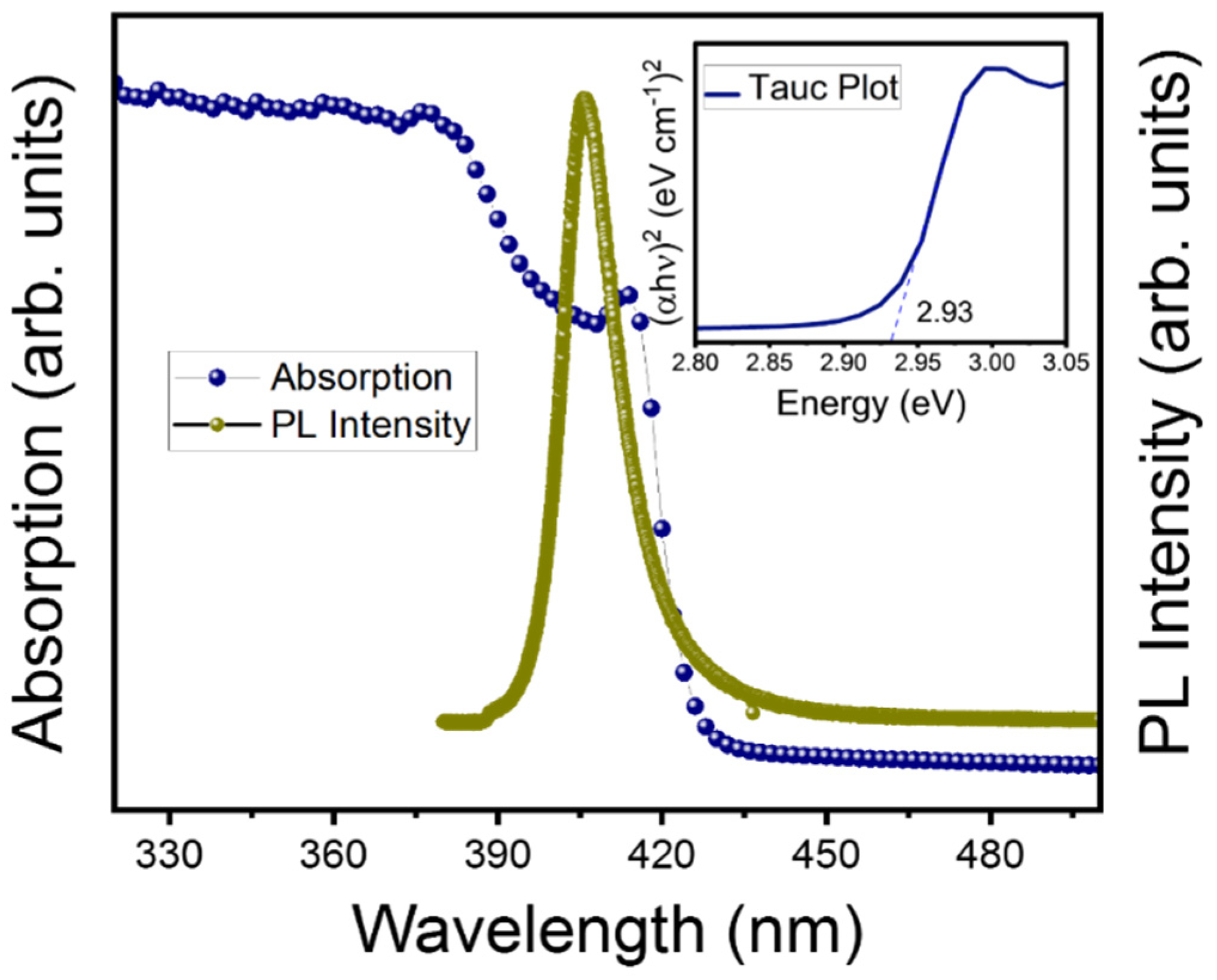
2.3. Characterization Techniques
3. Results and Discussions
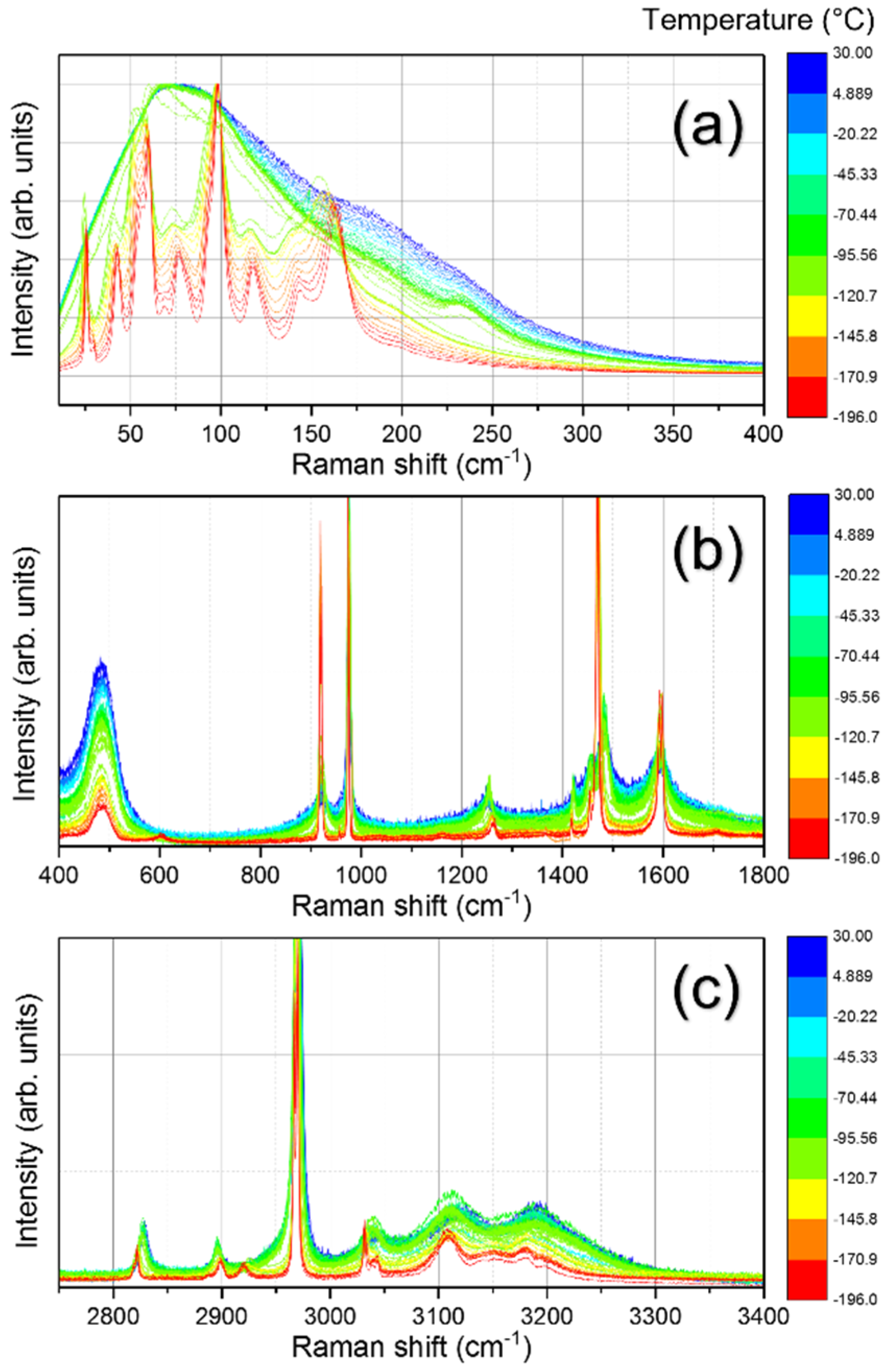
3.1. Structural Phase Transitions Probed by Raman Spectroscopy
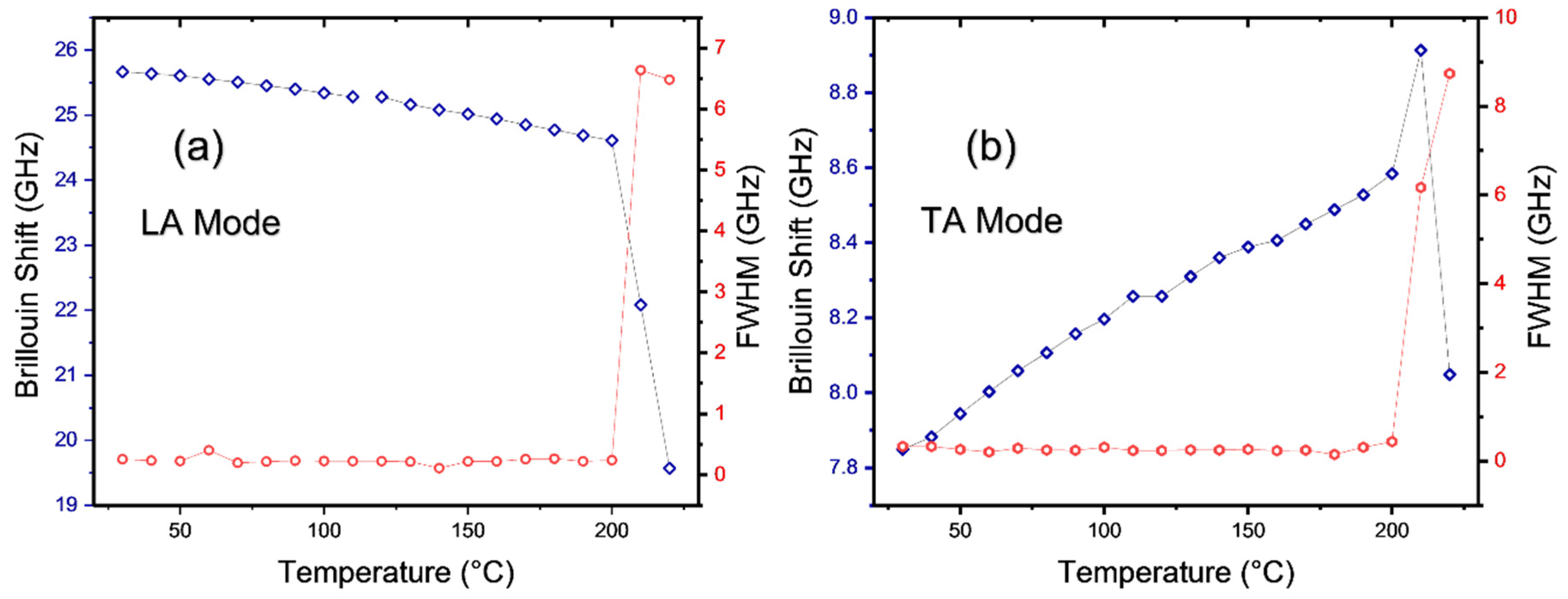
3.2. The Degradation Process of MAPbCl3 Probed by Raman and Brillouin Scattering
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Y.; Zhu, K. Organic-Inorganic Hybrid Lead Halide Perovskites for Optoelectronic and Electronic Applications. Chem. Soc. Rev. 2016, 45, 655–689. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K.; et al. Low Trap-State Density and Long Carrier Diffusion in Organolead Trihalide Perovskite Single Crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef] [PubMed]
- De Wolf, S.; Holovsky, J.; Moon, S.J.; Löper, P.; Niesen, B.; Ledinsky, M.; Haug, F.J.; Yum, J.H.; Ballif, C. Organometallic Halide Perovskites: Sharp Optical Absorption Edge and Its Relation to Photovoltaic Performance. J. Phys. Chem. Lett. 2014, 5, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Burschka, J.; Pellet, N.; Moon, S.J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential Deposition as a Route to High-Performance Perovskite-Sensitized Solar Cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Dou, L.; Yang, Y.M.; You, J.; Hong, Z.; Chang, W.H.; Li, G.; Yang, Y. Solution-Processed Hybrid Perovskite Photodetectors with High Detectivity. Nat. Commun. 2014, 5, 5404. [Google Scholar] [CrossRef]
- Tan, Z.K.; Moghaddam, R.S.; Lai, M.L.; Docampo, P.; Higler, R.; Deschler, F.; Price, M.; Sadhanala, A.; Pazos, L.M.; Credgington, D.; et al. Bright Light-Emitting Diodes Based on Organometal Halide Perovskite. Nat. Nanotechnol. 2014, 9, 687–692. [Google Scholar] [CrossRef]
