Predictions on Structural and Electronic Properties to Synthesize Bismuth-Carbon Compounds in Different Periodicities
Abstract
1. Introduction
2. Computational Detail
3. Results and Discussion
3.1. Structural Properties
- (A)
- Unit Cell of BiC
- (B)
- Super Cell of BiC
- (C)
- Unit Cell of BiC2 in 2D and 3D
- (D)
- Super Cell of BiC2 in 2D and 3D
- (E)
- Unit cell ofBiC3
- (F)
- Super Cell of BiC3
- (G)
- Unit Cell of Bi2C3
- (H)
- Super Cell of Bi2C3
- (I)
- Super Cell of BiC5
- (J)
- Unit Cell of Bi2C5
- (K)
- Super Cell of Bi2C5 in 2D
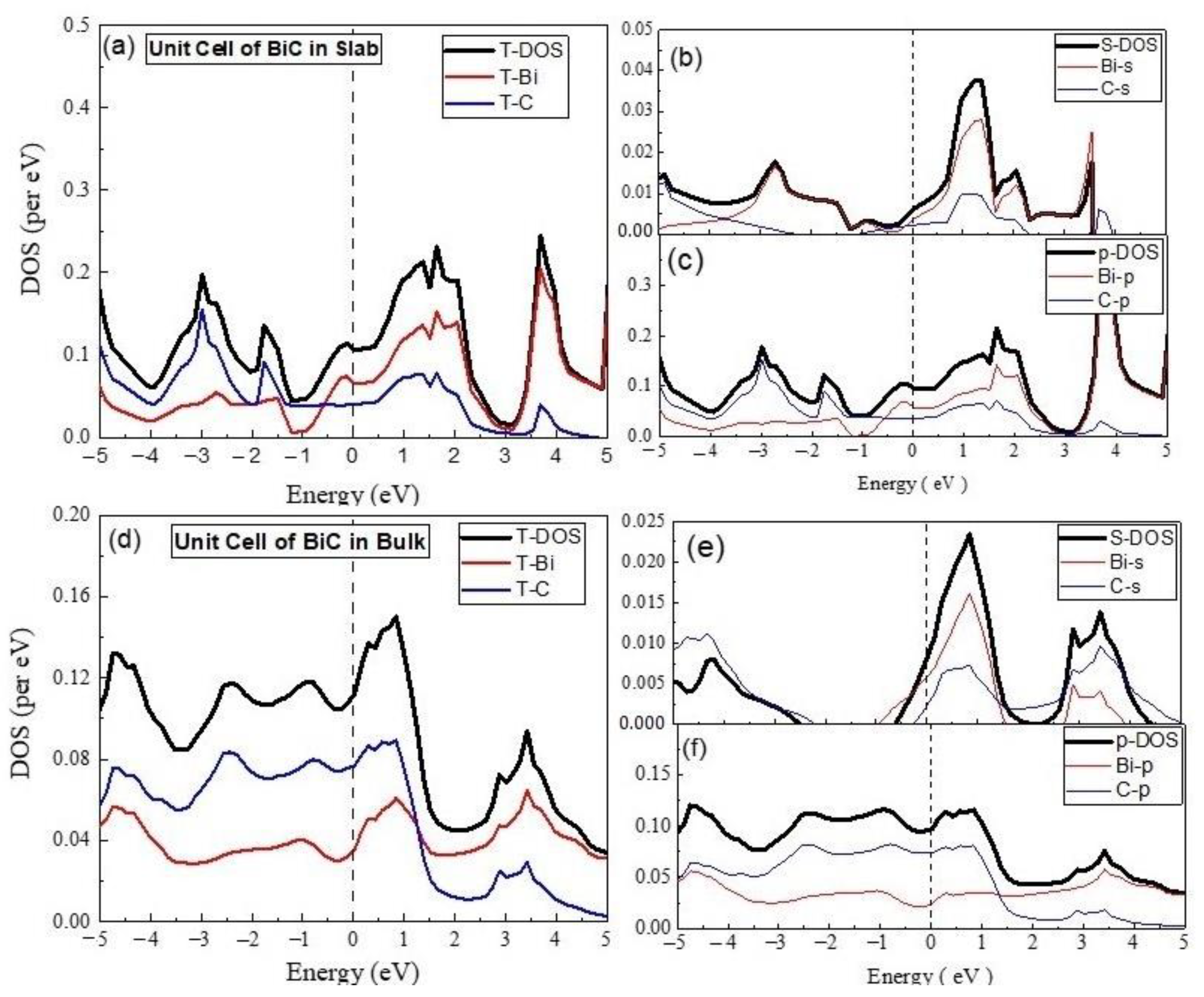
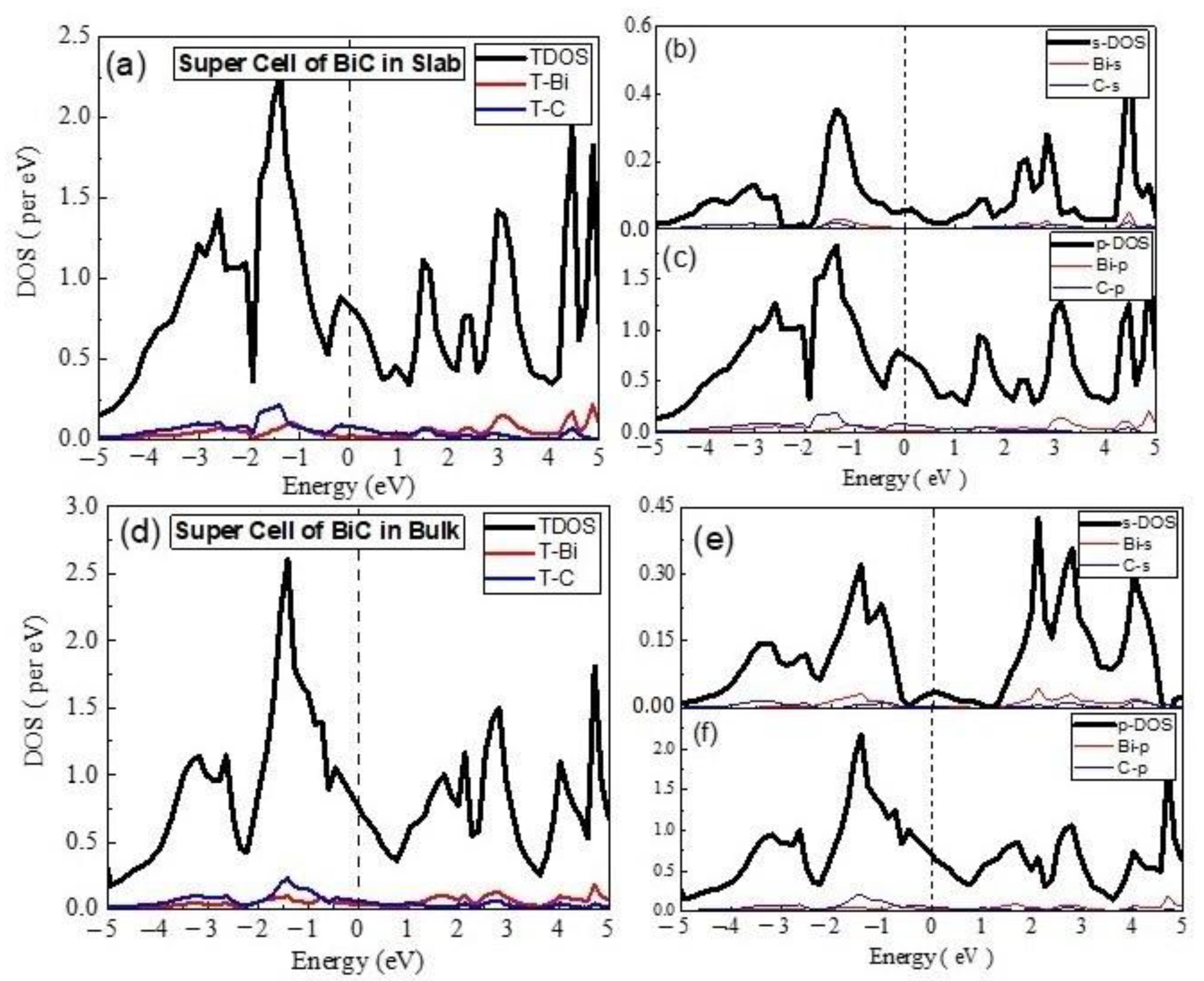
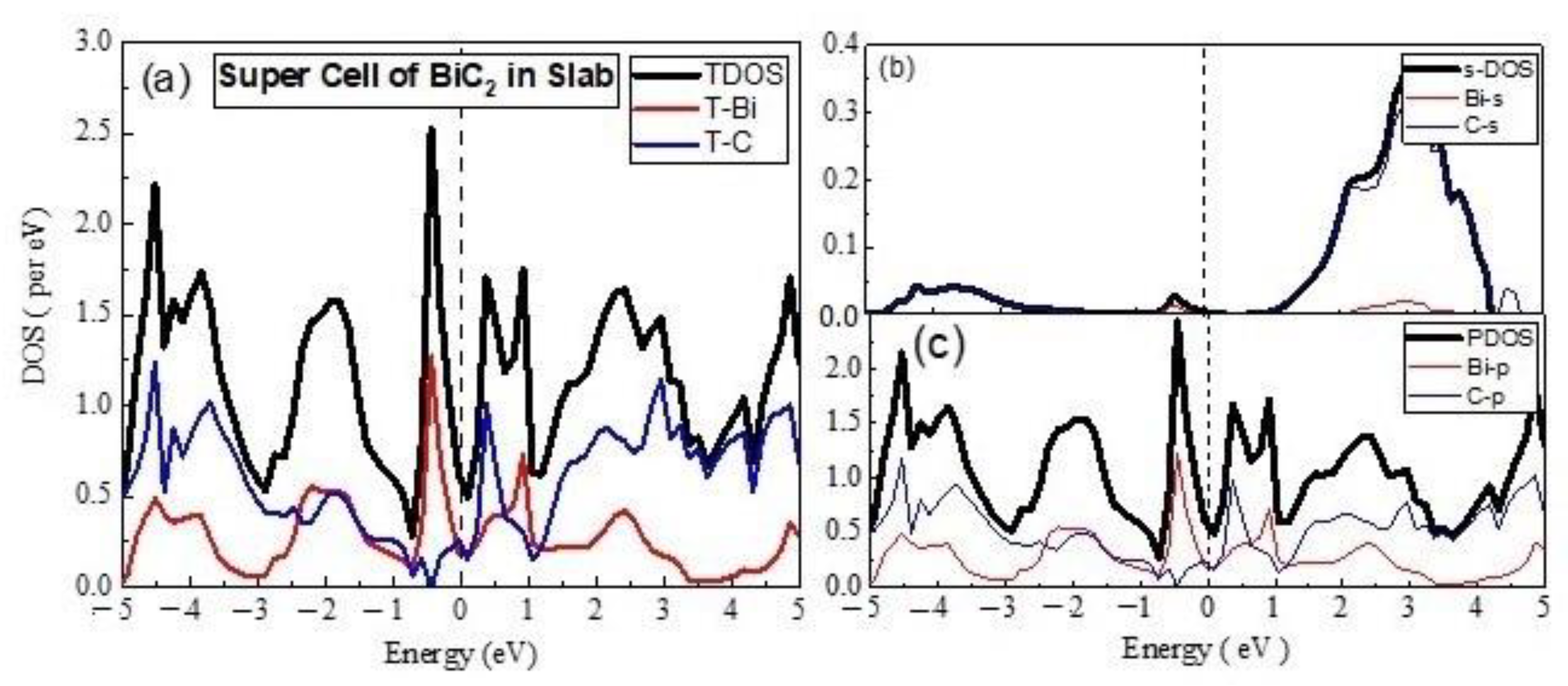
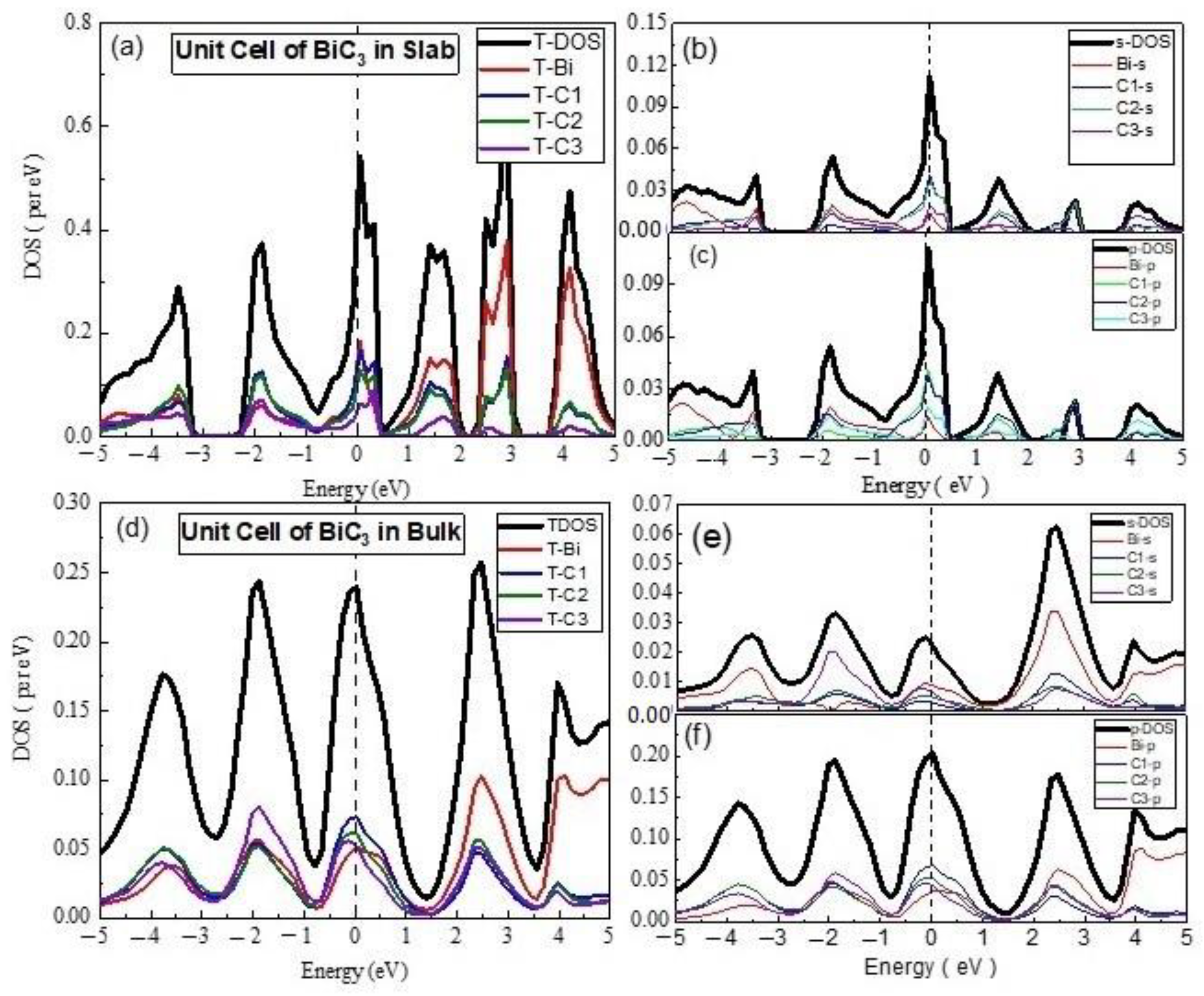
3.2. Electronics Properties
- (A)
- Unit Cell of BiC
- (B)
- Unit Cell of BiC2
- (C)
- Unit Cell of BiC3
- (D)
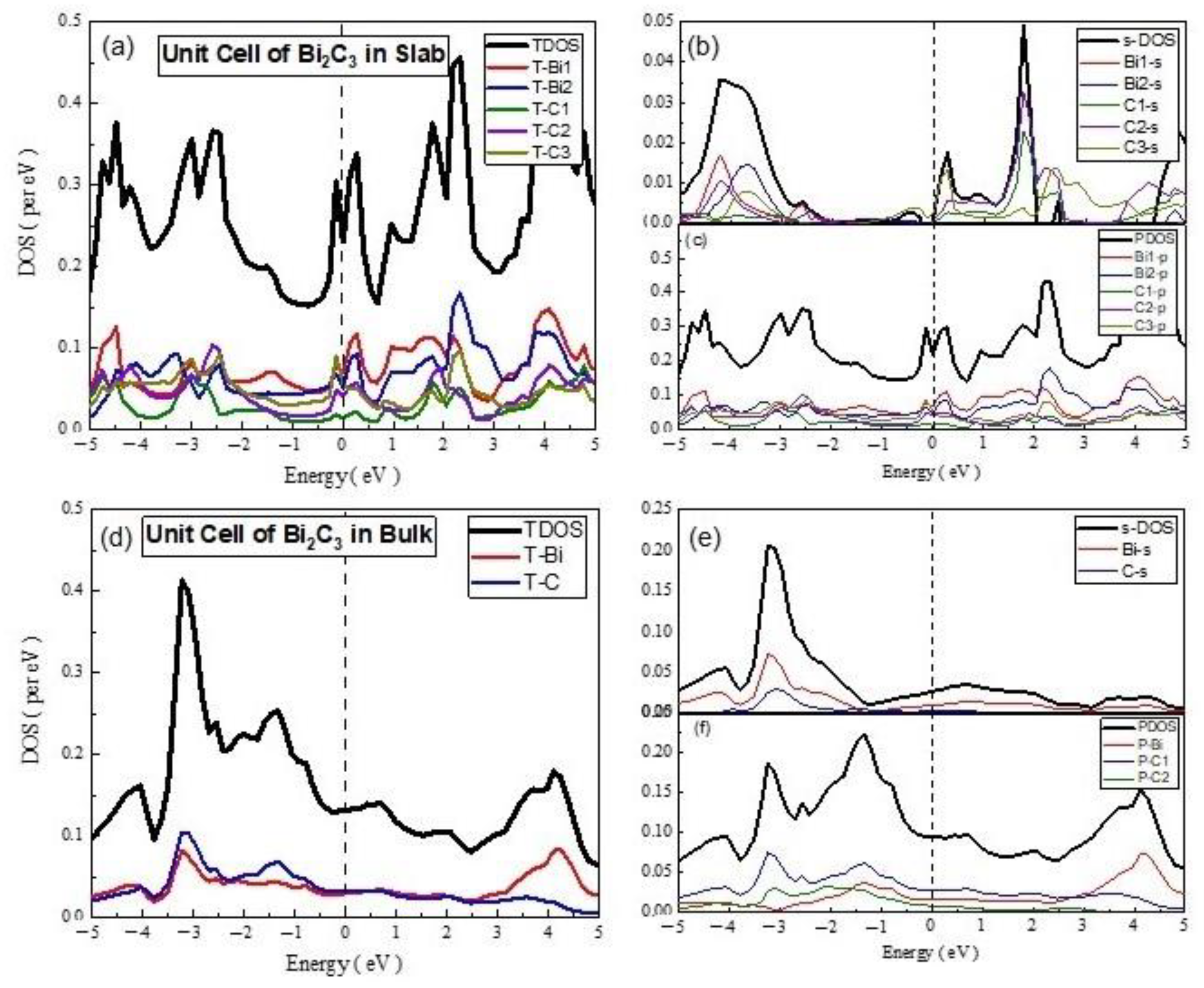
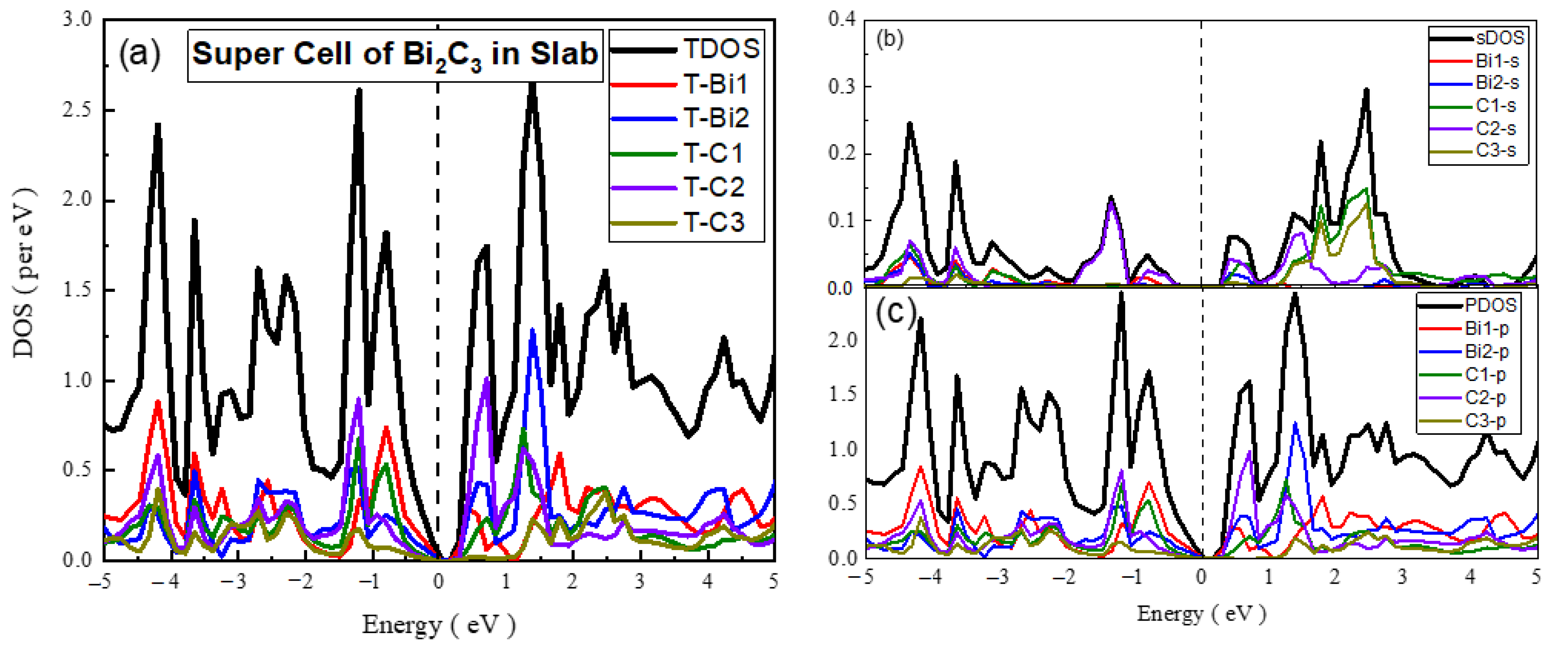
- Unit cell and Supercell of Bi2C3
- (E)
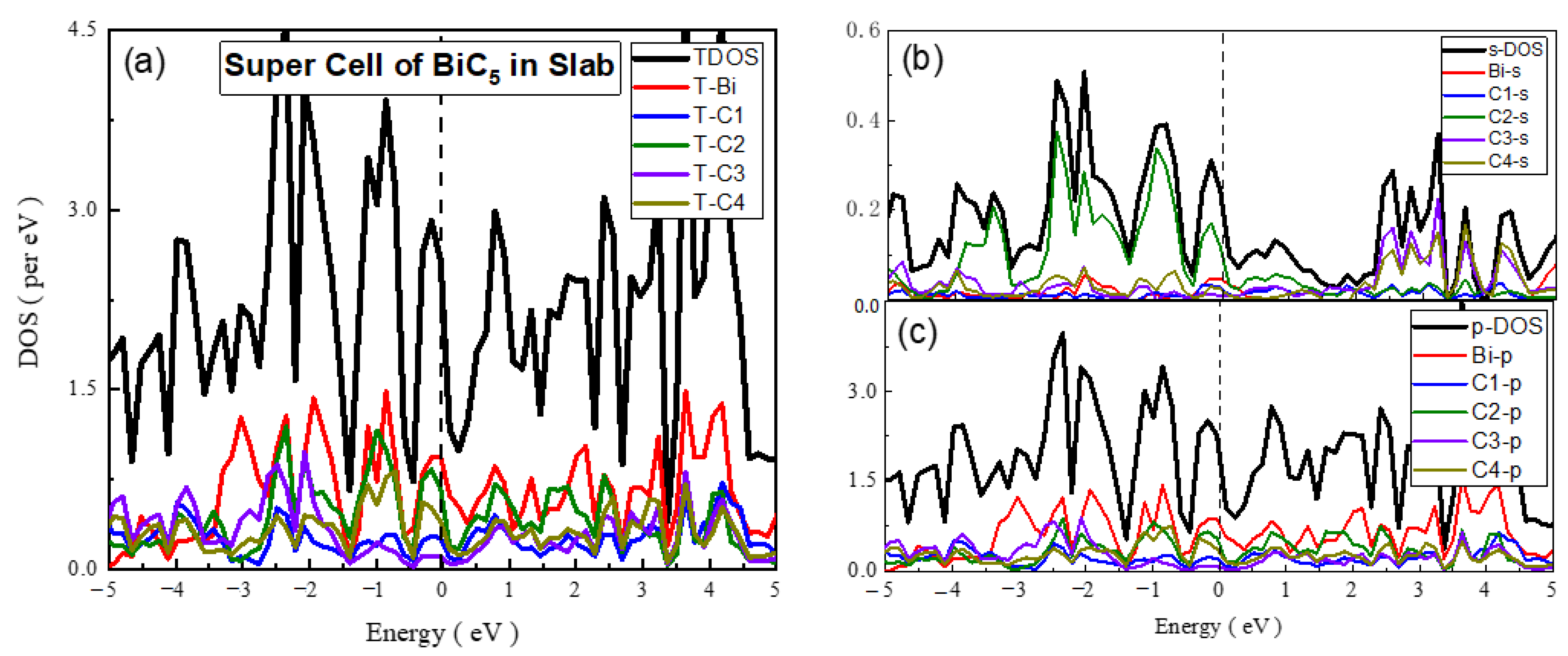
- Super Cell of BiC5
- (F)
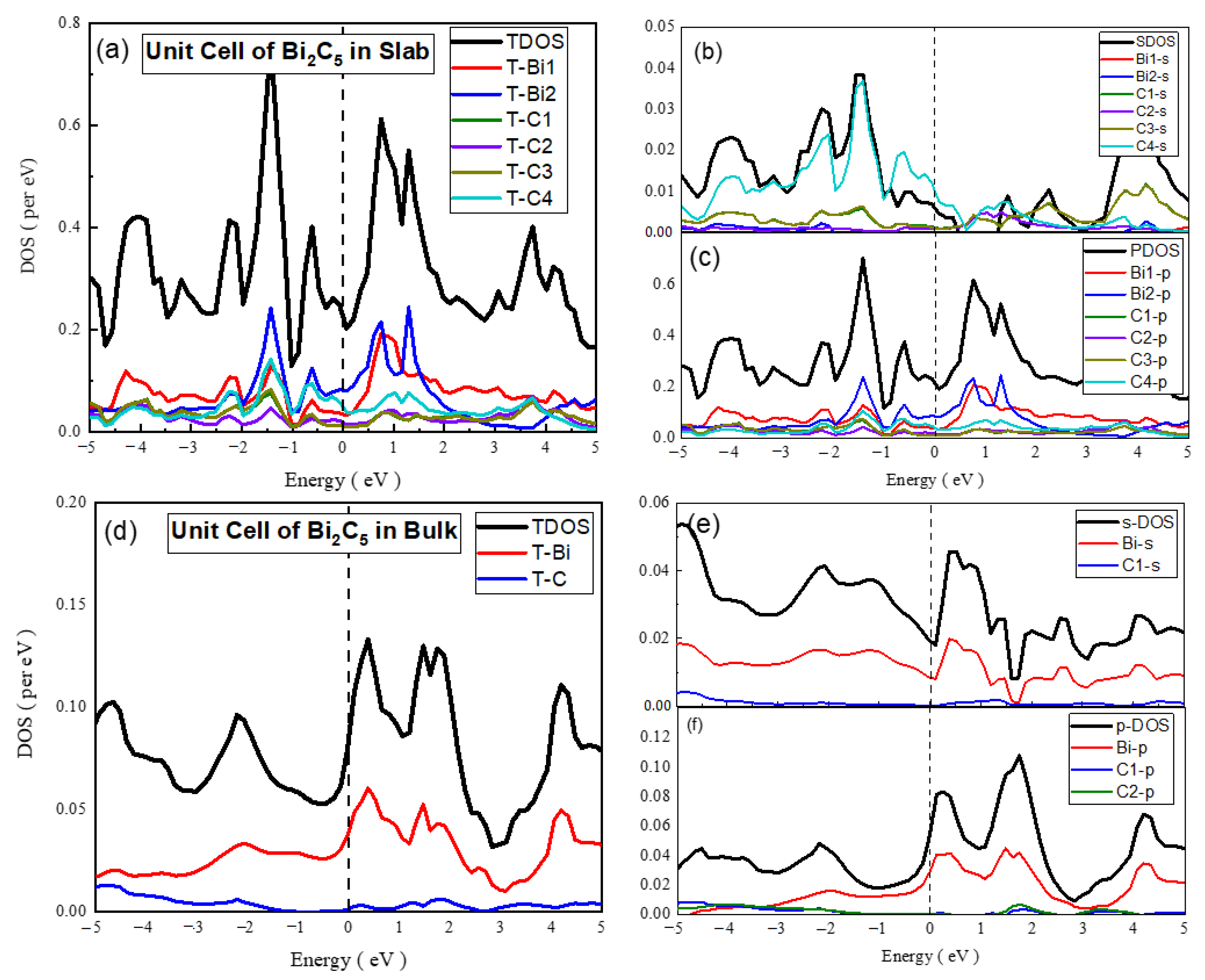
- Unit cell of Bi2C5
- (G)
- Unit cell of BiC5
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lamaoui, A.; Palacios-Santander, J.M.; Amine, A.; Cubillana-Aguilera, L. Computational approach and ultrasound Probe–Assisted synthesis of magnetic molecularly imprinted polymer for the electrochemical detection of bisphenol A. Mater. Sci. Eng. 2022, 277, 115568. [Google Scholar] [CrossRef]
- Babar, N.U.A.; Hussain, F.; Ashiq, M.N.; Joya, K.S. Engineered Modular Design of a Nanoscale CoNP/Aunano Hybrid Assembly for High-Performance Overall Water Splitting. ACS Appl. Energy Mater. 2021, 4, 8953–8968. [Google Scholar] [CrossRef]
