Theoretical Evaluation of Potential Cytotoxicity of Graphene Quantum Dot to Adsorbed DNA
Abstract
1. Introduction
2. Methods
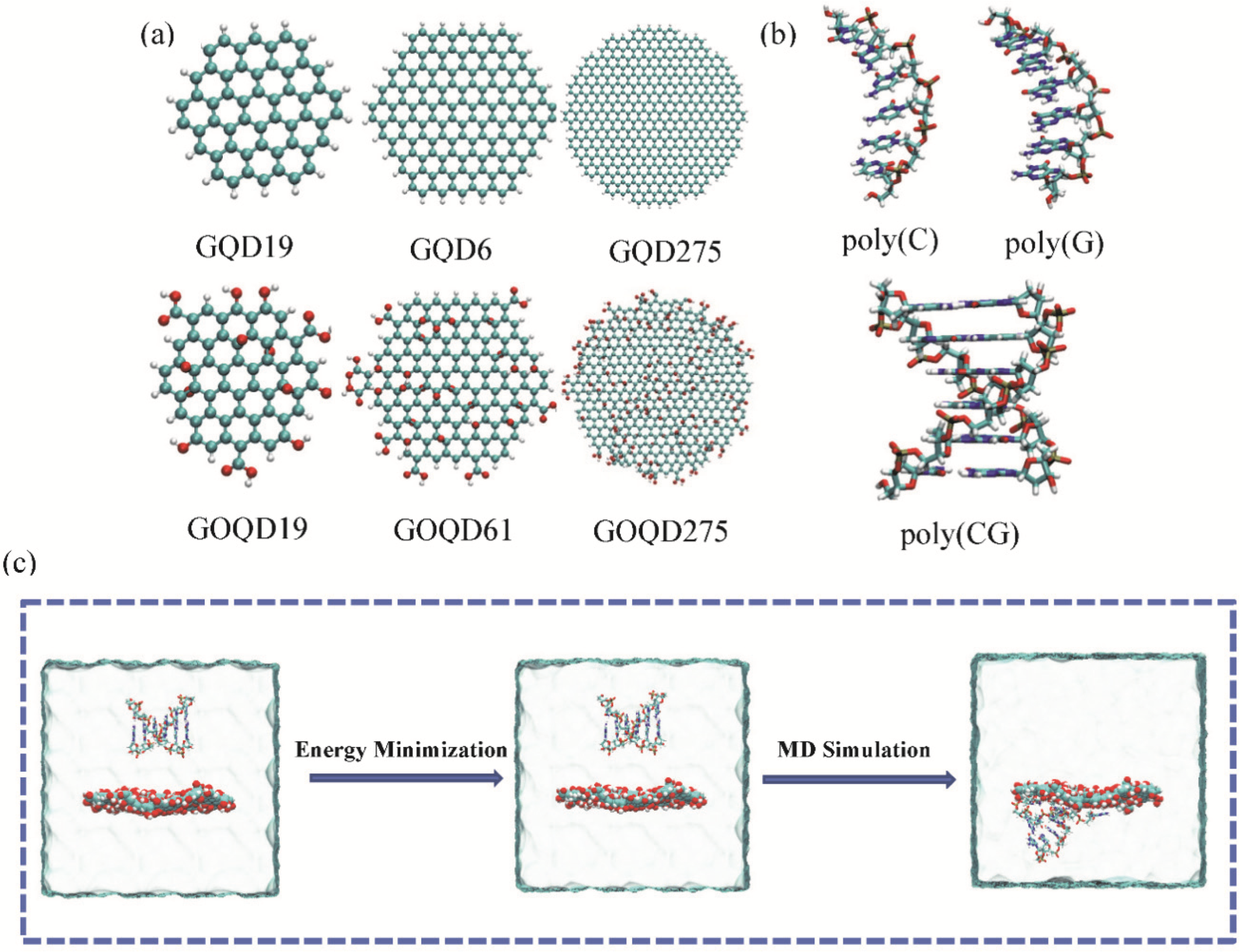
2.1. System Setup
2.2. MD Simulations
3. Results and Discussion
3.1. The Adsorption Behavior of DNA onto GQD and GOQD
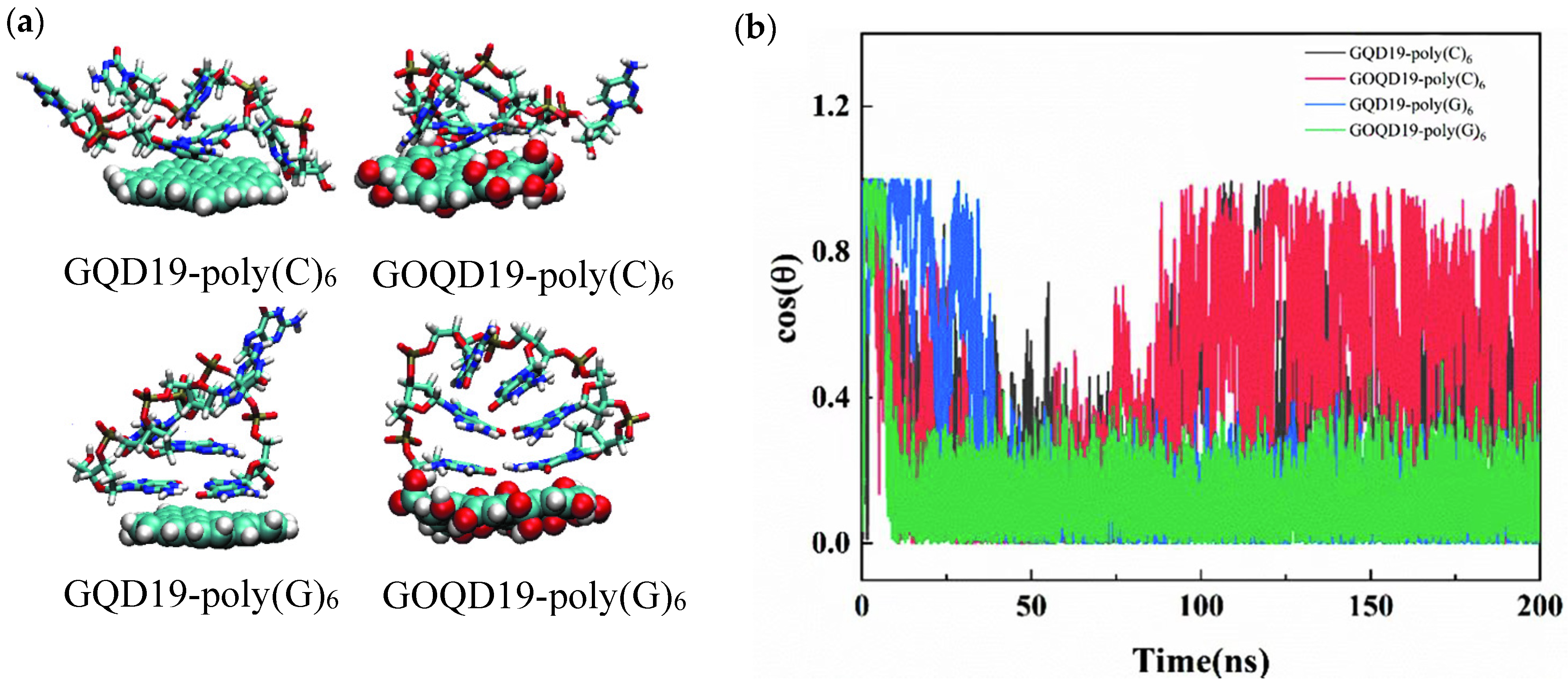
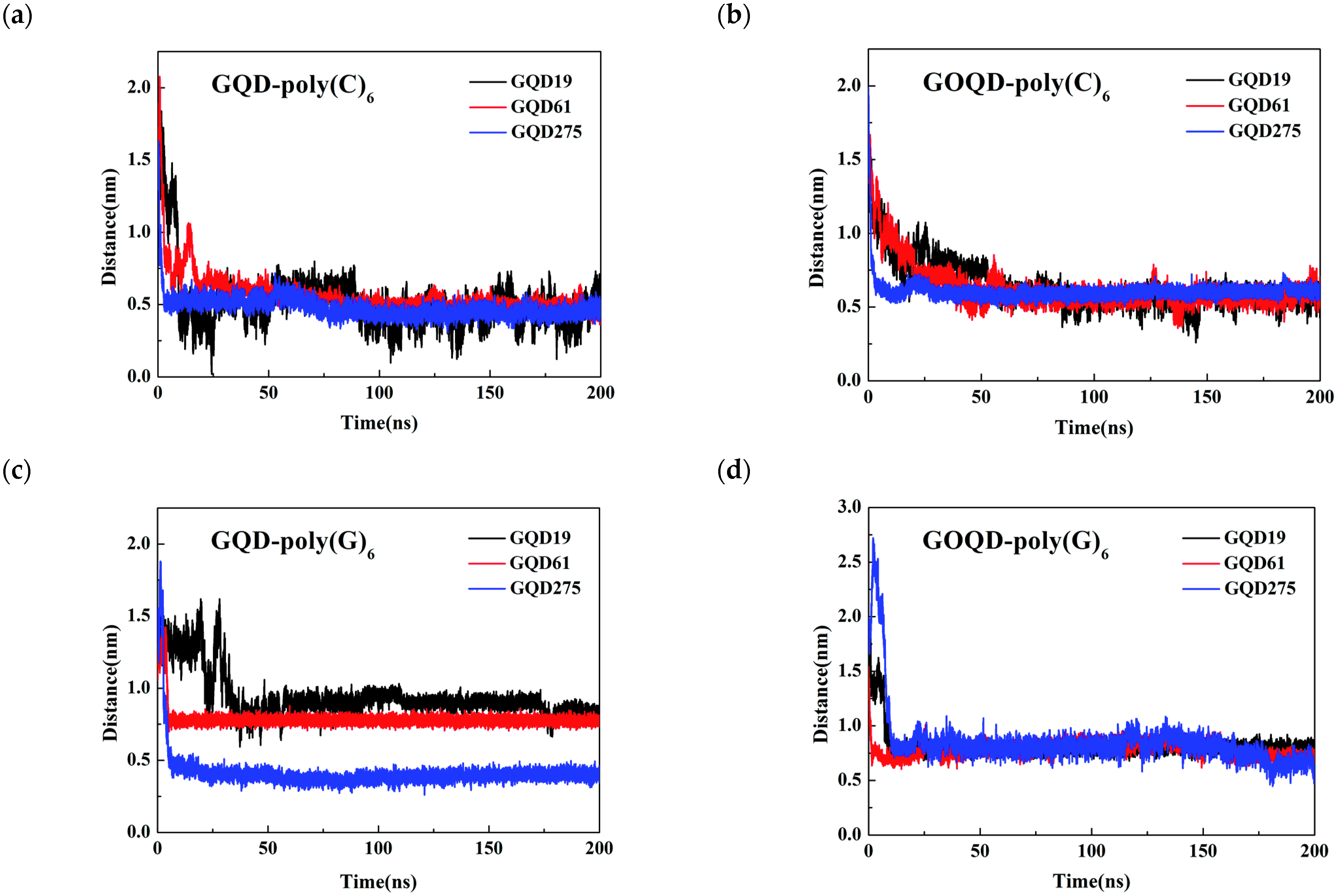
3.2. Structural Evolution of ssDNA Adsorbed onto GQD and GOQD
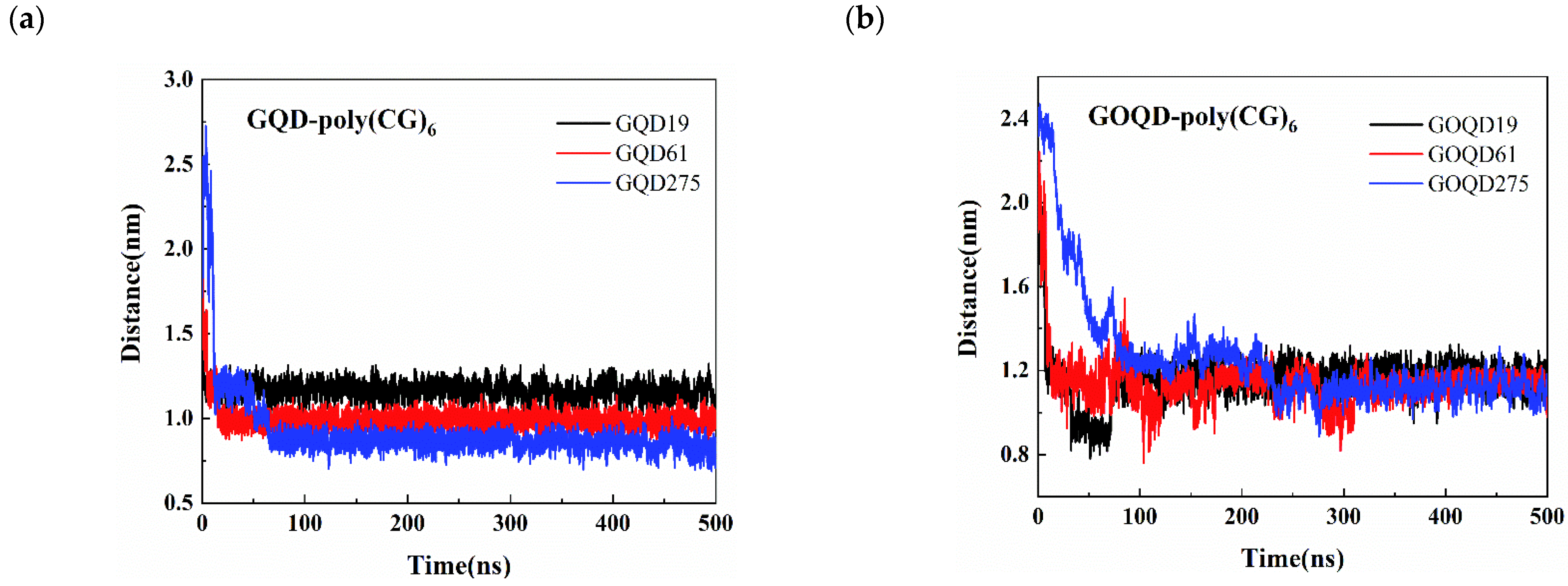
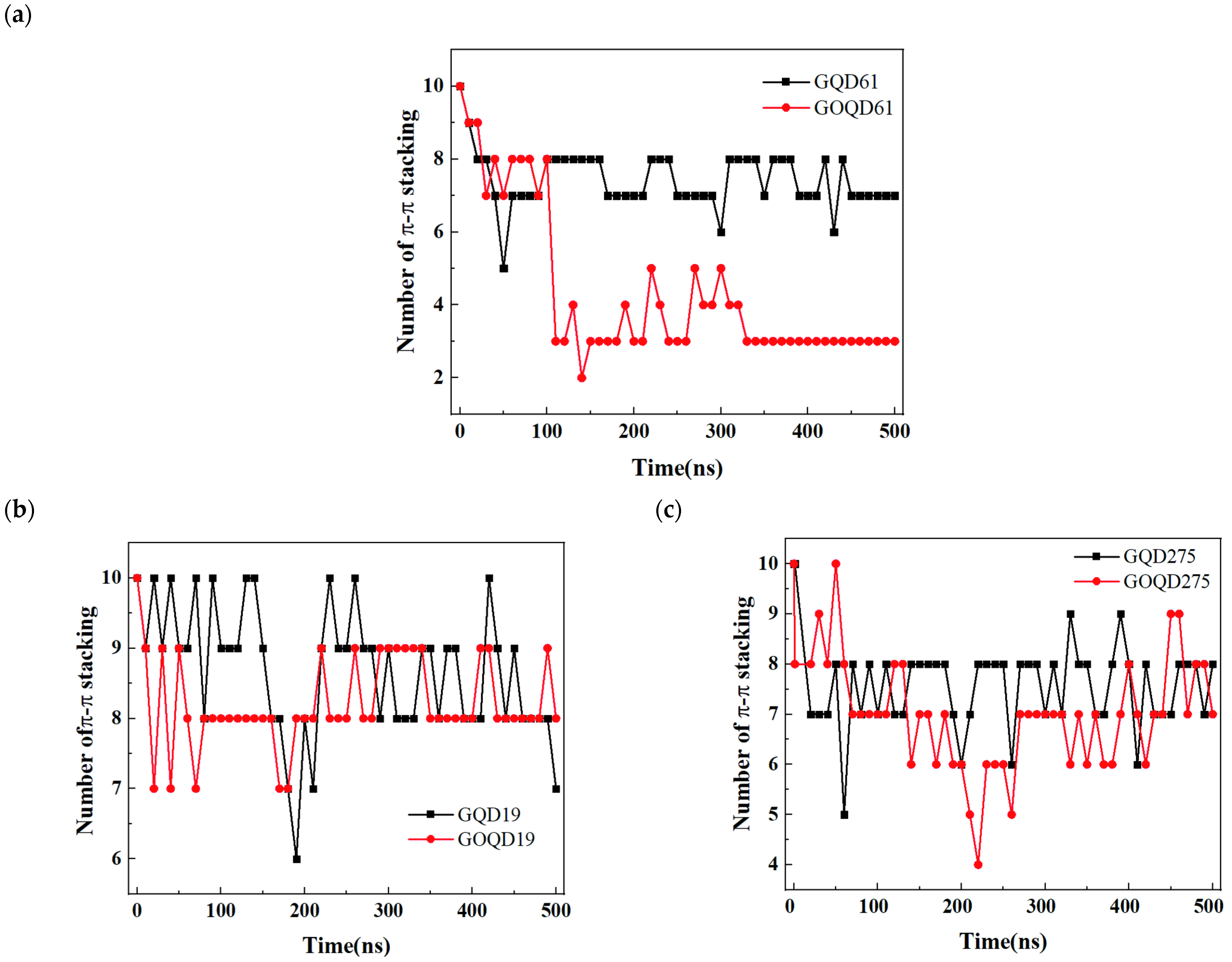
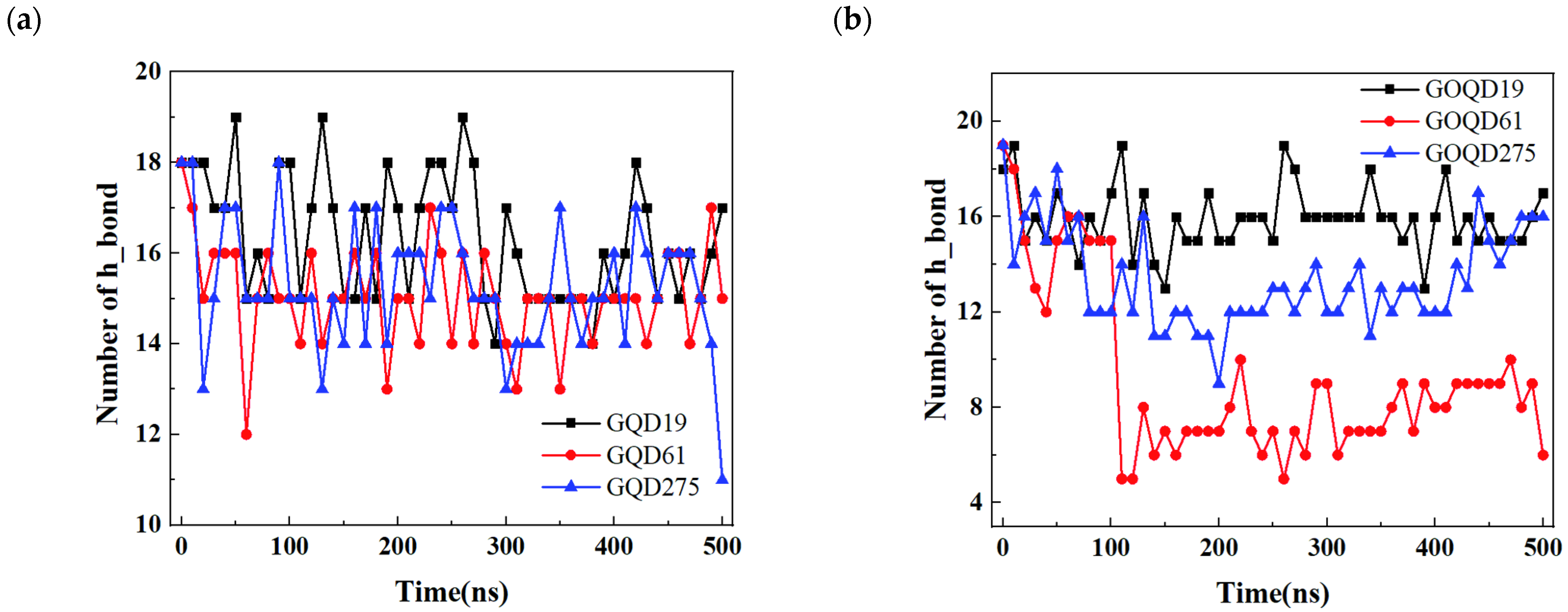
3.3. Structural Evolution of dsDNA Adsorbed onto GQD and GOQD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Yankowitz, M.; Chen, S.W.; Polshyn, H.; Zhang, Y.X.; Watanabe, K.; Taniguchi, T.; Graf, D.; Young, A.F.; Dean, C.R. Tuning superconductivity in twisted bilayer graphene. Science 2019, 363, 455–464. [Google Scholar] [CrossRef] [PubMed]
- McIver, J.W.; Schulte, B.; Stein, F.U.; Matsuyama, T.; Jotzu, G.; Meier, G.; Cavalleri, A. Light-induced anomalous Hall effect in graphene. Nat. Phys. 2020, 16, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Khatir, N.M.; Ahmadi, A.; Taghizade, N.; Motevali Khameneh, S.; Faghihnasiri, M. Electronic transport properties of nanoribbons of graphene and ψ-graphene-based lactate nanobiosensor. Superlattices Microstruct. 2020, 145, 106603. [Google Scholar] [CrossRef]
- Khatir, N.M.; Fatoorehchi, H.; Ahmadi, A.; Khoshnoodfar, A.; Faghihnasiri, M. Investigating the adsorption of the thyroid stimulating hormones molecules on graphene sheets by the density functional theory for possible nano-biosensor applications. Chem. Pet. Eng. 2021, 55, 385–392. [Google Scholar]
- Bacon, M.; Bradley, S.J.; Nann, T. Graphene quantum dots. Part. Part. Syst. Charact. 2014, 31, 415–428. [Google Scholar] [CrossRef]
- Haque, E.; Kim, J.; Malgras, V.; Reddy, K.R.; Ward, A.C.; You, J.; Bando, Y.; Hossain, M.S.A.; Yamauchi, Y. Recent advances in graphene quantum dots: Synthesis, properties, and applications. Small Methods 2018, 2, 1800050. [Google Scholar] [CrossRef]
