Effect of Fe-Doping on Thermal Expansion and Stability of Bismuth Magnesium Tantalate Pyrochlorere
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
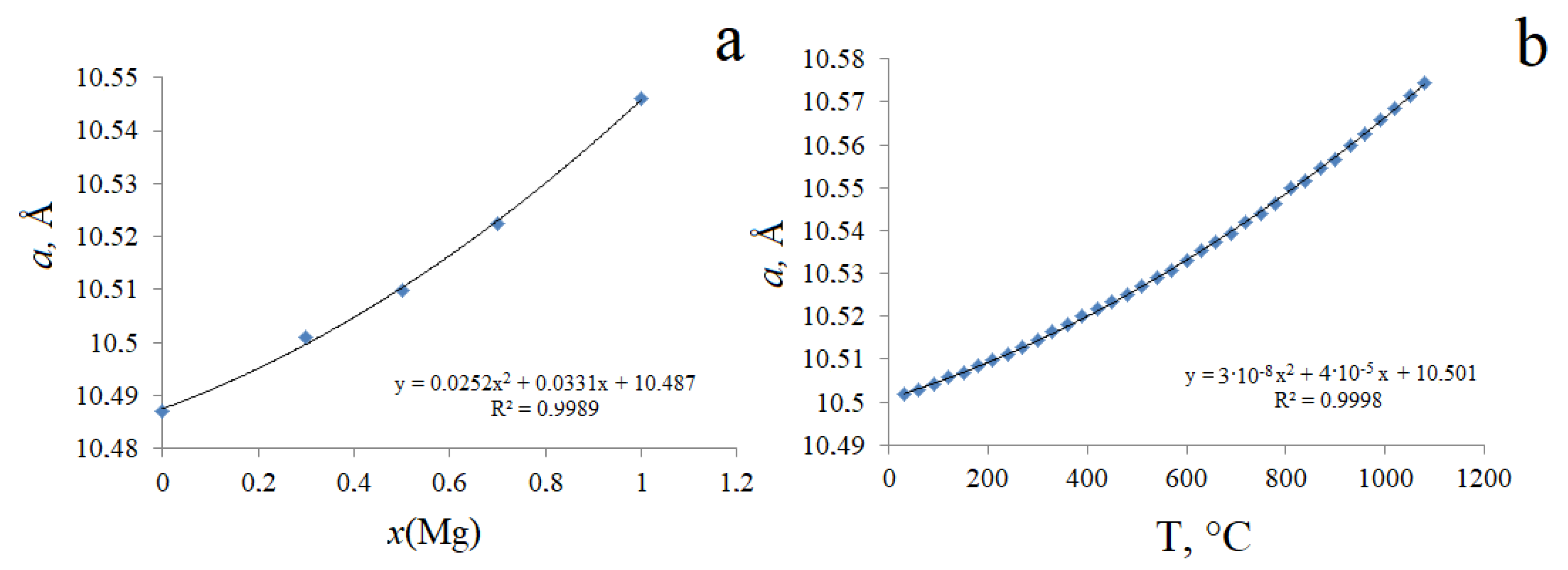
3.1. Morphology and Crystal Structure
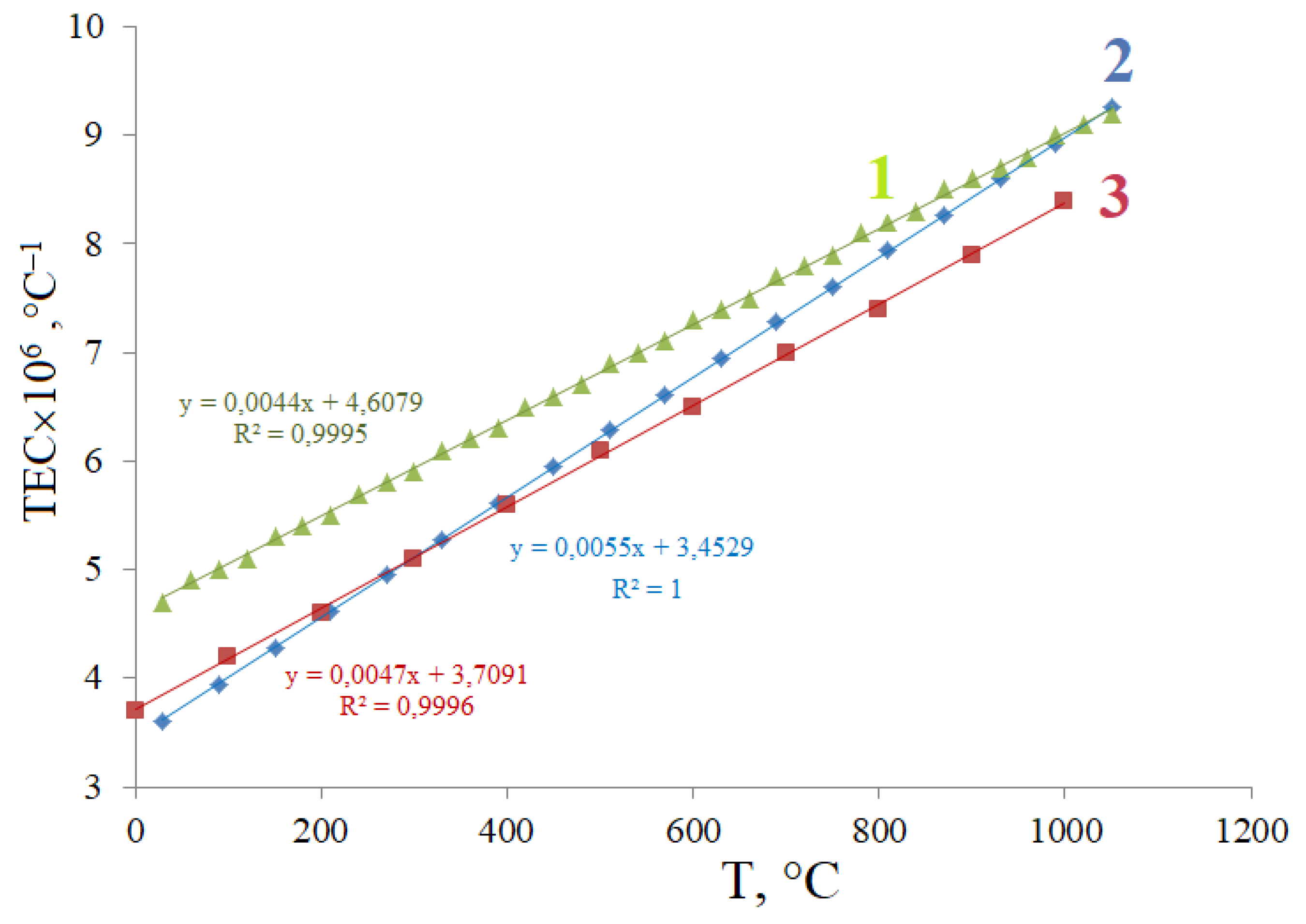
3.2. Thermal Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cann, D.P.; Randall, C.A.; Shrout, T.R. Investigation of the dielectric properties of bismuth pyrochlores. Solid State Commun. 1996, 100, 529–534. [Google Scholar] [CrossRef]
- Khaw, C.; Tan, K.; Lee, C. High temperature dielectric properties of cubic bismuth zinc tantalate. Ceram. Int. 2009, 35, 1473–1480. [Google Scholar] [CrossRef]
- Guo, Q.; Li, L.; Yu, S.; Sun, Z.; Zheng, H.; Li, J.; Luo, W. Temperature–stable dielectrics based on Cu–doped Bi2Mg2/3Nb4/3O7 pyrochlore ceramics for LTCC. Ceram. Int. 2018, 44, 333–338. [Google Scholar] [CrossRef]
- Gharbi, S.; Dhahri, R.; Rasheed, M.; Dhahri, E.; Barille, R.; Rguiti, M.; Tozri, A.; Berber, M.R. Effect of Bi substitution on nanostructural, morphologic, and electrical behavior of nanocrystalline La1-xBixNi0.5Ti0.5O3 (x = 0 and x = 0.2) for the electrical devices. Mater. Sci. Eng. B 2021, 270, 115191. [Google Scholar] [CrossRef]
- Dkhilalli, F.; Megdiche, S.; Guidara, K.; Rasheed, M.; Barillé, R.; Megdiche, M. AC conductivity evolution in bulk and grain boundary response of sodium tungstate Na2BiWO4. Ionics 2018, 24, 169–180. [Google Scholar] [CrossRef]
- Srihari, V.; Verma, A.K.; Pandey, K.K.; Vishwanadh, B.; Panchal, V.; Garg, N.; Errandonea, D. Making Yb2Hf2O7 Defect Fluorite Uncompressible by Particle Size Reduction. J. Phys. Chem. C 2021, 125, 27354–27362. [Google Scholar] [CrossRef]
