Investigation of the Neutralizing Behaviors of Cement-Based Materials Using a New pH Indicator Formulated from February Orchid Petals
Abstract
1. Introduction
2. Materials and Methods
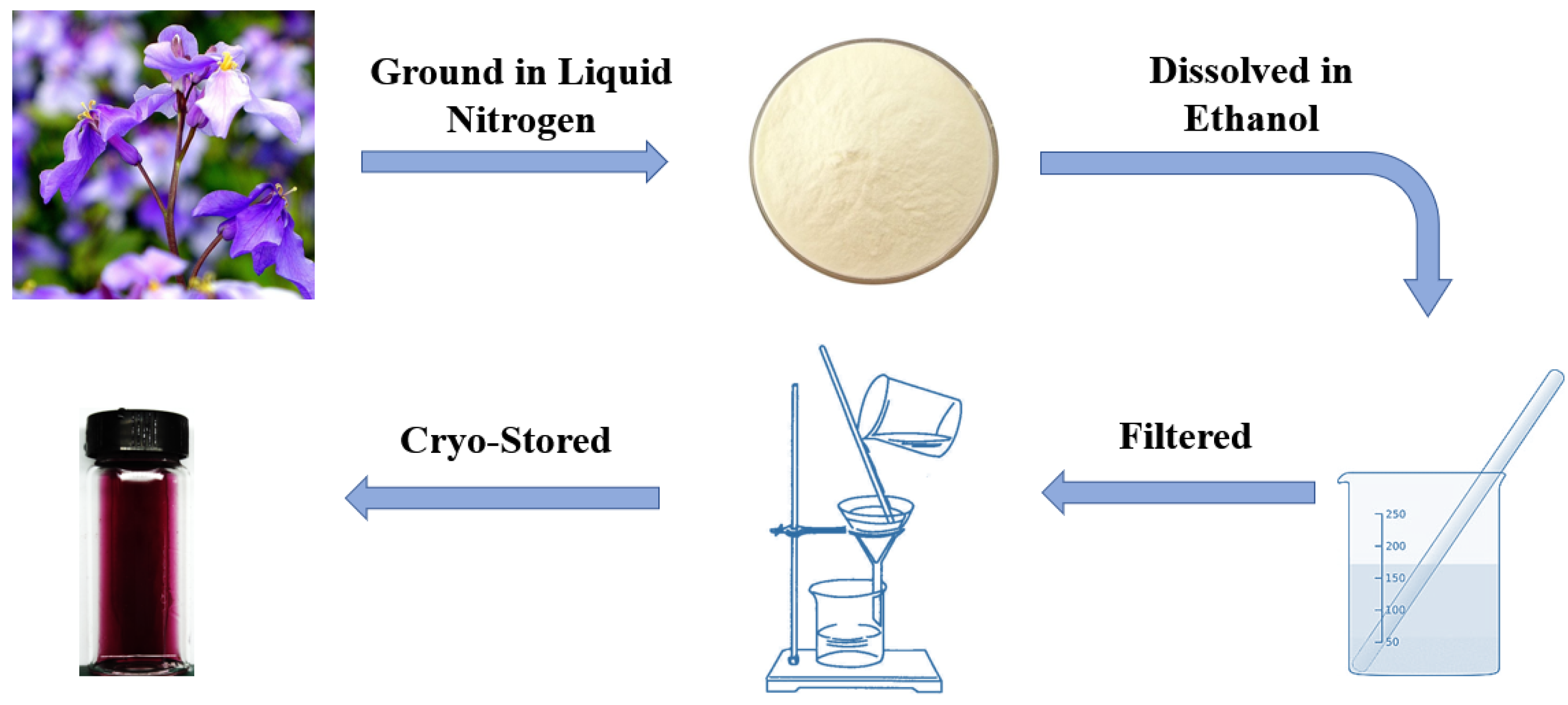
2.1. Formulation of Anthocyanin Indicator
2.2. Sample Preparation and Neutralization Test
2.3. Testing Methods
2.3.1. High-Performance Liquid Chromatograph (HPLC)
2.3.2. Indicator Spray Test
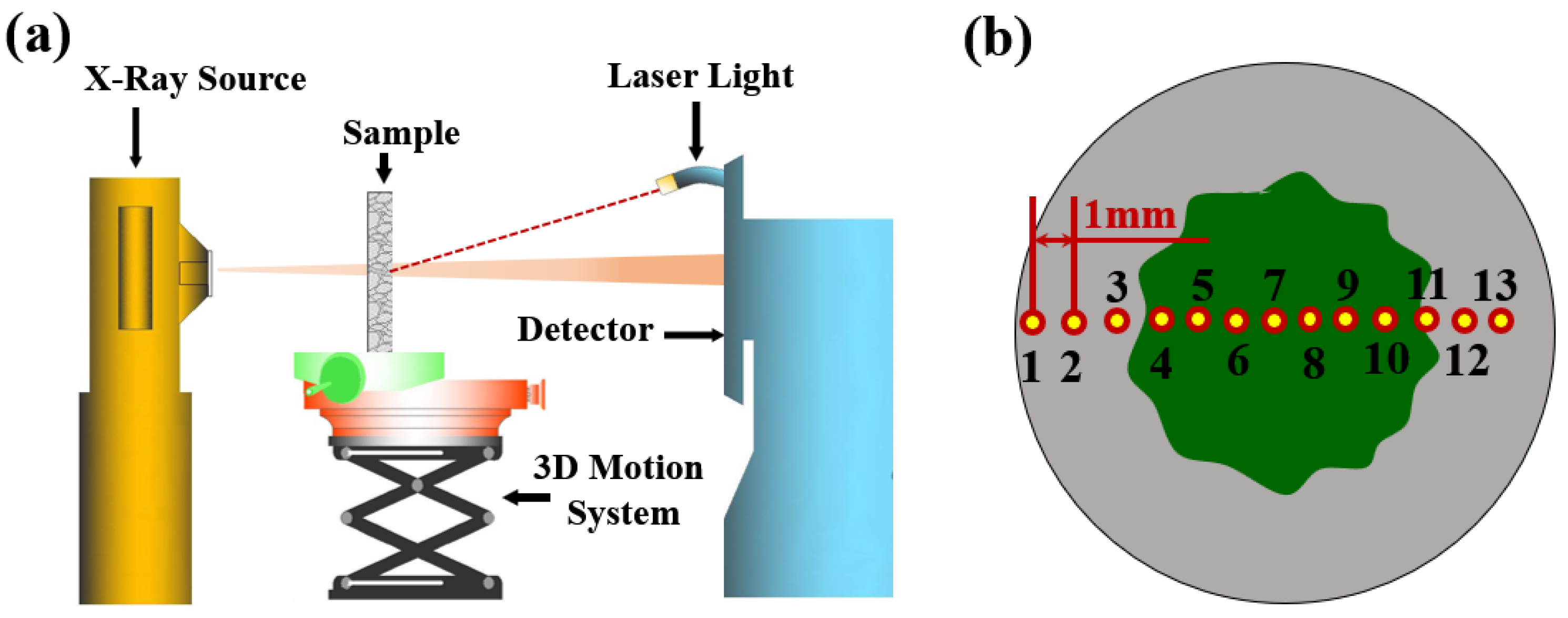
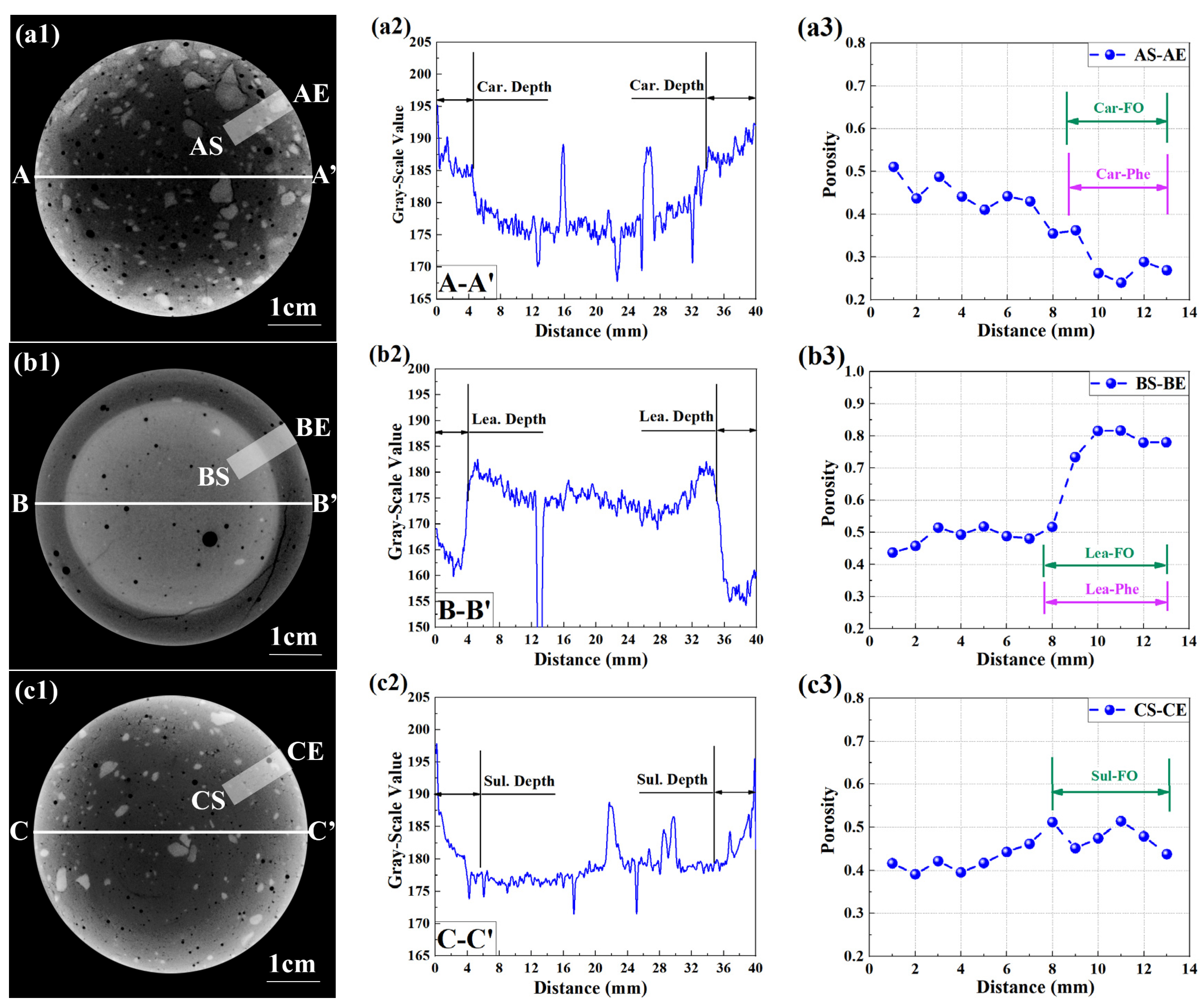
2.3.3. X-ray Computed Tomography (CT)
2.3.4. X-ray Attenuation Method (XRAM)
3. Results
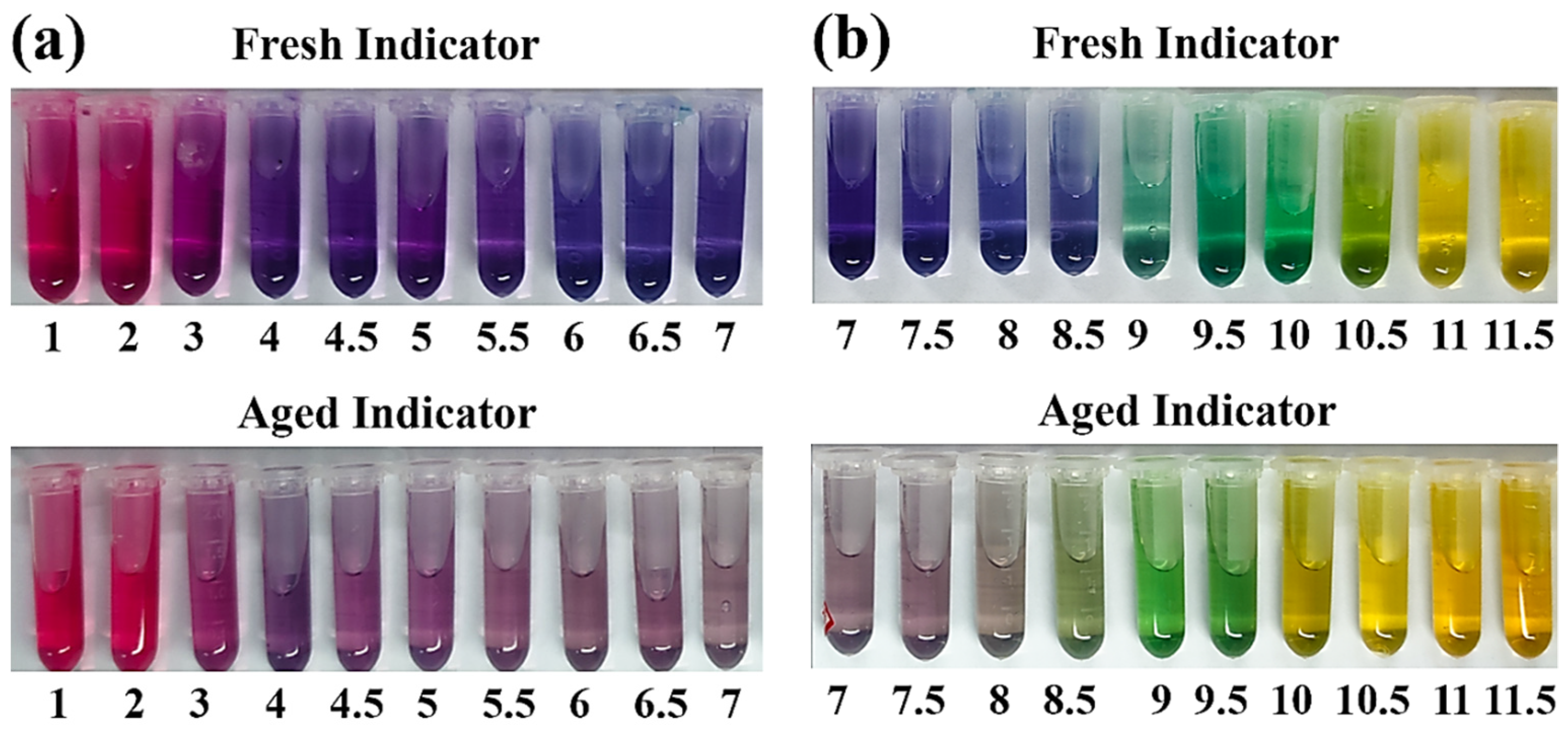
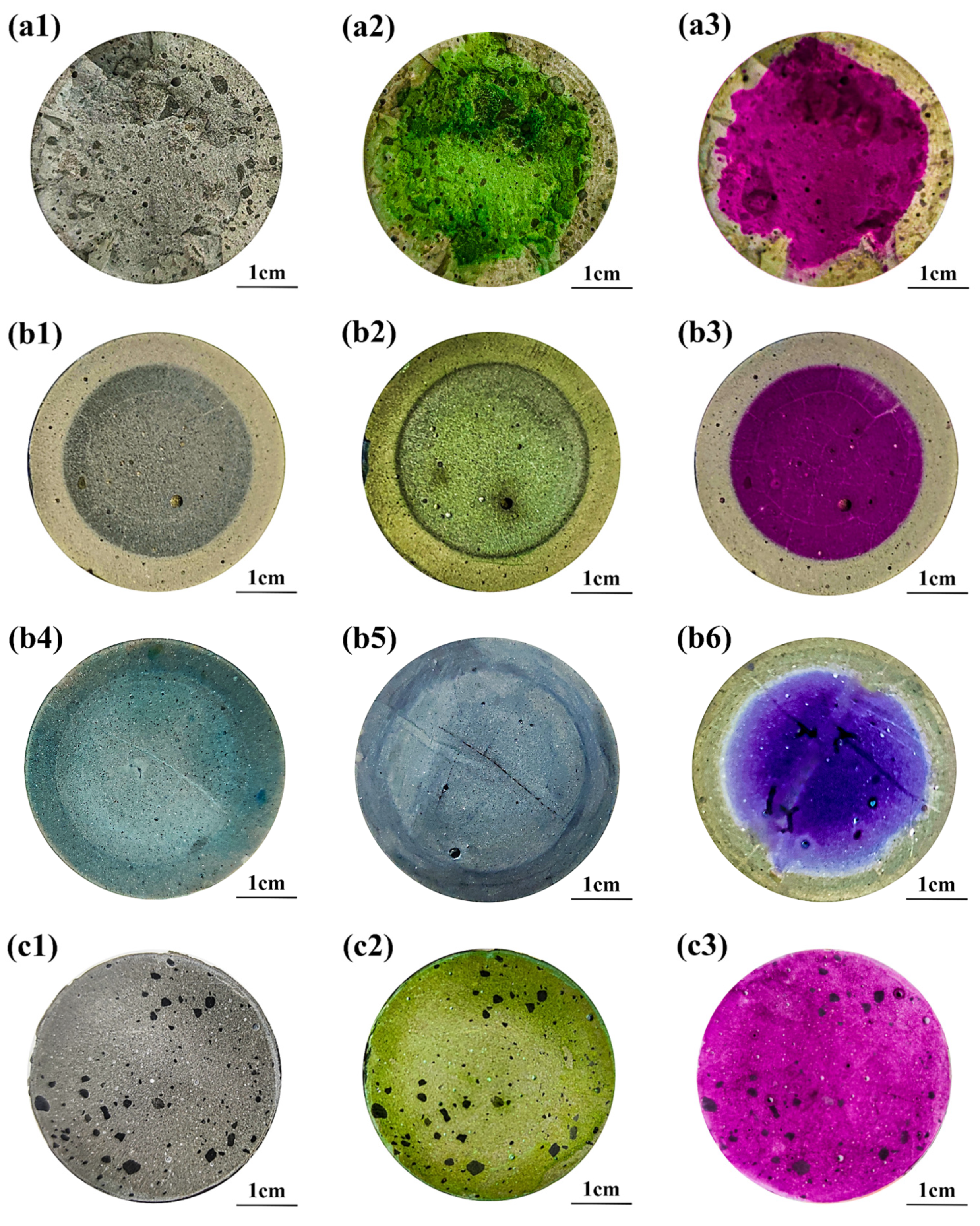
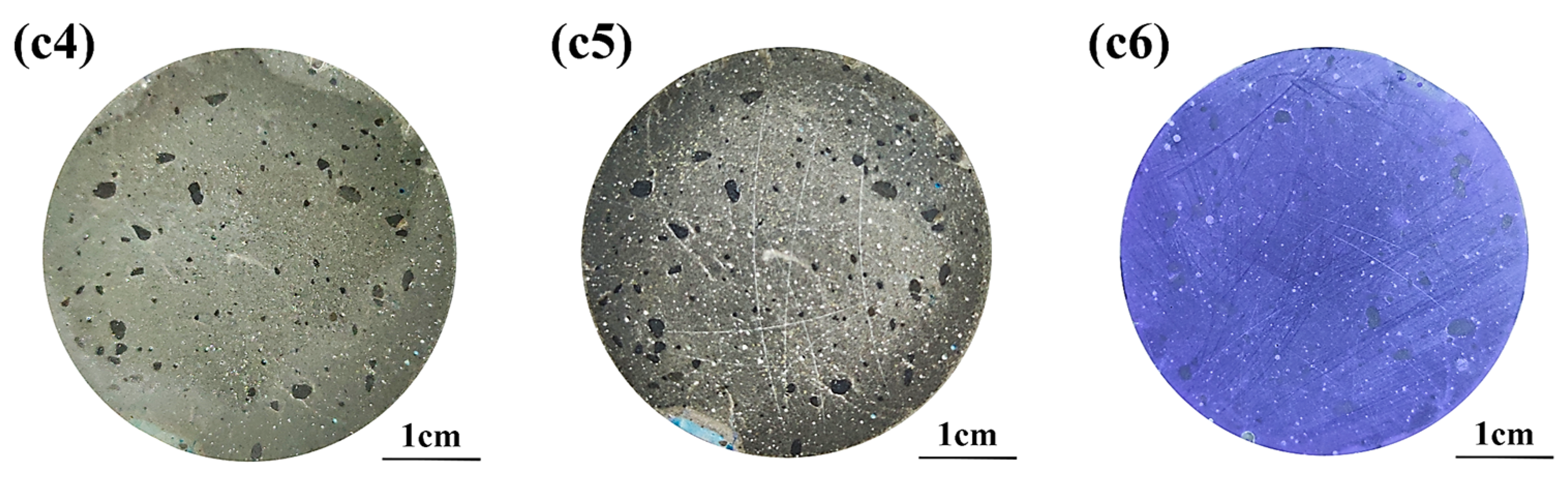
3.1. Discoloring Behavior of F. Orchid Indicator
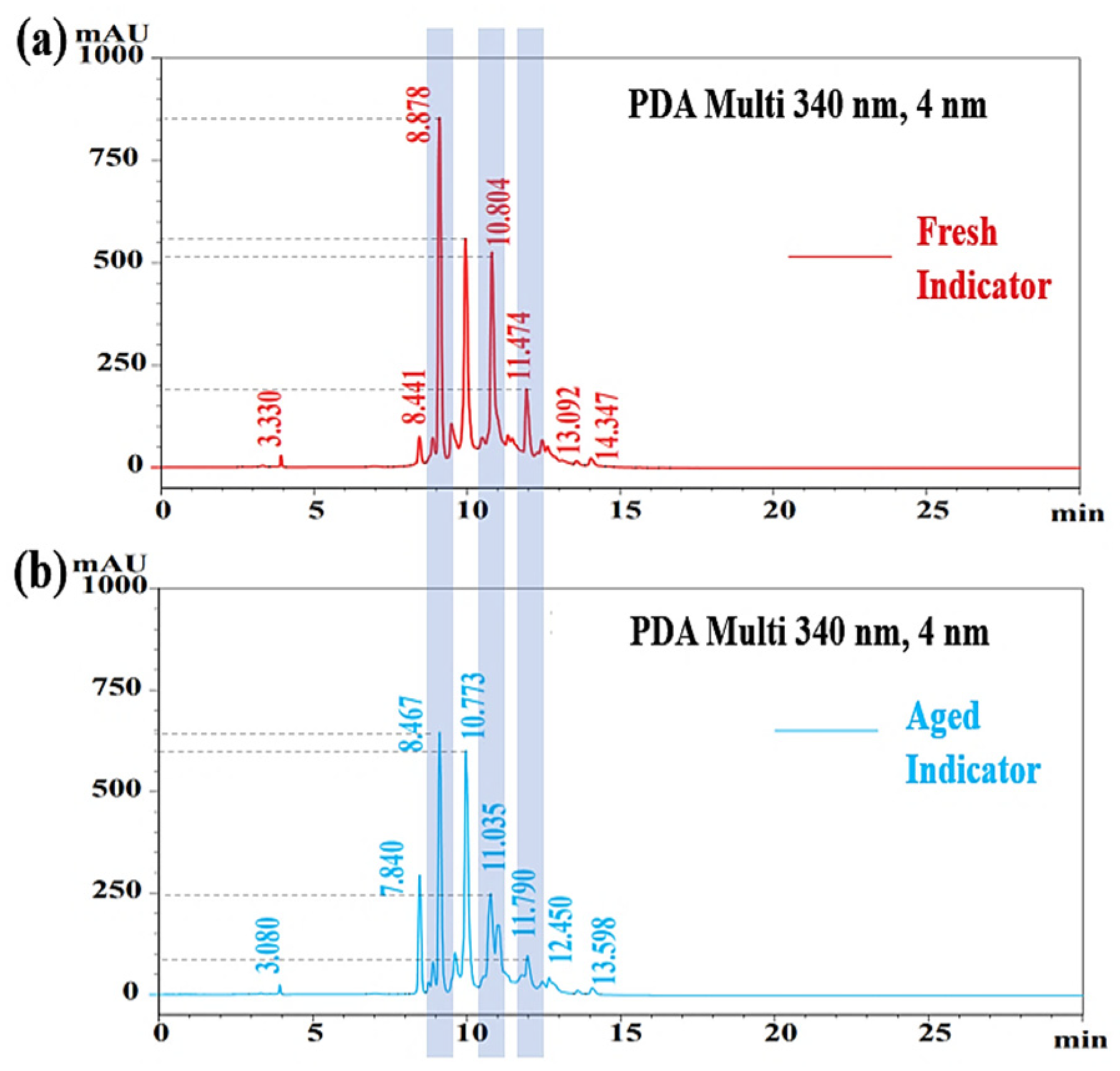
3.2. Chromatograms Obtained from HPLC
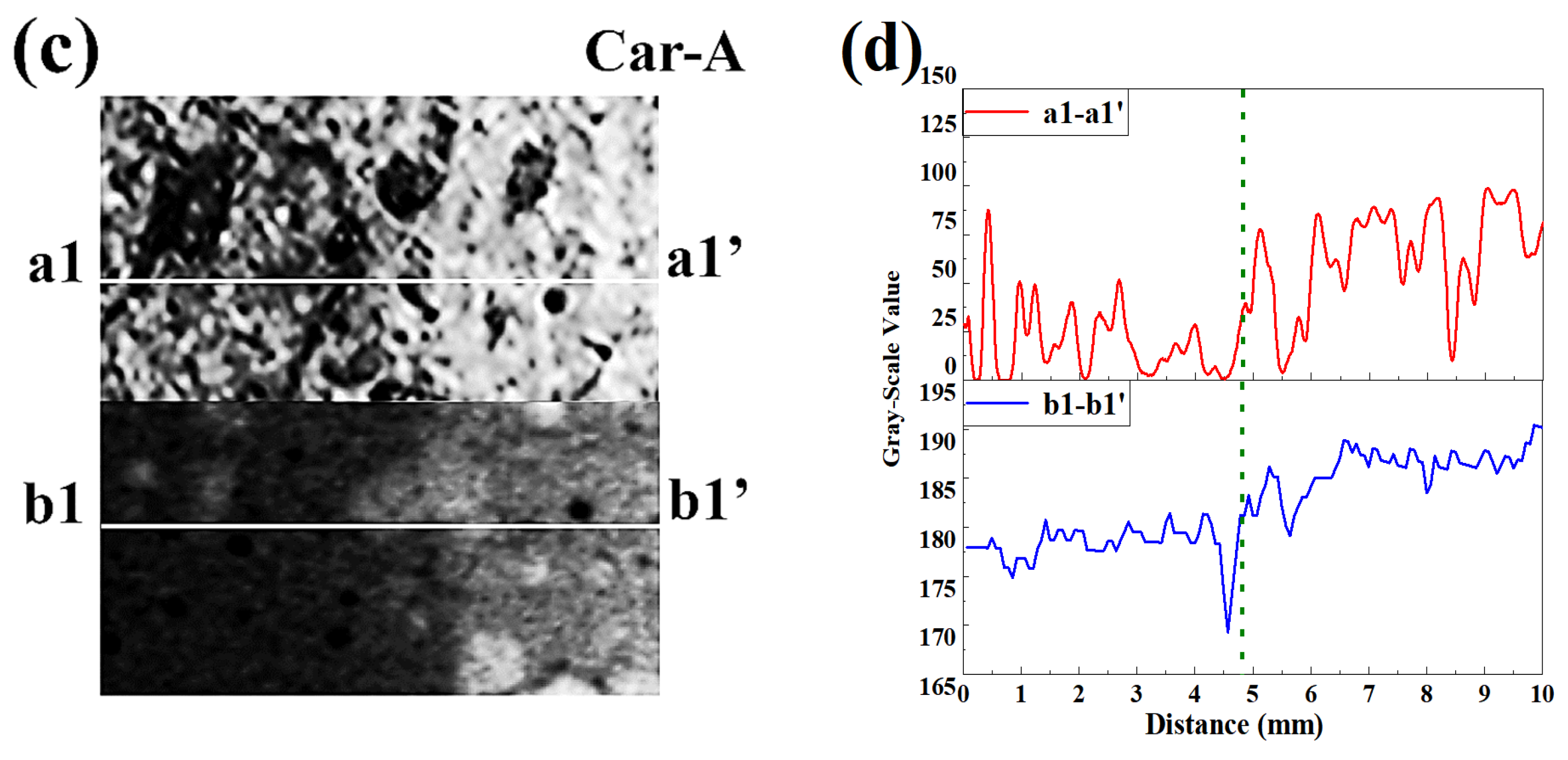
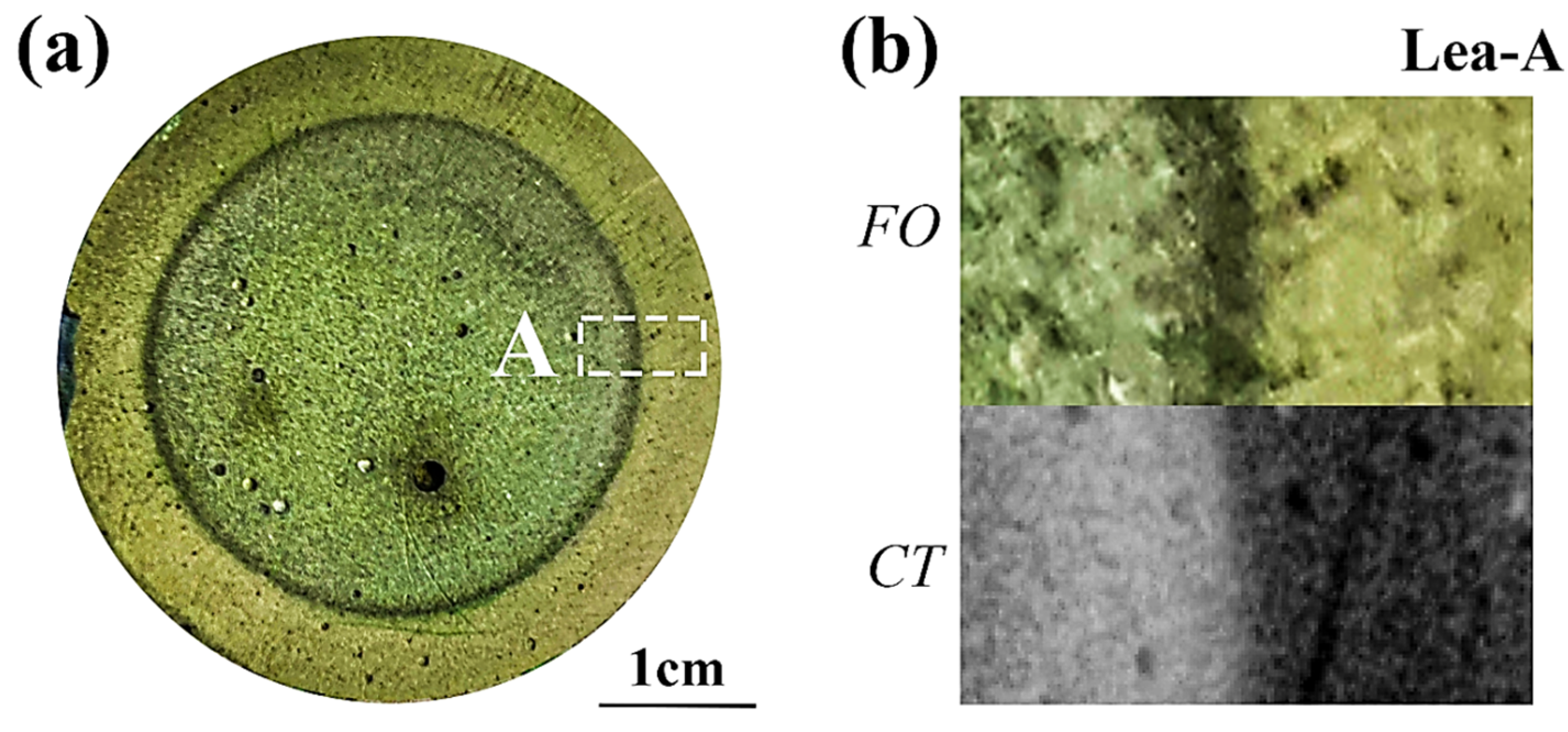
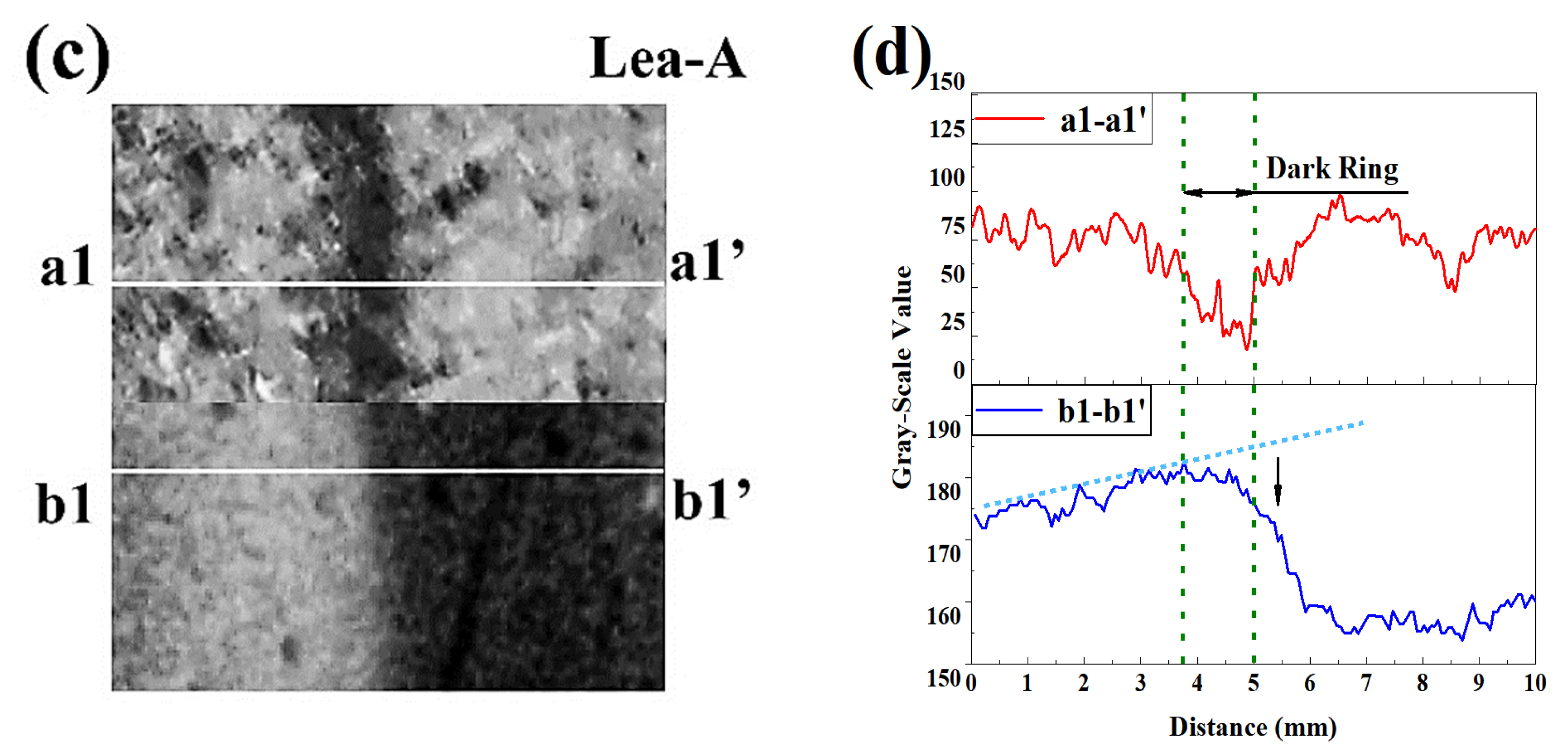
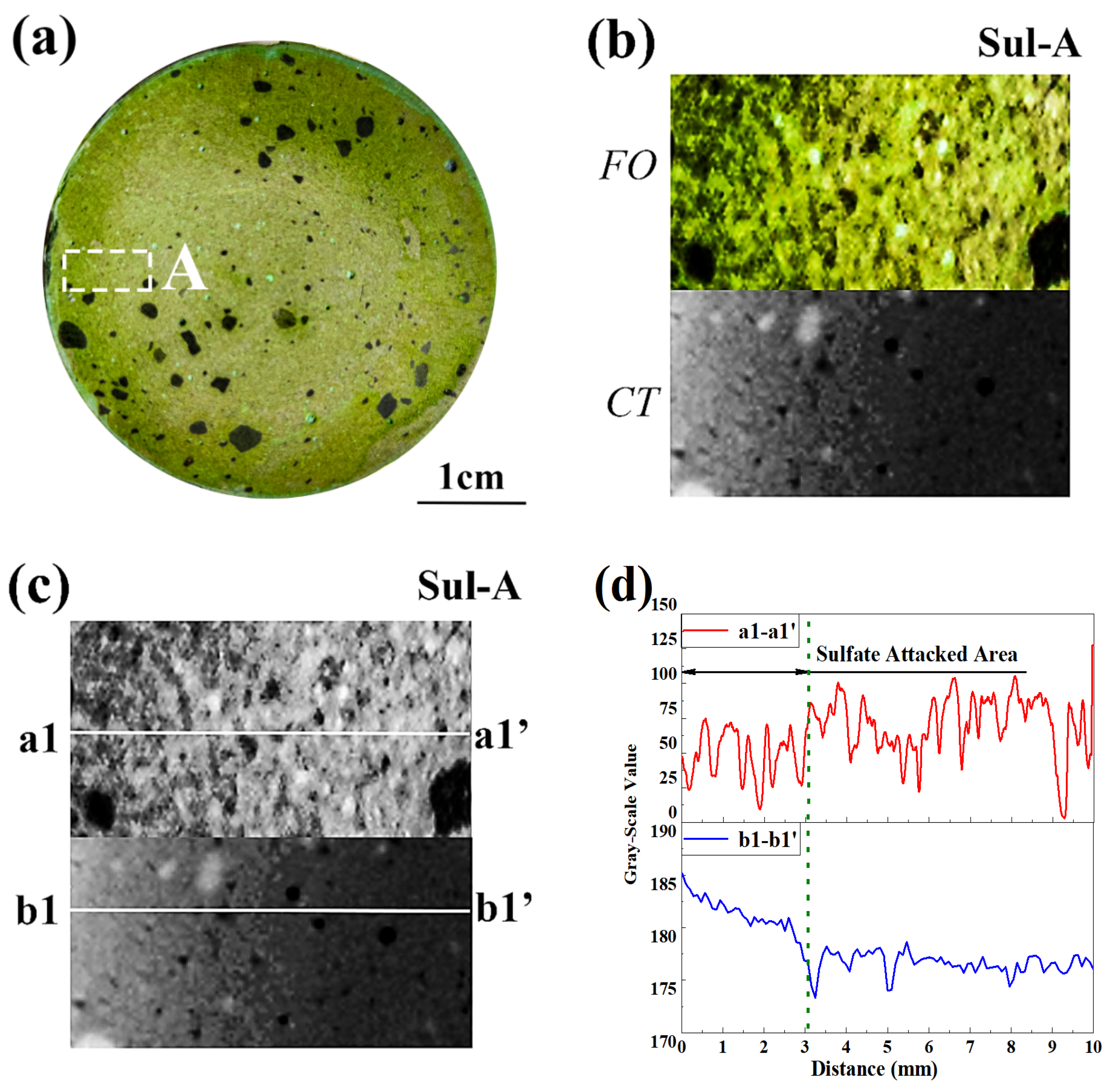
3.3. Neutralized Regions Characterized by Varying Indicators
3.4. Validation of F. Orchid Indicator’s Reliability
4. Discussion
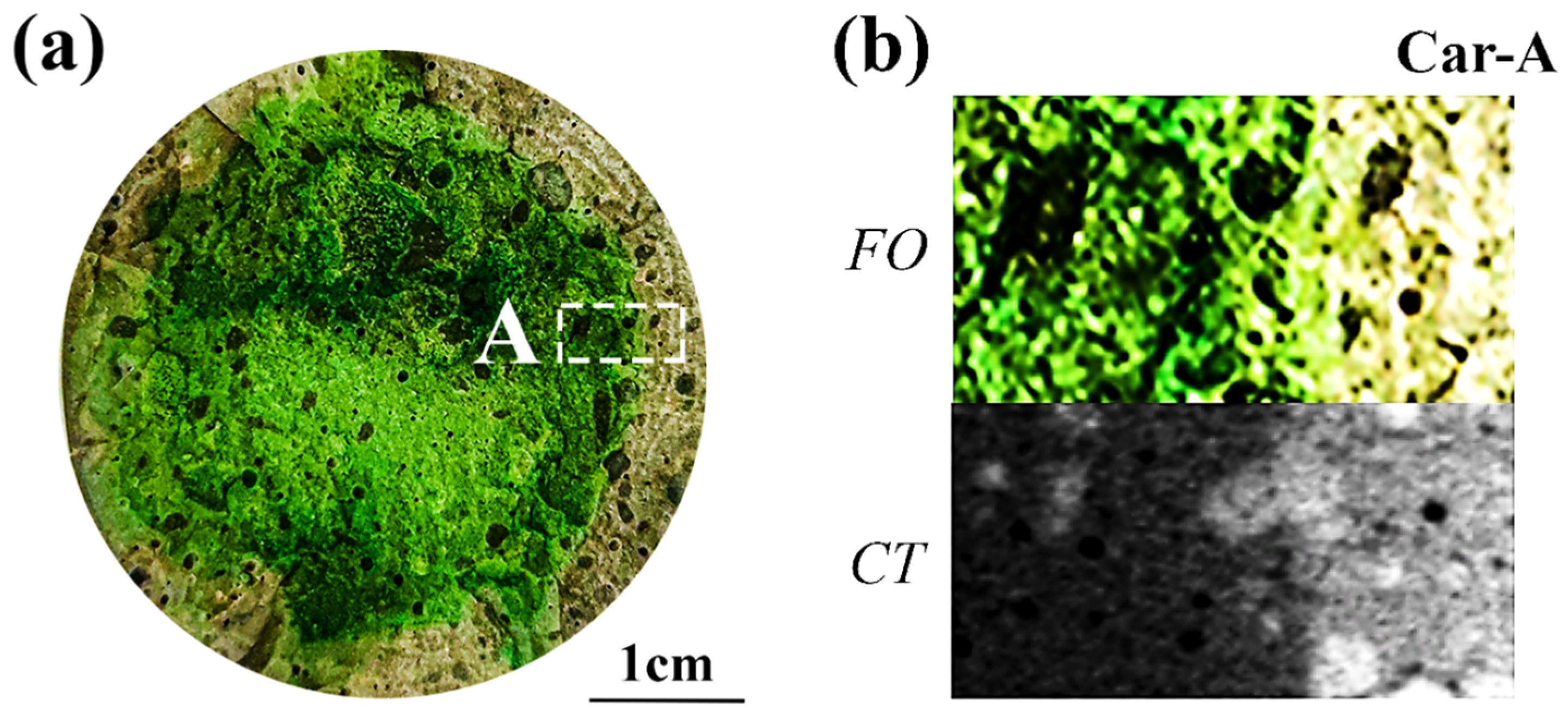
4.1. F. Orchid Indicator in Characterization of Neutralization Front
4.2. Applicability of F. Orchid Indicator in Neutralization Characterization
5. Conclusions and Future Work
5.1. Conclusions
5.2. Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lu, C.F.; Cai, C.X. Challenges and Countermeasures for Construction Safety during the Sichuan—Tibet Railway Project. Engineering 2019, 5, 833–838. [Google Scholar] [CrossRef]
- Pang, L.; Li, Q.W. Service life prediction of RC structures in marine environment using long term chloride ingress data: Comparison between exposure trials and real structure surveys. Constr. Build. Mater. 2016, 113, 979–987. [Google Scholar] [CrossRef]
- Li, K.F.; Zhang, D.D.; Li, Q.W.; Fan, Z.H. Durability for concrete structures in marine environments of HZM project: Design, assessment and beyond. Cem. Concr. Res. 2019, 115, 545–558. [Google Scholar] [CrossRef]
