Octahedral Tilting in Homologous Perovskite Series CaMoO3-SrMoO3-BaMoO3 Probed by Temperature-Dependent EXAFS Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
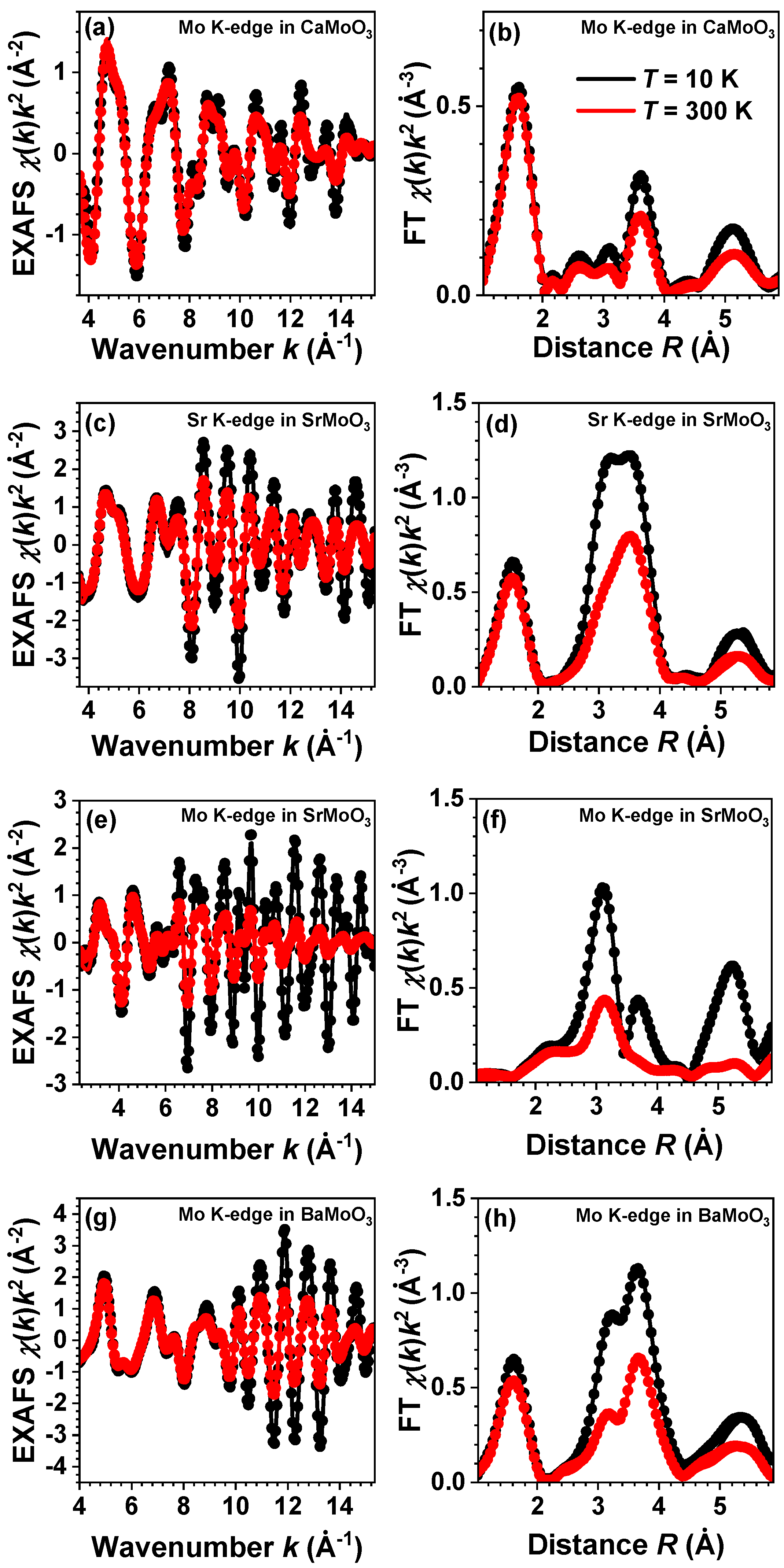
3. Results and Discussion
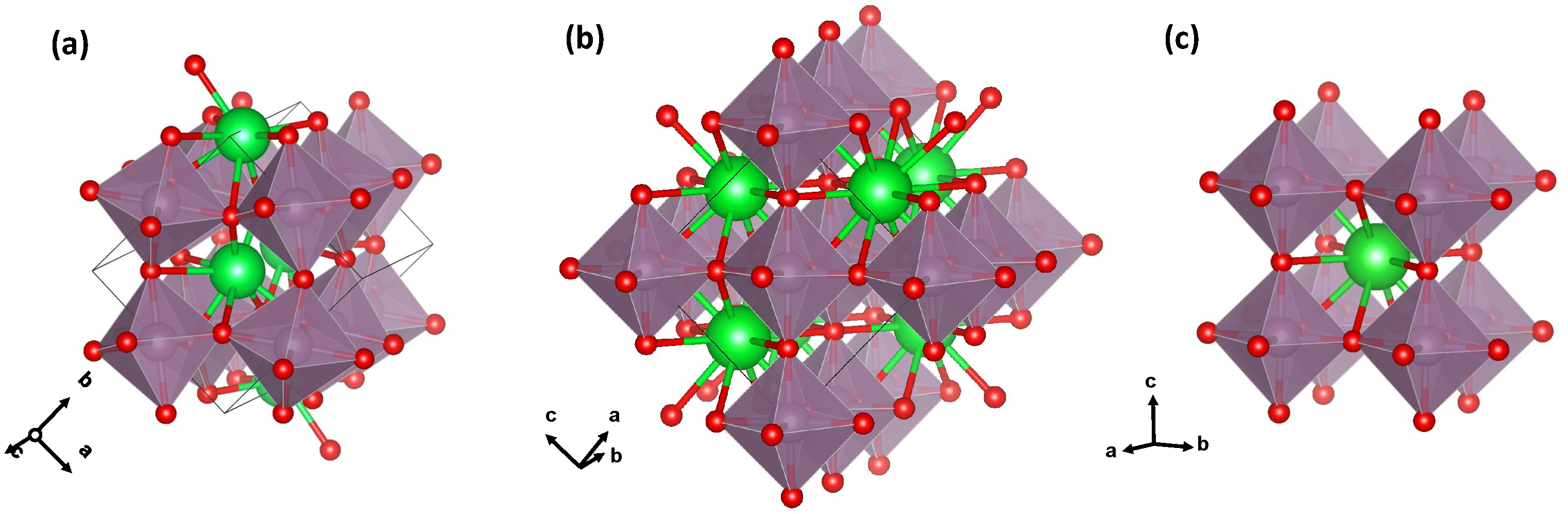
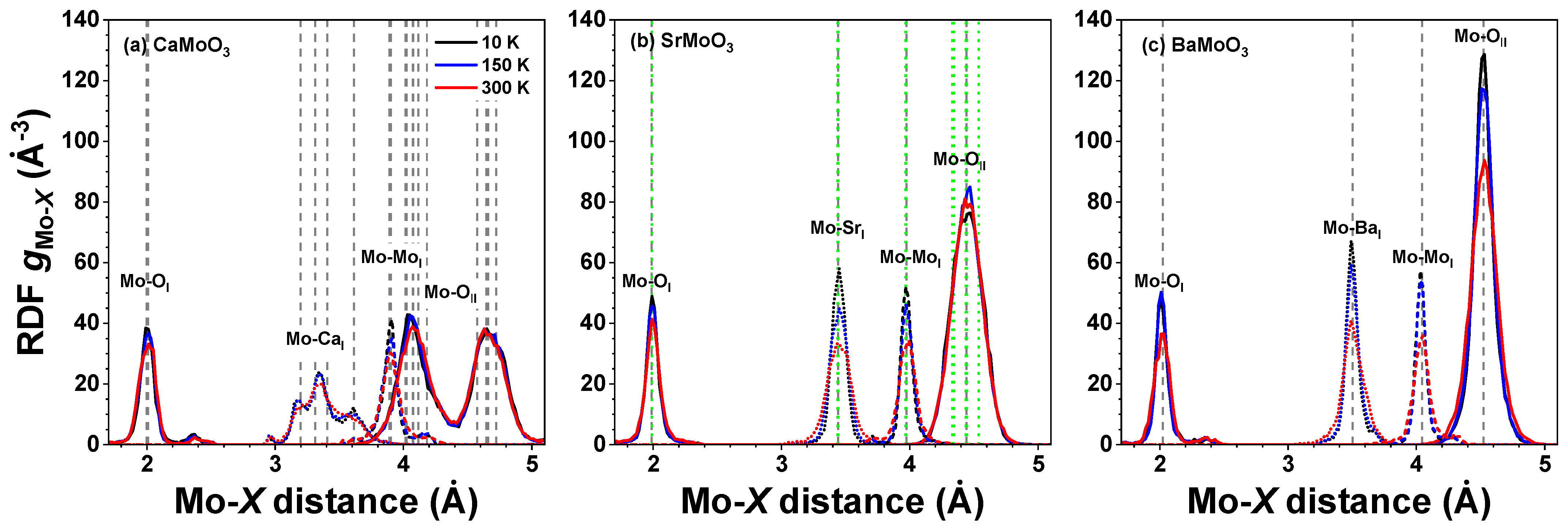
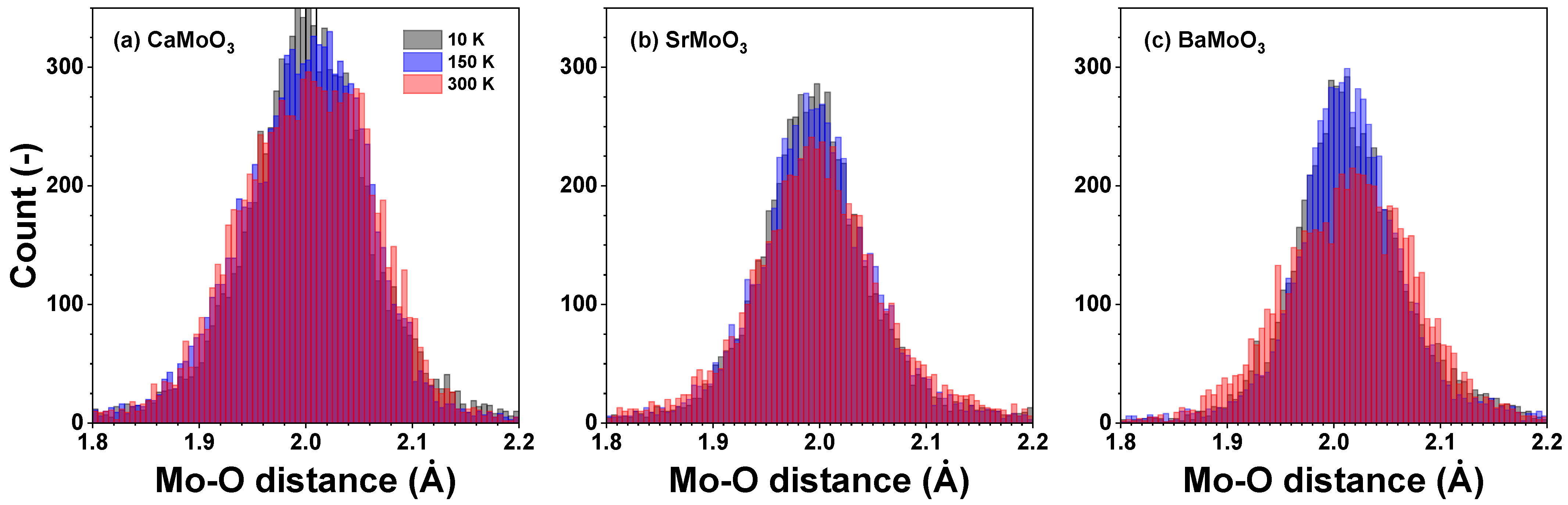
3.1. Analysis of RDFs
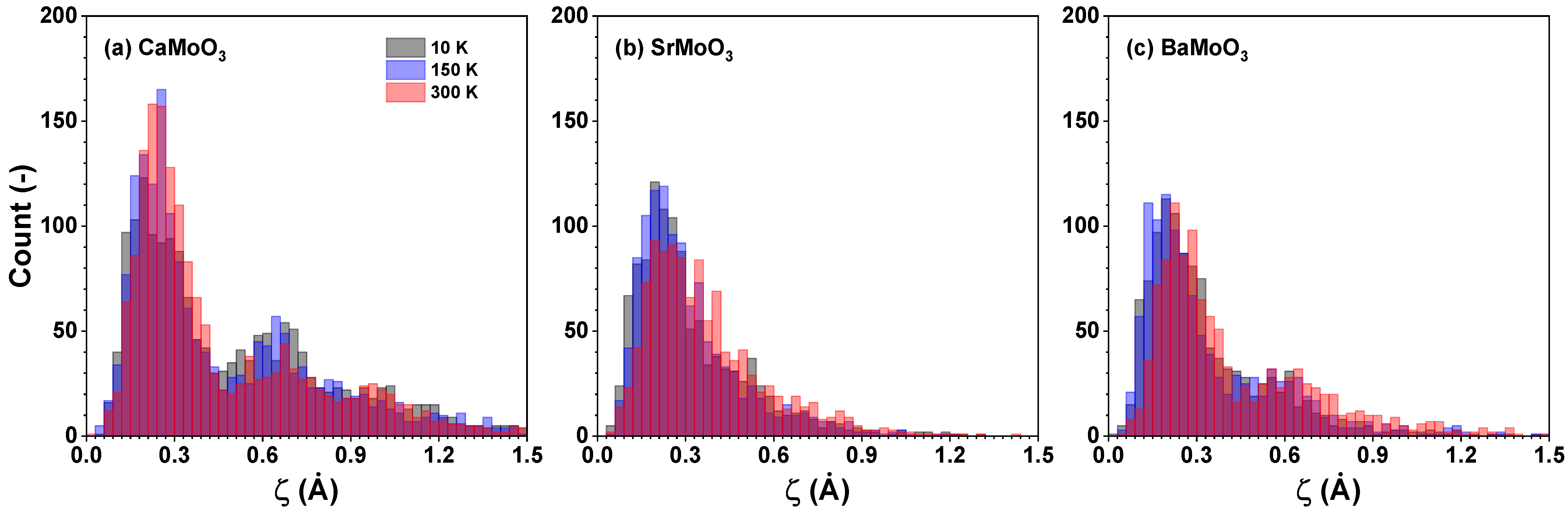
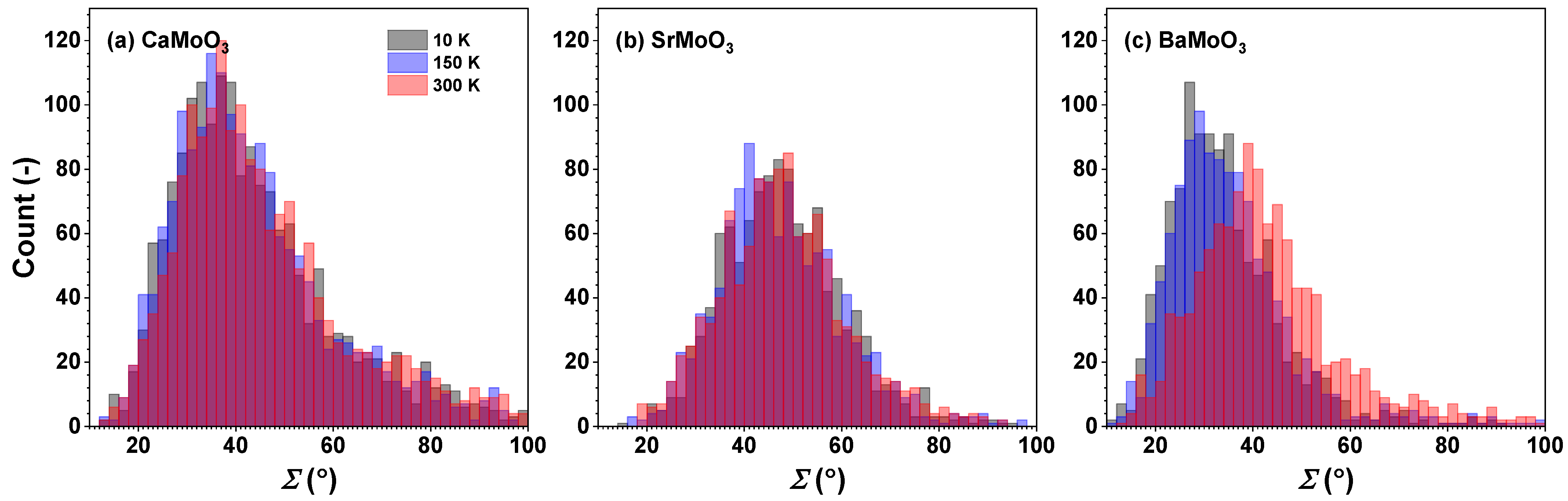
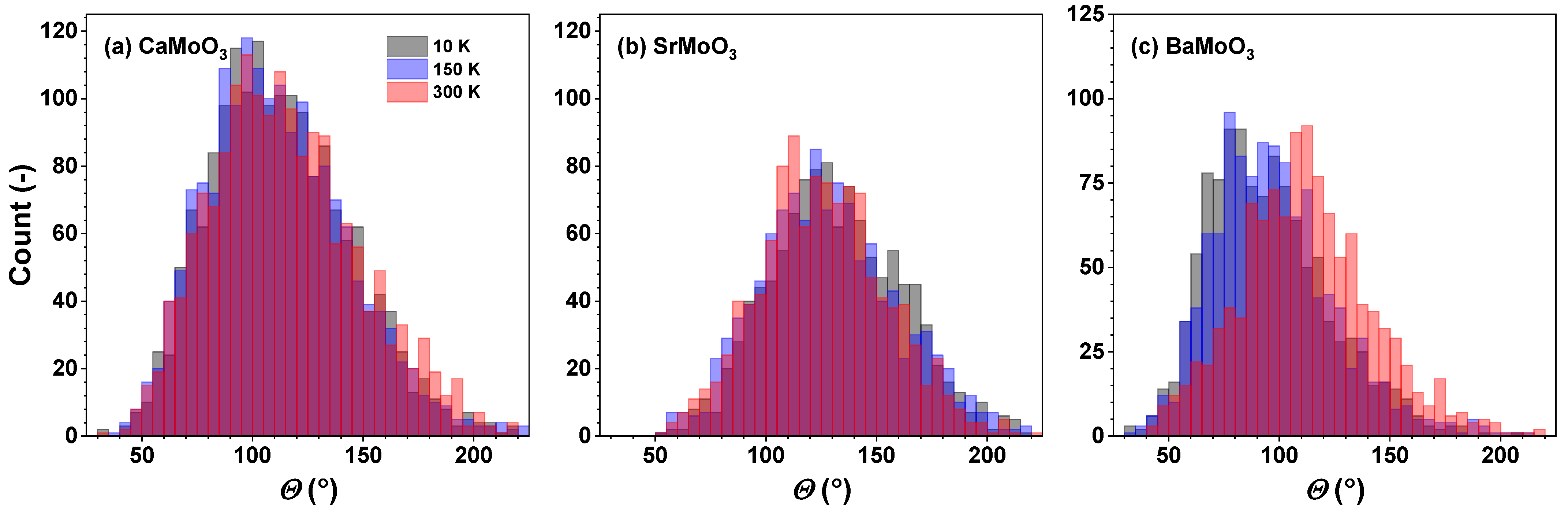
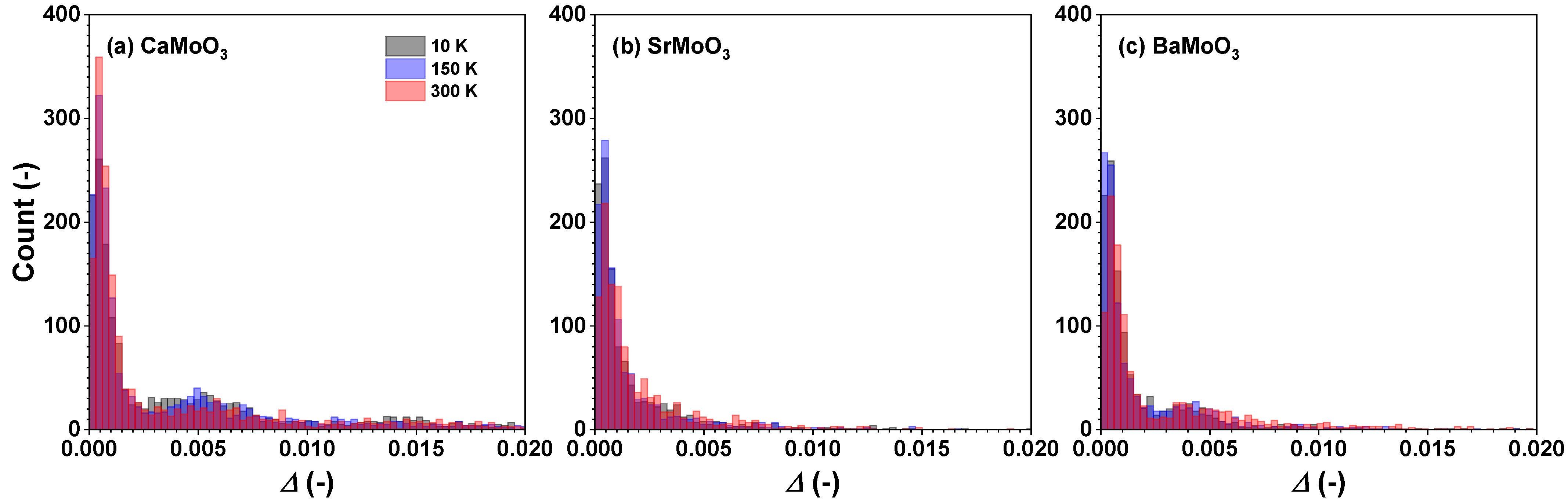
3.2. Polyhedral Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADF | angular distribution function |
| CA | configuration-averaged |
| EA | evolutionary algorithm |
| EXAFS | extended X-ray absorption fine structure |
| FT | Fourier transform |
| MT | muffin-tin |
| RDF | radial distribution function |
| RMC | reverse Monte Carlo |
| XAS | X-ray absorption spectroscopy |
References
- Ma, T.; Jacobs, R.; Booske, J.; Morgan, D. Discovery and engineering of low work function perovskite materials. J. Mater. Chem. C 2021, 9, 12778–12790. [Google Scholar] [CrossRef]
- Nagai, I.; Shirakawa, N.; Ikeda, S.i.; Iwasaki, R.; Nishimura, H.; Kosaka, M. Highest conductivity oxide SrMoO3 grown by a floating-zone method under ultralow oxygen partial pressure. Appl. Phys. Lett. 2005, 87, 024105. [Google Scholar] [CrossRef]