- Ozfidan, I.; Güçlü, A.D.; Korkusinski, M.; Hawrylak, P. Theory of optical properties of graphene quantum dots. Phys. Status Solidi Rapid Res. Lett. 2016, 10, 102–110. [Google Scholar] [CrossRef]
- Basko, D.M.; Duchemin, I.; Blase, X. Optical properties of graphene quantum dots: The role of chiral symmetry. 2D Mater. 2020, 7, 025041. [Google Scholar] [CrossRef]
- Kansara, V.; Shukla, R.; Flora, S.J.S.; Bahadur, P.; Tiwari, S. Graphene quantum dots: Synthesis, optical properties and navigational applications against cancer. Mater. Today Commun. 2022, 31, 103359. [Google Scholar] [CrossRef]
- Tabish, T.A.; Scotton, C.J.; Ferguson, D.C.J.; Lin, L.; der Veen, A.; Lowry, S.; Ali, M.; Jabeen, F.; Ali, M.; Winyard, P.G.; et al. Biocompatibility and toxicity of graphene quantum dots for potential application in photodynamic therapy. Nanomedicine 2018, 13, 1923–1937. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.E.; Jeong, J.-M.; Lim, H.S.; Lee, S.Y.; Cho, S.O. Ultraviolet/blue light emitting high-quality graphene quantum dots and their biocompatibility. Carbon 2020, 170, 213–219. [Google Scholar] [CrossRef]
- Ghaffarkhah, A.; Hosseini, E.; Kamkar, M.; Sehat, A.A.; Dordanihaghighi, S.; Allahbakhsh, A.; van der Kuur, C.; Arjmand, M. Synthesis, applications, and prospects of graphene quantum dots: A comprehensive review. Small 2022, 18, 2102683. [Google Scholar] [CrossRef] [PubMed]
- Dey, T.; Mukherjee, S.; Ghorai, A.; Das, S.; Ray, S.K. Surface state selective tunable emission of graphene quantum dots exhibiting novel thermal quenching characteristics. Carbon 2018, 140, 394–403. [Google Scholar] [CrossRef]
- Zhao, S.; Lavie, J.; Rondin, L.; Orcin-Chaix, L.; Diederichs, C.; Roussignol, P.; Chassagneux, Y.; Voisin, C.; Mullen, K.; Narita, A.; et al. Single photon emission from graphene quantum dots at room temperature. Nat. Commun. 2018, 9, 3470. [Google Scholar] [CrossRef]
