New Insights into the Mechanism of Nucleation of ZrO2 Inclusions at High Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Microstructure and Composition Characterization
2.3. Nucleation Calculation
2.4. First-Principles Calculation
3. Results
3.1. Inclusion Characterization
3.2. Classical Nucleation Calculation
3.3. First-Principles Calculations
3.3.1. Structures of (ZrO2)n Clusters
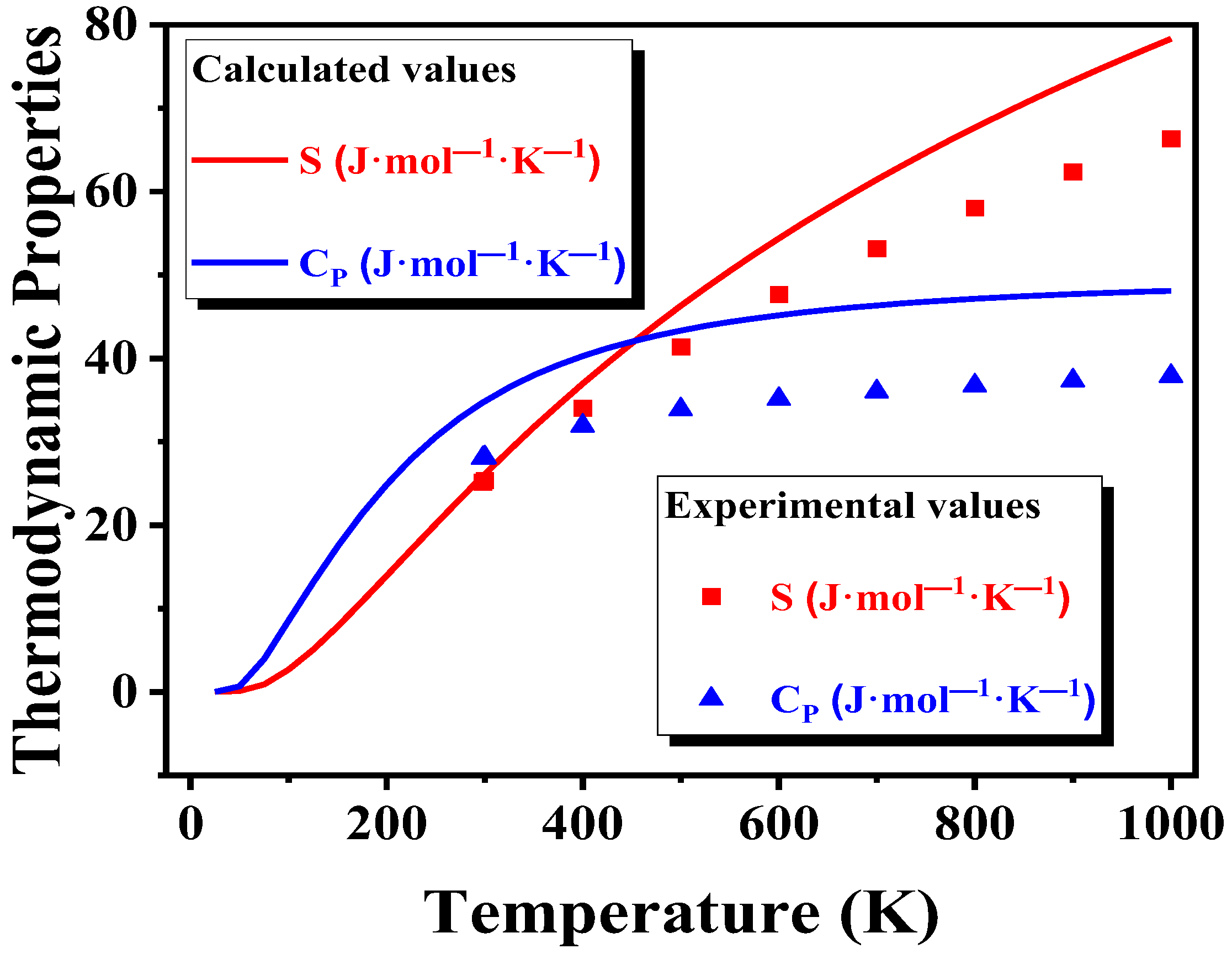
3.3.2. Thermodynamic Properties of the (ZrO2)n Clusters
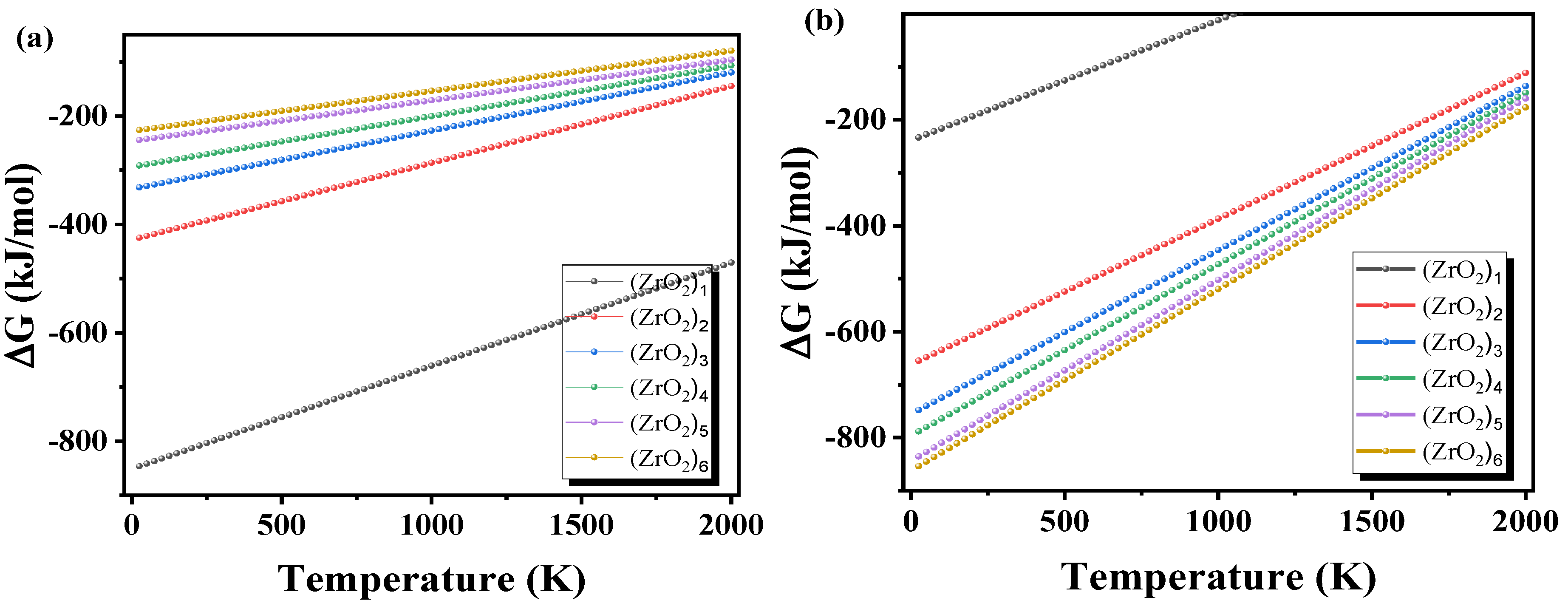
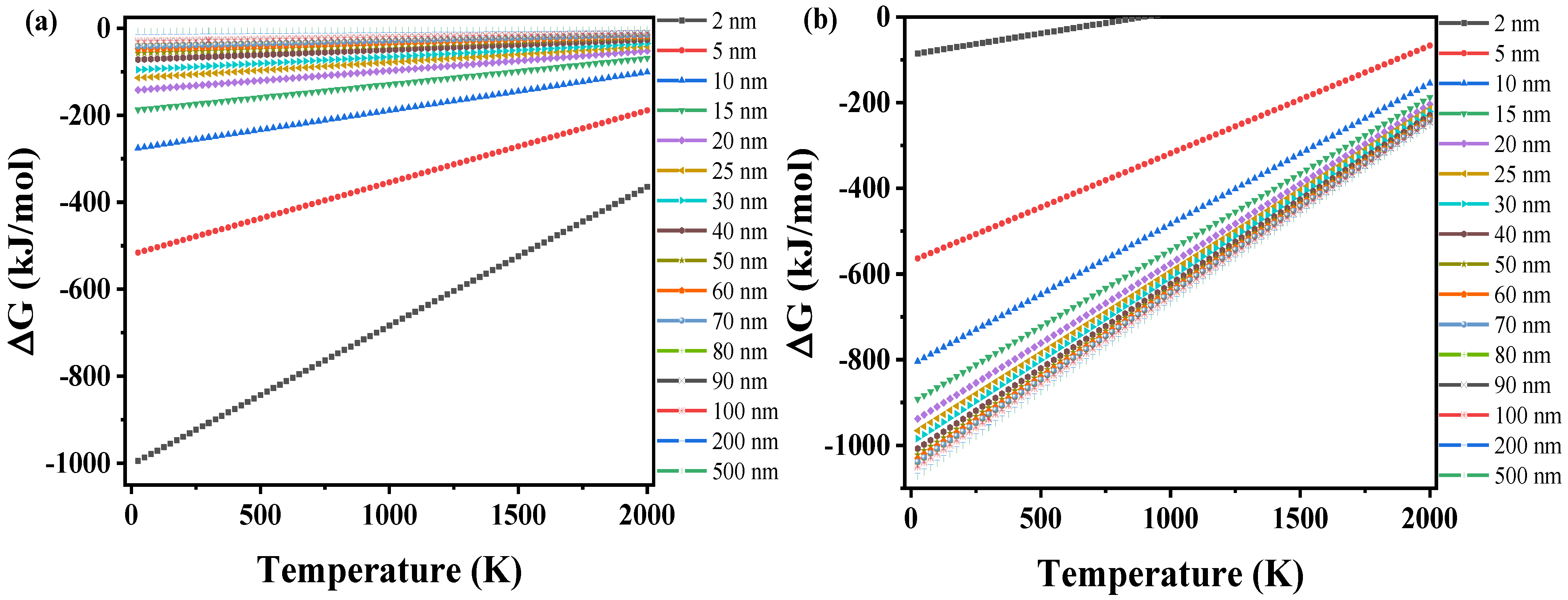
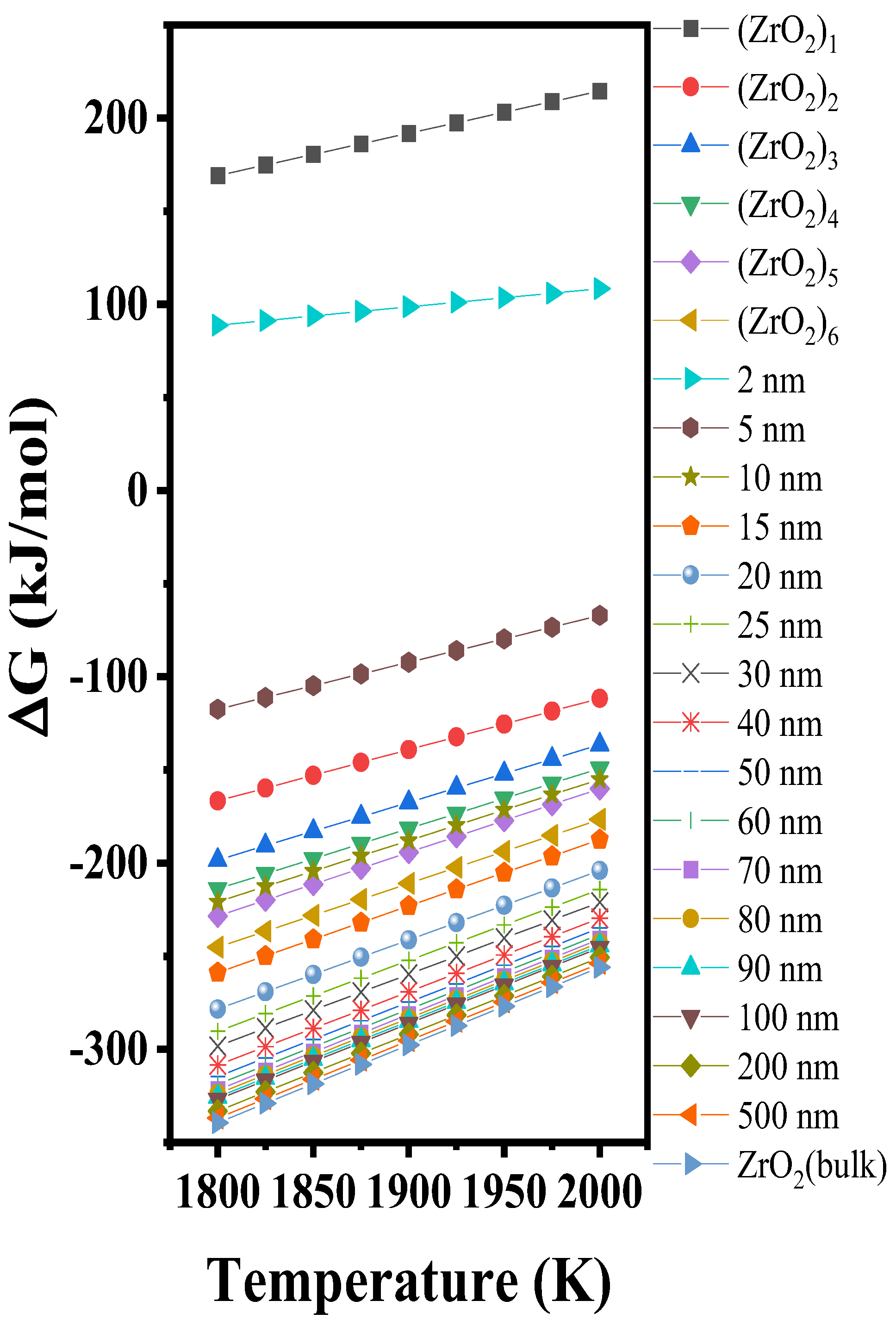
3.3.3. Gibbs Free Energy Changes of (ZrO2)n Clusters and Nanoparticles
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takamura, J.I.; Mizoguchi, S. Roles of oxides in steel performance. Proceedings of The Sixth International Iron and Steel Congress, ISIJ International, Nagoya, Japan, 21–26 October 1990; pp. 591–597. [Google Scholar]
- Wang, L.; Yang, S.; Li, J.; Chen, C.; Li, C.; Li, X. Study on the Capillary Interaction Between Particles on the Surface of High-Temperature Melts. Steel Res. Int. 2021, 92, 2100013. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Li, J.; Yang, S.; Chen, C.; Li, C.; Li, X. In-Situ Observation on the Agglomeration and Dispersion of Particles at the Interface of High-temperature Melts. ISIJ Int. 2021, 61, 753–762. [Google Scholar] [CrossRef]
