Effect of Chromium on Microstructure and Oxidation Wear Behavior of High-Boron High-Speed Steel at Elevated Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
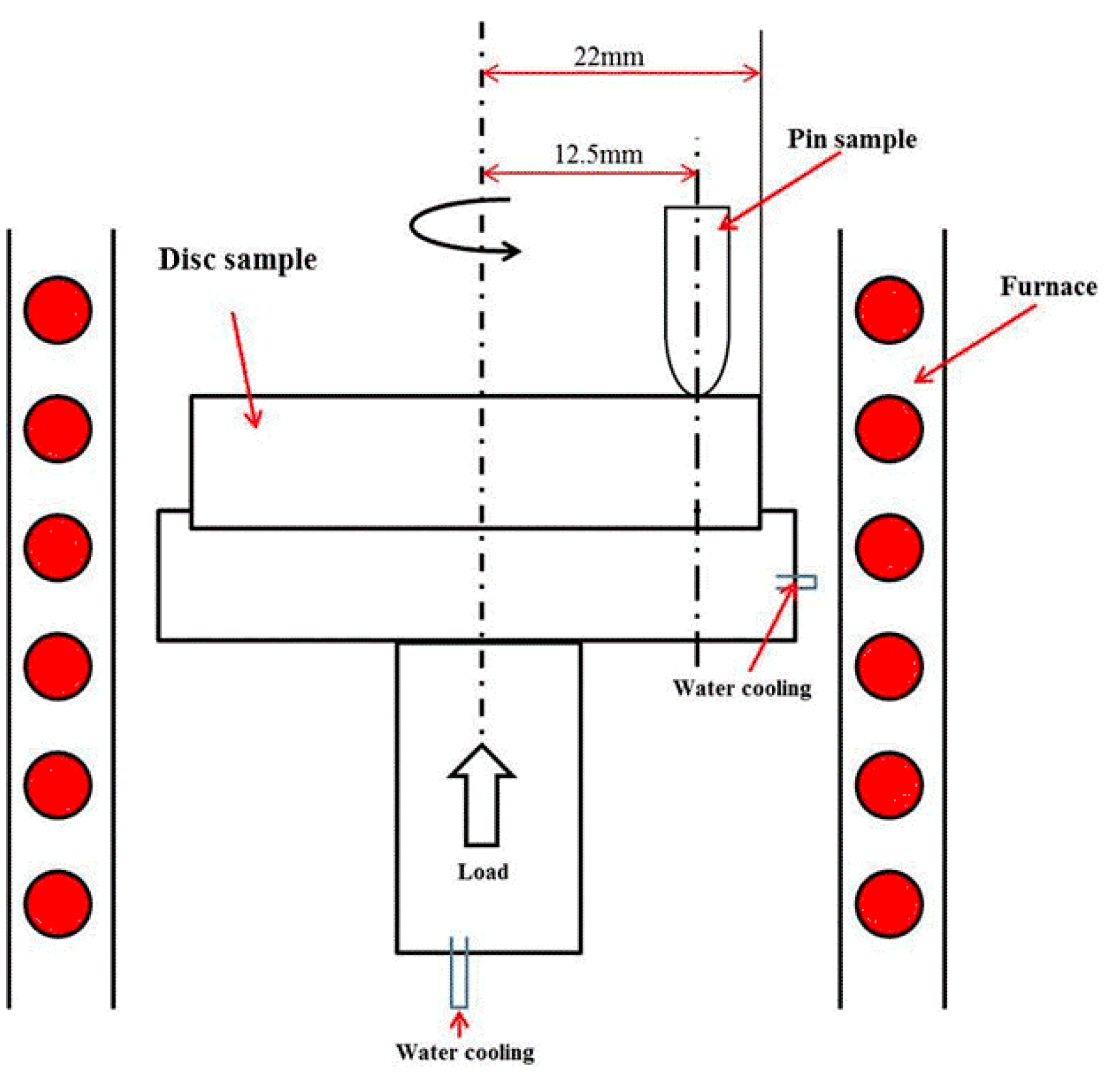
2.2. High Temperature Wear Experiment
3. Results and Discussion
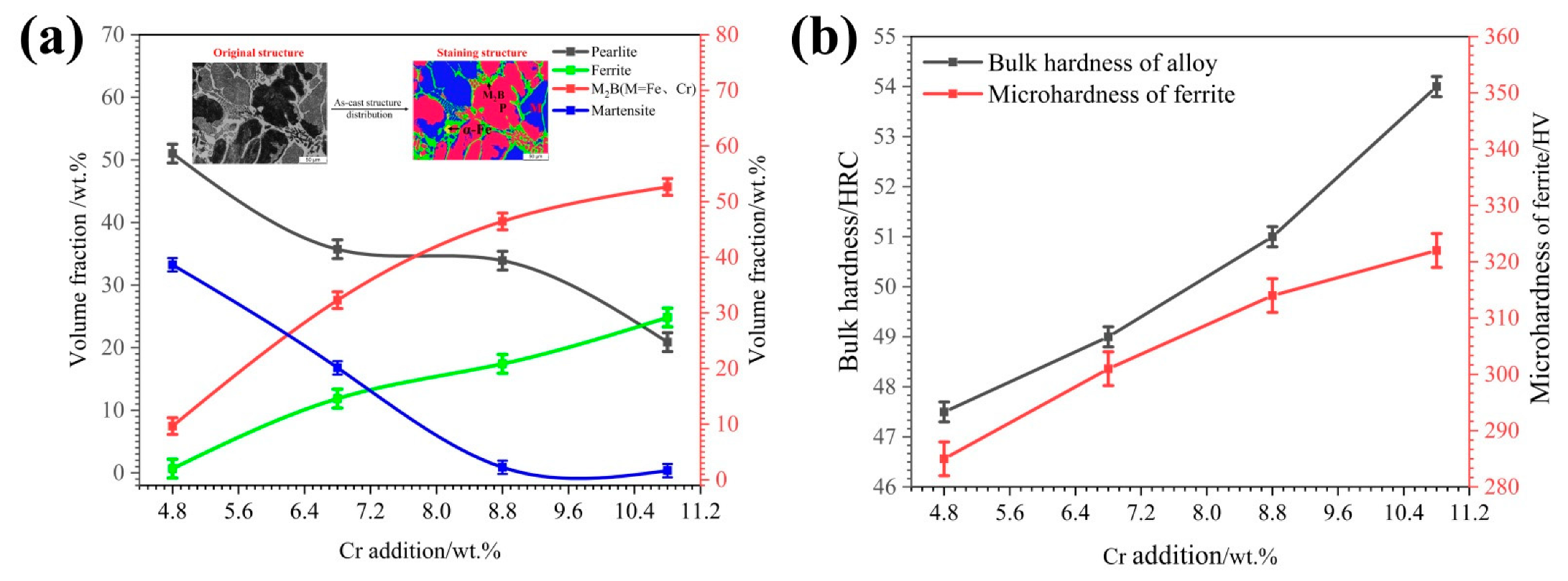
3.1. As-Cast Microstructure of HBHSSs with Various Cr Additions
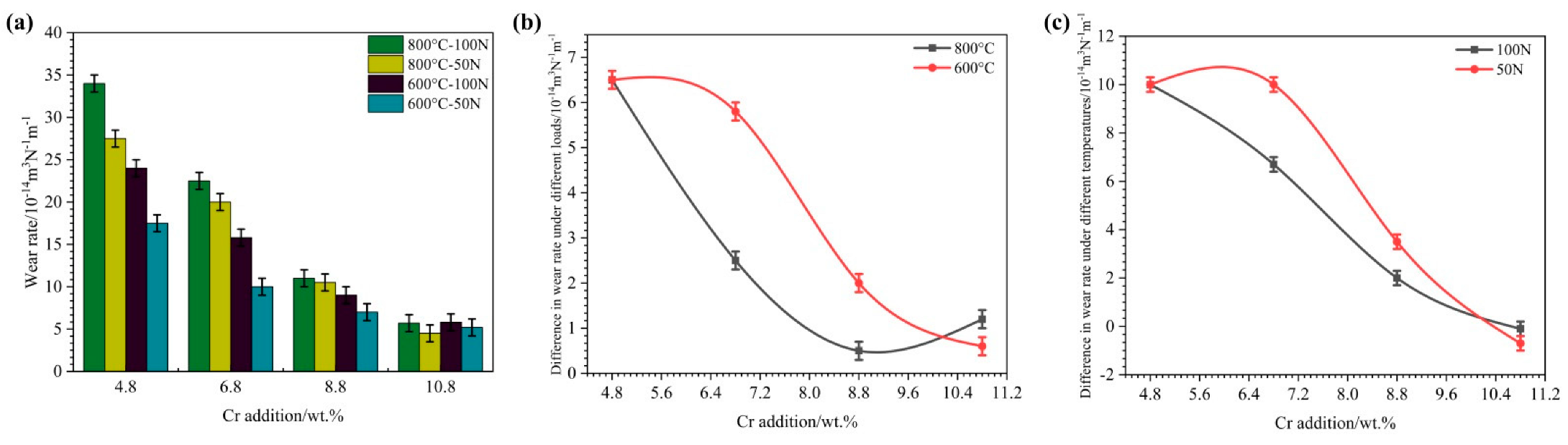
3.2. High-Temperature Oxidation Wear Rates of HBHSSs
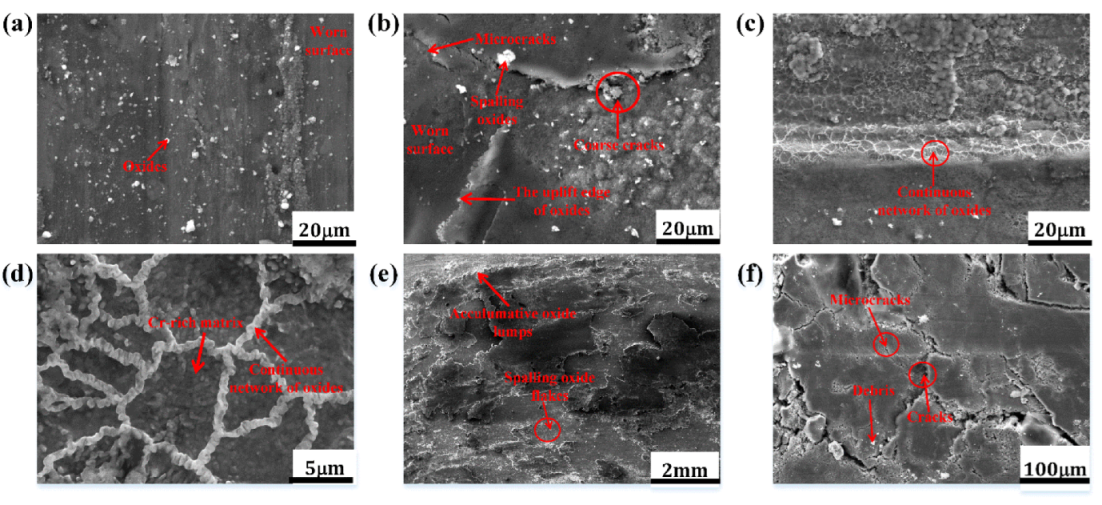
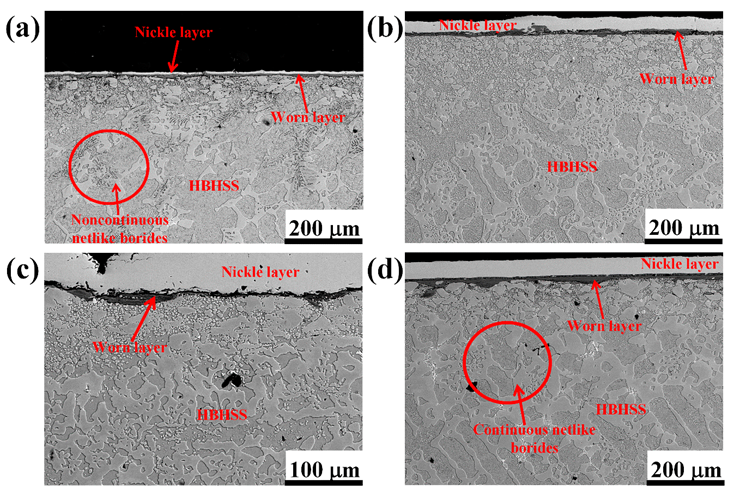
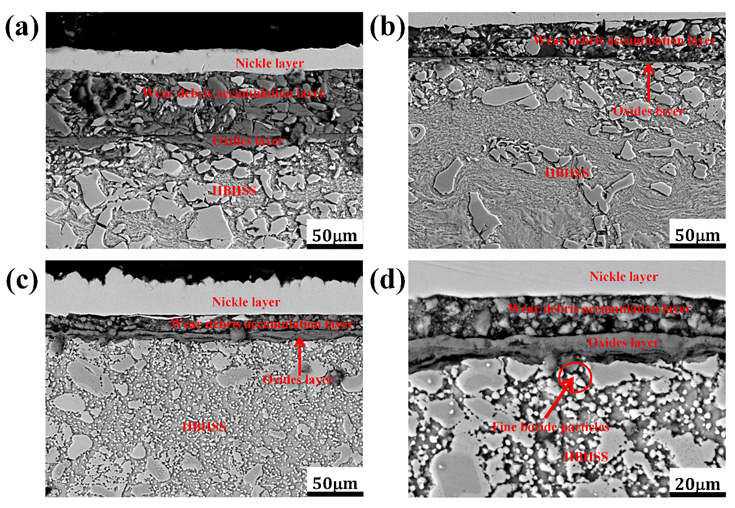
3.3. Surface and Cross-Sectional Morphologies of HBHSSs
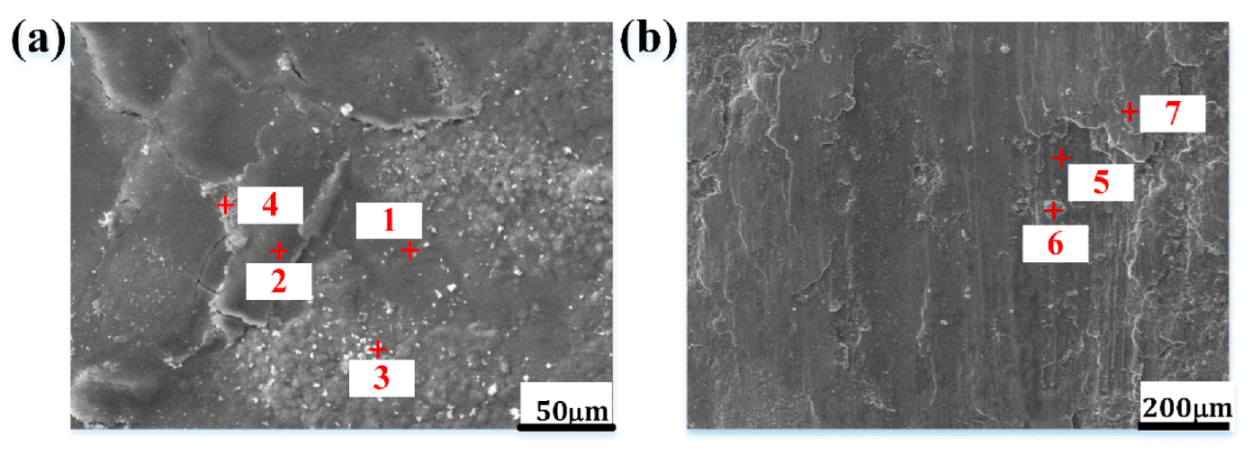
3.3.1. Surface Morphologies of HBHSSs
3.3.2. Cross-Sectional Morphologies of HBHSSs
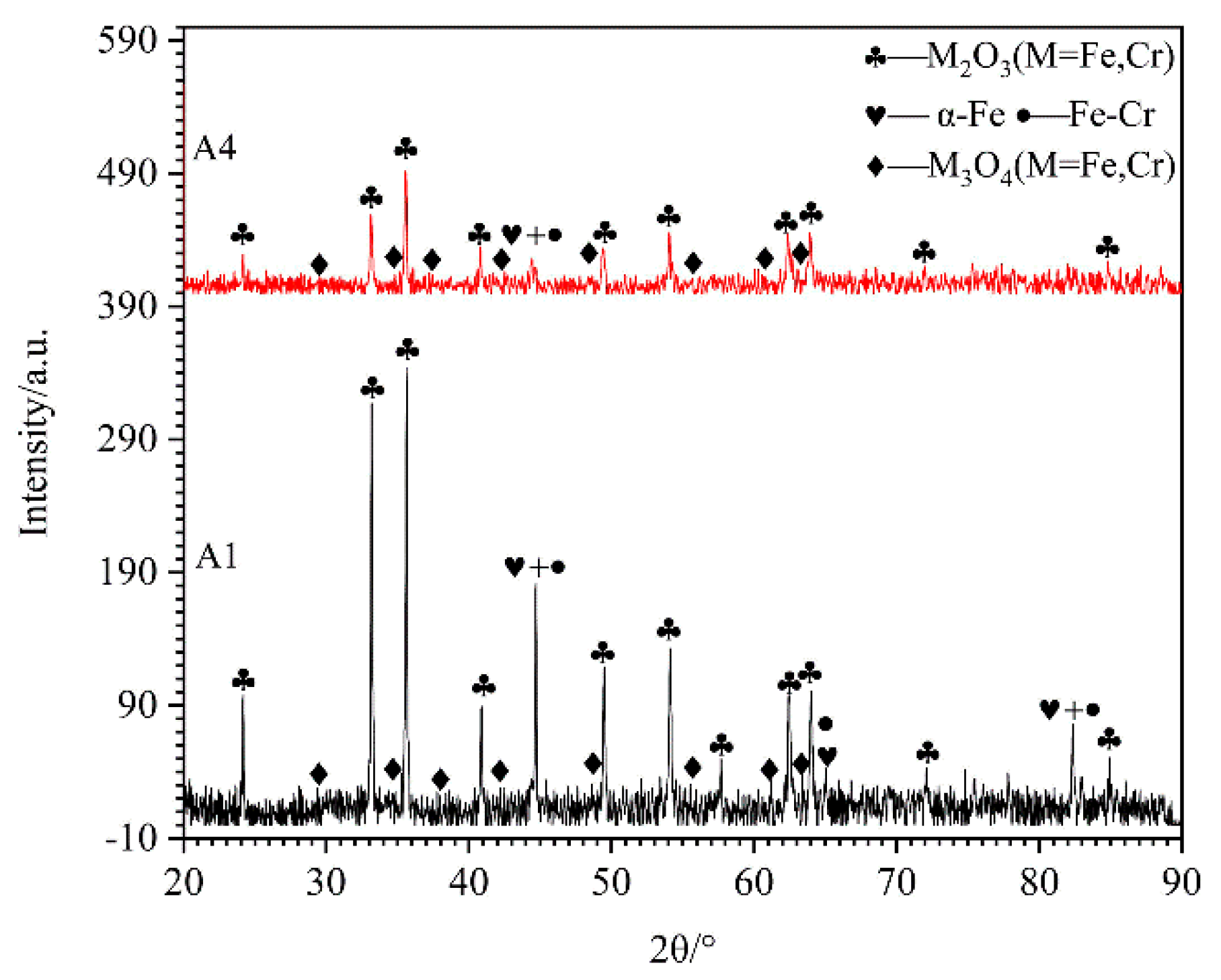
3.4. Microstructures and Compositions of Oxide Film Detected by XRD and EPMA
3.5. Wear Mechanism Analysis
4. Conclusions
- (1)
- The as-cast microstructure of HBHSSs is mainly composed of α-Fe, M2B (M = Fe,Cr) and a little Fe3 (C,B). The addition of Cr results in the transformation of martensite into ferrite and pearlite, and also refines the size of the grid-shaped borides.
- (2)
- After oxidation wear, oxide scales form and the oxidation wear resistance of HBHSSs can be gradually improved with increased additions of Cr. Meanwhile, an interaction between the oxidation wear temperature and applied load on HBHSSs occurs, and the temperature exhibits a larger influence on the oxidation wear properties.
- (3)
- The addition of Cr can reduce the thickness of the uniform and compact oxides and also inhibit the spallation and cracking of worn layers, which is attributed to improvements in microhardness and oxidation resistance of Cr in HBHSSs.
