Growth and Liquid-Phase Exfoliation of GaSe1−xSx Crystals
Abstract
1. Introduction
2. Materials and Methods
2.1. GaSe1−xSx Crystal Growth
2.2. Characterization of GaSe1−xSx Crystals
2.3. Photoconductivity Measurements
2.4. Exfoliation of GaSe1-xSx Crystals
3. Results
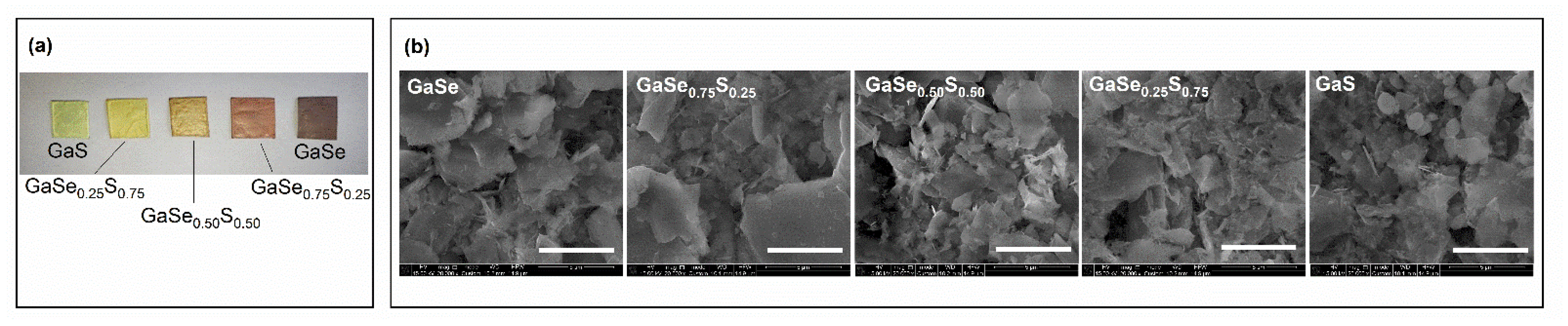
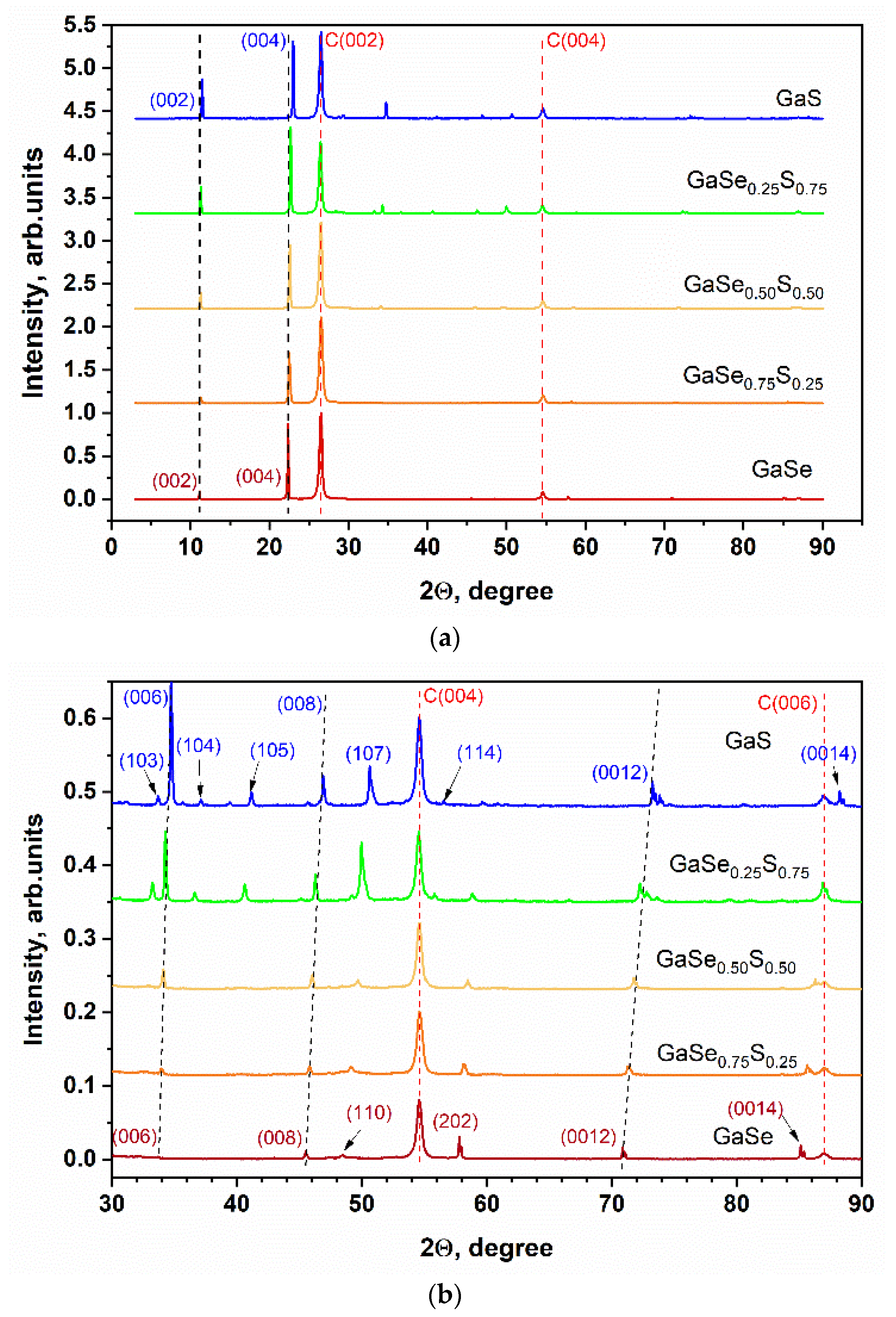
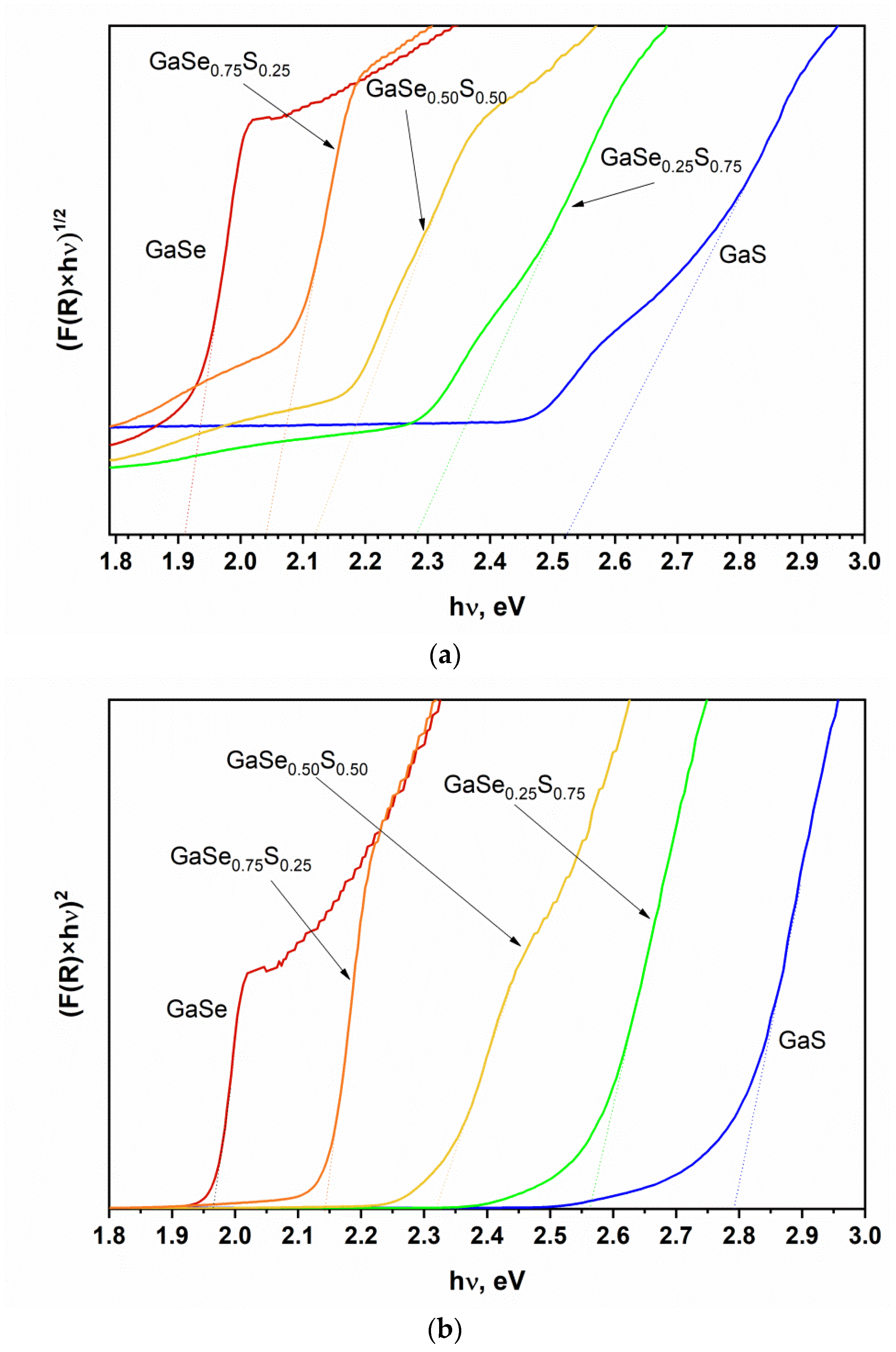
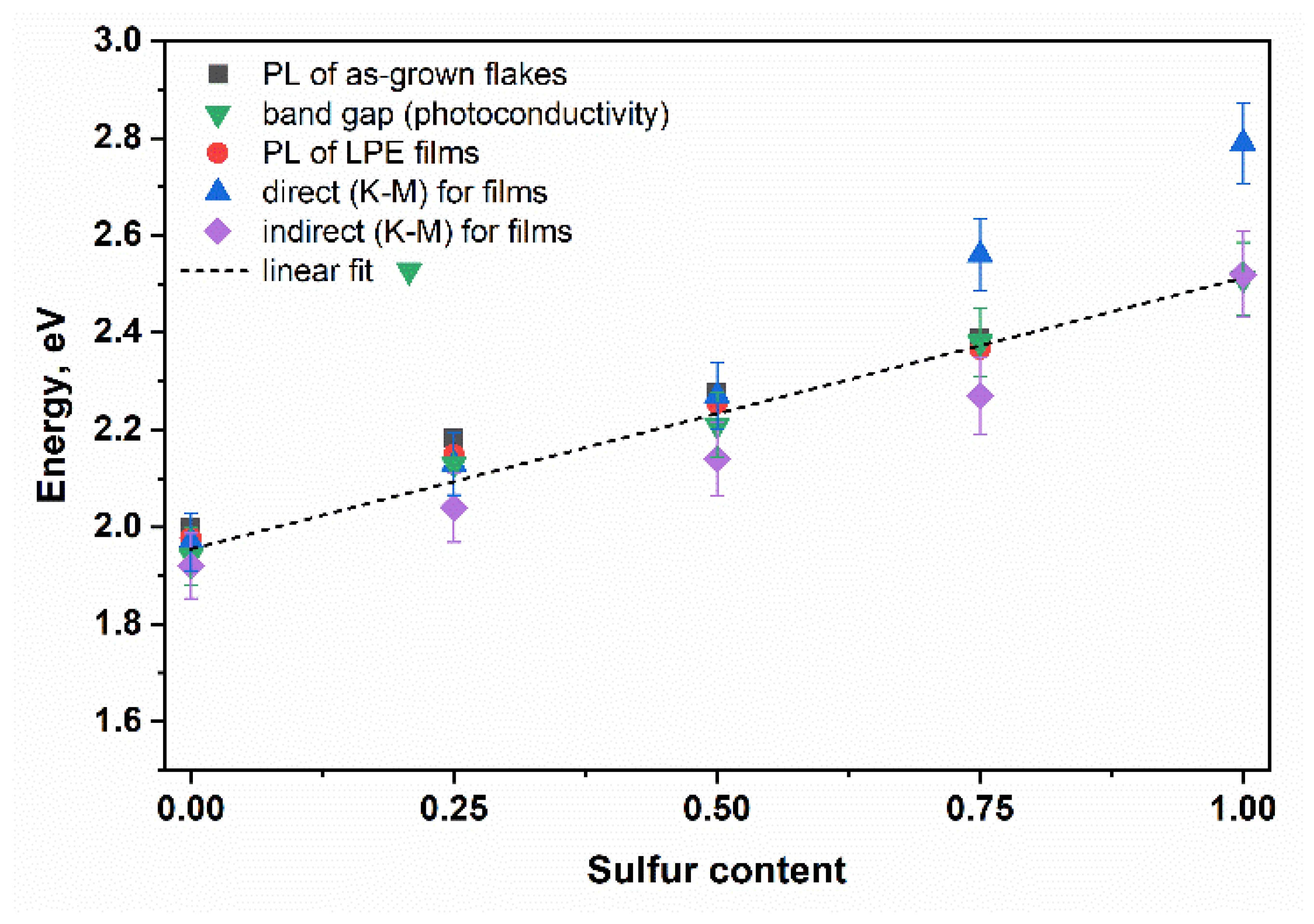
3.1. Characterization of As-Grown GaSe1−xSx Crystals
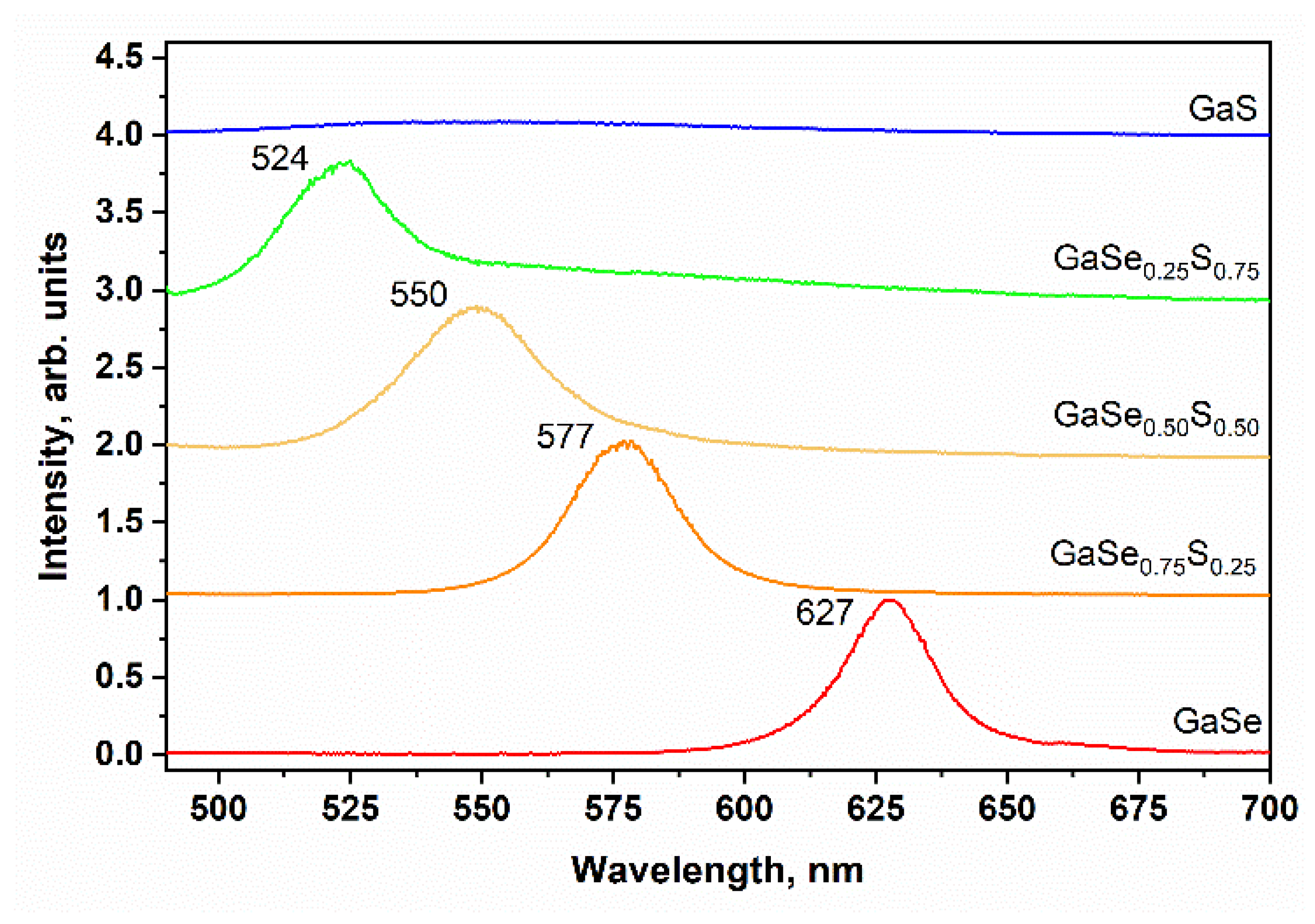
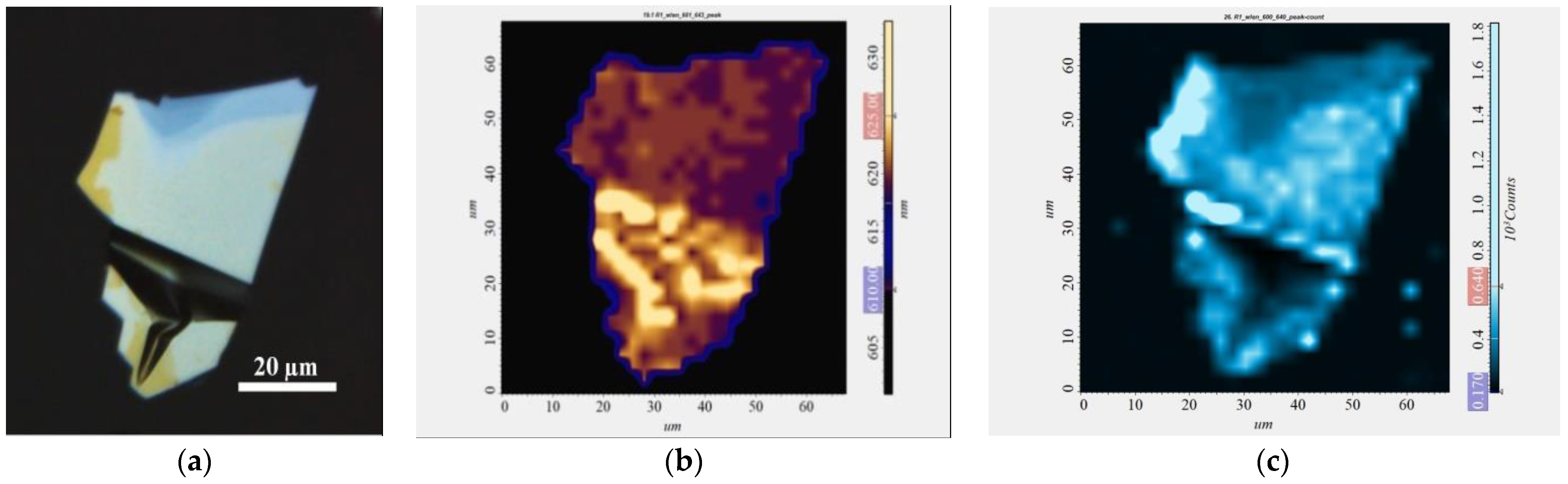
3.2. Characterization of Liquid-Phase Exfoliated GaSe1−xSx Flakes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, G.; Yang, L.; Yang, Z.; Wang, Y.; Jin, X.; Dai, J.; Wu, Q.; Liu, S.; Zhu, X.; Wang, X.; et al. A general ink formulation of 2D crystals for wafer-scale inkjet printing. Sci. Adv. 2020, 6, eaba5029. [Google Scholar] [CrossRef] [PubMed]
- Witomska, S.; Leydecker, T.; Ciesielski, A.; Samorì, P. Production and patterning of liquid phase–exfoliated 2D sheets for applications in optoelectronics. Adv. Funct. Mater. 2019, 29, 1901126. [Google Scholar] [CrossRef]
