Studying Performance and Kinetic Differences between Various Anode Electrodes in Proton Exchange Membrane Water Electrolysis Cell
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Electrode Fabrication
2.3. Characterization
3. Results and Discussions
3.1. Effects of Anode Electrode Fabrication Methods
3.2. Effects of Anode Electrode Ink Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pivovar, B. Catalysts for Fuel Cell Transportation and Hydrogen Related Uses. Nat. Catal. 2019, 2, 562–565. [Google Scholar] [CrossRef]
- Khan, M.A.; Al-Attas, T.; Roy, S.; Rahman, M.M.; Ghaffour, N.; Thangadurai, V.; Larter, S.; Hu, J.; Ajayan, P.M.; Kibria, M.G. Seawater electrolysis for hydrogen production: A solution looking for a problem? Energy Environ. Sci. 2021, 14, 4831–4839. [Google Scholar] [CrossRef]
- Hurtubia, B.; Sauma, E. Economic and environmental analysis of hydrogen production when complementing renewable energy generation with grid electricity. Appl. Energy 2021, 304, 117739. [Google Scholar] [CrossRef]
- Wang, W.; Xiong, F.; Zhu, S.; Chen, J.; Xie, J.; An, Q. Defect engineering in molybdenum-based electrode materials for energy storage. eScience 2022, 2, 278–294. [Google Scholar] [CrossRef]
- Tian, X.; Zhao, X.; Su, Y.-Q.; Wang, L.; Wang, H.; Dang, D.; Chi, B.; Liu, H.; Hensen, E.J.M.; Lou, X.W. Engineering bunched Pt-Ni alloy nanocages for efficient oxygen reduction in practical fuel cells. Science 2019, 366, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Yang, G.; Mo, J.; Li, Y.; Yu, S.; Cullen, D.A.; Retterer, S.T.; Toops, T.J.; Bender, G.; Pivovar, B.S.; et al. Novel Thin/Tunable Gas Diffusion Electrodes with Ultra-Low Catalyst Loading for Hydrogen Evolution Reactions in Proton Exchange Membrane Electrolyzer Cells. Nano Energy 2018, 47, 434–441. [Google Scholar] [CrossRef]
