Removal of Pharmaceuticals and Personal Care Products (PPCPs) by Free Radicals in Advanced Oxidation Processes
Abstract
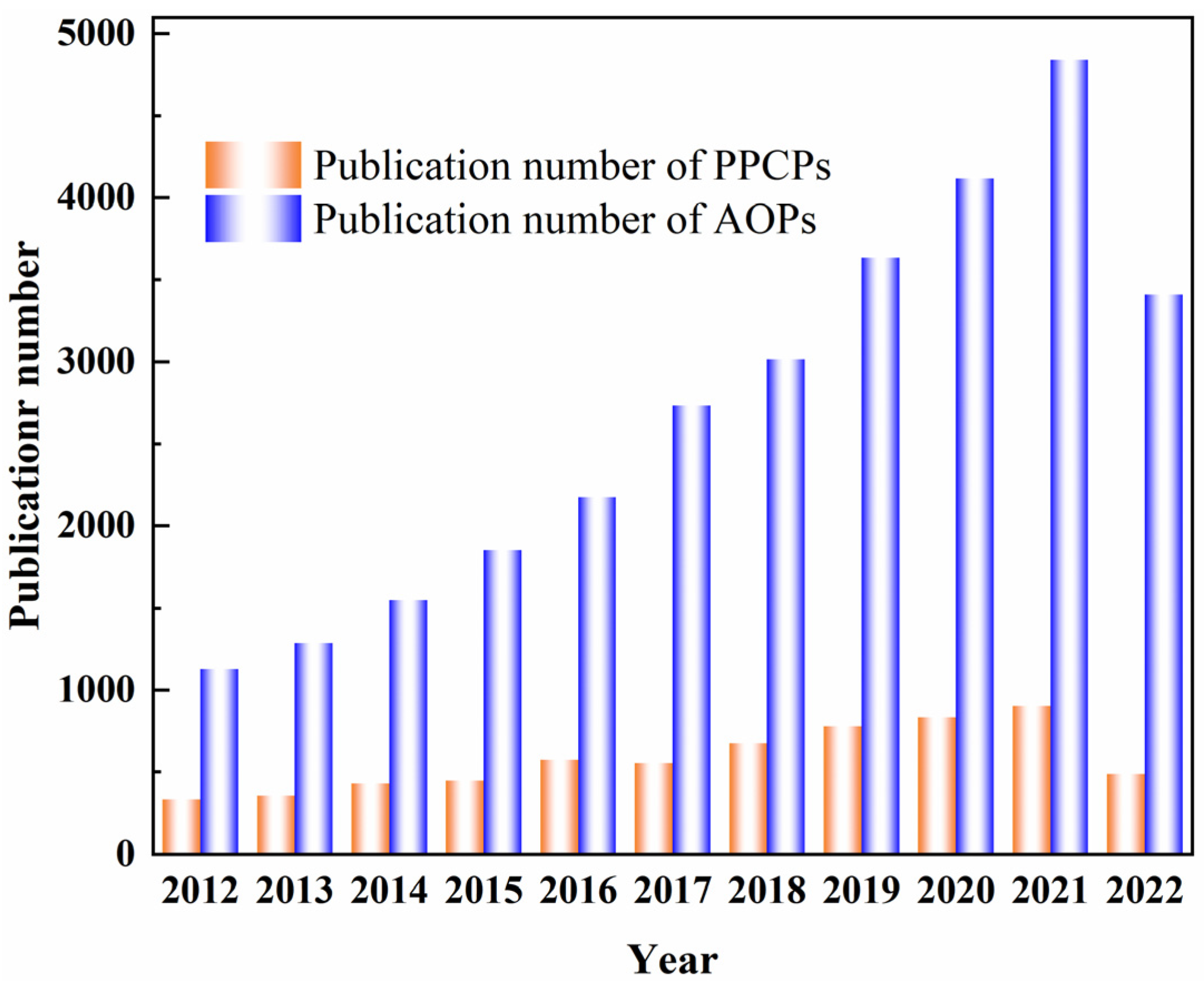
1. Introduction
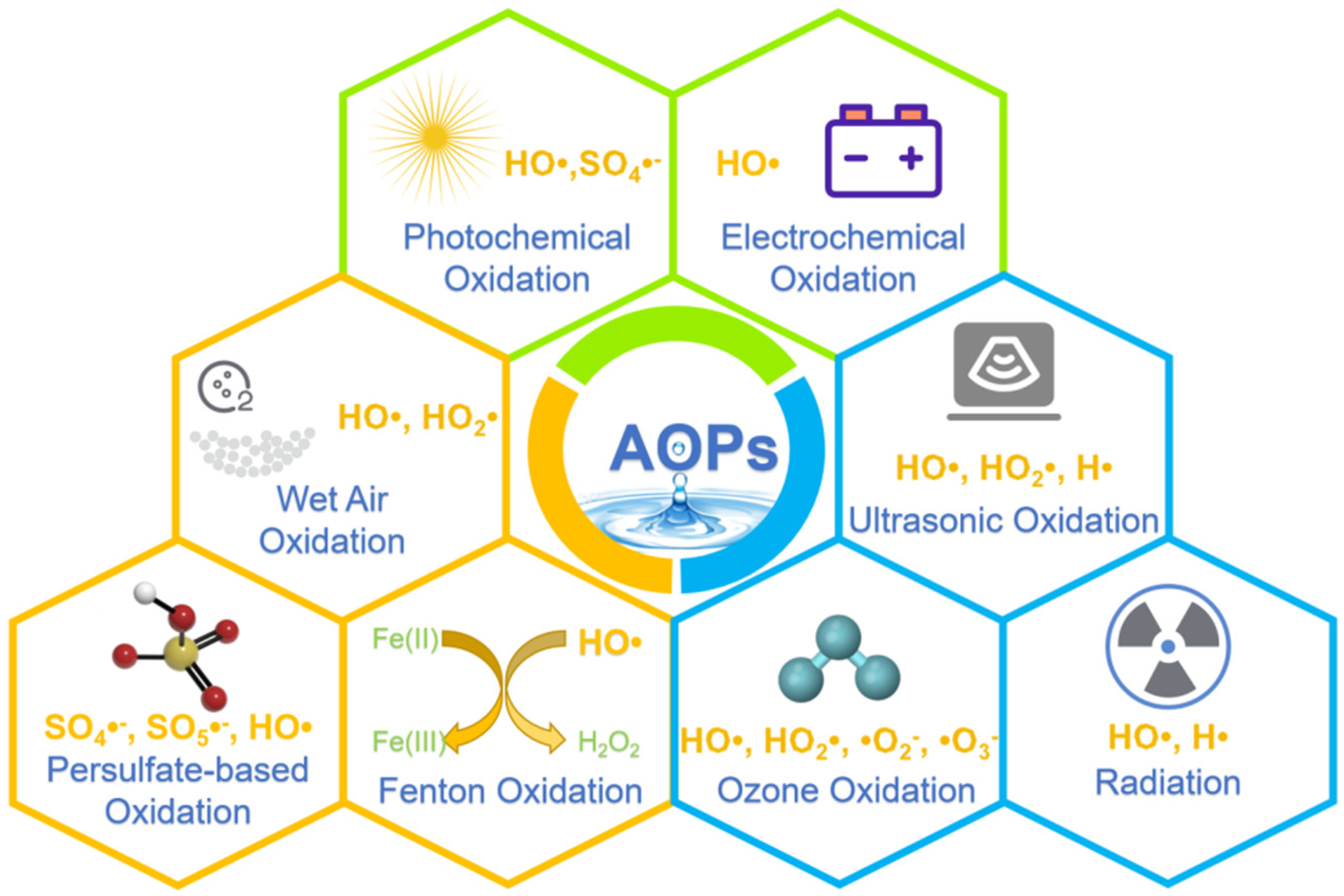
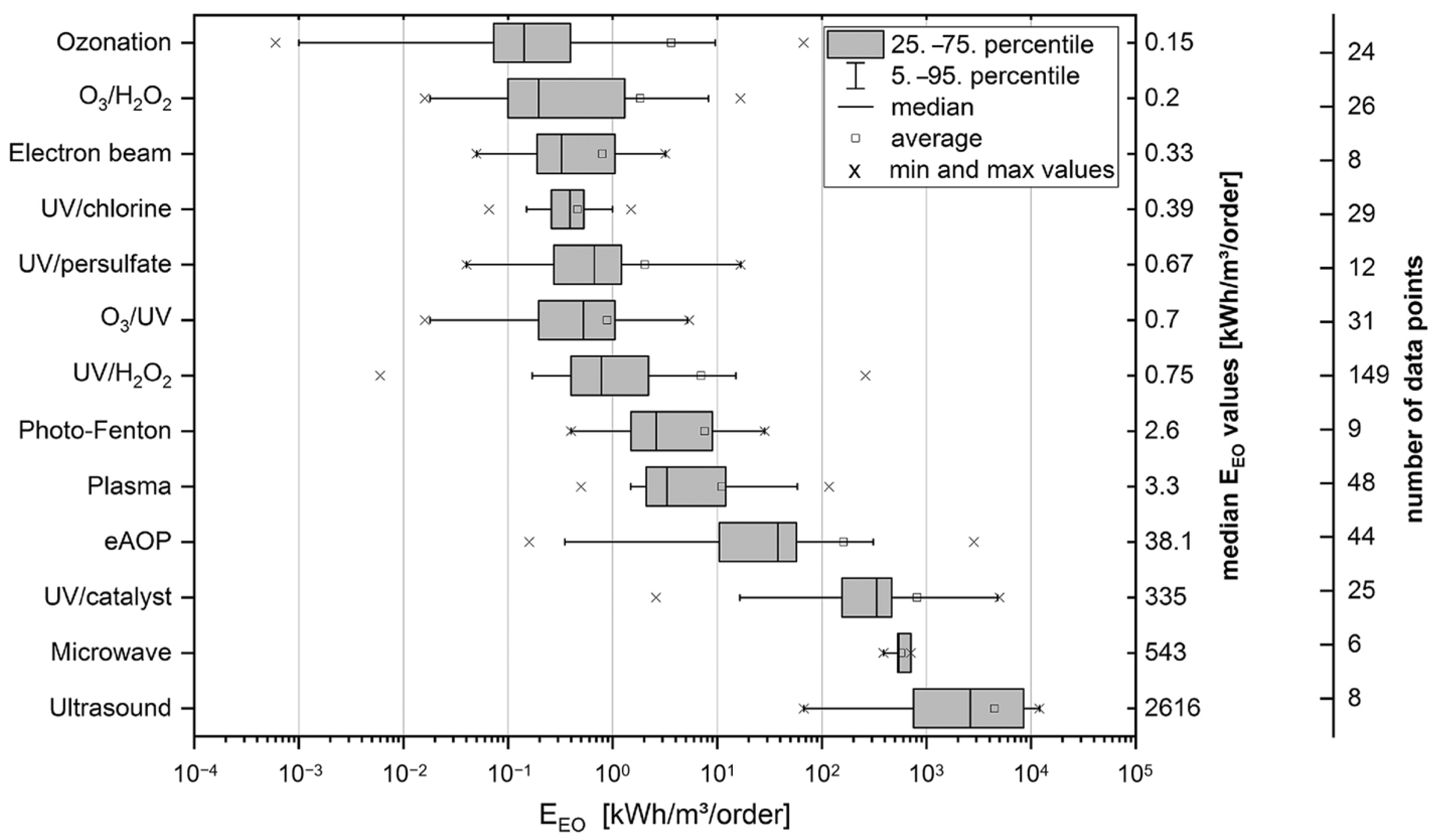
2. Various AOPs
2.1. Photochemical Oxidation
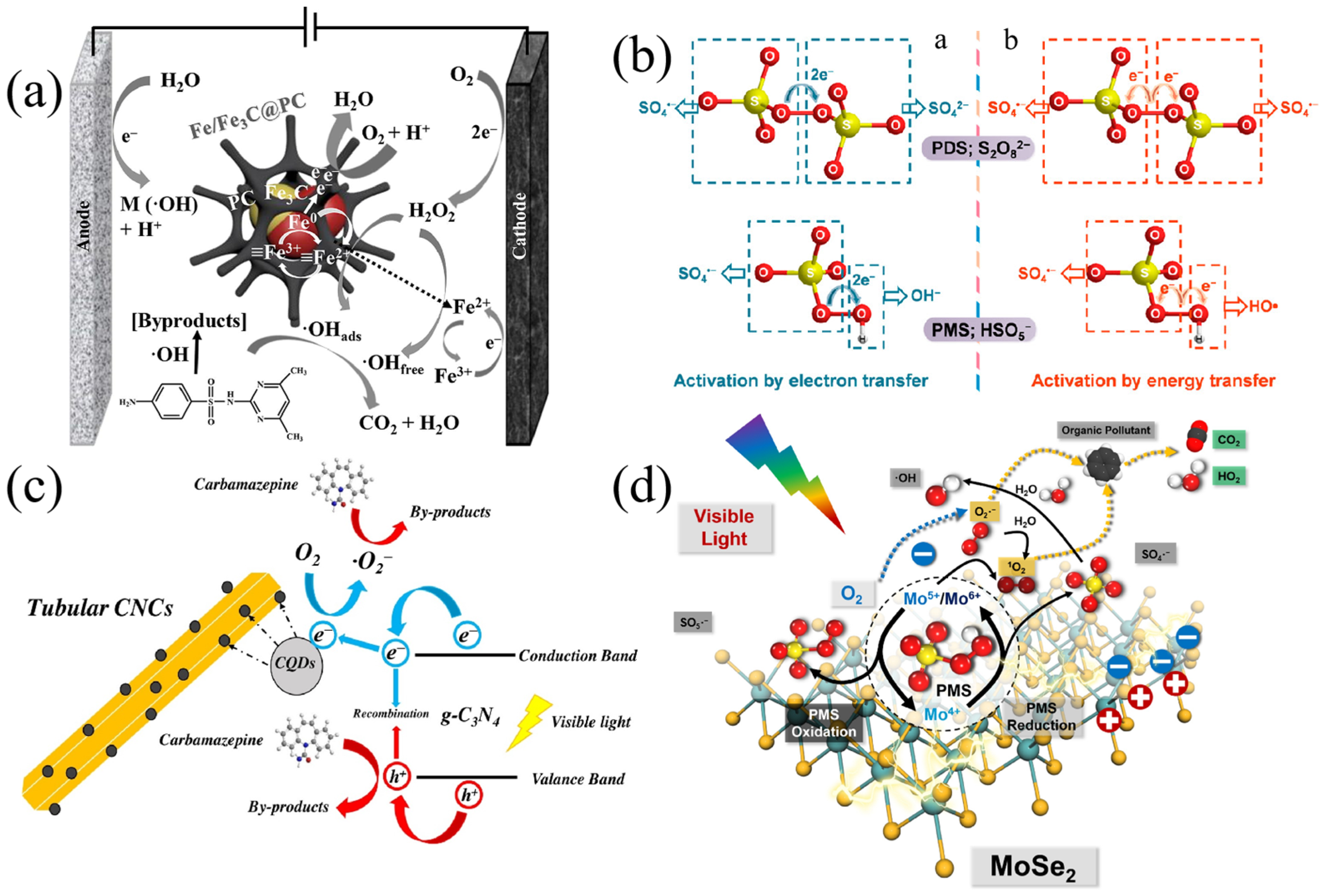
2.2. Electrochemical Oxidation
2.3. Wet Air Oxidation
2.4. Ultrasonic Oxidation
2.5. Gamma Ray/Electron Beam Radiation
2.6. Fenton Oxidation
2.7. The Ozone Oxidation Process
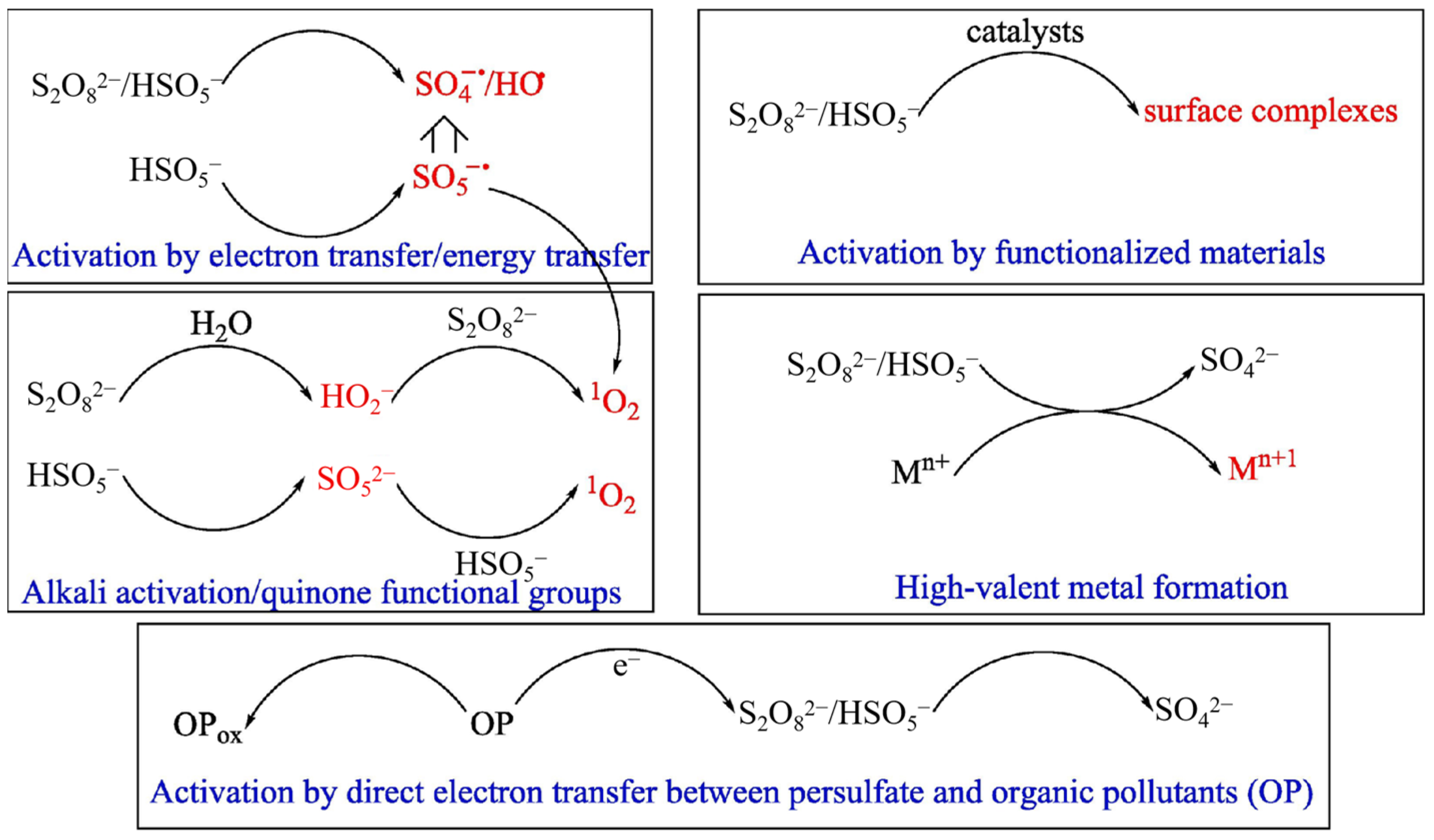
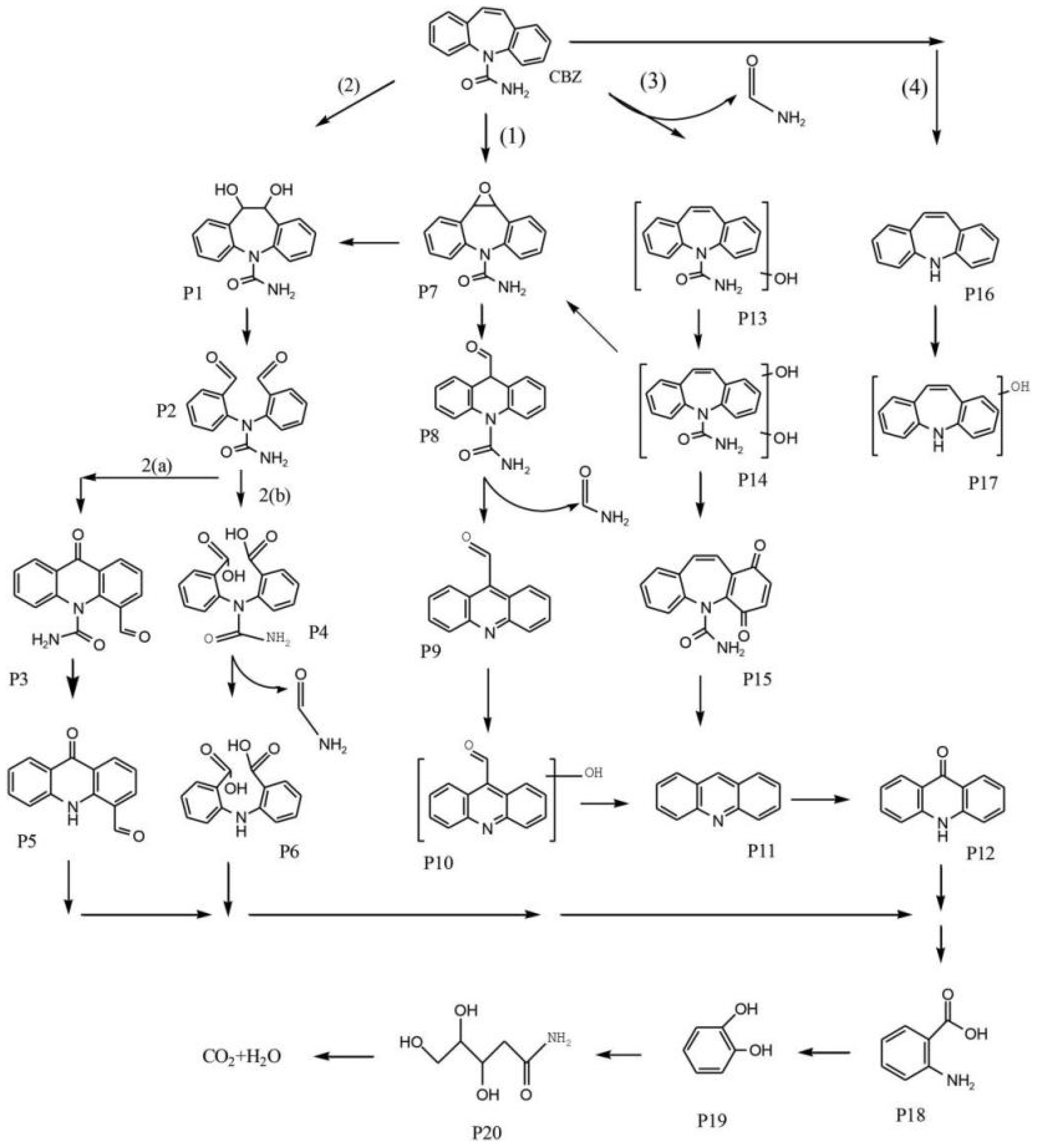
2.8. Persulfate-Based Oxidation
3. Different Free Radicals in the Removal of PPCPs
3.1. Hydroxyl Radical (HO·)
3.2. Sulfate Radical (SO4·−)
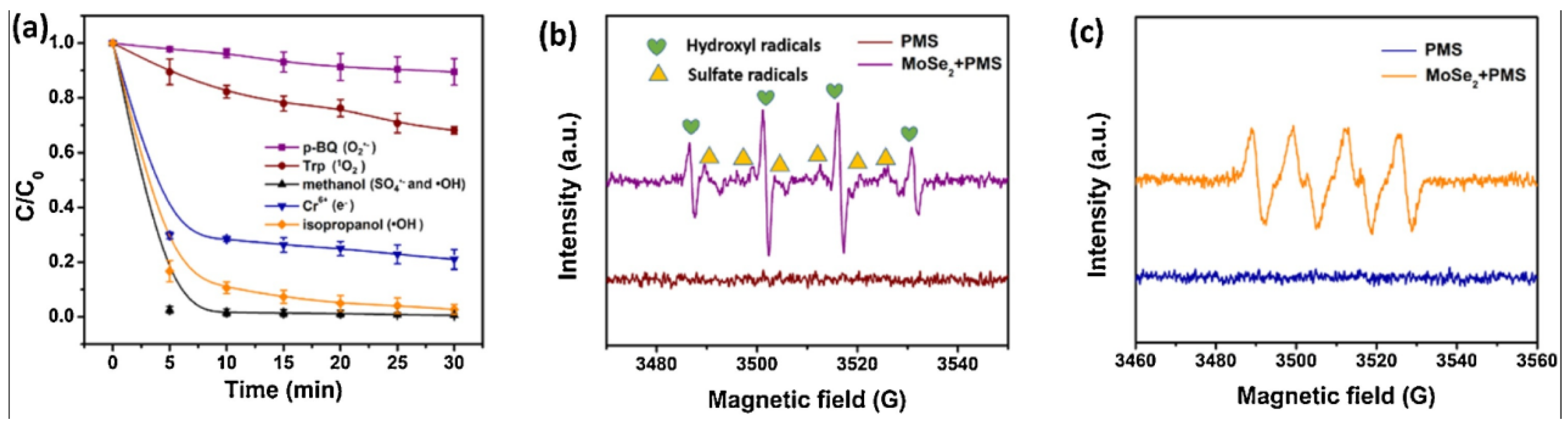
3.3. Superoxide Radical (O2·−)
3.4. Reactive Chlorine Species (RCS)
4. Perspective
- (1)
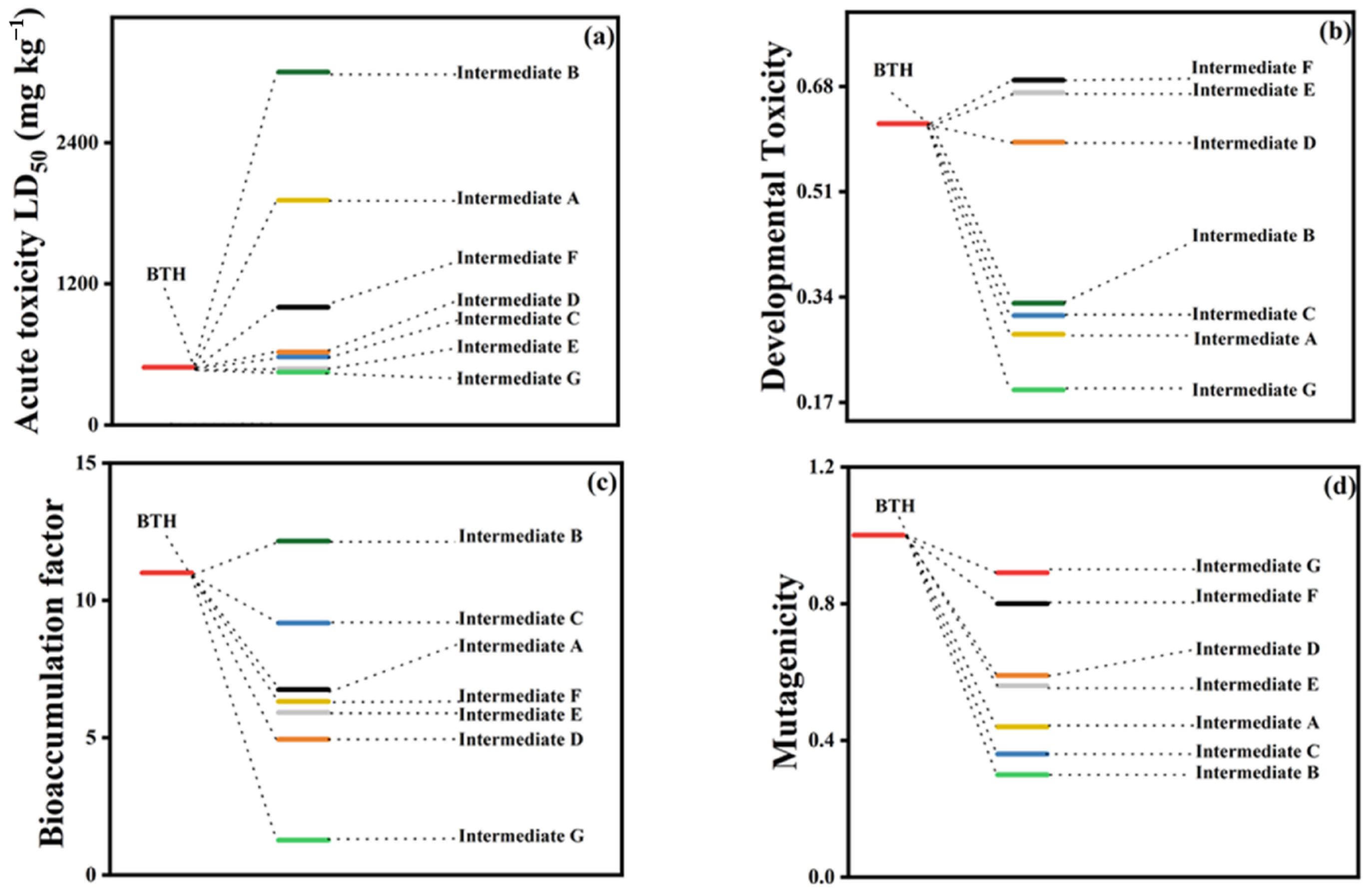
- To precisely identify the concentration of free radicals, more accurate approaches should be employed. The conventional radical identification methods are challenged because adding scavengers would cause negative effects on AOPs and influence the generation of free radicals. Other methods such as probe-based kinetic models, EPR and laser flash photolysis should be developed and employed to assist in identification of the free radicals in AOPs.
- (2)
- To achieve the practical application of AOPs, convenient and inexpensive approaches should be studied. At present, there are still some issues with AOPs, which need to be further studied. For example, the experimental operations are still complicated and have a high cost. Thus, while researchers focus on the efficiency of water treatment, convenient and inexpensive approaches should be studied to realize large-scale application.
- (3)
- To further study the reaction process and reveal the impacts of AOPs, the mechanism of AOPs in multiple mixture systems should be investigated in depth and the interference of impurity ions on the degradation reaction should be minimized.
- (4)
- To remove PPCPs from complex aqueous mixtures, a pilot-scale plant on the treatment of practical wastewater should be implemented instead of a laboratory scale experiment. Currently, most studies on the removal of PPCPs are focused on water environments containing limited given substances, which is unrealistic. Thus, practical wastewater (i.e., pharmaceutical discharges) should be used in later studies.
- (5)
- To selectively remove PPCPs from aqueous environments, AOPs should be further developed to adjust them to multiple water environments. In the later studies, the removal of PPCPs is expected to be achieved without affecting the existence of trace nutritious natural organic matters (NOMs) in water environments [181].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Noguera-Oviedo, K.; Aga, D.S. Lessons learned from more than two decades of research on emerging contaminants in the environment. J. Hazard. Mater. 2016, 316, 242–251. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef]
- Arpin-Pont, L.; Bueno, M.J.M.; Gomez, E.; Fenet, H. Occurrence of PPCPs in the marine environment: A review. Environ. Sci. Pollut. Res. 2016, 23, 4978–4991. [Google Scholar] [CrossRef]
- Caliman, F.A.; Gavrilescu, M. Pharmaceuticals, Personal Care Products and Endocrine Disrupting Agents in the Environment—A Review. Clean-Soil Air Water 2009, 37, 277–303. [Google Scholar]
- Ngo, T.H.; Van, D.A.; Tran, H.L.; Nakada, N.; Tanaka, H.; Huynh, T.H. Occurrence of pharmaceutical and personal care products in Cau River, Vietnam. Environ. Sci. Pollut. Res. 2021, 28, 12082–12091. [Google Scholar] [CrossRef]
- Spurgeon, D.; Wilkinson, H.; Civil, W.; Hutt, L.; Armenise, E.; Kieboom, N.; Sims, K.; Besien, T. Worst-case ranking of organic chemicals detected in groundwaters an surface waters in England. Sci. Total Environ. 2022, 835, 155101. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Lei, Y.; Liu, X.; Song, L.Y.; Hamid, N.; Zhang, R. Sulfonamide and tetracycline in landfill leachates from seven municipal solid waste (MSW) landfills: Seasonal variation and risk assessment. Sci. Total Environ. 2022, 825, 153936. [Google Scholar] [CrossRef]
- Pisetta, A.M.; Roveri, V.; Guimaraes, L.L.; de Oliveira, T.M.N.; Correia, A.T. First report on the occurrence of pharmaceuticals and cocaine in the coastal waters of Santa Catarina, Brazil, and its related ecological risk assessment. Environ. Sci. Pollut. Res. 2022, 29, 63099–63111. [Google Scholar] [CrossRef]
- Korkmaz, N.E.; Savun-Hekimoglu, B.; Aksu, A.; Burak, S.; Caglar, N.B. Occurrence, sources and environmental risk assessment of pharmaceuticals in the Sea of Marmara, Turkey. Sci. Total Environ. 2022, 819, 152996. [Google Scholar] [CrossRef]
- Anand, U.; Adelodun, B.; Cabreros, C.; Kumar, P.; Suresh, S.; Dey, A.; Ballesteros, F.; Bontempi, E. Occurrence, transformation, bioaccumulation, risk and analysis of pharmaceutical and personal care products from wastewater: A review. Environ. Chem. Lett. 2022. [Google Scholar] [CrossRef]
- Liu, J.L.; Wong, M.H. Pharmaceuticals and personal care products (PPCPs): A review on environmental contamination in China. Environ. Int. 2013, 59, 208–224. [Google Scholar] [CrossRef]
- Rahman, M.F.; Yanful, E.K.; Jasim, S.Y. Endocrine disrupting compounds (EDCs) and pharmaceuticals and personal care products (PPCPs) in the aquatic environment: Implications for the drinking water industry and global environmental health. J. Water Health 2009, 7, 224–243. [Google Scholar] [CrossRef]
- Pereira, A.L.; Barros, R.T.D.; Pereira, S.R. Pharmacopollution and Household Waste Medicine (HWM): How reverse logistics is environmentally important to Brazil. Environ. Sci. Pollut. Res. 2017, 24, 24061–24075. [Google Scholar] [CrossRef]
- Insani, W.N.; Qonita, N.A.; Jannah, S.S.; Nuraliyah, N.M.; Supadmi, W.; Gatera, V.A.; Alfian, S.D.; Abdulah, R. Improper disposal practice of unused and expired pharmaceutical products in Indonesian households. Heliyon 2020, 6, se04551. [Google Scholar] [CrossRef]
- Yang, L.; Wang, T.Y.; Zhou, Y.Q.; Shi, B.; Bi, R.; Meng, J. Contamination, source and potential risks of pharmaceuticals and personal products (PPCPs) in Baiyangdian Basin, an intensive human intervention area, China. Sci. Total Environ. 2021, 760, 144080. [Google Scholar] [CrossRef]
