Study on the Solidification Effect of Dredger Fill by Microbial-Induced Calcium Precipitation (MICP)
Abstract
1. Introduction
2. Materials and Methods
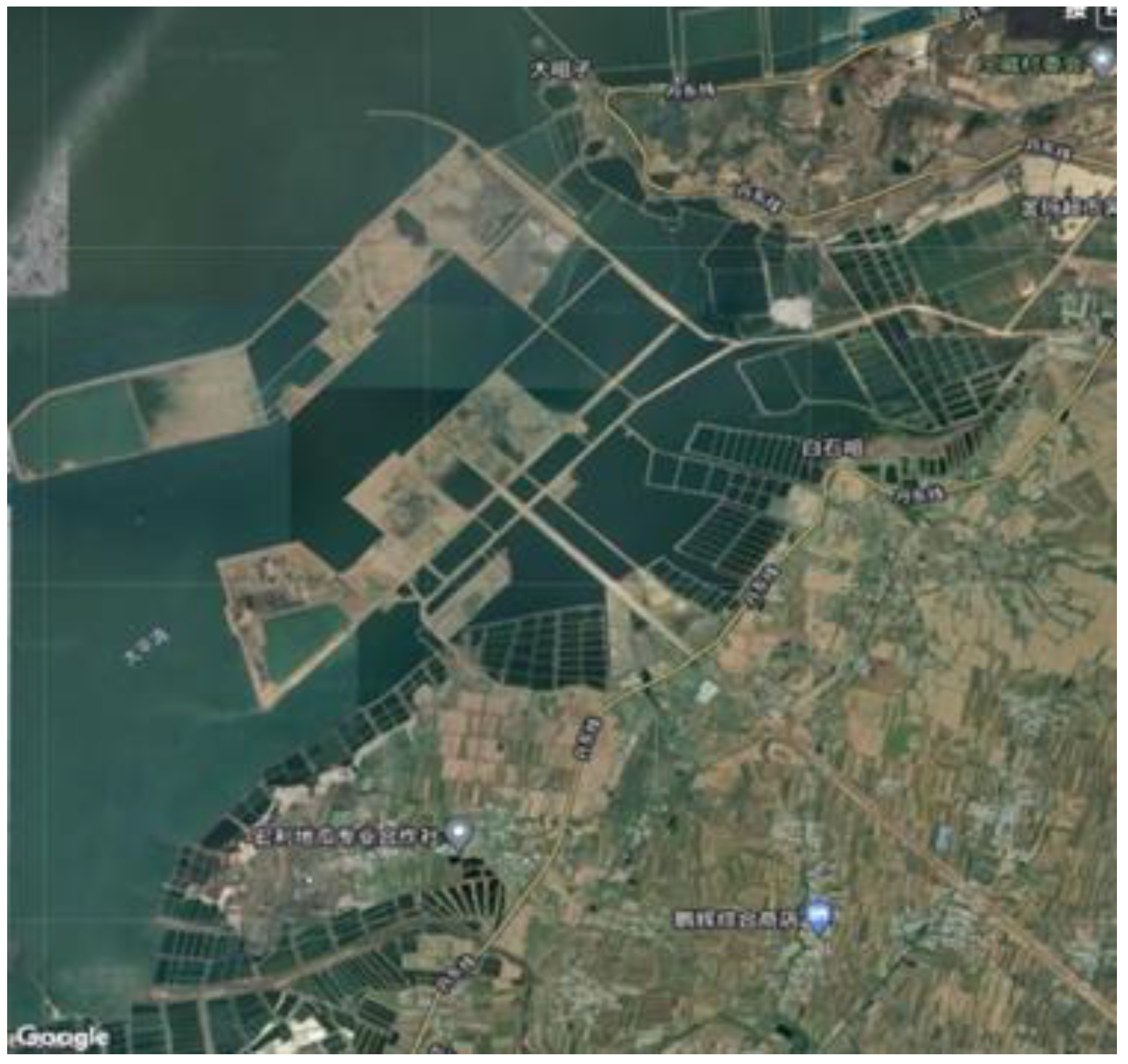
2.1. Soil
2.2. Urease Bacteria and Cementation Solution
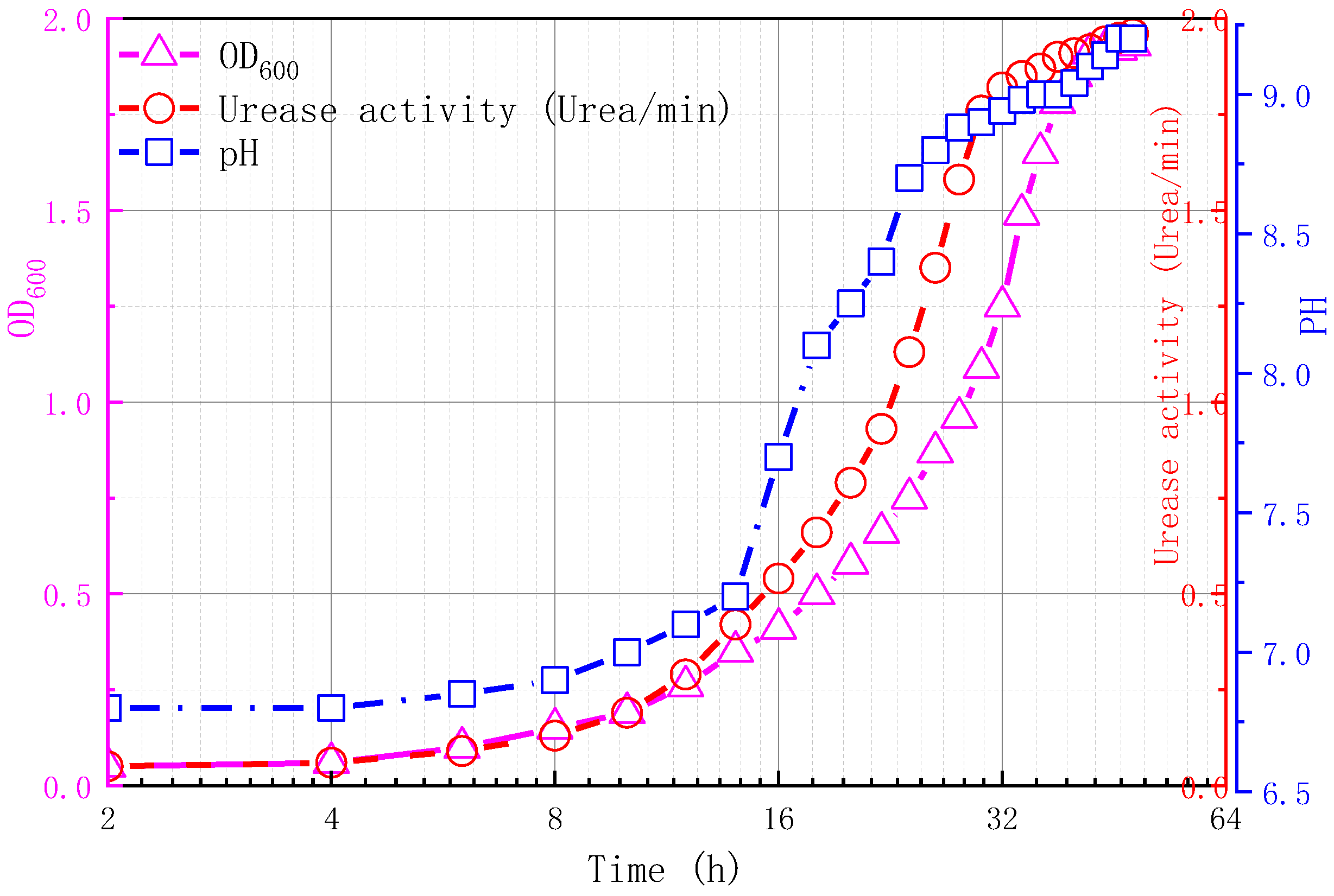
2.2.1. Bacterial Activation
2.2.2. Culture Medium and Cementation Solution
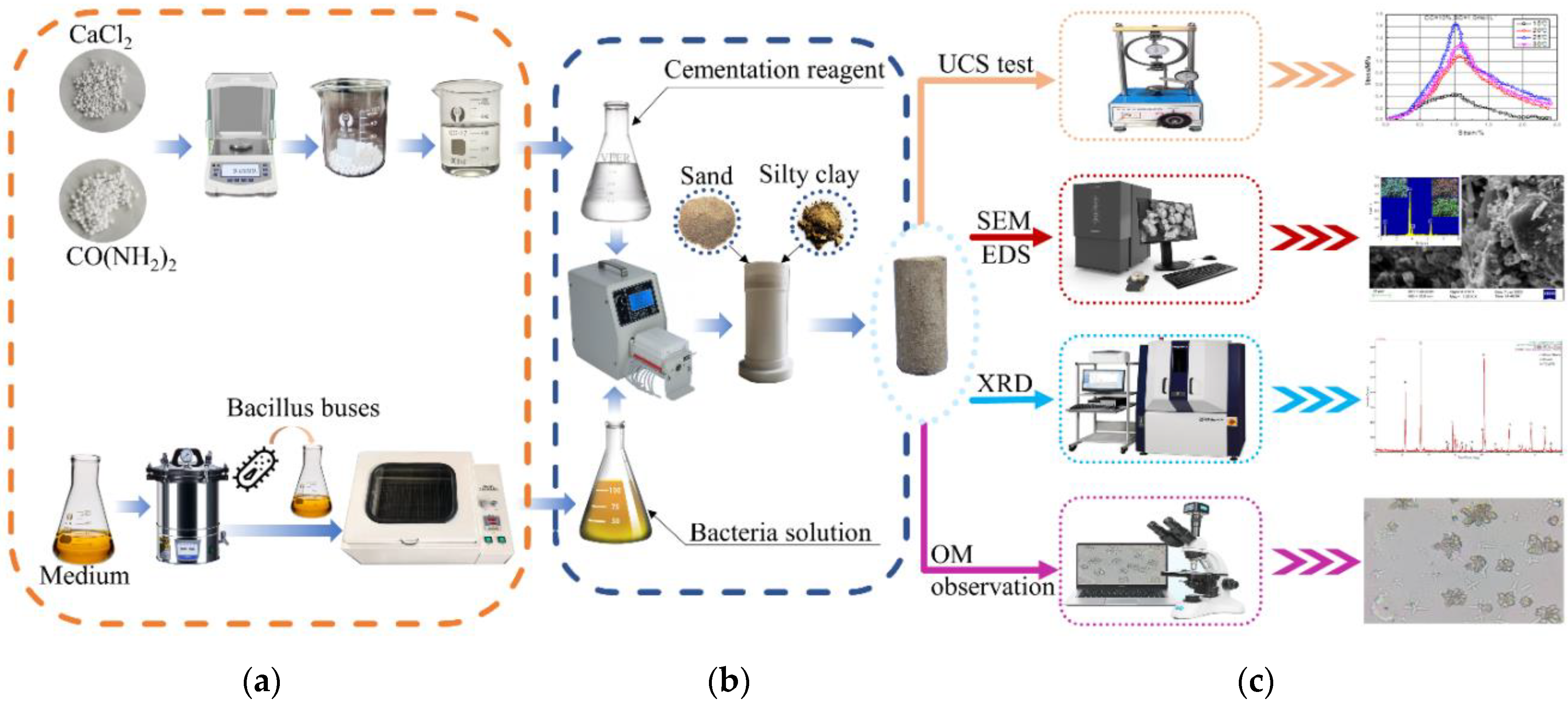