- Giampaoli, G.; Siritanon, T.; Day, B.; Li, J.; Subramanian, M.A. Temperature in-dependent low loss dielectrics based on quaternary pyrochlore oxides. Prog. Solid State Chem. 2018, 50, 16–23. [Google Scholar] [CrossRef]
- Subramanian, M.A.; Aravamudan, G.; Subba Rao, G.V. Oxide pyrochlores—A review. Prog. Solid State Chem. 1983, 15, 55–143. [Google Scholar] [CrossRef]
- Zhuk, N.; Krzhizhanovskaya, M.; Sekushin, N.; Kharton, V.; Makeev, B.; Belyy, V.; Korolev, R. Dielectric performance of pyrochlore-type Bi2MgNb2-xTaxO9 ceramics: The effects of tantalum doping. Ceram. Int. 2021, 47, 19424–19433. [Google Scholar] [CrossRef]
- Yu, S.; Li, L.; Zheng, H. BMN-based transparent capacitors with high dielectric tunability. J. Alloys Compd. 2017, 699, 68–72. [Google Scholar] [CrossRef]
- Sebastian, M.; Ubic, R.; Jantunen, H. Low-loss dielectric ceramic materials and their properties. Int. Mater. Rev. 2015, 60, 392–412. [Google Scholar] [CrossRef]
- Valant, M.; Babu, G.S.; Vrcon, M.; Kolodiazhnyi, T.; Axelsson, A.-K. Pyrochlore Range from Bi2O3-Fe2O3-TeO3 System for LTCC and Photocatalysis and the Crystal Structure of New Bi3(Fe0.56Te0.44)3O11. J. Am. Ceram. Soc. 2011, 95, 644–650. [Google Scholar] [CrossRef]
- Simonenko, N.P.; Sakharov, K.A.; Simonenko, E.P.; Sevastyanov, V.G.; Kuznetsov, N.T. Glycol–citrate synthesis of ultrafine lanthanum zirconate. Russ. J. Inorg. Chem. 2015, 60, 1452–1458. [Google Scholar] [CrossRef]
- Lufaso, M.W.; Vanderah, T.A.; Pazos, I.M.; Levin, I.; Roth, R.S.; Nino, J.C.; Provenzano, V.; Schenck, P.K. Phase formation, crystal chemistry, and properties in the system Bi2O3–Fe2O3–Nb2O5. J. Solid State Chem. 2006, 179, 3900–3910. [Google Scholar] [CrossRef]
- Jusoh, F.; Tan, K.; Zainal, Z.; Chen, S.; Khaw, C.; Lee, O. Novel pyrochlores in the Bi2O3-Fe2O3-Ta2O5 (BFT) ternary system: Synthesis, structural and electrical properties. J. Mater. Res. Technol. 2020, 9, 11022–11034. [Google Scholar] [CrossRef]