- Chen, H.Z.; Chen, W.F.; Guo, H.G.; Lin, H.; Zhang, Y.B. Pharmaceuticals and personal care products in the seawater around a typical subtropical tourist city of China and associated ecological risk. Environ. Sci. Pollut. Res. 2021, 28, 22716–22728. [Google Scholar] [CrossRef]
- Khan, S.A.; Hussain, D.; Abbasi, N.; Khan, T.A. Deciphering the adsorption potential of a functionalized green hydrogel nanocomposite for aspartame from aqueous phase. Chemosphere 2022, 289, 133232. [Google Scholar] [CrossRef]
- Subedi, B.; Lee, S.; Moon, H.B.; Kannan, K. Emission of artificial sweeteners, select pharmaceuticals, and personal care products through sewage sludge from wastewater treatment plants in Korea. Environ. Int. 2014, 68, 33–40. [Google Scholar] [CrossRef]
- Wang, Y.J.; Yin, T.R.; Kelly, B.C.; Gin, K.Y.H. Bioaccumulation behaviour of pharmaceuticals and personal care products in a constructed wetland. Chemosphere 2019, 222, 275–285. [Google Scholar] [CrossRef]
- Sugihara, K. Effect of Environmental Factors on the Ecotoxicity of Pharmaceuticals and Personal Care Products. Yakugaku Zasshi J. Pharm. Soc. Jpn. 2018, 138, 277–280. [Google Scholar] [CrossRef]
- Al-Mashaqbeh, O.; Alsafadi, D.; Dalahmeh, S.; Bartelt-Hunt, S.; Snow, D. Removal of Selected Pharmaceuticals and Personal Care Products in Wastewater Treatment Plant in Jordan. Water 2019, 11, 2004. [Google Scholar] [CrossRef]
- Ismanto, A.; Hadibarata, T.; Kristanti, R.A.; Maslukah, L.; Safinatunnajah, N.; Sathishkumar, P. The abundance of endocrine-disrupting chemicals (EDCs) in downstream of the Bengawan Solo and Brantas rivers located in Indonesia. Chemosphere 2022, 297, 134151. [Google Scholar] [CrossRef]
- Chaves, M.D.S.; Kulzer, J.; de Lima, P.D.P.; Barbosa, S.C.; Primel, E.G. Updated knowledge, partitioning and ecological risk of pharmaceuticals and personal care products in global aquatic environments. Environ. Sci. Process Impacts 2022. [Google Scholar] [CrossRef]
- Xie, J.Y.; Liu, Y.F.; Wu, Y.F.; Li, L.R.; Fang, J.; Lu, X.Q. Occurrence, distribution and risk of pharmaceutical and personal care products in the Haihe River sediments, China. Chemosphere 2022, 302, 134874. [Google Scholar] [CrossRef]
- Priya, A.K.; Gnanasekaran, L.; Rajendran, S.; Qin, J.Q.; Vasseghian, Y. Occurrences and removal of pharmaceutical and personal care products from aquatic systems using advanced treatment—A review. Environ. Res. 2022, 204, 112298. [Google Scholar] [CrossRef]
- Mendoza, A.; Acena, J.; Perez, S.; de Alda, M.L.; Barcelo, D.; Gil, A.; Valcarcel, Y. Pharmaceuticals and iodinated contrast media in a hospital wastewater: A case study to analyse their presence and characterise their environmental risk and hazard. Environ. Res. 2015, 140, 225–241. [Google Scholar] [CrossRef]
- Khan, S.A.; Hussain, D.; Khan, T.A. Mechanistic evaluation of metformin drug confiscation from liquid phase on itaconic acid/kaolin hydrogel nanocomposite. Environ. Sci. Pollut. Res. 2021, 28, 53298–53313. [Google Scholar] [CrossRef]
- Slosarczyk, K.; Jakobczyk-Karpierz, S.; Rozkowski, J.; Witkowski, A.J. Occurrence of Pharmaceuticals and Personal Care Products in the Water Environment of Poland: A Review. Water 2021, 13, 2283. [Google Scholar] [CrossRef]
- Caldas, S.S.; Arias, J.L.O.; Rombaldi, C.; Mello, L.L.; Cerqueira, M.B.R.; Martins, A.F.; Primel, E.G. Occurrence of Pesticides and PPCPs in Surface and Drinking Water in Southern Brazil: Data on 4-Year Monitoring. J. Braz. Chem. Soc. 2019, 30, 71–80. [Google Scholar] [CrossRef]
- Liu, S.; Wang, C.; Wang, P.F.; Chen, J.; Wang, X.; Yuan, Q.S. Anthropogenic disturbances on distribution and sources of pharmaceuticals and personal care products throughout the Jinsha River Basin, China. Environ. Res. 2021, 198, 110449. [Google Scholar] [CrossRef]
- Jiang, X.S.; Qu, Y.X.; Zhong, M.M.; Li, W.C.; Huang, J.; Yang, H.W.; Yu, G. Seasonal and spatial variations of pharmaceuticals and personal care products occurrence and human health risk in drinking water—A case study of China. Sci. Total Environ. 2019, 694, 133711. [Google Scholar] [CrossRef]
- Van, D.A.; Ngo, T.H.; Huynh, T.H.; Nakada, N.; Ballesteros, F.; Tanaka, H. Distribution of pharmaceutical and personal care products (PPCPs) in aquatic environment in Hanoi and Metro Manila. Environ. Monit. Assess. 2021, 193, 847. [Google Scholar] [CrossRef]
- Sharma, B.M.; Becanova, J.; Scheringer, M.; Sharma, A.; Bharat, G.K.; Whitehead, P.G.; Klanova, J.; Nizzetto, L. Health and ecological risk assessment of emerging contaminants (pharmaceuticals, personal care products, and artificial sweeteners) in surface and groundwater (drinking water) in the Ganges River Basin, India. Sci. Total Environ. 2019, 646, 1459–1467. [Google Scholar] [CrossRef]
- Zainab, S.M.; Junaid, M.; Rehman, M.Y.A.; Lv, M.; Yue, L.X.; Xu, N.; Malik, R.N. First insight into the occurrence, spatial distribution, sources, and risks assessment of antibiotics in groundwater from major urban-rural settings of Pakistan. Sci. Total Environ. 2021, 791, 148298. [Google Scholar] [CrossRef]
- Benotti, M.J.; Trenholm, R.A.; Vanderford, B.J.; Holady, J.C.; Stanford, B.D.; Snyder, S.A. Pharmaceuticals and Endocrine Disrupting Compounds in US Drinking Water. Environ. Sci. Technol. 2009, 43, 597–603. [Google Scholar] [CrossRef]
- Carballa, M.; Omil, F.; Lema, J.M. Calculation methods to perform mass balances of micropollutants in sewage treatment plants. application to pharmaceutical and personal care products (PPCPs). Environ. Sci. Technol. 2007, 41, 884–890. [Google Scholar] [CrossRef]
- Awfa, D.; Ateia, M.; Fujii, M.; Johnson, M.S.; Yoshimura, C. Photodegradation of pharmaceuticals and personal care products in water treatment using carbonaceous-TiO2 composites: A critical review of recent literature. Water Res. 2018, 142, 26–45. [Google Scholar] [CrossRef]
- Wick, A.; Fink, G.; Joss, A.; Siegrist, H.; Ternes, T.A. Fate of beta blockers and psycho-active drugs in conventional wastewater treatment. Water Res. 2009, 43, 1060–1074. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res. 2009, 43, 363–380. [Google Scholar] [CrossRef]
- Oluwole, A.O.; Omotola, E.O.; Olatunji, O.S. Pharmaceuticals and personal care products in water and wastewater: A review of treatment processes and use of photocatalyst immobilized on functionalized carbon in AOP degradation. BMC Chem. 2020, 14, 62. [Google Scholar] [CrossRef]