- Coleman, J.N.; Lotya, M.; O’neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J.; et al. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef]
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Strano, M.S.; Coleman, J.N. Liquid exfoliation of layered materials. Science 2013, 340, 72–75. [Google Scholar] [CrossRef]
- Alzakia, F.I.; Tan, S.C. Liquid-exfoliated 2D materials for optoelectronic applications. Adv. Sci. 2021, 8, 2003864. [Google Scholar] [CrossRef]
- Zappia, M.I.; Bianca, G.; Bellani, S.; Serri, M.; Najafi, L.; Oropesa-Nuñez, R.; Martín-García, B.; Bouša, D.; Sedmidubský, D.; Pellegrini, V.; et al. Solution-processed GaSe nanoflake-based films for photoelectrochemical water splitting and photoelectrochemical-type photodetectors. Adv. Funct. Mater. 2020, 30, 1909572. [Google Scholar] [CrossRef]
- Zappia, M.I.; Bianca, G.; Bellani, S.; Curreli, N.; Sofer, Z.; Serri, M.; Najafi, L.; Piccinni, M.; Oropesa-Nuñez, R.; Marvan, P.; et al. Two-dimensional gallium sulfide nanoflakes for UV-selective photoelectrochemical-type photodetectors. J. Phys. Chem. C 2021, 125, 11857–11866. [Google Scholar] [CrossRef]
- Luxa, J.; Wang, Y.; Sofer, Z.; Pumera, M. Layered post-transition-metal dichalcogenides (X−M−M−X) and their properties. Chem. A Eur. J. 2016, 22, 18810–18816. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Gu, Y.; Lin, Y.C.; Yu, Y.; Geohegan, D.B.; Xiao, K. Synthesis and emerging properties of 2D layered III-VI metal chalcogenides. Appl. Phys. Rev. 2019, 6, 041312. [Google Scholar] [CrossRef]
- Late, D.J.; Liu, B.; Luo, J.; Yan, A.; Matte, H.S.S.R.; Grayson, M.; Rao, C.N.R.; Dravid, V.P. GaS and GaSe ultrathin layer transistors. Adv. Mater. 2012, 24, 3549–3554. [Google Scholar] [CrossRef]
- Xiang, Q.; Meng, G.F.; Zhao, H.B.; Zhang, Y.; Li, H.; Ma, W.J.; Xu, J.Q. Au nanoparticle modified WO3 nanorods with their enhanced properties for photocatalysis and gas sensing. J. Phys. Chem. C 2010, 114, 2049–2055. [Google Scholar] [CrossRef]