- Majeed, M.H.; Aycibin, M.; Imer, A.G.; Muhammad, A.M.; Kareem, M.M. Influence of annealing process on structural, optical and electronic properties of nano-structured ZnO films synthesized by hydrothermal technique: Supported by DFT study. Mater. Sci. Eng. 2022, 282, 115793. [Google Scholar] [CrossRef]
- Khalid, H.H.; Erkan, S.; Bulut, N. Halogens Effect on Spectroscopy, Anticancer and Molecular Docking Studies for Platinum complexes. Optik 2021, 244, 166324. [Google Scholar] [CrossRef]
- Khan, T.M.; Khan, S.U.; Raffi, M.; Khan, R. Theoretical–Computational Study of Atmospheric DBD Plasma and Its Utility for Nanoscale Biocompatible Plasmonic Coating. Molecules 2021, 26, 5106. [Google Scholar] [CrossRef]
- Bahadar, A.; Mohammed, Z. Development of SWCNTs-reinforced EPDM/SBR matrices for shock absorbing applications. Mater. Res. Express 2020, 7, 025310. [Google Scholar] [CrossRef]
- Kishore, N.; Kumar, B.S.; Manoj, K.N.V.K.; Balamurugan, D.; Sriram, S. Mechanical properties of tungsten-rhenium, lanthanum and copper doped tungsten-rhenium nanostructures—A DFT study. Mater. Sci. Eng. 2022, 278, 115626. [Google Scholar] [CrossRef]
- Liu, P.F.; Bo, T.; Liu, Z.; Eriksson, O.; Wang, F.; Zhao, J.; Wang, B.T. Hexagonal M2C3 (M = As, Sb and Bi) monolayers: New functional materials with desirable band gaps and ultrahigh carrier mobility. J. Mater. Chem. 2018, 6, 12689–12697. [Google Scholar] [CrossRef]
- Wang, P.; Yan, G.; Zhu, X.; Du, Y.; Chen, D.; Zhang, J. Heterofullerene MC59 (M = B, Si, Al) as potential carriers for hydroxyurea drug delivery. Nanomaterials 2021, 11, 115. [Google Scholar] [CrossRef]