- Yi, Y.; Zhu, D.J.; Guo, S.C.; Zhang, Z.H.; Shi, C.J. A review on the deterioration and approaches to enhance the durability of concrete in the marine environment. Cem. Concr. Compos. 2020, 113, 103695. [Google Scholar] [CrossRef]
- Beushausen, H.; Torrent, R.; Alexander, M.G. Performance-based approaches for concrete durability: State of the art and future research needs. Cem. Concr. Res. 2019, 119, 11–20. [Google Scholar] [CrossRef]
- Ren, Y.P.; Huang, J.S.; Hong, Z.Y.; Lu, W.; Yin, J.; Zou, L.J.; Shen, X.H. Image-based concrete crack detection in tunnels using deep fully convolutional networks. Constr. Build. Mater. 2020, 234, 117367. [Google Scholar] [CrossRef]
- Park, S.E.; Eem, S.-H.; Jeon, H. Concrete crack detection and quantification using deep learning and structured light. Constr. Build. Mater. 2020, 252, 119096. [Google Scholar] [CrossRef]
- Wang, J.G.; Zhang, J.X.; Cao, D.D. Pore characteristics of recycled aggregate concrete and its relationship with durability under complex environmental factors. Constr. Build. Mater. 2021, 272, 121642. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, P.; Yu, Z.W. Effects of environmental factors on concrete carbonation depth and compressive strength. Materials 2018, 11, 2167. [Google Scholar] [CrossRef] [PubMed]
- Ming, J.; Wu, M.; Shi, J.J. Passive film modification by concrete carbonation: Re-visiting a corrosion-resistant steel with Cr and Mo. Cem. Concr. Compos. 2021, 123, 104178. [Google Scholar] [CrossRef]
- Overmann, S.; Lin, X.C.; Vollpracht, A. Investigations on the leaching behavior of fresh concrete–A review. Constr. Build. Mater. 2021, 272, 121390. [Google Scholar] [CrossRef]
- Liu, X.; Feng, P.; Li, W.; Geng, G.Q.; Huang, J.L.; Gao, Y.; Mu, S.; Hong, J.X. Effects of pH on the nano/micro structure of calcium silicate hydrate (CSH) under sulfate attack. Cem. Concr. Res. 2021, 140, 106306. [Google Scholar] [CrossRef]
