Ni/(R2O3,CaO) Nanocomposites Produced by the Exsolution of R1.5Ca0.5NiO4 Nickelates (R = Nd, Sm, Eu): Rare Earth Effect on the Catalytic Performance in the Dry Reforming and Partial Oxidation of Methane
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Characterization
2.3. Catalytic Experiments
3. Results and Discussion
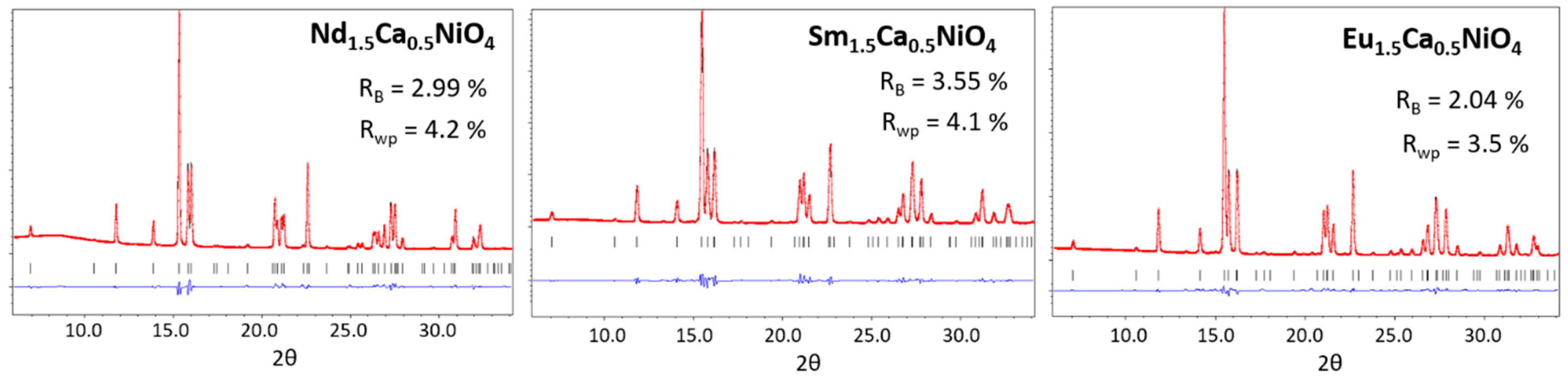
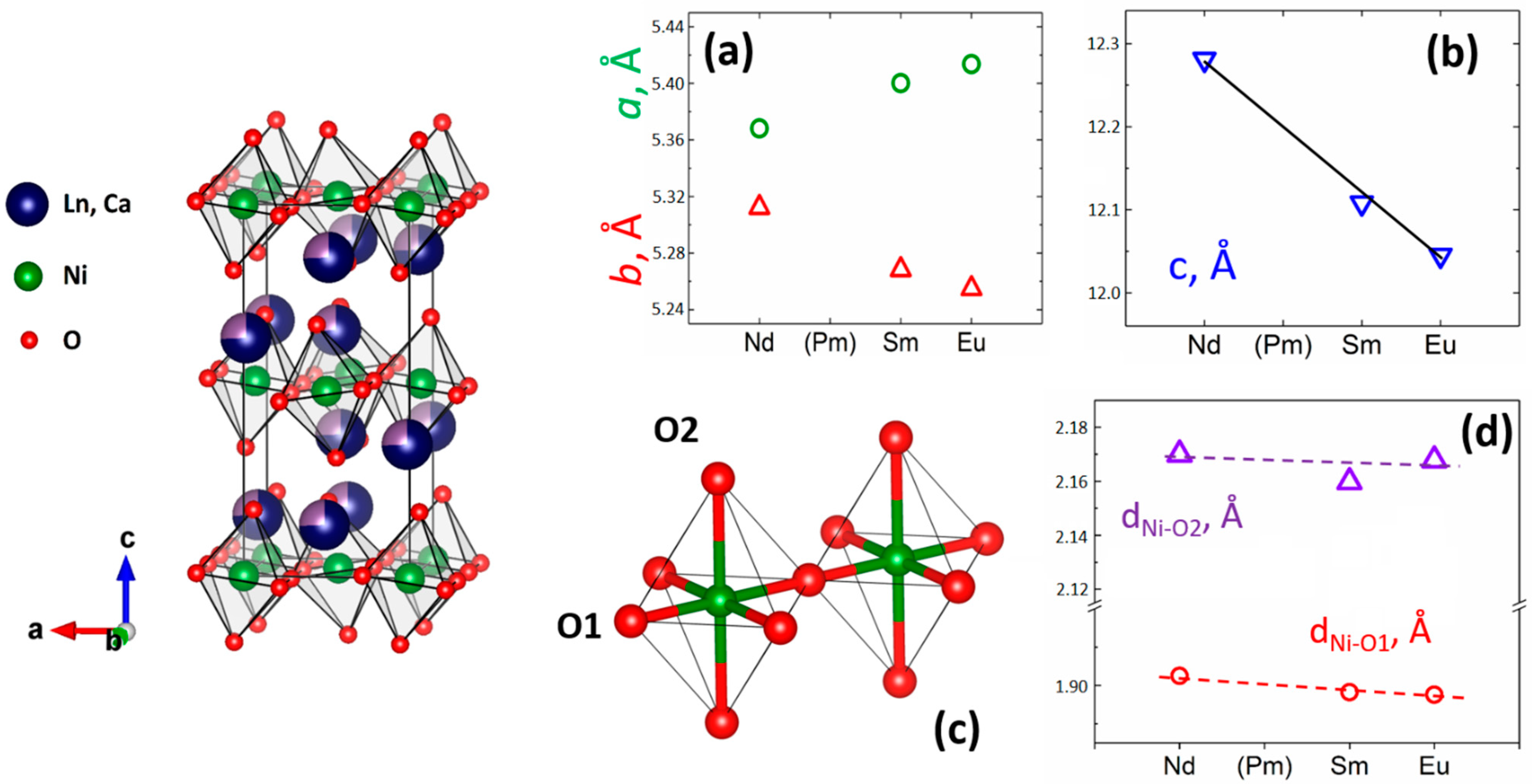
3.1. Synthesis of R1.5Ca0.5NiO4
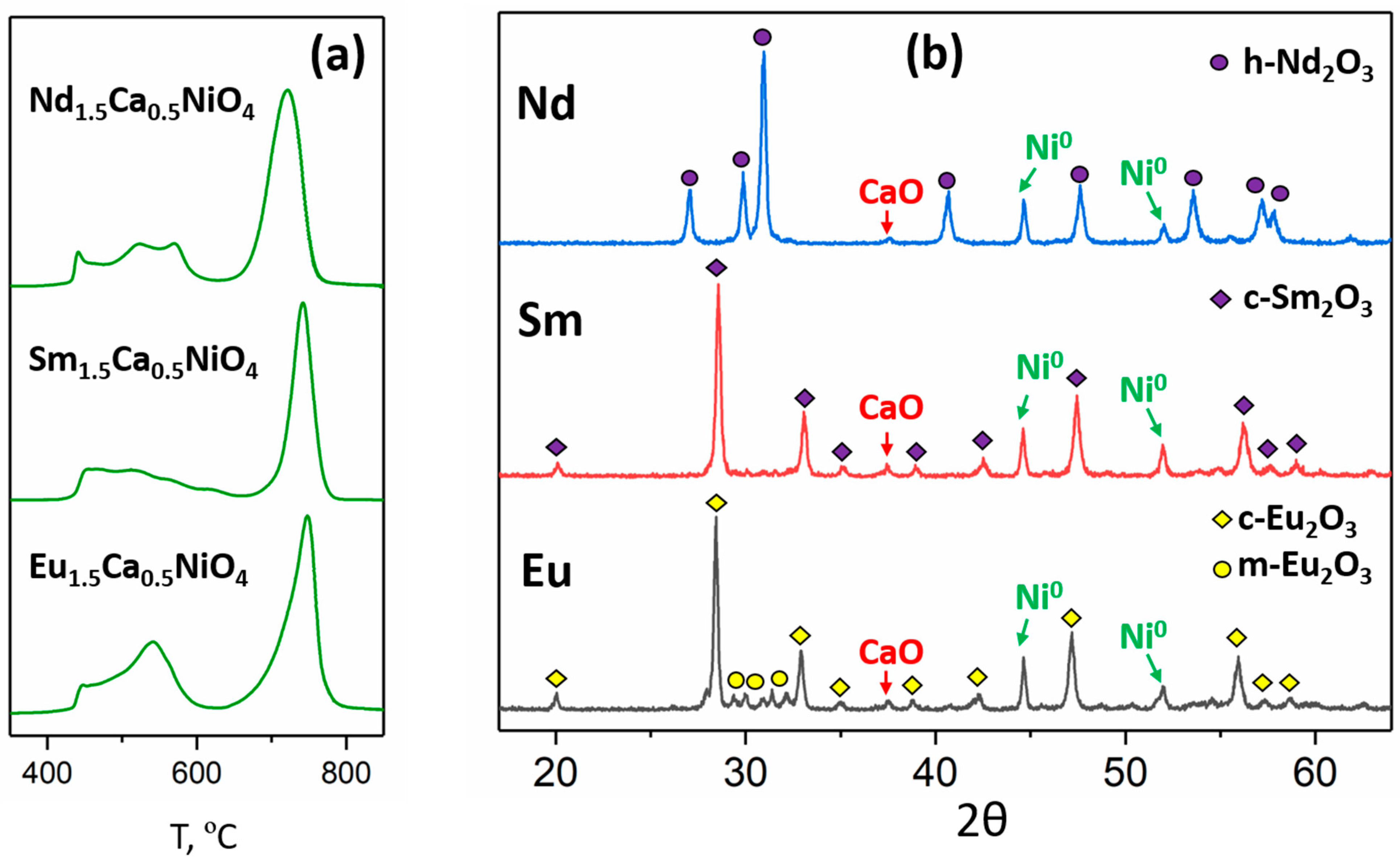
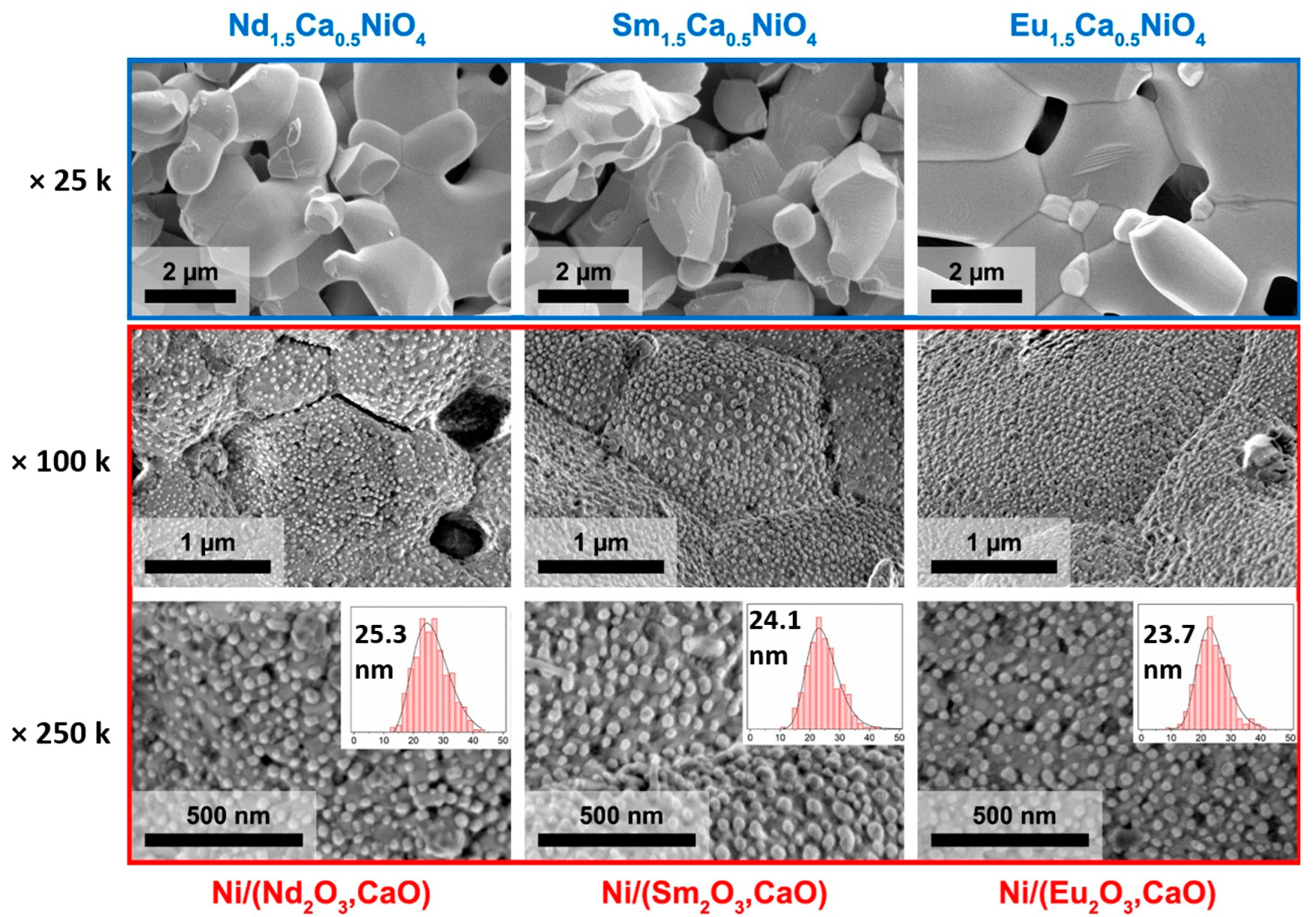
3.2. Synthesis of Ni/(R2O3,CaO) Composites
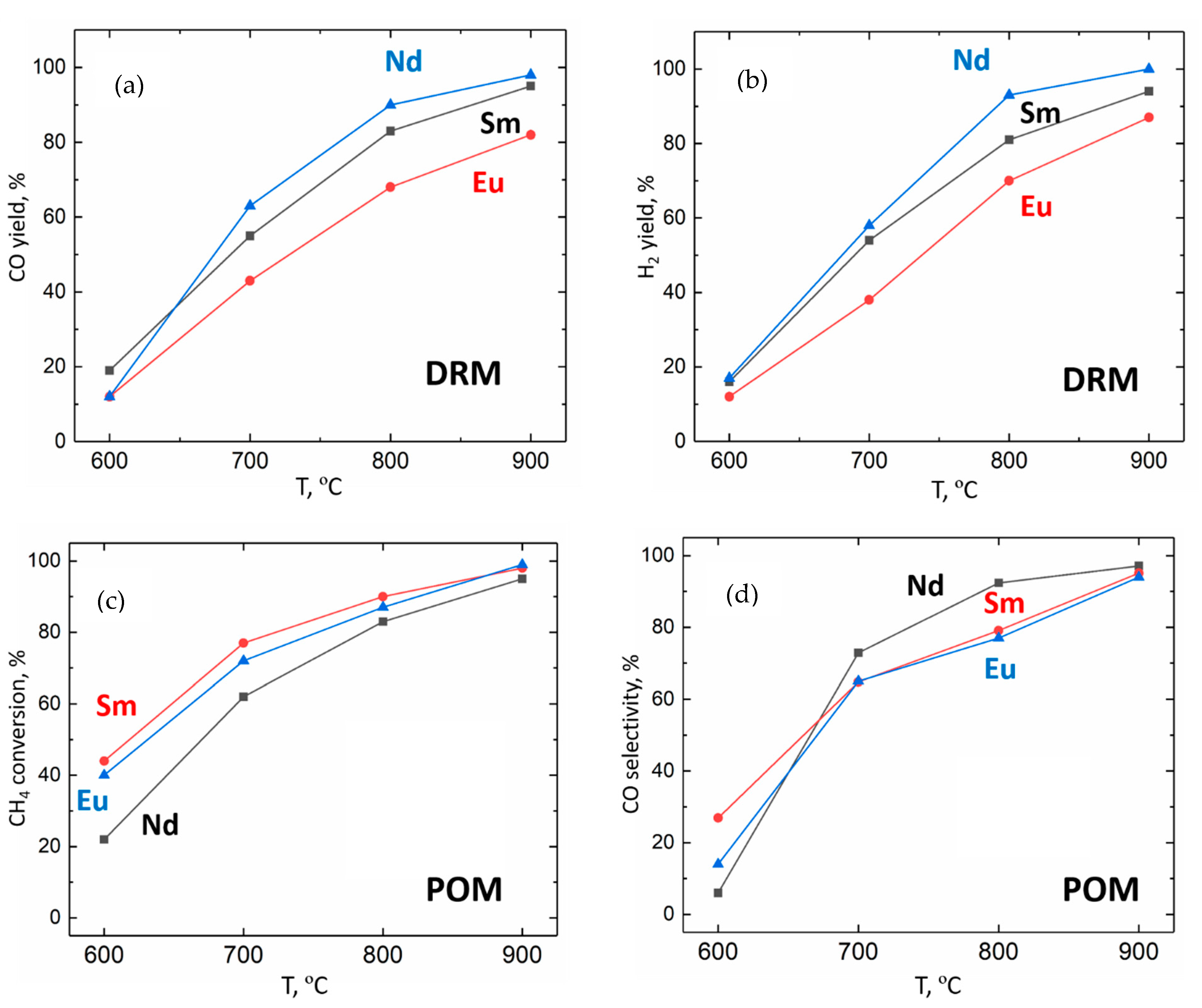
