Microstructure and Corrosion Behavior of Sn–Zn Alloys
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Phase Constitution and Microstructure of the Alloys before Corrosion
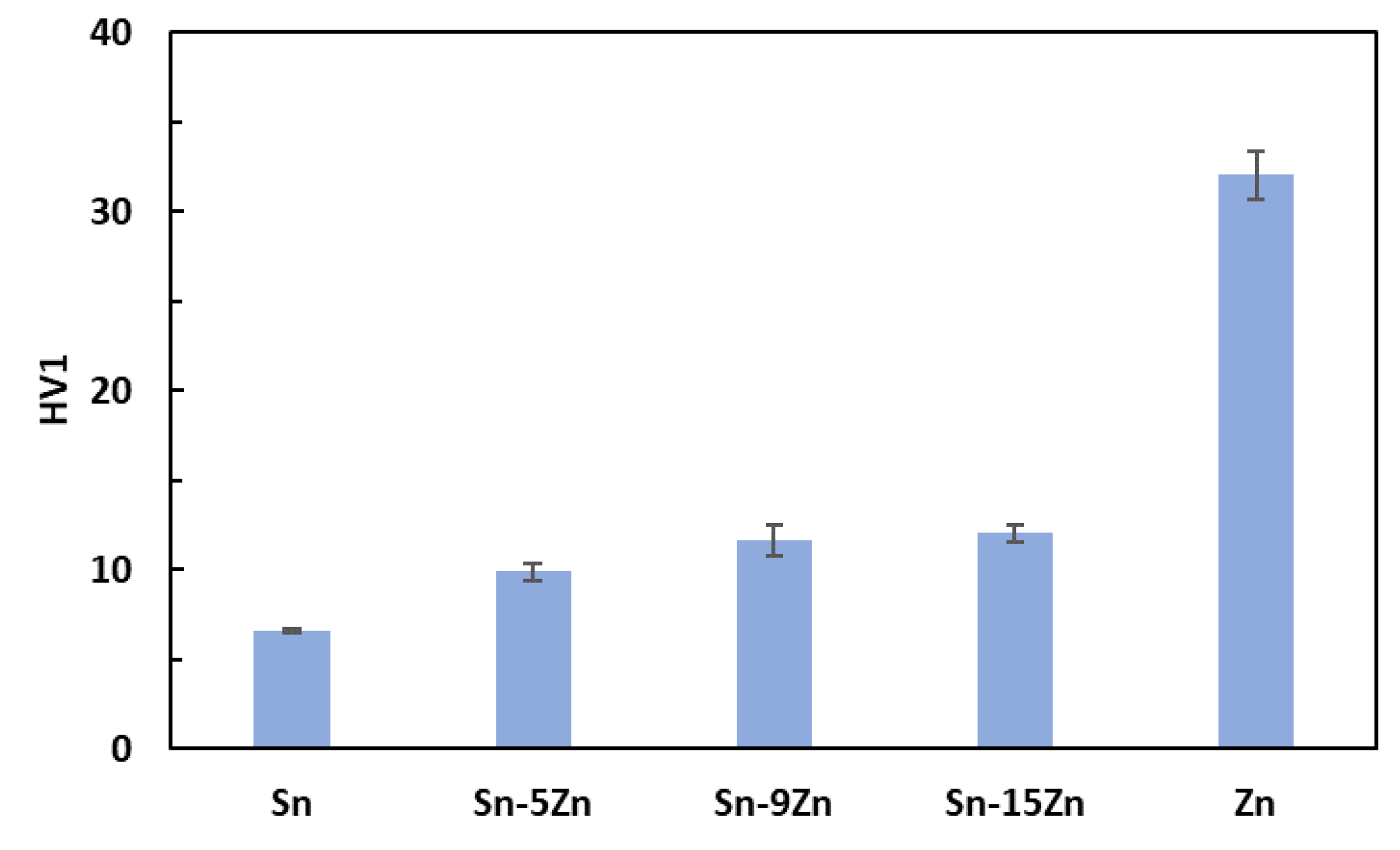
3.2. Hardness Measurements