- Lu, H.; Li, W.; Dong, H.; Wei, M. Graphene quantum dots for optical bioimaging. Small 2019, 15, 1902136. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Chen, S.Z.; Li, H.D.; Yuan, Y.P.; Zhang, Z.Y.; Xie, J.S.; Hwang, D.W.; Zhang, A.D.; Liu, M.L.; Zhou, X. Engineered paramagnetic graphene quantum dots with enhanced relaxivity for tumor imaging. Nano Lett. 2019, 19, 441–448. [Google Scholar] [CrossRef]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Carbon dots and graphene quantum dots in electrochemical biosensing. Nanomaterials 2019, 9, 634. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Revia, R.A.; Zhang, M. Graphene quantum dots and their applications in bioimaging, biosensing, and therapy. Adv. Mater. 2021, 33, 1904362. [Google Scholar] [CrossRef]
- Tabish, T.A.; Hayat, H.; Abbas, A.; Narayan, R.J. Graphene quantum dot–based electrochemical biosensing for early cancer detection. Curr. Opin. Electrochem. 2021, 30, 100786. [Google Scholar] [CrossRef]
- Sung, S.Y.; Su, Y.L.; Cheng, W.; Hu, P.F.; Chiang, C.S.; Chen, W.T.; Hu, S.H. Graphene quantum dots-mediated theranostic penetrative delivery of drug and photolytics in deep tumors by targeted biomimetic nanosponges. Nano Lett. 2019, 19, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Kadiyala, U.; Qu, Z.B.; Elvati, P.; Altheim, C.; Kotov, N.A.; Violi, A.; VanEpps, J.S. Anti-biofilm activity of graphene quantum dots via self-assembly with bacterial amyloid proteins. ACS Nano 2019, 13, 4278–4289. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.; Zhang, R.Y.; Zhao, D.H.; Zhang, X.S.; An, J.; Cheng, K.; Hou, X.L.; Song, X.L.; Zhao, Y.D.; Yang, X.Q. Ultrafast synthesis of gold nanosphere cluster coated by graphene quantum dot for active targeting PA/CT imaging and near-infrared laser/pH-triggered chemo-photothermal synergistic tumor therapy. Chem. Eng. J. 2019, 369, 87–99. [Google Scholar] [CrossRef]
- Liu, H.J.; Li, C.W.; Qian, Y.; Hu, L.; Fang, J.; Tong, W.; Nie, R.R.; Chen, Q.W.; Wang, H. Magnetic-induced graphene quantum dots for imaging-guided photothermal therapy in the second near-infrared window. Biomaterials 2020, 232, 119700. [Google Scholar] [CrossRef]