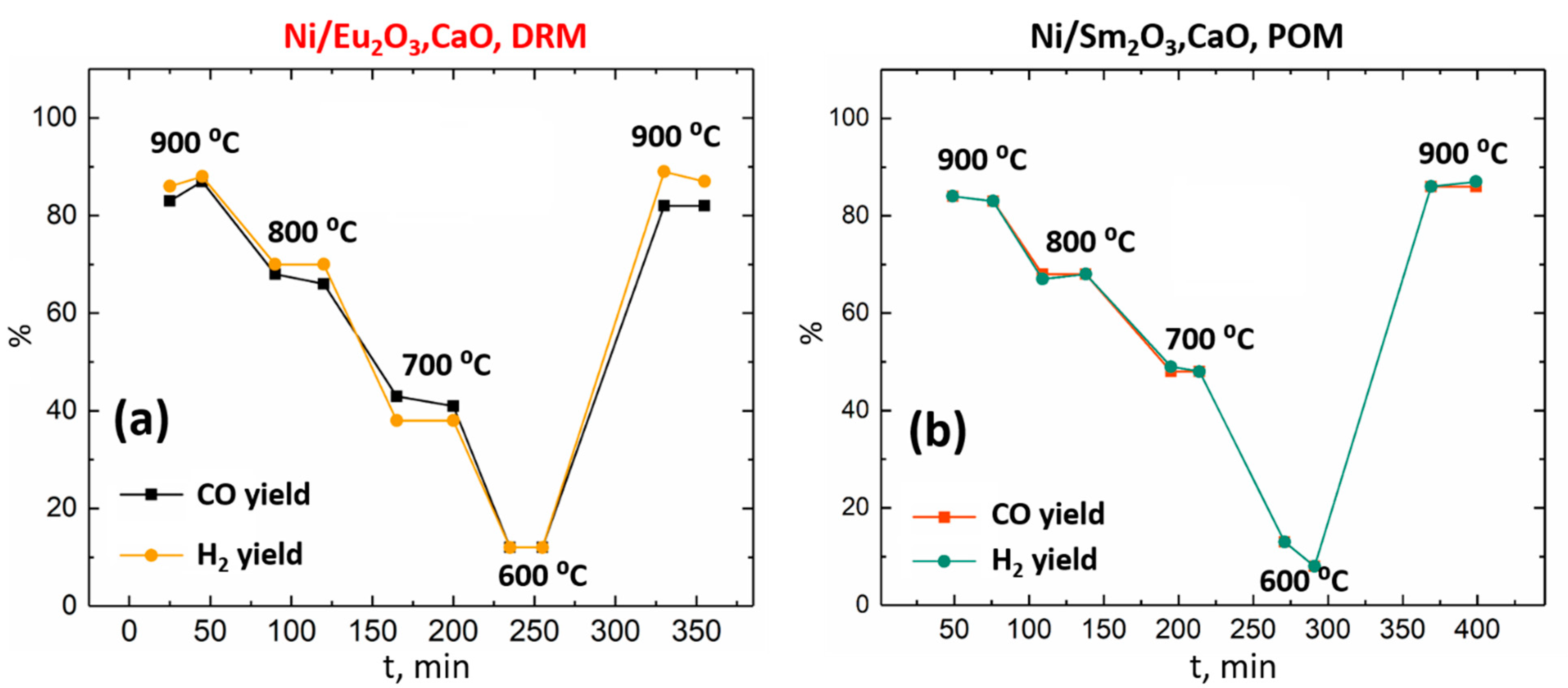
3.3. DRM and POM Catalytic Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, M.; Sun, Z.; Hu, Y.H. Catalysts for CO2 reforming of CH4: A review. J. Mater. Chem. A 2021, 9, 12495. [Google Scholar] [CrossRef]
- Zhang, X.; Vajglova, Z.; Maki-Arvela, P.; Peurla, M.; Palonen, H.; Murzin, D.Y.; Tungatarova, S.A.; Baizhumanova, T.S.; Aubakirov, Y.A. Mono- and Bimetallic Ni-Co Catalysts in Dry Reforming of Methane. ChemistrySelect 2021, 6, 3424–3434. [Google Scholar] [CrossRef]
- Arku, P.; Regmi, B.; Dutta, A. A review of catalytic partial oxidation of fossil fuels and biofuels: Recent advances in catalyst development and kinetic modeling. Chem. Eng. Res. Design 2018, 136, 385–402. [Google Scholar] [CrossRef]
- Alshihri, S.; Almegren, H. Advances in Selective Oxidation of Methane; InTechOpen, London, UK. 2020. Available online: https://www.intechopen.com/chapters/67664 (accessed on 14 October 2022).
