Preparation of Iron-Doped Titania Nanoparticles and Their UV-Blue Light-Shielding Capabilities in Polyurethane
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Fe-TiO2 Nanoparticles
2.2. Preparation of Fe-TiO2/PU Nanocomposites Films
2.3. Characterization
3. Results and Discussion
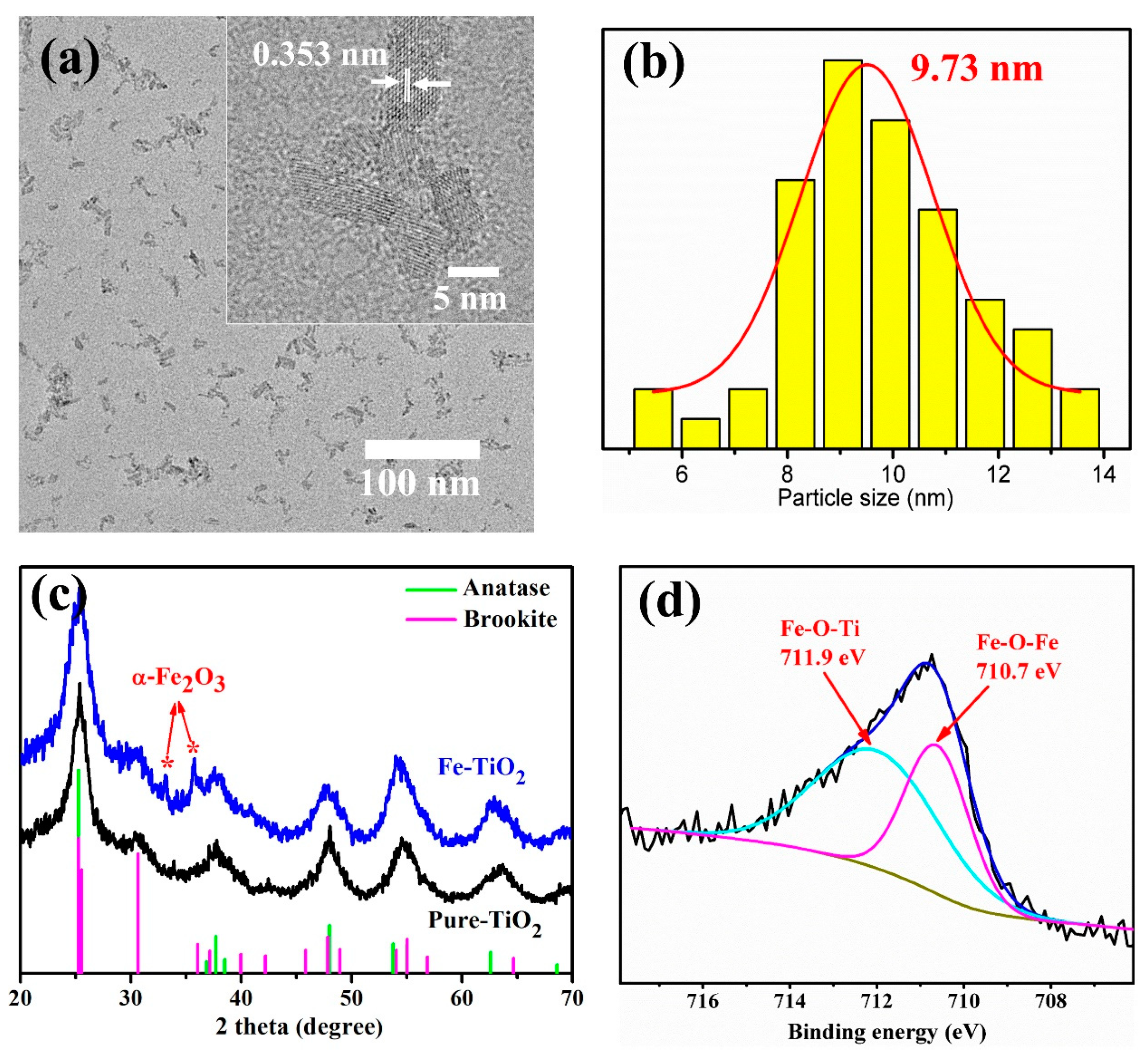
3.1. Structure of Fe-TiO2 Nanoparticles
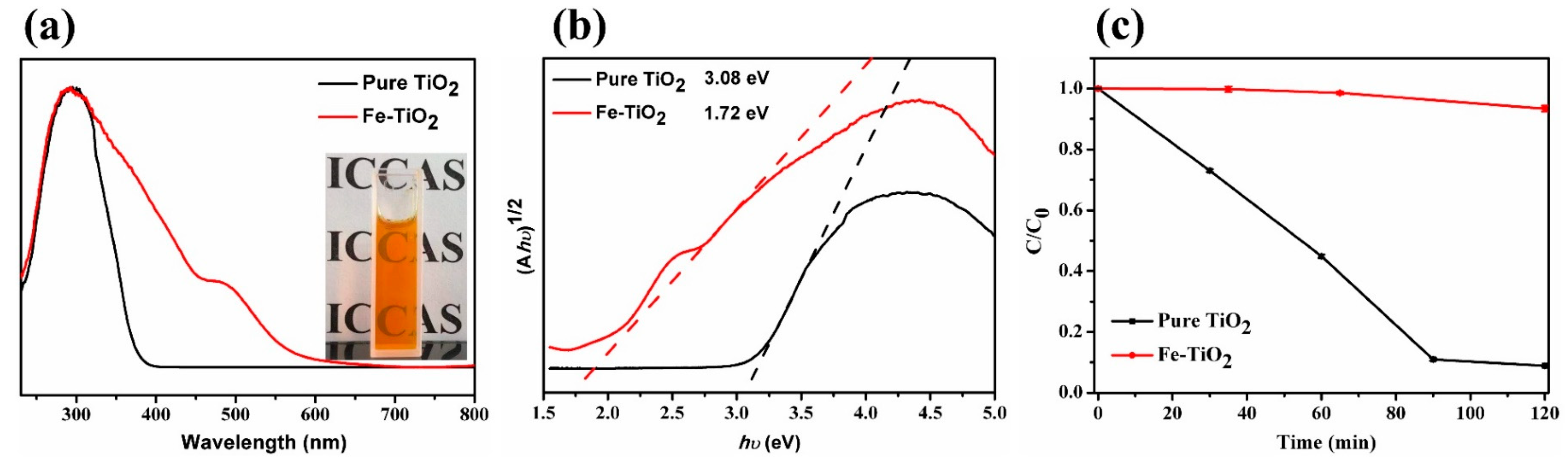
3.2. Light Absorption and Photocatalysis of Fe-TiO2 Nanoparticles
3.3. UV-Blue Light-Shielding Performance of Fe-TiO2/PU Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, R.E.; Kondratick, C.M.; Prakash, S.; Prakash, L. hRAD30 Mutations in the Variant Form of Xeroderma Pigmentosum. Science 1999, 285, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Kielbassa, C.; Roza, L.; Epe, B. Wavelength dependence of oxidative DNA damage induced by UV and visible light. Carcinogenesis 1997, 18, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhang, T.; Bryant, P.; Kurusingal, V.; Colwell, J.M.; Laycock, B. Degradation and stabilization of polyurethane elastomers. Prog. Polym. Sci. 2019, 90, 211–268. [Google Scholar]
- Qu, C.; Hu, J.; Liu, X.; Li, Z.; Ding, Y. Morphology and Mechanical Properties of Polyimide Films: The Effects of UV Irradiation on Microscale Surface. Materials 2017, 10, 1329. [Google Scholar] [CrossRef]
- Shi, Z.; Zou, C.; Zhou, F.; Zhao, J. Analysis of the Mechanical Properties and Damage Mechanism of Carbon Fiber/Epoxy Composites under UV Aging. Materials 2022, 15, 2919. [Google Scholar] [CrossRef] [PubMed]
- Mirhosseini, R.; Schubert, M.F.; Chhajed, S.; Cho, J.; Kim, J.K.; Schubert, E.F. Improved color rendering and luminous efficacy in phosphor-converted white light-emitting diodes by use of dual-blue emitting active regions. Opt. Express 2009, 17, 10806–10813. [Google Scholar] [CrossRef]