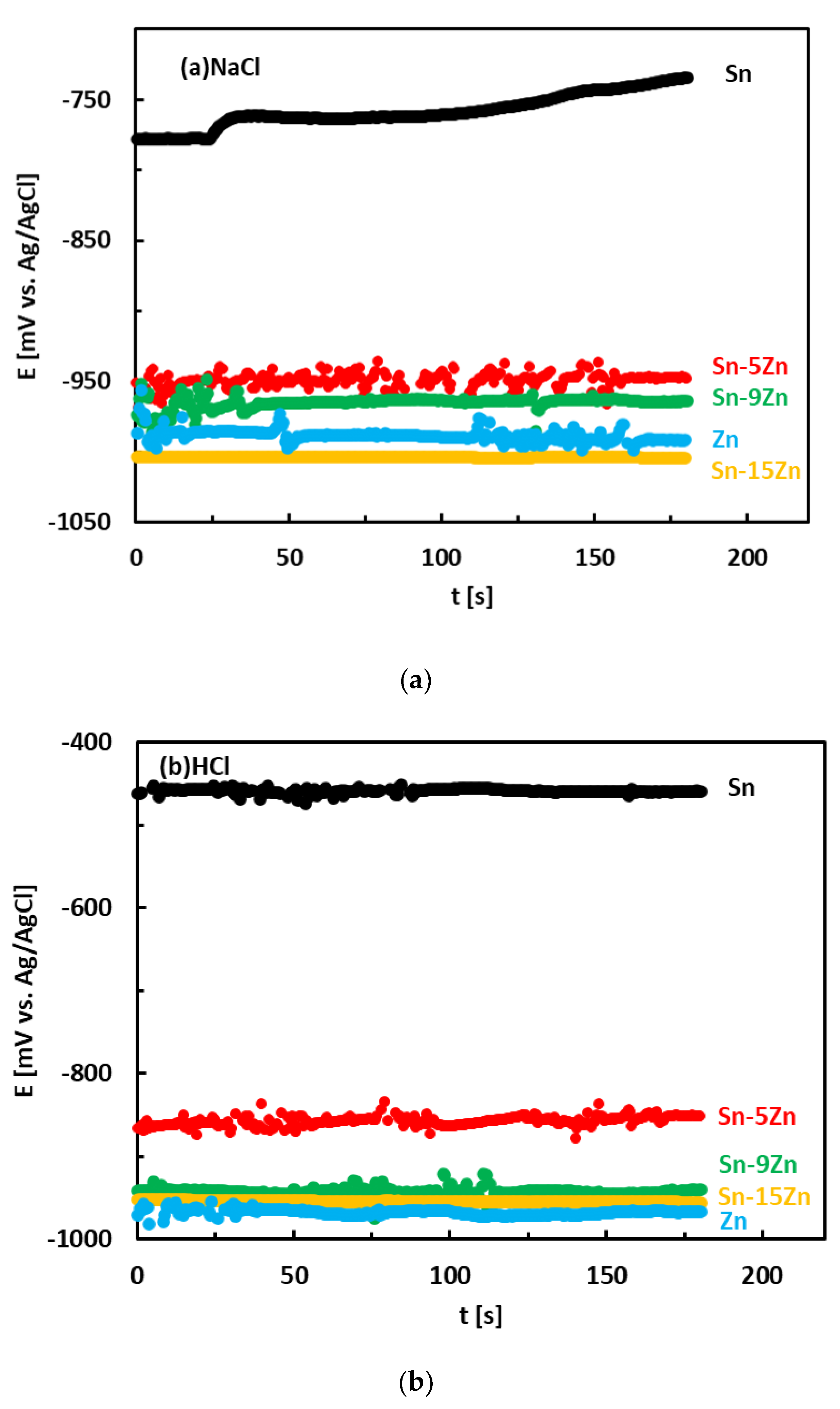
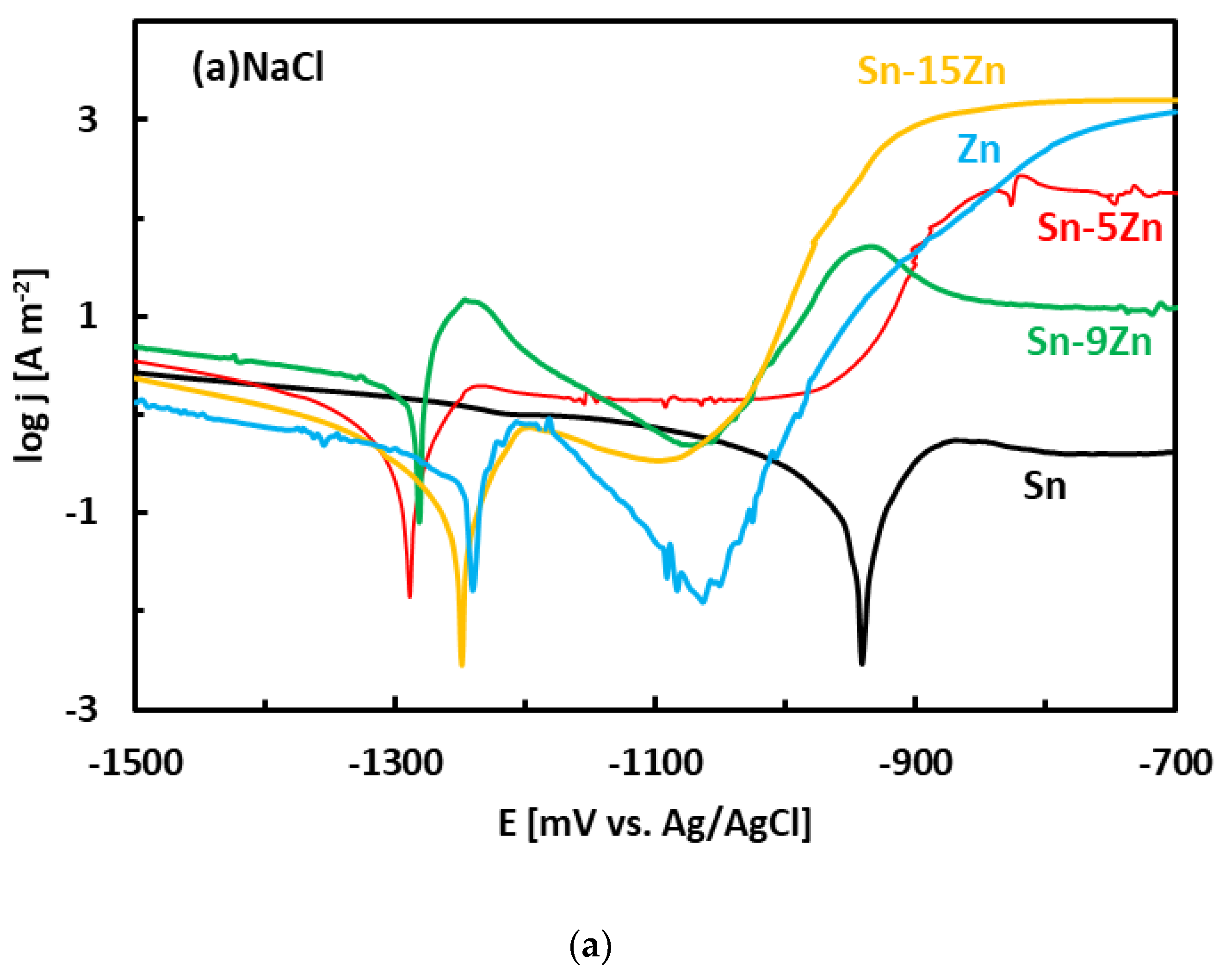
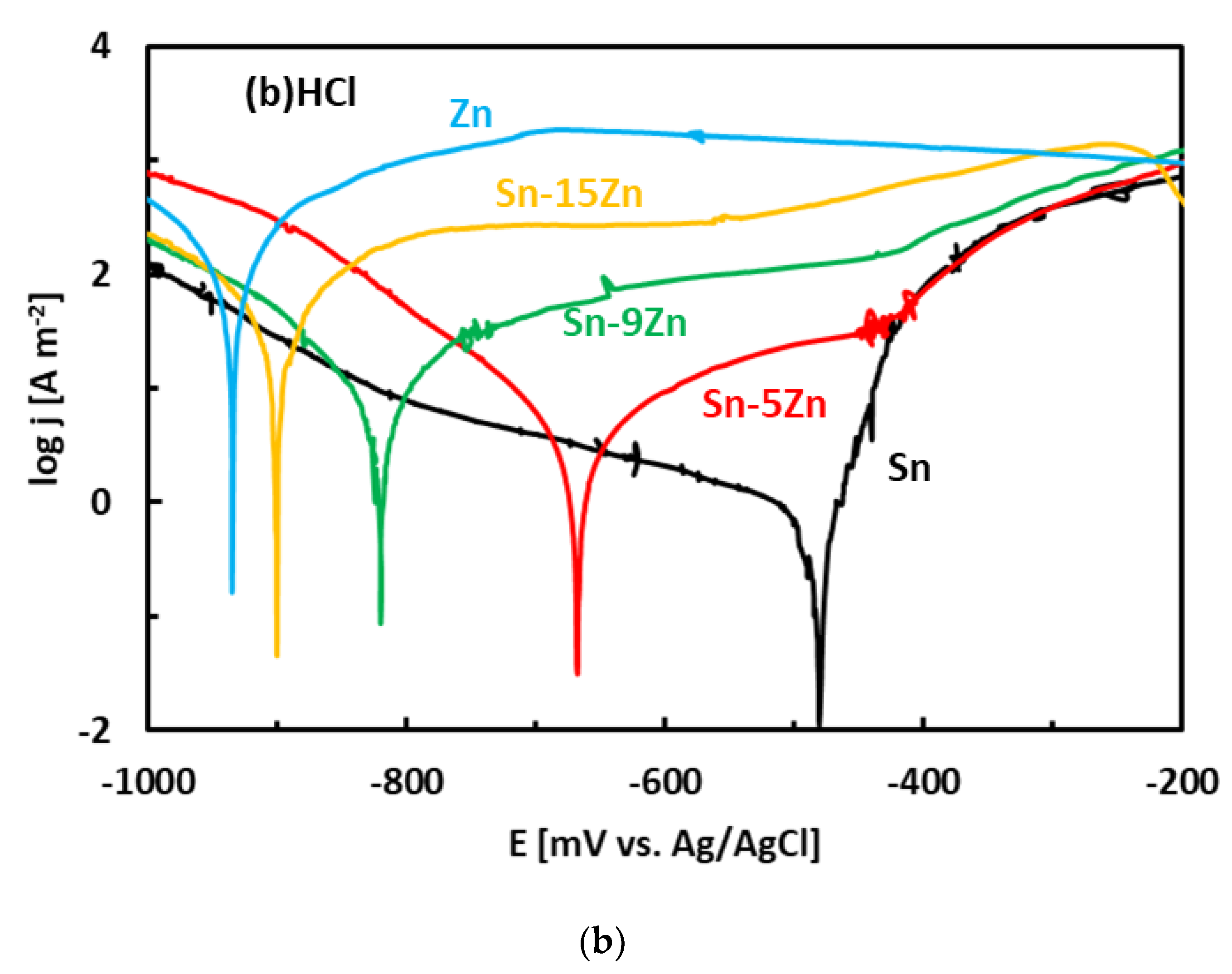
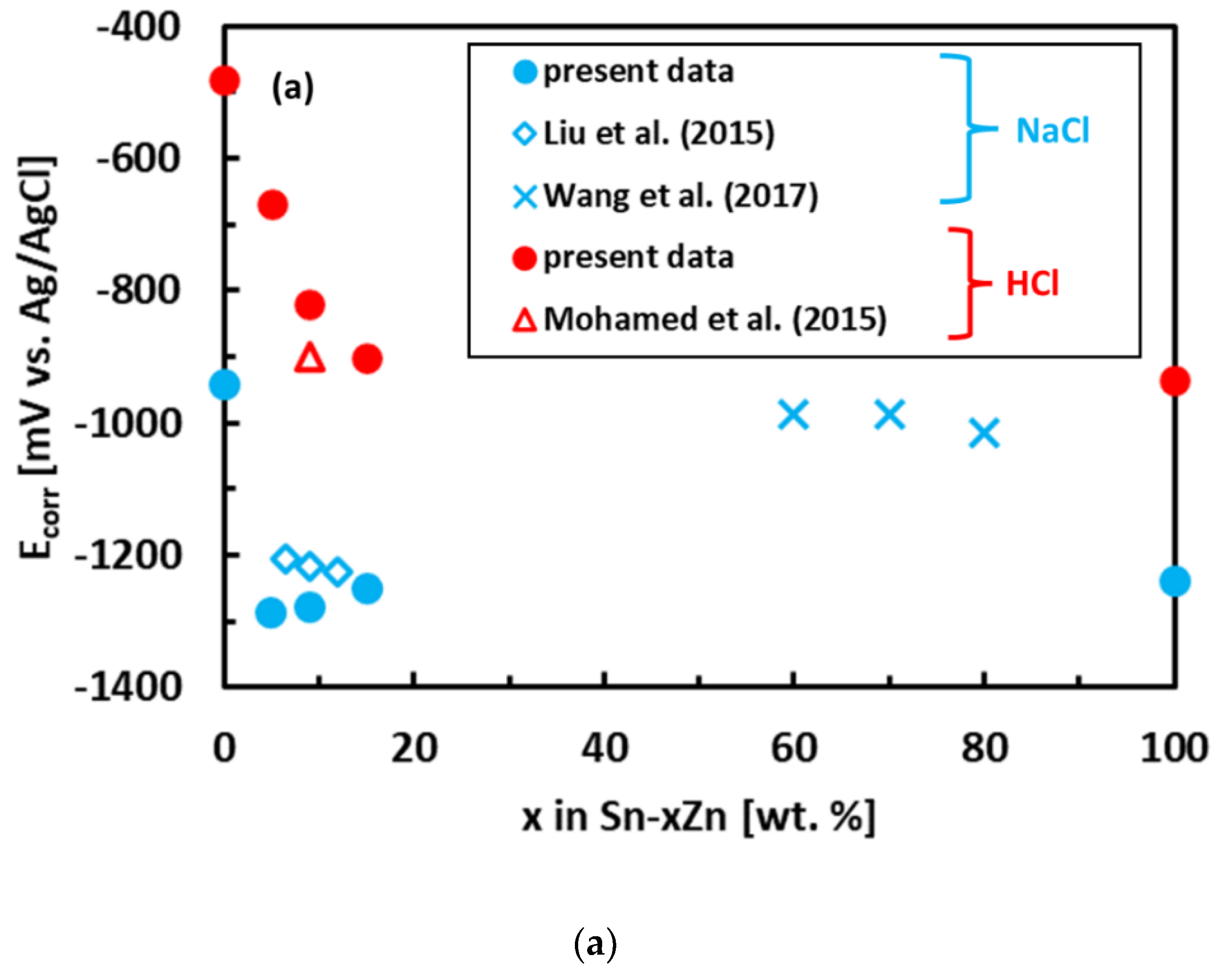
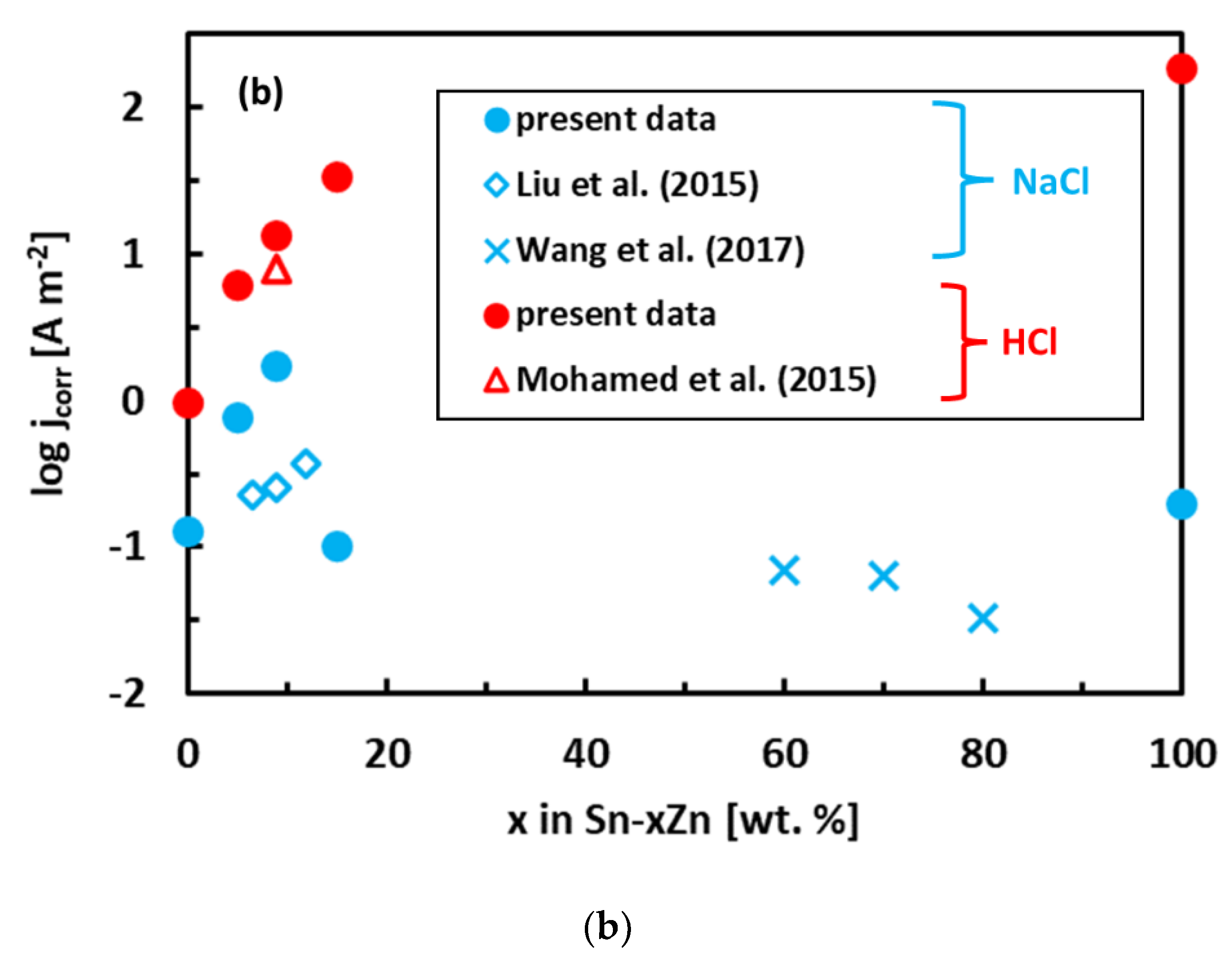
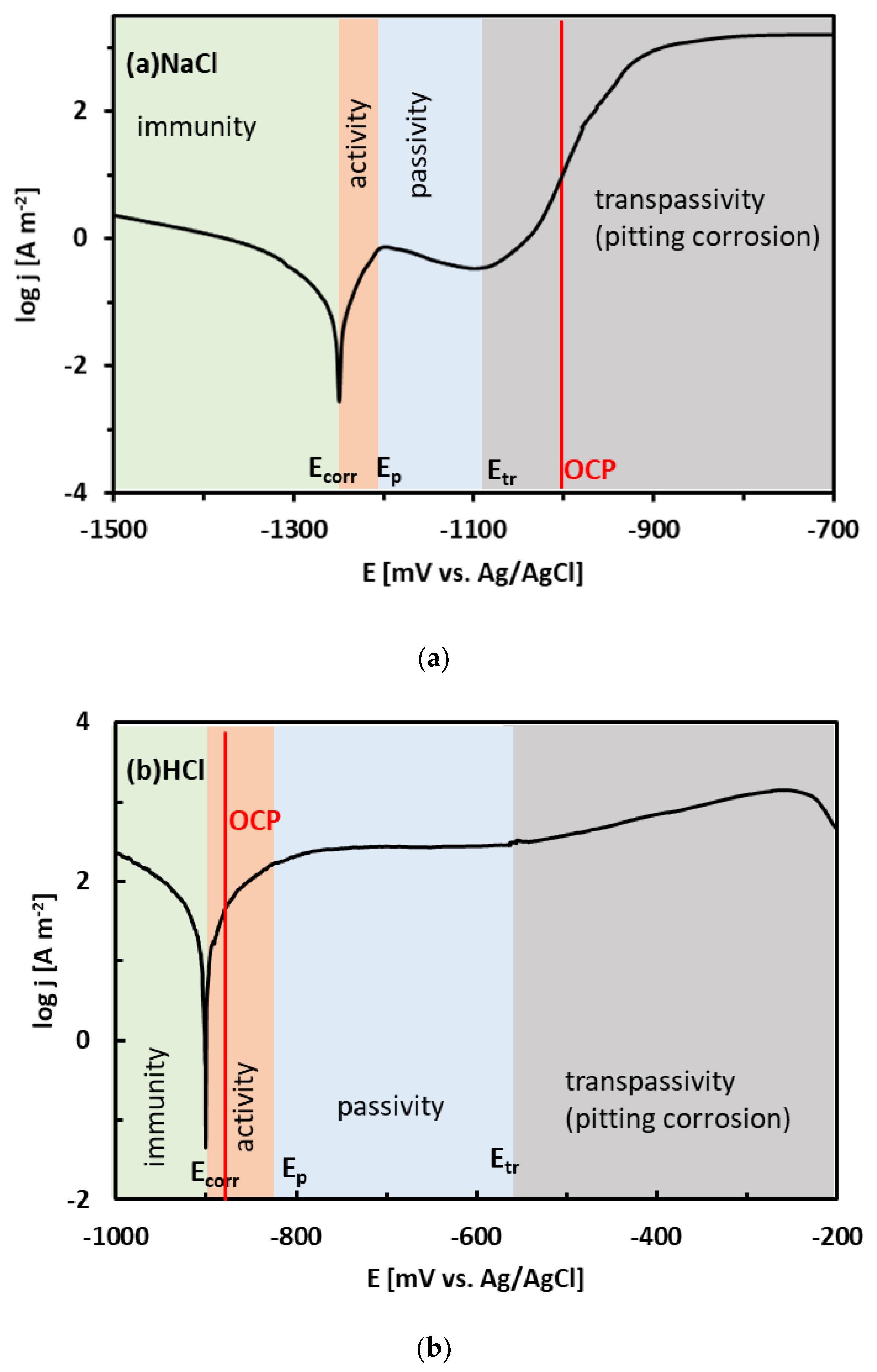