- Kamata, K.; Nakamura, T.; Sata, T. Synthesis and properties of the metallic molybdate (IV) CaMoO3. Chem. Lett. 1975, 4, 81–86. [Google Scholar] [CrossRef]
- Radetinac, A.; Takahashi, K.S.; Alff, L.; Kawasaki, M.; Tokura, Y. Single-crystalline CaMoO3 and SrMoO3 films grown by pulsed laser deposition in a reductive atmosphere. Appl. Phys. Express 2010, 3, 073003. [Google Scholar] [CrossRef]
- Stoner, J.L.; Murgatroyd, P.A.E.; O’Sullivan, M.; Dyer, M.S.; Manning, T.D.; Claridge, J.B.; Rosseinsky, M.J.; Alaria, J. Chemical Control of Correlated Metals as Transparent Conductors. Adv. Funct. Mater. 2019, 29, 1808609. [Google Scholar] [CrossRef]
- Brixner, L. X-ray study and electrical properties of system BaxSr(1-x)MoO3. J. Inorg. Nucl. Chem. 1960, 14, 225–230. [Google Scholar] [CrossRef]
- Ferretti, A.; Rogers, D.; Goodenough, J. The relation of the electrical conductivity in single crystals of rhenium trioxide to the conductivities of Sr2MgReO6 and NaxWO3. J. Phys. Chem. Solids 1965, 26, 2007–2011. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Yamanaka, S.; Kurosaki, K.; Maekawa, T.; Matsuda, T.; Kobayashi, S.i.; Uno, M. Thermochemical and thermophysical properties of alkaline-earth perovskites. J. Nucl. Mater. 2005, 344, 61–66. [Google Scholar] [CrossRef]