- Silva, R.V.; Neves, R.; de Brito, J.; Dhir, R.K. Carbonation behaviour of recycled aggregate concrete. Cem. Concr. Comp. 2015, 62, 22–32. [Google Scholar] [CrossRef]
- Hu, H.-H.; Zuo, X.-B.; Cui, D.; Tang, Y.-J. Experimental study on leaching-abrasion behavior of concrete in flowing solution with low velocity. Constr. Build. Mater. 2019, 224, 762–772. [Google Scholar] [CrossRef]
- Reeuwijk, N.M.; Venhuis, B.J.; de Kaste, D.; Hoogenboom, R.L.; Rietjens, I.M.; Martena, M.J. Active pharmaceutical ingredients detected in herbal food supplements for weight loss sampled on the Dutch market. Food Addit. Contam. Part A 2014, 31, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Giulietti, N.; Chiariotti, P.; Cosoli, G.; Mobili, A.; Pandarese, G.; Tittarelli, F.; Revel, G.M. Automated measurement system for detecting carbonation depth: Image-processing based technique applied to concrete sprayed with phenolphthalein. Measurement 2021, 175, 109142. [Google Scholar] [CrossRef]
- Cano-Barrita, P.F.J.; Balcom, B.J.; Castellanos, F. Carbonation front in cement paste detected by T2 NMR measurements using a low field unilateral magnet. Mater. Struct. 2017, 50, 150. [Google Scholar] [CrossRef]
- Gruyaert, E.; Van den Heede, P.; Maes, M.; De Belie, N. Investigation of the influence of blast-furnace slag on the resistance of concrete against organic acid or sulphate attack by means of accelerated degradation tests. Cem. Concr. Res. 2012, 42, 173–185. [Google Scholar] [CrossRef]
- Reis, R.; Camões, A.; Ribeiro, M. Using thymolphthalein for accelerated carbonation testing of high volume fly ash cementitious blends. In Service Life and Durability of Reinforced Concrete Structures; Springer: Cham, Switzerland, 2019; pp. 17–30. [Google Scholar]
- Danner, T.; Geiker, M.R. Long-term influence of concrete surface and crack orientation on self-healing and ingress in cracks–field observations. Nord. Concr. Res. 2018, 58, 1–16. [Google Scholar] [CrossRef]