2.3. Apparatus and Sample Preparation
2.4. Test Scheme
2.4.1. Unconfined Compressive Strength (UCS)
2.4.2. Content of Calcium Carbonate (CCC)
2.4.3. Permeability
2.4.4. Void Ratio
3. Results and Discussion
3.1. Relationship between UCS and CCC of MSDF
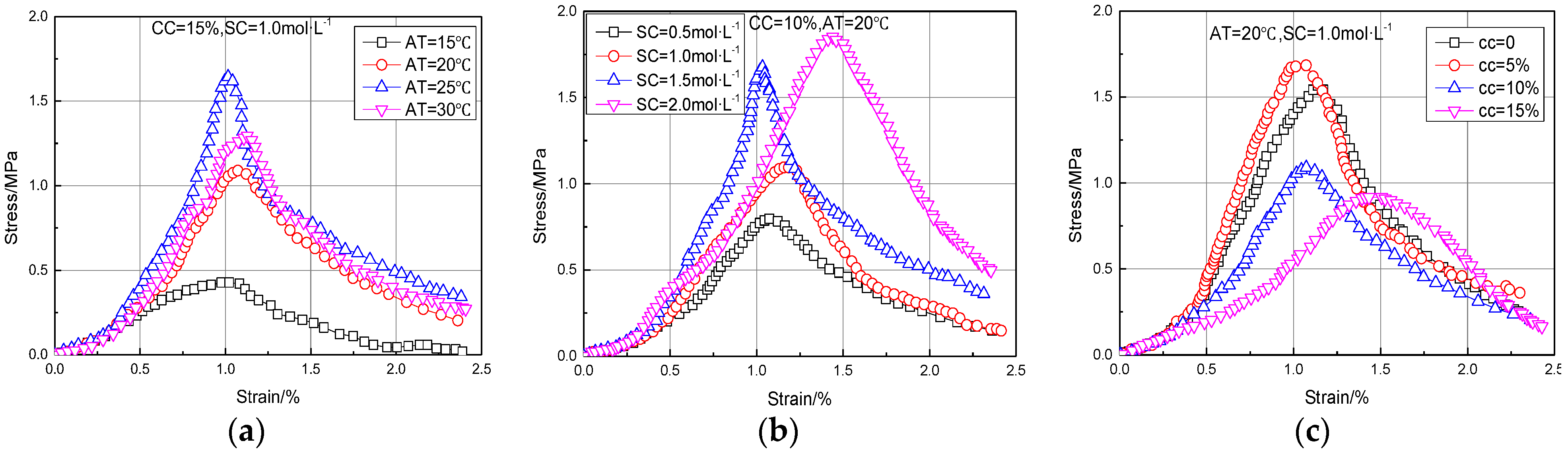
3.1.1. Stress–Strain Characteristics of MSDF
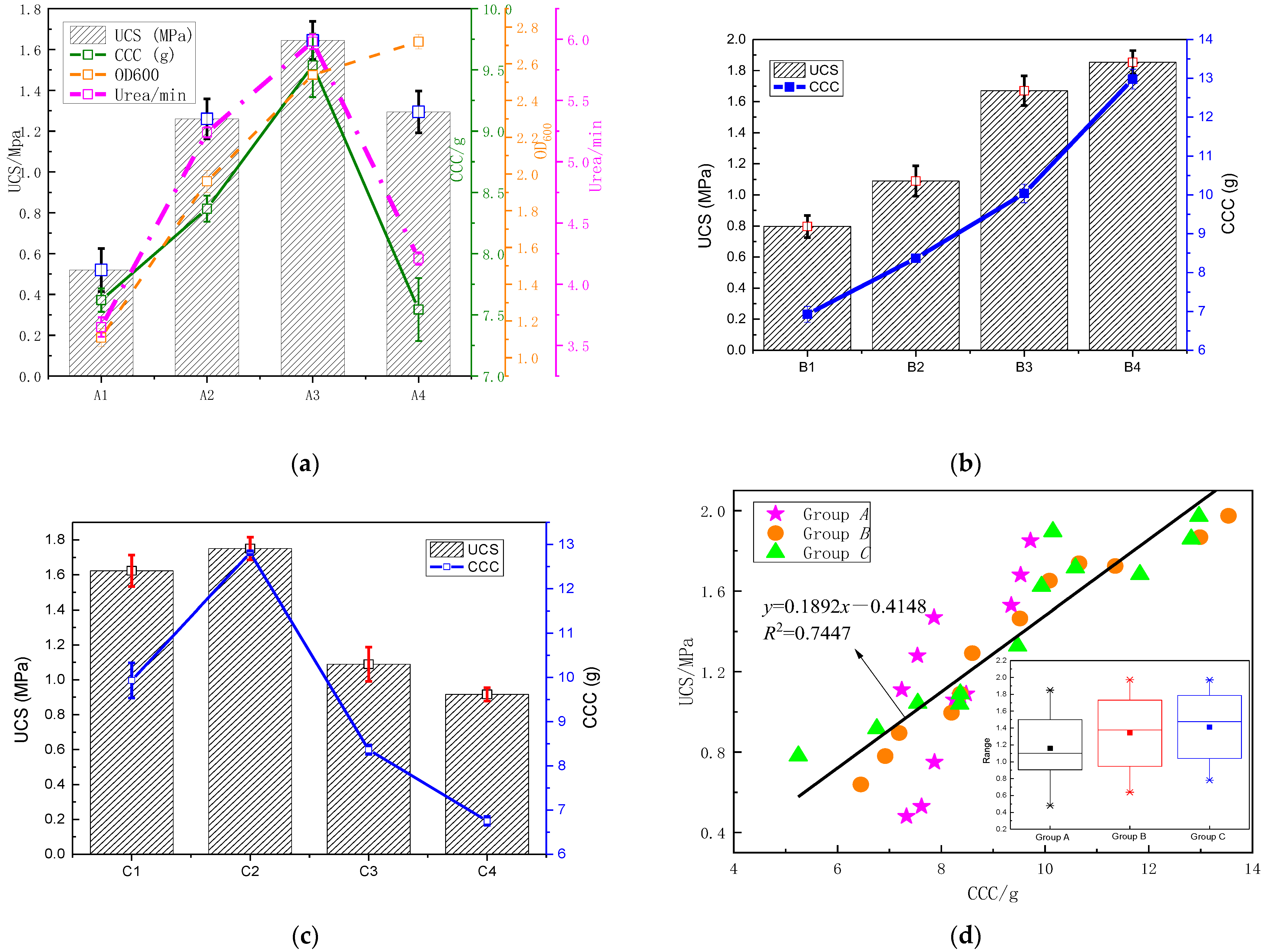
3.1.2. Relationship between UCS and CCC