- Abraham, S.; Bodnar, R.; Raines, J.; Wang, Y. Inclusion engineering and metallurgy of calcium treatment. J. Iron Steel Res. Int. 2018, 25, 133–145. [Google Scholar] [CrossRef]
- Liu, W.; Yang, S.F.; Li, J.S.; Wang, F.; Yang, H.B. Numerical model of inclusion separation from liquid metal with consideration of dissolution in slag. J. Iron Steel Res. Int. 2019, 26, 1147–1153. [Google Scholar] [CrossRef]
- Peng, B.W.; Li, F.J.; Zheng, S.B.; Li, H.G.; Zhai, Q.J. Inclusion control study in high aluminum steel. J. Iron Steel Res. Int. 2018, 25, 796–802. [Google Scholar] [CrossRef]
- Zhao, K.W.; Zeng, J.H.; Wang, X.H. Nonmetallic inclusion control of 350 km/h high speed rail steel. J. Iron Steel Res. Int. 2009, 16, 20–26. [Google Scholar] [CrossRef]
- Wang, L.; Yang, S.; Li, J.; Wu, T.; Liu, W.; Xiong, J. Improving Cleanliness of 95CrMo Drill Rod Steel by Slag Refining. Met. Mater. Trans. A 2016, 47, 99–107. [Google Scholar] [CrossRef]
- Yang, Y.; Zhan, D.; Lei, H.; Qiu, G.; Li, Y.; Jiang, Z.; Zhang, H. In situ observation of acicular ferrite nucleation and growth at different cooling rate in Ti-Zr deoxidized steel. Metall. Mater. Trans. B 2019, 50, 2536–2546. [Google Scholar] [CrossRef]