- Revert, A.B.; De Weerdt, K.; Hornbostel, K.; Geiker, M.R. Carbonation-induced corrosion: Investigation of the corrosion onset. Constr. Build. Mater. 2018, 162, 847–856. [Google Scholar] [CrossRef]
- Thiel, C.; Gehlen, C. On the determination of carbonation in cementitious materials. In Proceedings of the Rilem Spring Convention and Sustainable Materials, Systems and Structures Conference, Rovinj, Croatia, 18–22 March 2019; pp. 373–380. [Google Scholar]
- Seguí Femenias, Y.; Angst, U.; Elsener, B. pH-monitoring in mortar with thermally-oxidized iridium electrodes. RILEM Tech. Lett. 2017, 2, 59–66. [Google Scholar] [CrossRef]
- Skoulikidis, T.; Vassiliou, P.; Tsakona, K. Surface Consolidation of Pentelic Marble: Criteria for the selection of methods and materials-The Acropolis case (6 pp). Environ. Sci. Pollut. Res. 2005, 12, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.Y.; Lee, J.Y.; Chung, C.W. The application of various indicators for the estimation of carbonation and pH of cement based materials. J. Test. Eval. 2010, 38, 534–540. [Google Scholar]
- Herrera, R. Comparison of Methods to Determine the Carbonation Depth in Fly Ash Blended Cement Mortars. Ph.D. Thesis, Lakehead University, Thunder Bay, ON, Canada, 2014. [Google Scholar]
- Jeong, H.; Jung, B.J.; Kim, J.H.; Seo, S.Y.; Kim, K.S. Development and assessment of Nile blue-immobilized pH sensor to monitor the early stage of concrete carbonation. J. Build. Eng. 2022, 62, 105319. [Google Scholar] [CrossRef]
- Inserra, B.; Hayashi, K.; Marchisio, A.; Tulliani, J.M. Sol–gel-entrapped pH indicator for monitoring pH variations in cementitious materials. J. Appl. Biomater. Func. 2020, 18, 2280800020936540. [Google Scholar] [CrossRef]
- Vu, T.H.; Gowripalan, N.; Sirivivatnanon, V.; De Silva, P.; Kidd, P. Assessing Corrosion Resistance of Powder Form of Geopolymer Concrete. In Proceedings of the 29 Biennial Conference of the Concrete Institute of Australia, Sydney, Australia, 8–11 September 2019. [Google Scholar]