- (4)
- With increased temperatures and loads, more microcracks and spallation of oxides on HBHSSs appear due to a synergistic effect of the temperature and load, which may dominate the oxidation wear process and the failure of oxide films.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, S.D.; Fabijanic, D.; Annasamy, M.; Gallo, S.C.; Fordyce, I.; Paradowska, A.; Leary, M.; Easton, M.; Brandt, M. Microstructure, abrasive wear and corrosion characterisation of laser metal deposited Fe-30Cr-6Mo-10Ni-2.2C alloy. Wear 2019, 438–439, 203070. [Google Scholar] [CrossRef]
- Ling, Z.C.; Chen, W.P.; Lu, T.W.; Li, B.; Zhang, X.M. Interfacial morphology and tribo-corrosion behaviour of Fe-15.3 wt% Cr-3.1 wt% B-6.2 wt% Mo alloy in molten aluminium. Wear 2019, 430–431, 81–93. [Google Scholar] [CrossRef]
- Ji, X.L.; Luo, C.Y.; Sun, Y.; Zhao, J.H. Corrosive wear of multi-layer Fe-based coatings laser cladded from amorphous powders. Wear 2019, 438–439, 203113. [Google Scholar] [CrossRef]
- Wu, G.; Wang, Y. Fracture analysis of high chromium cast iron roll on CSP mill. Adv. Mater. Res. 2012, 548, 538–543. [Google Scholar] [CrossRef]
- Zamri, W.F.H.; Kosasih, P.B.; Tieu, A.K.; Zhu, Q.; Zhu, H. Variations in the microstructures and mechanical properties of the oxide layer on high speed steel hot rolling work rolls. J. Mater. Process. Technol. 2012, 212, 2597–2608. [Google Scholar] [CrossRef]
- Ren, X.Y.; Fu, H.G.; Xing, J.D.; Yi, Y.L. Research on high-temperature dry sliding friction wear behavior of Ca-Ti modified high boron high speed steel. Tribol. Int. 2019, 132, 165–176. [Google Scholar] [CrossRef]