- He, L.; Sun, X.M.; Zhu, F.P.; Ren, S.J.; Wang, S.G. OH-initiated transformation and hydrolysis of aspirin in AOPs system: DFT and experimental studies. Sci. Total Environ. 2017, 592, 33–40. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, T.J.; Zhang, Y.; Ge, F.; Steel, R.M.; Sun, L.Y. Advances in technologies for pharmaceuticals and personal care products removal. J. Mater. Chem. A 2017, 5, 12001–12014. [Google Scholar] [CrossRef]
- Fast, S.A.; Gude, V.G.; Truax, D.D.; Martin, J.; Magbanua, B.S. A Critical Evaluation of Advanced Oxidation Processes for Emerging Contaminants Removal. Environ. Process. 2017, 4, 283–302. [Google Scholar] [CrossRef]
- Somathilake, P.; Dominic, J.A.; Achari, G.; Langford, C.H.; Tay, J.H. Degradation of Carbamazepine by Photo-assisted Ozonation: Influence of Wavelength and Intensity of Radiation. Ozone Sci. Eng. 2018, 40, 113–121. [Google Scholar] [CrossRef]
- Villar-Navarro, E.; Levchuk, I.; Rueda-Marquez, J.J.; Homola, T.; Morinigo, M.A.; Vahala, R.; Manzano, M. Inactivation of simulated aquaculture stream bacteria at low temperature using advanced UVA- and solar-based oxidation methods. Sol. Energy 2021, 227, 477–489. [Google Scholar] [CrossRef]
- Agarkoti, C.; Gogate, P.R.; Pandit, A.B. Comparison of acoustic and hydrodynamic cavitation based hybrid AOPs for COD reduction of commercial effluent from CETP. J. Environ. Manag. 2021, 281, 111792. [Google Scholar] [CrossRef]
- Tian, D.Q.; Zhou, H.Y.; Zhang, H.; Zhou, P.; You, J.J.; Yao, G.; Pan, Z.C.; Liu, Y.; Lai, B. Heterogeneous photocatalyst-driven persulfate activation process under visible light irradiation: From basic catalyst design principles to novel enhancement strategies. Chem. Eng. J. 2022, 428, 131166. [Google Scholar] [CrossRef]
- Zhang, X.; Kamali, M.; Yu, X.B.; Costa, M.E.V.; Appels, L.; Cabooter, D.; Dewil, R. Kinetics and mechanisms of the carbamazepine degradation in aqueous media using novel iodate-assisted photochemical and photocatalytic systems. Sci. Total Environ. 2022, 825, 153871. [Google Scholar] [CrossRef]
- Gmurek, M.; Olak-Kucharczyk, M.; Ledakowicz, S. Photochemical decomposition of endocrine disrupting compounds—A review. Chem. Eng. J. 2017, 310, 437–456. [Google Scholar] [CrossRef]
- Xiong, Y.; Dai, X.L.; Liu, Y.Y.; Du, C.Y.; Yu, G.L.; Xia, Y. Insights into highly effective catalytic persulfate activation on oxygen-functionalized mesoporous carbon for ciprofloxacin degradation. Environ. Sci. Pollut. Res. 2022, 29, 59013–59026. [Google Scholar] [CrossRef]
- Sun, W.J.; Lv, H.X.; Ma, L.; Tan, X.D.; Jin, C.Y.; Wu, H.L.; Chen, L.L.; Liu, M.Y.; Wei, H.Z.; Sun, C.L. Use of catalytic wet air oxidation (CWAO) for pretreatment of high-salinity high-organic wastewater. J. Environ. Sci. 2022, 120, 105–114. [Google Scholar] [CrossRef]
- Mahamuni, N.N.; Adewuyi, Y.G. Advanced oxidation processes (AOPs) involving ultrasound for waste water treatment: A review with emphasis on cost estimation. Ultrason. Sonochem. 2010, 17, 990–1003. [Google Scholar] [CrossRef]
- Ang, W.L.; McHugh, P.J.; Symes, M.D. Sonoelectrochemical processes for the degradation of persistent organic pollutants. Chem. Eng. J. 2022, 444, 136573. [Google Scholar] [CrossRef]
- Gao, H.L.; Wen, Z.N.; Sun, B.C.; Zou, H.K.; Chu, G.W. Intensification of ozone mass transfer for wastewater treatment using a rotating bar reactor. Chem. Eng. Process. 2022, 176, 108946. [Google Scholar] [CrossRef]
- Chavez, A.M.; Gimeno, O.; Rey, A.; Pliego, G.; Oropesa, A.L.; Alvarez, P.M.; Beltran, F.J. Treatment of highly polluted industrial wastewater by means of sequential aerobic biological oxidation-ozone based AOPs. Chem. Eng. J. 2019, 361, 89–98. [Google Scholar] [CrossRef]
- Gholami, M.; Souraki, B.A.; Pendashteh, A. Electro-activated persulfate oxidation (EC/PS) for the treatment of real oilfield produced water: Optimization, developed numerical kinetic model, and comparison with thermal/EC/PS and EC systems. Process Saf. Environ. Protect. 2021, 153, 384–402. [Google Scholar] [CrossRef]
- Nashat, M.; Mossad, M.; El-Etriby, H.K.; Alalm, M.G. Optimization of electrochemical activation of persulfate by BDD electrodes for rapid removal of sulfamethazine. Chemosphere 2022, 286, 131579. [Google Scholar] [CrossRef]
- Khodadadi, T.; Solgi, E.; Mortazavi, S.; Nourmoradi, H. Comparison of advanced oxidation methods of Fenton, UV/Fenton, and O-3/Fenton in treatment of municipal wastewater. Desalin. Water Treat. 2020, 206, 108–115. [Google Scholar] [CrossRef]
- Rueda-Marquez, J.J.; Levchuk, I.; Manzano, M.; Sillanpaa, M. Toxicity Reduction of Industrial and Municipal Wastewater by Advanced Oxidation Processes (Photo-Fenton, UVC/H2O2, Electro-Fenton and Galvanic Fenton): A Review. Catalysts 2020, 10, 612. [Google Scholar] [CrossRef]
- Zhu, H.; Ning, S.Y.; Li, Z.Z.Q.; Wang, X.P.; Fujita, T.; Wei, Y.Z.; Yin, X.B. Synthesis of bimetallic NbCo-piperazine catalyst and study on its advanced redox treatment of pharmaceuticals and personal care products by activation of permonosulfate. Sep. Purif. Technol. 2022, 285, 120345. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, S.Z. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Bojanowska-Czajka, A. Application of Radiation Technology in Removing Endocrine Micropollutants from Waters and Wastewaters—A Review. Appl. Sci. 2021, 11, 12032. [Google Scholar] [CrossRef]
- Penalver, J.J.L.; Pacheco, C.V.G.; Polo, M.S.; Utrilla, J.R. Degradation of tetracyclines in different water matrices by advanced oxidation/reduction processes based on gamma radiation. J. Chem. Technol. Biotechnol. 2013, 88, 1096–1108. [Google Scholar] [CrossRef]
- Ghasemian, S.; Nasuhoglu, D.; Omanovic, S.; Yargeau, V. Photoelectrocatalytic degradation of pharmaceutical carbamazepine using Sb-doped Sn-80%-W-20%-oxide electrodes. Sep. Purif. Technol. 2017, 188, 52–59. [Google Scholar] [CrossRef]
- Prado, T.M.; Silva, F.L.; Carrico, A.; Lanza, M.R.D.; Fatibello, O.; Moraes, F.C. Photoelectrocatalytic degradation of caffeine using bismuth vanadate modified with reduced graphene oxide. Mater. Res. Bull. 2022, 145, 111539. [Google Scholar] [CrossRef]
- Lee, J.; von Gunten, U.; Kim, J.H. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef]