- Xu, K.; Yin, L.; Huang, Y.; Shifa, T.A.; Chu, J.; Wang, F.; Cheng, R.; Wang, Z.; He, J. Synthesis, properties and applications of 2D layered MIIIXVI (M = Ga, In; X = S, Se, Te) materials. Nanoscale 2016, 8, 16802–16818. [Google Scholar] [CrossRef]
- Qasrawi, A.F.; Al Garni, S.E.; Gasanly, N.M. Characterization of the MgO/GaSe0.5S0.5 heterojunction designed for visible light communications. Mater. Sci. Semicond. Process. 2015, 39, 377–383. [Google Scholar] [CrossRef]
- Karabulut, O.; Parlak, M.; Yilmaz, K.; Gasanly, N.M. Electrical and photoconductive properties of GaS0.75Se0.25 mixed crystals. Cryst. Res. Technol. 2005, 40, 253–258. [Google Scholar] [CrossRef]
- Ni, Y.; Wu, H.; Huang, C.; Mao, M.; Wang, Z.; Cheng, X. Growth and quality of gallium selenide (GaSe) crystals. J. Cryst. Growth 2013, 381, 10–14. [Google Scholar] [CrossRef]
- Abdullah, M.M.; Bhagavannarayana, G.; Wahab, M.A. Growth and characterization of GaSe single crystal. J. Cryst. Growth 2010, 312, 1534–1537. [Google Scholar] [CrossRef]