- Jusoh, F.; Tan, K.; Zainal, Z.; Chen, S.; Khaw, C.; Lee, O. Investigation of structural and dielectric properties of subsolidus bismuth iron niobate pyrochlores. J. Asian Ceram. Soc. 2020, 8, 957–969. [Google Scholar] [CrossRef]
- Zhuk, N.; Krzhizhanovskaya, M.; Koroleva, A.; Reveguk, A.; Sivkov, D.; Nekipelov, S. Thermal expansion, crystal structure, XPS and NEXAFS spectra of Fe-doped bismuth tantalate pyrochlore. Ceram. Int. 2022, 48, 14849–14855. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Krzhizhanovskaya, M.G.; Koroleva, A.V.; Nekipelov, S.V.; Kharton, V.V.; Sekushin, N.A. Thermal Expansion, XPS Spectra, and Structural and Electrical Properties of a New Bi2NiTa2O9 Pyrochlore. Inorg. Chem. 2021, 60, 4924–4934. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Krzhizhanovskaya, M.G.; Sekushin, N.A.; Kharton, V.V.; Koroleva, A.V.; Nekipelov, S.V.; Sivkov, D.V.; Sivkov, V.N.; Makeev, B.A.; Lebedev, A.M.; et al. Novel Ni-Doped Bismuth–Magnesium Tantalate Pyrochlores: Structural and Electrical Properties, Thermal Expansion, X-ray Photoelectron Spectroscopy, and Near-Edge X-ray Absorption Fine Structure Spectra. ACS Omega 2021, 6, 23262–23273. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Sekushin, N.A.; Semenov, V.G.; Fedorova, A.V.; Selyutin, A.A.; Krzhizhanovskaya, M.G.; Lutoev, V.P.; Makeev, B.A.; Kharton, V.V.; Sivkov, D.N.; et al. Dielectric properties, Mössbauer study, ESR spectra of Bi2FeTa2O9.5 with pyrochlore structure. J. Alloys Compd. 2022, 903, 163928. [Google Scholar] [CrossRef]
- Bruker AXS, Topas 5.0; General Profile and Structure Analysis Software for Powder Diffraction Data. Bruker AXS GmbH: Karlsruhe, Germany, 2014.