- Tung, F.I.; Zheng, L.J.; Hou, K.T.; Chiang, C.S.; Chen, M.H.; Liu, T.Y. One-stop radiotherapeutic targeting of primary and distant osteosarcoma to inhibit cancer progression and metastasis using 2DG-grafted graphene quantum dots. Nanoscale 2020, 12, 8809–8818. [Google Scholar] [CrossRef]
- Gong, L.; Sun, J.; Zheng, P.; Liu, Y.; Yang, G. Yellow fluorescent nitrogen and bromine co-doped graphene quantum dots for bioimaging. ACS Appl. Nano Mater. 2021, 4, 8564–8571. [Google Scholar] [CrossRef]
- Xue, Z.; Sun, Q.; Zhang, L.; Kang, Z.; Liang, L.; Wang, Q.; Shen, J.-W. Graphene quantum dot assisted translocation of drugs into a cell membrane. Nanoscale 2019, 11, 4503–4514. [Google Scholar] [CrossRef] [PubMed]
- Yew, Y.T.; Loo, A.H.; Sofer, Z.; Klimova, K.; Pumera, M. Coke-derived graphene quantum dots as fluorescence nanoquencher in DNA detection. Appl. Mater. Today 2017, 7, 138–143. [Google Scholar] [CrossRef]
- Agrawal, N.; Bhagel, D.; Mishra, P.; Prasad, D.; Kohli, E. Post-synthetic modification of graphene quantum dots bestows enhanced biosensing and antibiofilm ability: Efficiency facet. RSC Adv. 2022, 12, 12310–12320. [Google Scholar] [CrossRef]
- Iannazzo, D.; Espro, C.; Ferlazzo, A.; Celesti, C.; Branca, C.; Neri, G. Electrochemical and fluorescent properties of crown ether functionalized graphene quantum dots for potassium and sodium ions detection. Nanomaterials 2021, 11, 2897. [Google Scholar] [CrossRef]
- Ajgaonkar, R.; Lee, B.; Valimukhametova, A.; Nguyen, S.; Gonzalez-Rodriguez, R.; Coffer, J.; Akkaraju, G.R.; Naumov, A.V. Detection of pancreatic cancer miRNA with biocompatible nitrogen-doped graphene quantum dots. Materials 2022, 15, 5760. [Google Scholar] [CrossRef] [PubMed]
- Khatir, N.M.; Abdul-Malek, Z.; Banihashemian, S.M. Influences of magnetic fields on current–voltage characteristics of gold-DNA-gold structure with variable gaps. Mater. Sci. Semicond. Process. 2015, 36, 134–139. [Google Scholar] [CrossRef]