- Wang, T.; Li, J.; Zhao, Q.; Yin, Z.; Zhang, Y.; Chen, B.; Xie, Y.; Jie, W. High-quality GaSe single crystal grown by the bridgman method. Materials 2018, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Ho, C.H.; Shen, W.T.; Cheng, Z.H.; Huang, Y.S.; Tiong, K.K. Optical properties of GaSe1−xSx series layered semiconductors grown by vertical bridgman method. Mater. Chem. Phys. 2004, 88, 313–317. [Google Scholar] [CrossRef]
- Arancia, G.; Grandolfo, M.; Manfredotti, C.; Rizzo, A. Electron diffraction study of melt- and vapour-grown GaSe1−xSx single crystals. Phys. Status Solidi 1976, 33, 563–571. [Google Scholar] [CrossRef]
- Nazarov, M.M.; Sarkisov, S.Y.; Shkurinov, A.P.; Tolbanov, O.P. GaSe1−xSx and GaSe1−xTex thick crystals for broadband terahertz pulses generation. Appl. Phys. Lett. 2011, 99, 081105. [Google Scholar] [CrossRef]
- Zhang, H.-Z.; Kang, Z.-H.; Jiang, Y.; Gao, J.-Y.; Wu, F.-G.; Feng, Z.-S.; Andreev, Y.M.; Lanskii, G.V.; Morozov, A.N.; Sachkova, E.I.; et al. SHG phase matching in GaSe and mixed GaSe1_1-XS_x, X0.412, crystals at room temperature. Opt. Express 2008, 16, 9951. [Google Scholar] [CrossRef]