- Badar, M.S.; Kupwade-Patil, K.; Bernal, S.A.; Provis, J.L.; Allouche, E.N. Corrosion of steel bars induced by accelerated carbonation in low and high calcium fly ash geopolymer concretes. Constr. Build. Mater. 2014, 61, 79–89. [Google Scholar] [CrossRef]
- Vu, T.H.; Gowripalan, N.; De Silva, P.; Paradowska, A.; Garbe, U.; Kidd, P.; Sirivivatnanon, V. Assessing carbonation in one-part fly ash/slag geopolymer mortar: Change in pore characteristics using the state-of-the-art technique neutron tomography. Cem. Concr. Comp. 2020, 114, 103759. [Google Scholar] [CrossRef]
- Martin-del-Rio, J.J.; Alejandre, F.J.; Marquez, G.; Blasco, F.J. An argument for using alizarine yellow R and indigo carmine to determine in situ the degree of alkalinity in reinforced concrete. Constr. Build. Mater. 2013, 40, 426–429. [Google Scholar] [CrossRef]
- Bautista, A.; Velasco, F.; Torres-Carrasco, M. Influence of the alkaline reserve of chloride-contaminated mortars on the 6-year corrosion behavior of corrugated UNS S32304 and S32001 stainless steels. Metals 2019, 9, 686. [Google Scholar] [CrossRef]
- Cui, D.; Liu, W.; Wang, J.; Hu, J.; Shan, D.; Wan, Y.; Wang, Q.; Wang, J. Use of a novel pH indicator extracted from petals to investigate the carbonation behavior in cementitious materials. Cem. Concr. Comp. 2022, 134, 104804. [Google Scholar] [CrossRef]
- Chinchón-Payá, S.; Andrade, C.; Chinchón, S. Use of anthocyanin solutions in portland cement concrete to identify carbonation depth. Mater. Struct. 2020, 53, 101. [Google Scholar] [CrossRef]
- Vogler, N.; Lindemann, M.; Drabetzki, P.; Kühne, H.C. Alternative pH-indicators for determination of carbonation depth on cement-based concretes. Cem. Concr. Comp. 2020, 109, 103565. [Google Scholar] [CrossRef]
- Chinchón-Payá, S.; Andrade, C.; Chinchón, S. Indicator of carbonation front in concrete as substitute to phenolphthalein. Cem. Concr. Res. 2016, 82, 87–91. [Google Scholar] [CrossRef]
