The Role of Zn Substitution in Improving the Electrical Properties of CuI Thin Films and Optoelectronic Performance of CuI MSM Photodetectors
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
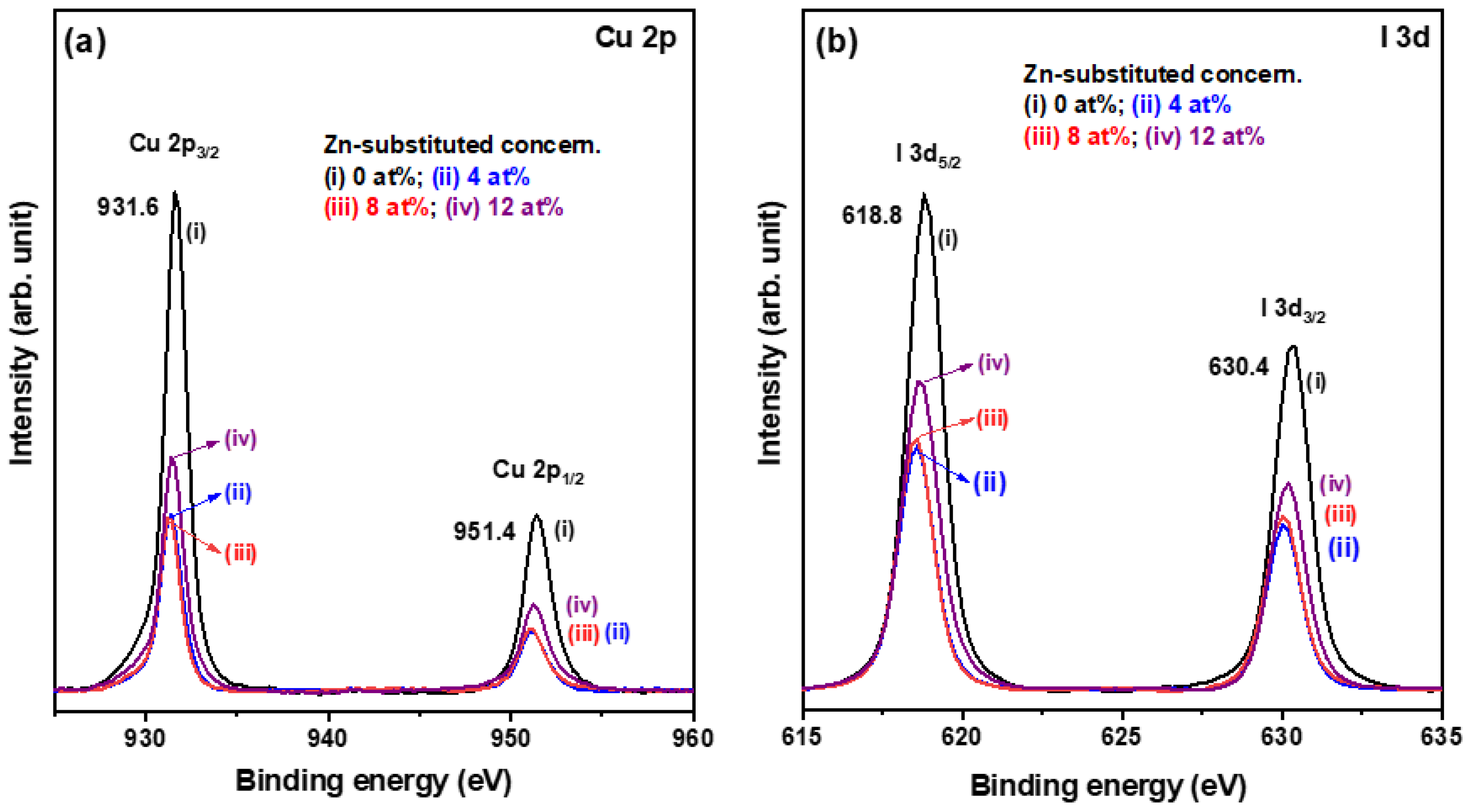
3.1. Physical Properties of Solution-Processed CuI-Based Thin Films
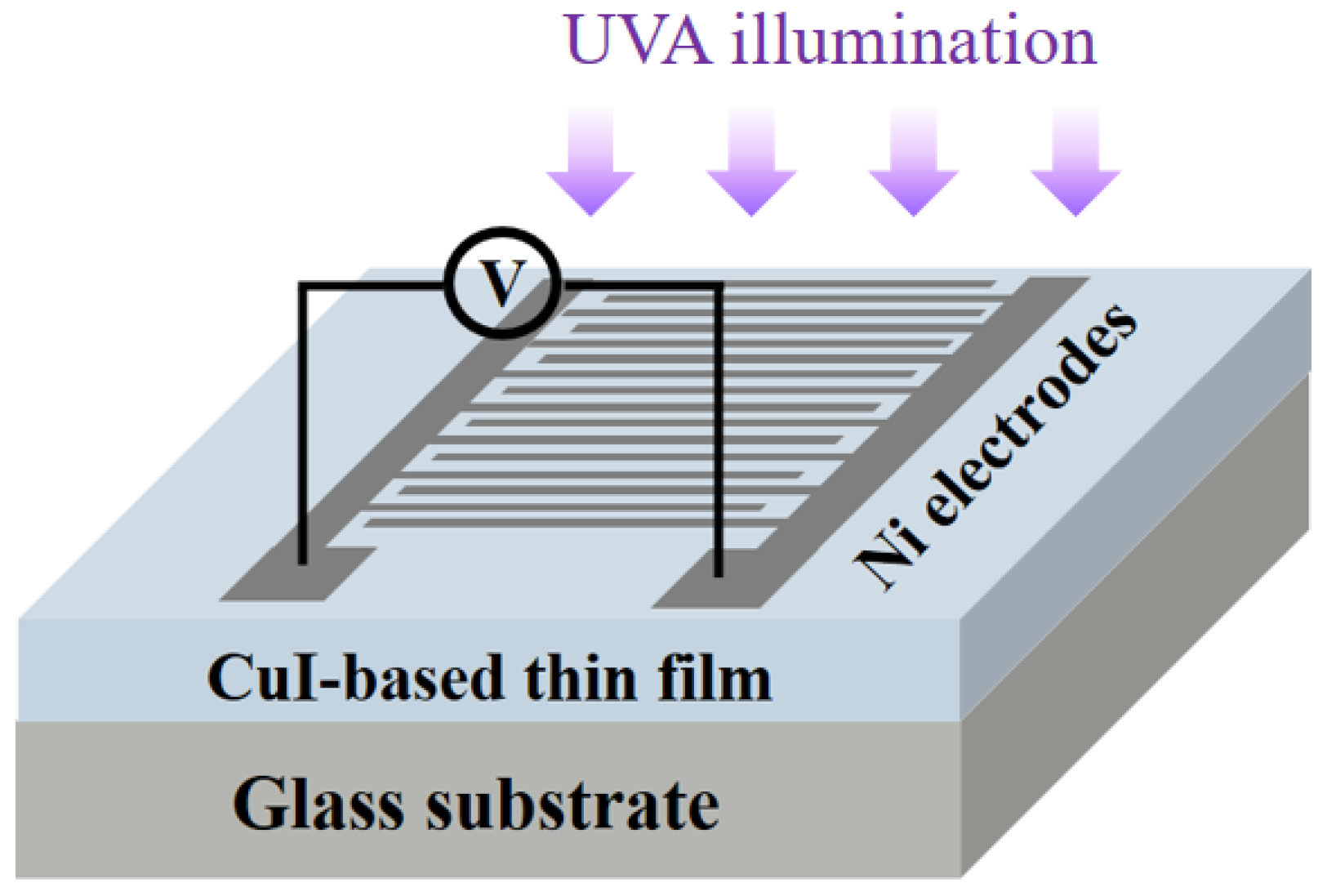
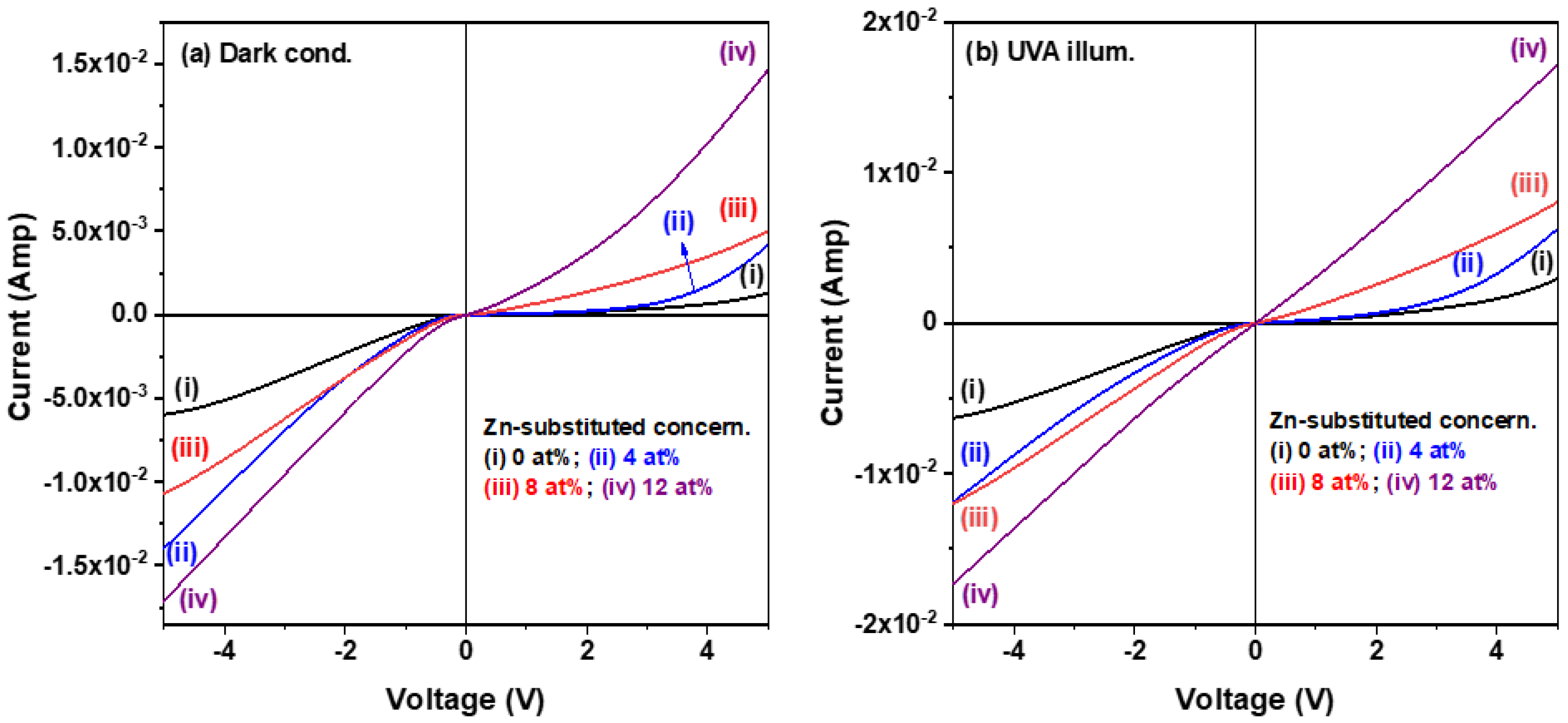
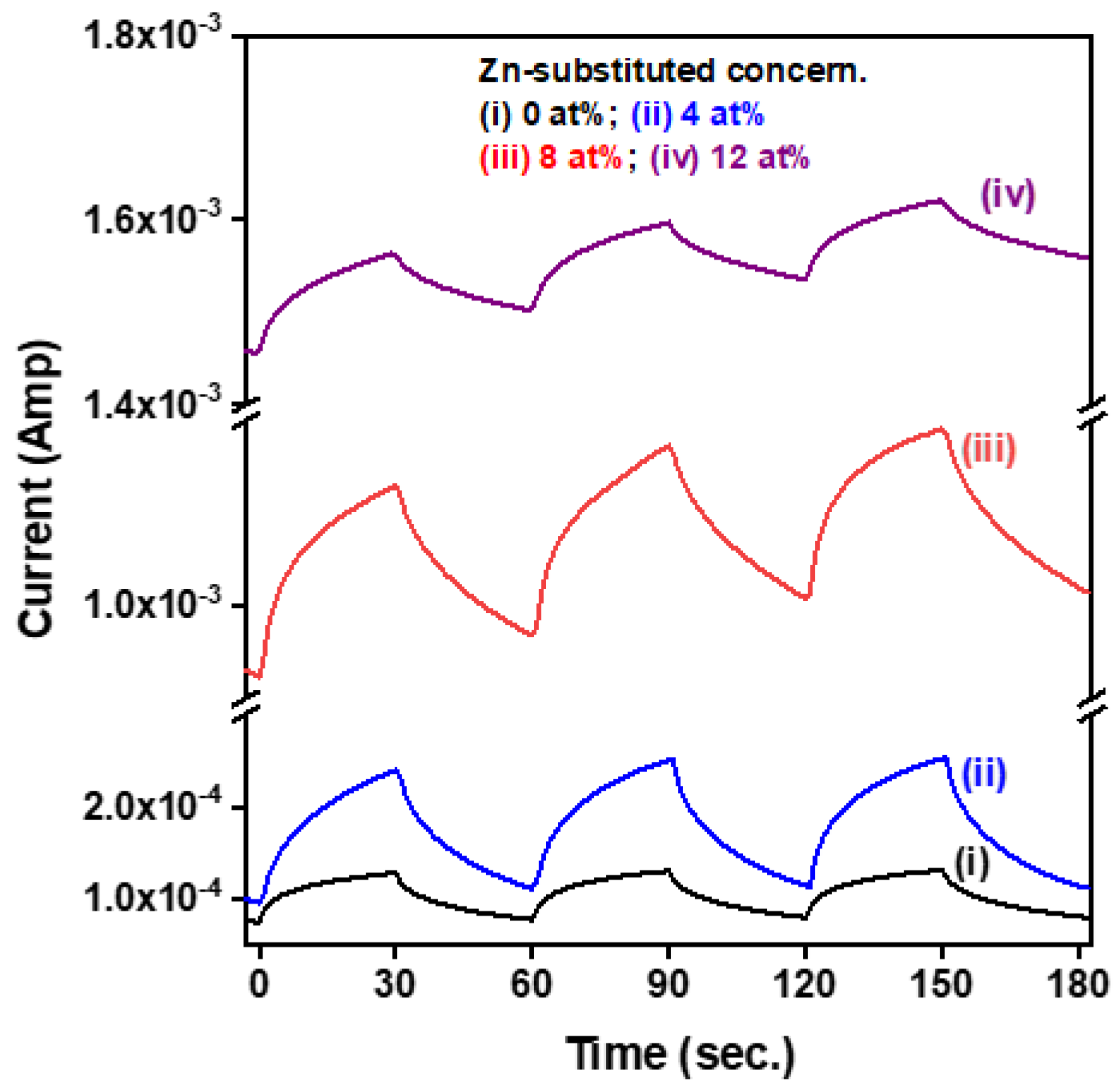
3.2. Optoelectronic Properties of the CuI-Based MSM Photodetectors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, A.; Zhu, H.; Kim, M.G.; Kim, J.; Noh, Y.Y. Engineering copper iodide (CuI) for multifunctional p-Type transparent semiconductors and conductors. Adv. Sci. 2021, 8, 2100546. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, M.; Schein, F.L.; Lorenz, M.; Böntgen, T.; Lenzner, J.; Wenckstern, H.V. Cuprous iodide−a p-type transparent semiconductor: History and novel applications. Phys. Status Solidi A 2013, 210, 1671–1703. [Google Scholar] [CrossRef]
- Peng, W.; Li, L.; Yu, S.; Zheng, H.; Yang, P. Structure, binding energy and optoelectrical properties of p-type CuI thin films: The effects of thickness. Appl. Surf. Sci. 2020, 502, 144424. [Google Scholar] [CrossRef]
- Yang, C.; Kneiβ, M.; Lorenz, M.; Grundmann, M. Room-temperature synthesized copper iodide thin film as degenerate p-type transparent conductor with a boosted figure of merit. Proc. Natl. Acad. Sci. USA 2016, 113, 12929–12933. [Google Scholar] [CrossRef] [PubMed]