- Kang, J.; Wells, S.A.; Sangwan, V.K.; Lam, D.; Liu, X.; Luxa, J.; Sofer, Z.; Hersam, M.C. Solution-based processing of optoelectronically active indium selenide. Adv. Mater. 2018, 30, 1802990. [Google Scholar] [CrossRef] [PubMed]
- Curreli, N.; Serri, M.; Spirito, D.; Lago, E.; Petroni, E.; Martín-García, B.; Politano, A.; Gürbulak, B.; Duman, S.; Krahne, R.; et al. Liquid phase exfoliated indium selenide based highly sensitive photodetectors. Adv. Funct. Mater. 2020, 30, 1908427. [Google Scholar] [CrossRef]
- Curreli, N.; Serri, M.; Zappia, M.I.; Spirito, D.; Bianca, G.; Buha, J.; Najafi, L.; Sofer, Z.; Krahne, R.; Pellegrini, V.; et al. Liquid-phase exfoliated gallium selenide for light-driven thin-film transistors. Adv. Electron. Mater. 2021, 7, 2001080. [Google Scholar] [CrossRef]
- Lim, S.Y.; Lee, J.U.; Kim, J.H.; Liang, L.; Kong, X.; Nguyen, T.T.H.; Lee, Z.; Cho, S.; Cheong, H. Polytypism in few-layer gallium selenide. Nanoscale 2020, 12, 8563–8573. [Google Scholar] [CrossRef]