- Liu, C.; Shviro, M.; Gago, A.S.; Zaccarine, S.F.; Bender, G.; Gazdzicki, P.; Morawietz, T.; Biswas, I.; Rasinski, M.; Everwand, A.; et al. Exploring the Interface of Skin-Layered Titanium Fibers for Electrochemical Water Splitting. Adv. Energy Mater. 2021, 11, 2002926. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, R.; Deng, P.; Li, J.; Huang, W.; Chen, Q.; Su, Y.-Q.; Jia, C.; Liu, Z.; Kang, Z.; et al. Rhodium decorated stable platinum nickel nanowires for effective ethanol oxidation reaction. Sci. China Mater. 2022. [Google Scholar] [CrossRef]
- Ling, J.; Kunwar, R.; Li, L.; Peng, S.; Misnon, I.I.; Ab Rahim, M.H.; Yang, C.-C.; Jose, R. Self-rechargeable energizers for sustainability. eScience 2022, 2, 347–364. [Google Scholar] [CrossRef]
- Tian, X.; Lu, X.F.; Xia, B.Y.; Lou, X.W. Advanced Electrocatalysts for the Oxygen Reduction Reaction in Energy Conversion Technologies. Joule 2020, 4, 45–68. [Google Scholar] [CrossRef]
- Kang, Z.; Alia, S.M.; Carmo, M.; Bender, G. In-situ and in-operando analysis of voltage losses using sense wires for proton exchange membrane water electrolyzers. J. Power Sources 2021, 481, 229012. [Google Scholar] [CrossRef]
- Liu, J.; Jia, K.; Chen, S.; Sun, J.; Gong, J. A Review of Research on the Estimation Methods and Influencing Factors of Urban Carbon Emission. J. Mod. Green Energy 2022, 1, 2. [Google Scholar]
- Jiménez-Arévalo, N.; Flores, E.; Giampietri, A.; Sbroscia, M.; Betti, M.G.; Mariani, C.; Ares, J.R.; Ferrer, I.J.; Leardini, F. Borocarbonitride Layers on Titanium Dioxide Nanoribbons for Efficient Photoelectrocatalytic Water Splitting. Materials 2021, 14, 5490. [Google Scholar] [CrossRef]
- Yu, Y.; Li, J.; Luo, J.; Kang, Z.; Jia, C.; Liu, Z.; Huang, W.; Chen, Q.; Deng, P.; Shen, Y.; et al. Mo-decorated cobalt phosphide nanoarrays as bifunctional electrocatalysts for efficient overall water/seawater splitting. Mater. Today Nano 2022, 18, 100216. [Google Scholar] [CrossRef]
