Preparation and Investigation of Spherical Powder Made from Corrosion-Resistant 316L Steel with the Addition of 0.2% and 0.5% Ag
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grabco, D.; Shikimaka, O.; Pyrtsac, C.; Prisacaru, A.; Barbos, Z.; Bivol, M.; Alexandrov, S.; Vilotic, D.; Vilotic, M. Microstructures generated in AISI 316L stainless steel by Vickers and Berkovich indentations. Mater. Sci. Eng. A 2021, 805, 140597. [Google Scholar] [CrossRef]
- Sheik, S.; Tirumalla, A.; Gurrala, A.K.; Mohammed, R. Effect of microstructural morphology on corrosion susceptibility of austenitic and super austenitic stainless steels. Mater. Today Proc. 2022, 66, 514–518. [Google Scholar] [CrossRef]
- Feng, W.; Wang, Z.; Sun, Q.; He, Y.; Sun, Y. Effect of thermomechanical processing via rotary swaging on grain boundary character distribution and intergranular corrosion in 304 austenitic stainless steel. J. Mater. Res. Technol. 2022, 19, 2470–2482. [Google Scholar] [CrossRef]
- Schillé, J.P.; Guo, Z.; Saunders, N.; Miodownik, A.P. Modeling phase transformations and material properties critical to processing simulation of steels. Mater. Manuf. Process. 2011, 26, 137–143. [Google Scholar] [CrossRef]
- Mehta, H.; Kaur, G.; Chaudhary, G.R.; Prabhakar, N.; Kaul, S.; Singhal, N.K. Evaluation of corrosion resistant, antimicrobial and cytocompatible behaviour of cobalt based metallosurfactants self-assembled monolayers on 316L stainless steel surface. Surf. Coat. Technol. 2022, 444, 128657. [Google Scholar] [CrossRef]