- Bubnova, R.S.; Firsova, V.; Filatov, S. Software for determining the thermal expansion tensor and the graphic representation of its characteristic surface (theta to tensor-TTT). Glas. Phys. Chem. 2013, 39, 347–350. [Google Scholar] [CrossRef]
- Zhuk, N.; Krzhizhanovskaya, M. Thermal expansion of bismuth magnesium tantalate and niobate pyrochlores. Ceram. Int. 2021, 47, 30099–30105. [Google Scholar] [CrossRef]
- Egorysheva, A.V.; Ellert, O.G.; Maksimov, Y.V.; Volodin, V.D.; Efimov, N.N.; Novotortsev, V.M. Subsolidus phase equilibria and magnetic characterization of the pyrochlore in the Bi2O3–Fe2O3–Sb2Ox system. J. Alloys Compd. 2013, 579, 311–314. [Google Scholar] [CrossRef]
- Lomakin, M.S.; Proskurina, O.V.; Sergeev, A.A.; Buryanenko, I.V.; Semenov, V.G.; Voznesenskiy, S.S.; Gusarov, V.V. Crystal structure and optical properties of the Bi–Fe–W–O pyrochlore phase synthesized via a hydrothermal method. J. Alloys Compd. 2021, 889, 161598. [Google Scholar] [CrossRef]