- Liu, D.S.; Liu, R.P.; W, Y.H.; Ma, Y.; Zhu, K. Microstructure and wear properties of Fe-15Cr-2.5Ti-2C-xB wt.% hardfacing alloys. Appl. Surf. Sci. 2013, 271, 253–259. [Google Scholar] [CrossRef]
- Xu, L.J.; Xing, J.D.; Wei, S.Z.; Zhang, Y.Z.; Long, R. Investigation on wear behaviors of high-vanadium high-speed steel compared with high-chromium cast iron under rolling contact condition. Mater. Sci. Eng. A 2006, 434, 63–70. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, D.X.; Long, R.; Wei, S.Z. Investigation of the damage in high vanadium high speed steel and high chrome cast iron roll under the laboratory condition. Foundry 2005, 54, 570–574. [Google Scholar]
- Ji, Y.; Li, Y.; Wei, S.; Long, R.; Xu, L. Influence of carbon content on wear properties of high-vanadium high-speed steel under dry sliding condition. J. Harbin Inst. Technol. 2006, 38, 116–119. [Google Scholar]
- Cao, Y.; Zhang, J.T.; Yin, F.X.; Liu, X.H. Microstructure and properties and its failure mechanism of high speed steel roll. Trans. Mater. Heat Treat. 2012, 33, 50–54. [Google Scholar]
- Ren, X.Y.; Fu, H.G.; Xing, J.D.; Yi, Y.L. Effect of solidification rate on microstructure and toughness of Ca-Ti modified high boron high speed steel. Mater. Sci. Eng. A 2019, 742, 617–627. [Google Scholar] [CrossRef]
- Yang, Y.W.; Fu, H.G.; Wang, K.M.; Zhu, L.L.; Jang, L. Thermodynamic calculation and analysis for aluminum-alloyed high boron high speed steel. Materialwiss. Werkstofftech. 2018, 49, 39–53. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.T.; Jiang, Y.H.; Li, L.; Li, Z.L. The microstructure and high-temperature tribology behavior of high boron HSS under different heat treatments. Ind. Lubr. Tribol. 2019, 71, 212–220. [Google Scholar] [CrossRef]