- Wang, S.; Mohammadi, M.; Dirba, I.; Hofmann, K.; Albert, B.; Alff, L.; Komissinskiy, P.; Molina-Luna, L. Molecular dynamics simulation of crystal structure and heat capacity in perovskite-type molybdates SrMoO3 and BaMoO3. Comput. Mater. Sci. 2021, 197, 110609. [Google Scholar] [CrossRef]
- Macquart, R.B.; Kennedy, B.J.; Avdeev, M. Neutron diffraction study of phase transitions in perovskite-type strontium molybdate SrMoO3. J. Solid State Chem. 2010, 183, 250–255. [Google Scholar] [CrossRef]
- de la Calle, C.; Alonso, J.; García-Hernández, M.; Pomjakushin, V. Neutron diffraction study and magnetotransport properties of stoichiometric CaMoO3 perovskite prepared by a soft-chemistry route. J. Solid State Chem. 2006, 179, 1636–1641. [Google Scholar] [CrossRef]
- Woodward, P.M. Octahedral tilting in perovskites. I. Geometrical considerations. Acta Cryst. B 1997, 53, 32–43. [Google Scholar] [CrossRef]
- Woodward, P.M. Octahedral Tilting in Perovskites. II. Structure Stabilizing Forces. Acta Cryst. B 1997, 53, 44–66. [Google Scholar] [CrossRef]
- Cammarata, A.; Rondinelli, J.M. Covalent dependence of octahedral rotations in orthorhombic perovskite oxides. J. Chem. Phys. 2014, 141, 114704. [Google Scholar] [CrossRef]