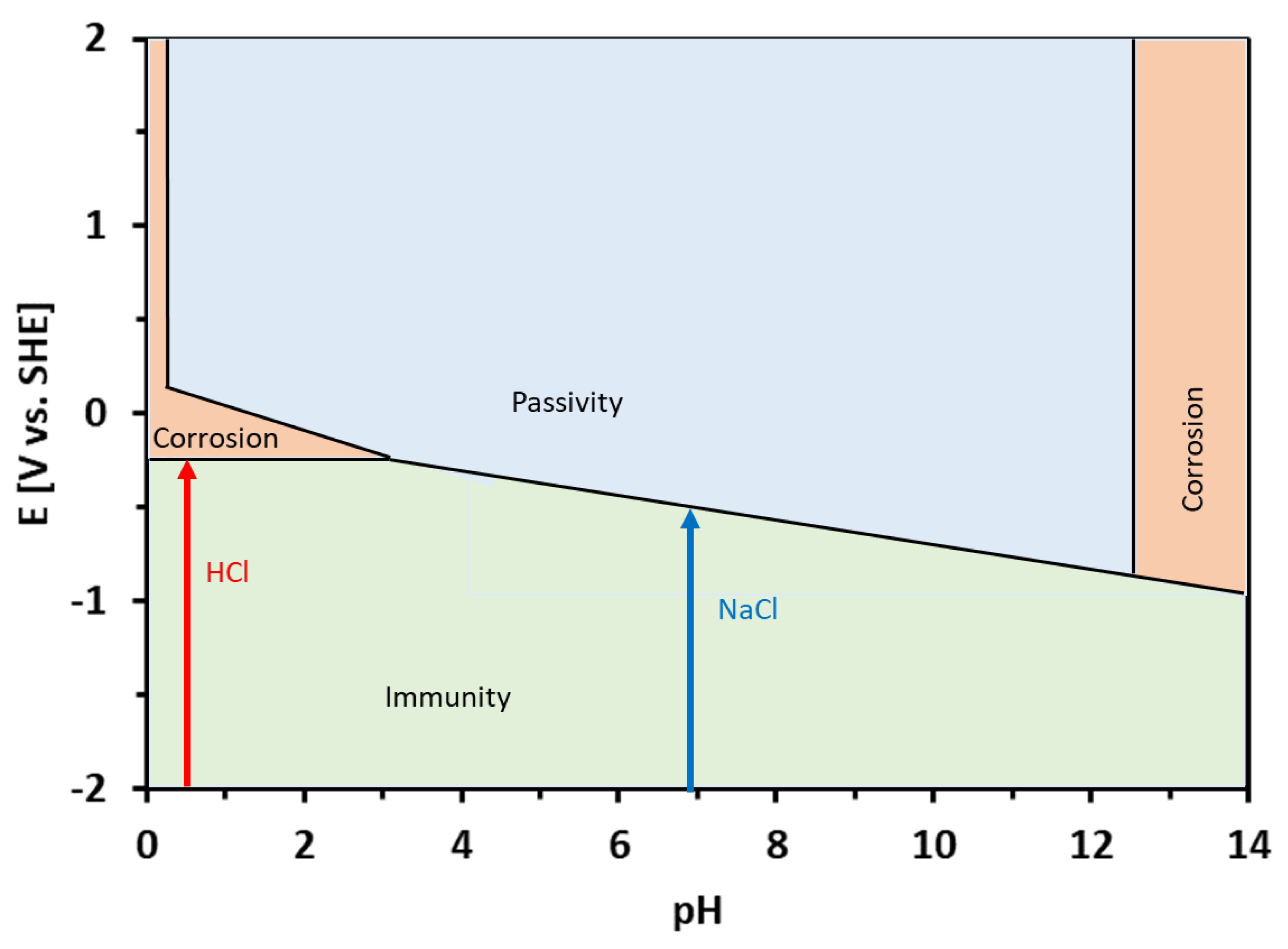
3.3. Corrosion Experiments
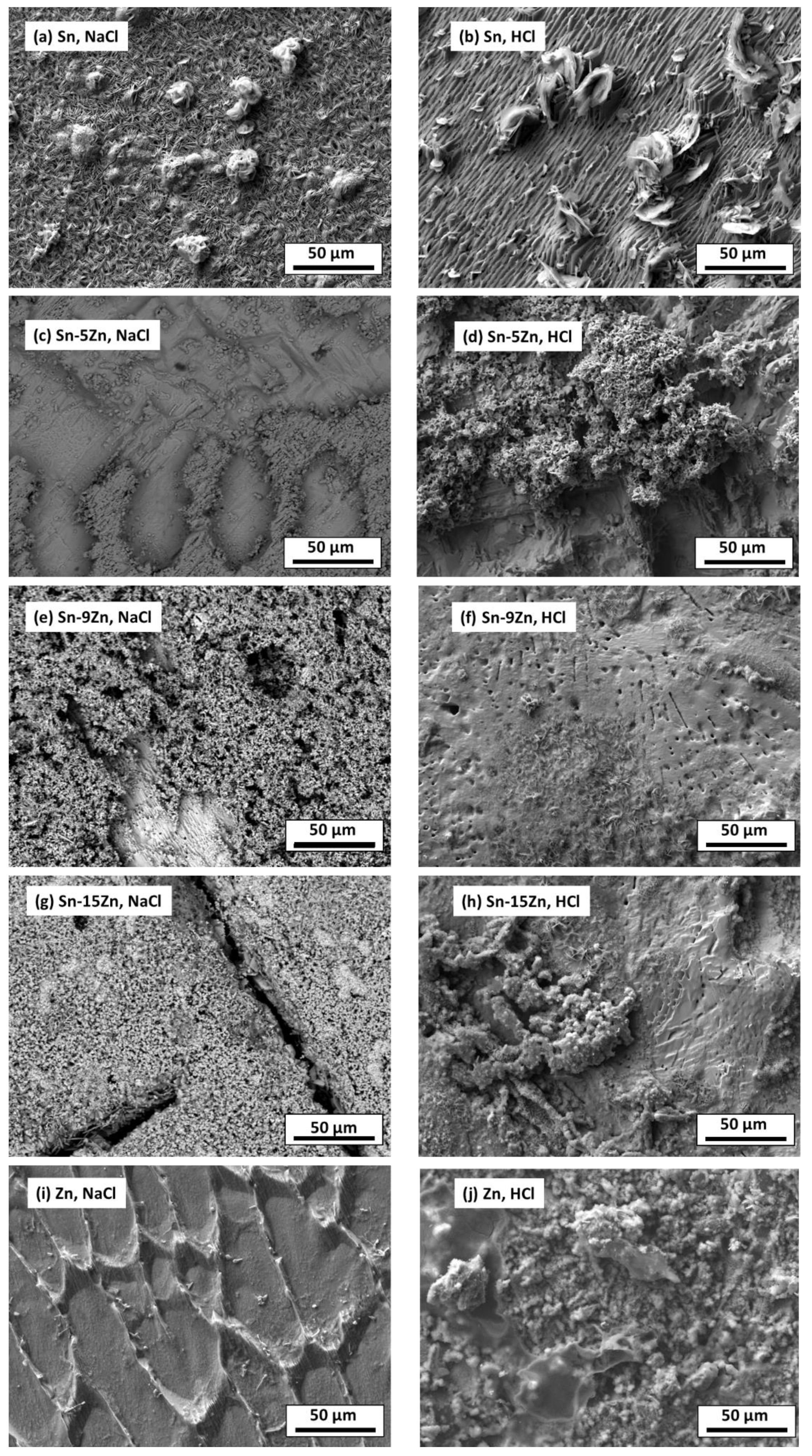
3.4. Inspection of Alloy Surfaces after Corrosion
4. Conclusions
- The Sn–xZn alloys were found to be composed of Sn and Zn. The intensity of Zn peaks increased with increasing Zn concentration in the alloy. The Sn–9Zn alloy had a eutectic microstructure. The Sn–5Zn and Sn–15Zn alloys were found to be composed of dendritic (Zn) or (Sn) and eutectic.