- Del Pozo-Zamudio, O.; Schwarz, S.; Sich, M.; Akimov, I.A.; Bayer, M.; Schofield, R.C.; Chekhovich, E.A.; Robinson, B.J.; Kay, N.D.; Kolosov, O.V.; et al. Photoluminescence of two-dimensional GaTe and GaSe films. 2D Mater. 2015, 2, 035010. [Google Scholar] [CrossRef]
- Jung, C.S.; Shojaei, F.; Park, K.; Oh, J.Y.; Im, H.S.; Jang, D.M. Red-to-ultraviolet emission tuning of two-dimensional gallium sulfide. ACS Nano 2015, 9, 9585–9593. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, B.M.; Manz, N.; Bethke, D.; Shaner, E.A.; Serov, A.; Kalugin, N.G. Role of humidity in oxidation of ultrathin GaSe. Mater. Res. Express 2019, 6, 085907. [Google Scholar] [CrossRef]

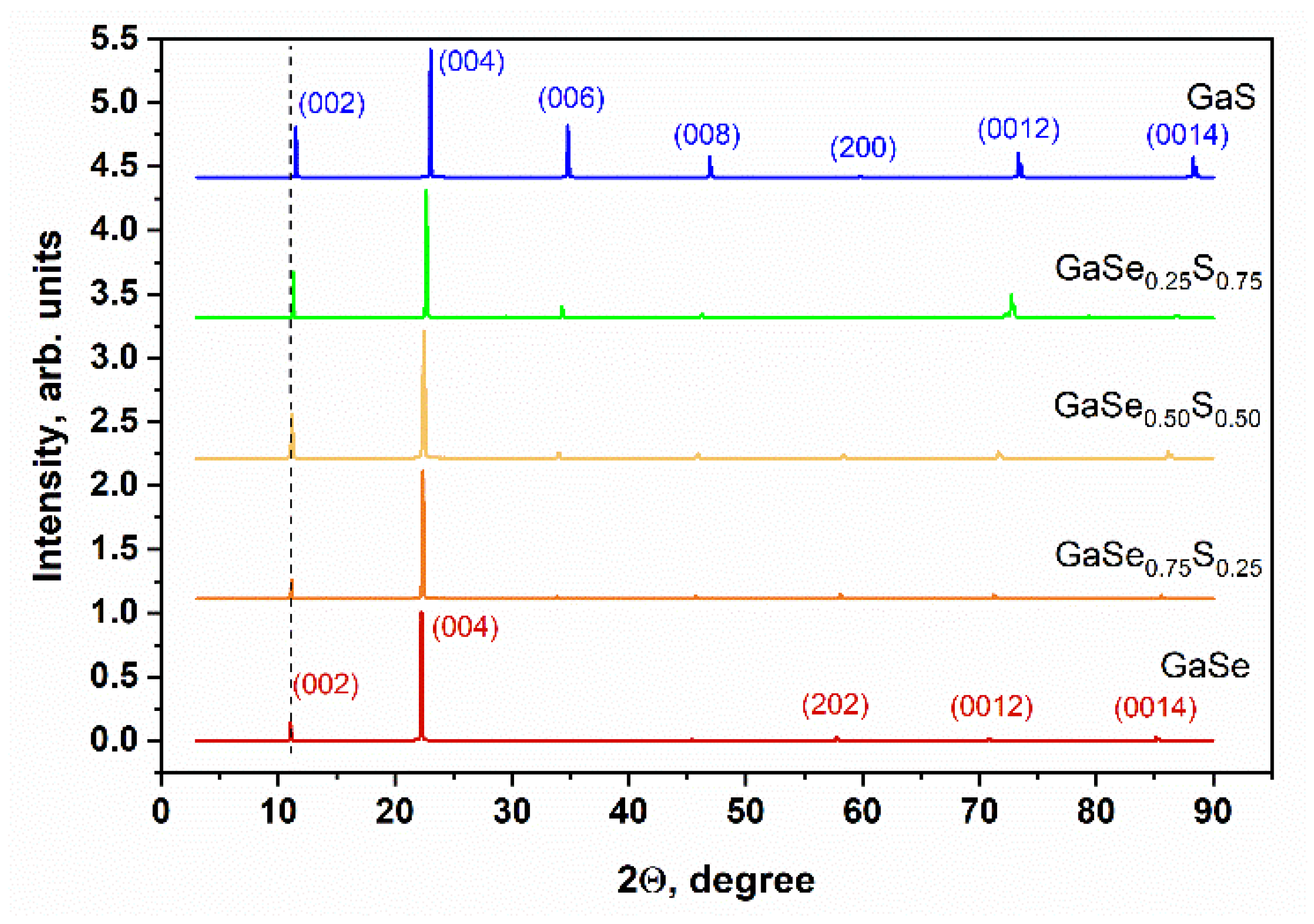
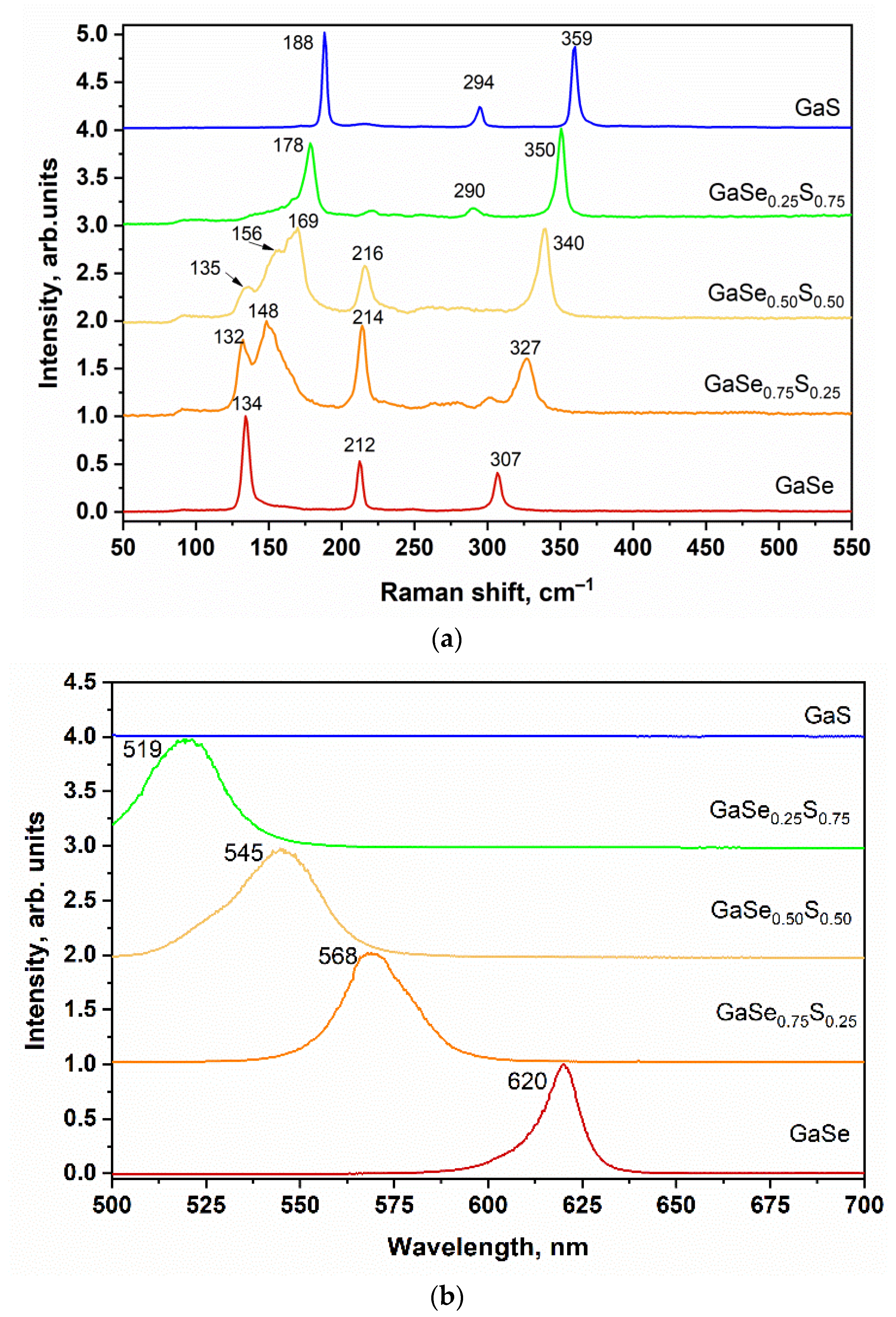

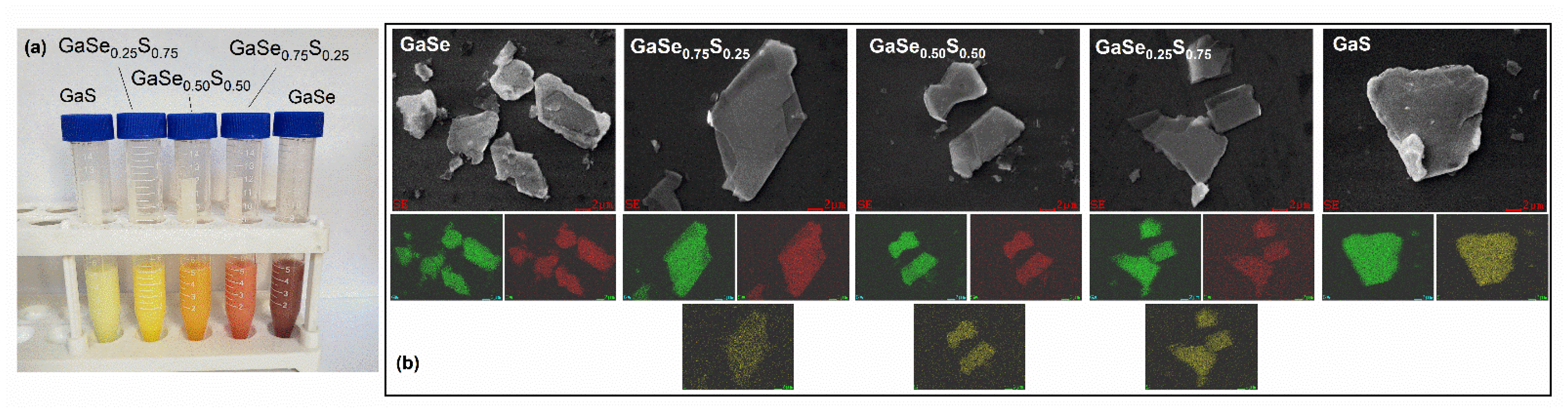
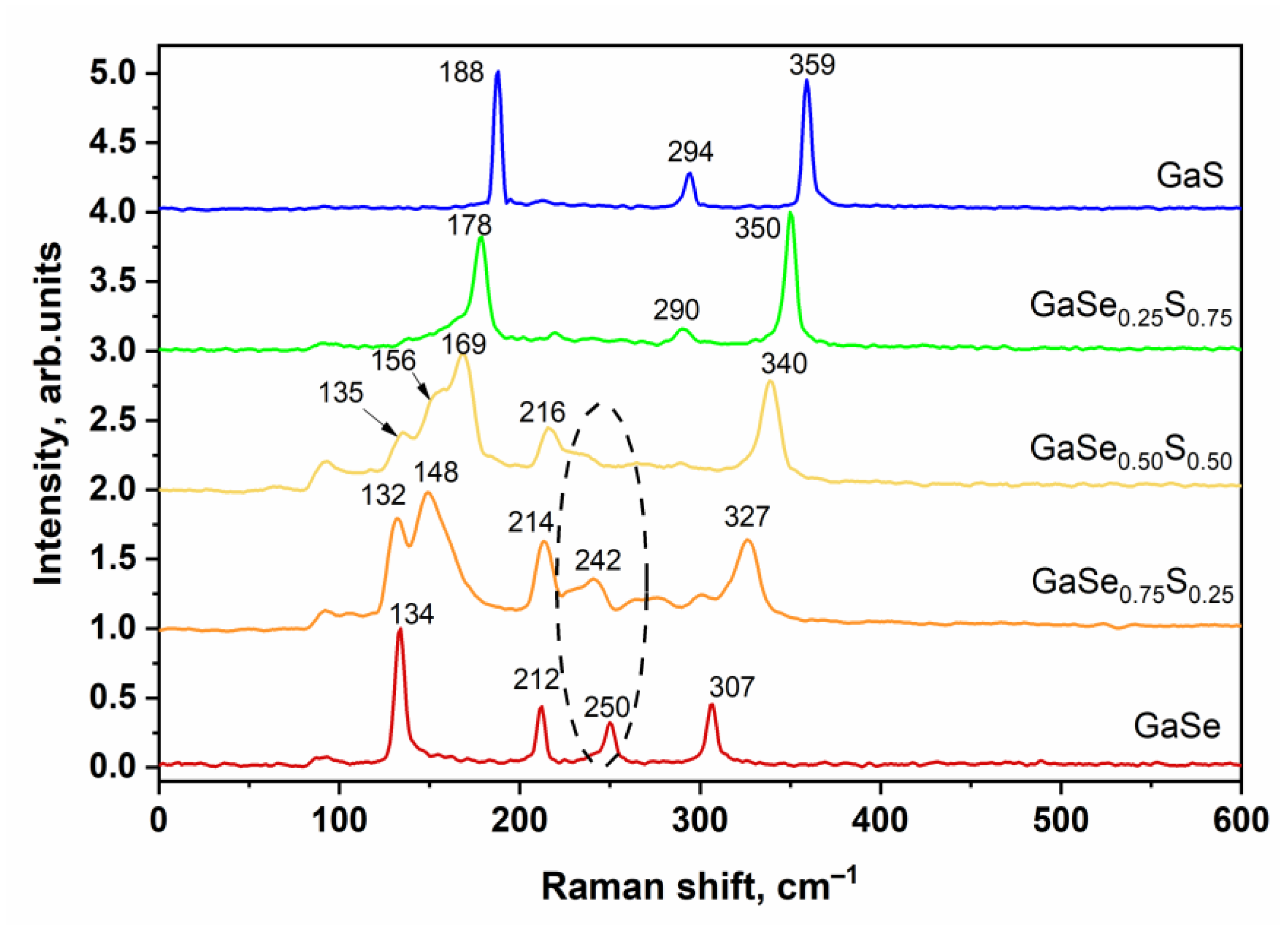
| Crystal Name | Ga, at.% | Se, at.% | S, at.% | d(004)—Spacing, Å |
|---|---|---|---|---|
| GaSe | 49.9 | 50.1 | - | 3.98 |
| GaSe0.75S0.25 | 49.7 | 38.7 | 11.6 | 3.95 |
| GaSe0.50S0.50 | 50.1 | 25.2 | 24.7 | 3.93 |
| GaSe0.25S0.75 | 49.5 | 12.1 | 38.4 | 3.91 |