- Zhang, Z.G.; An, J.; Han, Y.C.; Feng, L.; Li, X.F.; Xiong, S.W.; Xing, F.F.; Xin, M.H.; Li, Y.B.; Wang, Z.B. Advantages of an Orychophragmus violaceus-maize rotation in reducing greenhouse gas emissions and reactive nitrogen losses and increasing net ecosystem economic benefits on the North China Plain. J. Clean. Prod. 2021, 317, 128426. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Li, X.F.; Xiong, S.W.; An, J.; Han, Y.C.; Wang, G.P.; Feng, L.; Lei, Y.P.; Yang, B.F.; Xing, F.F.; et al. Orychophragmus violaceus as a winter cover crop is more conducive to agricultural sustainability than Vicia villosa in cotton-fallow systems. Arch. Agron. Soil Sci. 2022, 68, 1487–1500. [Google Scholar] [CrossRef]
- Choi, I.; Lee, J.Y.; Lacroix, M.; Han, J. Intelligent pH indicator film composed of agar/potato starch and anthocyanin extracts from purple sweet potato. Food Chem. 2017, 218, 122–128. [Google Scholar] [CrossRef]
- Paul, J.S.; Tiwari, K.; Jadhav, S. Long term preservation of commercial important fungi in glycerol at 4 °C. Int. J. Biol. Chem. 2015, 9, 79–85. [Google Scholar] [CrossRef]
- Noguchi, H.; Miyagi-Shiohira, C.; Kurima, K.; Kobayashi, N.; Saitoh, I.; Watanabe, M.; Noguchi, Y.; Matsushita, M. Islet culture/preservation before islet transplantation. Cell Med. 2015, 8, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Halász, K.; Csóka, L. Black chokeberry (Aronia melanocarpa) pomace extract immobilized in chitosan for colorimetric pH indicator film application. Food Packag. Shelf. 2018, 16, 185–193. [Google Scholar] [CrossRef]
- Mills, A.; Skinner, G.A. Water-based colourimetric optical indicators for the detection of carbon dioxide. Analyst 2010, 135, 1912–1917. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-J.; Zuo, X.-B.; Yin, G.-J.; Davoudi, H.; Li, X.-N. Influence of calcium leaching on chloride diffusivity in cement-based materials. Constr. Build. Mater. 2018, 174, 310–319. [Google Scholar] [CrossRef]