- Tsai, S.-C.; Li, M.-J.; Fang, H.-C.; Tu, C.-H.; Liu, C.-P. Efficiency enhancement of blue light emitting diodes by eliminating V-defects from InGaN/GaN multiple quantum well structures through GaN capping layer control. Appl. Surf. Sci. 2018, 439, 1127–1132. [Google Scholar] [CrossRef]
- Wu, J.; Seregard, S.; Algvere, P.V. Photochemical Damage of the Retina. Surv. Ophthalmol. 2006, 51, 461–481. [Google Scholar] [CrossRef] [PubMed]
- Yuda, E.; Ogasawara, H.; Yoshida, Y.; Hayano, J. Suppression of vagal cardiac modulation by blue light in healthy subjects. J. Physiol. Anthropol. 2016, 35, 24. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Yun, J.; Yoon, Y.D.; Park, S.I.; Seo, Y.J.; Park, W.S.; Chu, H.Y.; Park, K.H.; Lee, M.Y.; Lee, C.W.; et al. Blue light effect on retinal pigment epithelial cells by display devices. Integr. Biol. 2017, 9, 436–443. [Google Scholar] [CrossRef]
- Chen, J.; Yang, M.S.; Zhang, S.M. Immobilization of antioxidant on nanosilica and the aging resistance behavior in polypropylene. Compos. Part A Appl. Sci. Manuf. 2011, 42, 471–477. [Google Scholar] [CrossRef]
- Luo, F.; Chen, Z.; Chen, J.; Liu, P.; Ding, Y.; Zhang, S.; Gao, C.; Yang, M. Nanoparticle layer via UV-induced directional migration of iron-doped titania nanoparticles in polyvinyl butyral films and superior UV-stability. Polymer 2022, 254, 125107. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Ren, J.; Zhang, C. TiO2-KH550 Nanoparticle-Reinforced PVA/xylan Composite Films with Multifunctional Properties. Materials 2018, 11, 1589. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Lin, M.; Zhang, S.; Li, L.; Fu, Q.; Hou, J. A Self-Healing Coating with UV-Shielding Property. Coatings 2019, 9, 421. [Google Scholar] [CrossRef]