- Lang, X.J.; Ma, W.H.; Chen, C.C.; Ji, H.W.; Zhao, J.C. Selective Aerobic Oxidation Mediated by TiO2 Photocatalysis. Accounts Chem. Res. 2014, 47, 355–363. [Google Scholar] [CrossRef]
- Augugliaro, V.; Bellardita, M.; Loddo, V.; Palmisano, G.; Palmisano, L.; Yurdakal, S. Overview on oxidation mechanisms of organic compounds by TiO2 in heterogeneous photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 224–245. [Google Scholar] [CrossRef]
- Zhang, J.; Nosaka, Y. Generation of OH radicals and oxidation mechanism in photocatalysis of WO3 and BiVO4 powders. J. Photochem. Photobiol. A Chem. 2015, 303, 53–58. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, J.D.; Cao, H.B.; Guo, Z.; Rabeah, J.; Bruckner, A.; Xie, Y.B. The role of ozone and influence of band structure in WO3 photocatalysis and ozone integrated process for pharmaceutical wastewater treatment. J. Hazard. Mater. 2018, 360, 481–489. [Google Scholar] [CrossRef]
- Savun-Hekimoglu, B.; Eren, Z.; Ince, N.H. Photocatalytic Destruction of Caffeine on Sepiolite-Supported TiO2 Nanocomposite. Sustainability 2020, 12, 10314. [Google Scholar] [CrossRef]
- Lan, Y.C.; Lu, Y.L.; Ren, Z.F. Mini review on photocatalysis of titanium dioxide nanoparticles and their solar applications. Nano Energy 2013, 2, 1031–1045. [Google Scholar] [CrossRef]
- Ran, Z.L.; Fang, Y.H.; Sun, J.; Ma, C.; Li, S.F. Photocatalytic Oxidative Degradation of Carbamazepine by TiO2 Irradiated by UV Light Emitting Diode. Catalysts 2020, 10, 540. [Google Scholar] [CrossRef]
- Khraisheh, M.; Kim, J.; Campos, L.; Al-Muhtaseb, A.H.; Al-Hawari, A.; Al Ghouti, M.; Walker, G.M. Removal of pharmaceutical and personal care products (PPCPs) pollutants from water by novel TiO2-Coconut Shell Powder (TCNSP) composite. J. Ind. Eng. Chem. 2014, 20, 979–987. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, M.; Fang, L.P.; Lo, I.M.C. Visible-light-driven N-TiO2@SiO2@Fe3O4 magnetic nanophotocatalysts: Synthesis, characterization, and photocatalytic degradation of PPCPs. J. Hazard. Mater. 2019, 370, 108–116. [Google Scholar] [CrossRef]
- Cao, T.T.; Cui, H.; Zhou, D.D.; Ren, X.; Cui, C.W. Degradation mechanism of BPA under VUV irradiation: Efficiency contribution and DFT calculations. Environ. Sci. Pollut. Res. 2022. [Google Scholar] [CrossRef]
- Chuang, Y.H.; Chen, S.; Chinn, C.J.; Mitch, W.A. Comparing the UV/Monochloramine and UV/Free Chlorine Advanced Oxidation Processes (AOPs) to the UV/Hydrogen Peroxide AOP under Scenarios Relevant to Potable Reuse. Environ. Sci. Technol. 2017, 51, 13859–13868. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, S.Z. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Fu, H.; Gray, K.A. TiO2 (Core)/Crumpled Graphene Oxide (Shell) Nanocomposites Show Enhanced Photodegradation of Carbamazepine. Nanomaterials 2021, 11, 2087. [Google Scholar] [CrossRef]
- Wang, Y.; Penas-Garzon, M.; Rodriguez, J.J.; Bedia, J.; Belver, C. Enhanced photodegradation of acetaminophen over Sr@TiO2/UiO-66-NH2 heterostructures under solar light irradiation. Chem. Eng. J. 2022, 446, 137229. [Google Scholar] [CrossRef]
- Lozano, I.; Perez-Guzman, C.J.; Mora, A.; Mahlknecht, J.; Aguilar, C.L.; Cervantes-Aviles, P. Pharmaceuticals and personal care products in water streams: Occurrence, detection, and removal by electrochemical advanced oxidation processes. Sci. Total Environ. 2022, 827, 154348. [Google Scholar] [CrossRef]
- Guo, D.; Huang, Z.; Liu, Y.Y.; Zhang, Q.; Yang, Y.L.; Hong, J.M. Incorporation of single-atom copper into nitrogen-doped graphene for acetaminophen electrocatalytic degradation. Appl. Surf. Sci. 2022, 604, 154561. [Google Scholar] [CrossRef]
- Xia, Y.J.; Yan, Y.; Hu, L.B.; Dai, Q.Z.; Ma, X.J.; Lou, J.Q.; Xia, Y. Enhanced Mechanism of Electrochemical Oxidation of Antibiotic Norfloxacin using a Ti/SnO2-Sb2O3/alpha,beta-Co-PbO2 Electrode. J. Electrochem. Soc. 2021, 168, 106510. [Google Scholar] [CrossRef]
- Mishra, V.S.; Mahajani, V.V.; Joshi, J.B. Wet air oxidation. Ind. Eng. Chem. Res. 1995, 34, 2–48. [Google Scholar] [CrossRef]
- Luck, F. Wet air oxidation: Past, present and future. Catal. Today 1999, 53, 81–91. [Google Scholar] [CrossRef]
- Zhu, L.B.; Sheng, D. Study on wet oxidation of antibiotic wastewater. Oxid. Commun. 2016, 39, 1640–1645. [Google Scholar]
- Boucher, V.; Beaudon, M.; Ramirez, P.; Lemoine, P.; Volk, K.; Yargeau, V.; Segura, P.A. Comprehensive evaluation of non-catalytic wet air oxidation as a pretreatment to remove pharmaceuticals from hospital effluents. Environ. Sci. Wat. Res. Technol. 2021, 7, 1301–1314. [Google Scholar] [CrossRef]
- Boffito, D.C.; Crocella, V.; Pirola, C.; Neppolian, B.; Cerrato, G.; Ashokkumar, M.; Bianchi, C.L. Ultrasonic enhancement of the acidity, surface area and free fatty acids esterification catalytic activity of sulphated ZrO2-TiO2 systems. J. Catal. 2013, 297, 17–26. [Google Scholar] [CrossRef]
- Joseph, J.M.; Destaillats, H.; Hung, H.M.; Hoffmann, M.R. The sonochemical degradation of azobenzene and related azo dyes: Rate enhancements via Fenton’s reactions. J. Phys. Chem. A 2000, 104, 301–307. [Google Scholar] [CrossRef]
- Sierra, R.S.C.; Zuniga-Benitez, H.; Penuela, G.A. Elimination of cephalexin and doxycycline under low frequency ultrasound. Ultrason. Sonochem. 2021, 79, 105777. [Google Scholar] [CrossRef]
- Camargo-Perea, A.L.; Serna-Galvis, E.A.; Lee, J.; Torres-Palma, R.A. Understanding the effects of mineral water matrix on degradation of several pharmaceuticals by ultrasound: Influence of chemical structure and concentration of the pollutants. Ultrason. Sonochem. 2021, 73, 105500. [Google Scholar] [CrossRef]
- Chen, L.; Yin, W.T.; Shao, H.Y.; Tu, M.X.; Ren, Y.F.; Mao, C.K.; Huo, Z.H.; Xu, G. The performance and pathway of benzothiazole degradation by electron beam irradiation. Chemosphere 2022, 303, 134964. [Google Scholar] [CrossRef]
- Trojanowicz, M.; Bojanowska-Czajka, A.; Szreder, T.; Meczynska-Wielgosz, S.; Bobrowski, K.; Fornal, E.; Nichipor, H. Application of ionizing radiation for removal of endocrine disruptor bisphenol A from waters and wastewaters. Chem. Eng. J. 2021, 403, 126169. [Google Scholar] [CrossRef]
- Wang, J.L.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2020, 701, 135023. [Google Scholar] [CrossRef]
- Sonmez, G.; Bahadir, T.; Isik, M. Removal of selected pharmaceuticals from tap water by the Fenton process. Int. J. Environ. Anal. Chem. 2020. [Google Scholar] [CrossRef]