- NREL Efficiency Chart. Available online: http://www.nrel.gov/ncpv/images/efficiency_chart.jpg (accessed on 27 July 2022).
- Zhu, Y.; Liu, Y.; Miller, K.A.; Zhu, H.; Egap, E. Lead Halide Perovskite Nanocrystals as Photocatalysts for PET-RAFT Polymerization under Visible and Near-Infrared Irradiation. ACS Macro Lett. 2020, 9, 725–730. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Liao, Q.; Xu, Z.; Li, H.; Zheng, L.; Fu, H. Embedding Perovskite Nanocrystals into a Polymer Matrix for Tunable Luminescence Probes in Cell Imaging. Adv. Funct. Mater. 2017, 27, 1604382. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, K.; Yang, J.; Chen, X.; Xie, X.; Li, B.; Zhang, Z.; Liu, L.; Shan, C.; Shen, D. High-Performance Planar-Type Ultraviolet Photodetector Based on High-Quality CH3NH3PbCl3 Perovskite Single Crystals. ACS Appl. Mater. Interfaces 2019, 11, 34144–34150. [Google Scholar] [CrossRef]
- Quarti, C.; Mosconi, E.; Ball, J.M.; D’Innocenzo, V.; Tao, C.; Pathak, S.; Snaith, H.J.; Petrozza, A.; De Angelis, F. Structural and Optical Properties of Methylammonium Lead Iodide across the Tetragonal to Cubic Phase Transition: Implications for Perovskite Solar Cells. Energy Environ. Sci. 2016, 9, 155–163. [Google Scholar] [CrossRef]
- Anusca, I.; Balčiūnas, S.; Gemeiner, P.; Svirskas, Š.; Sanlialp, M.; Lackner, G.; Fettkenhauer, C.; Belovickis, J.; Samulionis, V.; Ivanov, M.; et al. Dielectric Response: Answer to Many Questions in the Methylammonium Lead Halide Solar Cell Absorbers. Adv. Energy Mater. 2017, 7, 1700600. [Google Scholar] [CrossRef]