- Imran, H.; Manikandan, P.N.; Dharuman, V. Graphene oxide supported liposomes for efficient label free electrochemical DNA biosensing. Sens. Actuator B Chem. 2018, 260, 841–851. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Wang, C.-F.; Lv, Y.-K.; Shen, S.-G. Fabrication of fluorescent biosensing platform based on graphene oxide-DNA and their application in biomolecule detection. Trac. Trends Anal. Chem. 2018, 106, 53–61. [Google Scholar] [CrossRef]
- Zeng, S.W.; Chen, L.; Wang, Y.; Chen, J.L. Exploration on the mechanism of DNA adsorption on graphene and graphene oxide via molecular simulations. J. Phys. D Appl. Phys. 2015, 48, 275402. [Google Scholar] [CrossRef]
- Gu, Z.L.; Zhao, L.; Liu, S.T.; Duan, G.X.; Perez-Aguilar, J.M.; Luo, J.D.; Li, W.F.; Zhou, R.H. Orientational binding of DNA guided by the C2N template. ACS Nano 2017, 11, 3198–3206. [Google Scholar] [CrossRef]
- Liang, L.; Kong, Z.; Kang, Z.; Wang, H.; Zhang, L.; Shen, J.-W. Theoretical evaluation on potential cytotoxicity of graphene quantum dots. ACS Biomater. Sci. Eng. 2016, 2, 1983–1991. [Google Scholar] [CrossRef]
- Kong, Z.; Hu, W.; Jiao, F.; Zhang, P.; Shen, J.; Cui, B.; Wang, H.; Liang, L. Theoretical evaluation of DNA genotoxicity of graphene quantum dots: A combination of density functional theory and molecular dynamics simulations. J. Phys. Chem. B 2020, 124, 9335–9342. [Google Scholar] [CrossRef]
- Liang, L.; Peng, X.; Sun, F.; Kong, Z.; Shen, J.-W. A review on the cytotoxicity of graphene quantum dots: From experiment to simulation. Nanoscale Adv. 2021, 3, 904–917. [Google Scholar] [CrossRef]
- Li, B.; Xie, X.; Duan, G.; Chen, S.H.; Meng, X.-Y.; Zhou, R. Binding patterns and dynamics of double-stranded DNA on the phosphorene surface. Nanoscale 2020, 12, 9430–9439. [Google Scholar] [CrossRef]
- Mehranfar, A.; Khavani, M.; Izadyar, M. A molecular dynamic study on the ability of phosphorene for designing new sensor for SARS-CoV-2 detection. J. Mol. Liq. 2022, 345, 117852. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.-Z.; Jiao, F.-F.; Xie, Z.-X.; Kong, Z.; Hu, W.; Shen, J.-W.; Liang, L.-J. Theoretical investigation of the mechanism of phospholipid extraction from the cell membrane using functionalized graphene quantum dots. Mater. Adv. 2022, 3, 6161–6170. [Google Scholar] [CrossRef]