- Kang, Z.; Chen, Y.; Wang, H.; Alia, S.M.; Pivovar, B.S.; Bender, G. Discovering and Demonstrating a Novel High-Performing 2D-Patterned Electrode for Proton-Exchange Membrane Water Electrolysis Devices. ACS Appl. Mater. Interfaces 2022, 14, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, Y.; Wei, X.; Wang, L.; She, H.; Huang, J.; Wang, Q. Preparation of double-layered Co−Ci/NiFeOOH co-catalyst for highly meliorated PEC performance in water splitting. Adv. Powder Mater. 2022, 1, 100024. [Google Scholar] [CrossRef]
- Kang, Z.; Yu, S.; Yang, G.; Li, Y.; Bender, G.; Pivovar, B.S.; Green, J.B., Jr.; Zhang, F.-Y. Performance improvement of proton exchange membrane electrolyzer cells by introducing in-plane transport enhancement layers. Electrochim. Acta 2019, 316, 43–51. [Google Scholar] [CrossRef]
- Darvishi, Y.; Hassan-Beygi, S.R.; Zarafshan, P.; Hooshyari, K.; Malaga-Toboła, U.; Gancarz, M. Numerical Modeling and Evaluation of PEM Used for Fuel Cell Vehicles. Materials 2021, 14, 7907. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, D.; Yu, Y.; Li, J.; Rao, P.; Jia, C.; Liu, Z.; Chen, Q.; Huang, W.; Luo, J.; et al. Bridge the activity and durability of Ruthenium for hydrogen evolution reaction with the Ru-O-C link. Chem. Eng. J. 2022, 433, 134421. [Google Scholar] [CrossRef]
- Laube, A.; Hofer, A.; Ressel, S.; Chica, A.; Bachmann, J.; Struckmann, T. PEM water electrolysis cells with catalyst coating by atomic layer deposition. Int. J. Hydrog. Energy 2021, 46, 38972–38982. [Google Scholar] [CrossRef]
- Stiber, S.; Sata, N.; Morawietz, T.; Ansar, S.A.; Jahnke, T.; Lee, J.K.; Bazylak, A.; Fallisch, A.; Gago, A.S.; Friedrich, K.A. A High-Performance, Durable and Low-Cost Proton Exchange Membrane Electrolyser with Stainless Steel Components. Energy Environ. Sci. 2021, 15, 109–122. [Google Scholar] [CrossRef]
