Abstract
In order to reduce the sintering temperature and improve the mechanical properties of B4C ceramics, ZrB2 was formed in situ using the SPS sintering method with ZrO2 and B4C as raw materials. Thermodynamic calculations revealed that CO pressure affected the formation of ZrB2 at temperatures from 814 °C to 1100 °C. The experimental results showed that the ZrB2 grain size was <5 µm and that the grains were uniformly distributed within the B4C ceramics. With an increase in ZrO2 content, the Vickers hardness and flexural strength of the B4C ceramics first increased and then decreased, while the fracture toughness continuously increased. When the content of ZrO2 was 15 wt%, the Vickers hardness, fracture toughness and flexural strength of B4C ceramics were 35.5 ± 0.63 GPa, 3.6 ± 0.24 MPa·m1/2 and 403 ± 10 MPa, respectively. These results suggest that ZrB2 inhibits B4C grain growth, eliminates crack tip stress, and provides fine grain to strengthen and toughen B4C ceramics.
1. Introduction
Due to their low density, high hardness and high elastic modulus, boron carbide (B4C) ceramics have been used in the manufacturing of armor, cutting tools and neutron absorbers [1,2,3,4,5]. However, further application of B4C ceramics is limited by the high sintering temperature (>2000 °C) and low fracture toughness (KIC < 2.2 MPa·m1/2), which can be attributed to the strong B-C covalent bonds, low atomic diffusion coefficient and low dislocation plasticity [6,7,8,9,10]. Therefore, it is essential to lower the sintering temperature and improve the mechanical properties of B4C ceramics.
The addition of second phases, such as SiC, Al2O3, TiC and TiO2, is an effective method of reducing the sintering temperature and improving the mechanical properties of B4C ceramics [11,12], but ultimately results in reduced material hardness. Moreover, brittle compounds are formed during the densification process, leading to a low fracture toughness. Transitional metal diborides (TiB2, ZrB2) are useful additives which have similar properties to B4C ceramics [13,14,15]. Among them, Zirconium Diboride (ZrB2) has a high melting point (3245 °C), high hardness (14–23 GPa) and high flexural strength (higher than 500 MPa), which could improve the flexural strength and fracture toughness of B4C ceramics [16,17].
Many reports have shown that the addition of ZrB2 could efficiently enhance the mechanical properties of B4C ceramics. For example, He et al. [18] found that ZrB2 promoted the sintering process and increased the ceramic’s high critical crack size, resulting in excellent thermal shock resistance. In order to inhibit the grain growth of B4C, Lin et al. [19] prepared B4C ceramics by pressureless sintering using ZrO2 as a sintering aid. The in situ formation of ZrB2 inhibited the abnormal growth of B4C grains, prolonged the crack extension path and improved the sintering activity. Guo et al. [20] prepared B4C-ZrB2 ceramics at low temperature, resulting in an average B4C grain size of 1–2 μm. The hardness and fracture toughness reached 33.0 ± 1.3 GPa and 4.1 ± 0.2 MPa·m1/2, respectively. Rehman et al. [21] prepared B4C ceramics by SPS at 1700 °C; the average grain size of ZrB2 was 1.3 ± 0.2 μm. The Vickers hardness, fracture toughness and flexural strength were 31.28 GPa, 4.2 MPa·m1/2 and 511 MPa, respectively. However, the role of ZrB2 as a sintering aid and grain growth inhibitor requires further investigation, and the effect of ZrB2 on the fracture mode transformation of B4C ceramics also needs to be clarified.
In this work, we aim to obtain densified B4C ceramics with the in situ formation of ZrB2 at 1700 °C using the spark plasma sintering (SPS) method, with ZrB2 grains homogeneously distributed in the B4C ceramics. The reaction between ZrO2 and B4C could effectively reduce the sintering temperature, and the pinning effect of ZrB2 is expected to inhibit the B4C grain growth, achieving desirable mechanical properties.
2. Materials and Methods
2.1. Materials and Processes
B4C powder (1 μm, analytically pure) and nano-ZrO2 (50 nm, purity > 99.9%) were used as raw materials. Nano-Y2O3 (50 nm, purity > 99.9%) was used as a sintering aid.