- Whitaker, M.J.; Marco, J.F.; Berry, F.J.; Raith, C.; Blackburn, E.; Greaves, C. Structural and magnetic characterisation of the pyrochlores Bi2−xFex(FeSb)O7, (x = 0.1, 0.2, 0.3), Nd1.8Fe0.2(FeSb)O7 and Pr2(FeSb)O7. J. Solid State Chem. 2013, 198, 316–322. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–766. [Google Scholar] [CrossRef]
- Jusoh, F.; Tan, K.; Zainal, Z.; Chen, S.; Khaw, C.; Lee, O.; Balachandran, R.; Murthy, H.A. Novel pyrochlore-structured bismuth iron antimonates: Structural, impedance and electrochemical studies. Results Phys. 2021, 27, 104542. [Google Scholar] [CrossRef]
- Filatov, S.K. Some structural-geometric regularities of crystal deformations under temperature, pressure, and chemical composition. Crystallography and Crystal Chemistry. Krist. Krist. 1973, 2, 5–12. (In Russian) [Google Scholar]
- Hazen, R.M. Temperature, pressure and composition: Structurally analogous variables. Phys. Chem. Miner. 1977, 1, 83–94. [Google Scholar] [CrossRef]
- Hazen, R.M.; Finger, L.W. Comparative Crystal Chemistry: Temperature, Pressure, and the Variation of Crystal Structure; John Wiley & Sons: New York, NY, USA, 1982. [Google Scholar]