- Zhao, X.C. Self-assembly of DNA segments on graphene and carbon nanotube arrays in aqueous solution: A molecular simulation study. J. Phys. Chem. C 2011, 115, 6181–6189. [Google Scholar] [CrossRef]
- Jeong, S.; Pinals, R.L.; Dharmadhikari, B.; Song, H.; Kalluri, A.; Debnath, D.; Qi, W.P.; Ham, M.; Patra, P.; Landry, M.P. Graphene quantum dot oxidation governs noncovalent biopolymer adsorption. Sci. Rep. 2020, 10, 7074. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Shen, Q.; Shen, J.-W.; Jin, L.; Zhang, L.; Sun, Q.; Hu, Q.; Liang, L. Understanding the size effect of graphene quantum dots on protein adsorption. Colloid Surf. B Biointerfaces 2019, 174, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Shen, X.; Dai, J.; Peng, G.; Liang, L.; Shen, J.-W.; Zhang, L. On the mechanism of graphene quantum dot encapsulation by chitosan: A molecular dynamics study. J. Mol. Liq. 2020, 320, 113453. [Google Scholar] [CrossRef]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of graphite oxide revisited. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- Medhekar, N.V.; Ramasubramaniam, A.; Ruoff, R.S.; Shenoy, V.B. Hydrogen bond networks in graphene oxide composite paper: Structure and mechanical properties. ACS Nano 2010, 4, 2300–2306. [Google Scholar] [CrossRef]
- Shih, C.J.; Lin, S.C.; Sharma, R.; Strano, M.S.; Blankschtein, D. Understanding the pH-dependent behavior of graphene oxide aqueous solutions: A comparative experimental and molecular dynamics simulation study. Langmuir 2012, 28, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Frish, M.; Trucks, G.W.; Schlegel, H.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, T.; Kudin, K.N.; Burant, J. Gaussian 03, Revision C.02; Gaussian Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Zhang, Y.; Pan, V.; Li, X.; Yang, X.; Li, H.; Wang, P.; Ke, Y. Dynamic DNA Structures. Small 2019, 15, 1900228. [Google Scholar] [CrossRef]