- Wongpanya, P.; Wongpinij, T.; Photongkam, P.; Siritapetawee, J. Improvement in corrosion resistance of 316 L stainless steel in simulated body fluid mixed with antiplatelet drugs by coating with Ti-doped DLC films for application in biomaterials. Corros. Sci. 2022, 208, 110611. [Google Scholar] [CrossRef]
- Zhang, D.H.; Meng, X.C.; Zuo, G.Z.; Huang, M.; Li, L.; Xu, W.; Li, C.L.; Tang, Z.L.; Yuan, J.S.; Liu, Y.B.; et al. Study of the corrosion characteristics of 304 and 316L stainless steel in the static liquid lithium. J. Nucl. Mater. 2021, 553, 153032. [Google Scholar] [CrossRef]
- Virtanen, S.; Milošev, I.; Gomez-Barrena, E.; Trebše, R.; Salo, J.; Konttinen, Y.T. Special modes of corrosion under physiological and simulated physiological conditions. Acta Biomater. 2008, 4, 468–476. [Google Scholar] [CrossRef]
- Brooks, E.K.; Brooks, R.P.; Ehrensberger, M.T. Effects of simulated inflammation on the corrosion of 316L stainless steel. Mater. Sci. Eng. C 2017, 71, 200–205. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Sreekumari, K.R.; Nandakumar, K.; Kikuchi, Y. Bacterial attachment to stainless steel welds: Significance of substratum microstructure. Biofouling 2001, 17, 303–316. [Google Scholar] [CrossRef]
- Baena, M.I.; Márquez, M.C.; Matres, V.; Botella, J.; Ventosa, A. Bactericidal activity of copper and niobium-alloyed austenitic stainless steel. Curr. Microbiol. 2006, 53, 491–495. [Google Scholar] [CrossRef]
- Sreekumari, K.R.; Takao, K.; Ujiro, T.; Kikuchi, Y. High nitrogen stainless steel as a preferred substratum for bacteria and other microfouling organisms. ISIJ Int. 2004, 44, 858–864. [Google Scholar] [CrossRef]