- Filatov, S.K.; Paufler, P.; Georgievskaya, M.I.; Levin, A.A.; Meyer, D.C.; Bubnova, R.S. Crystal formation from glass, crystal structure refinement and thermal behavior of K1-xRbxBSi2O6 boroleucite solid solutions from X-ray powder diffraction data. Z. Kristallogr. 2011, 226, 602. [Google Scholar] [CrossRef]
- Krzhizhanovskaya, M.G.; Bubnova, R.S.; Filatov, S.K.; Meyer, D.C.; Paufler, P. Crystal structure and thermal behaviour of (Rb,Cs)BSi2O6 solid solutions. Cryst. Res. Technol. 2006, 41, 285–292. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Krzhizhanovskaya, M.G.; Belyy, V.A.; Kharton, V.V.; Chichineva, A.I. Phase transformations and thermal expansion of α- and β-BiTaO4 and the high-temperature modification γ-BiTaO4. Chem. Mater. 2020, 32, 5493–5501. [Google Scholar] [CrossRef]
- Shukla, R.; Vasundhara, K.; Krishna, P.; Shinde, A.; Sali, S.; Kulkarni, N.; Achary, S.; Tyagi, A. High temperature structural and thermal expansion behavior of pyrochlore-type praseodymium zirconate. Int. J. Hydrogen. Energy 2015, 40, 15672–15678. [Google Scholar] [CrossRef]
- Raison, P.E.; Pavel, C.C.; Jardin, R.; Suard, E.; Haire, R.G.; Popa, K. Thermal expansion behavior of Ce2Zr2O7 up to 898 K in conjunction with structural analyses by neutron diffraction. Phys. Chem. Miner. 2010, 37, 555–559. [Google Scholar] [CrossRef]