- Francisco-López, A.; Charles, B.; Alonso, M.I.; Garriga, M.; Campoy-Quiles, M.; Weller, M.T.; Goñi, A.R. Phase Diagram of Methylammonium/Formamidinium Lead Iodide Perovskite Solid Solutions from Temperature-Dependent Photoluminescence and Raman Spectroscopies. J. Phys. Chem. C 2020, 124, 3448–3458. [Google Scholar] [CrossRef]
- Hsu, H.; Li, L.; Shellaiah, M.; Sun, K.W. Structural, Photophysical, and Electronic Properties Of CH3NH3PbCl3 Single crystals. Sci. Rep. 2019, 9, 13311. [Google Scholar] [CrossRef]
- Thu Nguyen, T.T.; Kim, Y.H.Y.Y.H.; Bae, S.; Bari, M.; Jung, H.R.; Jo, W.; Kim, Y.H.Y.Y.H.; Ye, Z.G.; Yoon, S. Raman Scattering Studies of the Structural Phase Transitions in Single-Crystalline CH3NH3PbCl3. J. Phys. Chem. Lett. 2020, 11, 3773–3781. [Google Scholar] [CrossRef]
- Mączka, M.; Ptak, M.; Vasconcelos, D.L.M.; Giriunas, L.; Freire, P.T.C.; Bertmer, M.; Banys, J.; Simenas, M. NMR and Raman Scattering Studies of Temperature-and Pressure-Driven Phase Transitions in CH3NH2NH2PbCl3 Perovskite. J. Phys. Chem. C 2020, 124, 26999–27008. [Google Scholar] [CrossRef]
- Lee, J.W.; Naqvi, F.H.; Ko, J.-H.; Kim, T.H.; Ahn, C.W. Acoustic Anomalies and the Critical Slowing-Down Behavior of MAPbCl3 Single Crystals Studied by Brillouin Light Scattering. Materials 2022, 15, 3692. [Google Scholar] [CrossRef]
- Chen, Y.; Hou, X.; Tao, S.; Fu, X.; Zhou, H.; Yin, J.; Wu, M.; Zhang, X. Synthesis, Crystal Structure and Photoresponse of Tetragonal Phase Single Crystal CH3NH3PbCl3. Chem. Commun. 2020, 56, 6404–6407. [Google Scholar] [CrossRef]
