Influence of rGO on the Crystallization Kinetics, Cytoxicity, and Electrical and Mechanical Properties of Poly (L-lactide-co-ε-caprolactone) Scaffolds
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of Scaffolds
2.2. Scanning Electron Microscopy (SEM)
2.3. Electrical Conductivity
2.4. Differential Scanning Calorimetry (DSC)
2.5. Mechanical Characterization
2.6. Cytotoxicity
3. Results and Discussion
3.1. Morphology
3.2. Electical Conductivity Studies
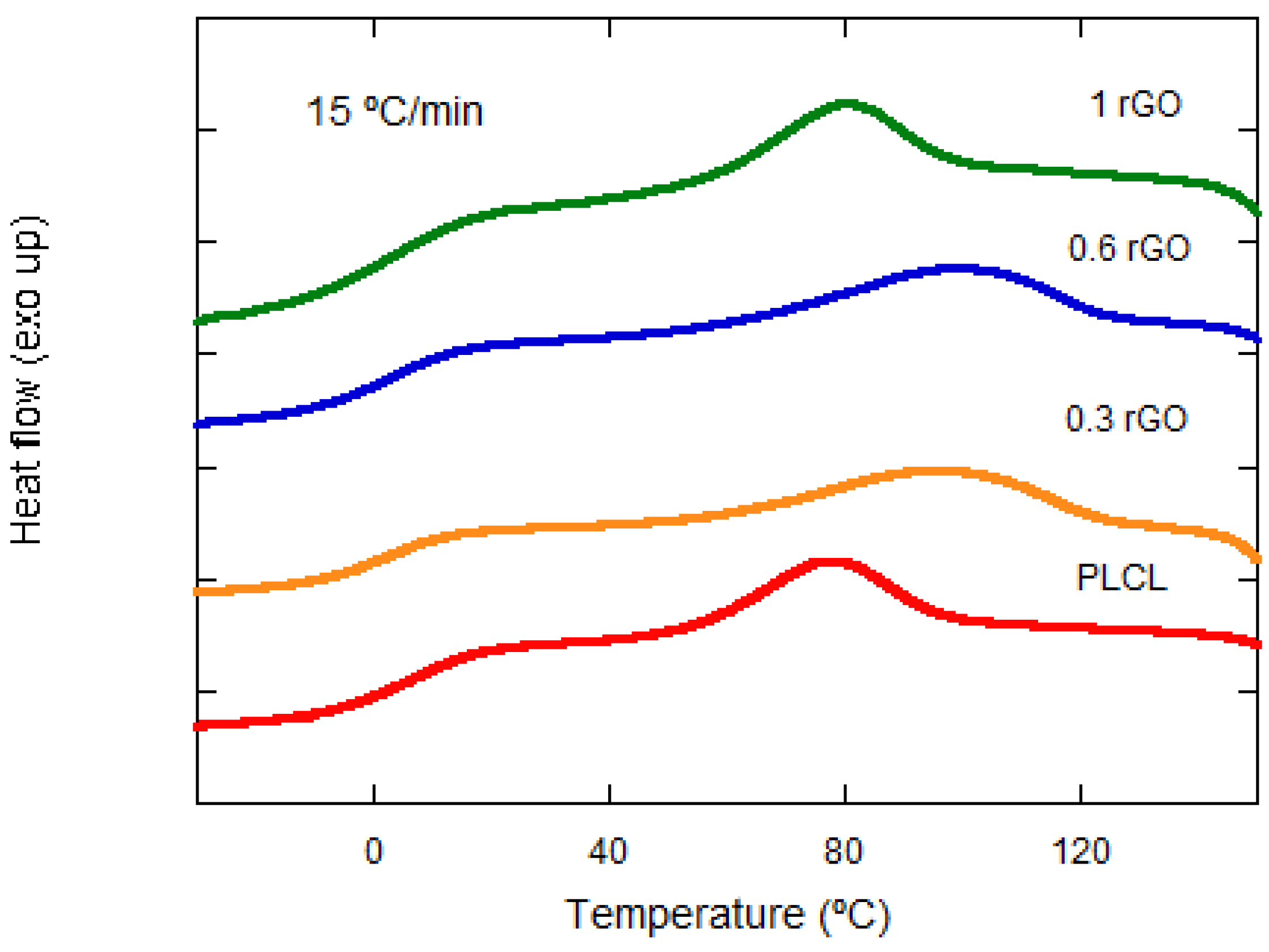
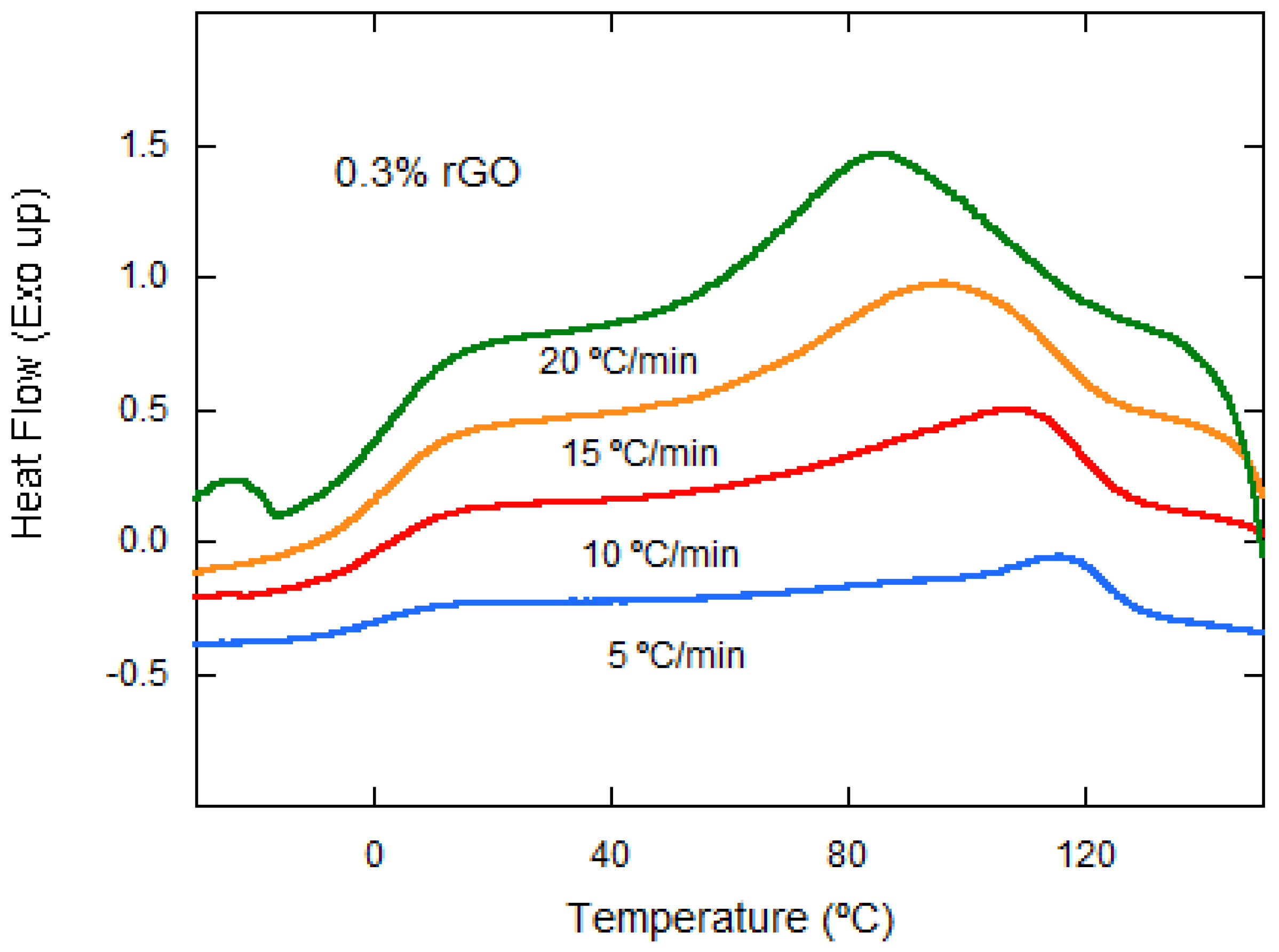
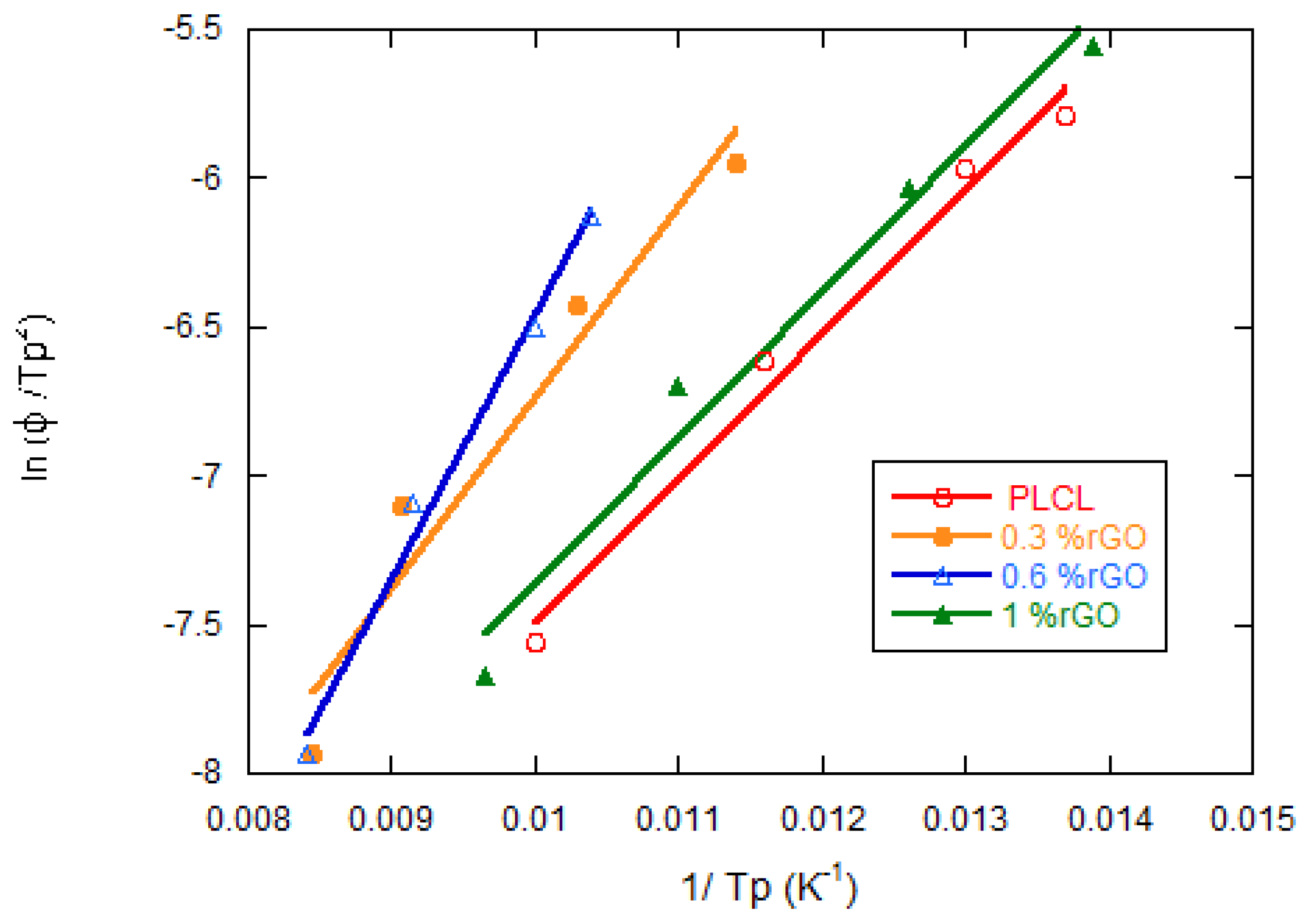
3.3. Thermal Properties
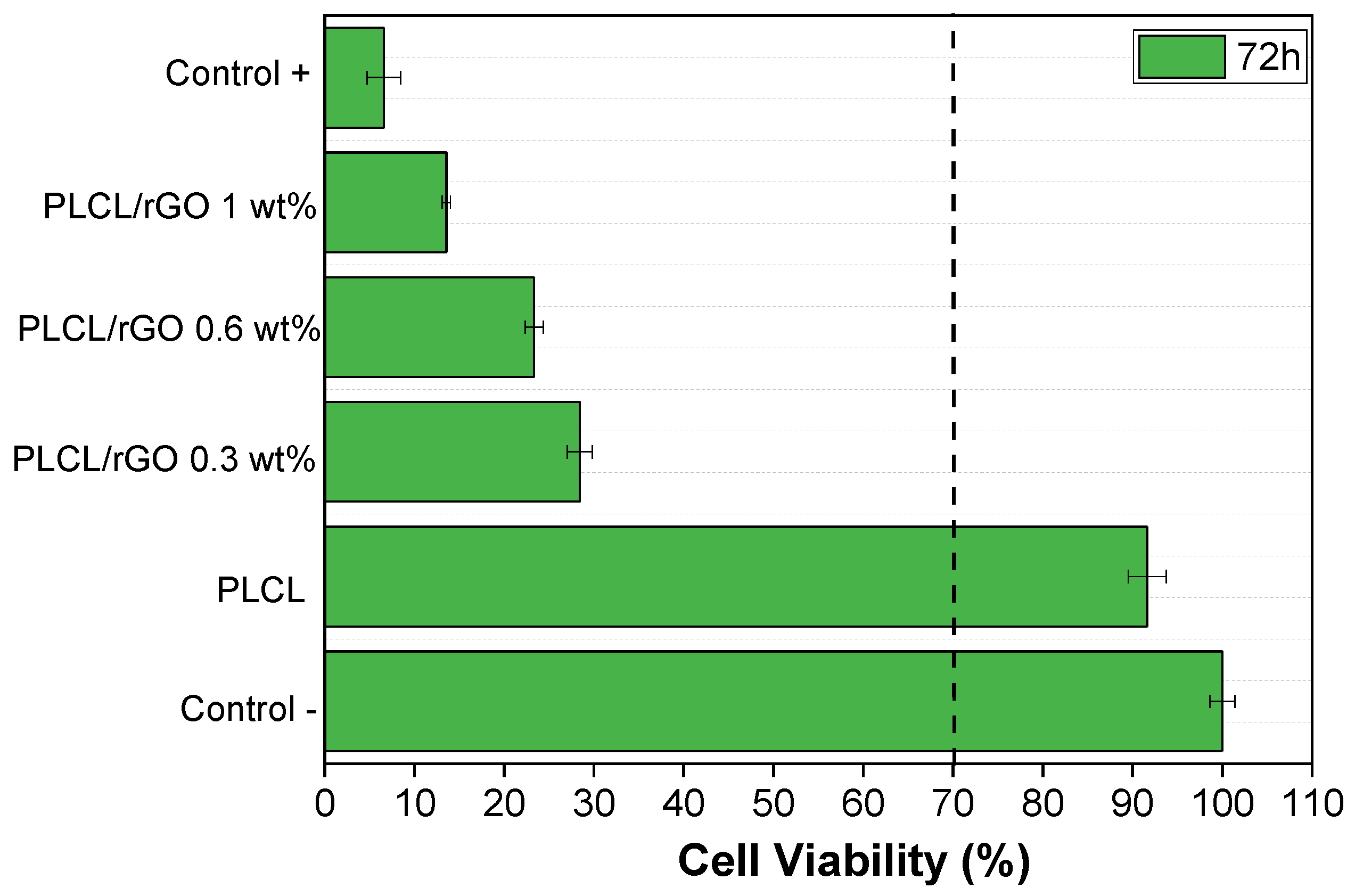
3.4. Cytotoxicity
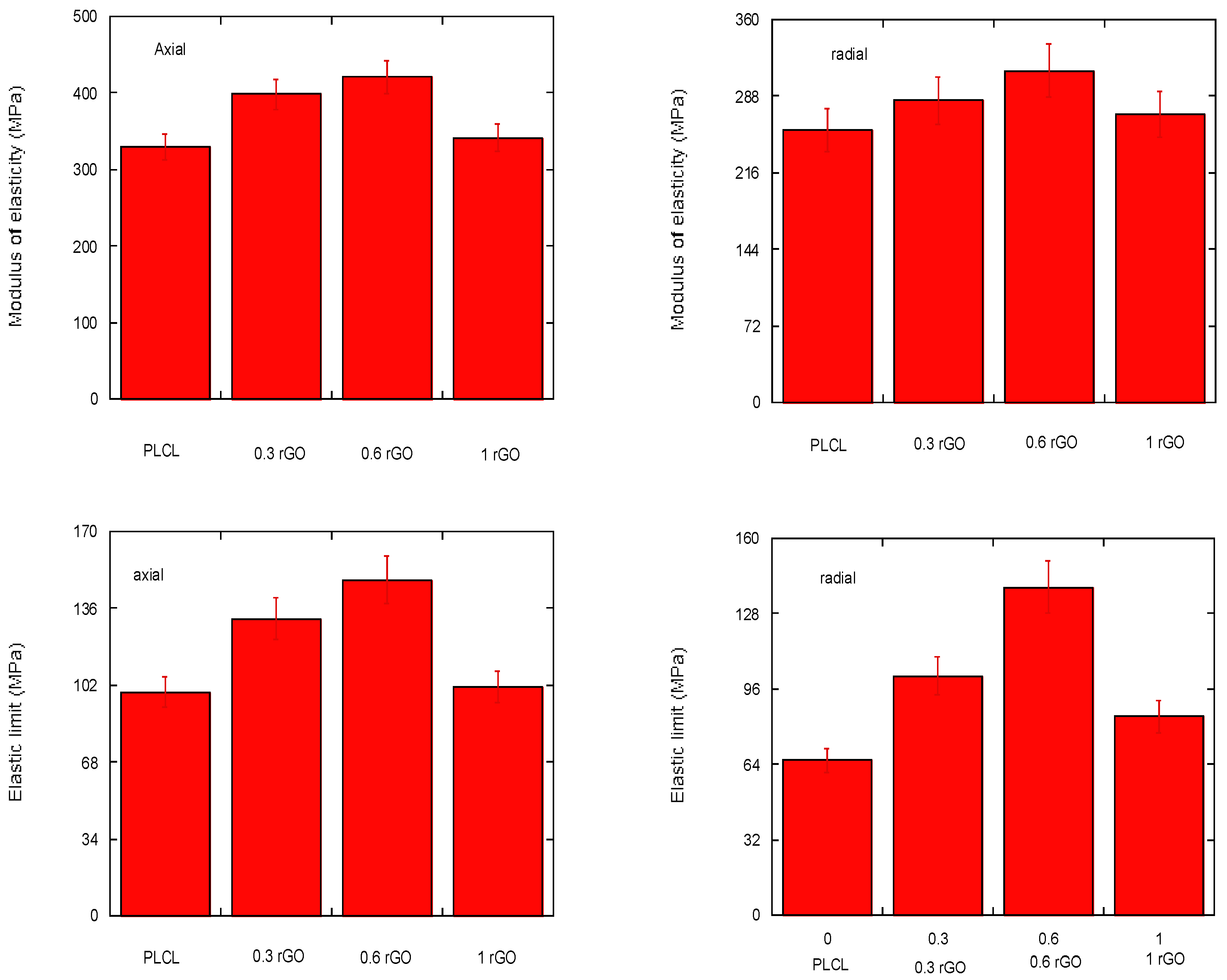
3.5. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Velasco Peña, M.A.; Garzón Alvarado, D.A. Scaffolds implants for the bone regeneration. Materials, techniques and modeling by means of reaction-diffusion systems. Rev. Cuba. Investig. Bioméd. 2010, 29, 140–154. [Google Scholar]
- He, X.; Fu, W.B.; Feng, B. Electrospun collagen-poly(L-lactic acid-co-ε-caprolactone) membranes for cartilage tissue engineering. J. Regen. Med. 2013, 8, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, B.V.; Dailing, E.A.; Brock, J.L.; Stansbury, J.W.; Randolph, M.A.; Anseth, K.S. A Biosynthetic Scaffold that Facilitates Chondrocyte-Mediated Degradation and Promotes