- Inudo, S.; Miyake, M.; Hirato, T. Electrical properties of CuI films prepared by spin coating. Phys. Status Solidi A 2013, 210, 2395–2398. [Google Scholar] [CrossRef]
- Huang, Y.; Tan, J.; Gao, G.; Xu, J.; Zhao, L.; Zhou, W.; Wang, Q.; Yuan, S.; Sun, J. Transparent p-type CuI film based self-powered ultraviolet photodetectors with ultrahigh speed, responsivity and detectivity. J. Mater. Chem. C 2022, 10, 13040–13046. [Google Scholar] [CrossRef]
- Liu, A.; Zhu, H.; Shim, K.I.; Hong, J.; Jung, H.; Han, J.W.; Noh, Y.Y. Key roles of trace oxygen treatment for high-performance Zn-doped CuI p-channel transistors. Adv. Electron. Mater. 2021, 7, 2000933. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Yang, W.; Fang, X. Solution-processed transparent Sn4+-doped CuI hybrid photodetectors with enhanced performance. Adv. Mater. Interfaces 2019, 6, 1900669. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Li, S.S. Native p-type transparent conductive CuI via intrinsic defects. J. Appl. Phys. 2011, 110, 054907. [Google Scholar] [CrossRef]
- Zhu, J.; Gu, M.; Pandey, R. Structural and electronic properties of CuI doped with Zn, Ga and Al. J. Phys. Chem. Solids 2013, 74, 1122–1126. [Google Scholar] [CrossRef]