- Megaw, H. Crystal Structures: A Working Approach; Studies in Physics and Chemistry; TechBooks: Fairfax, VA, USA, 1973. [Google Scholar]
- Guzmán-Verri, G.; Brierley, R.; Littlewood, P. Cooperative elastic fluctuations provide tuning of the metal–insulator transition. Nature 2019, 576, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, E.; Hampel, A.; Chikina, A.; Guedes, E.B.; Gatti, G.; Hunter, A.; Issing, J.; Biskup, N.; Varela, M.; Dreyer, C.E.; et al. Electronic structure of the highly conductive perovskite oxide SrMoO3. Phys. Rev. Mater. 2022, 6, 075002. [Google Scholar] [CrossRef]
- Hampel, A.; Lee-Hand, J.; Georges, A.; Dreyer, C.E. Correlation-induced octahedral rotations in SrMoO3. Phys. Rev. B 2021, 104, 035102. [Google Scholar] [CrossRef]
- Krayzman, V.; Levin, I.; Woicik, J.C.; Proffen, T.; Vanderah, T.A.; Tucker, M.G. A combined fit of total scattering and extended X-ray absorption fine structure data for local-structure determination in crystalline materials. J. Appl. Crystallogr. 2009, 42, 867–877. [Google Scholar] [CrossRef]
- Vailionis, A.; Boschker, H.; Siemons, W.; Houwman, E.P.; Blank, D.H.A.; Rijnders, G.; Koster, G. Misfit strain accommodation in epitaxial ABO3 perovskites: Lattice rotations and lattice modulations. Phys. Rev. B 2011, 83, 064101. [Google Scholar] [CrossRef]