- Wang, S.Z.; Wang, J.L. Trimethoprim degradation by Fenton and Fe(II)-activated persulfate processes. Chemosphere 2018, 191, 97–105. [Google Scholar] [CrossRef]
- El-Ghenymy, A.; Rodriguez, R.M.; Arias, C.; Centellas, F.; Garrido, J.A.; Cabot, P.L.; Brillas, E. Electro-Fenton and photoelectro-Fenton degradation of the antimicrobial sulfamethazine using a boron-doped diamond anode and an air-diffusion cathode. J. Electroanal. Chem. 2013, 701, 7–13. [Google Scholar] [CrossRef]
- Wang, J.L.; Bai, Z.Y. Fe-based catalysts for heterogeneous catalytic ozonation of emerging contaminants in water and wastewater. Chem. Eng. J. 2017, 312, 79–98. [Google Scholar] [CrossRef]
- Wang, J.L.; Chen, H. Catalytic ozonation for water and wastewater treatment: Recent advances and perspective. Sci. Total Environ. 2020, 704, 135249. [Google Scholar] [CrossRef]
- Yang, Y.L.; Fu, W.Y.; Chen, X.X.; Chen, L.; Hou, C.Y.; Tang, T.H.; Zhang, X.H. Ceramic nanofiber membrane anchoring nanosized Mn2O3 catalytic ozonation of sulfamethoxazole in water. J. Hazard. Mater. 2022, 436, 129168. [Google Scholar] [CrossRef]
- Paucar, N.E.; Kim, I.; Tanaka, H.; Sato, C. Ozone treatment process for the removal of pharmaceuticals and personal care products in wastewater. Ozone Sci. Eng. 2019, 41, 3–16. [Google Scholar] [CrossRef]
- Verinda, S.B.; Muniroh, M.; Yulianto, E.; Maharani, N.; Gunawan, G.; Amalia, N.F.; Hobley, J.; Usman, A.; Nur, M. Degradation of ciprofloxacin in aqueous solution using ozone microbubbles: Spectroscopic, kinetics, and antibacterial analysis. Heliyon 2022, 8, e10137. [Google Scholar] [CrossRef]
- Esquerdo, A.A.; Gadea, I.S.; Galvan, P.J.V.; Rico, D.P. Efficacy of atrazine pesticide reduction in aqueous solution using activated carbon, ozone and a combination of both. Sci. Total Environ. 2021, 764, 144301. [Google Scholar] [CrossRef]
- Azuma, T.; Usui, M.; Hayashi, T. Inactivation of Antibiotic-Resistant Bacteria in Wastewater by Ozone-Based Advanced Water Treatment Processes. Antibiotics 2022, 11, 210. [Google Scholar] [CrossRef]
- Johnson, R.L.; Tratnyek, P.G.; Johnson, R.O. Persulfate Persistence under Thermal Activation Conditions. Environ. Sci. Technol. 2008, 42, 9350–9356. [Google Scholar] [CrossRef]
- Li, N.; Wu, S.; Dai, H.X.; Cheng, Z.J.; Peng, W.C.; Yan, B.B.; Chen, G.Y.; Wang, S.B.; Duan, X.G. Thermal activation of persulfates for organic wastewater purification: Heating modes, mechanism and influencing factors. Chem. Eng. J. 2022, 450, 137976. [Google Scholar] [CrossRef]
- Liang, Z.L.; Peng, G.W.; Hu, J.P.; Hou, H.J.; Cai, C.; Yang, X.R.; Chen, S.J.; Liu, L.; Liang, S.; Xiao, K.K.; et al. Mechanochemically assisted persulfate activation for the facile recovery of metals from spent lithium ion batteries. Waste Manag. 2022, 150, 290–300. [Google Scholar] [CrossRef]
- Liu, X.T.; Zhang, X.H.; Shao, K.; Lin, C.Y.; Li, C.B.; Ge, F.Z.; Dong, Y.J. Fe-0-activated persulfate-assisted mechanochemical destruction of expired compound sulfamethoxazole tablets. RSC Adv. 2016, 6, 20938–20948. [Google Scholar] [CrossRef]
- Huang, W.Q.; Xiao, S.; Zhong, H.; Yan, M.; Yang, X. Activation of persulfates by carbonaceous materials: A review. Chem. Eng. J. 2021, 418, 129297. [Google Scholar] [CrossRef]
- Oyekunle, D.T.; Zhou, X.Q.; Shahzad, A.; Chen, Z.Q. Review on carbonaceous materials as persulfate activators: Structure-performance relationship, mechanism and future perspectives on water treatment. J. Mater. Chem. A 2021, 9, 8012–8050. [Google Scholar] [CrossRef]
- Huang, M.Q.; Wang, X.L.; Zhu, C.Y.; Zhu, F.X.; Liu, P.; Wang, D.X.; Fang, G.D.; Chen, N.; Gao, S.X.; Zhou, D.M. Efficient chlorinated alkanes degradation in soil by combining alkali hydrolysis with thermally activated persulfate. J. Hazard. Mater. 2022, 438, 129571. [Google Scholar] [CrossRef]
- Hong, Y.X.; Luo, Z.J.; Zhang, N.; Qu, L.L.; Zheng, M.; Suara, M.A.; Chelme-Ayala, P.; Zhou, X.T.; El-Din, M.G. Decomplexation of Cu(II)-EDTA by synergistic activation of persulfate with alkali and CuO: Kinetics and activation mechanism. Sci. Total Environ. 2022, 817, 152793. [Google Scholar] [CrossRef]
- Song, H.R.; Yan, L.X.; Jiang, J.; Ma, J.; Zhang, Z.X.; Zhang, J.M.; Liu, P.X.; Yang, T. Electrochemical activation of persulfates at BDD anode: Radical or nonradical oxidation? Water Res. 2018, 128, 393–401. [Google Scholar] [CrossRef]
- Zhi, D.; Lin, Y.H.; Jiang, L.; Zhou, Y.Y.; Huang, A.Q.; Yang, J.; Luo, L. Remediation of persistent organic pollutants in aqueous systems by electrochemical activation of persulfates: A review. J. Environ. Manag. 2020, 260, 110125. [Google Scholar] [CrossRef]
- Wang, C.W.; Liang, C.J. Oxidative degradation of TMAH solution with UV persulfate activation. Chem. Eng. J. 2014, 254, 472–478. [Google Scholar] [CrossRef]
- Dubois, V.; Rodrigues, C.S.D.; Alves, A.S.P.; Madeira, L.M. UV/Vis-Based Persulphate Activation for p-Nitrophenol Degradation. Catalysts 2021, 11, 480. [Google Scholar] [CrossRef]
- Anushree, C.; Krishna, D.N.G.; Philip, J. Efficient Dye Degradation via Catalytic Persulfate Activation using Iron Oxide-Manganese Oxide Core-Shell Particle Doped with Transition Metal Ions. J. Mol. Liq. 2021, 337, 116429. [Google Scholar] [CrossRef]
- Huang, M.J.; Han, Y.; Xiang, W.; Zhong, D.L.; Wang, C.; Zhou, T.; Wu, X.H.; Mao, J. In Situ-Formed Phenoxyl Radical on the CuO Surface Triggers Efficient Persulfate Activation for Phenol Degradation. Environ. Sci. Technol. 2021, 55, 15361–15370. [Google Scholar] [CrossRef]
- Cheng, X.; Guo, H.G.; Zhang, Y.L.; Wu, X.; Liu, Y. Non-photochemical production of singlet oxygen via activation of persulfate by carbon nanotubes. Water Res. 2017, 113, 80–88. [Google Scholar] [CrossRef]
- Deng, X.Y.; Zhao, Z.W.; Wang, C.; Chen, R.; Du, J.Y.; Shi, W.X.; Cui, F.Y. Insight into the nonradical mechanism of persulfate activation via visible-light for enhanced degradation of sulfonamides without catalyst. Appl. Catal. B-Environ. 2022, 316, 121653. [Google Scholar] [CrossRef]
- Li, J.; Zou, J.; Zhang, S.; Cai, H.; Huang, Y.; Lin, J.; Li, Q.; Yuan, B.; Ma, J. Sodium tetraborate simultaneously enhances the degradation of acetaminophen and reduces the formation potential of chlorinated by-products with heat-activated peroxymonosulfate oxidation. Water Res. 2022, 224, 119095. [Google Scholar] [CrossRef]