- Konnerth, H.; Matsagar, B.M.; Chen, S.S.; Prechtl, M.H.G.; Shieh, F.K.; Wu, K.C.W. Metal-organic framework (MOF)-derived catalysts for fine chemical production. Coord. Chem. Rev. 2020, 416, 213319. [Google Scholar] [CrossRef]
- Liao, Y.T.; Chi, N.V.; Ishiguro, N.; Young, A.P.; Tsung, C.K.; Wu, K.C.-W. Engineering a homogeneous alloy-oxide interface derived from metal-organic frameworks for selective oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid. Appl. Catal. B 2020, 270, 118805. [Google Scholar] [CrossRef]
- Elbadawi, A.H.; Ge, L.; Li, Z.; Liu, S.; Wang, S.; Zhu, Z. Catalytic partial oxidation of methane to syngas: Review of perovskite catalysts and membrane reactors. Catal. Rev. 2021, 63, 1–67. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Chen, M.; Liang, D.; Yang, Z.; Cheng, W.; Tang, Z.; Jun Wang, J.; Zhang, H. Recent advances during CH4 dry reforming for syngas production: A mini review. Int. J. Hydrogen Energy 2021, 46, 5852–5874. [Google Scholar] [CrossRef]
- Nishihata, Y.; Mizuki, J.; Akao, T.; Tanaka, H.; Uenishi, M.; Kimura, M.; Okamoto, T.; Hamada, N. Self−regeneration of a Pd−perovskite Catalyst for Automotive Emissions Control. Nature 2002, 418, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Tsekouras, G.; Miller, D.N.; MeΓnard, H.; Irvine, J.T.S. In situ growth of nanoparticles through control of non-stoichiometry. Nat. Chem. 2013, 5, 916–923. [Google Scholar]
- Neagu, D.; Oh, T.S.; Miller, D.N.; MeΓnard, H.; Bukhari, S.M.; Gamble, S.R.; Gorte, R.J.; Vohs, J.M.; Irvine, J.T.S. Nano-socketed nickel particles with enhanced coking resistance grown in situ by redox exsolution. Nat. Comm. 2015, 6, 8120. [Google Scholar] [CrossRef]
- Kwon, O.; Joo, S.; Choi, S.; Sengodan, S.; Kim, G. Review on exsolution and its driving forces in perovskites. J. Phys. Energy 2020, 2, 032001. [Google Scholar] [CrossRef]
- Kousi, K.; Neagu, D.; Bekris, L.; Papaioannou, E.I.; Metcalfe, I.S. Endogenous Nanoparticles Strain Perovskite Host Lattice Providing Oxygen Capacity and Driving Oxygen Exchange and CH4 Conversion to Syngas. Angew. Chem. Int. Ed. 2020, 59, 2510–2519. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Sayono, S.; Junzunza, J.; Pan, R.; Xu, M.; Pan, X.; Gilliard-AbdulAziz, K.L. The effects of stoichiometry on the properties of exsolved Ni-Fe alloy nanoparticles for dry methane reforming. AIChE J. 2020, 66, e17078. [Google Scholar] [CrossRef]
- Shlyakhtin, O.A.; Malyshev, S.A.; Loktev, A.S.; Mazo, G.N.; Garshev, A.V.; Chumakov, R.G.; Dedov, A.G. Synthesis and decomposition of Nd2−yCayCo1−xNixO4: The effect of resynthesis on the catalytic performance of decomposition products in the partial oxidation of methane. ACS Appl. Energy Mater. 2021, 4, 7661–7673. [Google Scholar] [CrossRef]
- Malyshev, S.A.; Shlyakhtin, O.A.; Loktev, A.S.; Mazo, G.N.; Timofeev, G.M.; Mukhin, I.E.; Kaplin, I.Y.; Svetogorov, R.D.; Valeev, R.G.; Dedov, A.G. Exsolution-like synthesis of Ni/(Nd2O3,CaO) nanocomposites from Nd2−xCaxNiO4 precursors for catalytic applications. J. Solid State Chem. 2022, 312, 123267. [Google Scholar] [CrossRef]
- Bhattar, S.; Ashraful Abedin, M.; Kanitkar, S.; Spivey, J.J. A review on dry reforming of methane over perovskite derived catalysts. Catal Today 2021, 365, 2–23. [Google Scholar] [CrossRef]
- Cihlar, J., Jr.; Vrba, R.; Castkova, K.; Cihlar, J. Effect of transition metal on stability and activity of La-Ca-M-(Al)-O (M = Co, Cr, Fe and Mn) perovskite oxides during partial oxidation of methane. Int. J. Hydrogen Energy 2017, 42, 19920–19934. [Google Scholar] [CrossRef]