- Cen, Q.; Fu, H. A study of heat treatment of high-boron high-speed steel roll. Materialwiss. Werkstofftech. 2013, 44, 612–617. [Google Scholar] [CrossRef]
- Qihong, C.; Zhan-Wen, W.; Yi, J.; Yehua, J.; Fei, L.; Hanguang, F. A study of centrifugal cast high boron high speed steel. Materialwiss. Werkstofftech. 2014, 45, 582–590. [Google Scholar] [CrossRef]
- Ma, S.Q.; Xing, J.D.; Guo, S.Q.; Bai, Y.; Fu, H.G.; Lyu, P.; Huang, Z.F.; Chen, W. Microstructural evolution and mechanical properties of the aluminum-alloyed Fe-1.50 wt%B-0.40 wt%C high-speed steel. Mater. Chem. Phys. 2017, 199, 356–369. [Google Scholar] [CrossRef]
- Ma, S.Q.; Pan, W.J.; Xing, J.D.; Guo, S.Q.; Fu, H.G.; Lyu, P. Microstructure and hardening behavior of Al-modified Fe-1.5 wt%B-0.4 wt%C high-speed steel during heat treatment. Mater. Charact. 2017, 132, 1–9. [Google Scholar] [CrossRef]
- Christodoulou, P.; Calos, N. A step towards designing Fe-Cr-B-C cast alloys. Mater. Sci. Eng. A 2001, 301, 103–117. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Li, J.; Li, B.H. Effects of hot rolling and titanium content on the microstructure and mechanical properties of high boron Fe-B alloys. Mater. Des. 2012, 36, 88–93. [Google Scholar] [CrossRef]
- Liu, Z.L.; Chen, X.; Li, Y.X.; Hu, K.H. Microstructure and mechanical properties of high boron white cast iron. Mater. Sci. Eng. A 2008, 486, 112–116. [Google Scholar] [CrossRef]
- Yi, Y.L.; Li, Q.; Long, S.L.; Lv, Z.; Li, S.J.; Liu, Y.Z.; Zheng, B.C.; Li, W. Effect of matrix microstructure on the matrix/M2B wear interaction and its quantitative characterization in an Fe-2wt%B alloy. Wear 2021, 472–473, 203608. [Google Scholar] [CrossRef]