- Xia, M.; Gu, M.; Liu, X.; Liu, B.; Huang, S.; Ni, C. Electrical and luminescence properties of Zn2+ doped CuI thin films. J. Mater. Sci. Mater. Electron. 2015, 26, 2629–2633. [Google Scholar] [CrossRef]
- Liu, A.; Zhu, H.; Park, W.T.; Kim, S.J.; Kim, H.; Kim, M.G.; Noh, Y.Y. High-performance p-channel transistors with transparent Zn doped-CuI. Nat. Com. 2020, 11, 4309. [Google Scholar] [CrossRef] [PubMed]
- Tsay, C.Y.; Tsai, H.M.; Chen, Y.C. Improved optoelectronic characteristics of Ga-In co-doped ZnO UV photodetectors by asymmetric metal contact structure. Crystals 2022, 12, 746. [Google Scholar] [CrossRef]
- Sang, L.; Liao, M.; Sumiya, M. A comprehensive review of semiconductor ultraviolet photodetectors: From thin film to one-dimensional nanostructures. Sensors 2013, 13, 10482–10518. [Google Scholar] [CrossRef]
- Alaie, Z.; Nejad, S.M.; Yousefi, M.H. Recent advances in ultraviolet photodetectors. Mater. Sci. Semicond. Process. 2015, 29, 16–55. [Google Scholar] [CrossRef]
- Tsay, C.Y.; Chen, Y.C.; Tsai, H.M.; Hsu, C.L. Performance improvement in amorphous zinc tin oxide ultraviolet photodetectors using an asymmetric pair of interdigitated electrodes. Physic B 2022, 640, 413970. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Prentice Hall: Hoboken, NJ, USA, 2001; p. 170. ISBN 0-201-61091-4. [Google Scholar]
- Krishnaiah, M.; Kumar, A.; Mishra, D.; Kushwaha, A.K.; Jin, S.H.; Park, J.T. Solution-processed CuI films towards flexible visible-photodetectors: Role of annealing temperature on Cu/I ratio and photo detective properties. J. Alloys Compd. 2021, 887, 161326. [Google Scholar] [CrossRef]