- Feng, J.; Xiao, B.; Zhou, R.; Pan, W. Thermal expansions of Ln2Zr2O7 (Ln = La, Nd, Sm, and Gd) pyrochlore. J. Appl. Phys. 2012, 111, 103535. [Google Scholar] [CrossRef]
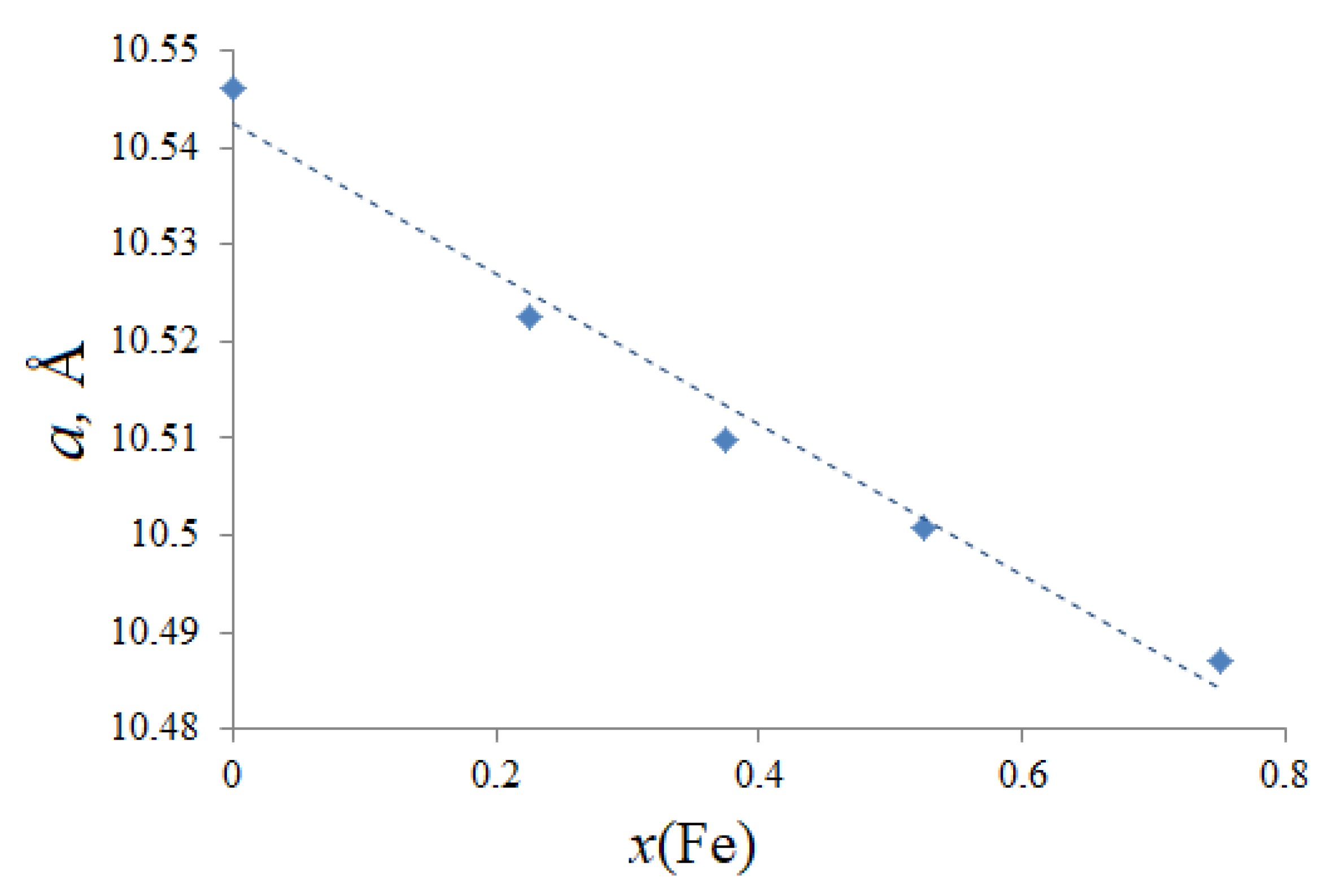

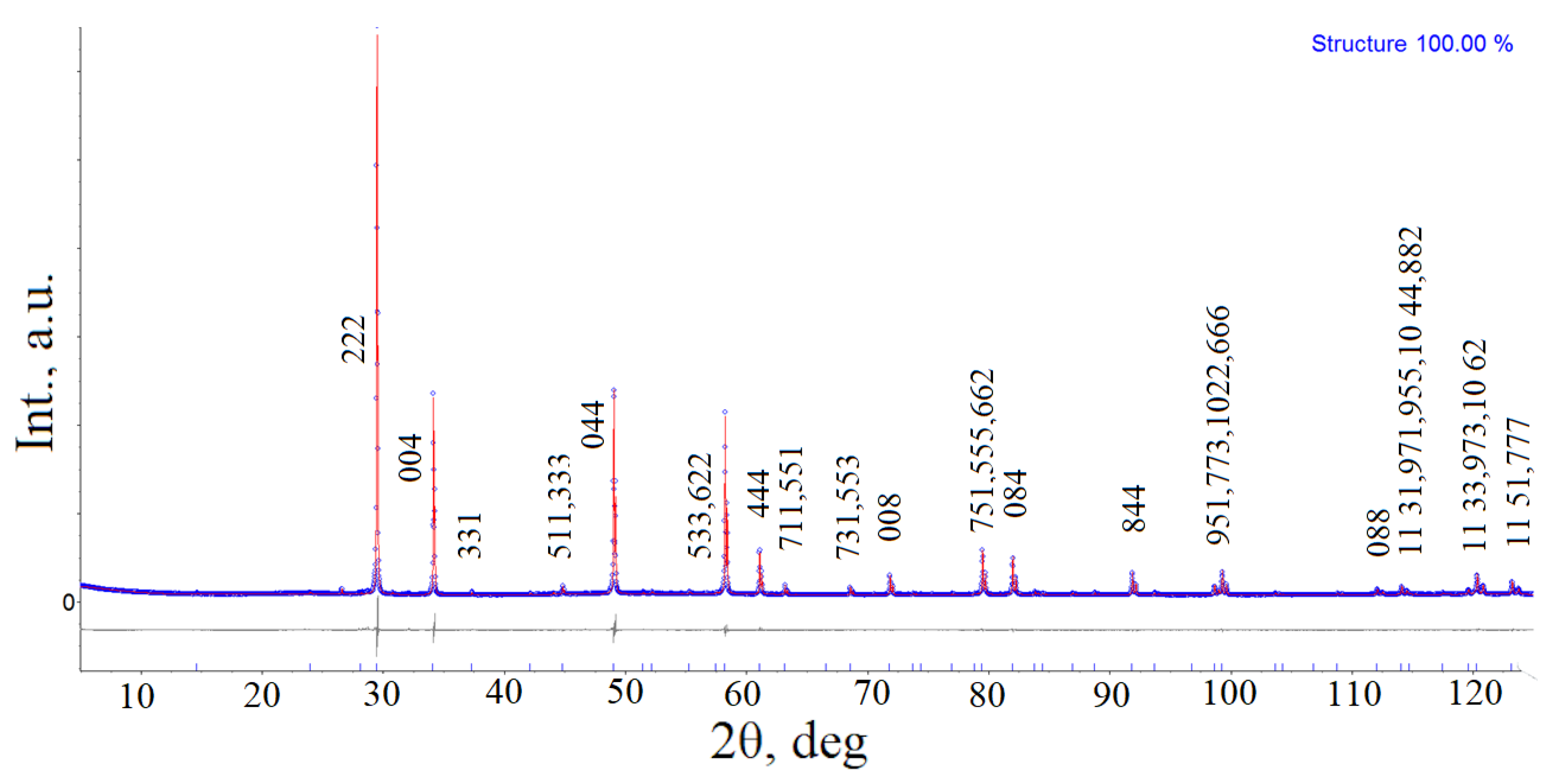
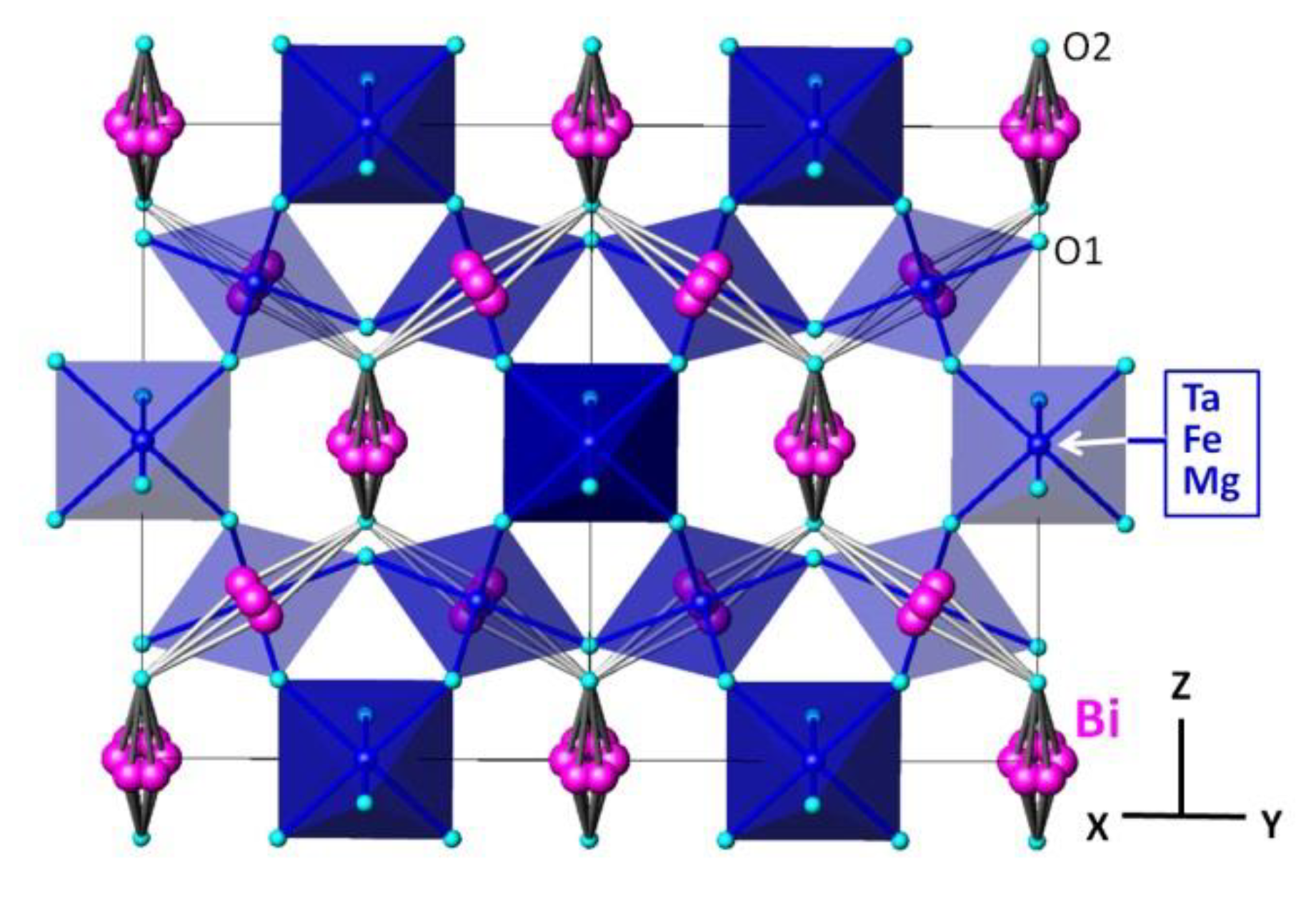
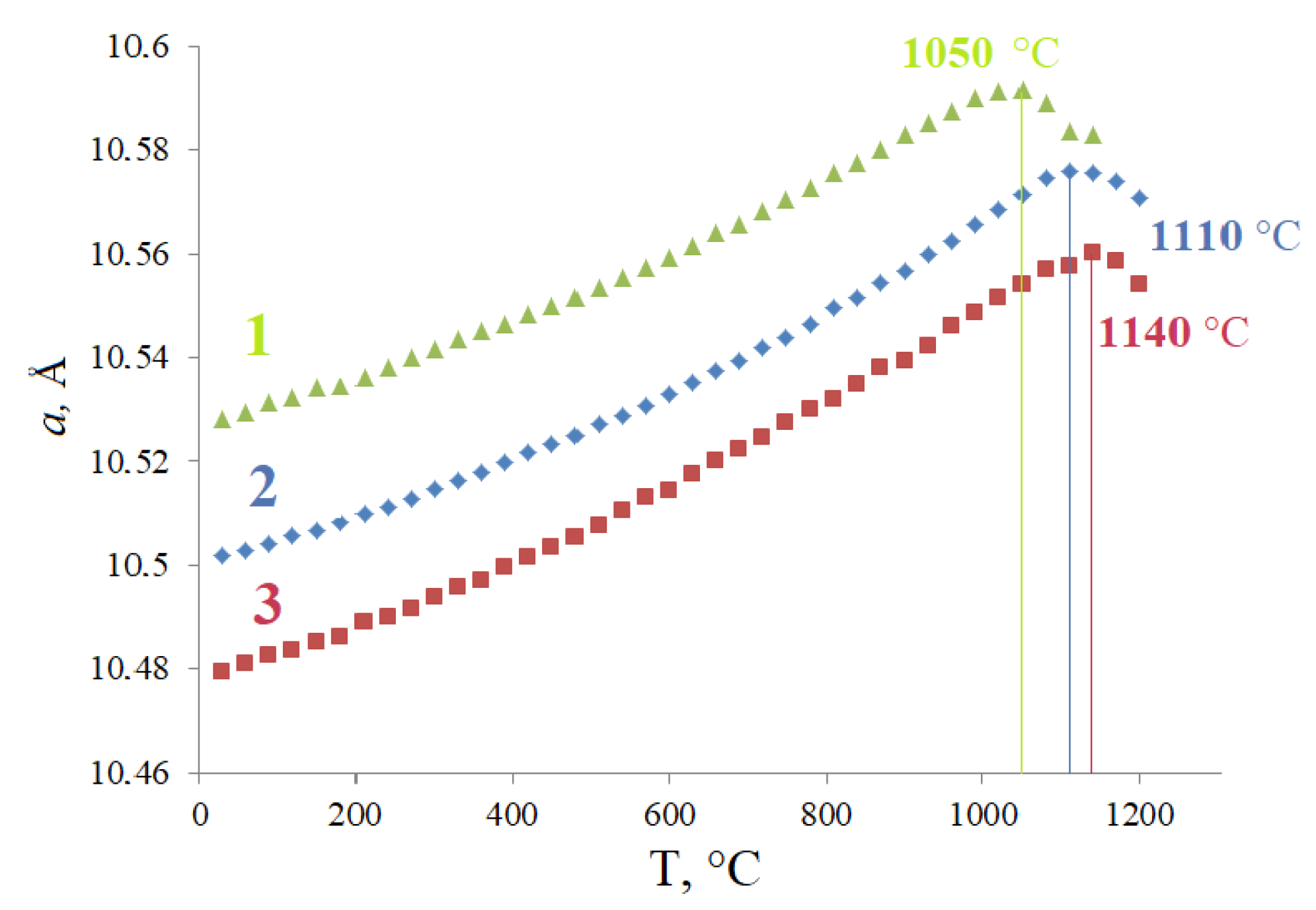
| a (Å) | 10.51039(4) |
| α, β, γ (°) | 90, 90, 90 |
| V (Å3) | 1161.064(12) |
| Dcalc (g/cm3) | 7.523(2) |
| RB (%) | 0.57 |
| Rwp (%) | 3.63 |
| Rp (%) | 2.63 |
| Rexp (%) | 2.16 |
| GOF | 1.68 |
| Atom | Wyckoff Site | x | y | z | SOF | Biso,Å2 |
|---|---|---|---|---|---|---|
| Bi | 96g | 0 | −0.0252(1) | 0.0252(1) | 0.113(1) | 1.00(7) |
| Ta | 16b | 0.5000 | 0.5000 | 0.5000 | 0.667(6) | 0.62(3) |
| Fe | 16b | 0.5000 | 0.5000 | 0.5000 | 0.167(6) | 0.62(3) |
| Mg | 16b | 0.5000 | 0.5000 | 0.5000 | 0.167(6) | 0.62(3) |
| O1 | 48f | 0.1250 | 0.1250 | 0.4306(5) | 1 | 1.9(2) |
| O2 | 8a | 0.1250 | 0.1250 | 0.1250 | 0.69(3) | 1.9(2) |
| Bond | Length (Å) |
|---|---|
| Bi1–O1 × 2 | 2.3062(2) |
| –O1 × 2 | 2.344(4) |
| –O1 × 2 | 2.683(3) |
| –O2 × 2 | 2.983(4) |