Articular Cartilage Extracellular Matrix Deposition. Regen. Eng. Transl. Med. 2015, 1, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Wang, C.; Peng, S.; Shuai, C.; Yang, W.; Zhao, Z. A co-dispersed nanosystem of strontium-anchored reduced graphene oxide to enhance the bioactivity and mechanical property of polymer scaffolds. Mater. Chem. Front. 2021, 5, 2373–2386. [Google Scholar] [CrossRef]
- Fischbach, C.; Chen, R.; Matsumoto, T. Engineering tumors with 3D scaffolds. Nat. Methods 2007, 4, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, S.; Diban, N.; Bianchi, F.; Ye, H.; Urtiaga, A. Evidences of the Effect of GO and rGO in PCL Membranes. Macromol. Biosci. 2018, 18, e1800195. [Google Scholar] [CrossRef]
- Pattison, M.; Wurster, S.; Webster, T.J.; Haberstroh, K.M. Three-dimensional, nano-structured PLGA scaffolds for bladder tissue replacement applications. Biomaterials 2005, 26, 2491–2500. [Google Scholar] [CrossRef]
- Zhang, D.; Ni, N.; Chen, J. Electrospun SF/PLCL nanofibrous membrane: A potential scaffold for retinal progenitor cell proliferation and differentiation. Sci. Rep. 2015, 5, 14326. [Google Scholar] [CrossRef]
- Puppi, D.; Chiellini, F.; Piras, A.M. Polymeric materialsfor bone and cartilage repair. Prog. Polym. Sci. 2010, 35, 403–440. [Google Scholar] [CrossRef]