- Vitova, T.; Mangold, S.; Paulmann, C.; Gospodinov, M.; Marinova, V.; Mihailova, B. X-ray absorption spectroscopy of Ru-doped relaxor ferroelectrics with a perovskite-type structure. Phys. Rev. B 2014, 89, 144112. [Google Scholar] [CrossRef]
- Levin, I.; Krayzman, V.; Woicik, J.C. Local structure in perovskite BaSrTiO3: Reverse Monte Carlo refinements from multiple measurement techniques. Phys. Rev. B 2014, 89, 024106. [Google Scholar] [CrossRef]
- Levin, I.; Krayzman, V.; Playford, H.; Woicik, J.; Maier, R.; Lu, Z.; Bruma, A.; Eremenko, M.; Tucker, M. The mediation of bond strain by vacancies and displacive disorder in A-site-deficient perovskites. Acta Mater. 2021, 207, 116678. [Google Scholar] [CrossRef]
- Timoshenko, J.; Kuzmin, A.; Purans, J. EXAFS study of hydrogen intercalation into ReO3 using the evolutionary algorithm. J. Phys. Condens. Matter 2014, 26, 055401. [Google Scholar] [CrossRef] [PubMed]
- Welter, E.; Chernikov, R.; Herrmann, M.; Nemausat, R. A beamline for bulk sample x-ray absorption spectroscopy at the high brilliance storage ring PETRA III. AIP Conf. Proc. 2019, 2054, 040002. [Google Scholar] [CrossRef]
- Kuzmin, A.; Chaboy, J. EXAFS and XANES analysis of oxides at the nanoscale. IUCrJ 2014, 1, 571–589. [Google Scholar] [CrossRef]