- Liao, K.H.; Ou, K.L.; Cheng, H.C.; Lin, C.T.; Peng, P.W. Effect of silver on antibacterial properties of stainless steel. Appl. Surf. Sci. 2010, 256, 3642–3646. [Google Scholar] [CrossRef]
- Chiang, W.C.; Tseng, I.S.; Møller, P.; Hilbert, L.R.; Tolker-Nielsen, T.; Wu, J.K. Influence of silver additions to type 316 stainless steels on bacterial inhibition, mechanical properties, and corrosion resistance. Mater. Chem. Phys. 2010, 119, 123–130. [Google Scholar] [CrossRef]
- Yang, S.M.; Chen, Y.C.; Pan, Y.T.; Lin, D.Y. Effect of silver on microstructure and antibacterial property of 2205 duplex stainless steel. Mater. Sci. Eng. C 2016, 63, 376–383. [Google Scholar] [CrossRef]
- Sreekumari, K.R.; Nandakumar, K.; Takao, K.; Kikuchi, Y. Silver containing stainless steel as a new outlook to abate bacterial adhesion and microbiologically influenced corrosion. ISIJ Int. 2003, 43, 1799–1806. [Google Scholar] [CrossRef]
- Kaplan, M.A.; Ivannikov, A.Y.; Konushkin, S.V.; Nasakina, E.O.; Baikin, A.S.; Kartabaeva, B.B.; Gorbenko, A.D.; Kolmakov, A.G.; Sevostyanov, M.A. Study of the Structure, Mechanical Characteristics, and Antibacterial Properties of Corrosion-Resistant Steel Alloyed with Silver and Titanium. Dokl. Chem. 2022, 502 Pt 2, 37–44. [Google Scholar] [CrossRef]
- Yap, C.Y.; Chua, C.K.; Dong, Z.L.; Liu, Z.H.; Zhang, D.Q.; Loh, L.E.; Sing, S.L. Review of selective laser melting: Materials and applications. Appl. Phys. Rev. 2015, 2, 241101. [Google Scholar] [CrossRef]