- Wagner, C.D.; Riggs, W.M.; Davis, L.E.; Moulder, J.F.; Muilenberg, G.E. Handbook of X-ray Photoelectron Spectroscopy, 1st ed.; Perkin-Elmer Corp.: Eden Prairie, MN, USA, 1979; pp. 82, 124. [Google Scholar]
- Ghazal, N.; Madkour, M.; Nazeer, A.A.; Obayya, S.S.A.; Mohamed, S.A. Electrochemical capacitive performance of thermally evaporated Al-doped CuI thin films. RSC Adv. 2021, 11, 39262–39269. [Google Scholar] [CrossRef]
- Cota-Leal, M.; Cabrera-German, D.; Sotelo-Lerma, M.; Martínez-Gil, M.; García-Valenzuela, J.A. High-transparent and conductive CuI films obtained by a redirected low-cost and electroless two-step route: Chemical solution deposition of CuS2 and subsequent iodination. Mater. Sci. Semicond. Process. 2019, 95, 59–67. [Google Scholar] [CrossRef]
- Vasquez, R.P. CuI by XPS. Surf. Sci. Spectra 1993, 2, 149–154. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Y.; Lin, Z.; Huang, J.; Chen, X.; Pan, D.; Huang, F. Growth strategy and physical properties of the high mobility P-type CuI crystal. Cryst. Growth Des. 2010, 10, 2057–2060. [Google Scholar] [CrossRef]
- Sirimanne, P.M.; Rusop, M.; Shirate, T.; Soga, T.; Jimbo, T. Characterization of transparent conducting CuI thin films prepared by pulse laser deposition technique. Chem. Phys. Lett. 2002, 366, 485–489. [Google Scholar] [CrossRef]