The compositions of the samples are shown in Table 1. The raw materials were mixed with stainless steel balls in anhydrous ethanol with a ball-to-material ratio of 5:1 and a mixing time of 6 h at 250 r/min. After mixing, the mixtures were dried in a drying oven at 80 °C for 24 h and then passed through a 200-mesh sieve. Then, the mixtures were sintered by SPS (Dr. Sinter-3.20 MKII, Sumitomo, Tokyo, JPN) under vacuum. The heating rate was maintained at 100 °C/min and a uniaxial load of 5 kN was applied during the heating stage to ensure good contact conductivity with the graphite indenter. After the temperature rose to 1600 °C, the uniaxial load was slowly increased to 15 kN. Then, the sintering temperature was increased to 1700 °C for 6 min. The sintered ceramics were cooled in the furnace to about 150 °C and the samples were taken out to obtain B4C ceramics.

Table 1.
Compositions of samples.
2.2. Characterizations
The bulk density of B4C ceramics was measured using the Archimedes method. The Vickers hardness and fracture toughness were measured by the indentation method using a Vickers hardness tester (HV-50A, Huayin, Laizhou, China). The spacing between each indentation was ensured to be greater than 100 μm to avoid crack deformation or the overlapping of extended areas caused by indentation. Five effective indentations were also used to calculate the mean and error of Vickers hardness and fracture toughness. The B4C ceramics were processed into long strip samples of 2 mm × 3 mm × 20 mm using a diamond wire cutting machine, and the bending strength of the samples was measured using a three-point bending test with a span of 16 mm and a loading rate of 0.5 mm/min. The mean values of flexural strength and error analyses were calculated with 3 sets of valid data. The phase compositions were characterized by X-ray diffractometer (XRD, X’Pert MPD Pro, Philips, Amsterdam, The Netherlands). The voltage and current of the Cu Kα1 radiation were 40 kV and 40 mA, respectively. The scanning range (2θ) was 10°–90°. The microscopic morphologies were characterized using a field-emission scanning electron microscope (FESEM, Nova NanoSem 400, Thermo, Waltham, MA, USA).
3. Results and Discussion
3.1. Thermodynamic Calculations
The in situ generation of ZrB2 from B4C and nano-ZrO2 is represented in Reaction (1):
According to thermodynamic calculations by FactSage, the variation in Gibbs’ free energy of reaction (1) as a function of temperature at different PCO (pressure of CO) and as a function of PCO at different temperatures is shown in Figure 1. Figure 1a shows that in the standard state (PCO = 0.986 atm), a temperature above 1212 °C is favorable for the formation of ZrB2. It is expected that reaction (1) proceeds more rapidly when the reaction is carried out under vacuum. Therefore, pure ZrB2 can be synthesized at a temperature as low as 814 °C when PCO = 10−5 atm, according to Figure 1a. However, the CO generated by the sample surrounded by graphite die cannot be expelled in time, leading to PCO > 10−4 atm; thus, the reaction will proceed at a temperature higher than 814 °C. Figure 1b further illustrates that reaction (1) needs to proceed at temperatures above 1100 °C when PCO is in the range of 0–0.1 atm. In the experiments, the vacuum pump worked continuously and the vacuum level in the furnace was as low as 8 × 10−5 atm during SPS, maintaining the PCO at a low level and thus promoting the low-temperature densification of B4C ceramics.
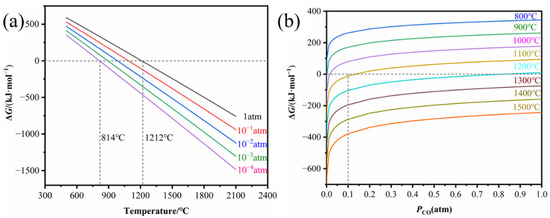
Figure 1.
Gibbs’ free energy change of reaction (1). (a) Variation in temperature at different PCO; (b) variation with PCO at different temperatures.
3.2. XRD Phase Analysis
The XRD patterns of B4C ceramics prepared by SPS with different ZrB2 contents are shown in Figure 2. The ceramics are composed of B4C and ZrB2, and no residual nano-ZrO2 phase can be detected, indicating that nano-ZrO2 and B4C completely reacted to form the ZrB2 phase. The B4C in the samples all had the rhombohedral crystal structure (PDF #35-0798). The diffraction peaks of samples at 2θ of 21.21°, 32.64°, and 41.69° were all taken from the ZrB2 crystal (PDF #89-3930), and correspond to the (110), (200) and (211) crystal planes of its hexagonal crystal structure, respectively. The intensity of the ZrB2 diffraction peak increases with the addition of nano-ZrO2, while the intensity of the B4C diffraction peak is the opposite, which is consistent with the expected results. The XRD results indicate that nano-ZrO2 can react with B4C in situ to form ZrB2, which can promote the diffusion effect between B4C particles during sintering and achieve the densification of B4C ceramics.