- Maalej, A.; Abid, Y.; Kallel, A.; Daoud, A.; Lautié, A.; Romain, F. Phase Transitions and Crystal Dynamics in the Cubic Perovskite CH3NH3PbCl3. Solid State Commun. 1997, 103, 279–284. [Google Scholar] [CrossRef]
- Alvarez-Galván, M.C.; Alonso, J.A.; López, C.A.; López-Linares, E.; Contreras, C.; Lázaro, M.J.; Fauth, F.; Martínez-Huerta, M.V. Crystal Growth, Structural Phase Transitions, and Optical Gap Evolution of CH3NH3Pb(Br1−xClx)3 Perovskites. Cryst. Growth Des. 2019, 19, 918–924. [Google Scholar] [CrossRef]
- Onoda-Yamamuro, N.; Matsuo, T.; Suga, H. Calorimetric and IR Spectroscopic Studies of Phase Transitions in Methylammonium Trihalogenoplumbates (II). J. Phys. Chem. Solids 1990, 51, 1383–1395. [Google Scholar] [CrossRef]
- Leguy, A.M.A.; Goñi, A.R.; Frost, J.M.; Skelton, J.; Brivio, F.; Rodríguez-Martínez, X.; Weber, O.J.; Pallipurath, A.; Alonso, M.I.; Campoy-Quiles, M.; et al. Dynamic Disorder, Phonon Lifetimes, and the Assignment of Modes to the Vibrational Spectra of Methylammonium Lead Halide Perovskites. Phys. Chem. Chem. Phys. 2016, 18, 27051–27066. [Google Scholar] [CrossRef] [PubMed]
- Poglitsch, A.; Weber, D. Dynamic Disorder in Methylammoniumtrihalogenoplumbates (II) Observed by Millimeter-Wave Spectroscopy. J. Chem. Phys. 1987, 87, 6373–6378. [Google Scholar] [CrossRef]
- Niemann, R.G.; Kontos, A.G.; Palles, D.; Kamitsos, E.I.; Kaltzoglou, A.; Brivio, F.; Falaras, P.; Cameron, P.J. Halogen Effects on Ordering and Bonding of CH3NH3+ in CH3NH3PbX3 (X = Cl, Br, I) Hybrid Perovskites: A Vibrational Spectroscopic Study. J. Phys. Chem. C 2016, 120, 2509–2519. [Google Scholar] [CrossRef]
- Niu, G.; Guo, X.; Wang, L. Review of Recent Progress in Chemical Stability of Perovskite Solar Cells. J. Mater. Chem. A 2015, 3, 8970–8980. [Google Scholar] [CrossRef]