- Chai, F.; Yang, C.F.; Su, H.; Zhang, Y.Q.; Xu, Z.; Yang, Y.H. Effect of Zr addition to Ti-killed steel on inclusion formation and microstructural evolution in welding induced coarse-grained heat affected zone. Acta Metall. Sin. Engl. Lett. 2008, 21, 220–226. [Google Scholar] [CrossRef]
- Guo, A.M.; Li, S.R.; Guo, J.; Li, P.H.; Ding, Q.F.; Wu, K.M.; He, X.L. Effect of zirconium addition on the impact toughness of the heat affected zone in a high strength low alloy pipeline steel. Mater. Charact. 2008, 59, 134–139. [Google Scholar] [CrossRef]
- Wu, H.B.; Hou, M.; Liang, G.L.; Tang, D. Effect of zirconium on the low-temperature toughness of CGHAZ in F40 ship plates containing titanium. J. Univ. Sci. Technol. Beijing 2012, 34, 137–142. [Google Scholar]
- Min, J.; Hu, Z.; Wang, X.; Pak, J.J. Characterization of microstructure and non-metallic inclusions in Zr-Al deoxidized low carbon steel. ISIJ Int. 2013, 53, 1386–1391. [Google Scholar]
- Bhadeshia, H.K.D.H.; Honeycombe, R.W.K. Steels Micro-Structure and Properties, 3rd ed.; Elsevier Ltd.: Oxford, UK, 2006; Volume 7, pp. 1–360. [Google Scholar]
- He, K.; Baker, T.N. Zr-contaning precipitates in a Ti-Nb micro-alloyed HSLA steel containing 0.016% Zr addition. Mater. Sci. Eng. 1996, 215, 57–66. [Google Scholar]
- Sarma, D.S.; Karasev, A.V.; Jonsson, P.G. On the role of nonmetallic inclusions in the nucleation of acicular ferrite in steels. ISIJ Int. 2009, 49, 1063–1074. [Google Scholar] [CrossRef]