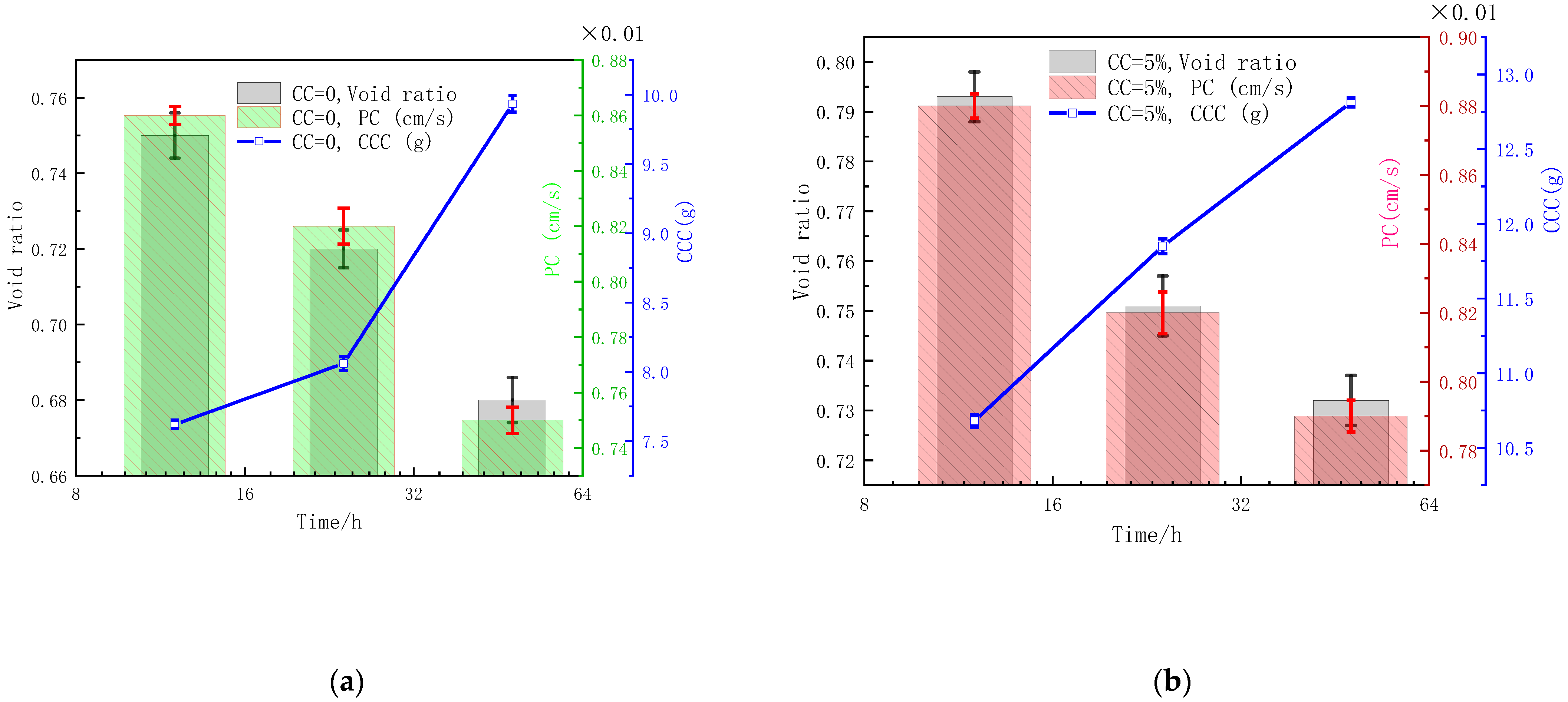
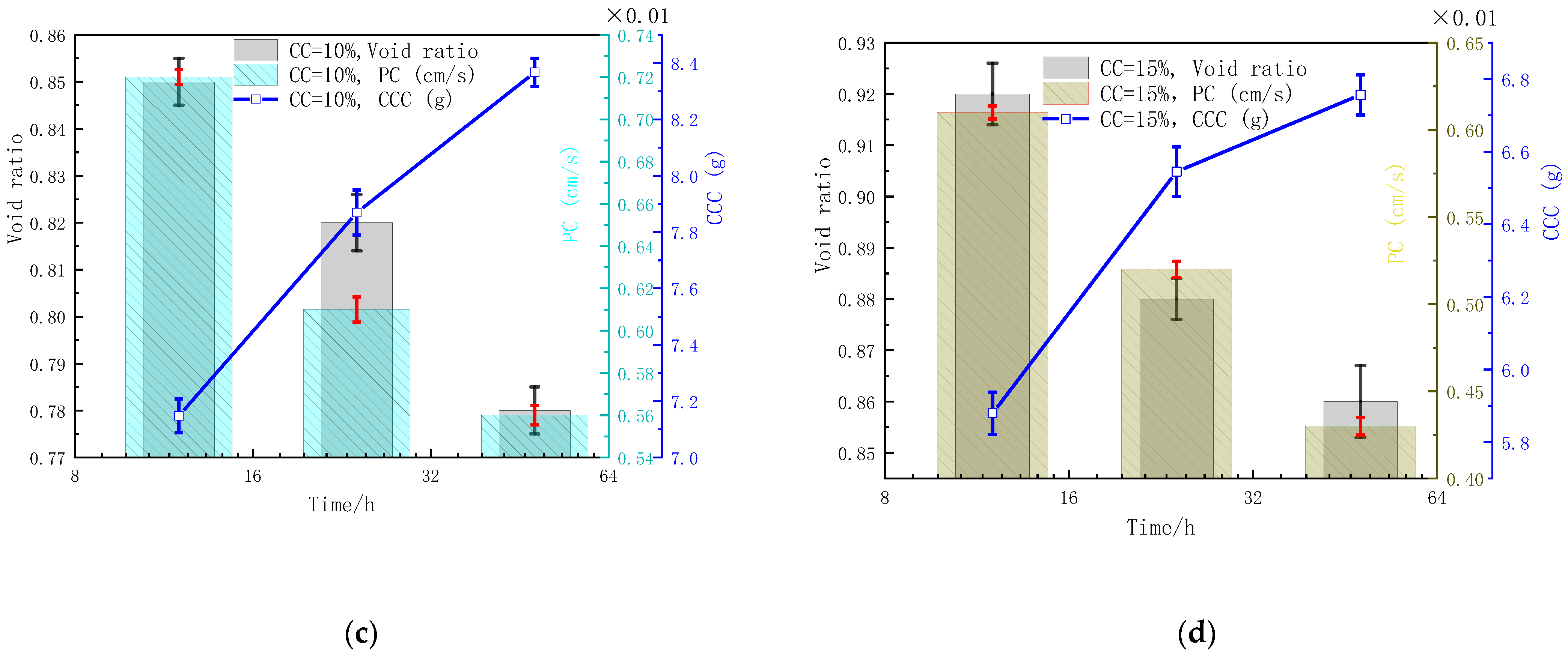
3.2. Influences of CC on Void Ratio, Permeability, and CCC
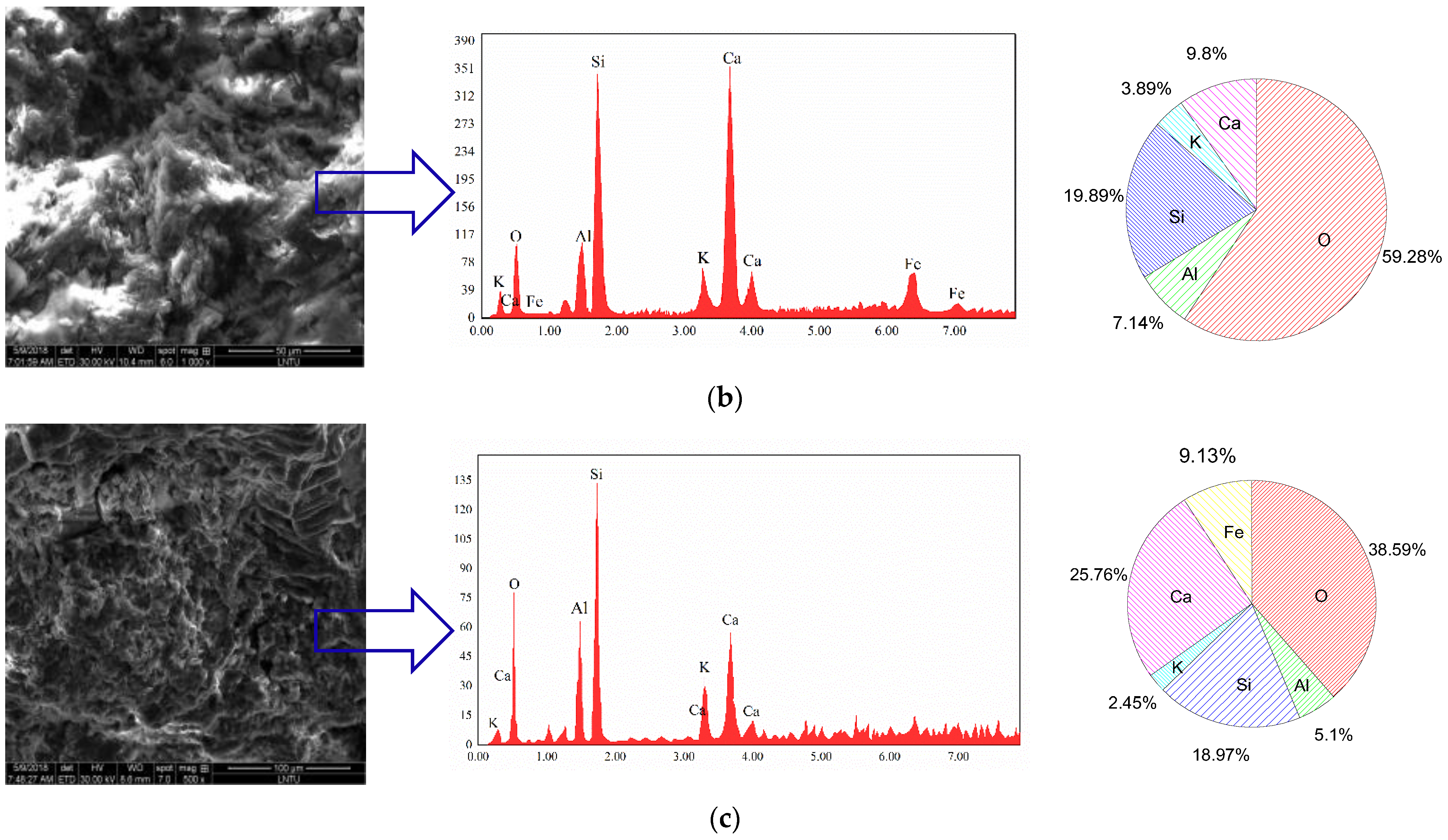
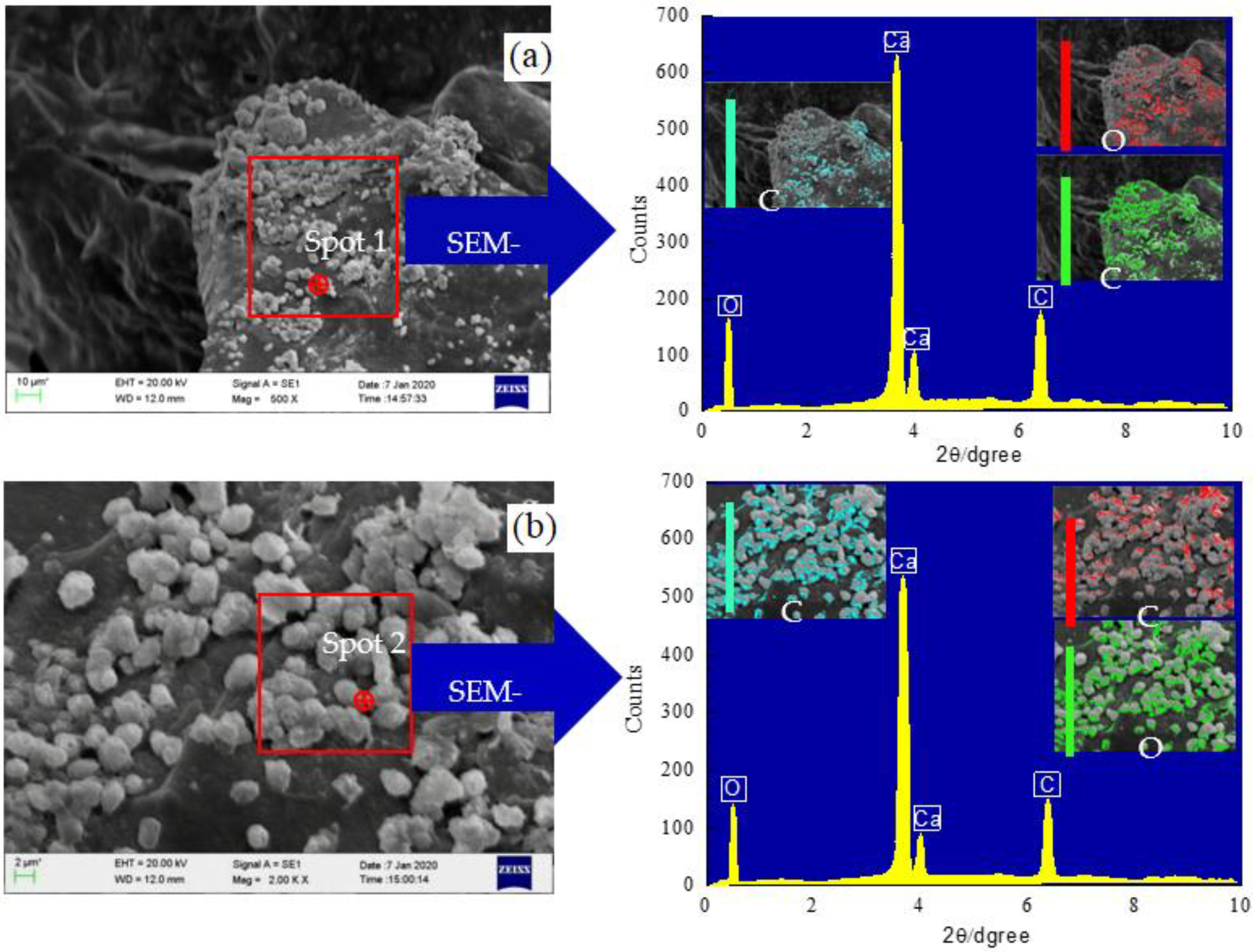
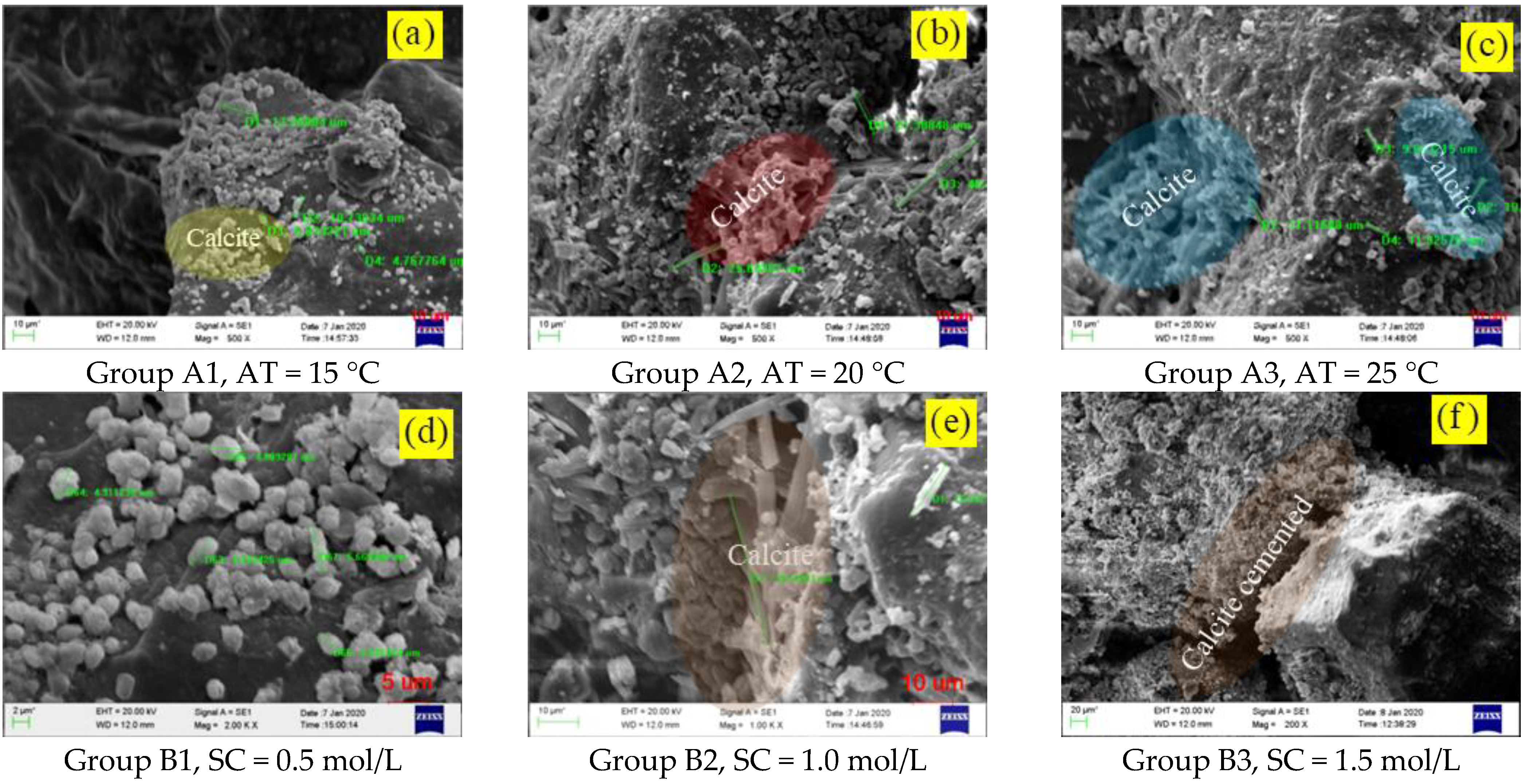
3.3. SEM–EDS and XRD Analysis
3.3.1. SEM–EDS Analysis
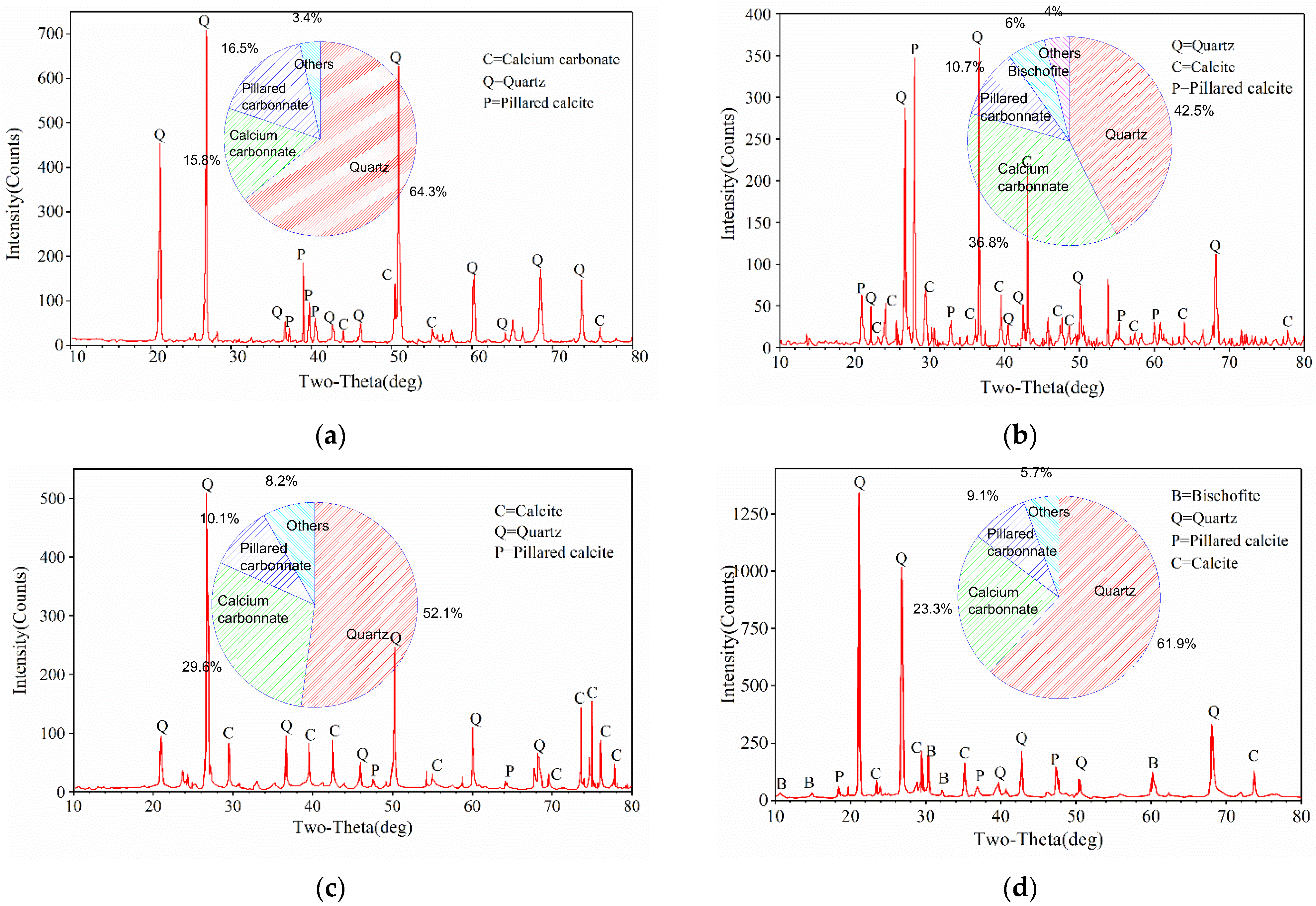
3.3.2. XRD Analysis