- Seyedsalehi, A.; Daneshmandi, L.; Barajaa, M.; Riordan, J.; Laurencin, C.T. Fabrication and characterization of mechanically competent 3D printed polycaprolactone reduced graphene oxide scaffolds. Sci. Rep. 2020, 10, 22210. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, L.Y.; Yang, X.B.; Weng, J. Preparation of bioactive porous HA/PCL composite scaffolds. Appl. Surf. Sci. 2008, 255, 2942–2946. [Google Scholar] [CrossRef]
- Qiu, Z.; Pan, H. Preparation, crystallization and hydrolytic degradation of biodegradable poly (l-lactide)/polyhedral oligomeric silsesquioxanes nanocomposite. Compos. Sci. Technol. 2010, 70, 1089. [Google Scholar] [CrossRef]
- Zhou, W.; Duan, M.; Wang, M.; Cheung, W. Crystallization kinetics of poly(L-lactide)/carbonated hydroxyapatite nanocomposite microspheres. J. Appl. Polym. Sci. 2009, 113, 4100. [Google Scholar] [CrossRef]
- Nejati, E.; Firouzdor, V.; Eslaminejad, M.; Bagheri, F. Needle-like nano hydroxyapatite/poly (l-lactide acid) composite scaffold for bone tissue engineering application. Mater. Sci. Eng. C 2009, 29, 942. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, Z.; Yan, S.; Yang, W. Crystallization Behavior of Biodegradable Poly(L-lactide)/Multiwalled Carbon Nanotubes Nanocomposites from the Amorphous State. Polym. Eng. Sci. 2011, 51, 1564–1573. [Google Scholar] [CrossRef]