- Guo, Q.Y.; Song, B.; Song, M.M. Effects of Ti, Al and Zr deoxidation on morphology of sulfides in medium-sulfur non-quenched and tempered steel. Trans. Mater. Heat Treat. 2019, 40, 133–139. [Google Scholar]
- Li, Y.; Wan, X.L.; Cheng, L.; Wu, K.M. First-principles calculation of the interaction of Mn with ZrO2 and its effect on the formation of ferrite in high-strength low-alloy steels. Scr. Mater. 2014, 75, 78–81. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L. Thermodynamics dependent on size in nucleation process of inclusions and calculation of critical nucleus radius in clean molten steel. Iron Steel 2012, 47, 22–26. [Google Scholar] [CrossRef]
- Wang, G.C.; Wang, Q.; Li, S.L.; Ai, X.G.; Fan, C.G. Evidence of Multi-step Nucleation Leading to Various Crystallization Pathways from an Fe-O-Al Melt. Sci. Rep. 2014, 4, 5082. [Google Scholar] [CrossRef]
- Lei, H.; Xiao, Y.; Wang, G.; Zhang, H.; Jin, W.; Zhang, L. Thermodynamic insight into the growth of nanoscale particle of Al-deoxidation in Fe–O–Al melt. Sci. Rep. 2020, 10, 16909. [Google Scholar] [CrossRef]
- Zhao, D.; Bao, W.; Li, H.; Zheng, S.; Chou, K.-C. Cluster-assisted nucleation mechanism of titanium oxides in Fe-Ti supercooled alloys. J. Alloys Compd. 2018, 744, 797–800. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, W.; He, L.; Li, H.; Zheng, S. Study on the growth and morphology evolution of titanium oxide clusters in molten iron with molecular dynamics simulation. RSC Adv. 2019, 9, 32620–32627. [Google Scholar] [CrossRef] [PubMed]