- Misra, R.K.; Aharon, S.; Li, B.; Mogilyansky, D.; Visoly-Fisher, I.; Etgar, L.; Katz, E.A. Temperature- and Component-Dependent Degradation of Perovskite Photovoltaic Materials under Concentrated Sunlight. J. Phys. Chem. Lett. 2015, 6, 326–330. [Google Scholar] [CrossRef]
- Wu, B.; Nguyen, H.T.; Ku, Z.; Han, G.; Giovanni, D.; Mathews, N.; Fan, H.J.; Sum, T.C. Discerning the Surface and Bulk Recombination Kinetics of Organic–Inorganic Halide Perovskite Single Crystals. Adv. Energy Mater. 2016, 6, 1600551. [Google Scholar] [CrossRef]
- Jung, H.R.; Bari, M.; Cho, Y.; Kim, Y.S.; Nguyen, T.T.T.; Kim, Y.; Yoon, S.; Jo, Y.C.; Kim, J.H.; Yuldashev, S.; et al. Exotic Optoelectronic Behaviors in CH3NH3PbCl3 perovskite Single Crystals: Co-Existence of Free and Bound Excitons with Structural Phase Transitions. Appl. Phys. Lett. 2021, 118, 143301. [Google Scholar] [CrossRef]
- Kontos, A.G.; Manolis, G.K.; Kaltzoglou, A.; Palles, D.; Kamitsos, E.I.; Kanatzidis, M.G.; Falaras, P. Halogen-NH2+ Interaction, Temperature-Induced Phase Transition, and Ordering in (NH2CHNH2)PbX3 (X = Cl, Br, I) Hybrid Perovskites. J. Phys. Chem. C 2020, 124, 8479–8487. [Google Scholar] [CrossRef]
- Hassan, F.; Jae, N.; Ko, H.; Heon, T.; Chang, K.; Ahn, W.; Hwang, Y.; Sheraz, M. A-Site Cation Effect on Optical Phonon Modes and Thermal Stability in Lead - Based Perovskite Bromide Single Crystals Using Raman Spectroscopy. J. Korean Phys. Soc. 2022, 81, 230–240. [Google Scholar]
- Chi, L.; Swainson, I.; Cranswick, L.; Her, J.-H.; Stephens, P.; Knop, O.; Chi, L.; Swainson, I.; Cranswick, L.; Her, J.-H.; et al. The Ordered Phase of Methylammonium Lead Chloride CH3ND3PbCl3. J. Solid State Chem. 2005, 178, 1376–1385. [Google Scholar] [CrossRef]