- Roy, T.D.; Simon, J.L.; Ricci, J.L.; Rekow, E.D.; Thompson, V.P.; Parsons, J.R. Performance of degradable composite bone repair products made via three-dimensional fabrication techniques. J. Biomed. Mater. Res. A 2003, 66, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.M.; Orton, D.G.; Hollister, S.J.; Feinberg, S.E.; Halloran, J.W. Mechanical and in vivo performance of hydroxyapatite implants with controlled architectures. Biomaterials 2002, 23, 1283–1293. [Google Scholar] [CrossRef]
- Lewandrowski, K.U.; Gresser, J.D.; Bronde, S.; Silva, A.E.; Wise, D.L.; Trantolo, D.J. Developing porosity of poly (propylene glycol-co-fumaric acid) bone graft substitutes and the effect on osteointegration: A preliminary histology study in rats. J. Biomater. Sci. Polym. Ed. 2000, 11, 879–889. [Google Scholar] [CrossRef]
- Díaz, E.; Martín, J.; León, J. Carbon nanotube reinforced poly(l-lactide) scaffolds: In vitro degradation, conductivity, mechanical and thermal properties. Compos. Interfaces 2021, 28, 511–525. [Google Scholar] [CrossRef]
- Shioyama, H. The interactions of two chemical species in the interlayer spacing of graphite. Synth. Met. 2000, 114, 1–15. [Google Scholar] [CrossRef]
- Du, X.S.; Xiao, M.; Meng, Y.Z.; Hay, A.S. Direct synthesis of poly(arylenedisulfide)/carbon nanosheet composites via the oxidation with graphite oxide. Carbon 2005, 43, 195. [Google Scholar] [CrossRef]