- Kolmakov, A.G.; Ivannikov, A.Y.; Kaplan, M.A.; Kirsankin, A.A.; Sevostyanov, M.A. Corrosion-resistant steels in additive manufacturing. Izv. Ferr. Metall. 2021, 64, 619–650. [Google Scholar] [CrossRef]
- Vukkum, V.B.; Gupta, R.K. Review on corrosion performance of laser powder-bed fusion printed 316L stainless steel: Effect of processing parameters, manufacturing defects, post-processing, feedstock, and microstructure. Mater. Des. 2022, 221, 110874. [Google Scholar] [CrossRef]
- Rodrigues, T.A.; Farias, F.W.C.; Zhang, K.; Shamsolhodaei, A.; Shen, J.; Zhou, N.; Schell, N.; Capek, J.; Polatidis, E.; Santos, T.G.; et al. Wire and Arc Additive Manufacturing of Stainless Steel 316L/Inconel 625 Functionally Graded Material: Development and Characterization. J. Mater. Res. Technol. 2022, V21, 237–251. [Google Scholar] [CrossRef]
- Revilla, R.I.; Van Calster, M.; Raes, M.; Arroud, G.; Andreatta, F.; Pyl, L.; Guillaume, P.; De Graeve, I. Microstructure and corrosion behavior of 316L stainless steel prepared using different additive manufacturing methods: A comparative study bringing insights into the impact of microstructure on their passivity. Corros. Sci. 2020, 176, 108914. [Google Scholar] [CrossRef]
- Vinoth, V.; Sathiyamurthy, S.; Natarajan, U.; Venkatkumar, D.; Prabhakaran, J.; Prakash, K.S. Examination of microstructure properties of AISI 316L stainless steel fabricated by wire arc additive manufacturing. Mater. Today Proc. 2022, 66 Pt 3, 702–706. [Google Scholar] [CrossRef]