- Valderrama, G.; Kiennemann, A.; Urbina de Navarro, C.; Goldwasser, M.R. LaNi1−xMnxO3 perovskite-type oxides as catalysts precursors for dry reforming of methane. Appl. Catal. A 2018, 565, 26–33. [Google Scholar] [CrossRef]
- Shah, M.; Das, S.; Nayak, A.K.; Mondal, P.; Bordoloi, A. Smart designing of metal-support interface for imperishable dry reforming catalyst. Appl. Catal. A 2018, 556, 137.e54. [Google Scholar] [CrossRef]
- Tathod, A.P.; Hayek, N.; Shpasser, D.; David, S.A.; Simakov, D.S.A.; Gazit, O.M. Mediating interaction strength between nickel and zirconia using a mixed oxide nanosheets interlayer for methane dry reforming. Appl. Catal. B 2019, 249, 106–115. [Google Scholar] [CrossRef]
- Enger, B.C.; Lodeng, R.; Holmen, A. A review of catalytic partial oxidation of methane to synthesis gas with emphasis on reaction mechanisms over transition metal catalysts. Appl. Catal. A 2008, 346, 1–27. [Google Scholar] [CrossRef]
- Al-Sayari, S.A. Recent Developments in the Partial Oxidation of Methane to Syngas. Open Catalysis J. 2013, 6, 17–28. [Google Scholar] [CrossRef]
- Salaev, M.A.; Liotta, L.F.; Vodyankina, O.V. Lanthanoid-containing Ni-based catalysts for dry reforming of methane: A review. Int. J. Hydrogen Energy 2022, 47, 4489–4535. [Google Scholar] [CrossRef]
- Ozazuwa, O.U.; Abidin, S.Z. An overview on the role of lanthanide series (rare earth metals) in H2 and syngas production from CH4 reforming processes. Chem. Eng. Sci. 2020, 227, 115863. [Google Scholar] [CrossRef]
- Abdulrasheed, A.; Abdul Jalil, A.; Gambo, Y.; Ibrahim, M.; Umar Hambali, H.; Shahul Hamid, M.Y. A review on catalyst development for dry reforming of methane to syngas: Recent advances. Renew. Sust. Energy Revs. 2019, 108, 175–193. [Google Scholar] [CrossRef]
- Song, X.; Dong, X.; Yin, S.; Wang, M.; Li, M.; Wang, H. Effects of Fe partial substitution of La2NiO4/LaNiO3 catalyst precursors prepared by wet impregnation method for the dry reforming of methane. Appl. Catal. A 2016, 526, 132–138. [Google Scholar] [CrossRef]
- Bekheet, M.F.; Nezhad, P.D.K.; Bonmassar, N.; Schlicker, L.; Gili, A.; Praetz, S.; Gurlo, A.; Doran, A.; Gao, Y.; Heggen, M.; et al. Steering the Methane Dry Reforming Reactivity of Ni/La2O3 Catalysts by Controlled In Situ Decomposition of Doped La2NiO4 Precursor Structures. ACS Catal. 2021, 11, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Dama, S.; Ghodke, S.; Bobade, R.; Gurav, H.; Chilukuri, S. Tuning the dimensionality of layered Srn+1Tin−xNixO3n+1 perovskite structures for improved activity in syngas generation. J. Catalysis 2018, 360, 27–39. [Google Scholar] [CrossRef]
- Takeda, Y.; Nishijima, M.; Imanishi, N.; Kanno, R.; Yamamoto, O. Crystal chemistry and transport properties of Nd2−xAxNiO4 (A = Ca, Sr, or Ba, 0 ≤ x ≤ 1.4). J. Solid State Chem. 1992, 96, 72–83. [Google Scholar] [CrossRef]
- Song, J.; Ning, D.; Boukamp, B.; Bassat, J.-M.; Bouwmeester, H.J.M. Structure, electrical conductivity and oxygen transport properties of Ruddlesden–Popper phases Lnn+1NinO3n+1 (Ln = La, Pr and Nd; n = 1, 2 and 3). J. Mater. Chem. A 2020, 8, 22206. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.T. Effect of lanthanide (Ln = La, Nd, and Pr) doping on electrochemical performance of Ln2NiO4+δ - YSZ composite cathodes for solid oxide fuel cells. Ceram. Intern. 2021, 47, 2493–2498. [Google Scholar] [CrossRef]