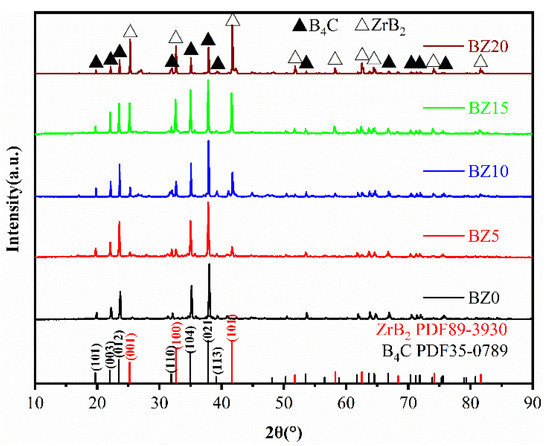
Figure 2.
XRD patterns of B4C ceramics with different nano-ZrO2 contents.
3.3. Microstructural Analysis
The BSE images of B4C ceramics and the grain size of ZrB2 prepared by SPS are shown in Figure 3. The distribution of B, C and Ti elements in sample BZ15 are shown in Figure 3f–i. Combined with XRD, it can be deduced that the dark gray phase is B4C, and the bright white phase is ZrB2. Figure 3a shows that the addition of 3 wt% nano-Y2O3 can achieve a polished surface of B4C ceramics with no obvious pores without the addition of nano-ZrO2. Figure 3b–e show that the amount of ZrB2 produced gradually increases as the addition of nano-ZrO2 increases from 5 wt% to 20 wt%, and the average particle size of ZrB2 increases from 1.03 μm to 1.37 μm. The in situ reaction of nano-ZrO2 to generate ZrB2 facilitates the flowability between B4C particles and promotes the densification and pore discharge of B4C ceramics, such that there are no obvious pores on the polished surface. In addition, ZrB2 not only has small grain size, but is also uniformly distributed within the B4C ceramics, which will effectively improve the mechanical properties.
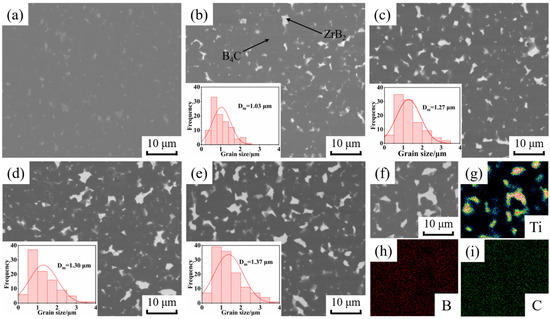
Figure 3.
BSE images of B4C ceramics and grain size of ZrB2. (a) BZ0, (b) BZ5, (c) BZ10, (d) BZ15, (e) BZ20 (f) BSE image of BZ15; element of Ti (g), B (h) and C (i).
BSE diagrams of the fracture morphology of B4C ceramics with different nano-ZrO2 contents are shown in Figure 4. The fracture mode is mainly through crystal fracture, and the fracture surface is relatively neat and smooth. This indicates a favorable degree of connection between the grains after SPS. In addition, there are more pores in the BZ0 sample, but the number of pores decreases significantly with the addition of nano-ZrO2. This indicates that nano-ZrO2 promotes the densification of B4C ceramics.
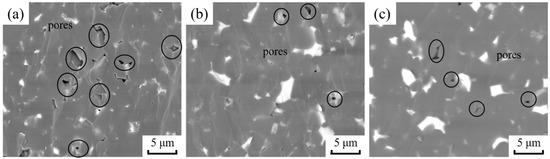
Figure 4.
BSE diagrams of B4C ceramics’ fracture morphology. (a) BZ0; (b) BZ10; (c) BZ20.