- Wang, W.; Li, K.; Ding, L.; Yu, S.; Xie, Z.; Cullen, D.A.; Yu, H.; Bender, G.; Kang, Z.; Wrubel, J.A.; et al. Exploring the Impacts of Conditioning on Proton Exchange Membrane Electrolyzers by In Situ Visualization and Electrochemistry Characterization. ACS Appl. Mater. Interfaces 2022, 14, 9002–9012. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Wang, M.; Yang, Y.; Wang, H.; Liu, Y.; Mo, J.; Li, J.; Deng, P.; Jia, C.; Tian, X. Performance improvement induced by membrane treatment in proton exchange membrane water electrolysis cells. Int. J. Hydrogen Energy 2022, 47, 5807–5816. [Google Scholar] [CrossRef]
- Ayers, K. High efficiency PEM water electrolysis: Enabled by advanced catalysts, membranes, and processes. Curr. Opin. Chem. Eng. 2021, 33, 100719. [Google Scholar] [CrossRef]
- Kang, Z.; Mo, J.; Yang, G.; Li, Y.; Talley, D.A.; Han, B.; Zhang, F.-Y. Performance Modeling and Current Mapping of Proton Exchange Membrane Electrolyzer Cells with Novel Thin/Tunable Liquid/Gas Diffusion Layers. Electrochim. Acta 2017, 255, 405–416. [Google Scholar] [CrossRef]
- Schuler, T.; Kimura, T.; Schmidt, T.J.; Büchi, F.N. Towards a Generic Understanding of Oxygen Evolution Reaction Kinetics in Polymer Eectrolyte Water Electrolysis. Energy Environ. Sci. 2020, 13, 2153–2166. [Google Scholar] [CrossRef]
- Kang, Z.; Schuler, T.; Chen, Y.; Wang, M.; Zhang, F.-Y.; Bender, G. Effects of interfacial contact under different operating conditions in proton exchange membrane water electrolysis. Electrochim. Acta 2022, 429, 140942. [Google Scholar] [CrossRef]
- Wang, Q.; Tong, X.; Ricote, S.; Sažinas, R.; Hendriksen, P.V.; Chen, M. Nano-LaCoO3 infiltrated BaZr0.8Y0.2O3−δ electrodes for steam splitting in protonic ceramic electrolysis cells. Adv. Powder Mater. 2022, 1, 100003. [Google Scholar] [CrossRef]
- Zhao, G.; Ma, W.; Wang, X.; Xing, Y.; Hao, S.; Xu, X. Self-water-absorption-type two-dimensional composite photocatalyst with high-efficiency water absorption and overall water-splitting performance. Adv. Powder Mater. 2022, 1, 100008. [Google Scholar] [CrossRef]
- Buryakovskaya, O.A.; Vlaskin, M.S.; Grigorenko, A.V. Effect of Thermal Treatment of Aluminum Core-Shell Particles on Their Oxidation Kinetics in Water for Hydrogen Production. Materials 2021, 14, 6493. [Google Scholar] [CrossRef]