- Timoshenko, J.; Kuzmin, A.; Purans, J. Reverse Monte Carlo modeling of thermal disorder in crystalline materials from EXAFS spectra. Comp. Phys. Commun. 2012, 183, 1237–1245. [Google Scholar] [CrossRef]
- Anspoks, A.; Marini, C.; Miyanaga, T.; Joseph, B.; Kuzmin, A.; Purans, J.; Timoshenko, J.; Bussmann-Holder, A. Local structure of A-atom in ABO3 perovskites studies by RMC-EXAFS. Rad. Phys. Chem. 2020, 175, 108072. [Google Scholar] [CrossRef]
- Timoshenko, J.; Anspoks, A.; Kalinko, A.; Kuzmin, A. Thermal disorder and correlation effects in anti-perovskite-type copper nitride. Acta Mater. 2017, 129, 61–71. [Google Scholar] [CrossRef]
- Kotomin, E.A.; Kuzmin, A.; Purans, J.; Timoshenko, J.; Piskunov, S.; Merkle, R.; Maier, J. Theoretical and experimental studies of charge ordering in CaFeO3 and SrFeO3 crystals. Phys. Status Solidi 2022, 259, 2100238. [Google Scholar] [CrossRef]
- Timoshenko, J.; Kuzmin, A. Wavelet data analysis of EXAFS spectra. Comp. Phys. Commun. 2009, 180, 920–925. [Google Scholar] [CrossRef]
- Ankudinov, A.L.; Ravel, B.; Rehr, J.J.; Conradson, S.D. Real-space multiple-scattering calculation and interpretation of x-ray-absorption near-edge structure. Phys. Rev. B 1998, 58, 7565–7576. [Google Scholar] [CrossRef]