- Pikalov, S.M.; Vedmid’, L.B.; Filonova, E.A.; Pikalova, E.Y.; Lyagaeva, J.G.; Danilov, N.A.; Murashkina, A.A. High-temperature behavior of calcium substituted layered neodymium nickelates. J. Alloys Compd. 2019, 801, 558–567. [Google Scholar] [CrossRef]
- Tarutin, A.P.; Gorshkov, M.Y.; Bainov, I.N.; Vdovin, G.K.; Vylkov, A.I.; Lyagaeva, J.G.; Medvedev, D.A. Barium-doped nickelates Nd2–xBaxNiO4+δ as promising electrode materials for protonic ceramic electrochemical cells. Ceram. Intern. 2020, 46, 24355–24364. [Google Scholar] [CrossRef]
- Gao, Z.; Ding, X.; Ding, D.; Ding, L. Infiltrated Pr2NiO4 as promising bi-electrode for symmetrical solid oxide fuel cells. Int. J. Hydrogen Energy 2018, 43, 8953–8961. [Google Scholar] [CrossRef]
- Dedov, A.G.; Loktev, A.S.; Komissarenko, D.A.; Mazo, G.N.; Shlyakhtin, O.A.; Parkhomenko, K.V.; Kiennemann, A.A.; Roger, A.-C.; Ishmurzin, A.V.; Moiseev, I.I. Partial oxidation of methane to produce syngas over a neodymium–calcium cobaltate-based catalyst. Appl. Catal. A 2015, 489, 140–146. [Google Scholar] [CrossRef]
- Galayda, A.P.; Volkova, N.E.; Gavrilova, L.Y.; Balymov, K.G.; Cherepanov, V.A. Phase equilibria, structure and properties of intermediate phases in the Sm2O3–Fe2O3–CoO and Sm2O3–CaO–CoO systems. J. Alloys Compd. 2017, 718, 288–297. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Ceretti, M.; Wahyudi, O.; Andre, G.; Meven, M.; Villesuzanne, A.; Paulus, W. (Pr,Nd)2NiO4+δ: Reaction intermediates and redox behavior explored by in situ neutron poeder diffraction during electrochemical oxygen intercalation. Inorg. Chem. 2018, 57, 4657–4666. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, J.; Liu, J.; Wang, C.; Huang, Q.; Xu, X.; Peng, H.; Liu, W.; Wang, X.; Zhou, W. Methane dry reforming over Ni/Mg-Al-O: On the significant promotional effects of rare earth Ce and Nd metal oxides. J. CO2 Util. 2018, 25, 242–253. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malyshev, S.A.; Shlyakhtin, O.A.; Loktev, A.S.; Mazo, G.N.; Timofeev, G.M.; Mukhin, I.E.; Svetogorov, R.D.; Roslyakov, I.V.; Dedov, A.G. Ni/(R2O3,CaO) Nanocomposites Produced by the Exsolution of R1.5Ca0.5NiO4 Nickelates (R = Nd, Sm, Eu): Rare Earth Effect on the Catalytic Performance in the Dry Reforming and Partial Oxidation of Methane. Materials 2022, 15, 7265. https://doi.org/10.3390/ma15207265
Malyshev SA, Shlyakhtin OA, Loktev AS, Mazo GN, Timofeev GM, Mukhin IE, Svetogorov RD, Roslyakov IV, Dedov AG. Ni/(R2O3,CaO) Nanocomposites Produced by the Exsolution of R1.5Ca0.5NiO4 Nickelates (R = Nd, Sm, Eu): Rare Earth Effect on the Catalytic Performance in the Dry Reforming and Partial Oxidation of Methane. Materials. 2022; 15(20):7265. https://doi.org/10.3390/ma15207265
Chicago/Turabian StyleMalyshev, Sergey A., Oleg A. Shlyakhtin, Alexey S. Loktev, Galina N. Mazo, Grigoriy M. Timofeev, Igor E. Mukhin, Roman D. Svetogorov, Ilya V. Roslyakov, and Alexey G. Dedov. 2022. "Ni/(R2O3,CaO) Nanocomposites Produced by the Exsolution of R1.5Ca0.5NiO4 Nickelates (R = Nd, Sm, Eu): Rare Earth Effect on the Catalytic Performance in the Dry Reforming and Partial Oxidation of Methane" Materials 15, no. 20: 7265. https://doi.org/10.3390/ma15207265
APA StyleMalyshev, S. A., Shlyakhtin, O. A., Loktev, A. S., Mazo, G. N., Timofeev, G. M., Mukhin, I. E., Svetogorov, R. D., Roslyakov, I. V., & Dedov, A. G. (2022). Ni/(R2O3,CaO) Nanocomposites Produced by the Exsolution of R1.5Ca0.5NiO4 Nickelates (R = Nd, Sm, Eu): Rare Earth Effect on the Catalytic Performance in the Dry Reforming and Partial Oxidation of Methane. Materials, 15(20), 7265. https://doi.org/10.3390/ma15207265