- Jian, Y.X.; Huang, Z.F.; Xing, J.D.; Liu, X.T.; Sun, L.; Zheng, B.C.; Wang, Y. Investigation on two-body abrasive wear behavior and mechanism of Fe-3.0 wt% B cast alloy with different chromium content. Wear 2016, 362–363, 68–77. [Google Scholar] [CrossRef]
- Ma, S.D.; Zhang, J.J. Wear resistant high boron cast alloy—A review. Rev. Adv. Mater. Sci. 2016, 44, 54–62. [Google Scholar]
- Yi, Y.L.; Xing, J.D.; Yu, L.L.; Fu, H.G.; Wan, M.J.; Lu, Y.F.; Jian, Y.X.; Zheng, Q.L.; Chen, Y. Effect of casting thickness on microstructure, mechanical properties and abrasion resistance of Fe-B cast alloy. Tribol. Int. 2018, 122, 179–188. [Google Scholar] [CrossRef]
- Yi, Y.L.; Xing, J.D.; Ren, X.Y.; Fu, H.G.; Li, Q.; Yi, D.W. Investigation on abrasive wear behavior of Fe-B alloys containing various molybdenum contents. Tribol. Int. 2019, 135, 237–245. [Google Scholar] [CrossRef]
- Prosviryakov, A.; Mondoloni, B.; Churyumov, A.; Pozdniakov, A. Microstructure and Hot Deformation Behaviour of a Novel Zr-Alloyed High-Boron Steel. Metals 2019, 9, 218. [Google Scholar] [CrossRef] [Green Version]
- Moon, J.; Jang, J.H.; Kim, S.D.; Lee, T.H.; Ha, H.Y.; Lee, C.H.; Hong, H.U. Different aspect of solidification cracking susceptibility and hot ductility behavior of borated stainless steels and the effects of boron content. Mater. Charact. 2020, 164, 110319. [Google Scholar] [CrossRef]
- Rojacz, H.; Pahr, H.; Baumgartner, S.; Varga, M. High temperature abrasion resistance of differently welded structural steels. Tribol. Int. 2017, 113, 487–499. [Google Scholar] [CrossRef]