- Grech, I.S.; Sullivan, J.H.; Lancaster, R.J.; Plummer, J.; Lavery, N.P. The optimisation of hot isostatic pressing treatments for enhanced mechanical and corrosion performance of stainless steel 316L produced by laser powder bed fusion. Addit. Manuf. 2022, 58, 103072. [Google Scholar]
- Dawes, J.; Bowerman, R.; Trepleton, R. Introduction to the additive manufacturing powder metallurgy supply chain. Johns. Matthey Technol. Rev. 2015, 59, 243–256. [Google Scholar] [CrossRef]
- Egger, G.; Gygax, P.E.; Glardon, R.; Karapatis, N.P. Optimization of powder layer density in selective laser sintering. Solid Free. Fabr. Symp. 1999, 2, 255–263. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.W.; Stucker, B. Additive manufacturing technologies: Rapid prototyping to direct digital manufacturing. Addit. Manuf. Technol. Rapid Prototyp. Direct Digit. Manuf. 2010, 36–100. [Google Scholar] [CrossRef]
- Miao, G.; Du, W.; Pei, Z.; Ma, C. A Literature Review on Powder Spreading in Additive Manufacturing. Addit. Manuf. 2022, 58, 103029. [Google Scholar] [CrossRef]
- Zhai, W.; Zhou, W.; Nai, S.M.L.; Wei, J. Characterization of nanoparticle mixed 316 L powder for additive manufacturing. J. Mater. Sci. Technol. 2020, 47, 162–168. [Google Scholar] [CrossRef]
- Ganesan, V.V.; Amerinatanzi, A.; Jain, A. Discrete Element Modeling (DEM) simulations of powder bed densification using horizontal compactors in metal additive manufacturing. Powder Technol. 2022, 405, 117557. [Google Scholar] [CrossRef]
- Song, R.B.; Xiang, J.Y.; Hou, D.P. Characteristics of mechanical properties and microstructure for 316L austenitic stainless steel. J. Iron Steel Res. Int. 2011, 18, 53–59. [Google Scholar] [CrossRef]