- Suito, H.; Ohta, H. Characteristics of Particle Size Distribution in Early Stage of Deoxidation. ISIJ Int. 2006, 46, 33–41. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Yang, S.; Chen, C.; Jin, H.; Li, X. Nucleation and Ostwald Growth of Particles in Fe-O-Al-Ca Melt. Sci. Rep. 2018, 8, 1135. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, L.; Chen, C.; Li, J.; Li, X. Effect of Mg Treatment on the Nucleation and Ostwald Growth of Inclusions in Fe-O-Al-Mg Melt. Materials 2020, 13, 3355. [Google Scholar] [CrossRef] [PubMed]
- Humenik, M.; Kingery, W.D. Metal-Ceramic Interactions: III, Surface Tension and Wettability of Metal-Ceramic Systems. J. Am. Ceram. Soc. 1954, 37, 18–23. [Google Scholar] [CrossRef]
- Hino, M.; Ito, K. Thermodynamic Data for Steelmaking, 2nd ed.; Tohoku University Press: Tohoku, Japan, 2010; pp. 247–264. [Google Scholar]
- Ohta, H.; Suito, H. Efects of Dissolved Oxygen and Size Distribution on Particle Coarsening of Deoxidation Product. ISIJ Int. 2006, 46, 42–49. [Google Scholar] [CrossRef]
- Sakata, K.; Suito, H. Dispersion of fine primary inclusions of MgO and ZrO2 in Fe-10 mass pct Ni alloy and the solidification structure. Met. Mater. Trans. B 1999, 30, 1053–1063. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1997, 77, 3865. [Google Scholar] [CrossRef]