- Xia, M.; Gu, M.; Liu, X.; Liu, B.; Huang, S.; Ni, C. Luminescence characteristics of CuI film by iodine annealing. J. Mater. Sci. Mater. Electron. 2015, 26, 5092–5096. [Google Scholar] [CrossRef]
- Lin, G.; Zhao, F.; Zhao, Y.; Zhang, D.; Yang, L.; Xue, X.; Wang, X.; Qu, C.; Li, Q.; Zhang, L. Luminescence properties and mechanisms of CuI thin films fabricated by vapor iodization of copper films. Materials 2016, 9, 990. [Google Scholar] [CrossRef]
- Amor, M.B.; Boukhachem, A.; Boubaker, K.; Amlouk, M. Structural, optical and electrical studies on Mg-doped NiO thin films for sensitivity applications. Mater. Sci. Semicond. Process. 2014, 27, 994–1006. [Google Scholar] [CrossRef]
- Tsay, C.Y.; Yu, S.H. Melioration of electrical and optical properties of Al and B co-doped ZnO transparent semiconductor thin films. Coatings 2021, 11, 1259. [Google Scholar] [CrossRef]
- Krishnaiah, M.; Kumar, A.; Kushwaha, A.K.; Song, J.; Jin, S.H. Thickness dependent photodetection properties of solution-processed CuI films: Towards cost-effective flexible visible photodetectors. Mater. Lett. 2021, 305, 130815. [Google Scholar] [CrossRef]



| Concern. of Zn Substituting (at%) | Relative Intensity of (II(111)/ICuI(111) + I(111)) | Average Crystalline Size (nm) | Optical Bandgap (eV) | Urbach Energy (meV) | Carrier Concern. (cm−3) | Hall Mobility (cm2/Vs) | Electrical Resistivity (Ω·cm) |