- Rahman, S.; Nawaz, R.; Khan, J.A.; Ullah, H.; Irfan, M.; Glowacz, A.; Lyp-Wronska, K.; Wzorek, L.; Khan, M.K.A.; Jalalah, M.; et al. Synthesis and Characterization of Carbon and Carbon-Nitrogen Doped Black TiO2 Nanomaterials and Their Application in Sonophotocatalytic Remediation of Treated Agro-Industrial Wastewater. Materials 2021, 14, 6175. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.I.; Li, W.; Huang, C.P.; Jung, O.; Ni, C. Study of Nd3+, Pd2+, Pt4+, and Fe3+ dopant effect on photoreactivity of TiO2 nanoparticles. Proc. Natl. Acad. Sci. USA 2002, 99, 6482. [Google Scholar] [CrossRef] [PubMed]
- Livraghi, S.; Paganini, M.C.; Giamello, E.; Selloni, A.; di Valentin, C.; Pacchioni, G. Origin of Photoactivity of Nitrogen-Doped Titanium Dioxide under Visible Light. J. Am. Chem. Soc. 2006, 128, 15666–15671. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.A.; Limas-Ballesteros, R.; López, T.; Moreno, A.; Gómez, R.; Novaro, O.; Bokhimi, X. Quantitative Determination of Titanium Lattice Defects and Solid-State Reaction Mechanism in Iron-Doped TiO2 Photocatalysts. J. Phys. Chem. B 2001, 105, 9692–9698. [Google Scholar] [CrossRef]
- Han, C.; Wang, F.; Gao, C.; Liu, P.; Ding, Y.; Zhang, S.; Yang, M. Transparent epoxy–ZnO/CdS nanocomposites with tunable UV and blue light-shielding capabilities. J. Mater. Chem. C 2015, 3, 5065–5072. [Google Scholar] [CrossRef]
- Demir, M.M.; Wegner, G. Challenges in the Preparation of Optical Polymer Composites With Nanosized Pigment Particles: A Review on Recent Efforts. Macromol. Mater. Eng. 2012, 297, 838–863. [Google Scholar] [CrossRef]