- Tang, Y.-J.; Zuo, X.-B.; Yin, G.-J.; He, S.-L.; Ayinde, O. Influence of slag on leaching behavior of cement mortar lined in ductile iron pipe under a flowing solution. Mater. Des. 2017, 114, 612–622. [Google Scholar] [CrossRef]
- Morandeau, A.; Thiéry, M.; Dangla, P. Impact of accelerated carbonation on OPC cement paste blended with fly ash. Cem. Concr. Res. 2015, 67, 226–236. [Google Scholar] [CrossRef]
- Morandeau, A.; Thiery, M.; Dangla, P. Investigation of the carbonation mechanism of CH and CSH in terms of kinetics, microstructure changes and moisture properties. Cem. Concr. Res. 2014, 56, 153–170. [Google Scholar] [CrossRef]
- Talukdar, S.; Banthia, N.; Grace, J. Carbonation in concrete infrastructure in the context of global climate change–Part 1: Experimental results and model development. Cem. Concr. Comp. 2012, 34, 924–930. [Google Scholar] [CrossRef]
- Cui, D.; Sun, W.; Banthia, N. Use of tomography to understand the influence of preconditioning on carbonation tests in cement-based materials. Cem. Concr. Comp. 2018, 88, 52–63. [Google Scholar] [CrossRef]
- Turcry, P.; Oksri-Nelfia, L.; Younsi, A.; Aït-Mokhtar, A. Analysis of an accelerated carbonation test with severe preconditioning. Cem. Concr. Res. 2014, 57, 70–78. [Google Scholar] [CrossRef]
- Cui, D.; Banthia, N.; Wang, Q.N.; Sun, W. Investigation on porosity of partly carbonated paste specimens blended with fly ash through dual CT scans. Constr. Build. Mater. 2019, 196, 692–702. [Google Scholar] [CrossRef]
- Yin, G.-J.; Zuo, X.-B.; Li, X.-N.; Zou, Y.-X. An integrated macro-microscopic model for concrete deterioration under external sulfate attack. Eng. Fract. Mech. 2020, 240, 107345. [Google Scholar] [CrossRef]
- Yin, G.-J.; Zuo, X.-B.; Tang, Y.-J.; Ayinde, O.; Wang, J.-L. Numerical simulation on time-dependent mechanical behavior of concrete under coupled axial loading and sulfate attack. Ocean Eng. 2017, 142, 115–124. [Google Scholar] [CrossRef]