- Deshpande, M.P.; Sakariya, P.N.; Bhatt, S.V.; Garg, N.; Patel, K.; Chaki, S.H. Characterization of Bi2S3 nanorods prepared at room temperature. Mater. Sci. Semicond. Process. 2014, 21, 180–185. [Google Scholar] [CrossRef]
- Bafekry, A.; Faraji, M.; Fadlallah, M.M.; Jappor, H.R.; Hieu, N.N.; Ghergherehchi, M.; Feghhi, S.A.H.; Gogova, D. Prediction of two-dimensional bismuth-based chalcogenides Bi2X3 (X = S, Se, Te) monolayers with orthorhombic structure: A first-principles study. J. Phys. Appl. Phys. 2021, 54, 395103. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.Y.; Rajput, S.; Gilks, D.; Lari, L.; Galindo, P.L.; Weinert, M.; Lazarov, V.K.; Li, L. Tuning Dirac states by strain in the topological insulator Bi2Se3. Nat. Phys. 2014, 10, 294–299. [Google Scholar] [CrossRef]
- Ma, F.; Zhou, M.; Jiao, Y.; Gao, G.; Gu, Y.; Bilic, A.; Chen, H.; Du, A. Single layer bismuth iodide: Computational exploration of structural, electrical, mechanical and optical properties. Sci. Rep. 2015, 5, 17558. [Google Scholar] [CrossRef]
- Liu, C.; Wan, W.; Ma, J.; Guo, W.; Yao, Y. Robust ferroelectricity in two-dimensional SbN and BiP. Nanoscale 2018, 10, 7984–7990. [Google Scholar] [CrossRef]
- Liu, H.Y.; Yang, C.L.; Wang, M.S.; Ma, X.G. Two-dimensional BiP3 with high carrier mobility and moderate band gap for hydrogen generation from water splitting. Appl. Surf. Sci. 2020, 501, 144263. [Google Scholar] [CrossRef]
- Barhoumi, M.; Lazaar, K.; Said, M. A first principle study of the pristine InBi honeycomb film functionalized with fluorine atoms. J. Fluor. Chem. 2018, 212, 171–179. [Google Scholar] [CrossRef]