- Yousefi, N.; Gudarzi, M.; Zheng, Q.; Aboutalebi, S.H.; Sharifb, F.; Kim, J.K. Self-alignment and high electrical conductivity of ultralarge graphene oxide–polyurethane nanocomposites. J. Mater. Chem. 2012, 22, 12709–12717. [Google Scholar] [CrossRef]
- Wu, Z.S.; Ren, W.C.; Gao, L.B.; Zhao, J.P.; Chen, Z.P.; Liu, B.L.; Tang, D.M.; Yu, B.; Jiang, C.B.; Cheng, H.M. Synthesis of Graphene sheets with high electrical conductivity and good termal stability by hydrogen arc discharge exfoliation. ACS Nano 2009, 3, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Ikehara, T.; Nishi, T. Crystallization behaviour of biodegradable poly (ethylene succinate) from the amorphous state. Polymer 2003, 44, 5429–5437. [Google Scholar] [CrossRef]
- Wang, H.; Qiu, Z. Crystallization behaviors of biodegradable poly(L-lactic acid)/graphene oxide nanocomposites from the amorphous state. Thermochim. Acta 2011, 526, 229–236. [Google Scholar] [CrossRef]
- Achilias, D.S.; Papageorgiou, G.Z.; Karayannidis, G.P. Isothermal and nonisothermal crystallization kinetics of poly(propylene terephthalate. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 3775–3796. [Google Scholar] [CrossRef]
- Zhang, R.; Zheng, H.; Lou, X.; Ma, D. Crystallization characteristics of polypropylene and low ethylene content polypropylene copolymer with and without nucleating agents. J. Appl. Polym. Sci. 1994, 51, 51–56. [Google Scholar] [CrossRef]