- Stelmachowski, P.; Duch, J.; Sebastián, D.; Lázaro, M.J.; Kotarba, A. Carbon-Based Composites as Electrocatalysts for Oxygen Evolution Reaction in Alkaline Media. Materials 2021, 14, 4984. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Fang, J.; Ai, X.; Huang, D.; Zhong, Z.; Yang, X.; Wang, L. Comparative study of alkaline water electrolysis, proton exchange membrane water electrolysis and solid oxide electrolysis through multiphysics modeling. Appl. Energy 2022, 312, 118788. [Google Scholar] [CrossRef]
- Kang, Z.; Pak, M.; Bender, G. Introducing a novel technique for measuring hydrogen crossover in membrane-based electrochemical cells. Int. J. Hydrogen Energy 2021, 46, 15161–15167. [Google Scholar] [CrossRef]
- Pham, C.V.; Escalera-López, D.; Mayrhofer, K.; Cherevko, S.; Thiele, S. Essentials of High Performance Water Electrolyzers–From Catalyst Layer Materials to Electrode Engineering. Adv. Energy Mater. 2021, 11, 2101998. [Google Scholar] [CrossRef]
- Xie, Z.; Yu, S.; Ma, X.; Li, K.; Ding, L.; Wang, W.; Cullen, D.A.; Meyer, H.M.; Yu, H.; Tong, J.; et al. MoS2 nanosheet integrated electrodes with engineered 1T-2H phases and defects for efficient hydrogen production in practical PEM electrolysis. Appl. Catal. B Environ. 2022, 313, 121458. [Google Scholar] [CrossRef]
- Jiang, G.; Yu, H.; Yao, D.; Li, Y.; Chi, J.; Zhang, H.; Shao, Z. Boosting the oxygen evolution stability and activity of a heterogeneous IrRu bimetallic coating on a WO3 nano-array electrode for PEM water electrolysis. J. Mater. Chem. A 2022, 10, 11893–11903. [Google Scholar] [CrossRef]
- Peng, X.; Taie, Z.; Liu, J.; Zhang, Y.; Peng, X.; Regmi, Y.N.; Fornaciari, J.C.; Capuano, C.; Binny, D.; Kariuki, N.N.; et al. Hierarchical electrode design of highly efficient and stable unitized regenerative fuel cells (URFCs) for long-term energy storage. Energy Environ. Sci. 2020, 13, 4872–4881. [Google Scholar] [CrossRef]