- Zhao, A.; Zhang, L.; Guo, Y.; Li, H.; Ruan, S.; Zeng, Y. Emerging members of two-dimensional materials: Bismuth-based ternary compounds. 2D Mater. 2020, 8, 012004. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Y.; Jing, Y.; Heine, T.; Li, Y. β-PdBi2 monolayer: Two-dimensional topological metal with superior catalytic activity for carbon dioxide electroreduction to formic acid. Mater. Today Adv. 2020, 8, 100091. [Google Scholar] [CrossRef]
- Song, Z.; Liu, C.C.; Yang, J.; Han, J.; Ye, M.; Fu, B.; Yang, Y.; Niu, Q.; Lu, J.; Yao, Y. Quantum spin Hall insulators and quantum valley Hall insulators of BiX/SbX (X = H, F, Cl and Br) monolayers with a record bulk band gap. NPG Asia Mater. 2014, 6, e147. [Google Scholar] [CrossRef]
- Cates, N.C.; de la Torre, J.C.R.; Aina, S.; Lobera, M.P.; Bernechea, M. Bismuth-based nanomaterials for energy applications. In Sustainable Material Solutions for Solar Energy Technologies; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–35. [Google Scholar]
- Fornaro, L.; Cuna, A.; Noguera, A.; Perez, M.; Mussio, L. Growth of bismuth tri-iodide platelets for room temperature X-ray detection. IEEE Trans. Nucl. Sci. 2004, 51, 2461–2465. [Google Scholar] [CrossRef]
- Domnich, V.; Reynaud, S.; Haber, R.A.; Chhowalla, M. Boron carbide: Structure, properties, and stability under stress. J. Am. Ceram. Soc. 2011, 94, 3605–3628. [Google Scholar] [CrossRef]
- Qian, S.; Sheng, X.; Zhou, Y.; Yan, X.; Chen, Y.; Huang, Y.; Huang, X.; Feng, E.; Huang, W. Two-dimensional Be2C with octacoordinate carbons and negative Poisson’s ratio. J. Phys. Chem. 2018, 122, 7959–7967. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, H.; Li, Z. First-principles study of two dimensional C3N and its derivatives. RSC Adv. 2020, 10, 33469–33474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jiao, Y.; He, T.; Bottle, S.; Frauenheim, T.; Du, A. Predicting two-dimensional C3B/C3N van der Waals p–n heterojunction with strong interlayer electron coupling and enhanced photocurrent. J. Phys. Chem. Lett. 2018, 9, 858–862. [Google Scholar] [CrossRef]