TEM images of BZ15 are shown in Figure 5. Figure 5a shows the interface between B4C and ZrB2 in the B4C ceramics. The HRTEM image shown in Figure 5b indicates that the grain boundary between B4C (d = 0.3781 nm, (012) zone axis) and ZrB2 (d = 0.2166 nm, (101) zone axis) is clean. This result indicates that the grain boundary strength between B4C and ZrB2 is strong. It is well-known that a high binding force between the matrix phase and second phase has a significant effect on mechanical properties. Therefore, this suggests that excellent mechanical properties can be obtained from these B4C ceramic samples.
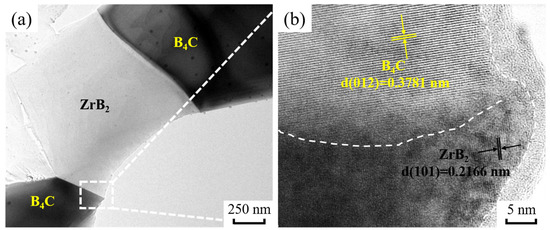
Figure 5.
TEM diagrams of BZ15. (a) TEM; (b) HRTEM.
3.4. Mechanical Properties of the B4C Ceramics
The relative density and bulk density of B4C ceramics prepared by SPS with different ZrO2 contents are shown in Figure 6. The relative densities measured are all above 98.3%, and increase and then decrease with increasing ZrO2 content. The bulk density continues to increase, between 2.52 g/cm3 and 2.83 g/cm3. The relative density reaches a maximum of 99.6% when the content of ZrO2 is 5 wt%; this can be attributed to the in situ reaction of nano-ZrO2 to generate ZrB2, which that promotes the flowability between B4C particles and obtains dense ceramics. When the content of ZrO2 is higher than 5 wt%, the CO gas generated by the sintering reaction also increases, causing the relative density to decrease. The observed increase in bulk density can be explained by the fact that the theoretical density of ZrB2 (6.1 g/cm3) is greater than that of B4C, meaning that an increase in ZrB2 content leads to a gradual increase in the bulk density of the ceramics.

Figure 6.
Relative density and bulk density of B4C ceramics at different ZrB2 contents.
The Vickers hardness, fracture toughness and flexural strength of B4C ceramics prepared by SPS at different ZrO2 contents are shown in Figure 7. From Figure 7a, it can be seen that with the increase of ZrO2 content, the Vickers hardness shows a trend of first increasing and then decreasing, and the fracture toughness gradually increases. The Vickers hardness is maintained above 35.5 GPa with a of ZrO2 content of 5–15 wt%, but decreases to 32.3 GPa with a ZrO2 content of 20 wt%. Fracture toughness gradually increases from 2.3 MPa·m1/2 (BZ0) to 3.96 MPa·m1/2 (BZ20). This is because an increase in the relative density of B4C ceramics leads to an increase in Vickers hardness, but the presence of pores can also have a negative effect on Vickers hardness. The ZrB2 generated in situ from nano-ZrO2 has a pinning effect, which inhibits the growth of B4C particles. At the same time, the residual stresses present during cooling can block the crack expansion of B4C ceramics due to the difference in thermal expansion coefficients of B4C and ZrB2. Both processes are beneficial for the improvement of the fracture toughness of B4C ceramics. As can be seen in Figure 7b, the bending strength of the B4C ceramics shows a trend of increasing and then decreasing with an increase in ZrO2 content. The bending strength reaches a maximum value of 404 MPa at 15 wt% ZrO2, which is 60.3% higher than the BZ0 sample (252 MPa). The addition of nano-ZrO2 promotes the sintering between B4C particles and inhibits their further growth. Furthermore, the bending strength of ZrB2 is greater than that of B4C, ultimately improving the bending strength of the B4C ceramics. However, when the content of ZrO2 reaches 20 wt%, the generation of CO gas leads to an increase in the number of pores and a decrease in the density, which results in a lower bending strength. When the content of ZrO2 is 15 wt%, the Vickers hardness, fracture toughness and bending strength of sample BZ15 are 35.5 GPa, 3.6 MPa·m1/2 and 404 MPa, respectively, with optimal overall performance.

Figure 7.
Mechanical properties of B4C ceramics with different nano-ZrO2 contents. (a) Vickers hardness and fracture toughness; (b) bending strength.