- Brito, J.; Restivo, J.; Sousa, J.P.S.; Spera, N.C.M.; Falcão, D.S.; Rocha, A.; Pinto, A.; Pereira, M.F.R.; Soares, O.S.G.P. Implementation of Transition Metal Phosphides as Pt-Free Catalysts for PEM Water Electrolysis. Energies 2022, 15, 1821. [Google Scholar] [CrossRef]
- Zhao, Z.L.; Wang, Q.; Huang, X.; Feng, Q.; Gu, S.; Zhang, Z.; Xu, H.; Zeng, L.; Gu, M.; Li, H. Boosting the Oxygen Evolution Reaction Using Defect-Rich Ultra-Thin Ruthenium Oxide Nanosheets in Acidic Media. Energy Environ. Sci. 2020, 13, 5143–5151. [Google Scholar] [CrossRef]
- Zhu, Y.; Lin, Q.; Zhong, Y.; Tahini, H.A.; Shao, Z.; Wang, H. Metal oxide-based materials as an emerging family of hydrogen evolution electrocatalysts. Energy Environ. Sci. 2020, 13, 3361–3392. [Google Scholar] [CrossRef]
- Wang, W.; Xie, Z.; Li, K.; Yu, S.; Ding, L.; Zhang, F.-Y. Recent progress in in-situ visualization of electrochemical reactions in electrochemical energy devices. Curr. Opin. Electrochem. 2022, 35, 101088. [Google Scholar] [CrossRef]
- Xie, Z.; Yu, S.; Yang, G.; Li, K.; Ding, L.; Wang, W.; Zhang, F.-Y. Optimization of catalyst-coated membranes for enhancing performance in proton exchange membrane electrolyzer cells. Int. J. Hydrogen Energy 2021, 46, 1155–1162. [Google Scholar] [CrossRef]
- Yu, S.; Li, K.; Wang, W.; Xie, Z.; Ding, L.; Kang, Z.; Wrubel, J.; Ma, Z.; Bender, G.; Yu, H.; et al. Tuning Catalyst Activation and Utilization Via Controlled Electrode Patterning for Low-Loading and High-Efficiency Water Electrolyzers. Small. 2022, 18, 2107745. [Google Scholar] [CrossRef]
- Yang, G.; Yu, S.; Kang, Z.; Li, Y.; Bender, G.; Pivovar, B.S.; Green, J.B.; Cullen, D.A.; Zhang, F.Y. Building Electron/Proton Nanohighways for Full Utilization of Water Splitting Catalysts. Adv. Energy Mater. 2020, 10, 1903871. [Google Scholar] [CrossRef]
- Liu, H.; Kang, X.; Zhao, T.; Zhang, Z.; Ge, S.; Hu, S.; Luo, Y.; Yang, F.; Li, S.-H.; Sun, C. Engineering membrane electrode assembly for advanced polymer electrolyte water electrolyzer. Sci. China Mater. 2022, 1–30. [Google Scholar] [CrossRef]