- The binary Sn–Zn alloys were prone to pitting corrosion in NaCl solution. There was an intermediate decrease in current density observed at potentials from −1200 to −1000 mV vs. Ag/AgCl, indicating a passivation behavior. This transient decrease was, however, later followed by an abrupt increase of current density at potentials greater than −950 mV vs. Ag/AgCl. In the NaCl solution, the Sn–xZn alloys were subject to pitting corrosion immediately after their immersion in the electrolyte.
- The corrosion resistance of the Sn–Zn alloys increased with increasing Sn concentration. The corrosion potentials and corrosion rates of the Sn–Zn alloys in HCl were significantly higher compared to NaCl.
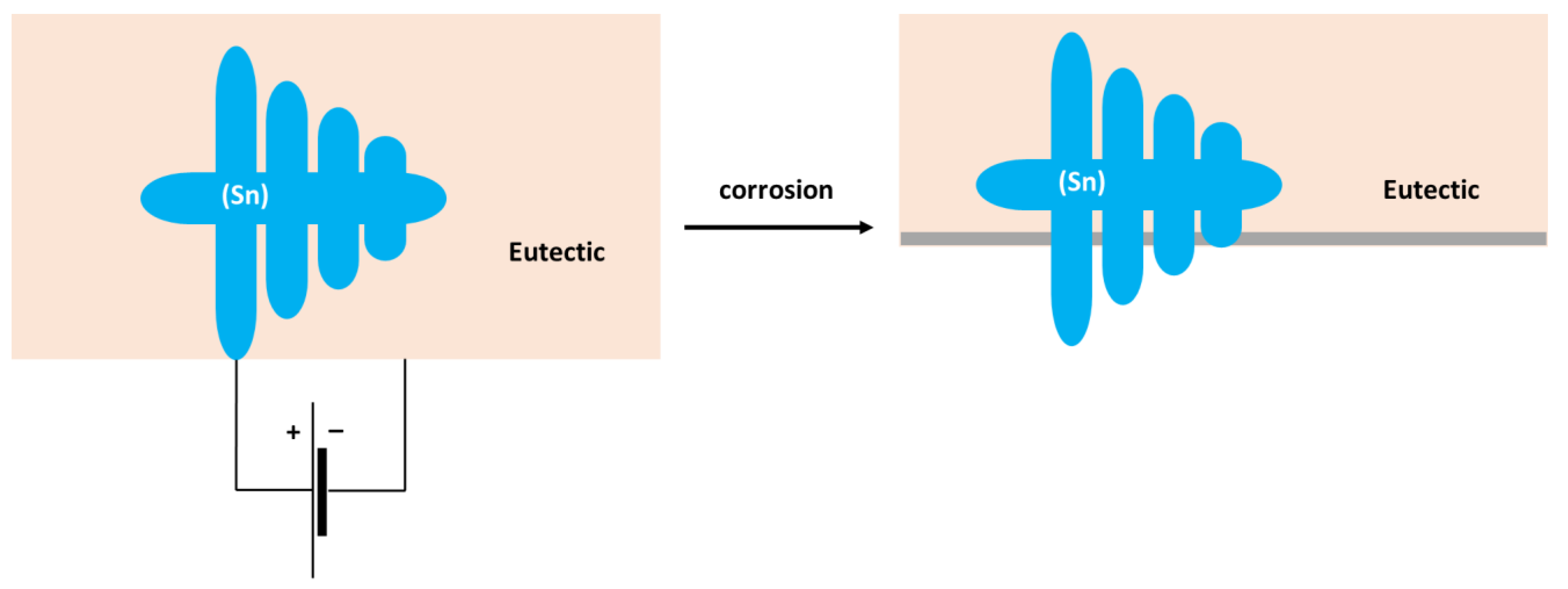
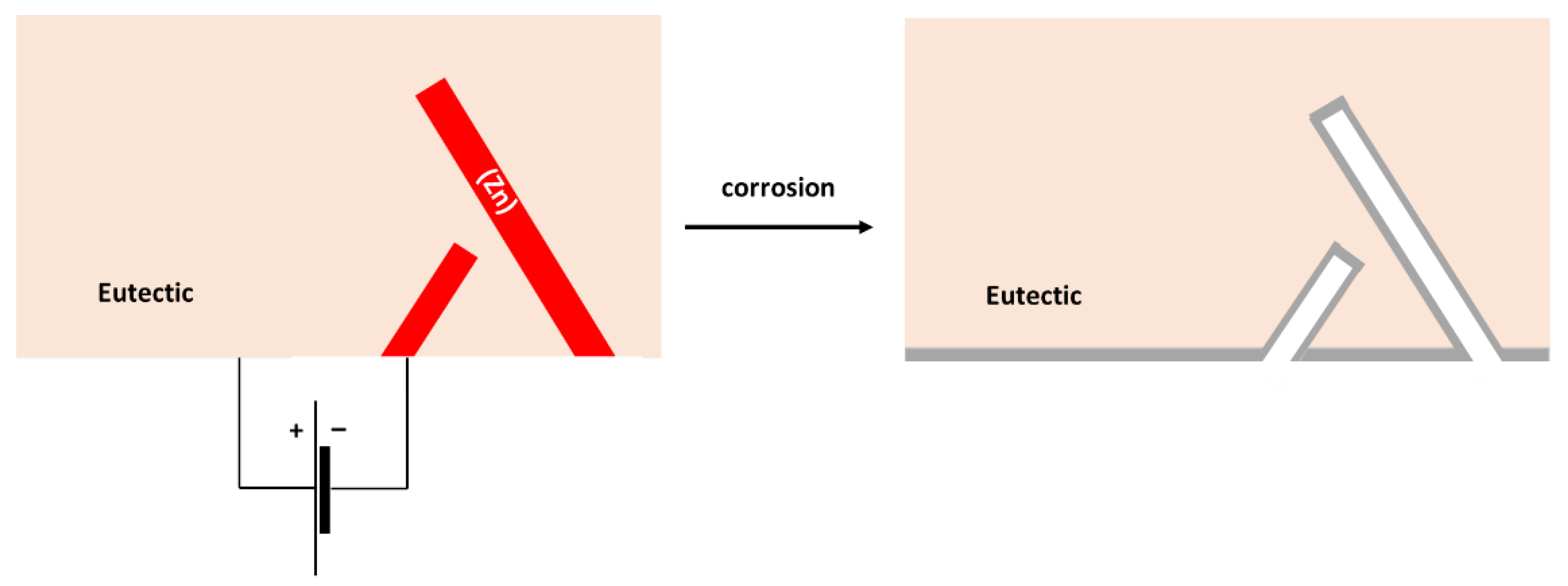
- The corrosion of the binary Sn–Zn alloys was found to take place by a galvanic mechanism. The (Sn) dendrites in the Sn–5Zn alloys were nobler compared to the eutectic. The eutectic played the role of an anode and was preferentially attacked by corrosion. In the Sn–15Zn alloy, the needle-like (Zn) was preferentially dissolved, leaving large cavities in the corroded alloy. The eutectic played the role of the local cathode in the Sn–15Zn alloy because of the higher Sn concentration. The eutectic was covered with a layer of corrosion products.