3.5. Densification and Toughening Mechanism Analysis
Crack propagation within the BZ15 sample following the Vickers hardness indentation tests is shown in Figure 8. There are obvious branching and deflection phenomena in crack extension, which can increase the energy required for crack extension and thus improve the fracture toughness of B4C ceramics. In addition, the fracture mode of the B4C ceramics changes from a traditional transgranular fracture to a hybrid mode of intergranular and transgranular fracture, which is beneficial for the improvement of fracture toughness.

Figure 8.
BSE diagrams of the crack expansion path of sample BZ15. (a) Partial view; (b) Partial enlarged view.
The different coefficients of thermal expansion between the matrix and the second phase in ceramics could induce residual stress during the cooling process. The effect of this is more obvious when the strength and elastic modulus of the second phase are higher than those of the matrix. Since the thermal expansion coefficients of B4C and ZrB2 are quite different, the interface between ZrB2 and B4C will therefore be subject to residual stress (pi) during the cooling process [22].
where Δα is the difference between the coefficients of thermal expansion of B4C and ZrB2, ΔT is the temperature difference between the cooling stages of SPS, ν is Poisson’s ratio, E is elastic modulus, and the subscripts p and m stand for ZrB2 and B4C, respectively.
In this condition, σr (radial stress) and σt (tangential stress) are generated in the matrix at a distance r from the particles [22].
and
where R is the radius of the particle and r is the distance from a point in the stress field to the center of the particle. It can be seen that both σr and σt are proportional to (R/r)3.
When a crack passes through this stress field, a deflection will occur, as shown in Figure 9. When second phase particles are encountered ahead of the crack, the radial tensile stress σr increases in the matrix (σr is maximal at the interface), causing the crack to deflect in the direction of the particles and reach the particle/matrix interface before propagating along the original extension direction. The combined effect of σr and σt lengthens the crack expansion path in the matrix and consumes the energy for crack expansion. Therefore, the crack deflection caused by the residual stress field produces an absolute toughening effect. Figure 8 shows that the crack extension undergoes deflection and bypass, and that there is a secondary crack generation on the primary crack, which increases the path of crack extension and consumes the energy of crack extension. If the crack propagation path is more tortuous, and the volume fraction of the second phase component which leads to crack deflection is higher, then the effect of crack deflection and toughening will be significant.

Figure 9.
Schematic diagram of residual stress and crack extension in B4C ceramics.
3.6. Comparison of Properties of B4C Ceramics in Recent Years
The Vickers hardness and fracture toughness of B4C ceramics prepared under different sintering methods in recent years are shown in Figure 10 [20,21,23,24,25,26,27,28,29,30,31,32,33]. The different sintering methods described are pressureless sintering, hot-pressure sintering and spark plasma sintering. Although the Vickers hardness and fracture toughness of B4C ceramics are high, the sintering temperature of dense B4C ceramics is almost above 1800 °C. In this study, B4C ceramics are successfully prepared at 1700 °C by in situ reaction using nano-ZrO2 as a sintering aid. The maximum values of Vickers hardness and fracture toughness are 35.5 GPa and 3.6 MPa·m1/2, respectively. The in situ-generated ZrB2 inhibits the growth of B4C grains, deflecting and bifurcating the cracks, thus enhancing the mechanical properties of B4C ceramics.
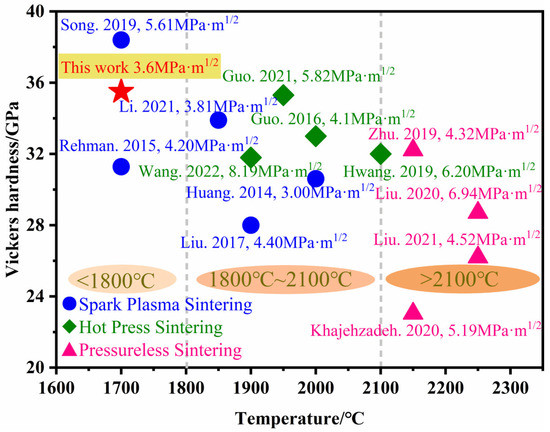
Figure 10.
Comparison of the performance of B4C ceramics in recent years [20,21,23,24,25,26,27,28,29,30,31,32,33].