- Park, J.; Kang, Z.; Bender, G.; Ulsh, M.; Mauger, S.A. Roll-to-roll production of catalyst coated membranes for low-temperature electrolyzers. J. Power Sources 2020, 479, 228819. [Google Scholar] [CrossRef]
- Young, J.L.; Kang, Z.; Ganci, F.; Madachy, S.; Bender, G. PEM electrolyzer characterization with carbon-based hardware and material sets. Electrochem. Commun. 2021, 124, 106941. [Google Scholar] [CrossRef]
- Kang, Z.; Wang, H.; Liu, Y.; Mo, J.; Wang, M.; Li, J.; Tian, X. Exploring and understanding the internal voltage losses through catalyst layers in proton exchange membrane water electrolysis devices. Appl. Energy 2022, 317, 119213. [Google Scholar] [CrossRef]
- Xie, J.; Garzon, F.; Zawodzinski, T.; Smith, W. Ionomer Segregation in Composite MEAs and Its Effect on Polymer Electrolyte Fuel Cell Performance. J. Electrochem. Soc. 2004, 151, A1084–A1093. [Google Scholar] [CrossRef]
- Kang, Z.; Mo, J.; Yang, G.; Zhang, F.-Y.; Reterer, S.T.; Cullen, D.A. Micro/nano manufacturing of novel multifunctional layers for hydrogen production from water splitting. In Proceedings of the 2017 IEEE 12th International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Los Angeles, CA, USA, 9–12 April 2017. [Google Scholar]
- Kang, Z.; Alia, S.M.; Young, J.L.; Bender, G. Effects of Various Parameters of Different Porous Transport Layers in Proton Exchange Membrane Water Electrolysis. Electrochim. Acta 2020, 354, 136641. [Google Scholar] [CrossRef]
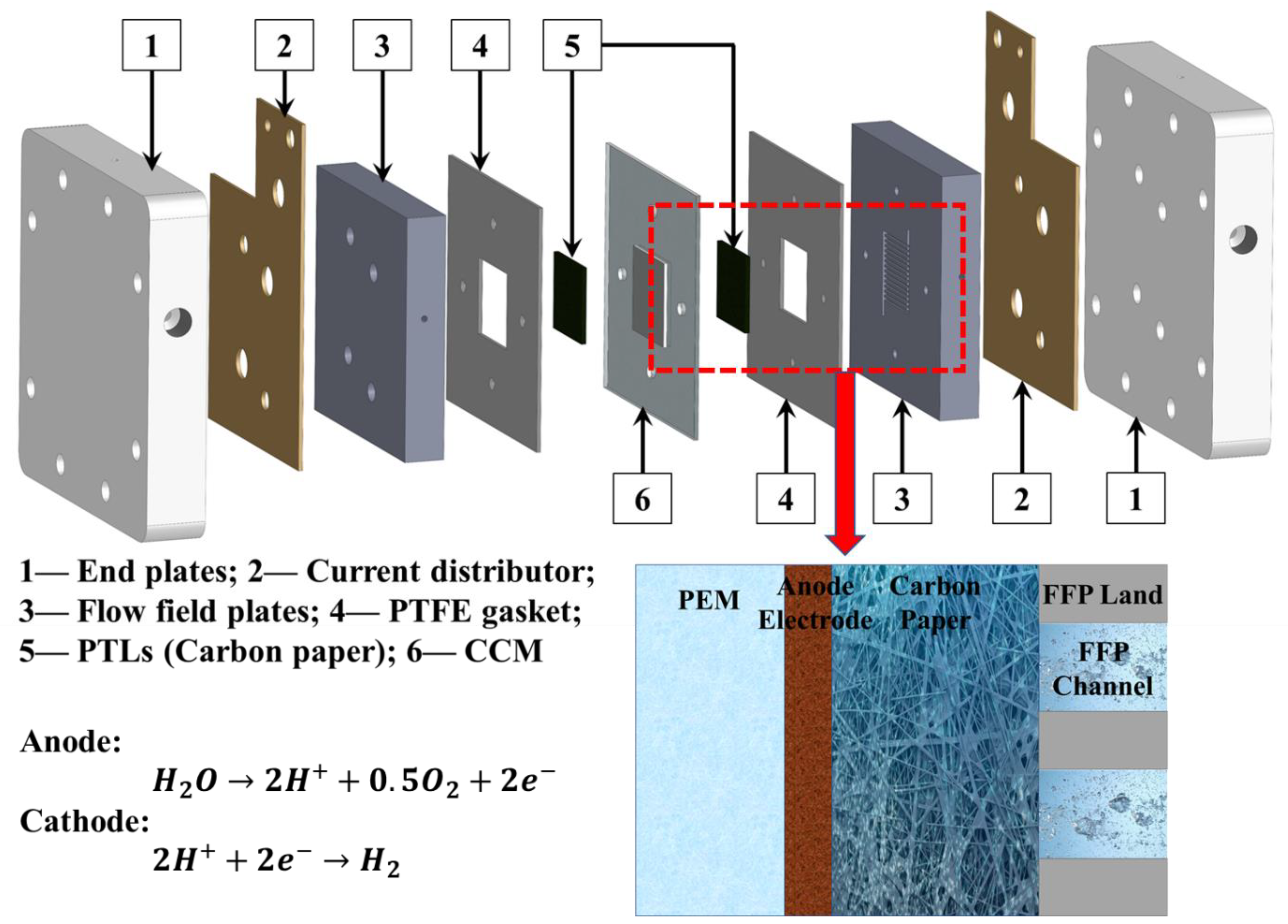


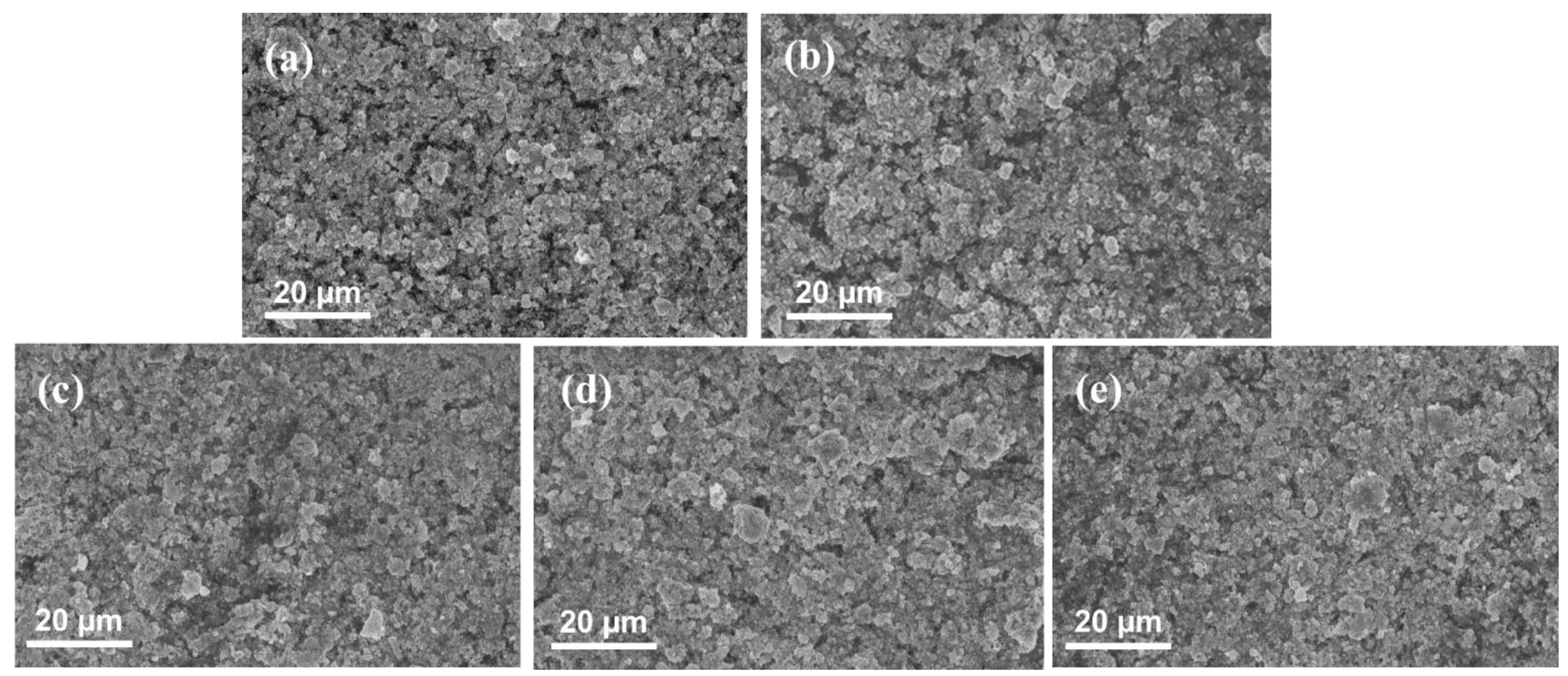
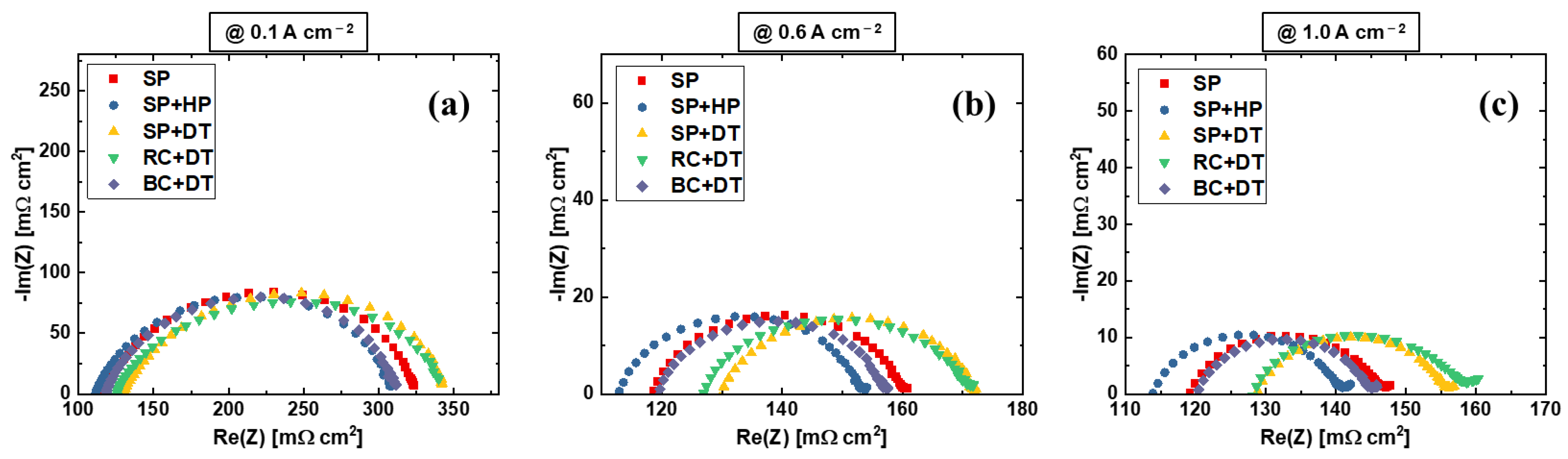
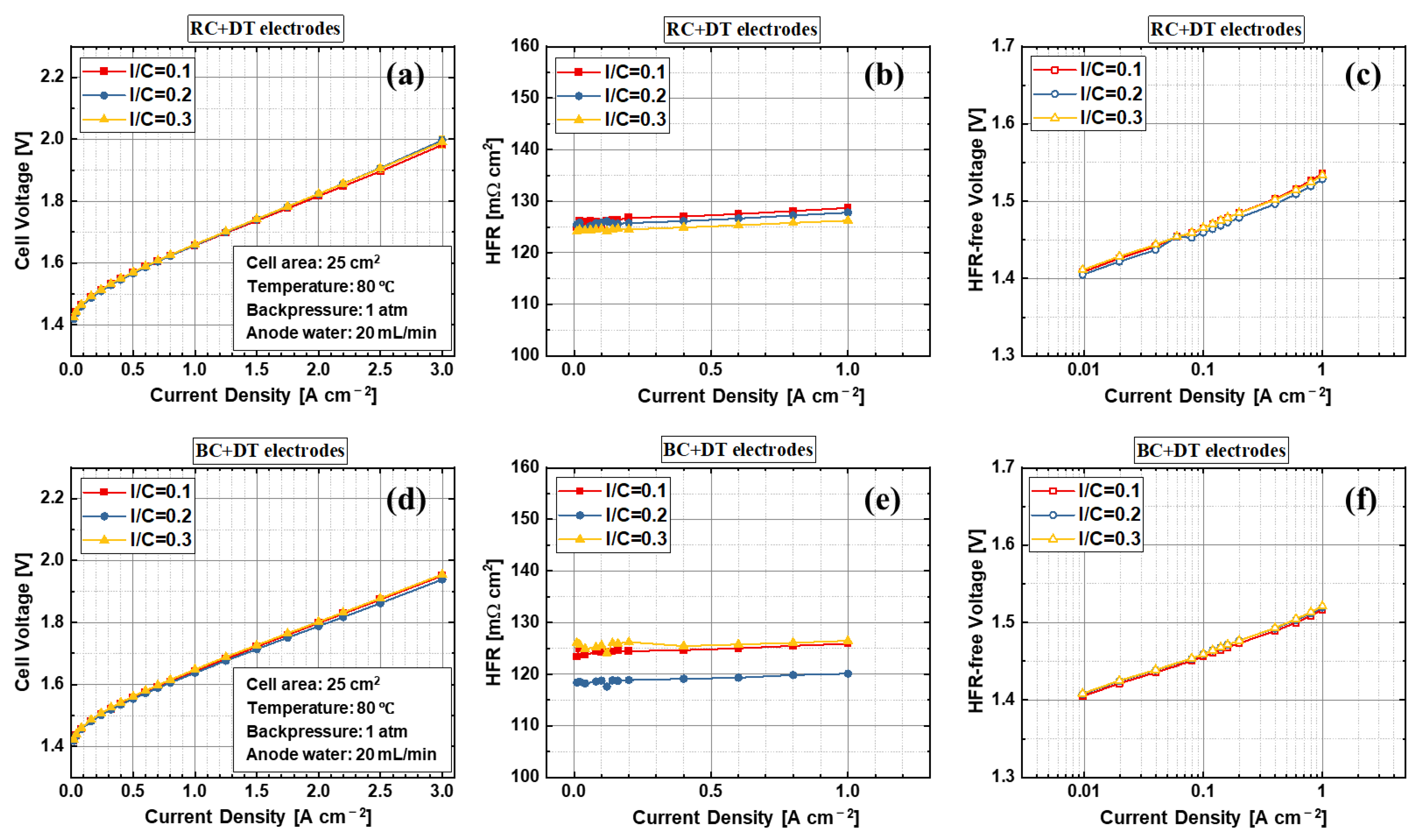

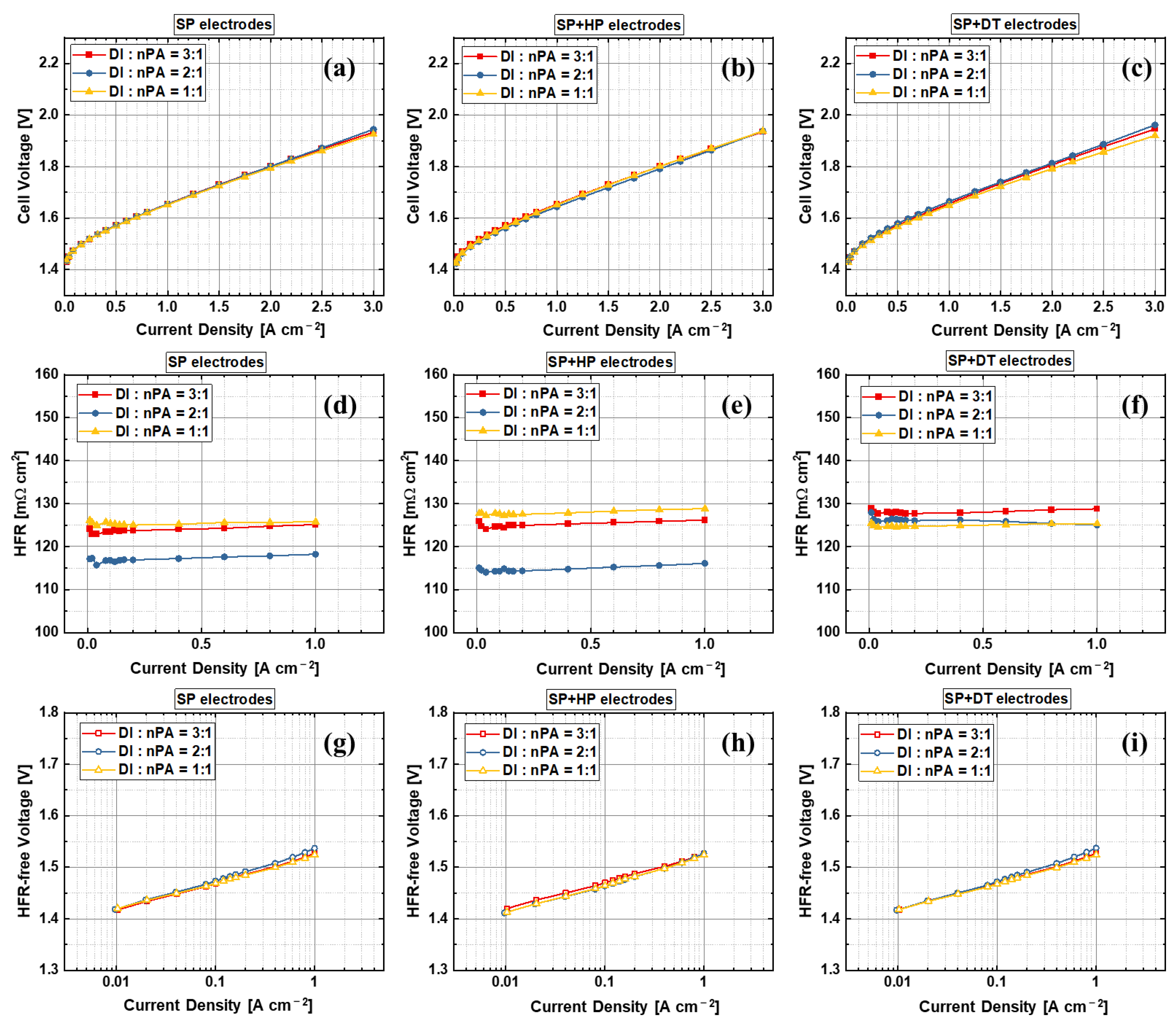
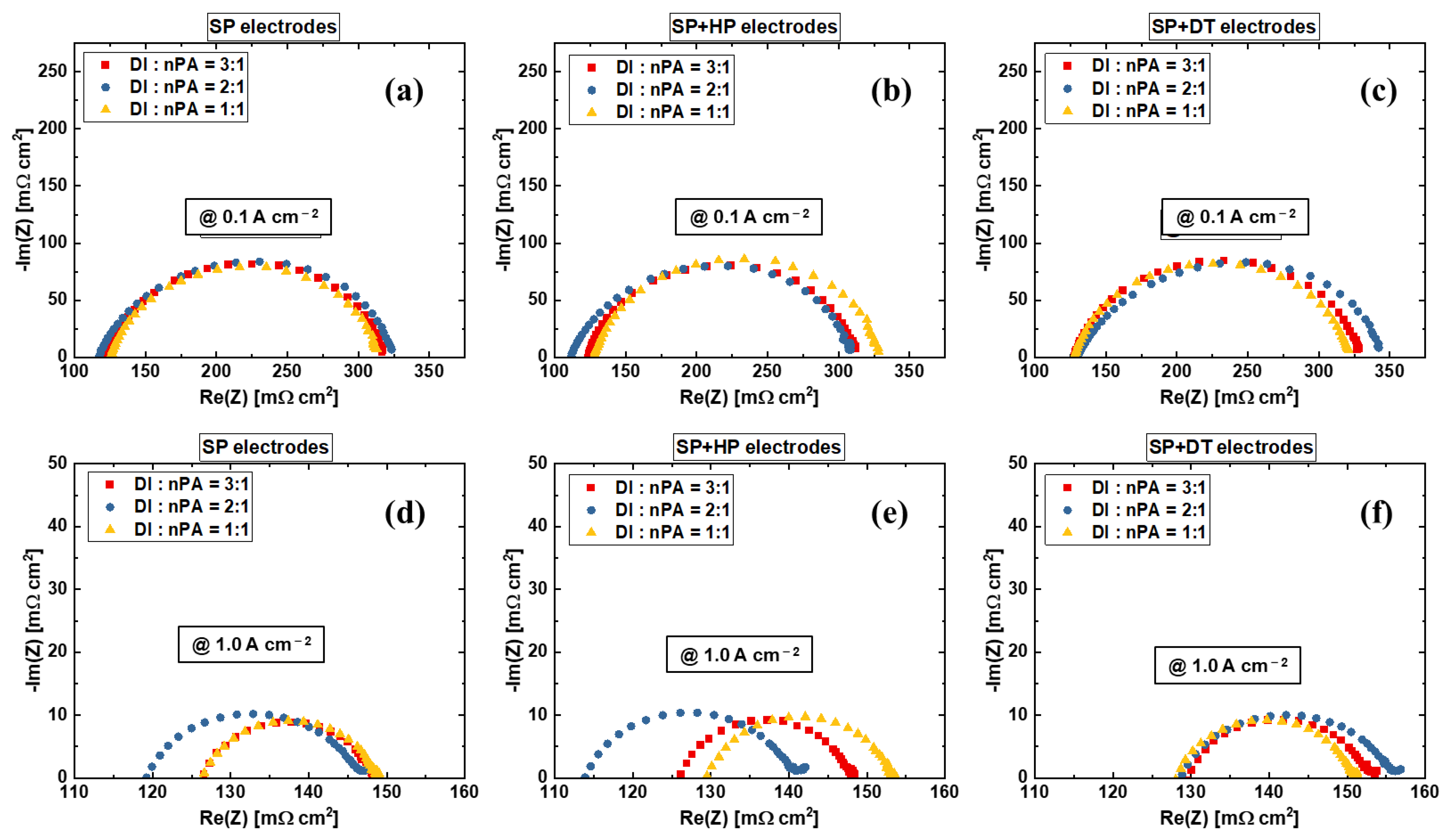
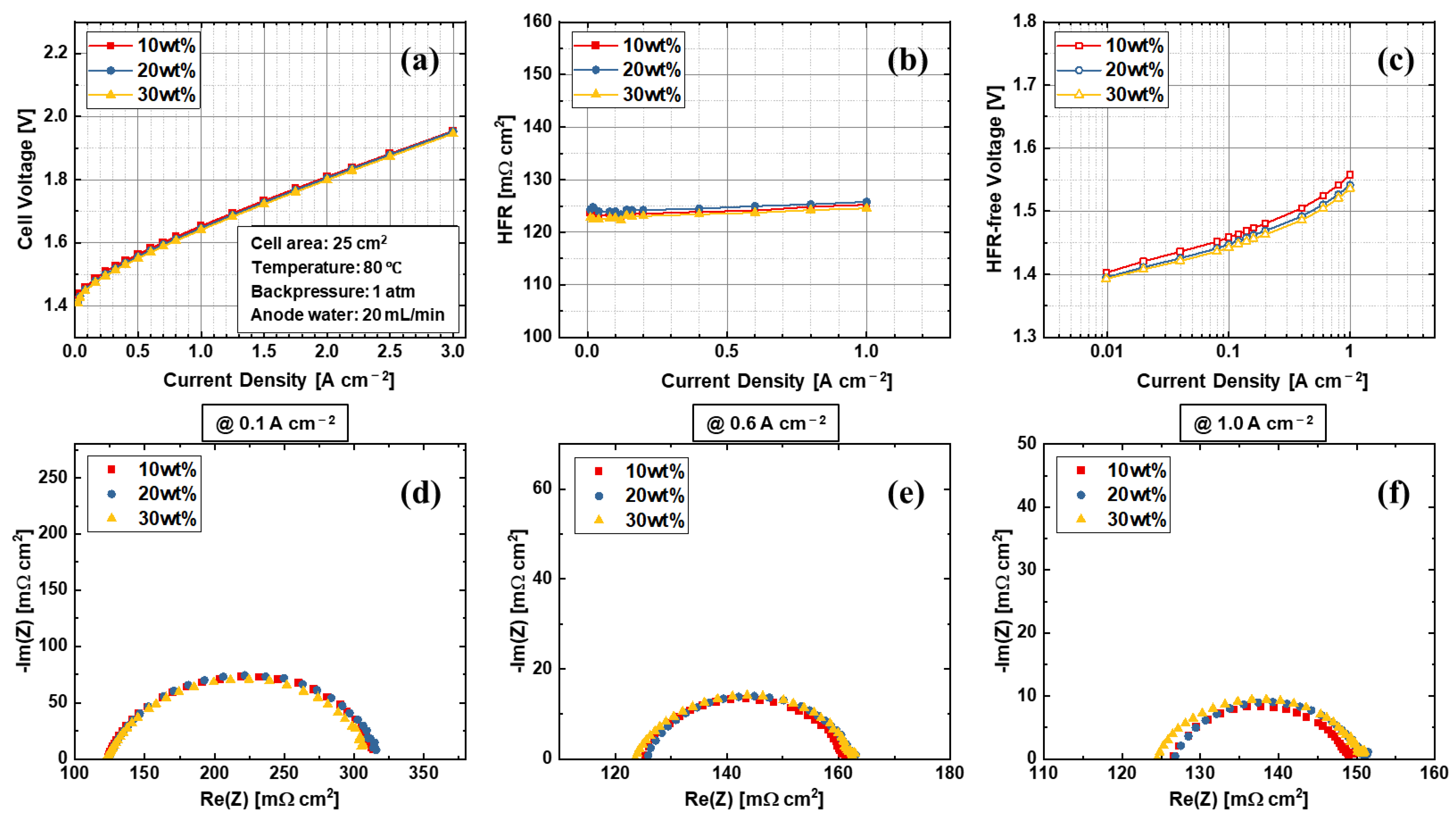
| Label | Substrate | Coating Technique | Post-Coating Process | Ir Loading [mg cm−2] | Pt Loading [mg cm−2] |