- Weng, M.T.; Cai, M.Q.; Xie, Z.Q.; Dong, C.Y.; Zhang, Y.; Song, Z.J.; Shi, Y.J.; Jin, M.C.; Wang, Q.; Wei, Z.S. Hydrodynamic cavitation-enhanced heterogeneous activation of persulfate for tetracycline degradation: Synergistic effects, degradation mechanism and pathways. Chem. Eng. J. 2022, 431, 134238. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hubner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Constantin, M.A.; Chiriac, F.L.; Gheorghe, S.; Constantin, L.A. Degradation of Carbamazepine from Aqueous Solutions via TiO2-Assisted Photo Catalyze. Toxics 2022, 10, 168. [Google Scholar] [CrossRef]
- Yang, L.W.; Jia, Y.Y.; Peng, Y.Q.; Zhou, P.; Yu, D.A.; Zhao, C.L.; He, J.J.; Zhan, C.A.L.; Lai, B. Visible-light induced activation of persulfate by self-assembled EHPDI/TiO2 photocatalyst toward efficient degradation of carbamazepine. Sci. Total Environ. 2021, 783, 146996. [Google Scholar] [CrossRef]
- Zhou, S.Q.; Xia, Y.; Li, T.; Yao, T.; Shi, Z.; Zhu, S.M.; Gao, N.Y. Degradation of carbamazepine by UV/chlorine advanced oxidation process and formation of disinfection by-products. Environ. Sci. Pollut. Res. 2016, 23, 16448–16455. [Google Scholar] [CrossRef]
- Feijoo, S.; Kamali, M.; Pham, Q.K.; Assoumani, A.; Lestremau, F.; Cabooter, D.; Dewil, R. Electrochemical Advanced Oxidation of Carbamazepine: Mechanism and optimal operating conditions. Chem. Eng. J. 2022, 446, 137114. [Google Scholar] [CrossRef]
- De la Cruz, N.; Gimenez, J.; Esplugas, S.; Grandjean, D.; de Alencastro, L.F.; Pulgarin, C. Degradation of 32 emergent contaminants by UV and neutral photo-fenton in domestic wastewater effluent previously treated by activated sludge. Water Res. 2012, 46, 1947–1957. [Google Scholar] [CrossRef]
- Yentur, G.; Dukkanci, M. Synergistic effect of sonication on photocatalytic oxidation of pharmaceutical drug carbamazepine. Ultrason. Sonochem. 2021, 78, 105749. [Google Scholar] [CrossRef]
- Zheng, M.; Xu, G.; Zhao, L.; Pei, J.C.; Wu, M.H. Comparison of EB-radiolysis and UV/H2O2-degradation of CBZ in pure water and solutions. Nucl. Sci. Tech. 2015, 26, 020302. [Google Scholar]
- Mugunthan, E.; Saidutta, M.B.; Jagadeeshbabu, P.E. Photocatalytic activity of ZnO-WO3 for diclofenac degradation under visible light irradiation. J. Photochem. Photobiol. A Chem. 2019, 383, 111993. [Google Scholar]
- Finkbeiner, P.; Franke, M.; Anschuetz, F.; Ignaszak, A.; Stelter, M.; Braeutigam, P. Sonoelectrochemical degradation of the anti-inflammatory drug diclofenac in water. Chem. Eng. J. 2015, 273, 214–222. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, G.M.; Xian, G.; Zhang, N.; Li, J.W. A High-Efficiency CuO/CeO2 Catalyst for Diclofenac Degradation in Fenton-Like System. Front. Chem. 2019, 7, 796. [Google Scholar] [CrossRef]
- Gao, G.Y.; Chu, W.; Chen, Z.L.; Shen, J.M. Catalytic ozonation of diclofenac with iron silicate-loaded pumice in aqueous solution. Water Sci. Technol. Water Supply 2017, 17, 1458–1467. [Google Scholar] [CrossRef]
- Xian, G.; Zhang, G.M.; Chang, H.Z.; Zhang, Y.; Zou, Z.G.; Li, X.Y. Heterogeneous activation of persulfate by Co3O4-CeO2 catalyst for diclofenac removal. J. Environ. Manag. 2019, 234, 265–272. [Google Scholar] [CrossRef]
- Makropoulou, T.; Kortidis, I.; Davididou, K.; Motaung, D.E.; Chatzisymeon, E. Photocatalytic facile ZnO nanostructures for the elimination of the antibiotic sulfamethoxazole in water. J. Water Process. Eng. 2020, 36, 101299. [Google Scholar] [CrossRef]
- Wan, J.T.; Jin, C.J.; Liu, B.H.; She, Z.L.; Gao, M.C.; Wang, Z.Y. Electrochemical oxidation of sulfamethoxazole using Ti/SnO2-Sb/Co-PbO2 electrode through ANN-PSO. J. Serb. Chem. Soc. 2019, 84, 713–727. [Google Scholar] [CrossRef]
- Al-Hamadani, Y.A.J.; Chu, K.H.; Flora, J.R.V.; Kim, D.H.; Jang, M.; Sohn, J.; Joo, W.; Yoon, Y. Sonocatalytical degradation enhancement for ibuprofen and sulfamethoxazole in the presence of glass beads and single-walled carbon nanotubes. Ultrason. Sonochem. 2016, 32, 440–448. [Google Scholar] [CrossRef]
- Rostami, F.; Ramavandi, B.; Arfaeinia, H.; Nasrzadeh, F.; Hashemi, S. Sulfamethoxazole antibiotic removal from aqueous solution and hospital wastewater using photo-Fenton process. Desalin. Water Treat. 2020, 184, 388–394. [Google Scholar] [CrossRef]
- Wang, S.Z.; Wang, J.L. Synergistic effect of PMS activation by Fe-0@Fe3O4 anchored on N, S, O co-doped carbon composite for degradation of sulfamethoxazole. Chem. Eng. J. 2022, 427, 131960. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, T.H.; Cha, S.M.; Yu, S. Degradation of sulfamethoxazole by ionizing radiation: Identification and characterization of radiolytic products. Chem. Eng. J. 2017, 313, 556–566. [Google Scholar] [CrossRef]
- Gligorovski, S.; Strekowski, R.; Barbati, S.; Vione, D. Environmental Implications of Hydroxyl Radicals (center dot OH). Chem. Rev. 2015, 115, 13051–13092. [Google Scholar] [CrossRef]
- Sirtori, C.; Aguera, A.; Carra, I.; Perez, J.A.S. Identification and monitoring of thiabendazole transformation products in water during Fenton degradation by LC-QTOF-MS. Anal. Bioanal. Chem. 2014, 406, 5323–5337. [Google Scholar] [CrossRef]
- Babu, S.G.; Ashokkumar, M.; Neppolian, B. The Role of Ultrasound on Advanced Oxidation Processes. Top. Curr. Chem. 2016, 374, 75. [Google Scholar] [CrossRef]
- Cui, T.Y.; Xiao, Z.H.; Wang, Z.B.; Liu, C.; Song, Z.L.; Wang, Y.P.; Zhang, Y.T.; Li, R.Y.; Xu, B.B.; Qi, F.; et al. FeS2/carbon felt as an efficient electro-Fenton cathode for carbamazepine degradation and detoxification: In-depth discussion of reaction contribution and empirical kinetic model. Environ. Pollut. 2021, 282, 117023. [Google Scholar] [CrossRef]
- Du, X.; Fu, W.; Su, P.; Cai, J.; Zhou, M. Internal-micro-electrolysis-enhanced heterogeneous electro-Fenton process catalyzed by Fe/Fe3C@PC core-shell hybrid for sulfamethazine degradation. Chem. Eng. J. 2020, 398, 125681. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Xu, L.J.; Wang, J.L. Fenton-like degradation of 2,4-dichlorophenol using Fe3O4 magnetic nanoparticles. Appl. Catal. B Environ. 2012, 123, 117–126. [Google Scholar] [CrossRef]
- He, S.; Chen, Y.; Li, X.; Zeng, L.; Zhu, M. Heterogeneous Photocatalytic Activation of Persulfate for the Removal of Organic Contaminants in Water: A Critical Review. ACS EST Engg. 2022, 2, 527–546. [Google Scholar] [CrossRef]