- Rehr, J.J.; Albers, R.C. Theoretical approaches to x-ray absorption fine structure. Rev. Mod. Phys. 2000, 72, 621–654. [Google Scholar] [CrossRef]
- Hedin, L.; Lundqvist, B.I. Explicit local exchange-correlation potentials. J. Phys. C Solid State Phys. 1971, 4, 2064. [Google Scholar] [CrossRef]
- Yang, Y.; Kawazoe, Y. Characterization of zero-point vibration in one-component crystals. EPL 2012, 98, 66007. [Google Scholar] [CrossRef]
- Ertl, A.; Hughes, J.; Pertlik, F.; Foit, F.; Wright, S.; Brandstatter, F.; Marler, B. Polyhedron distortions in tourmaline. Canad. Mineral. 2002, 40, 153–162. [Google Scholar] [CrossRef]
- Ketkaew, R.; Tantirungrotechai, Y.; Harding, P.; Chastanet, G.; Guionneau, P.; Marchivie, M.; Harding, D.J. OctaDist: A tool for calculating distortion parameters in spin crossover and coordination complexes. Dalton Trans. 2021, 50, 1086–1096. [Google Scholar] [CrossRef]
- Cumby, J.; Attfield, J.P. Ellipsoidal analysis of coordination polyhedra. Nat. Commun. 2017, 8, 14235. [Google Scholar] [CrossRef]
- Hui, Q.; Tucker, M.G.; Dove, M.T.; Wells, S.A.; Keen, D.A. Total scattering and reverse Monte Carlo study of the 105 K displacive phase transition in strontium titanate. J. Phys. Condens. Matter 2005, 17, S111–S124. [Google Scholar] [CrossRef]