The phase-field model of Roy shows that interfacial stress is an important factor affecting the interfacial stress distribution at interfaces and the phase-field solution [34]. Therefore, the effect of interfacial stress between B4C and ZrB2, as well as the ZrO2 phase transition process on the evolution of the nano-microstructure, must be studied in further detail. In addition, Mahdi investigated the effect of high pressure on the phase transformation [35], which may offer some insight into the effect of residual stress on ZrB2 grain growth due to the difference in the coefficients of thermal expansion between B4C and ZrB2.
4. Conclusions
In this study, B4C ceramics were prepared by the in situ generation of ZrB2 using SPS at low temperature. The effects of ZrB2 on the microstructure and mechanical properties of the B4C ceramics were characterized and tested. The following conclusions were drawn.
(1) The ceramics were composed of B4C and ZrB2. The nano-ZrO2 reacted to form fine-crystalline ZrB2, which was uniformly distributed in the B4C ceramics. The in situ generation of ZrB2 contributed to the flowability between B4C particles, improving the sintering activity and achieving the densification of the B4C ceramics at low temperature.
(2) The relative density, Vickers hardness and bending strength of the B4C ceramics first increased and then decreased with the increase of ZrB2 content, and the bulk density and fracture toughness increased continuously. The relative density was above 98.3% and the bulk density was 2.52–2.83 g/cm3. When the content of ZrO2 was 15 wt%, the Vickers hardness, fracture toughness and bending strength were 35.5 ± 0.63 GPa, 3.6 ± 0.24 MPa·m1/2 and 403 ± 10 MPa, respectively, with the best overall performance.
(3) The pinning effect of ZrB2 particles changed the fracture mode of the B4C ceramics from traditional transgranular fracture to a hybrid mode of intergranular and transgranular fracture, which improved the bending strength of the ceramics. The high bonding strength between powders and the residual stress between B4C and ZrB2 led to crack deflection and crack branching during the crack propagation, which resulted in the high mechanical properties of the B4C ceramics.
Author Contributions
Formal analysis, W.Y., J.Y., X.L., P.C., Y.Z. and B.Z.; investigation, W.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 51972242 and 51774218), the Science Fund for Creative Research Groups of the National Natural Science Foundation of Hubei Province (Grant No.2020CFA038) and the Key Research and Development Project of Hubei Province (Grant No. 2020BAA028) and the Young Top-notch Talent Cultivation Program of Hubei Province.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study can be made available by the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sha, W.; Liu, Y.; Zhou, Y.; Huang, Y.; Huang, Z. Effect of Carbon Content on Mechanical Properties of Boron Carbide Ceramics Composites Prepared by Reaction Sintering. Materials 2022, 15, 6028. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.M.; Guo, D.; Song, S.; Cheng, C.; Han, J.; Wang, X.; An, Q.; Chen, M. Dislocation-mediated shear amorphization in boron carbide. Sci. Adv. 2021, 7, eabc6714. [Google Scholar] [CrossRef] [PubMed]
- Thévenot, F. Boron carbide—A comprehensive review. J. Eur. Ceram. Soc. 1990, 6, 205–225. [Google Scholar] [CrossRef]
- Chang, Y.; Sun, X.; Ma, M.; Mu, C.; Li, P.; Li, L.; Li, M.; Nie, A.; Xiang, J.; Zhao, Z. Application of hard ceramic materials B4C in energy storage: Design B4C@ C core-shell nanoparticles as electrodes for flexible all-solid-state micro-supercapacitors with ultrahigh cyclability. Nano Energy 2020, 75, 104947. [Google Scholar] [CrossRef]