- Kojio, K.; Furukawa, M.; Nonaka, Y.; Nakamura, S. Control of Mechanical Properties of Thermoplastic Polyurethane Elastomers by Restriction of Crystallization of Soft Segment. Materials 2010, 3, 5097–5110. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Su, J.; Li, T.; Ma, P.; Bai, H.; Xie, Y.; Chen, M.; Dong, W. A Novel UV-Shielding and Transparent Polymer Film: When Bioinspired Dopamine–Melanin Hollow Nanoparticles Join Polymers. ACS Appl. Mater. Interfaces 2017, 9, 36281–36289. [Google Scholar] [CrossRef] [PubMed]
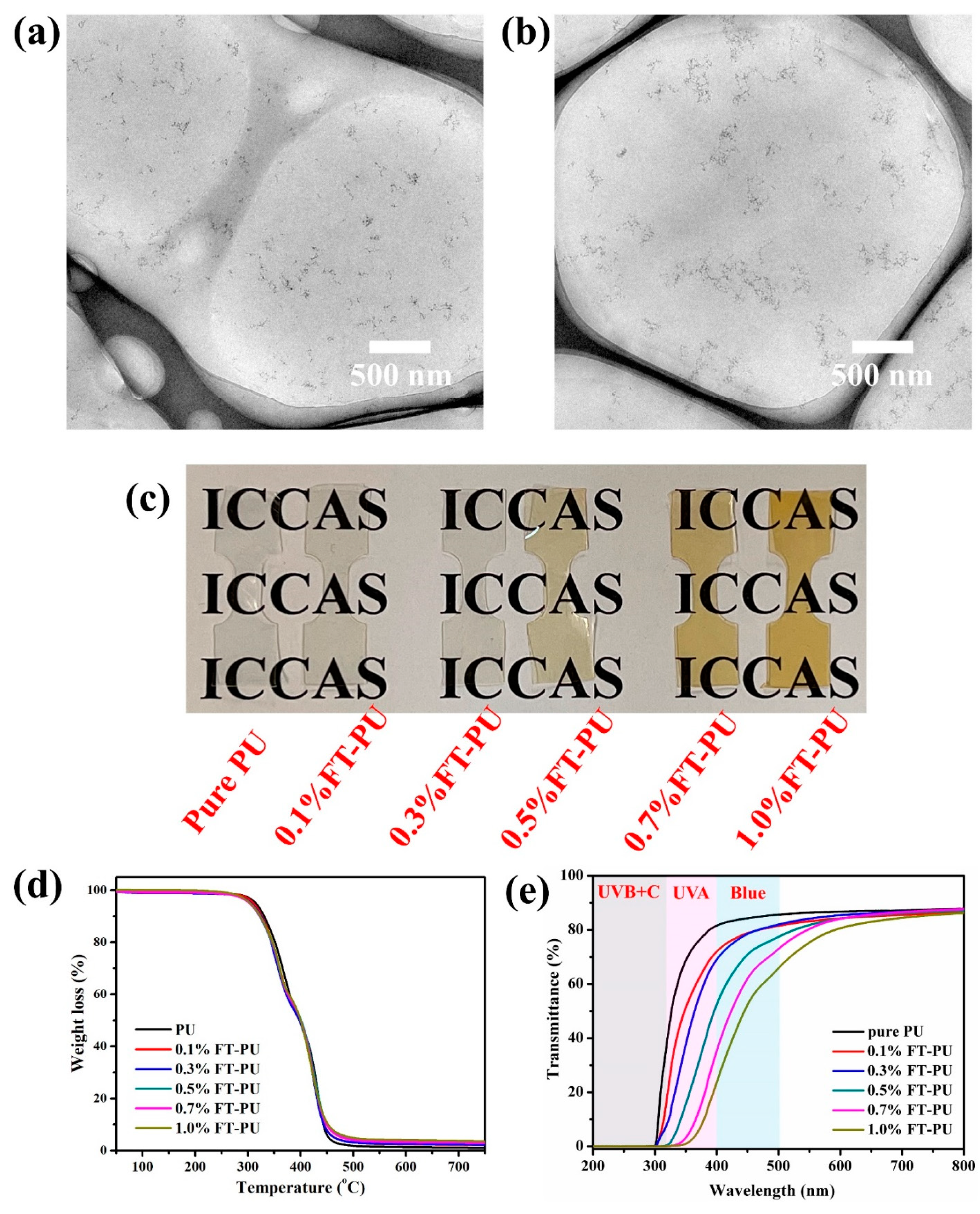
| Sample | UVB (280–320 nm) | UVA (320–400 nm) | Blue (400–500 nm) | Ave-T (500–800 nm) |
|---|---|---|---|---|
| PU | 89.84 | 30.92 | 15.92 | 86.92 |
| 0.1%FT-PU | 96.56 | 46.50 | 21.92 | 84.66 |
| 0.3%FT-PU | 98.00 | 56.59 | 22.58 | 85.71 |
| 0.5%FT-PU | 99.91 | 76.13 | 31.32 | 84.47 |
| 0.7%FT-PU | 99.97 | 88.98 | 41.02 | 84.12 |
| 1.0%FT-PU | 99.93 | 93.91 | 50.73 | 80.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baimanova, R.; Luo, F.; Yang, M. Preparation of Iron-Doped Titania Nanoparticles and Their UV-Blue Light-Shielding Capabilities in Polyurethane. Materials 2022, 15, 7370. https://doi.org/10.3390/ma15207370
Baimanova R, Luo F, Yang M. Preparation of Iron-Doped Titania Nanoparticles and Their UV-Blue Light-Shielding Capabilities in Polyurethane. Materials. 2022; 15(20):7370. https://doi.org/10.3390/ma15207370
Chicago/Turabian StyleBaimanova, Regina, Fushuai Luo, and Mingshu Yang. 2022. "Preparation of Iron-Doped Titania Nanoparticles and Their UV-Blue Light-Shielding Capabilities in Polyurethane" Materials 15, no. 20: 7370. https://doi.org/10.3390/ma15207370
APA StyleBaimanova, R., Luo, F., & Yang, M. (2022). Preparation of Iron-Doped Titania Nanoparticles and Their UV-Blue Light-Shielding Capabilities in Polyurethane. Materials, 15(20), 7370. https://doi.org/10.3390/ma15207370