|---|---|---|---|---|---|---|---|
| 0 | 0.250 | 7.23 | 3.00 | 81.6 | 4.45 × 1018 | 2.86 | 4.86 × 10−1 |
| 4 | 0.237 | 7.89 | 3.00 | 77.1 | −4.41 × 1018 | 7.93 | 1.79 × 10−1 |
| 8 | 0.184 | 8.83 | 3.01 | 76.5 | −4.34 × 1018 | 8.45 | 1.71 × 10−1 |
| 12 | 0.096 | 10.57 | 3.01 | 72.8 | −4.01 × 1018 | 9.44 | 1.65 × 10−1 |
| Parameter | * Mathematical Relation | Zn Substituted Concentration (at%) | |||
|---|---|---|---|---|---|
| 0 | 4 | 8 | 12 | ||
| Response current (Amp) | ΔI = Ilight − Idark | 5.28 × 10−5 | 1.39 × 10−4 | 2.05 × 10−4 | 9.33 × 10−5 |
| Sensitivity (unit less) | S = Ilight − Idark/Idark | 0.68 | 1.25 | 0.21 | 0.06 |
| Responsivity (mA/Watt) | R = ΔI/PA0 | 186 | 488 | 722 | 328 |
| Specific detectivity (Jones) | D = R × (A0/2eIdark)1/2 | 1.37 × 108 | 3.01 × 108 | 1.51 × 108 | 5.50 × 107 |
| External Quantum Efficiency (%) | EQE = (hc/eλ) × R | 62.2 | 164 | 242 | 110 |
| Sensing Layer | Electrodes/ Substrate | Method/ Temp. (°C) | Sensitivity (Unit Less) | Responsivity (mA/Watt) | Detectivity (×109 Jones) | Illumin. Wavelength | Reference |
|---|---|---|---|---|---|---|---|
| ZnO | In/quartz | sol-gel/450 | 4.6 | 0.51 | 4.57 | 350 nm | [8] |
| ZnO | Al/glass | sol-gel/500 | 0.19 | 3.25 | 0.03 | UVA | [13] |
| ZTO | Al/glass | sol-gel/500 | 2.95 | 0.14 | <0.01 | UVA | [16] |