- Mączka, M.; Ptak, M. Temperature-Dependent Raman Studies of FAPbBr3 and MAPbBr3 Perovskites: Effect of Phase Transitions on Molecular Dynamics and Lattice Distortion. Solids 2022, 3, 111–121. [Google Scholar] [CrossRef]
- Kim, Y.; Bae, S.; Park, J.; Nguyen, T.T.T.; Jung, H.R.; Jo, W.; Kim, Y.H.; Raebiger, H.; Yoon, S. Parallel Alignment of Methylammonium Cations in an Orthorhombic CH3NH3PbCl3Single Crystal Observed by Polarized Micro-Raman Scattering Spectroscopy. Chem. Mater. 2022, 34, 2972–2980. [Google Scholar] [CrossRef]
- Nakada, K.; Matsumoto, Y.; Shimoi, Y.; Yamada, K.; Furukawa, Y. Temperature-Dependent Evolution of Raman Spectra of Methylammonium Lead Halide Perovskites, CH3NH3PbX3 (X = I, Br). Molecules 2019, 24, 626. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Fang, Y.; Fan, X.; Zhang, B.; Kuo, J.L.; White, T.J.; Chow, G.M.; Yan, J.; Shen, Z.X. Hydrogen-Bonding Evolution during the Polymorphic Transformations in CH3NH3PbBr3: Experiment and Theory. Chem. Mater. 2017, 29, 5974–5981. [Google Scholar] [CrossRef]
- Park, B.W.; Jain, S.M.; Zhang, X.; Hagfeldt, A.; Boschloo, G.; Edvinsson, T. Resonance Raman and Excitation Energy Dependent Charge Transfer Mechanism in Halide-Substituted Hybrid Perovskite Solar Cells. ACS Nano 2015, 9, 2088–2101. [Google Scholar] [CrossRef] [PubMed]
- Nagabhushana, G.P.; Shivaramaiah, R.; Navrotsky, A. Direct Calorimetric Verification of Thermodynamic Instability of Lead Halide Hybrid Perovskites. Proc. Natl. Acad. Sci. USA 2016, 113, 7717–7721. [Google Scholar] [CrossRef]