- Spencer, K.; Conlon, K.T.; Bréchet, Y.; Embury, J.D. The strain induced martensite transformation in austenitic stainless steels: Part 2–Effect of internal stresses on mechanical response. Mater. Sci. Technol. 2009, 25, 18–28. [Google Scholar] [CrossRef]
- Nanda, T.; Kumar, B.R.; Singh, V. A Thermal Cycling Route for Processing Nano-grains in AISI 316L Stainless Steel for Improved Tensile Deformation Behaviour. Def. Sci. J. 2016, 66, 529–535. [Google Scholar] [CrossRef]



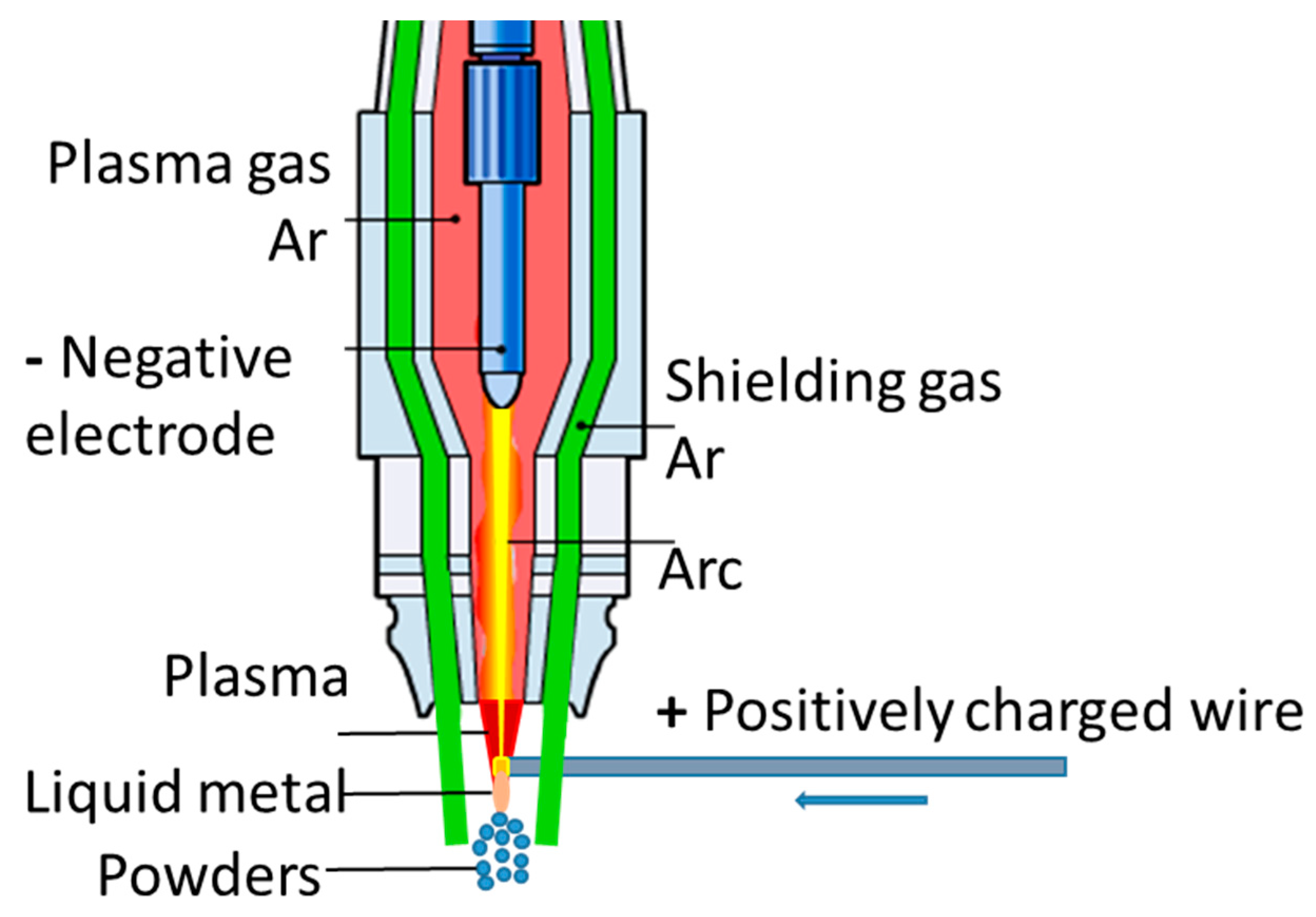

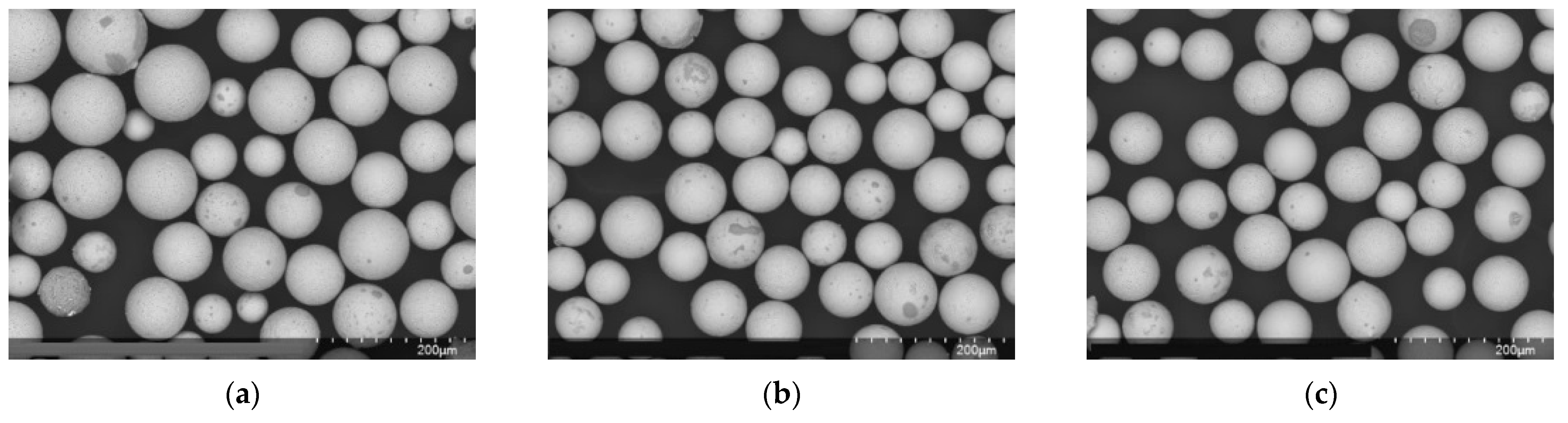


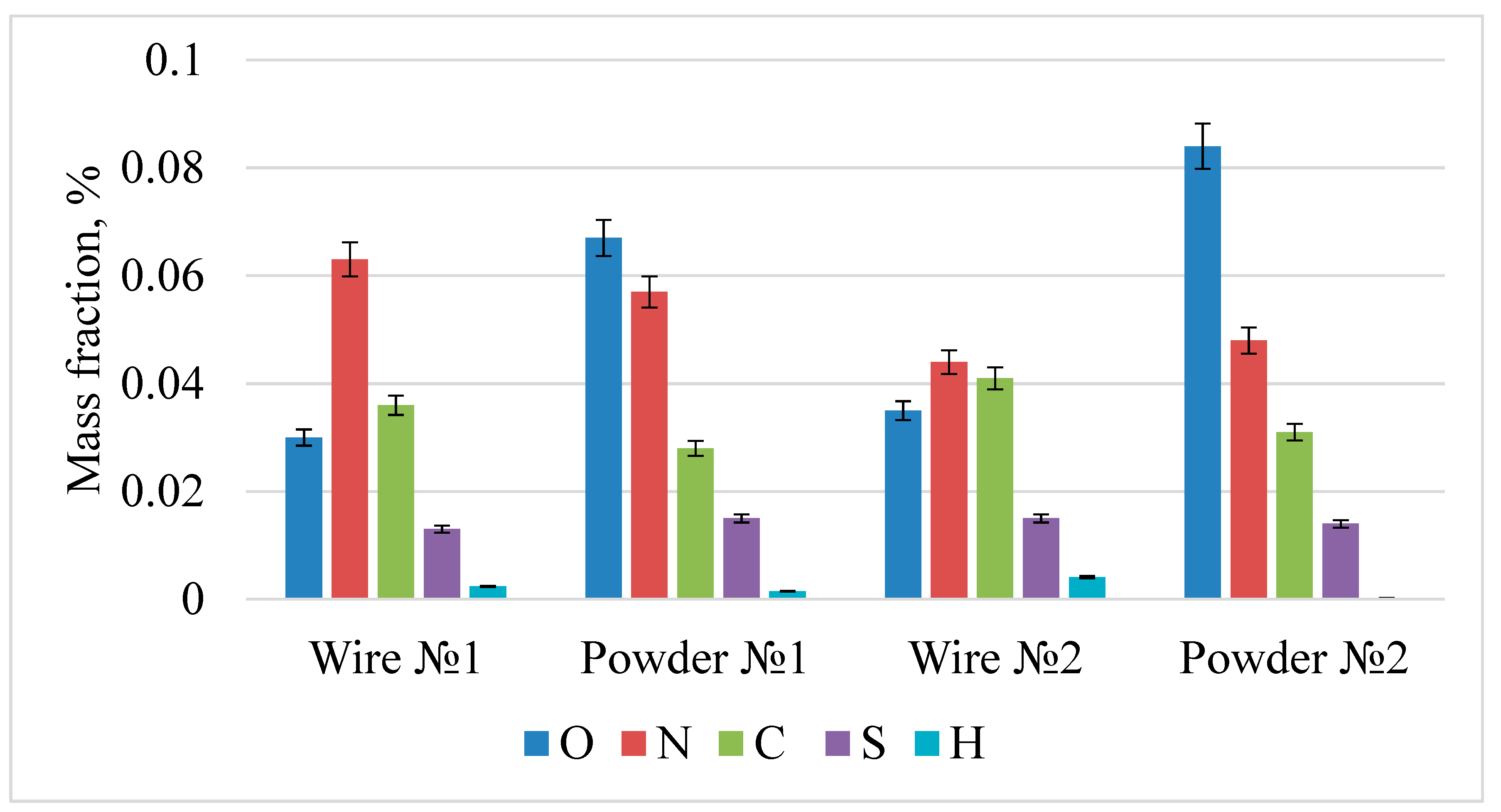
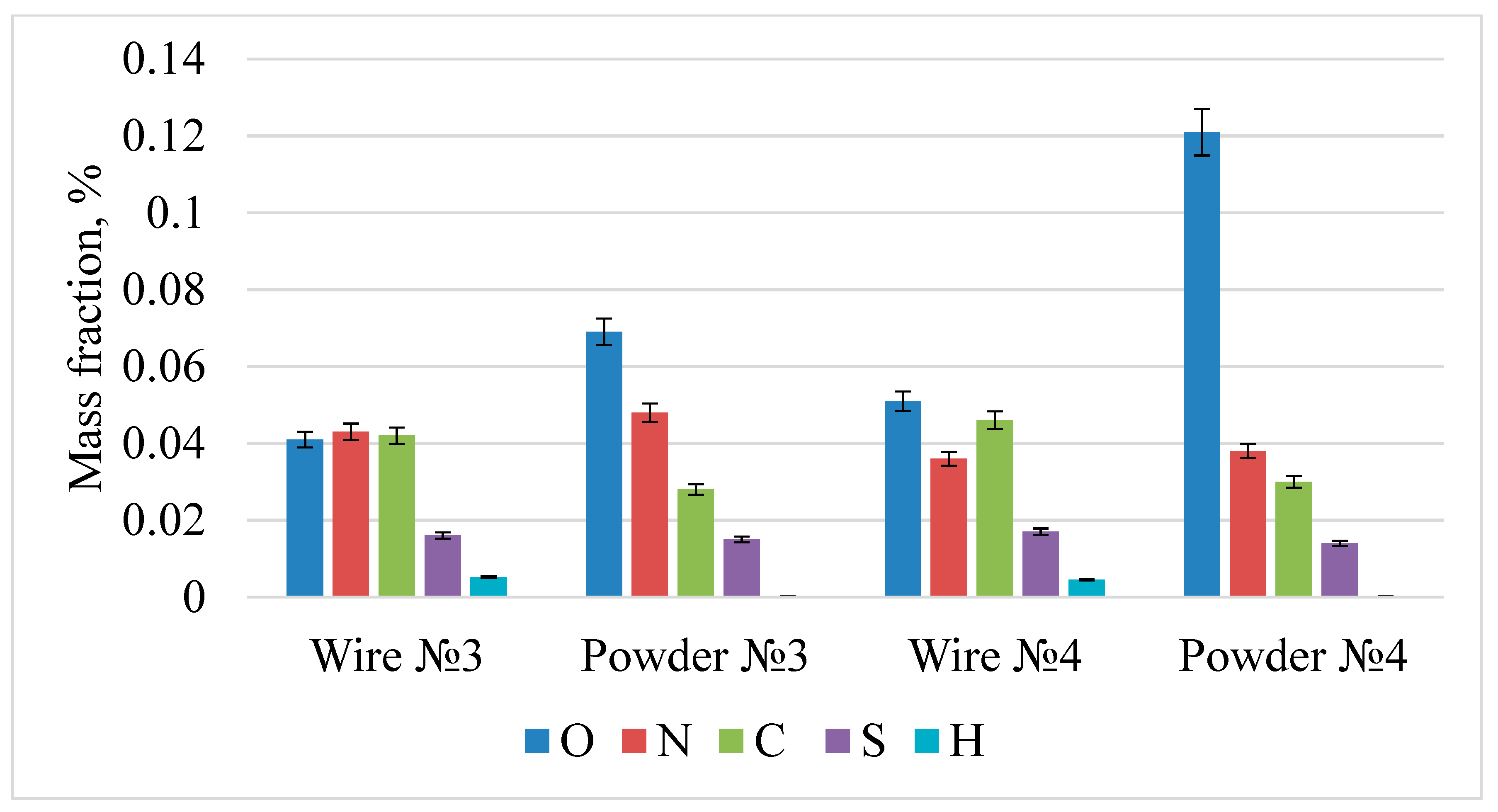
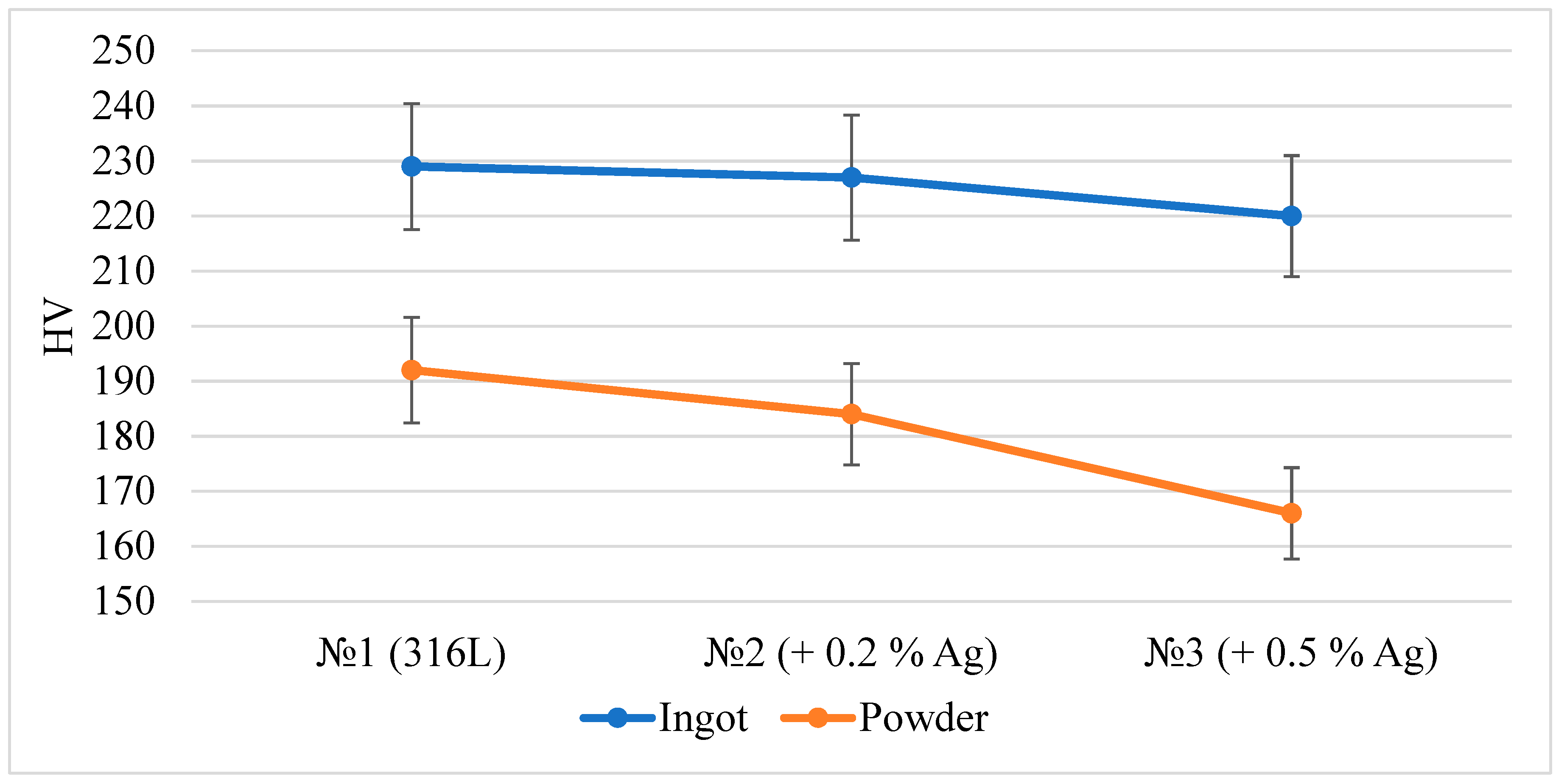
| Steel | C, % | Cr, % | Ni, % | Ag, % | Si, % | Mn, % | Mo, % | Si, % |
|---|---|---|---|---|---|---|---|---|
| №1 | 0.023 | 17 | 10 | 0 | 0.5 | 1.5 | 2 | 0.5 |
| №2 | 0.023 | 17 | 10 | 0.2 | 0.5 | 1.5 | 2 | 0.5 |
| №3 | 0.023 | 17 | 10 | 0.5 | 0.5 | 1.5 | 2 | 0.5 |
| Composition | Crystal Lattice Parameters (Å) | Phase Composition | Volume Fraction, % | Weight Fraction, % |
|---|---|---|---|---|
| №1 (316L) | 3.59605 ± 0.00004 | γ–Fe | 100 | 100 |
| №2 (+0.2% Ag) | 3.59792 ± 0.00003 | γ–Fe | 100 | 100 |
| №3 (0.5% Ag) | 3.59930 ± 0.00002 | γ–Fe | 100 | 100 |
| Composition | Crystal Lattice Parameters (Å) | Phase Composition | Volume Fraction, % | Weight Fraction, % |
|---|---|---|---|---|
| №1 (316L) | 3.59442 ± 0.00008 | γ–Fe | 85.3 ± 0.1 | 85.6 ± 0.1 |