3.3.3. Effect of Cementation Solution Concentration on Mineralization
4. Conclusions
- Under different conditions, the stress–strain curves of MSDF showed the characteristics of strain softening with obvious peak stress, which was the UCS value. The research showed that UCS had an obvious linear relationship with CCC, which can be expressed as y = 0.1892x − 0.4148.
- The environmental temperature mainly affected urease activity, the concentration of cement solution mainly affected the total amount of calcium, and the content of clay mainly affected the particle size distribution, which led to the difference of microorganism-induced DF particle size. The optimum ore-forming conditions were 25 °C, a cementation solution concentration of 2 mol/L, and a 5% SC content.
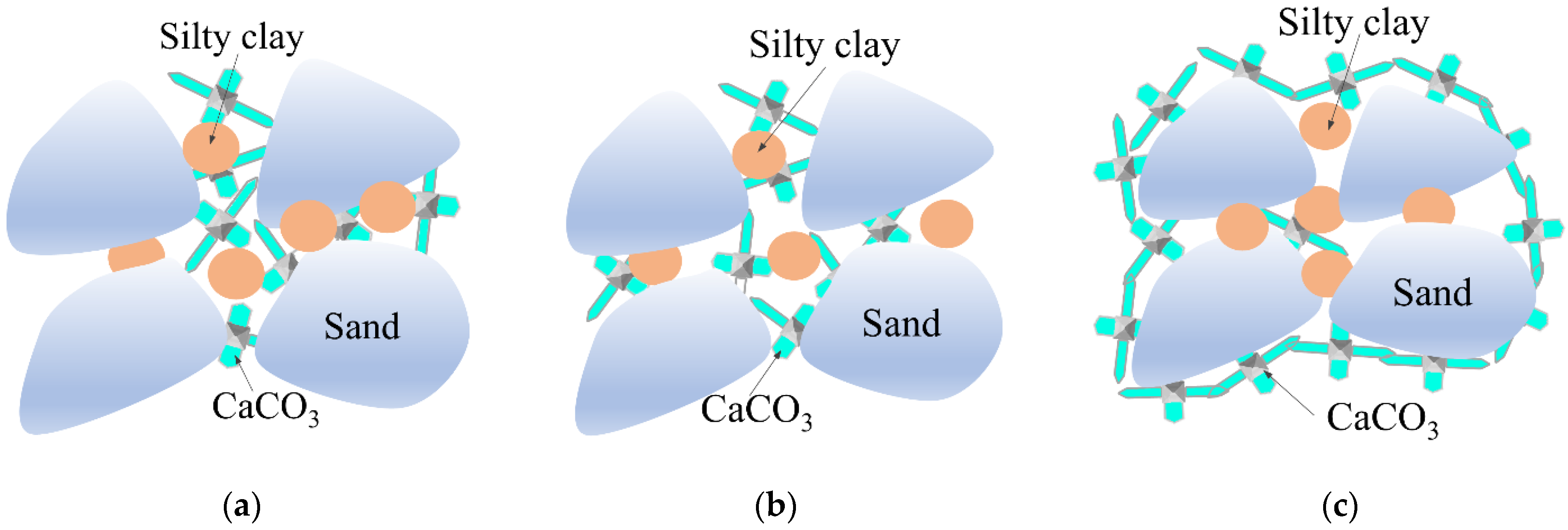
- The cementation mode between DF particles could generally be divided into three types: calcite precipitation, calcite cruciform cementation, and calcite wrapped cementation. The urease produced by Sporosarcina pasteurii microorganisms was dispersed between DF and silty clay particles, which played a positive role in enriching CaCO3 precipitation in a certain nutrient solution environment.