- Wu, M.; Yang, G.; Wang, M.; Wang, W.; Zhang, W.; Feng, J.; Liu, T. Nonisothermal crystallization kinetics of ZnO nanorod filled polyamide composites. Mater. Chem. Phys. 2008, 109, 547–555. [Google Scholar] [CrossRef]
- Huang, J.H.; Hung, K.; Tseng, T.; Kang, C. Nonisothermal crystallization of poly(ethylene-co-glycidyl methacrylate)/clay nanocomposites. J. Appl. Polym. Sci. 2006, 100, 1335–1343. [Google Scholar] [CrossRef]
- Supaphol, P.; Dangseeyun, N.; Srimoaon, P. Non-isothermal melt-crystallization kinetics of poly(trimethylene terephthalate). Polym. Test 2004, 23, 817–826. [Google Scholar]
- Khanna, Y. A barometer of crystallization rates of polymeric materials. Eng. Sci. 1990, 30, 1615–1619. [Google Scholar] [CrossRef]
- Kissinger, H. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217–221. [Google Scholar] [CrossRef]
- Sartoneva, R.; Kuismanen, K.; Juntunen, M.; Karjalainen, S.; Hannula, M.; Kyllönen, L.; Hyttinen, J.; Huhtala, H.; Paakinaho, K.; Miettinen, S. Porous poly- l -lactide-co-ε-caprolactone scaffold: A novel biomaterial for vaginal tissue engineering. R. Soc. Open Sci. 2018, 5, 180811. [Google Scholar] [CrossRef] [PubMed]
- Díaz, E.; Iglesias, N.; Ribeiro, S.; Lanceros-Méndez, S. Cytocompatible scaffolds of poly(L-lactide)/reduced graphene oxide for tissue engineering. Journal of Biomaterials Science. Polym. Ed. 2021, 32, 1406–1419. [Google Scholar]