- Lv, B.; Hu, X.; Liu, X.; Zhang, Z.; Song, J.; Luo, Z.; Gao, Z. Thermal transport properties of novel two-dimensional CSe. Phys. Chem. Chem. Phys. 2020, 22, 17833–17841. [Google Scholar] [CrossRef]
- Zhang, Q.; Feng, Y.; Chen, X.; Zhang, W.; Wu, X.; Wu, L.; Wang, Y. Structural and electronic anisotropy, negative Poisson’s ratio, strain-sensitive Dirac-like cone in monolayer α-CSe: Tailoring electronic properties. Comput. Mater. Sci. 2019, 168, 87–95. [Google Scholar] [CrossRef]
- Ande, C.K.; Sluiter, M.H. First-Principles calculations on stabilization of iron carbides (Fe3C, Fe5C2 and η-Fe2C) in steels by common alloying elements. Metall. Mater. Trans. 2012, 43, 4436–4444. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Le, M.Q. Mechanical properties of various two-dimensional silicon carbide sheets: An atomistic study. Superlattices Microstruct. 2016, 98, 102–115. [Google Scholar] [CrossRef]
- Chabi, S.; Guler, Z.; Brearley, A.J.; Benavidez, A.D.; Luk, T.S. The Creation of True Two-Dimensional Silicon Carbide. Nanomaterials 2021, 11, 1799. [Google Scholar] [CrossRef]
- Wellmann, P.; Neubauer, G.; Fahlbusch, L.; Salamon, M.; Uhlmann, N. Growth of SiC bulk crystals for application in power electronic devices–process design, 2D and 3D X-ray in situ visualization and advanced doping. Cryst. Res. Technol. 2015, 50, 2–9. [Google Scholar] [CrossRef]
- Kurtoglu, M.; Naguib, M.; Gogotsi, Y.; Barsoum, M.W. First principles study of two-dimensional early transition metal carbides. Mrs Commun. 2012, 2, 133–137. [Google Scholar] [CrossRef]