- Wang, G.; Xiao, Y.; Zhao, C.; Jing, L.; Shang, D. Atomic Cluster Aggregates in Nucleation of Solid Alumina Inclusion in the Aluminum Deoxidation for Liquid Iron. Metall. Mater. Trans. B 2017, 49, 282–290. [Google Scholar] [CrossRef]
- Wang, G.; Xiao, Y.; Song, Y.; Zhou, H.; Tian, Q.; Li, F. A density functional study on the aggregation of alumina clusters. Res. Chem. Intermed. 2017, 43, 1447–1463. [Google Scholar] [CrossRef]
- Xiao, Y.; Lei, H.; Yang, B.; Wang, G.; Wang, Q.; Jin, W. Nucleation and growth for magnesia inclusion in Fe-O-Mg melt. RSC Adv. 2018, 8, 38336–38345. [Google Scholar] [CrossRef]
- Xiao, Y.; Lei, H.; Yang, B.; Zhao, Y.; Wang, Q.; Wang, G. Thermodynamic insight into the growth of Calcia inclusions at the nanoscale: The case of Fe-O-Ca melt. RSC Adv. 2019, 9, 11135–11141. [Google Scholar] [CrossRef]
- Aihara, J.-I. Reduced HOMOLUMO Gap as an Index of Kinetic Stability for Polycyclic Aromatic Hydrocarbons. J. Phys. Chem. A 1999, 103, 7487–7495. [Google Scholar] [CrossRef]
- Chase, M.W. NIST-JANAF Thermochemical Tables; American Chemical Society: Washington, DC, USA, 1998; Volume 2, pp. 1880–1881. [Google Scholar]
- Byun, J.S.; Shim, J.H.; Cho, Y.W.; Lee, D.N. Non-metallic particle and intragranular nucleation of ferrite in Ti-killed C-Mn steel. Acta Mater. 2003, 51, 1593–1606. [Google Scholar] [CrossRef]