- Ketcham, R.A.; Hanna, R.D. Beam hardening correction for X-ray computed tomography of heterogeneous natural materials. Comput. Geosci. 2014, 67, 49–61. [Google Scholar] [CrossRef]
- Cui, D.; Wei, H.; Zuo, X.B.; Zheng, K.; Wang, Q.N. Use of Graphene Oxide to Improve the Durability and Mechanical Properties of Mortar Immersed in Flowing River for Three Years. Nanomaterials 2020, 10, 2385. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Zuo, X.; Zheng, K.; Talukdar, S. Tomography-based investigation on the carbonation behavior through the surface-opening cracks of sliced paste specimen. Materials 2020, 13, 1804. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, D.; Shi, X.; Liu, W.; Zheng, K.; Yin, G.; Wang, J.; Han, G.; Wan, Y.; Wang, J.; Li, W. Investigation of the Neutralizing Behaviors of Cement-Based Materials Using a New pH Indicator Formulated from February Orchid Petals. Materials 2022, 15, 8033. https://doi.org/10.3390/ma15228033
Cui D, Shi X, Liu W, Zheng K, Yin G, Wang J, Han G, Wan Y, Wang J, Li W. Investigation of the Neutralizing Behaviors of Cement-Based Materials Using a New pH Indicator Formulated from February Orchid Petals. Materials. 2022; 15(22):8033. https://doi.org/10.3390/ma15228033
Chicago/Turabian StyleCui, Dong, Xiaohan Shi, Wenya Liu, Keren Zheng, Guangji Yin, Jing Wang, Guantong Han, Yi Wan, Junsong Wang, and Wenting Li. 2022. "Investigation of the Neutralizing Behaviors of Cement-Based Materials Using a New pH Indicator Formulated from February Orchid Petals" Materials 15, no. 22: 8033. https://doi.org/10.3390/ma15228033
APA StyleCui, D., Shi, X., Liu, W., Zheng, K., Yin, G., Wang, J., Han, G., Wan, Y., Wang, J., & Li, W. (2022). Investigation of the Neutralizing Behaviors of Cement-Based Materials Using a New pH Indicator Formulated from February Orchid Petals. Materials, 15(22), 8033. https://doi.org/10.3390/ma15228033