- Kumar, V.; Rajput, K.; Roy, D.R. Monolayer Bi2C3: A promising sensor for environmentally toxic NCGs with high sensitivity and selectivity. Appl. Surf. Sci. 2020, 534, 147609. [Google Scholar] [CrossRef]
- Okur, H.E.; Bulut, N.; Ates, T.; Kaygili, O. Structural and optical characterization of Sm-doped ZnO nanoparticles. Bull. Mater. Sci. 2019, 42, 199. [Google Scholar] [CrossRef]
- Khera, E.A.; Ullah, H.; Imran, M.; Niaz, N.A.; Hussain, F.; Khalil, R.A.; Kim, S. ab initio study of oxygen vacancy effects on structural, electronic and thermoelectric behavior of AZr1−xMxO3 (A = Ba, Ca, Sr; M = Al, Cu, x = 0.25) for application of memory devices. J. Mol. Graph. Model. 2021, 103, 107825. [Google Scholar] [CrossRef] [PubMed]
- Te Velde, G.T.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; van Gisbergen, S.J.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Wang, Y. Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys. Rev. 1996, 54, 16533. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Tong, T.; Zhu, B.; Jiang, C.; Cheng, B.; Yu, J. Mechanistic insight into the enhanced photocatalytic activity of single-atom Pt, Pd or Au-embedded g-C3N4. Appl. Surf. Sci. 2018, 433, 1175–1183. [Google Scholar] [CrossRef]
- Aktürk, O.Ü.; Tomak, M. Bismuth doping of graphene. Appl. Phys. Lett. 2010, 96, 081914. [Google Scholar] [CrossRef]
- Zhan, L.B.; Yang, C.L.; Wang, M.S.; Ma, X.G. Two-dimensional Bi2Se3 monolayer with high mobility and enhanced optical absorption in the UV–visible light region. Phys. Low-Dimens. Syst. Nanostruct. 2020, 124, 114272. [Google Scholar] [CrossRef]