- Hart, K.; Foloppe, N.; Baker, C.M.; Denning, E.J.; Nilsson, L.; MacKerell, A.D. Optimization of the CHARMM additive force field for DNA: Improved treatment of the BI/BII conformational equilibrium. J. Chem. Theory Comput. 2012, 8, 348–362. [Google Scholar] [CrossRef]
- Boonstra, S.; Onck, P.R.; van der Giessen, E. CHARMM TIP3P water model suppresses peptide folding by solvating the unfolded state. J. Phys. Chem. B 2016, 120, 3692–3698. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Tanugi, D.; Grossman, J.C. Water desalination across nanoporous graphene. Nano Lett. 2012, 12, 3602–3608. [Google Scholar] [CrossRef] [PubMed]
- Salomon-Ferrer, R.; Case, D.A.; Walker, R.C. An overview of the Amber biomolecular simulation package. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2013, 3, 198–210. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M.J. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Zhao, D.H.; Li, L.B.; Zhou, J. Simulation insight into the cytochrome c adsorption on graphene and graphene oxide surfaces. Appl. Surf. Sci. 2018, 428, 825–834. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.J.; Wang, H.B.; Li, L.H.; Zhao, W.J.; Shen, J.W.; Liang, L.J. Study on the adsorption orientation of DNA on two-dimensional MoS2 surface via molecular dynamics simulation: A vertical orientation phenomenon. Chem. Phys. 2020, 529, 110546. [Google Scholar] [CrossRef]
- Antony, J.; Grimme, S. Structures and interaction energies of stacked graphene-nucleobase complexes. Phys. Chem. Chem. Phys. 2008, 10, 2722–2729. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhu, L.; Chen, J.-F.; Dai, L. Can graphene quantum dots cause DNA damage in cells? Nanoscale 2015, 7, 9894. [Google Scholar] [CrossRef]


| Name of System | GQD Type | DNA Type | Number of Na+ | Number of Water Molecules | Simulation Time (ns) |
|---|---|---|---|---|---|
| GQD19-poly(C)6 | GQD19 | poly(C)6 | 5 | 6112 | 200 |