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Liu, D.; Ge, H.; Shen, Y.; Zhang, K. Experimental Investigation on Organic Matter Orientation Characteristics of Terrestrial and Marine Shale in China. Geofluids 2021, 2021, 8895350. [Google Scholar] [CrossRef]
- Wu, Y.; Tran, Q.C.; Zhang, X.; Lu, Y.; Xu, J.; Zhang, H. Experimental investigation on the treatment of different typical marine dredged sludges by flocculant and vacuum preloading. Arab. J. Geosci. 2022, 15, 642. [Google Scholar] [CrossRef]
- Wang, J.; Wu, L.; Yang, T.; Yan, X.; Pei, X.; Huang, X.; Long, G.; Xue, R. Laboratory experiments on HMC coupling mechanisms in innovative clean foundation treatments for Zn-contaminated dredger fills. Sci. Total Environ. 2020, 702, 134939. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, J.; Pei, X.; Fogg, G.E.; Yang, T.; Yan, X.; Huang, X.; Liu, Z.; Qi, R. Distribution and origination of zinc contamination in newly reclaimed heterogeneous dredger fills: Field investigation and numerical simulation. Mar. Pollut. Bull. 2019, 149, 110496. [Google Scholar] [CrossRef] [PubMed]
- Vivier, L.; Cyrus, D.P. Dune Mining and the Nhlabane Estuary, South Africa: The Effect of a Dredger Crossing on the Zoobenthic Community. Mar. Pollut. Bull. 1999, 39, 308–314. [Google Scholar] [CrossRef]
- Liu, J.; Lei, H.; Zheng, G.; Zhou, H.; Zhang, X. Laboratory model study of newly deposited dredger fills using improved multiple-vacuum preloading technique. J. Rock Mech. Geotech. Eng. Engl. Ed. 2017, 9, 924–935. [Google Scholar] [CrossRef]
- Uścinowicz, S.; Jegliński, W.; Miotk-Szpiganowicz, G.; Nowak, J.; Pączek, U.; Przezdziecki, P.; Szefler, K.; Poręba, G. Impact of sand extraction from the bottom of the southern Baltic Sea on the relief and sediments of the seabed. Oceanologia 2014, 56, 857–880. [Google Scholar] [CrossRef]
- Wang, J.; Cai, Y.; Fu, H.; Hu, X.; Cai, Y.; Lin, H.; Zheng, W. Experimental study on a dredged fill ground improved by a two-stage vacuum preloading method. Soils Found. 2018, 58, 766–775. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Y.; Chen, Y.; Xiao, H. Deformation and failure mechanism of rapid stabilization for dredger fill in road engineering. Arab. J. Geosci. 2020, 13, 266. [Google Scholar] [CrossRef]
- Wang, X.; Ding, H.; Meng, Q.; Wei, H.; Wu, Y.; Zhang, Y. Engineering characteristics of coral reef and site assessment of hydraulic reclamation in the South China Sea. Constr. Build. Mater. 2021, 300, 124263. [Google Scholar] [CrossRef]
- Yu, Q.; Yan, X.; Wang, Q.; Yang, T.; Kong, Y.; Huang, X.; Mehmood, Q. X-ray computed tomography-based evaluation of the physical properties and compressibility of soil in a reclamation area. Geoderma 2017, 375, 114524. [Google Scholar] [CrossRef]
- Tang, L.; Song, J.; Chen, H.; Wang, Y.; Yin, J.; Ye, J. Impacts of organic content and pH on consolidation of clayey dredger fill by vacuum preloading method. Geosci. J. 2017, 21, 765–778. [Google Scholar] [CrossRef]
- Mitchell, J.K.; Santamarina, J.C. Biological considerations in geotechnical engineering. J. Geotech. Geoenviron. Eng. 2005, 131, 1222–1233. [Google Scholar] [CrossRef]
- Cheng, L.; Cord-Ruwisch, R. In situ soil cementation with ureolytic bacteria by surface percolation. Ecol. Eng. 2012, 42, 64–72. [Google Scholar] [CrossRef]
- Chu, J.; Ivanov, V.; Naeimi, M.; Stabnikov, V.; Liu, H.-L. Optimization of calcium-based bioclogging and biocementation of sand. Acta Geotech. 2014, 9, 277–285. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M.A.; Cord-Ruwisch, R.; Addis, M.; Haranto, T.; Elms, C. Soil stabilisation by microorganism induced calcium carbonate precipitation: Investigation of some important physical and environmental aspects. In Proceedings of the 7th International Congress on Environmental Geotechnics, Melbourne, Australia, 10–14 November 2014. [Google Scholar]
- Zamarreňo, D.V.; May, E.; Inkpen, R. Influence of environmental temperature on biocalcification by non-sporing freshwater bacteria. Geomicrobiol. J. 2009, 26, 298–309. [Google Scholar] [CrossRef]
- Cacchio, P.; Ercole, C.; Cappuccio, G.; Lepidi, A. Calcium Carbonate Precipitation by Bacterial Strains Isolated from a Limestone Cave and from a Loamy Soil. Geomicrobiol. J. 2003, 20, 85–98. [Google Scholar] [CrossRef]
- Mahawish, A.; Bouazza, A.; Gates, W.P. Effect of particle size distribution on the bio-cementation of coarse aggregates. Acta Geotech. 2018, 13, 1019–1025. [Google Scholar] [CrossRef]
- Sohail, M.G.; Al Disi, Z.; Zouari, N.; Al Nuaimi, N.; Kahraman, R.; Gencturk, B.; Rodrigues, D.F.; Yildirim, Y. Bio self-healing concrete using MICP by an indigenous Bacillus cereus strain isolated from Qatari soil. Constr. Build. Mater. 2022, 328, 126943. [Google Scholar] [CrossRef]
- Burbank, M.B.; Weaver, T.J.; Green, T.L.; Williams, B.C.; Crawford, R.L. Precipitation of calcite by indigenous microorganisms to strengthen liquefiable soils. Geomicrobiol. J. 2011, 28, 301–312. [Google Scholar] [CrossRef]
- Gomez, M.G.; Anderson, C.M.; DeJong, J.T.; Nelson, D.C.; Lau, X.H. Stimulating in situ soil bacteria for bio-cementation of sands. In Proceedings of the Geo-Congress 2014: Geo-Characterization and Modeling for Sustainability, Atlanta, GA, USA, 23–26 February 2014; pp. 1674–1682. [Google Scholar]
- Gomez, M.G.; Graddy, C.M.; DeJong, J.T.; Nelson, D.C.; Tsesarsky, M. Stimulation of native microorganisms for biocementation in samples recovered from field-scale treatment depths. J. Geotech. Geoenviron. Eng. 2018, 144, 04017098. [Google Scholar] [CrossRef]
- Graddy, C.M.; Gomez, M.G.; Kline, L.M.; Morrill, S.R.; DeJong, J.T.; Nelson, D.C. Diversity of sporosarcina-like bacterial strains obtained from meter-scale augmented and stimulated biocementation experiments. Environ. Sci. Technol. 2018, 52, 3997–4005. [Google Scholar] [CrossRef]
- Nafisi, A.; Liu, Q.; Montoya, B.M. Effect of stress path on the shear response of bio-cemented sands. Acta Geotech. 2021, 16, 3239–3251. [Google Scholar] [CrossRef]
- Gowthaman, S.; Nakashima, K.; Kawasaki, S. Freeze-thaw durability and shear responses of cemented slope soil treated by microbial induced carbonate precipitation. Soils Found. 2020, 60, 840–855. [Google Scholar] [CrossRef]
- Gowthaman, S.; Iki, T.; Nakashima, K.; Ebina, K.; Kawasaki, S. Feasibility study for slope soil stabilization by microbial induced carbonate precipitation (MICP) using indigenous bacteria isolated from cold subarctic region. SN Appl. Sci. 2019, 1, 1480. [Google Scholar] [CrossRef]
- Sharma, M.; Satyam, N.; Reddy, K.R. Liquefaction Resistance of Biotreated Sand Before and After Exposing to Weathering Conditions. Indian Geotech. J. 2022, 52, 328–340. [Google Scholar] [CrossRef]
- Van Paassen, L.A. Biogrout, Ground Improvement by Microbial Induced Carbonate Precipitation. Doctoral Thesis, Technische Universiteit Delft, Delft, The Netherlands, 2009. [Google Scholar]
- Whiffin, V.S. Microbial CaCO3 precipitation for the production of biocement. Ph.D. Thesis, Murdoch University, Dubai, United Arab Emirates, 2004. [Google Scholar]
- Sharma, M.; Satyam, N.; Reddy, K.R. Large-scale spatial characterization and liquefaction resistance of sand by hybrid bacteria induced biocementation. Eng. Geol. 2022, 302, 106635. [Google Scholar] [CrossRef]
- Wit, L.D.; Talmon, A.M.; Rhee, C.V. 3D CFD simulations of trailing suction hopper dredger plume mixing: A parameter study of near-field conditions influencing the suspended sediment source flux. Mar. Pollut. Bull. 2014, 88, 47–61. [Google Scholar] [CrossRef]
- Su, F.; Yang, Y.; Qi, Y.; Zhang, H. Combining microorganismly induced calcite precipitation (MICP) with zeolite: A new technique to reduce ammonia emission and enhance soil treatment ability of MICP technology. J. Environ. Chem. Eng. 2022, 10, 107770. [Google Scholar] [CrossRef]
- GB/T 50123-2019; Standard for Soil Test Methods. China Planning Press: Beijing, China, 2019.