| CuI | Ni/SiO2-Si | spin-coating/175 | 403 | 65.2 | 16.6 | Blue-LED | [29] |
| CuI | Ni/PI | spin-coating/175 | 264 | 45.6 | 6.56 | Blue-LED | [18] |
| CuI:Zn | Ni/glass | spin-coating/150 | 0.21 | 722 | 0.15 | UVA | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsay, C.-Y.; Chen, Y.-C.; Tsai, H.-M.; Sittimart, P.; Yoshitake, T. The Role of Zn Substitution in Improving the Electrical Properties of CuI Thin Films and Optoelectronic Performance of CuI MSM Photodetectors. Materials 2022, 15, 8145. https://doi.org/10.3390/ma15228145
Tsay C-Y, Chen Y-C, Tsai H-M, Sittimart P, Yoshitake T. The Role of Zn Substitution in Improving the Electrical Properties of CuI Thin Films and Optoelectronic Performance of CuI MSM Photodetectors. Materials. 2022; 15(22):8145. https://doi.org/10.3390/ma15228145
Chicago/Turabian StyleTsay, Chien-Yie, Yun-Chi Chen, Hsuan-Meng Tsai, Phongsaphak Sittimart, and Tsuyoshi Yoshitake. 2022. "The Role of Zn Substitution in Improving the Electrical Properties of CuI Thin Films and Optoelectronic Performance of CuI MSM Photodetectors" Materials 15, no. 22: 8145. https://doi.org/10.3390/ma15228145
APA StyleTsay, C.-Y., Chen, Y.-C., Tsai, H.-M., Sittimart, P., & Yoshitake, T. (2022). The Role of Zn Substitution in Improving the Electrical Properties of CuI Thin Films and Optoelectronic Performance of CuI MSM Photodetectors. Materials, 15(22), 8145. https://doi.org/10.3390/ma15228145