- Moder, J.; Grun, F.; Summer, F.; Gasperlmair, T.; Andritschky, M. Effect of temperature on wear and tribofilm formation in highly-loaded DLC-steel line contacts. Tribol. Int. 2018, 123, 120–129. [Google Scholar] [CrossRef]
- Lee, J.H.; Oh, J.C.; Park, J.W.; Lee, H.C.; Lee, S. Effects of tempering temperature on wear resistance and surface roughness of a high speed steel roll. ISIJ Int. 2001, 41, 859–865. [Google Scholar] [CrossRef]
- Hao, L.; Wu, H.; Wei, D.B.; Cheng, X.W.; Zhao, J.W.; Luo, S.Z.; Jiang, L.Z.; Jiang, Z.Y. Wear and friction behavior of high-speed steel and infinite chill material for rolling ferritic stainless steels. Wear 2017, 376–377, 1580–1585. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.Q.; Xing, J.D.; Liu, G.F.; Yi, D.W.; Fu, H.G.; Zhang, J.J.; Li, Y.F. Effect of chromium concentration on microstructure and properties of Fe-3.5 B alloy. Mater. Sci. Eng. A 2010, 527, 6800–6808. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.X.; Zhang, H.M. Microstructure and mechanical properties of high boron white cast iron with about 4 wt% chromium. J. Mater. Sci. 2010, 46, 957–963. [Google Scholar] [CrossRef]
- Zhou, C.J.; Xing, J.D.; Xiao, B.; Feng, J.; Xie, X.J.; Chen, Y.H. First principles study on the structural properties and electronic structure of X2B (X = Cr, Mn, Fe, Co, Ni, Mo and W) compounds. Comput. Mater. Sci. 2009, 44, 1056–1064. [Google Scholar] [CrossRef]
- Xiao, B.; Feng, J.; Zhou, C.T.; Xing, J.D.; Xie, X.J.; Cheng, Y.H.; Zhou, R. The elasticity, bond hardness and thermodynamic properties of X2B (X = Cr, Mn, Fe, Co, Ni, Mo, W) investigated by DFT theory. Phys. B 2010, 405, 1274–1278. [Google Scholar] [CrossRef]