- Liu, P.; Shao, G.H.; Huang, R.P. Mechanical Properties and constitutive model of MICP-cemented sand. J. Southeast Univ. (Nat. Sci. Ed.) 2019, 49, 720–726. [Google Scholar]
- Zhao, Q.; Li, L.; Li, C.; Li, M.; Amini, F.; Zhang, H. Factors affecting improvement of engineering properties of MICP-treated soil catalyzed by bacteria and urease. J. Mater. Civ. Eng. 2014, 26, 04014094. [Google Scholar] [CrossRef]
- Lin, H.; Suleiman, M.T.; Brown, D.G. Investigation of pore-scale CaCO3 distributions and their effects on stiffness and permeability of sands treated by microbially induced carbonate precipitation (MICP). Soils Found. 2020, 60, 944–961. [Google Scholar] [CrossRef]
- Yang, A.; Zhou, J.; Kong, L. Experimental study on soft soil solidification of dredger fill in Tianjin Binhai New Area. Geotech. Mech. 2013, 34, 2442–2448. [Google Scholar] [CrossRef]
- Tang, C.; Gu, K. Strength characteristics of soft soil strengthened with polypropylene fiber and cement. J. Civ. Eng. 2011, 44, 5–8. [Google Scholar] [CrossRef]

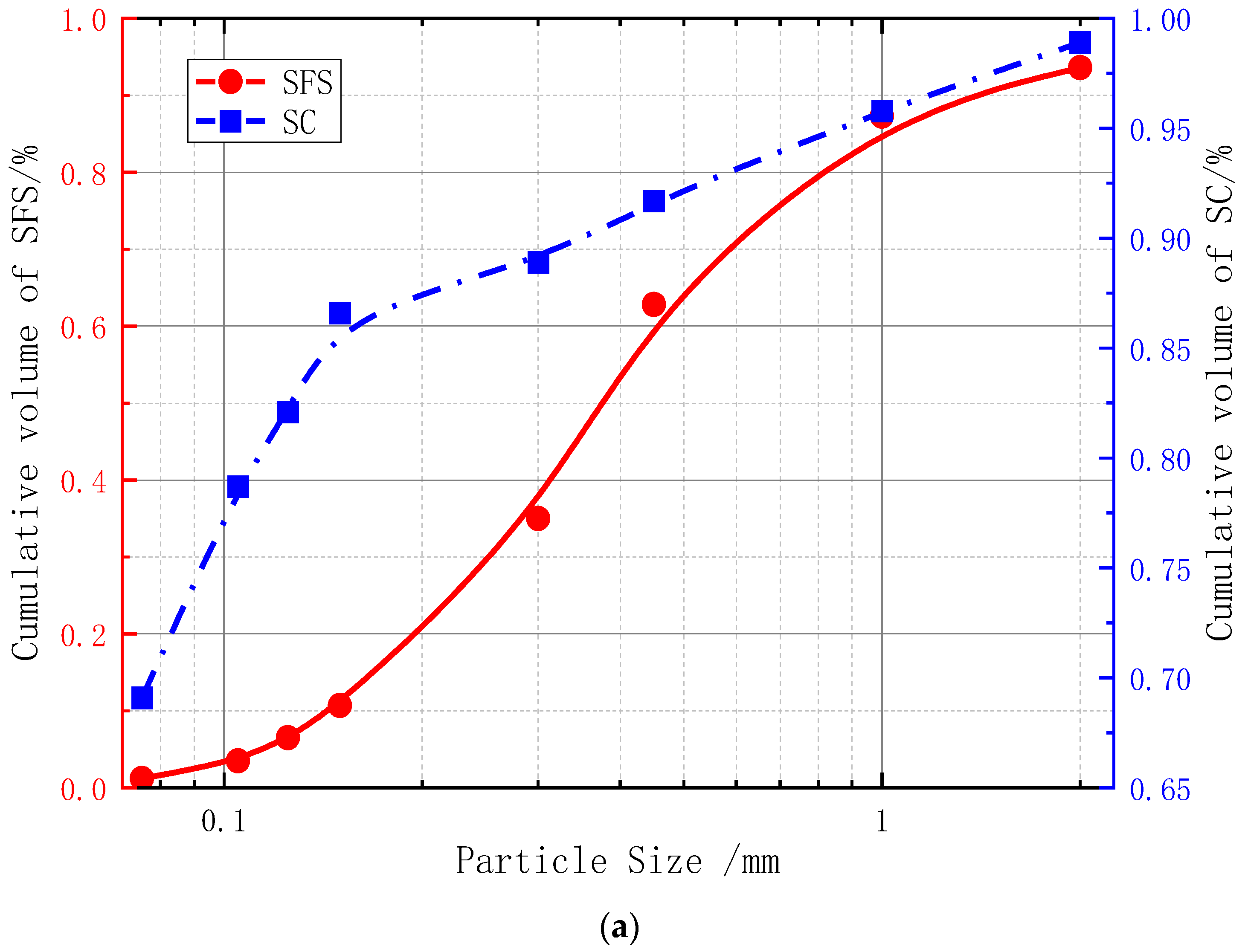


| Soil | Void Ratio | Moisture Content % | |||
|---|---|---|---|---|---|
| SFS | 1.58 | 1.80 | 1.62 | 1.14 | 35.12 |
| SC | 1.25 | 2.68 | 1.76 | 1.25 | 36.75 |
| Group | Specimen | Ambient Temperature °C | Cementation Solution Concentration mol/L | Clay Content % |
|---|---|---|---|---|
| A | A1 | 15 | 1.0 | 10 |
| A2 | 20 | |||
| A3 | 25 | |||
| A4 | 30 | |||
| B | B1 | 20 | 0.5 | |
| B2 | 1.0 | |||
| B3 | 1.5 | |||
| B4 | 2.0 | |||
| C | C1 | 1.0 | 0 | |
| C2 | 5 | |||
| C3 | 10 | |||
| C4 | 15 |
| CC/% | 12–24 h | 24–48 h | ||||
|---|---|---|---|---|---|---|
| Void Ratio 10−3/h | PC 10−5 cm/s/h | CCC 10−2 g/h | Void Ratio 10−3/h | PC 10−5 cm/s/h | CCC 10−2 g/h | |
| 0 | −2.5 | −3.33 | 3.67 | −1.67 | −2.92 | 7.81 |
| 5 | −3.5 | −5.01 | 9.75 | −0.79 | −1.25 | 4.01 |
| 10 | −2.5 | −9.17 | 6.00 | −1.67 | −2.08 | 2.07 |
| 15 | −3.3 | −7.52 | 5.55 | −0.83 | −3.75 | 0.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Tian, L.; Xu, Y.; Tian, Z.; Zhang, Z. Study on the Solidification Effect of Dredger Fill by Microbial-Induced Calcium Precipitation (MICP). Materials 2022, 15, 7891. https://doi.org/10.3390/ma15227891
Li J, Tian L, Xu Y, Tian Z, Zhang Z. Study on the Solidification Effect of Dredger Fill by Microbial-Induced Calcium Precipitation (MICP). Materials. 2022; 15(22):7891. https://doi.org/10.3390/ma15227891
Chicago/Turabian StyleLi, Jun, Lijun Tian, Yan Xu, Zefeng Tian, and Zhendong Zhang. 2022. "Study on the Solidification Effect of Dredger Fill by Microbial-Induced Calcium Precipitation (MICP)" Materials 15, no. 22: 7891. https://doi.org/10.3390/ma15227891
APA StyleLi, J., Tian, L., Xu, Y., Tian, Z., & Zhang, Z. (2022). Study on the Solidification Effect of Dredger Fill by Microbial-Induced Calcium Precipitation (MICP). Materials, 15(22), 7891. https://doi.org/10.3390/ma15227891