| GaS | 49.1 | - | 50.9 | 3.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aitzhanov, M.; Guseinov, N.; Nemkayeva, R.; Sagidolda, Y.; Tolepov, Z.; Prikhodko, O.; Mukhametkarimov, Y. Growth and Liquid-Phase Exfoliation of GaSe1−xSx Crystals. Materials 2022, 15, 7080. https://doi.org/10.3390/ma15207080
Aitzhanov M, Guseinov N, Nemkayeva R, Sagidolda Y, Tolepov Z, Prikhodko O, Mukhametkarimov Y. Growth and Liquid-Phase Exfoliation of GaSe1−xSx Crystals. Materials. 2022; 15(20):7080. https://doi.org/10.3390/ma15207080
Chicago/Turabian StyleAitzhanov, Madi, Nazim Guseinov, Renata Nemkayeva, Yerulan Sagidolda, Zhandos Tolepov, Oleg Prikhodko, and Yerzhan Mukhametkarimov. 2022. "Growth and Liquid-Phase Exfoliation of GaSe1−xSx Crystals" Materials 15, no. 20: 7080. https://doi.org/10.3390/ma15207080
APA StyleAitzhanov, M., Guseinov, N., Nemkayeva, R., Sagidolda, Y., Tolepov, Z., Prikhodko, O., & Mukhametkarimov, Y. (2022). Growth and Liquid-Phase Exfoliation of GaSe1−xSx Crystals. Materials, 15(20), 7080. https://doi.org/10.3390/ma15207080