- The chemical composition of the corrosion products formed on the Sn–Zn alloys changed with the Zn weight fraction. Sn and the Sn–5Zn alloy formed reaction products rich in Sn. Alloys with a higher concentration of Zn formed corrosion products with a high Zn atomic fraction. The corrosion products formed on the surface of the binary Sn–Zn alloys in the HCl solution were prone to spallation. As a result of rapid growth, the corrosion products contained defects. The corrosion products formed in the acidic solution spalled off and left the underlying substrate prone to further corrosion.
- In the NaCl solution, the Sn–15Zn alloy had the lowest corrosion rate. In the HCl environment, however, the Sn–5Zn alloy was found to have the lowest corrosion rate. The eutectic solder had worse corrosion resistance in both environments. Although the eutectic Sn–9Zn alloy is suitable for soldering, one must consider its worse corrosion resistance. The corrosion resistance of Sn–Zn alloys in acidic environments can be increased by reducing the weight of zinc in the alloy. The hypoeutectic Sn–5Zn was more corrosion-resistant in HCl compared to the Sn–9Zn alloy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, S.; Huang, C.M.; Pecht, M. A review of lead-free solders for electronics applications. Microelectron. Reliab. 2017, 75, 77–95. [Google Scholar] [CrossRef]
- Zhong, S.J.; Zhang, L.; Li, M.L.; Long, W.M.; Wang, F.J. Development of lead-free interconnection materials in electronic industry during the past decades: Structure and properties. Mater. Des. 2022, 215, 110439. [Google Scholar] [CrossRef]
- Ogunseitan, O.A. Public health and environmental benefits of adopting lead-free solders. JOM 2007, 59, 12–17. [Google Scholar] [CrossRef]
- Chada, S. Topics in lead-free solders: Restriction of hazardous substances recast (RoHS2). JOM 2013, 65, 1348–1349. [Google Scholar] [CrossRef]
- George, E.; Pecht, M. RoHS compliance in safety and reliability critical electronics. Microelectron. Reliab. 2016, 65, 1–7. [Google Scholar] [CrossRef]
- Farina, S.; Morando, C. Comparative corrosion behaviour of different Sn–based solder alloys. J. Mater. Sci. Mater. Electron. 2015, 26, 464–471. [Google Scholar] [CrossRef]
- Méndez, C.M.; Scheiber, V.L.; Rozicki, R.S.; Kociubczyk, A.I.; Ares, A.E. Electrochemical behavior of Sn–Zn alloys with different grain structures in chloride-containing solutions. Arab. J. Chem. 2018, 11, 1084–1096. [Google Scholar] [CrossRef]
- Salhi, Y.; Cherrouf, S.; Cherkaoui, M.; Abdelouahdi, K. Electrodeposition of nanostructured Sn–Zn coatings. Appl. Surf. Sci. 2016, 367, 64–69. [Google Scholar] [CrossRef]
- Dubent, S.; Mertens, M.L.A.D.; Saurat, M. Electrodeposition, characterization and corrosion behaviour of tin–20 wt% zinc coatings electroplated from a non-cyanide alkaline bath. Mater. Chem. Phys. 2010, 120, 371–380. [Google Scholar] [CrossRef]
- Kazimiererczak, H.; Ozga, P.; Jalowiec, A.; Kowalik, R. Tin–zinc alloy electrodeposition from aqueous citrate baths. Surf. Coat. Technol. 2014, 240, 311–319. [Google Scholar] [CrossRef]
- Wang, K.; Pickering, H.W.; Weil, K.G. EQCM studies of the electrodeposition and corrosion of tin–zinc coatings. Electrochim. Acta 2001, 46, 3835–3840. [Google Scholar] [CrossRef]
- Hu, C.-C.; Wang, C.-K.; Lee, G.-L. Composition control of tin–zinc deposits using experimental strategies. Electrochim. Acta 2006, 51, 3692–3698. [Google Scholar] [CrossRef]
- Ashiru, O.A.; Shirokoff, J. Electrodeposition and characterization of tin-zinc alloy coatings. Appl. Surf. Sci. 1996, 103, 159–169. [Google Scholar] [CrossRef]
- Liu, J.C.; Wang, Z.H.; Xie, J.Y.; Ma, J.S.; Zhang, G.; Suganuma, K. Understanding corrosion mechanism of Sn–Zn alloys in NaCl solution via corrosion products characterization. Mater. Corros. 2016, 67, 522–530. [Google Scholar] [CrossRef]
- Liu, J.C.; Park, S.W.; Nagao, S.; Nogi, M.; Koga, H.; Ma, J.-S.; Zhang, G.; Suganuma, K. The role of Zn precipitates and Cl- anions in pitting corrosion of Sn–Zn solder alloys. Corros. Sci. 2015, 92, 263–271. [Google Scholar] [CrossRef]
- Liu, J.-C.; Zhang, G.; Nagao, S.; Jiu, J.-T.; Nogi, M.; Sugahara, T.; Ma, J.-S.; Suganuma, K. Metastable pitting and its correlation with electronic properties of passive films on Sn–xZn solder alloys. Corros. Sci. 2015, 99, 154–163. [Google Scholar] [CrossRef]
- Popov, B.N. Thermodynamics in the Electrochemical Reactions of Corrosion. In Corrosion Engineering; Elsevier: Amsterdam, The Netherlands, 2015; pp. 29–92. [Google Scholar] [CrossRef]
- Mohd Nazeri, M.F.; Yahaya, M.Z.; Gursel, A.; Cheani, F.; Masri, M.N.; Mohamad, A.A. Corrosion characterization of Sn–Zn solder: A review. Solder. Surf. Mt. Technol. 2019, 31, 52–67. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, C.; Jiu, J.; Nagao, S.; Nogi, M.; Koga, H.; Zhang, H.; Zhang, G.; Suganuma, K. Electrochemical behavior of Zn-xSn high-temperature solder alloys in 0.5 M NaCl solution. J. Alloys Compd. 2017, 716, 231–239. [Google Scholar] [CrossRef]
- Wei, X.; Huang, H.; Zhou, L.; Zhang, M.; Liu, X. On the advantages of using a hypoeutectic Sn–Zn as lead-free solder material. Mater. Lett. 2007, 61, 655–658. [Google Scholar] [CrossRef]
- El-Daly, A.A.; Hammad, A.E.; Al-Ganainy, G.S.; Ibrahiem, A.A. Design of lead-free candidate alloys for low-temperature soldering applications based on the hypoeutectic Sn–6.5Zn alloy. Mater. Des. 2014, 56, 594–603. [Google Scholar] [CrossRef]
- Nazeri, M.F.M.; Mohamad, A.A. Corrosion measurement of Sn–Zn lead-free solders in 6 M KOH solution. Measur. 2014, 47, 820–826. [Google Scholar] [CrossRef]
- Nazeri, M.F.M.; Ismail, A.B.; Mohamad, A.A. Effect of polarizations on Sn–Zn solders alloys in alkaline electrolyte. J. Alloy Compd. 2014, 606, 278–287. [Google Scholar] [CrossRef]
- Mori, M.; Miura, K.; Sasaki, T.; Ohtsuka, T. Corrosion of tin alloys in sulfuric and nitric acids. Corros. Sci. 2002, 44, 887–898. [Google Scholar] [CrossRef]
- Jumali, N.; Mohamad, A.A.; Mohd Nazeri, M.F. Corrosion properties of Sn–9Zn solder in acidic solution. Mater. Sci. Forum 2017, 888, 365–372. [Google Scholar] [CrossRef]
- Nordarina, J.; Mohd, H.Z.; Ahmad, A.M.; Muhammad, F.M.N. Corrosion Behaviour of Sn–based Lead-Free Solders in Acidic Solution. IOP Conf. Ser. Mater. Sci. Eng. 2018, 318, 012003. [Google Scholar] [CrossRef]
- McKinley, C.; Ghahreman, A. Hydrochloric acid regeneration in hydrometallurgical processes: A review. Miner. Process. Extr. Metall. 2018, 127, 157–168. [Google Scholar] [CrossRef]
- Koelz, H.R. Gastric acid in vertebrates. Scand. J. Gastroenterol. 1992, 27, 2–6. [Google Scholar] [CrossRef]
- Soll, A.H.; Walsh, J.H. Regulation of gastric acid secretion. Annu. Rev. Physiol. 1979, 41, 35–53. [Google Scholar] [CrossRef]
- Garcia, L.R.; Osorio, W.R.; Peixoto, L.C.; Garcia, A. Mechanical properties of Sn–Zn lead-free solder alloys based on the microstructure array. Mater. Charact. 2010, 61, 212–220. [Google Scholar] [CrossRef]
- Garcia, L.R.; Osorio, W.R.; Peixoto, L.C.; Garcia, A. Wetting behavior and mechanical properties of Sn–Zn and Sn–Pb solder alloys. J. Electron. Mater. 2009, 38, 2405–2414. [Google Scholar] [CrossRef]
- Pietrzak, K.; Klasik, A.; Maj, M.; Wojciechowski, A.; Sobczak, N. Microstructural Aspects of fatigue parameters of lead-free Sn–Zn solders with various Zn content. Arch. Foundry Eng. 2017, 17, 131–136. [Google Scholar] [CrossRef]
- ISO 17475:2005 Corrosion of Metals and Alloys—Electrochemical Test Methods—Guidelines for Conducting Potentiostatic and Potentiodynamic Polarization Measurements. Available online: https://www.iso.org/standard/31392.html (accessed on 9 September 2022).