- Jian, Y.X.; Huang, Z.F.; Xing, J.D.; Wang, B.Y. Effects of chromium addition on fracture toughness and hardness of oriented bulk Fe2B crystals. Mater. Charact. 2015, 110, 138–144. [Google Scholar] [CrossRef]
- Jian, Y.X.; Huang, Z.F.; Xing, J.D.; Guo, X.Z.; Wang, Y.; Lv, Z. Effects of Mn addition on the two-body abrasive wear behavior of Fe-3.0 wt% B alloy. Tribol. Int. 2016, 103, 243–251. [Google Scholar] [CrossRef]
- Jian, Y.X.; Huang, Z.F.; Xing, J.D.; Li, J.F. Effects of chromium additions on the three-body abrasive wear behavior of Fe-3.0 wt% B alloy. Wear 2017, 378–379, 165–173. [Google Scholar] [CrossRef]
- Tian, S.; Liao, Z.L.; Guo, W.C.; He, Q.L.; Wang, H.; Wang, W.M. Effects of theTiB2-SiC Volume Ratio and Spark Plasma Sintering Temperature on the Properties and Microstructure of TiB2-BN-SiC Composite Ceramics. Crystals 2021, 12, 29. [Google Scholar] [CrossRef]
- Samal, S.; Cibulková, J.; Čtvrtlík, R.; Tomáštík, J.; Václavek, L.; Kopeček, J.; Šittner, P. Tribological Behavior of NiTi Alloy Produced by Spark Plasma Sintering Method. Coatings 2021, 11, 1246. [Google Scholar] [CrossRef]




| Samples | Cr | B | Al | W | Mn | Mo | Si | C | V | Ti |
|---|---|---|---|---|---|---|---|---|---|---|
| A1 | 4.80 | 1.50 | 1.20 | 1.20 | 0.65 | 0.65 | 0.60 | 0.55 | 0.50 | 0.08 |
| A2 | 6.80 | 1.50 | 1.20 | 1.20 | 0.65 | 0.65 | 0.60 | 0.55 | 0.50 | 0.08 |
| A3 | 8.80 | 1.50 | 1.20 | 1.20 | 0.65 | 0.65 | 0.60 | 0.55 | 0.50 | 0.08 |
| A4 | 10.80 | 1.50 | 1.20 | 1.20 | 0.65 | 0.65 | 0.60 | 0.55 | 0.50 | 0.08 |
| M2 | 4.50 | — | 1.20 | 5.80 | 0.23 | 4.80 | 0.60 | 0.90 | 1.80 | 0.08 |