- Dualeh, A.; Gao, P.; Seok, S.I.; Nazeeruddin, M.K.; Grätzel, M. Thermal Behavior of Methylammonium Lead-Trihalide Perovskite Photovoltaic Light Harvesters. Chem. Mater. 2014, 26, 6160–6164. [Google Scholar] [CrossRef]
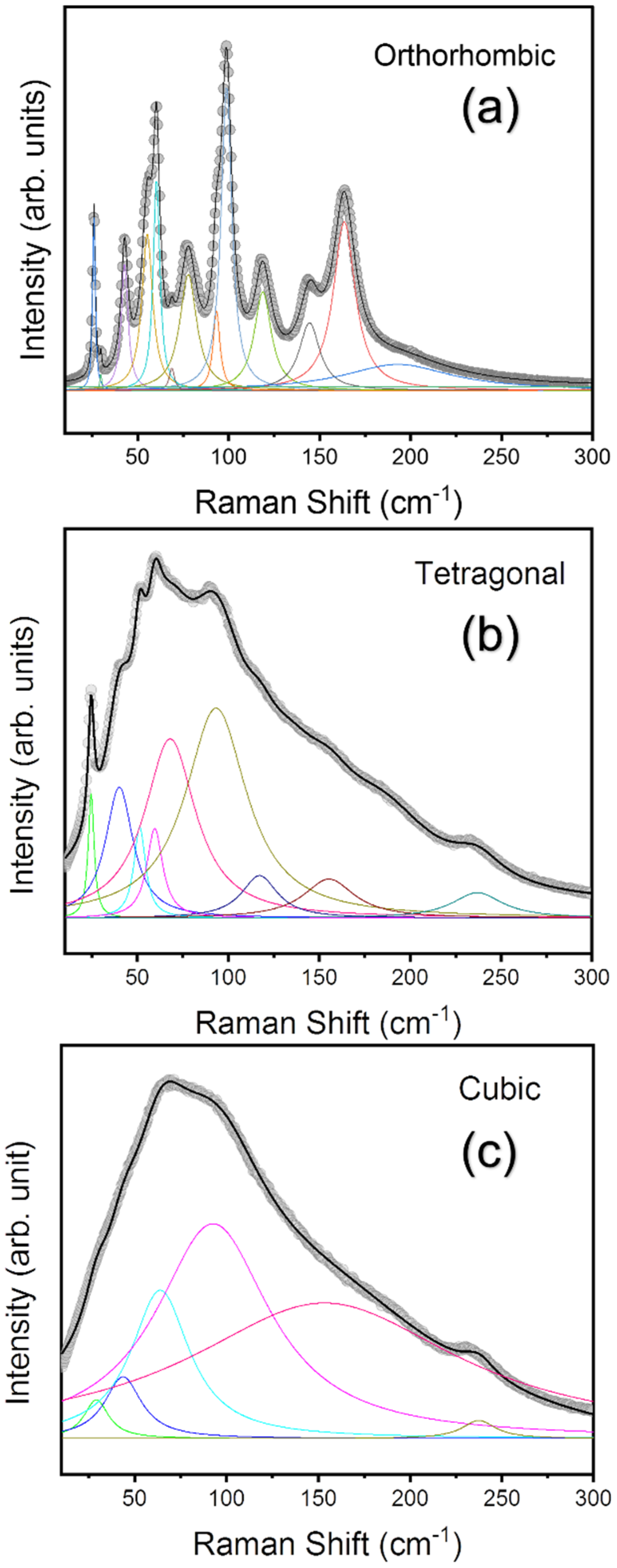

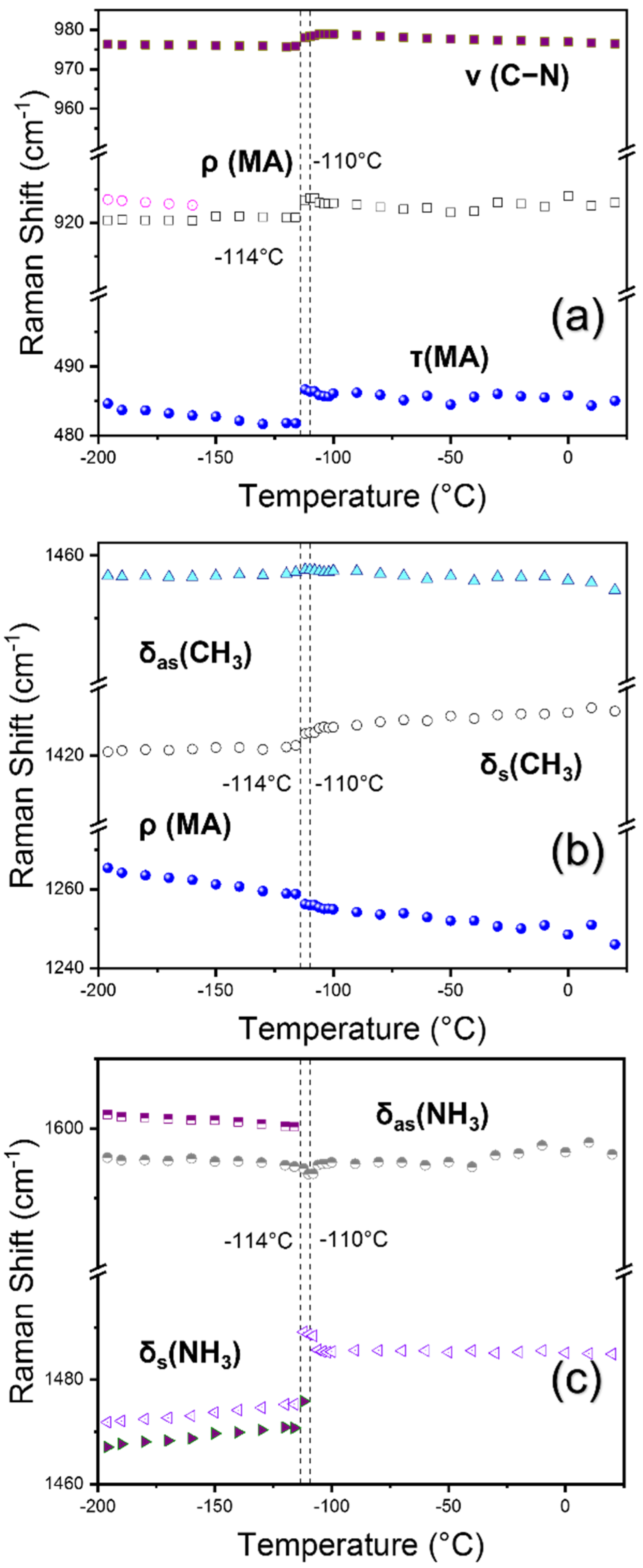
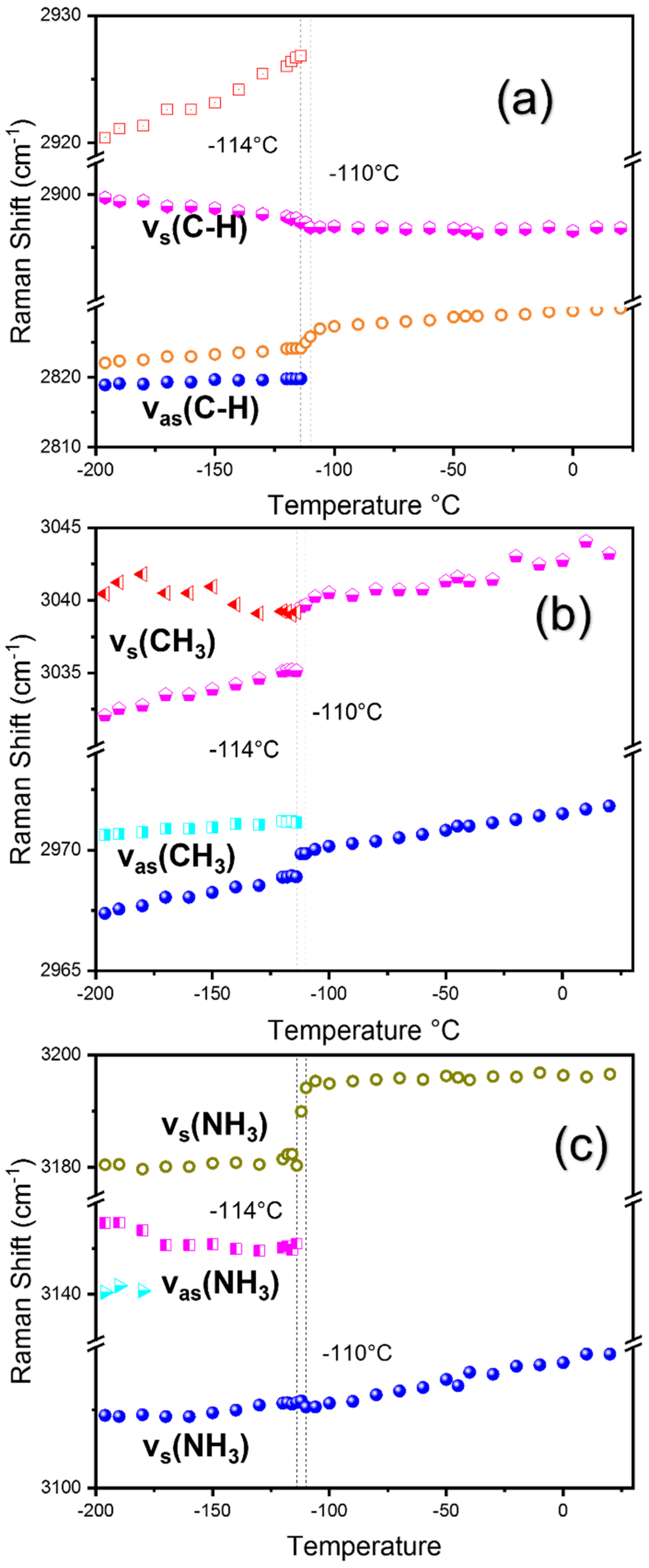

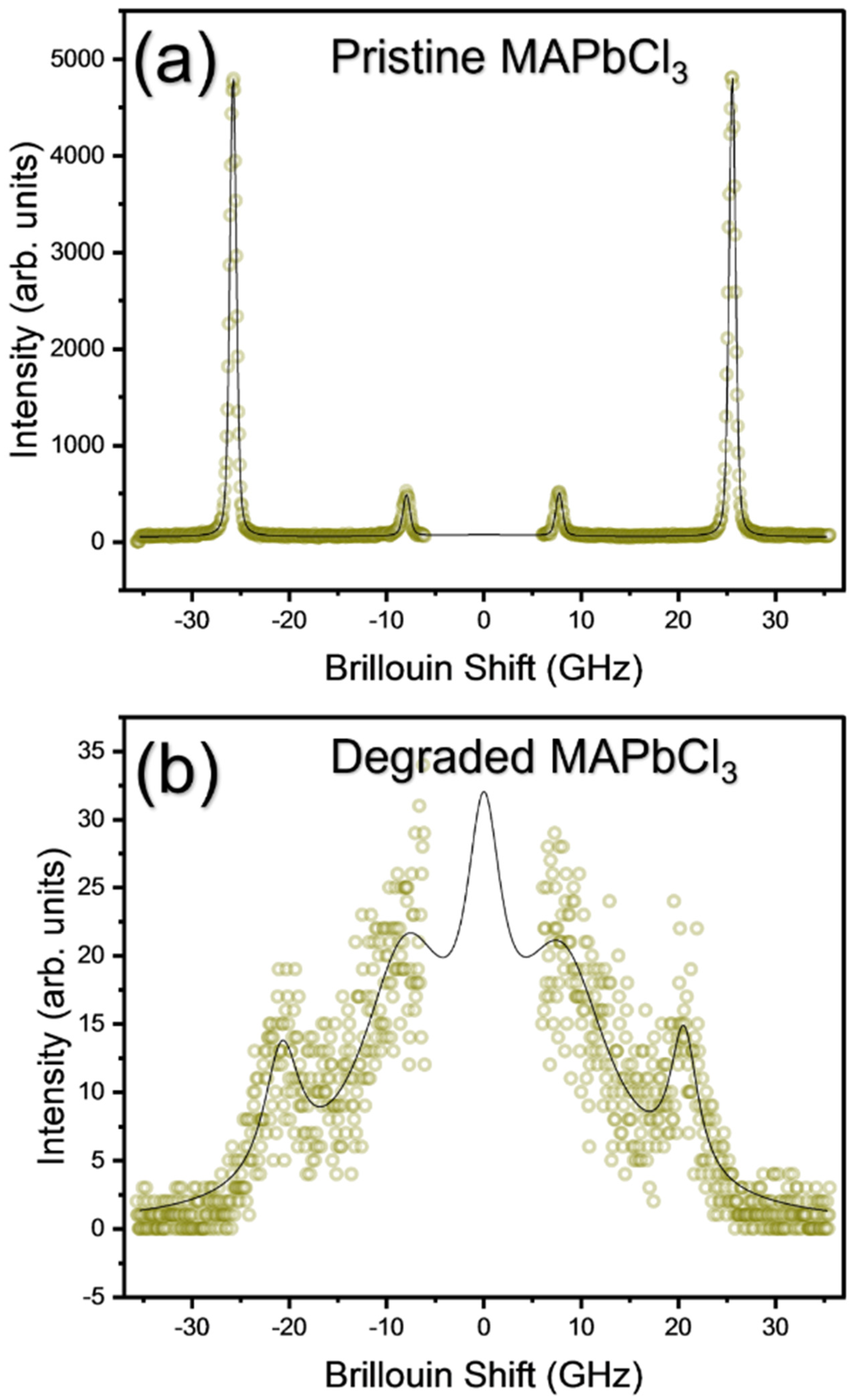
| Reports | Cubic to Tetra | Tetra to Ortho | Technique Used | Sample |
|---|---|---|---|---|
| Our work | 167.2 K (−110 °C) | 161 K (−114 °C) | Raman spectroscopy | Single crystal |
| Nguyen et al. [17] | Elusive | 160 K (−113 °C) | Raman spectroscopy | Single crystal |
| Alvarez-Galván et al. [21] | 167.5 K | X-ray diffraction | Crystalline powder | |
| Onoda -Yamamur et al. [22] | 177 K | 171.5 K | Calorimetric and IR spectroscopy | Single crystal |
| Poglitsch et al. [24] | 178.8 K | 172.9 K | X-ray diffraction | Single crystal |
| Leguy et al. [23] | 179 K | 172 K | Raman and terahertz spectroscopy | Single crystal |
| Niemann et al. [25] | 177.2 K | 171.5 K | Raman and IR spectroscopy | Film |
| Hsu et al. [15] | 185 K | 175 K | X-ray diffraction | Single crystal |
| Mode | Assignment |
|---|---|
| 26 s | Lattice libration [17] |
| 29 vw | Octahedra twist/MA+ motion [20,23] |
| 42 m | Octahedra twist/MA+ motion [20,23] |
| 55 sh | Octahedra twist/PbCl6 motion [20,23] |
| 60 vs | Octahedra twist/PbCl6 motion [20,23] |
| 68 vw | |
| 77 m | Octahedra distortion/PbCl6 motion [20,23] |
| 93 sh | δs (Cl–Pb–Cl) [25] |
| 98 vs | δs (Cl–Pb–Cl) [25] |
| 119 m | δas (Cl–Pb–Cl) [5] |
| 144 m | νs (Cl−Pb−Cl) [5] |
| 164 s | νas (Pb–Cl) [25] |