- McCafferty, E. Chapter 7 kinetics of corrosion. In Introduction to Corrosion Science; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- The Nuclear Forensics International Technical Working Group Guidelines on Powder X-ray Diffraction (XRD)–General Overview. Available online: http://www.nf-itwg.org/pdfs/ITWG-INFL-PXRD.pdf (accessed on 9 September 2022).
- Newman, J.A.; Schmitt, P.D.; Toth, S.J.; Deng, F.; Zhang, S.; Simpson, G.J. Parts per million powder x-ray diffraction. Anal. Chem. 2015, 87, 10950–10955. [Google Scholar] [CrossRef] [PubMed]
- Stanjek, H.; Häusler, W. Basics of X-ray Diffraction. Hyperfine Interact. 2004, 154, 107–119. [Google Scholar] [CrossRef]
- Han, K.; Ohnuma, I.; Okuda, K.; Kainuma, R. Experimental determination of phase diagram in the Zn-Fe binary system. J. Alloys Compd. 2018, 737, 490–504. [Google Scholar] [CrossRef]
- Kaya, M.; Hussaini, S.; Kursunoglu, S. Critical review on secondary zinc resources and their recycling technologies. Hydrometallurgy 2020, 195, 105362. [Google Scholar] [CrossRef]
- Yahaya, M.Z.; Mohamad, A.A. Hardness testing of lead-free solders: A review. Solder. Surf. Mt. Technol. 2017, 29, 203–224. [Google Scholar] [CrossRef]
- Islam, R.A.; Wu, B.Y.; Alam, M.O.; Chan, Y.C.; Jillek, W. Investigations on microhardness of Sn–Zn based lead-free solder alloys as replacement of Sn–Pb solder. J. Alloys Compd. 2005, 392, 149–158. [Google Scholar] [CrossRef]
- Drapala, J.; Kroupa, A.; Smetana, B.; Burkovic, R.; Lasek, S.; Musiol, J. Study of Zn–Sn–Al alloys for high-temperature solders. In Proceedings of the METAL 2009-18th International Conference on Metallurgy and Materials, Hradec nad Moravicí, Czech Republic, 19–21 May 2008; pp. 1–8. Available online: http://metal2014.tanger.cz/files/proceedings/metal_09/Lists/Papers/137.pdf (accessed on 9 September 2022).
- Billah, M.M.; Shorowordi, K.M.; Sharif, A. Effect of micron size Ni particle addition in Sn–8Zn-3Bi lead-free solder alloy on the microstructure, thermal and mechanical properties. J. Alloys Compd. 2014, 585, 32–39. [Google Scholar] [CrossRef]
- Popov, B.N. Passivity. In Corrosion Engineering; Elsevier: Amsterdam, The Netherlands, 2015; pp. 143–179. [Google Scholar] [CrossRef]
- Duriska, L.; Cernickova, I.; Priputen, P.; Palcut, M. Aqueous corrosion of aluminum-transition metal alloys composed of structurally complex phases: A review. Materials 2021, 14, 5418. [Google Scholar] [CrossRef]
- Mohamed, M.N.; Aziz, N.A.; Mohamad, A.A.; Firdaus, M.; Nazeri, M. Polarization Study of Sn–9Zn and Sn–37Pb Solders in Hydrochloric Acid Solution. Int. J. Electroact. Mater. 2015, 3, 28–32. Available online: https://electroactmater.com/index.php/volume-3-2015?id=60&subid=261 (accessed on 9 September 2022).
- Popov, B.N. Electrochemical Kinetics of Corrosion. In Corrosion Engineering; Elsevier: Amsterdam, The Netherlands, 2015; pp. 93–142. [Google Scholar] [CrossRef]
- ASTM G 102-89; ASTM-Standards, Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. ASTM International: West Conshohocken, PA, USA, 1999; Volume 89, pp. 1–7. Available online: http://www.astm.org/Standards/G102.htm (accessed on 9 September 2022).
- Al-Hinai, A.T.; Al-Hinai, M.H.; Dutta, J. Application of Eh-pH diagram for room temperature precipitation of zinc stannate microcubes in an aqueous media. Mater. Res. Bull. 2014, 49, 645–650. [Google Scholar] [CrossRef]
- Li, S.; Xingxing, W.; Yongtao, J.; Zhongying, L. Corrosion behavior of Sn–based lead-free solder alloys: A review. J. Mater. Sci. Mater. Electron. 2020, 31, 9076–9090. [Google Scholar] [CrossRef]
- Špoták, M.; Drienovsky, M.; Rízeková Trnková, L.; Palcut, M. Corrosion of candidate lead-free solder alloys in saline solution. In Proceedings of the 24th International Conference on Metallurgy and Materials METAL, Brno, Czech Republic, 3–5 June 2015; pp. 1650–1656. Available online: https://www.confer.cz/metal/2015/2346-corrosion-of-candidate-lead-free-solder-alloys-in-saline-solution (accessed on 9 September 2022).