| Temperature/°C | Load/N | Sliding Speed r/min | Wear Time/min | Gliding Distance/m |
|---|---|---|---|---|
| 600,800 | 50,100 | 100 | 30 | 236 |
| Point | O | Fe | Cr | Si | Mn | Ni | Mo |
|---|---|---|---|---|---|---|---|
| 1 | 29.19 | 66.60 | 2.64 | 0.81 | 0.76 | — | — |
| 2 | 30.18 | 62.96 | 3.88 | 1.07 | — | 1.91 | — |
| 3 | 36.55 | 60.63 | 0.62 | 0.03 | 0.74 | — | 1.42 |
| 4 | 34.47 | 58.99 | 3.61 | 1.37 | 0.73 | 0.86 | — |
| 5 | 8.97 | 72.17 | 10.53 | 1.49 | 2.06 | 1.32 | — |
| 6 | 19.55 | 50.28 | 3.85 | — | 12.67 | — | 11.47 |
| 7 | 10.73 | 32.29 | 11.78 | 0.92 | 1.10 | 36.20 | 1.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, P.; Ma, S.; Jiao, M.; Lv, P.; Xing, J.; Xu, L.; Huang, Z. Effect of Chromium on Microstructure and Oxidation Wear Behavior of High-Boron High-Speed Steel at Elevated Temperatures. Materials 2022, 15, 557. https://doi.org/10.3390/ma15020557
Guo P, Ma S, Jiao M, Lv P, Xing J, Xu L, Huang Z. Effect of Chromium on Microstructure and Oxidation Wear Behavior of High-Boron High-Speed Steel at Elevated Temperatures. Materials. 2022; 15(2):557. https://doi.org/10.3390/ma15020557
Chicago/Turabian StyleGuo, Pengjia, Shengqiang Ma, Ming Jiao, Ping Lv, Jiandong Xing, Liujie Xu, and Zhifu Huang. 2022. "Effect of Chromium on Microstructure and Oxidation Wear Behavior of High-Boron High-Speed Steel at Elevated Temperatures" Materials 15, no. 2: 557. https://doi.org/10.3390/ma15020557
APA StyleGuo, P., Ma, S., Jiao, M., Lv, P., Xing, J., Xu, L., & Huang, Z. (2022). Effect of Chromium on Microstructure and Oxidation Wear Behavior of High-Boron High-Speed Steel at Elevated Temperatures. Materials, 15(2), 557. https://doi.org/10.3390/ma15020557