| 2.87512 ± 0.00011 | α–Fe | 13.8 ± 0.1 | 13.5 ± 0.1 | |
| 8.81800 ± 0.00166 | σ–NiCr | 0.9 ± 0.1 | 0.8 ± 0.1 | |
| 4.57800 ± 0.00159 | ||||
| №2 (+ 0.2%Ag) | 3.59488 ± 0.00009 | γ–Fe | 82.9 ± 0.2 | 83.3 ± 0.2 |
| 2.87552 ± 0.00012 | α–Fe | 15.8 ± 0.1 | 15.5 ± 0.1 | |
| 8.81800 ± 0.00168 | σ–NiCr | 1.3 ± 0.1 | 1.3 ± 0.1 | |
| 4.57800 ± 0.00163 | ||||
| №3 (+ 0.5%Ag) | 3.59497 ± 0.00008 | γ–Fe | 74.2 ± 0.1 | 74.7 ± 0.1 |
| 2.87599 ± 0.00008 | α–Fe | 24.1 ± 0.1 | 23.7 ± 0.1 | |
| 8.80994 ± 0.00167 | σ–NiCr | 1.7 ± 0.1 | 1.6 ± 0.1 | |
| 4.59121 ± 0.00162 |
| Composition | Crystal Lattice Parameters (Å) | Phase Composition | Volume Fraction, % | Weight Fraction, % |
|---|---|---|---|---|
| №1 (316L) | 3.59564 ± 0.00002 | γ–Fe | 89.7 ± 0.2 | 91.6 ± 0.1 |
| 2.87621 ± 0.00012 | α–Fe | 4.6 ± 0.1 | 4.6 ± 0.1 | |
| 8.43767 ± 0.00166 | Fe3O4 | 5.7 ± 0.2 | 3.7 ± 0.1 | |
| №2 (+0.2% Ag) | 3.59521 ± 0.00002 | γ–Fe | 86.3 ± 0.2 | 89.4 ± 0.2 |
| 2.87393 ± 0.00010 | α–Fe | 4.4 ± 0.1 | 4.4 ± 0.1 | |
| 8.43871 ± 0.00158 | Fe3O4 | 9.4 ± 0.2 | 6.2 ± 0.1 | |
| №3 (+0.5% Ag) | 3.59495 ± 0.00002 | γ–Fe | 92.5 ± 0.2 | 93.6 ± 0.1 |
| 2.87435 ± 0.00012 | α–Fe | 4.7 ± 0.1 | 4.6 ± 0.1 | |
| 8.43300 ± 0.00165 | Fe3O4 | 2.8 ± 0.2 | 1.8 ± 0.1 |
| Material | №1 (316L) | №2 (+0.2% Ag) | №3 (+0.5% Ag) |
|---|---|---|---|
| Ingot | 0 | 0.1979 ± 0.073 | 0.4972 ± 0.171 |
| Wire | 0 | 0.1975 ± 0.071 | 0.49614 ± 0.173 |