- Phillpot, S.R.; Wolf, D.; Gleiter, H. Molecular dynamics study of the synthesis and characterization of a fully dense, three dimensional nanocrystalline material. J. Appl. Phys. 1995, 78, 847–861. [Google Scholar] [CrossRef]
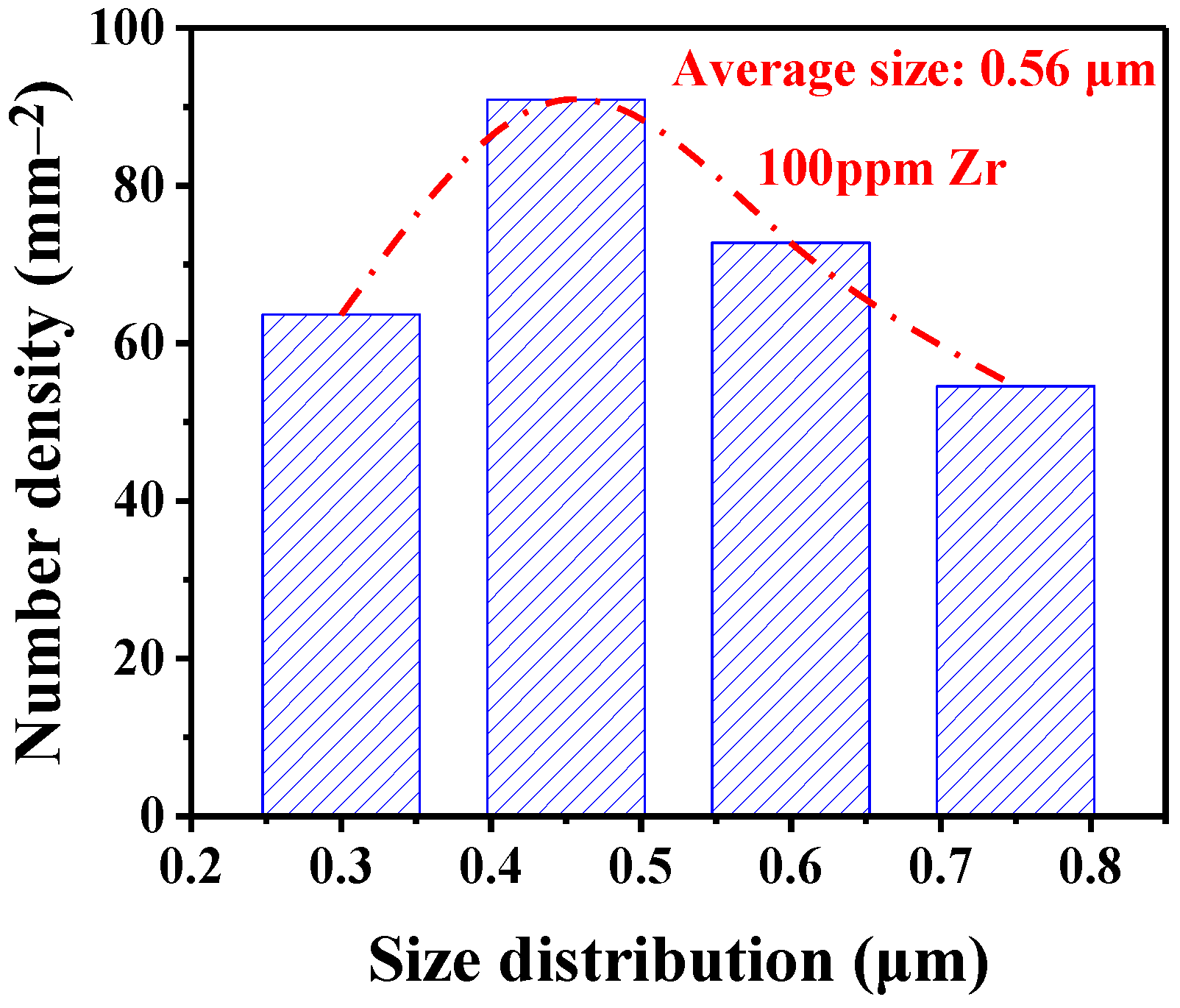
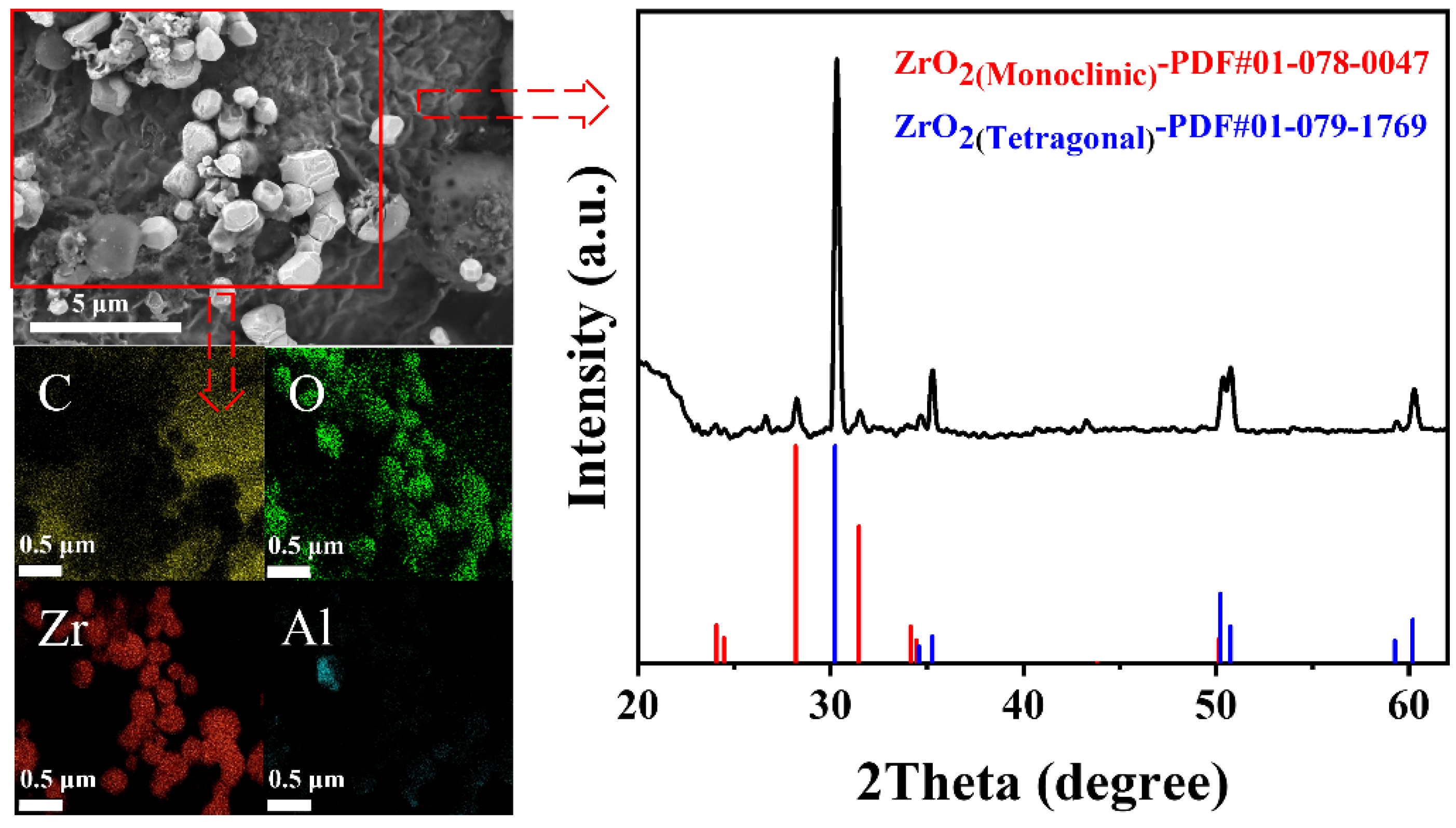
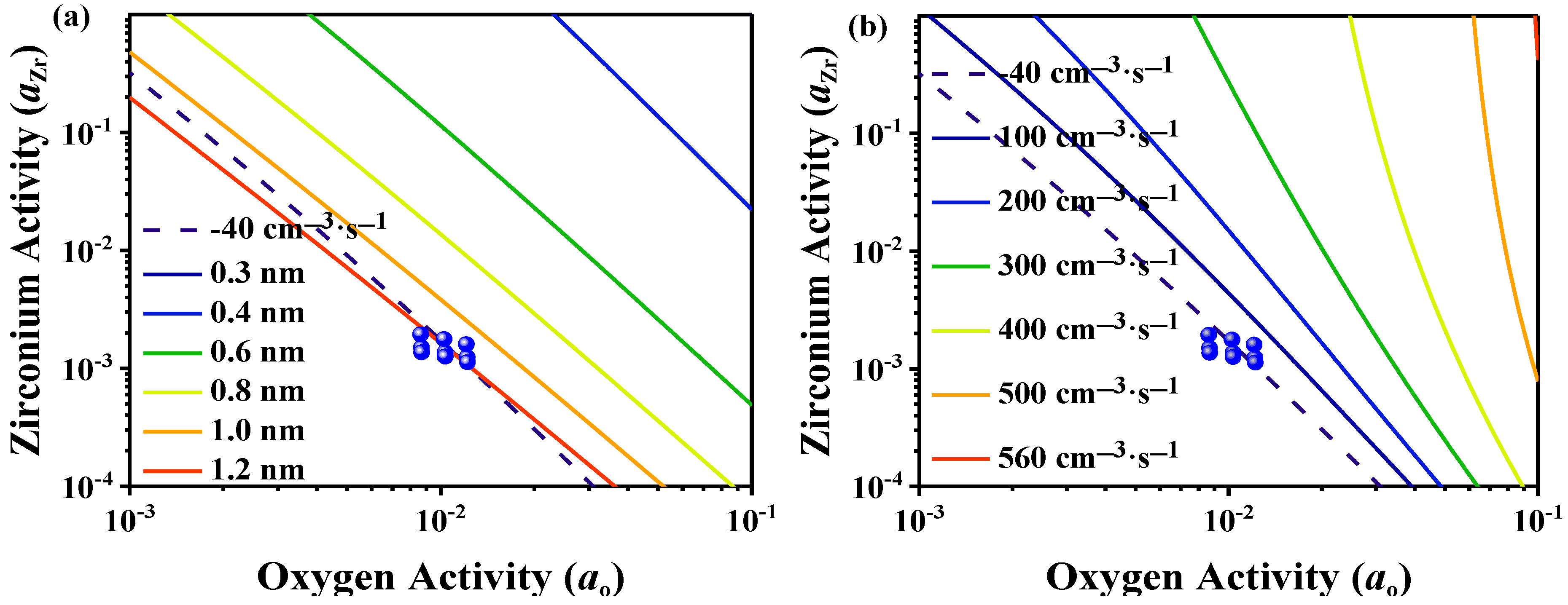
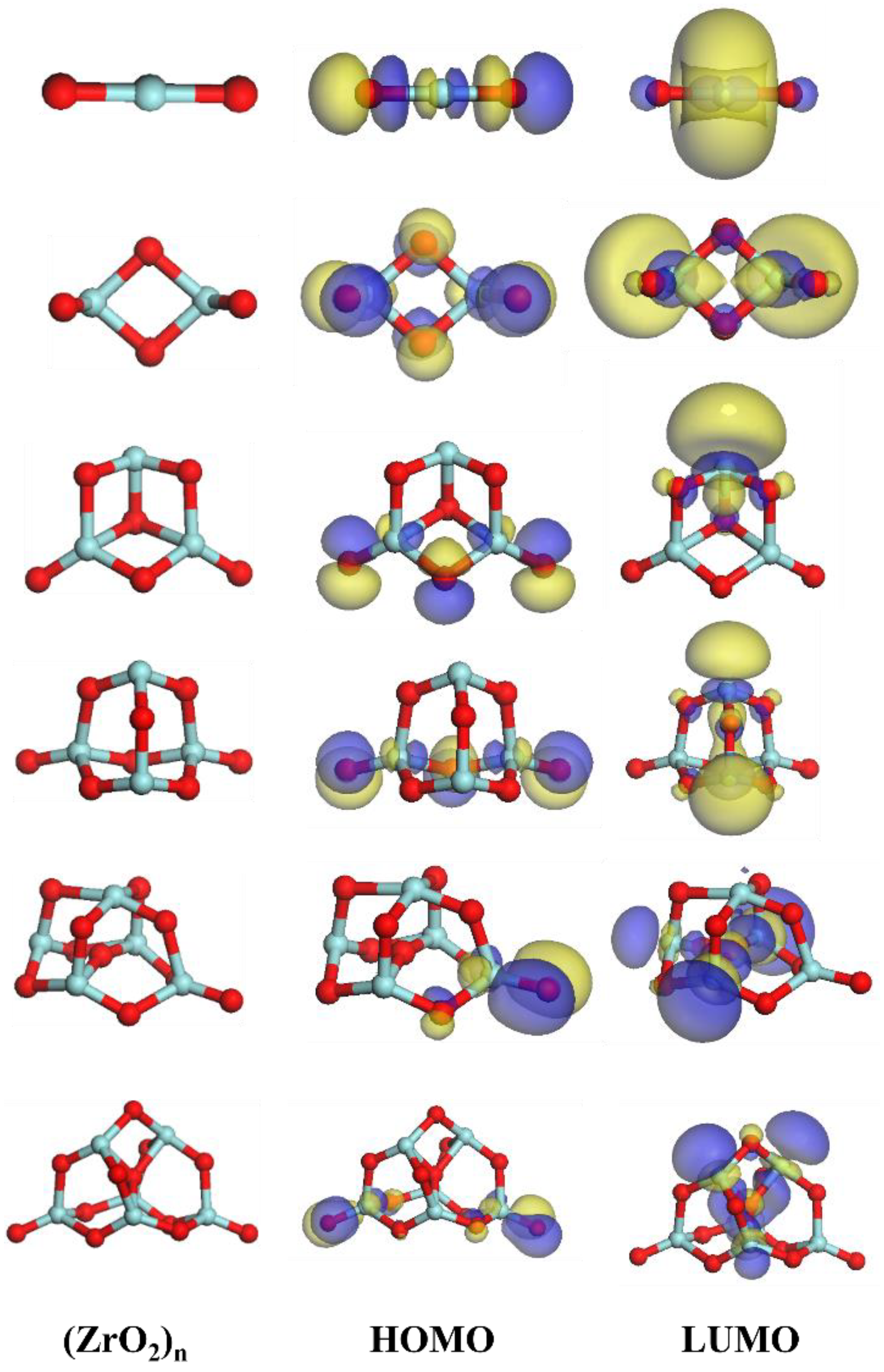

| Composition | C | Si | Mn | P | S | Cr | Al | Cu | Ni | Ti | N | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amount | 0.016 | 0.0033 | 0.01 | 0.0053 | 0.0017 | 0.0107 | 0.003 | 0.0037 | 0.0038 | 0.001 | 0.002 | Bal. |
| Zr Addition | Holding Time/s | [O]/ppm | [N]/ppm | [Zr]/ppm |
|---|---|---|---|---|
| 125 | 21 | 24 | ||
| 100 ppm | 120 | 89 | 28 | 31 |
| 106 | 34 | 22 |
| Inclusion | Θ (deg) | γSV (J/m2) | VO (m3/mol) | logKeq |
|---|---|---|---|---|
| ZrO2 | 111 [27] | 1.395 [27] | 10.5× 10−6 | −57,000/T + 21.8 [28] |
| Zr | O | |
|---|---|---|
| O | −23 | −0.17 |
| Zr | 0.032 | −4 |
| Clusters | Bond Length(Zr-O) (nm) | eV | |
|---|---|---|---|
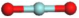 (ZrO2)1 | 0.189 | 0.38 | −5.55 |
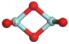 (ZrO2)2 | 0.192 | 0.48 | −7.05 |
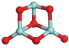 (ZrO2)3 | 0.204 | 0.60 | −7.38 |
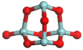 (ZrO2)4 | 0.20 | 0.66 | −7.52 |