|---|---|---|---|---|---|
| SP | Nafion 115 | Ultrasonic spray | Null | 0.35 ± 0.03 | 0.2 ± 0.03 |
| SP + HP | Nafion 115 | Ultrasonic spray | Hot press | 0.35 ± 0.03 | |
| SP + DT | PTFE | Ultrasonic spray | Decal transfer | 0.34 ± 0.04 | |
| RC + DT | PTFE | Rod coating | Decal transfer | 0.36 ± 0.05 | |
| BC + DT | PTFE | Blade coating | Decal transfer | 0.35 ± 0.04 |
| Cell Voltage@2.0 A cm−2 [V] | Avg. HFR [mΩ cm2] | HFR-Free Voltage@0.1 A cm−2 [V] | Tafel Slopes [mV/dec] | |
|---|---|---|---|---|
| SP | 1.80 | 117 ± 0.6 | 1.47 | 58 |
| SP + HP | 1.79 | 115 ± 0.6 | 1.46 | 57 |
| SP + DT | 1.81 | 126 ± 0.7 | 1.47 | 59 |
| RC + DT | 1.82 | 126 ± 0.8 | 1.46 | 60 |
| BC + DT | 1.79 | 119 ± 0.7 | 1.46 | 56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Z.; Fan, Z.; Zhang, F.; Zhang, Z.; Tian, C.; Wang, W.; Li, J.; Shen, Y.; Tian, X. Studying Performance and Kinetic Differences between Various Anode Electrodes in Proton Exchange Membrane Water Electrolysis Cell. Materials 2022, 15, 7209. https://doi.org/10.3390/ma15207209
Kang Z, Fan Z, Zhang F, Zhang Z, Tian C, Wang W, Li J, Shen Y, Tian X. Studying Performance and Kinetic Differences between Various Anode Electrodes in Proton Exchange Membrane Water Electrolysis Cell. Materials. 2022; 15(20):7209. https://doi.org/10.3390/ma15207209
Chicago/Turabian StyleKang, Zhenye, Zihao Fan, Fan Zhang, Zhenyu Zhang, Chao Tian, Weina Wang, Jing Li, Yijun Shen, and Xinlong Tian. 2022. "Studying Performance and Kinetic Differences between Various Anode Electrodes in Proton Exchange Membrane Water Electrolysis Cell" Materials 15, no. 20: 7209. https://doi.org/10.3390/ma15207209
APA StyleKang, Z., Fan, Z., Zhang, F., Zhang, Z., Tian, C., Wang, W., Li, J., Shen, Y., & Tian, X. (2022). Studying Performance and Kinetic Differences between Various Anode Electrodes in Proton Exchange Membrane Water Electrolysis Cell. Materials, 15(20), 7209. https://doi.org/10.3390/ma15207209