- Zhao, C.; Liao, Z.; Liu, W.; Liu, F.; Ye, J.; Liang, J.; Li, Y. Carbon quantum dots modified tubular g-C3N4 with enhanced photocatalytic activity for carbamazepine elimination: Mechanisms, degradation pathway and DFT calculation. J. Hazard. Mater. 2020, 381, 120957. [Google Scholar] [CrossRef]
- Dong, C.; Wang, Z.; Ye, Z.; He, J.; Zheng, Z.; Gong, X.; Zhang, J.; Lo, I.M.C. Superoxide radicals dominated visible light driven peroxymonosulfate activation using molybdenum selenide (MoSe2) for boosting catalytic degradation of pharmaceuticals and personal care products. Appl. Catal. B Environ. 2021, 296, 120223. [Google Scholar] [CrossRef]
- Wang, L.; Li, B.Q.; Dionysiou, D.D.; Chen, B.Y.; Yang, J.; Li, J. Overlooked Formation of H2O2 during the Hydroxyl Radical-Scavenging Process When Using Alcohols as Scavengers. Environ. Sci. Technol. 2022, 56, 3386–3396. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D. Degradation of organic contaminants in water with sulfate radicals generated by the conjunction of peroxymonosulfate with cobalt. Environ. Sci. Technol. 2003, 37, 4790. [Google Scholar] [CrossRef]
- Hu, P.D.; Long, M.C. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Appl. Catal. B Environ. 2016, 181, 103–117. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Barbati, S.; Doumenq, P.; Chiron, S. Sulfate radical anion oxidation of diclofenac and sulfamethoxazole for water decontamination. Chem. Eng. J. 2012, 197, 440–447. [Google Scholar] [CrossRef]
- Norman, R.O.C.; Storey, P.M.; West, P.R. Electron spin resonance studies. Part XXV. Reactions of the sulphate radical anion with organic compounds. J. Chem. Soc. B Phys. Org. 1970, 1087–1095. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Zhou, C.Y.; Xiong, W.P.; Zeng, G.M.; Huang, D.L.; Zhang, C.; Wang, W.J.; Song, B.A.; Tang, X.; et al. Recent advances in application of graphitic carbon nitride-based catalysts for degrading organic contaminants in water through advanced oxidation processes beyond photocatalysis: A critical review. Water Res. 2020, 184, 116200. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, J.S.; Lin, H.J.; Yuan, J.Y. “Cooking carbon in a solid salt”: Synthesis of porous heteroatom-doped carbon foams for enhanced organic pollutant degradation under visible light. Appl. Mater. Today 2018, 12, 168–176. [Google Scholar] [CrossRef]
- Watts, R.J.; Ahmad, M.; Hohner, A.K.; Teel, A.L. Persulfate activation by glucose for in situ chemical oxidation. Water Res. 2018, 133, 247–254. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, B.T.; Teng, Y.G.; Zhao, J.J.; Kuang, L.L.; Sun, X.J. Carbon nanofibers supported Co/Ag bimetallic nanoparticles for heterogeneous activation of peroxymonosulfate and efficient oxidation of amoxicillin. J. Hazard. Mater. 2020, 400, 123290. [Google Scholar] [CrossRef]
- Gao, L.W.; Guo, Y.; Zhan, J.H.; Yu, G.; Wang, Y.J. Assessment of the validity of the quenching method for evaluating the role of reactive species in pollutant abatement during the persulfate-based process. Water Res. 2022, 221, 118730. [Google Scholar] [CrossRef]
- Fang, G.D.; Dionysiou, D.D.; Al-Abed, S.R.; Zhou, D.M. Superoxide radical driving the activation of persulfate by magnetite nanoparticles: Implications for the degradation of PCBs. Appl. Catal. B Environ. 2013, 129, 325–332. [Google Scholar] [CrossRef]
- Baum, R.M. Superoxide theory of oxygen-toxicity is center of heated debate. Chem. Eng. News 1984, 62, 20–26. [Google Scholar] [CrossRef]
- Sawyer, D.T.; Valentine, J.S. How super is superoxide. Accounts Chem. Res. 1981, 14, 393–400. [Google Scholar] [CrossRef]
- Frimer, A.A.; Farkashsolomon, T.; Aljadeff, G. Mechanism of the superoxide anion radical (o-2-.) mediated oxidation of diarylmethanes. J. Org. Chem. 1986, 51, 2093–2098. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, H.; Yan, S.; Song, W. Kinetics studies and mechanistic considerations on the reactions of superoxide radical ions with dissolved organic matter. Water Res. 2018, 149, 56–64. [Google Scholar] [CrossRef]
- Sharma, V.K.; Triantis, T.M.; Antoniou, M.G.; He, X.X.; Pelaez, M.; Han, C.S.; Song, W.H.; O’Shea, K.E.; de la Cruz, A.A.; Kaloudis, T.; et al. Destruction of microcystins by conventional and advanced oxidation processes: A review. Sep. Purif. Technol. 2012, 91, 3–17. [Google Scholar] [CrossRef]
- Yang, X.; Sun, J.L.; Fu, W.J.; Shang, C.; Li, Y.; Chen, Y.W.; Gan, W.H.; Fang, J.Y. PPCP degradation by UV/chlorine treatment and its impact on DBP formation potential in real waters. Water Res. 2016, 98, 309–318. [Google Scholar] [CrossRef]
- Hirakawa, T.; Nosaka, Y. Properties of O-2(center dot-) and OH center dot formed in TiO2 aqueous suspensions by photocatalytic reaction and the influence of H2O2 and some ions. Langmuir 2002, 18, 3247–3254. [Google Scholar] [CrossRef]
- Minakata, D.; Kamath, D.; Maetzold, S. Mechanistic Insight into the Reactivity of Chlorine-Derived Radicals in the Aqueous-Phase UV Chlorine Advanced Oxidation Process: Quantum Mechanical Calculations. Environ. Sci. Technol. 2017, 51, 6918–6926. [Google Scholar] [CrossRef]
- Xiang, Y.Y.; Fang, J.Y.; Shang, C. Kinetics and pathways of ibuprofen degradation by the UV/chlorine advanced oxidation process. Water Res. 2016, 90, 301–308. [Google Scholar] [CrossRef]
- Guo, K.H.; Wu, Z.H.; Shang, C.; Yao, B.; Hou, S.D.; Yang, X.; Song, W.H.; Fang, J.Y. Radical Chemistry and Structural Relationships of PPCP Degradation by UV/Chlorine Treatment in Simulated Drinking Water. Environ. Sci. Technol. 2017, 51, 10431–10439. [Google Scholar] [CrossRef]
- Wang, Z.Y.; An, N.; Shao, Y.S.; Gao, N.Y.; Du, E.D.; Xu, B. Experimental and simulation investigations of UV/persulfate treatment in presence of bromide: Effects on degradation kinetics, formation of brominated disinfection byproducts and bromate. Sep. Purif. Technol. 2020, 242, 116767. [Google Scholar] [CrossRef]
- Lei, Y.; Lei, X.; Westerhoff, P.; Tong, X.Y.; Ren, J.N.; Zhou, Y.J.; Cheng, S.S.; Ouyang, G.F.; Yang, X. Bromine Radical (Br(center dot)and Br-2(center dot-)) Reactivity with Dissolved OrganicMatter and Brominated Organic Byproduct Formation br. Environ. Sci. Technol. 2022, 56, 5189–5199. [Google Scholar] [CrossRef]
- Liu, Y.Q.; He, X.X.; Duan, X.D.; Fu, Y.S.; Fatta-Kassinos, D.; Dionysiou, D.D. Significant role of UV and carbonate radical on the degradation of oxytetracycline in UV-AOPs: Kinetics and mechanism. Water Res. 2016, 95, 195–204. [Google Scholar] [CrossRef]