| GQD61-poly(C)6 | GQD61 | poly(C)6 | 5 | 7403 | 200 |
| GQD275-poly(C)6 | GQD275 | poly(C)6 | 5 | 12,047 | 200 |
| GOQD19-poly(C)6 | GOQD19 | poly(C)6 | 5 | 6100 | 200 |
| GOQD61-poly(C)6 | GOQD61 | poly(C)6 | 5 | 7387 | 200 |
| GOQD275-poly(C)6 | GOQD275 | poly(C)6 | 5 | 11,941 | 200 |
| GQD19-poly(G)6 | GQD19 | poly(G)6 | 5 | 6102 | 200 |
| GQD61-poly(G)6 | GQD61 | poly(G)6 | 5 | 7397 | 200 |
| GQD275-poly(G)6 | GQD275 | poly(G)6 | 5 | 12,029 | 200 |
| GOQD19-poly(G)6 | GOQD19 | poly(G)6 | 5 | 6099 | 200 |
| GOQD61-poly(G)6 | GOQD61 | poly(G)6 | 5 | 7384 | 200 |
| GOQD275-poly(G)6 | GOQD275 | poly(G)6 | 5 | 11,925 | 200 |
| GQD19-poly(CG)6 | GQD19 | poly(CG)6 | 10 | 6050 | 500 |
| GQD61-poly(CG)6 | GQD61 | poly(CG)6 | 10 | 7330 | 500 |
| GQD275-poly(CG)6 | GQD275 | poly(CG)6 | 10 | 11,959 | 500 |
| GOQD19-poly(CG)6 | GOQD19 | poly(CG)6 | 10 | 6039 | 500 |
| GOQD61-poly(CG)6 | GOQD61 | poly(CG)6 | 10 | 7310 | 500 |
| GOQD275-poly(CG)6 | GOQD275 | poly(CG)6 | 10 | 11,891 | 500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, L.; Shen, X.; Zhou, M.; Chen, Y.; Lu, X.; Zhang, L.; Wang, W.; Shen, J.-W. Theoretical Evaluation of Potential Cytotoxicity of Graphene Quantum Dot to Adsorbed DNA. Materials 2022, 15, 7435. https://doi.org/10.3390/ma15217435
Liang L, Shen X, Zhou M, Chen Y, Lu X, Zhang L, Wang W, Shen J-W. Theoretical Evaluation of Potential Cytotoxicity of Graphene Quantum Dot to Adsorbed DNA. Materials. 2022; 15(21):7435. https://doi.org/10.3390/ma15217435
Chicago/Turabian StyleLiang, Lijun, Xin Shen, Mengdi Zhou, Yijian Chen, Xudong Lu, Li Zhang, Wei Wang, and Jia-Wei Shen. 2022. "Theoretical Evaluation of Potential Cytotoxicity of Graphene Quantum Dot to Adsorbed DNA" Materials 15, no. 21: 7435. https://doi.org/10.3390/ma15217435
APA StyleLiang, L., Shen, X., Zhou, M., Chen, Y., Lu, X., Zhang, L., Wang, W., & Shen, J.-W. (2022). Theoretical Evaluation of Potential Cytotoxicity of Graphene Quantum Dot to Adsorbed DNA. Materials, 15(21), 7435. https://doi.org/10.3390/ma15217435