- Qu, Z.; Yu, C.; Wei, Y.; Su, X.; Du, A. Thermal conductivity of boron carbide under fast neutron irradiation. J. Adv. Ceram. 2022, 11, 482–494. [Google Scholar] [CrossRef]
- Domnich, V.; Reynaud, S.; Haber, R.A.; Chhowalla, M. Boron carbide: Structure, properties, and stability under stress. J. Am. Ceram. Soc. 2011, 94, 3605–3628. [Google Scholar] [CrossRef]
- Zan, Y.; Zhou, Y.; Zhao, H.; Liu, Z.; Wang, Q.; Wang, D.; Wang, W.; Xiao, B.; Ma, Z. Enhancing high-temperature strength of (B4C+ Al2O3)/Al designed for neutron absorbing materials by constructing lamellar structure. Compos. Part B Eng. 2020, 183, 107674. [Google Scholar] [CrossRef]
- Paidar, M.; Bokov, D.; Mehrez, S.; Ojo, O.O.; Ramalingam, V.V.; Memon, S. Improvement of mechanical and wear behavior by the development of a new tool for the friction stir processing of Mg/B4C composite. Surf. Coat. Technol. 2021, 426, 127797. [Google Scholar] [CrossRef]
- Demirskyi, D.; Vasylkiv, O. Analysis of the high-temperature flexural strength behavior of B4C–TaB2 eutectic composites produced by in situ spark plasma sintering. Mater. Sci. Eng. A 2017, 697, 71–78. [Google Scholar] [CrossRef]
- Zhang, W. A review of tribological properties for boron carbide ceramics. Prog. Mater. Sci. 2021, 116, 100718. [Google Scholar] [CrossRef]
- Zhao, X.; Zou, J.; Ji, W.; Wang, A.; He, Q.; Xiong, Z.; Wang, W.; Fu, Z. Processing and mechanical properties of B4C-SiCw ceramics densified by spark plasma sintering. J. Eur. Ceram. Soc. 2022, 42, 2004–2014. [Google Scholar] [CrossRef]
- Jin, X.; Tang, C.; Li, Q.; Wang, D.; Ding, X.; Ran, S. Densification and strengthening mechanism in spark plasma sintering of B4C–VB2 ceramics via carbide boronizing. Ceram. Int. 2022, 48, 26452–26459. [Google Scholar] [CrossRef]
- Ren, D.; Deng, Q.; Wang, J.; Yang, J.; Li, Y.; Shao, J.; Li, M.; Zhou, J.; Ran, S.; Du, S. Synthesis and properties of conductive B4C ceramic composites with TiB2 grain network. J. Am. Ceram. Soc. 2018, 101, 3780–3786. [Google Scholar] [CrossRef]
- Tu, R.; Li, N.; Li, Q.; Zhang, S.; Zhang, L.; Goto, T. Effect of microstructure on mechanical, electrical and thermal properties of B4C-HfB2 composites prepared by arc melting. J. Eur. Ceram. Soc. 2016, 36, 3929–3937. [Google Scholar] [CrossRef]
- Yu, H.; Namini, A.S.; Shakeri, M.S.; Delbari, S.A.; Van Le, Q.; Cha, J.H.; Kim, S.Y.; Jang, H.W.; Lee, S.-H.; Swiatkowska-Warkocka, Z. HRTEM study and mechanical properties of ZrB2–SiC composite: An insight into in-situ carbon formation over the SPS process. Int. J. Refract. Met. Hard Mater. 2022, 104, 105789. [Google Scholar] [CrossRef]
- Cheng, X.; He, R.; Qu, Z.; Ai, S.; Fang, D. High temperature flexural strength of B4C–ZrB2 ceramic at 1000–1600 °C in air. Ceram. Int. 2015, 41, 14574–14578. [Google Scholar] [CrossRef]
- Yang, Q.; Hwang, C.; Khan, A.U.; Domnich, V.; Gronske, E.D.; Haber, R.A. Anisotropy and residual stress in B4C-ZrB2 eutectic. Mater. Charact. 2019, 155, 109797. [Google Scholar] [CrossRef]
- He, R.; Jing, L.; Qu, Z.; Zhou, Z.; Ai, S.; Kai, W. Effects of ZrB2 contents on the mechanical properties and thermal shock resistance of B4C–ZrB2 ceramics. Mater. Des. 2015, 71, 56–61. [Google Scholar] [CrossRef]
- Lin, X.; Ai, S.; Feng, Y.; Gao, D.; Guo, X.; Liu, Y.; Xie, B.; Gong, H.; Zhang, Y. Fabrication and properties of in-situ pressureless-sintered ZrB2/B4C composites. Ceram. Int. 2017, 43, 15593–15596. [Google Scholar] [CrossRef]
- Guo, W.; Wu, L.; You, Y.; Lin, H.; Zhang, G. Three-step reactive hot pressing of B4C–ZrB2 ceramics. J. Eur. Ceram. Soc. 2016, 36, 951–957. [Google Scholar] [CrossRef]