- Jabeen, A.; Alkhedher, M.; Majid, A.; Al Hassan, N. On downscaling of the tantalum oxides from three to zero dimensions. Mater. Sci. Semicond. Process. 2022, 151, 107014. [Google Scholar] [CrossRef]
- Anasori, B.; Xie, Y.; Beidaghi, M.; Lu, J.; Hosler, B.C.; Hultman, L.; Kent, P.R.C.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional, ordered, double transition metals carbides (MXenes). ACS Nano 2015, 9, 9507–9516. [Google Scholar] [CrossRef] [PubMed]
- Taghizade, N.; Faizabadi, E. Spin-filtering effects and negative differential resistance in N/B-doped zigzag silicon carbide nanoribbons with asymmetric edge hydrogenation. Mater. Sci. Eng. 2021, 271, 115253. [Google Scholar] [CrossRef]


| Compounds | Lattice Constant | Lattice Angles | Area or Volume | Symmetry |
|---|---|---|---|---|
| U.C. of BiC in Slab | a = 3, b = 3 | 9 Å2 | ||
| U.C. of BiC in Bulk | a = 3, b = 3, c = 3 | = = = 90° | 27 Å3 | Cubic |
| S.C. BiC in Slab | a = 6, b = 6 | 36 Å2 | ||
| S.C BiC in Bulk | a = 6, b = 6, c = 6 | = = = 90° | 216 Å3 | Cubic |
| U.C. of BiC2 in Slab | a = 3, b = 3 | 9 Å2 | ||
| U.C. of BiC2 in Bulk | a = 3, b = 3, c = 3 | = = = 90° | 27 Å3 | Cubic |
| S.C. of BiC2 of 2 × 2 Slab | a = 6, b = 6 | 36 Å2 | ||
| S.C. of BiC2 of 2 × 2 mono Slab | a = 6, b = 6 | 36 Å2 | ||
| S.C. of BiC2 of 4 × 4 mono Slab | a = 12, b = 12 | 144 Å2 | ||
| S.C. of BiC2 of 4 × 4 bi-layer Slab | a = 12, b = 12 | 144 Å2 | ||
| S.C. of BiC2 of 4 × 4 two bi-layer Slab | a = 12, b = 12 | 144 Å2 | ||
| U.C. of BiC3 in Slab | a = 3, b = 3 | 9 Å2 | ||
| U.C. of BiC3 in Bulk | a = 3, b = 3, c = 3 | = = = 90° | 27 Å3 | Cubic |
| S.C. of BiC3 in Slab | a = 6, b = 6 | 36 Å2 | ||
| S.C. of BiC3 in Bulk | a = 6, b = 6, c = 6 | = = = 90° | 216 Å3 | Cubic |