- Eckold, P.; Rolff, M.; Niewa, R.; Hügel, W. Synthesis, characterization and in situ Raman detection of Sn3O2(OH)2-xClx phases as intermediates in tin corrosion. Corros. Sci. 2015, 98, 399–405. [Google Scholar] [CrossRef]
- Fromhold, A.T.; Noh, S.J. The transport of ions and electrons through microscopically inhomogeneous passive films: Breakdown implications. Corros. Sci. 1989, 29, 237–255. [Google Scholar] [CrossRef]
- Miao, W.; Cole, I.S.; Neufeld, A.K.; Furman, S. Pitting Corrosion of Zn and Zn-Al Coated Steels in pH 2 to 12 NaCl Solutions. J. Electrochem. Soc. 2007, 154, C7. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, C.; Liu, J.; Zhang, G.; Suganuma, K. Corrosion mechanism of Zn-30Sn high-temperature, lead-free solder in neutral NaCl solution. Corros. Sci. 2018, 140, 40–50. [Google Scholar] [CrossRef]
- Yue, R.; Huang, H.; Ke, G.; Zhang, H.; Pei, J.; Xue, G.; Yuan, G. Microstructure, mechanical properties and in vitro degradation behavior of novel Zn-Cu-Fe alloys. Mater. Charact. 2017, 134, 114–122. [Google Scholar] [CrossRef]
- Shi, Z.; Li, C.; Li, M.; Li, X.; Wang, L. Second phase refining induced optimization of Fe alloying in Zn: Significantly enhanced strengthening effect and corrosion uniformity. Int. J. Miner. Metall. Mater. 2022, 29, 796–806. [Google Scholar] [CrossRef]

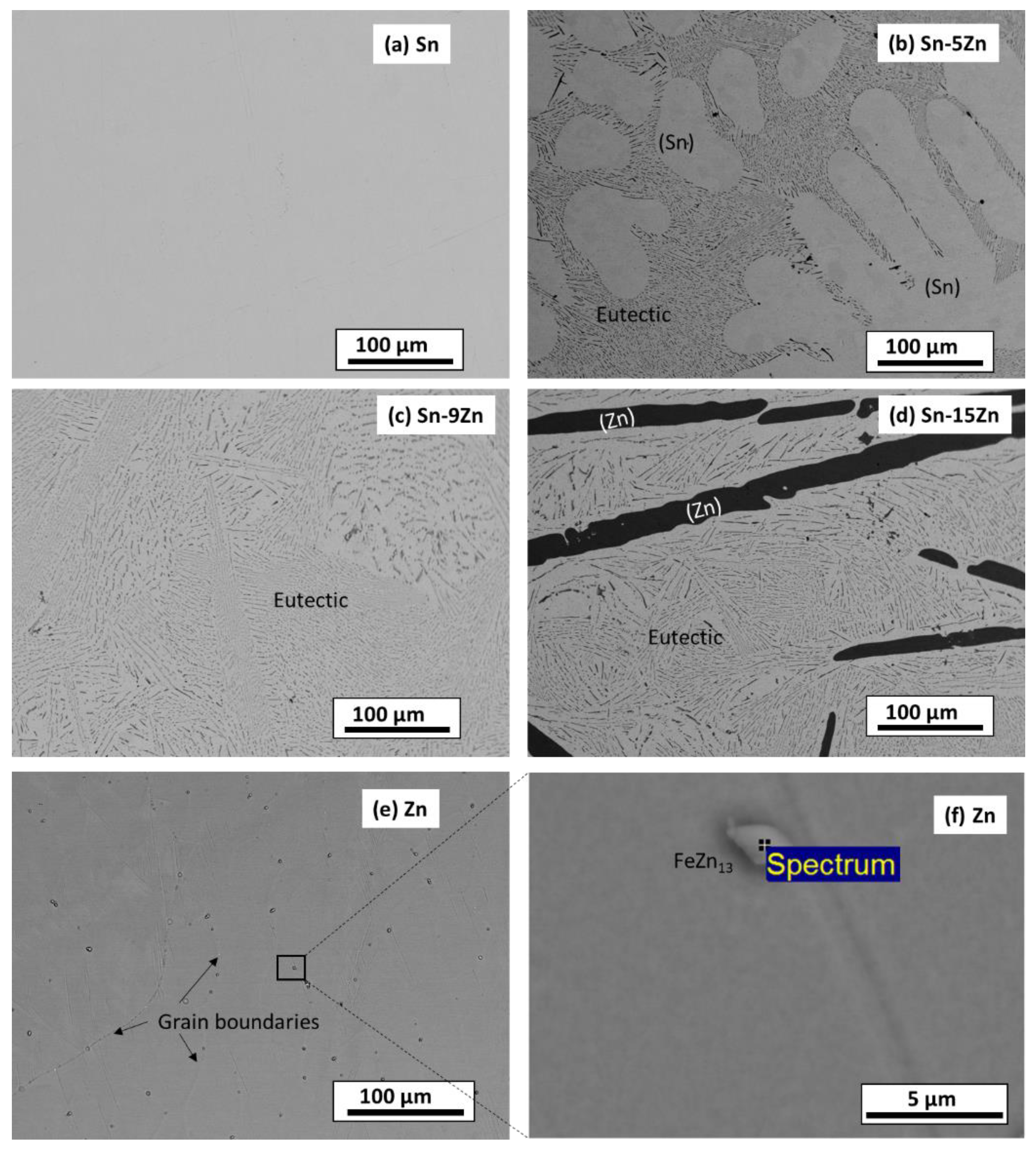
| Constituent | Chemical Composition [wt.%] | ||||
|---|---|---|---|---|---|
| Sn | Zn | Fe | P | Ti | |
| (Sn) | 100.0 | 0.0 | - | - | - |
| Eutectic | 90.9 | 9.1 | - | - | - |
| (Zn) | 0.0 | 100.0 | - | - | - |
| FeZn13 | - | 95.1 | 3.9 | 0.7 | 0.3 |
| 3.5 wt.% NaCl | ||||||
|---|---|---|---|---|---|---|
| Material | Ew | ρ | OCP | Ecorr | jcorr | CR |
| [kg mol−1] | [kg m−3] | [mV vs. Ag/AgCl] | [mV vs. Ag/AgCl] | [A m−2] | [mm/year] | |
| Sn | 0.05935 | 7265 | −735 | −941 | 0.126 | 0.336 |
| Sn–5Zn | 0.05703 | 7259 | −949 | −1288 | 0.759 | 1.95 |
| Sn–9Zn | 0.05530 | 7254 | −964 | −1279 | 1.74 | 4.33 |
| Sn–15Zn | 0.05288 | 7246 | −1004 | −1249 | 0.100 | 0.239 |
| Zn | 0.03269 | 7140 | −992 | −1240 | 0.194 | 0.291 |
| 1 wt.% HCl | ||||||
| Material | Ew | ρ | OCP | Ecorr | jcorr | CR |
| [kg mol−1] | [kg m−3] | [mV vs. Ag/AgCl] | [mV vs. Ag/AgCl] | [A m−2] | [mm/year] | |
| Sn | 0.05935 | 7265 | −460 | −481 | 0.955 | 2.55 |
| Sn–5Zn | 0.05703 | 7259 | −853 | −668 | 6.03 | 15.5 |
| Sn–9Zn | 0.05530 | 7254 | −940 | −820 | 13.2 | 32.9 |
| Sn–15Zn | 0.05288 | 7246 | −956 | −900 | 33.9 | 80.8 |
| Zn | 0.03269 | 7140 | −967 | −935 | 186 | 279 |
| Chemical Composition [at.%] | ||||
|---|---|---|---|---|
| Material | Sn | Zn | Cl | O |
| Sn | 38.8 ± 13.2 | - | 14.9 ± 6.5 | 46.3 ± 7.3 |
| Sn–5Zn | 54.6 ± 14.7 | 2.6 ± 1.4 | 1.6 ± 0.9 | 41.4 ± 11.6 |
| Sn–9Zn | 10.8 ± 3.2 | 12.2 ± 2.9 | 4.7 ± 1.4 | 72.4 ± 1.1 |
| Sn–15Zn | 6.5 ± 4.0 | 16.7 ± 4.0 | 6.3 ± 1.7 | 70.5 ± 2.4 |
| Zn | - | 35.6 ± 6.0 | 4.5 ± 1.7 | 60.0 ± 5.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerhátová, Ž.; Babincová, P.; Drienovský, M.; Pašák, M.; Černičková, I.; Ďuriška, L.; Havlík, R.; Palcut, M. Microstructure and Corrosion Behavior of Sn–Zn Alloys. Materials 2022, 15, 7210. https://doi.org/10.3390/ma15207210
Gerhátová Ž, Babincová P, Drienovský M, Pašák M, Černičková I, Ďuriška L, Havlík R, Palcut M. Microstructure and Corrosion Behavior of Sn–Zn Alloys. Materials. 2022; 15(20):7210. https://doi.org/10.3390/ma15207210
Chicago/Turabian StyleGerhátová, Žaneta, Paulína Babincová, Marián Drienovský, Matej Pašák, Ivona Černičková, Libor Ďuriška, Róbert Havlík, and Marián Palcut. 2022. "Microstructure and Corrosion Behavior of Sn–Zn Alloys" Materials 15, no. 20: 7210. https://doi.org/10.3390/ma15207210
APA StyleGerhátová, Ž., Babincová, P., Drienovský, M., Pašák, M., Černičková, I., Ďuriška, L., Havlík, R., & Palcut, M. (2022). Microstructure and Corrosion Behavior of Sn–Zn Alloys. Materials, 15(20), 7210. https://doi.org/10.3390/ma15207210