| Powder | 0 | 0.1956 ± 0.075 | 0.4851 ± 0.178 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaplan, M.A.; Gorbenko, A.D.; Ivannikov, A.Y.; Konushkin, S.V.; Kirsankin, A.A.; Baikin, A.S.; Sergienko, K.V.; Nasakina, E.O.; Mikhailova, A.V.; Rumyantsev, B.A.; et al. Preparation and Investigation of Spherical Powder Made from Corrosion-Resistant 316L Steel with the Addition of 0.2% and 0.5% Ag. Materials 2022, 15, 7887. https://doi.org/10.3390/ma15227887
Kaplan MA, Gorbenko AD, Ivannikov AY, Konushkin SV, Kirsankin AA, Baikin AS, Sergienko KV, Nasakina EO, Mikhailova AV, Rumyantsev BA, et al. Preparation and Investigation of Spherical Powder Made from Corrosion-Resistant 316L Steel with the Addition of 0.2% and 0.5% Ag. Materials. 2022; 15(22):7887. https://doi.org/10.3390/ma15227887
Chicago/Turabian StyleKaplan, Mikhail A., Artem D. Gorbenko, Alexander Y. Ivannikov, Sergey V. Konushkin, Andrey A. Kirsankin, Alexander S. Baikin, Konstantin V. Sergienko, Elena O. Nasakina, Anna V. Mikhailova, Boris A. Rumyantsev, and et al. 2022. "Preparation and Investigation of Spherical Powder Made from Corrosion-Resistant 316L Steel with the Addition of 0.2% and 0.5% Ag" Materials 15, no. 22: 7887. https://doi.org/10.3390/ma15227887
APA StyleKaplan, M. A., Gorbenko, A. D., Ivannikov, A. Y., Konushkin, S. V., Kirsankin, A. A., Baikin, A. S., Sergienko, K. V., Nasakina, E. O., Mikhailova, A. V., Rumyantsev, B. A., Gorudko, I. V., Kolmakov, A. G., Simakin, A. V., Gudkov, S. V., Oshkukov, S. A., & Sevostyanov, M. A. (2022). Preparation and Investigation of Spherical Powder Made from Corrosion-Resistant 316L Steel with the Addition of 0.2% and 0.5% Ag. Materials, 15(22), 7887. https://doi.org/10.3390/ma15227887