- Bocharov, D.; Krack, M.; Rafalskij, Y.; Kuzmin, A.; Purans, J. Ab initio molecular dynamics simulations of negative thermal expansion in ScF3: The effect of the supercell size. Comp. Mater. Sci. 2020, 171, 109198. [Google Scholar] [CrossRef]
- Siwach, P.K.; Singh, H.K.; Srivastava, O.N. Low field magnetotransport in manganites. J. Phys. Condens. Matter 2008, 20, 273201. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakradze, G.; Kuzmin, A. Octahedral Tilting in Homologous Perovskite Series CaMoO3-SrMoO3-BaMoO3 Probed by Temperature-Dependent EXAFS Spectroscopy. Materials 2022, 15, 7619. https://doi.org/10.3390/ma15217619
Bakradze G, Kuzmin A. Octahedral Tilting in Homologous Perovskite Series CaMoO3-SrMoO3-BaMoO3 Probed by Temperature-Dependent EXAFS Spectroscopy. Materials. 2022; 15(21):7619. https://doi.org/10.3390/ma15217619
Chicago/Turabian StyleBakradze, Georgijs, and Alexei Kuzmin. 2022. "Octahedral Tilting in Homologous Perovskite Series CaMoO3-SrMoO3-BaMoO3 Probed by Temperature-Dependent EXAFS Spectroscopy" Materials 15, no. 21: 7619. https://doi.org/10.3390/ma15217619
APA StyleBakradze, G., & Kuzmin, A. (2022). Octahedral Tilting in Homologous Perovskite Series CaMoO3-SrMoO3-BaMoO3 Probed by Temperature-Dependent EXAFS Spectroscopy. Materials, 15(21), 7619. https://doi.org/10.3390/ma15217619