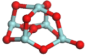 (ZrO2)5 | 0.206 | 0.70 | −7.68 |
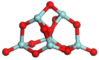 (ZrO2)6 | 0.203 | 0.93 | −7.75 |
| Clusters | (ZrO2)1 | (ZrO2)2 | (ZrO2)3 | (ZrO2)4 | (ZrO2)5 | (ZrO2)6 |
|---|---|---|---|---|---|---|
| LUMO | −0.15927 | −0.12413 | −0.15856 | −0.15835 | −0.11532 | −0.13762 |
| HOMO | −0.18301 | −0.2138 | −0.21179 | −0.19151 | −0.20248 | −0.22429 |
| LUMO-HUMO | 0.023742 | 0.089665 | 0.05323 | 0.033158 | 0.087153 | 0.086679 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, L.; Chen, C.; Yang, S.; Li, X. New Insights into the Mechanism of Nucleation of ZrO2 Inclusions at High Temperature. Materials 2022, 15, 7960. https://doi.org/10.3390/ma15227960
Li Y, Wang L, Chen C, Yang S, Li X. New Insights into the Mechanism of Nucleation of ZrO2 Inclusions at High Temperature. Materials. 2022; 15(22):7960. https://doi.org/10.3390/ma15227960
Chicago/Turabian StyleLi, Yutang, Linzhu Wang, Chaoyi Chen, Shufeng Yang, and Xiang Li. 2022. "New Insights into the Mechanism of Nucleation of ZrO2 Inclusions at High Temperature" Materials 15, no. 22: 7960. https://doi.org/10.3390/ma15227960
APA StyleLi, Y., Wang, L., Chen, C., Yang, S., & Li, X. (2022). New Insights into the Mechanism of Nucleation of ZrO2 Inclusions at High Temperature. Materials, 15(22), 7960. https://doi.org/10.3390/ma15227960