| <Bi1VIII–O> | 2.57905 |
| Ta1–O1 × 6 | 1.9959(16) |
| <Ta1VI–O> | 2.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuk, N.A.; Krzhizhanovskaya, M.G.; Nekipelov, S.V.; Sivkov, V.N.; Sivkov, D.V. Effect of Fe-Doping on Thermal Expansion and Stability of Bismuth Magnesium Tantalate Pyrochlorere. Materials 2022, 15, 7668. https://doi.org/10.3390/ma15217668
Zhuk NA, Krzhizhanovskaya MG, Nekipelov SV, Sivkov VN, Sivkov DV. Effect of Fe-Doping on Thermal Expansion and Stability of Bismuth Magnesium Tantalate Pyrochlorere. Materials. 2022; 15(21):7668. https://doi.org/10.3390/ma15217668
Chicago/Turabian StyleZhuk, Nadezhda A., Maria G. Krzhizhanovskaya, Sergey V. Nekipelov, Viktor N. Sivkov, and Danil V. Sivkov. 2022. "Effect of Fe-Doping on Thermal Expansion and Stability of Bismuth Magnesium Tantalate Pyrochlorere" Materials 15, no. 21: 7668. https://doi.org/10.3390/ma15217668
APA StyleZhuk, N. A., Krzhizhanovskaya, M. G., Nekipelov, S. V., Sivkov, V. N., & Sivkov, D. V. (2022). Effect of Fe-Doping on Thermal Expansion and Stability of Bismuth Magnesium Tantalate Pyrochlorere. Materials, 15(21), 7668. https://doi.org/10.3390/ma15217668