| U.C. of Bi2C3 in Slab | a = 3, b = 3 | 9 Å2 | ||
| U.C. of Bi2C3 in Bulk | a = 3, b = 3, c = 3 | = = = 90° | 27 Å3 | Cubic |
| S.C. of Bi2C3 of Slab | a = 6, b = 6 | 36 Å2 | ||
| S.C. of Bi2C3 10 × 10 Slab | a = 30, b = 30 | 900 Å2 | ||
| S.C. of Bi2C3 10 × 10 2-Slab | a = 30, b = 30 | 900 Å2 | ||
| S.C. of BiC5 Slab | a = 9, b = 9 | 81 Å2 | ||
| U.C. of Bi2C5 in Slab | a = 3, b = 3 | 9 Å2 | ||
| U.C of Bi2C5 in Bulk | a = 3, b = 3, c = 3 | = = = 90° | 27 Å3 | Cubic |
| U.C. of Bi2C5 in Slab | a = 9, b = 9 | 81 Å2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majid, A.; Younes, T.M.; Jabeen, A.; Batool, H.; Alkhedher, M.; ElDin, S.M. Predictions on Structural and Electronic Properties to Synthesize Bismuth-Carbon Compounds in Different Periodicities. Materials 2022, 15, 8150. https://doi.org/10.3390/ma15228150
Majid A, Younes TM, Jabeen A, Batool H, Alkhedher M, ElDin SM. Predictions on Structural and Electronic Properties to Synthesize Bismuth-Carbon Compounds in Different Periodicities. Materials. 2022; 15(22):8150. https://doi.org/10.3390/ma15228150
Chicago/Turabian StyleMajid, Abdul, Tariq M. Younes, Alia Jabeen, Hira Batool, Mohammad Alkhedher, and Sayed M. ElDin. 2022. "Predictions on Structural and Electronic Properties to Synthesize Bismuth-Carbon Compounds in Different Periodicities" Materials 15, no. 22: 8150. https://doi.org/10.3390/ma15228150
APA StyleMajid, A., Younes, T. M., Jabeen, A., Batool, H., Alkhedher, M., & ElDin, S. M. (2022). Predictions on Structural and Electronic Properties to Synthesize Bismuth-Carbon Compounds in Different Periodicities. Materials, 15(22), 8150. https://doi.org/10.3390/ma15228150