- Arafat, M.T.; Savalani, M.M.; Gibson, I. Improving the Mechanical Properties in Tissue Engineered Scaffolds. ASME Int. Mech. Eng. Congr. Expo. 2008, 48630, 3–6. [Google Scholar]
- Díaz, E.; Sandonis, I.; Valle, M.B. In Vitro Degradation of Poly(caprolactone)/nHA Composites. J. Nanomater. 2014, 2014, 185. [Google Scholar] [CrossRef]

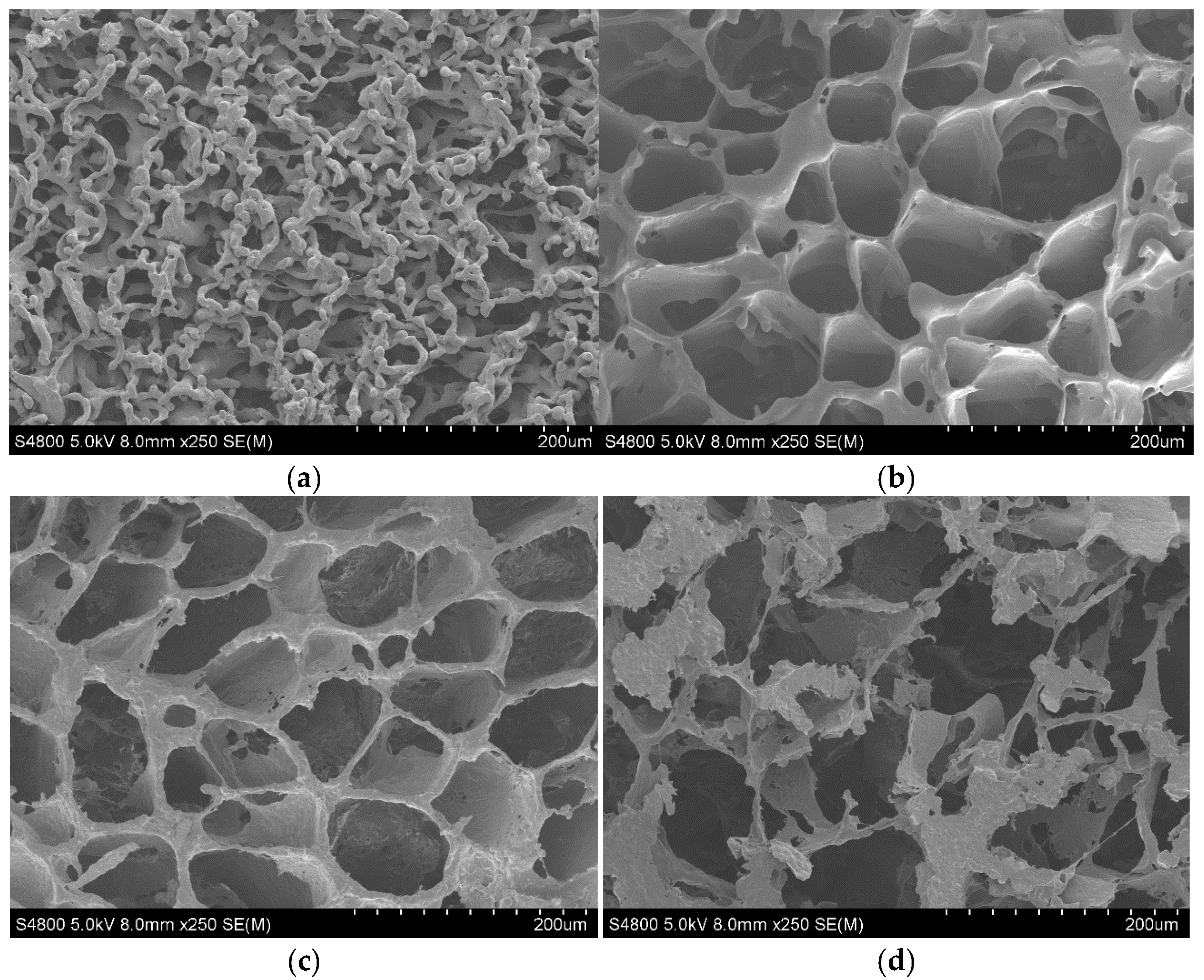
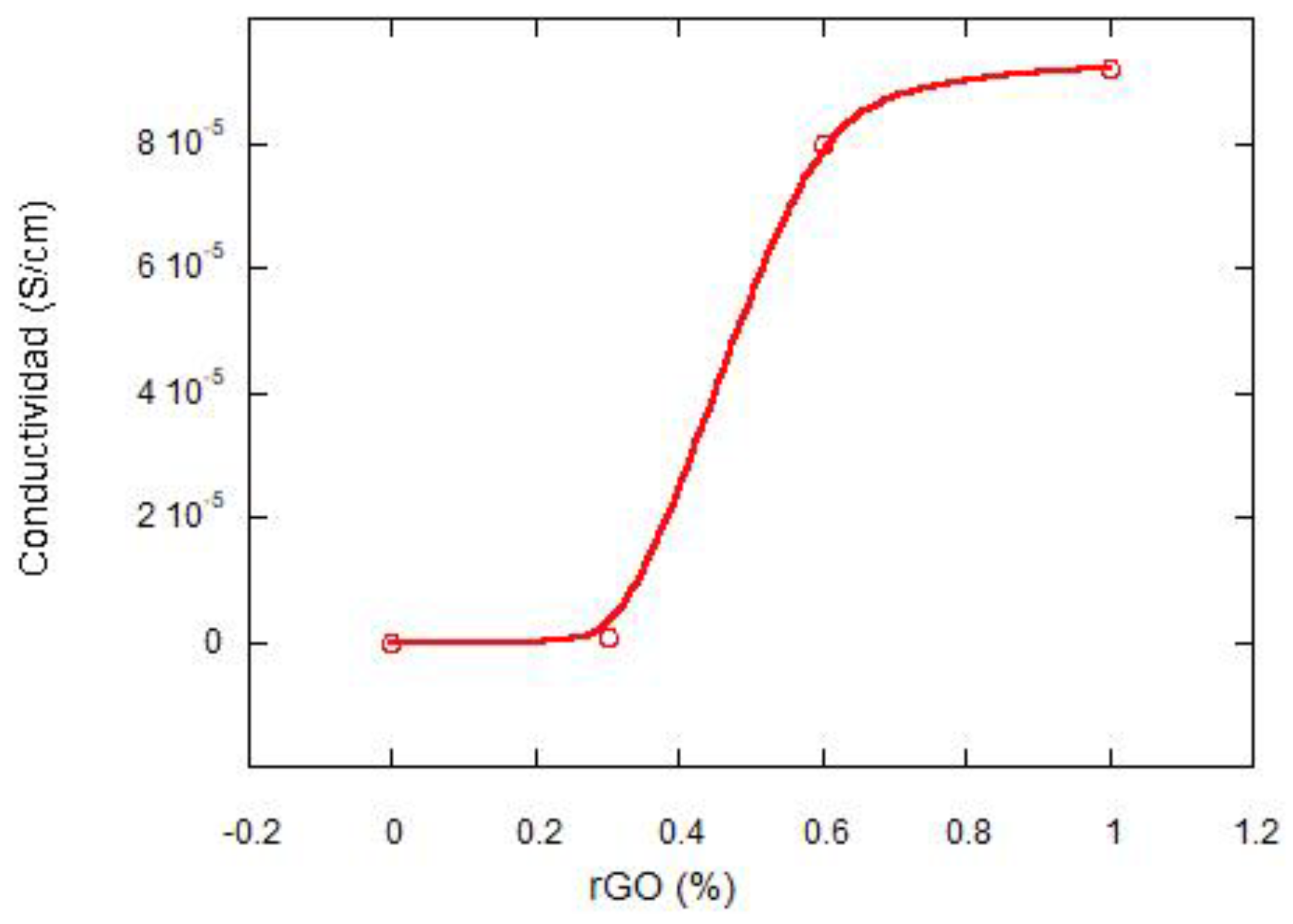
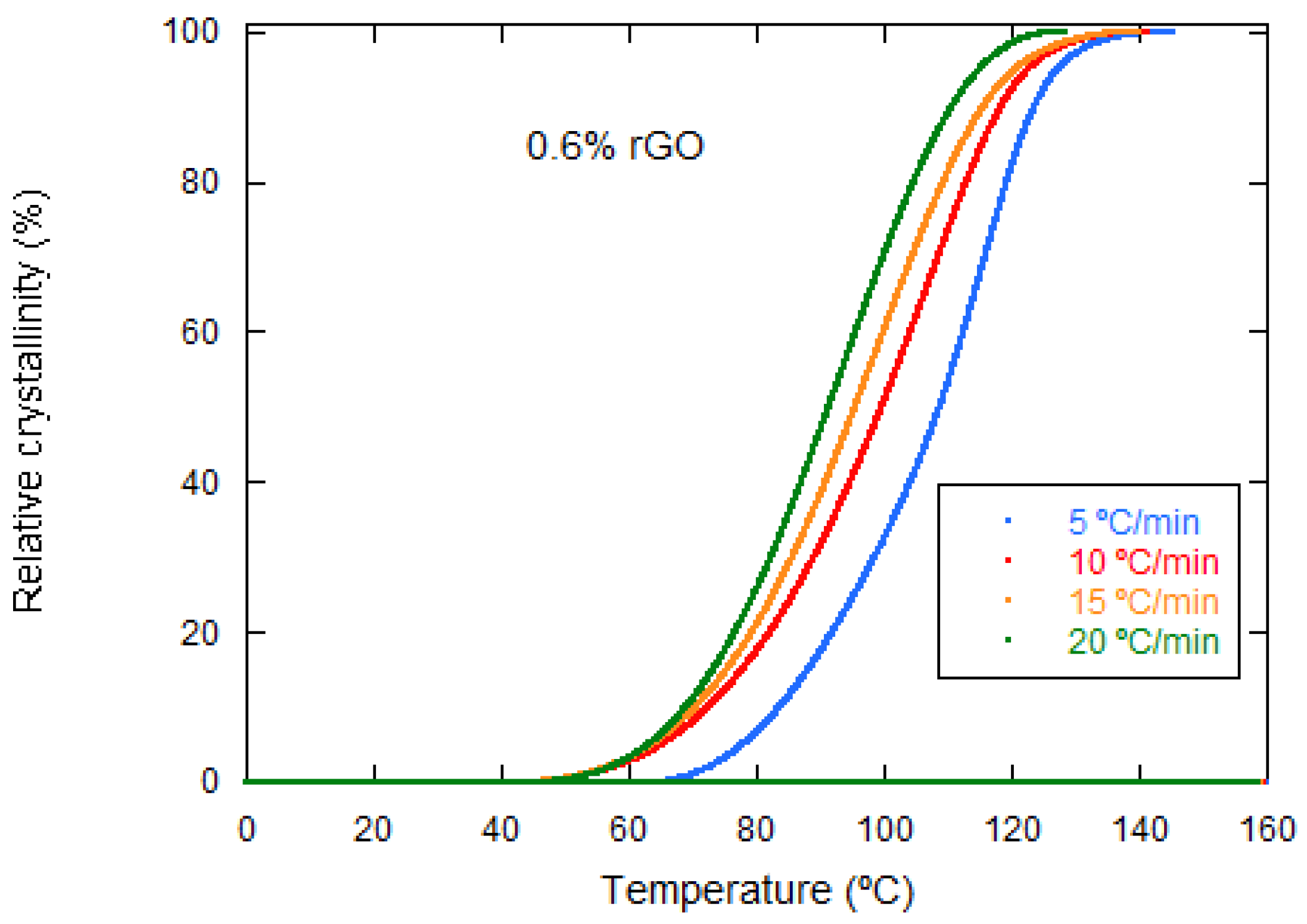
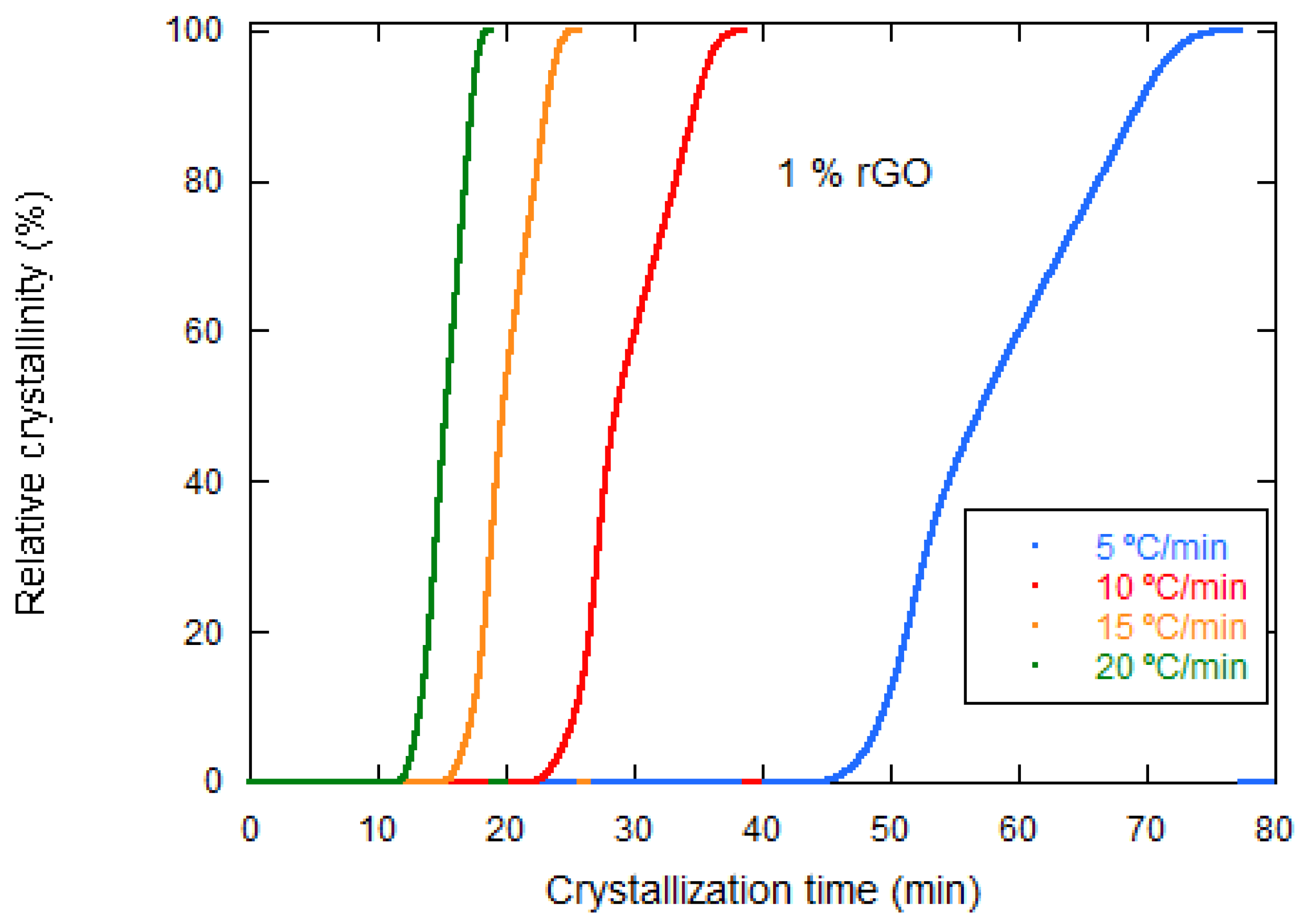
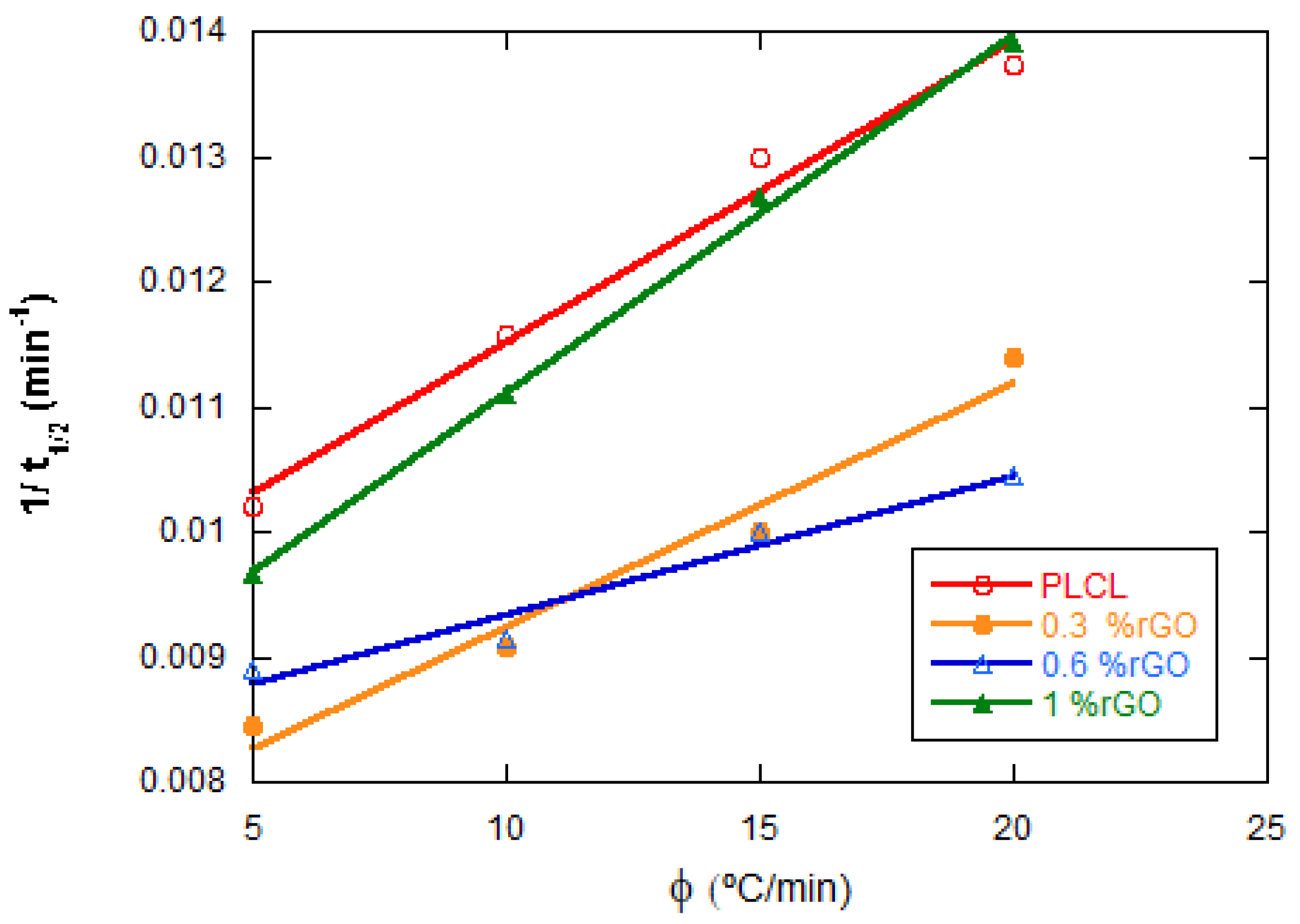
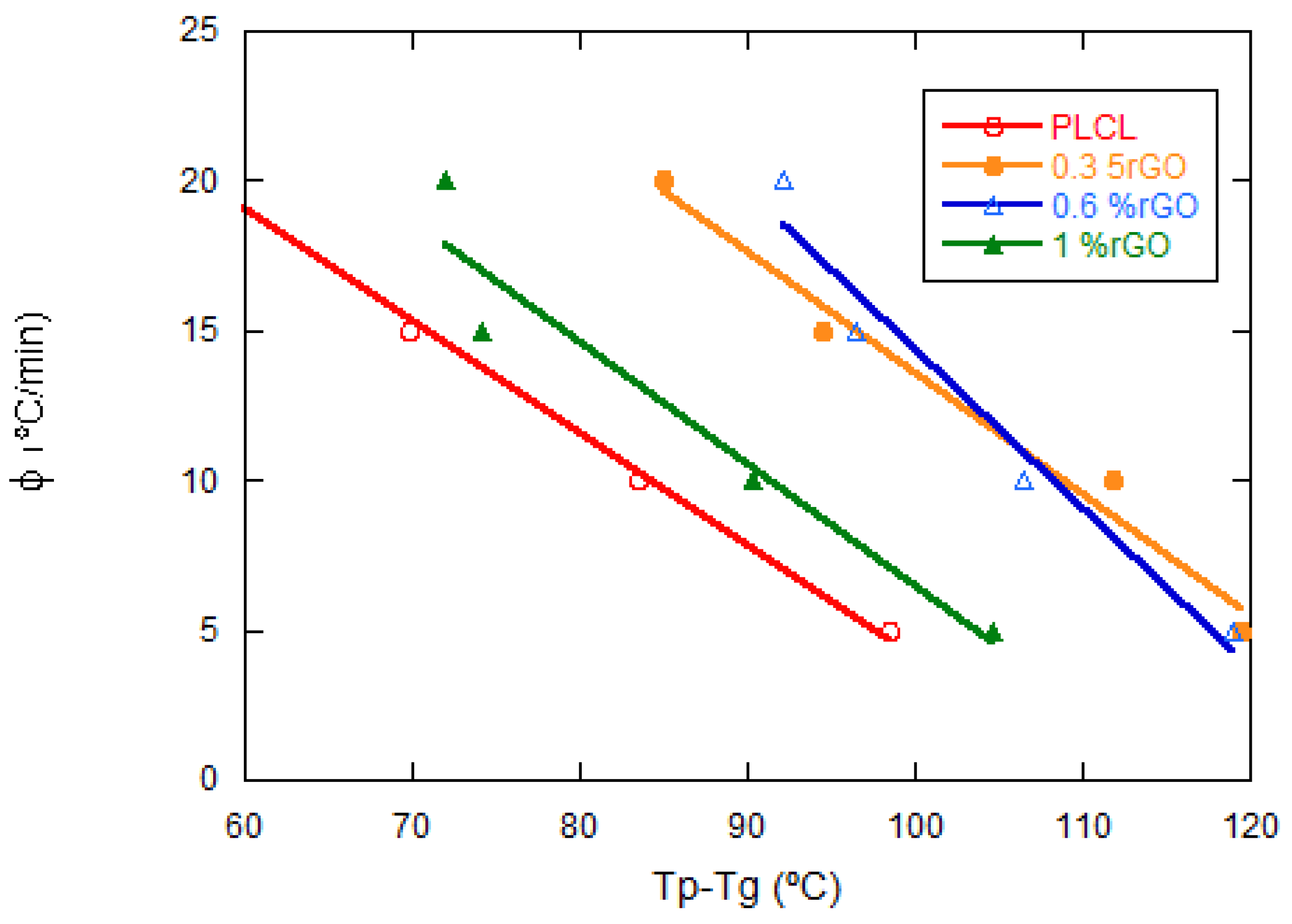
| rGO Content (wt%) | Conductivity (S·cm−1) Perpendicular to Pores | Conductivity (S·cm−1) Parallel to Pores |
|---|---|---|
| 0 | 1 × 10−12 | 1 × 10−12 |
| 0.3 | 2 × 10−6 | 5 × 10−7 |
| 0.6 | 3.5 × 10−5 | 8 × 10−5 |
| 1 | 7 × 10−8 | 9.2 × 10−5 |
| Samples | Φ (°C/min) | Tp (°C) | ΔHc (J/g) | t1/2 (min) |
|---|---|---|---|---|
| Neat PLCL | 5 | 98.13 | 20.10 | 10.15 |
| 10 | 86.31 | 19.50 | 5.26 | |
| 15 | 76.71 | 13.52 | 4.25 | |
| 20 | 72.77 | 5.27 | 3.46 | |
| PLCL/0.3% rGO | 5 | 118.38 | 8.47 | 13.45 |
| 10 | 110.10 | 12.53 | 6.60 | |
| 15 | 96.46 | 13.60 | 4.42 | |
| 20 | 87.54 | 12.79 | 3.36 | |
| PLCL/0.6% rGO | 5 | 118.36 | 6.55 | 13.92 |
| 10 | 109.38 | 13.44 | 6.87 | |
| 15 | 99.87 | 15.51 | 4.63 | |
| 20 | 95.71 | 12.27 | 3.45 | |
| PLCL/1% rGO | 5 | 103.57 | 18.95 | 13.17 |