- Gao, J.; Duan, X.D.; O'Shea, K.; Dionysiou, D.D. Degradation and transformation of bisphenol A in UV/Sodium percarbonate: Dual role of carbonate radical anion. Water Res. 2020, 171, 115394. [Google Scholar] [CrossRef]
- Zhu, S.M.; Tian, Z.C.; Wang, P.; Zhang, W.Q.; Bu, L.J.; Wu, Y.T.; Dong, B.Z.; Zhou, S.Q. The role of carbonate radicals on the kinetics, radical chemistry, and energy requirement of UV/chlorine and UV/H2O2 processes. Chemosphere 2021, 278, 130499. [Google Scholar] [CrossRef]
- Li, Y.; Dong, H.; Xiao, J.; Li, L.; Chu, D.; Hou, X.; Xiang, S.; Dong, Q.; Zhang, H. Advanced oxidation processes for water purification using percarbonate: Insights into oxidation mechanisms, challenges, and enhancing strategies. J. Hazard. Mater. 2022, 442, 130014. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Li, J.; Song, W.; Ma, R.; Yang, J.X.; Zhang, X.L.; Huang, F.; Dong, W.Y. Rapid degradation of atrazine by a novel advanced oxidation process of bisulfite/chlorine dioxide: Efficiency, mechanism, pathway. Chem. Eng. J. 2022, 445, 136558. [Google Scholar] [CrossRef]
- Cheng, S.S.; Zhang, X.R.; Song, W.H.; Pan, Y.H.; Lambropoulou, D.; Zhong, Y.; Du, Y.; Nie, J.X.; Yang, X. Photochemical oxidation of PPCPs using a combination of solar irradiation and free available chlorine. Sci. Total Environ. 2019, 682, 629–638. [Google Scholar] [CrossRef]
- Yang, Z.C.; Qian, J.S.; Shan, C.; Li, H.C.; Yin, Y.Y.; Pan, B.C. Toward Selective Oxidation of Contaminants in Aqueous Systems. Environ. Sci. Technol. 2021, 55, 14494–14514. [Google Scholar] [CrossRef]

| Location | Aquatic Environment | Chemical | Category | Concentration | Ref. |
|---|---|---|---|---|---|
| Poland | Groundwater | N,Ndiethyl-meta-toluamide(DEET) | Mosquito and insect repellants | 17.28 μg/L | [28] |
| 17β-oestradiol | Hormones | 48 ng/L | |||
| Surface water | CAF | Stimulants | 29.9955 μg/L | ||
| Bisphenol A (BPA) | Hormones | 3.113 μg/L | |||
| Drinking water | Azithromycin (AZM) | Antibiotics | 193 ng/L | ||
| Paracetamol | Non-steroidal anti-inflammatory drugs | 173 ng/L | |||
| IBP | Non-steroidal anti-inflammatory drugs | 224 ng/L | |||
| CAF | Stimulants | 159 ng/L | |||
| Brazil | Surface water | Avobenzone (ABZ) | Sunscreen agents | 340 ng/L | [29] |
| Glibenclamide (GBC) | Hypoglycemic drugs | 50–120 ng/L | |||
| Drinking water | Nimesulide (NI) | Non-steroidal anti-inflammatory drugs | 181 ng/L | ||
| Methylparaben | Preservatives | 234 ng/L | |||
| ABZ | Sunscreen agents | 290 ng/L | |||
| China | Surface water | SMX | Antibiotics | <LOQ–2.92 ng/L | [30] |
| 4-n-nonylphenol | Hormones | 9.90–457.40 ng/L | |||
| Salicylic acid (SA) | Analgesics | 2.92–34.12 ng/L | |||
| Drinking water | Sulfamethoxypyridazine | Antibacterial | 107.14 ng/L | [31] | |
| Lincomycin | Antibiotics | 1.00–29.32 ng/L | |||
| SMX | Antibiotics | <LOQ–2.18 ng/L | |||
| Vietnam | Surface water | CBZ | Antiepileptics | <LOQ–57.4 ng/L | [32] |
| LCM | Antibiotics | <LOQ–378 ng/L | |||
| SMZ | Antibiotics | 3.65–2778 ng/L | |||
| India | Groundwater | Ketoprofen (KPF) | Non-steroidal anti-inflammatory drugs | <LOQ–23.4 ng/L | [33] |
| IBP | Non-steroidal anti-inflammatory drugs | <LOQ–49.4 ng/L | |||
| CAF | Stimulant | 15.2–262 ng/L | |||
| Pakistan | Groundwater | Tigecycline | Antibiotics | 21.3 ng/L | [34] |
| Ciprofloxacin (CIP) | Antibiotics | 18.2 ng/L | |||
| United States | Drinking water | CBZ | Antiepileptic | 51 ng/L | [35] |
| DCF | Non-steroidal anti-inflammatory drugs | 1.2 ng/L | |||
| SMX | Antibiotics | 110 ng/L | |||
| Naproxen | Non-steroidal anti-inflammatory drugs | 32 ng/L |
| PPCP | AOPs | Dominant Radicals | Concentration of PPCPs | Reaction Time (min) | pH | Removal Rate (%) | Ref. |
|---|---|---|---|---|---|---|---|
| CBZ | Photochemical oxidation | HO·, O2·− | 8.75 mg/L | 45 | 6 | 99.77 | [124] |
| Persulfate-based + photochemical oxidation | SO4·−, HO·, O2·− | 5 ppm | 30 | 7 | 97.1 | [125] | |
| UV/chlorine oxidation | HO·, Cl· | 40 μM | 60 | 7 | 83.9 | [126] | |
| Electrochemical oxidation | HO·, SO4·− | 1 μM | 5 | 2 | 100 | [127] | |
| Photo Fenton oxidation | HO· | 285 ng/L | 30 | 6–7 | 94 | [128] | |
| Photo-assisted ozone oxidation | HO· | 5 mg/L | 2 | 7 ± 0.2 | >99.99 | [44] | |
| Sono-photocatalytic oxidation | O2·− | 10 ppm | 240 | 7 | 93.37 | [129] | |
| Electron beam radiation | HO·, H· | 75 mg/L | / | 6.3 | 99.9 | [130] | |
| DCF | Photochemical oxidation | HO· | 20 mg/L | 270 | 5 | 76 | [131] |
| Sonoelectrochemical oxidation | HO· | 50 μg/L | 5 | 4 | 96.8 | [132] | |
| Fenton-like oxidation | HO· | 20 mg/L | 60 | 5 | 86.62 | [133] | |
| Ozone oxidation | HO· | 29.6 mg/L | 60 | 7 | 73.3 | [134] | |
| Persulfate-based oxidation | SO4·− | 20 mg/L | 15 | 5 | 90 | [135] | |
| SMX | Photochemical oxidation | HO·, O2·− | 10 mg/L | 60 | 7.5 | 84 | [136] |
| Electrochemical oxidation | HO· | 74.45 mg/L | 51.49 | 4.78 | 100 | [137] | |
| Ultrasonic oxidation | HO· | 10 μM | 60 | 7 | 97 | [138] | |
| Photo-Fenton oxidation | HO· | 25 mg/L | 15 | 3 | 98.06 | [139] | |
| Persulfate-based oxidation | SO4·− | 0.04 mM | 120 | 3.4 | 100 | [140] | |
| Gamma ray radiation | HO· | 39.48 μM | / | 6.7 | 88.6 | [141] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, J.; Li, Y.; Song, Q.; Wang, L.; Luo, T.; Gao, C.; Liu, L.; Yang, S. Removal of Pharmaceuticals and Personal Care Products (PPCPs) by Free Radicals in Advanced Oxidation Processes. Materials 2022, 15, 8152. https://doi.org/10.3390/ma15228152
Jiao J, Li Y, Song Q, Wang L, Luo T, Gao C, Liu L, Yang S. Removal of Pharmaceuticals and Personal Care Products (PPCPs) by Free Radicals in Advanced Oxidation Processes. Materials. 2022; 15(22):8152. https://doi.org/10.3390/ma15228152
Chicago/Turabian StyleJiao, Jiao, Yihua Li, Qi Song, Liujin Wang, Tianlie Luo, Changfei Gao, Lifen Liu, and Shengtao Yang. 2022. "Removal of Pharmaceuticals and Personal Care Products (PPCPs) by Free Radicals in Advanced Oxidation Processes" Materials 15, no. 22: 8152. https://doi.org/10.3390/ma15228152
APA StyleJiao, J., Li, Y., Song, Q., Wang, L., Luo, T., Gao, C., Liu, L., & Yang, S. (2022). Removal of Pharmaceuticals and Personal Care Products (PPCPs) by Free Radicals in Advanced Oxidation Processes. Materials, 15(22), 8152. https://doi.org/10.3390/ma15228152