| 193 w | νs (Pb–Cl) [25] |
| 237 w | R of MA+ cation [20] |
| 484 m | τ (MA) [17] |
| 920 s | ρ (MA) [17] |
| 923 sh | ρ (MA) |
| 976 vs | ν (C−N) [25] |
| 1265 w | ρ (MA) [23,25] |
| 1421 w | δs (CH3) [16,25] |
| 1457 m | δas (CH3) [16,25] |
| 1467 w | δs (NH3) [16] |
| 1472 vs | δs (NH3) [25] |
| 1596 m | δas (NH3) [23,25] |
| 1602 m | δas (NH3) |
| 2819 sh | νas (C–H) [23] |
| 2822 w | νas (C–H) [23] |
| 2900 w | νs (C–H) [23] |
| 2920 w | |
| 2967 sh | νas (CH3) |
| 2971 vs | νas (CH3) [25] |
| 3032 m | νs (CH3) [17] |
| 3040 sh | νs (CH3) |
| 3109 m | νs (NH3) [23] |
| 3140 w | νas (NH3) [17] |
| 3156 w | νas (NH3) [17] |
| 3180 w | νs (NH3) [17] |
| 3201 w |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naqvi, F.H.; Ko, J.-H. Structural Phase Transitions and Thermal Degradation Process of MAPbCl3 Single Crystals Studied by Raman and Brillouin Scattering. Materials 2022, 15, 8151. https://doi.org/10.3390/ma15228151
Naqvi FH, Ko J-H. Structural Phase Transitions and Thermal Degradation Process of MAPbCl3 Single Crystals Studied by Raman and Brillouin Scattering. Materials. 2022; 15(22):8151. https://doi.org/10.3390/ma15228151
Chicago/Turabian StyleNaqvi, Furqanul Hassan, and Jae-Hyeon Ko. 2022. "Structural Phase Transitions and Thermal Degradation Process of MAPbCl3 Single Crystals Studied by Raman and Brillouin Scattering" Materials 15, no. 22: 8151. https://doi.org/10.3390/ma15228151
APA StyleNaqvi, F. H., & Ko, J.-H. (2022). Structural Phase Transitions and Thermal Degradation Process of MAPbCl3 Single Crystals Studied by Raman and Brillouin Scattering. Materials, 15(22), 8151. https://doi.org/10.3390/ma15228151