- Rehman, S.S.; Ji, W.; Fu, Z.; Wang, W.; Wang, H.; Asif, M.; Zhang, J. In situ synthesis and sintering of B4C/ZrB2 composites from B4C and ZrH2 mixtures by spark plasma sintering. J. Eur. Ceram. Soc. 2015, 35, 1139–1145. [Google Scholar] [CrossRef]
- Wachtman, J.B.; Cannon, W.R.; Matthewson, M.J. Mechanical Properties of Ceramics; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Li, P.; Ma, M.; Wu, Y.; Zhang, X.; Chang, Y.; Zhuge, Z.; Sun, L.; Hu, W.; Yu, D.; Xu, B. Preparation of dense B4C ceramics by spark plasma sintering of high-purity nanoparticles. J. Eur. Ceram. Soc. 2021, 41, 3929–3936. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, Z.-H.; Hu, Z.-Y.; Yin, S.-P.; Wang, H.; Ma, Z.-W. Microstructure and mechanical properties of super-hard B4C ceramic fabricated by spark plasma sintering with (Ti3SiC2+ Si) as sintering aid. Ceram. Int. 2019, 45, 8790–8797. [Google Scholar] [CrossRef]
- Liu, G.; Chen, S.; Zhao, Y.; Fu, Y.; Wang, Y. The effect of transition metal carbides MeC (Me = Ti, Zr, Nb, Ta, and W) on mechanical properties of B4C ceramics fabricated via pressureless sintering. Ceram. Int. 2020, 46, 27283–27291. [Google Scholar] [CrossRef]
- Wang, A.; Hu, L.; Guo, W.; Zhao, X.; Shi, Y.; He, Q.; Wang, W.; Wang, H.; Fu, Z. Synergistic effects of TiB2 and graphene nanoplatelets on the mechanical and electrical properties of B4C ceramic. J. Eur. Ceram. Soc. 2022, 42, 869–876. [Google Scholar] [CrossRef]
- Liu, G.; Chen, S.; Zhao, Y.; Fu, Y.; Wang, Y. Effect of Ti and its compounds on the mechanical properties and microstructure of B4C ceramics fabricated via pressureless sintering. Ceram. Int. 2021, 47, 13756–13761. [Google Scholar] [CrossRef]
- Hwang, C.; DiPietro, S.; Xie, K.Y.; Yang, Q.; Celik, A.M.; Khan, A.U.; Domnich, V.; Walck, S.; Hemker, K.J.; Haber, R.A. Small amount TiB2 addition into B4C through sputter deposition and hot pressing. J. Am. Ceram. Soc. 2019, 102, 4421–4426. [Google Scholar] [CrossRef]
- Guo, W.; Wang, A.; He, Q.; Tian, T.; Liu, C.; Hu, L.; Shi, Y.; Liu, L.; Wang, W.; Fu, Z. Microstructure and mechanical properties of B4C-TiB2 ceramic composites prepared via a two-step method. J. Eur. Ceram. Soc. 2021, 41, 6952–6961. [Google Scholar] [CrossRef]
- Khajehzadeh, M.; Ehsani, N.; Baharvandi, H.R.; Abdollahi, A.; Bahaaddini, M.; Tamadon, A. Thermodynamical evaluation, microstructural characterization and mechanical properties of B4C-TiB2 nanocomposite produced by in-situ reaction of nano-TiO2. Ceram. Int. 2020, 46, 26970–26984. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, D.; Li, J.; Huang, Q.; Ran, S. Densification of high-strength B4C-TiB2 composites fabricated by pulsed electric current sintering of TiC-B mixture. Scr. Mater. 2017, 135, 15–18. [Google Scholar] [CrossRef]
- Huang, S.; Vanmeensel, K.; Vleugels, J. Powder synthesis and densification of ultrafine B4C-ZrB2 composite by pulsed electrical current sintering. J. Eur. Ceram. Soc. 2014, 34, 1923–1933. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, F.; Wang, Y.; Cheng, H.; Luo, D.; Zhao, Y. Mechanical properties and microstructure evolution of pressureless-sintered B4C–SiC ceramic composite with CeO2 additive. Ceram. Int. 2019, 45, 15108–15115. [Google Scholar] [CrossRef]
- Roy, A.M. Influence of interfacial stress on microstructural evolution in NiAl alloys. JETP Lett. 2020, 112, 173–179. [Google Scholar] [CrossRef]
- Javanbakht, M. High pressure phase evolution under hydrostatic pressure in a single imperfect crystal due to nanovoids. Materialia 2021, 20, 101199. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).