| 10 | 89.91 | 19.10 | 6.74 | |
| 15 | 78.92 | 13.56 | 4.72 | |
| 20 | 71.86 | 5.591 | 3.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, E.; León, J.; Murillo-Marrodán, A.; Ribeiro, S.; Lanceros-Méndez, S. Influence of rGO on the Crystallization Kinetics, Cytoxicity, and Electrical and Mechanical Properties of Poly (L-lactide-co-ε-caprolactone) Scaffolds. Materials 2022, 15, 7436. https://doi.org/10.3390/ma15217436
Díaz E, León J, Murillo-Marrodán A, Ribeiro S, Lanceros-Méndez S. Influence of rGO on the Crystallization Kinetics, Cytoxicity, and Electrical and Mechanical Properties of Poly (L-lactide-co-ε-caprolactone) Scaffolds. Materials. 2022; 15(21):7436. https://doi.org/10.3390/ma15217436
Chicago/Turabian StyleDíaz, Esperanza, Joseba León, Alberto Murillo-Marrodán, Sylvie Ribeiro, and Senentxu Lanceros-Méndez. 2022. "Influence of rGO on the Crystallization Kinetics, Cytoxicity, and Electrical and Mechanical Properties of Poly (L-lactide-co-ε-caprolactone) Scaffolds" Materials 15, no. 21: 7436. https://doi.org/10.3390/ma15217436
APA StyleDíaz, E., León, J., Murillo-Marrodán, A., Ribeiro, S., & Lanceros-Méndez, S. (2022). Influence of rGO on the Crystallization Kinetics, Cytoxicity, and Electrical and Mechanical Properties of Poly (L-lactide-co-ε-caprolactone) Scaffolds. Materials, 15(21), 7436. https://doi.org/10.3390/ma15217436







