Modeling the Hydrogen Redistribution at the Grain Boundary of Misoriented Bicrystals in Austenite Stainless Steel
Abstract
:1. Introduction
2. Governing Equations of Hydrogen Diffusion
3. Crystallographic Constitutive Model
4. Finite Element Model
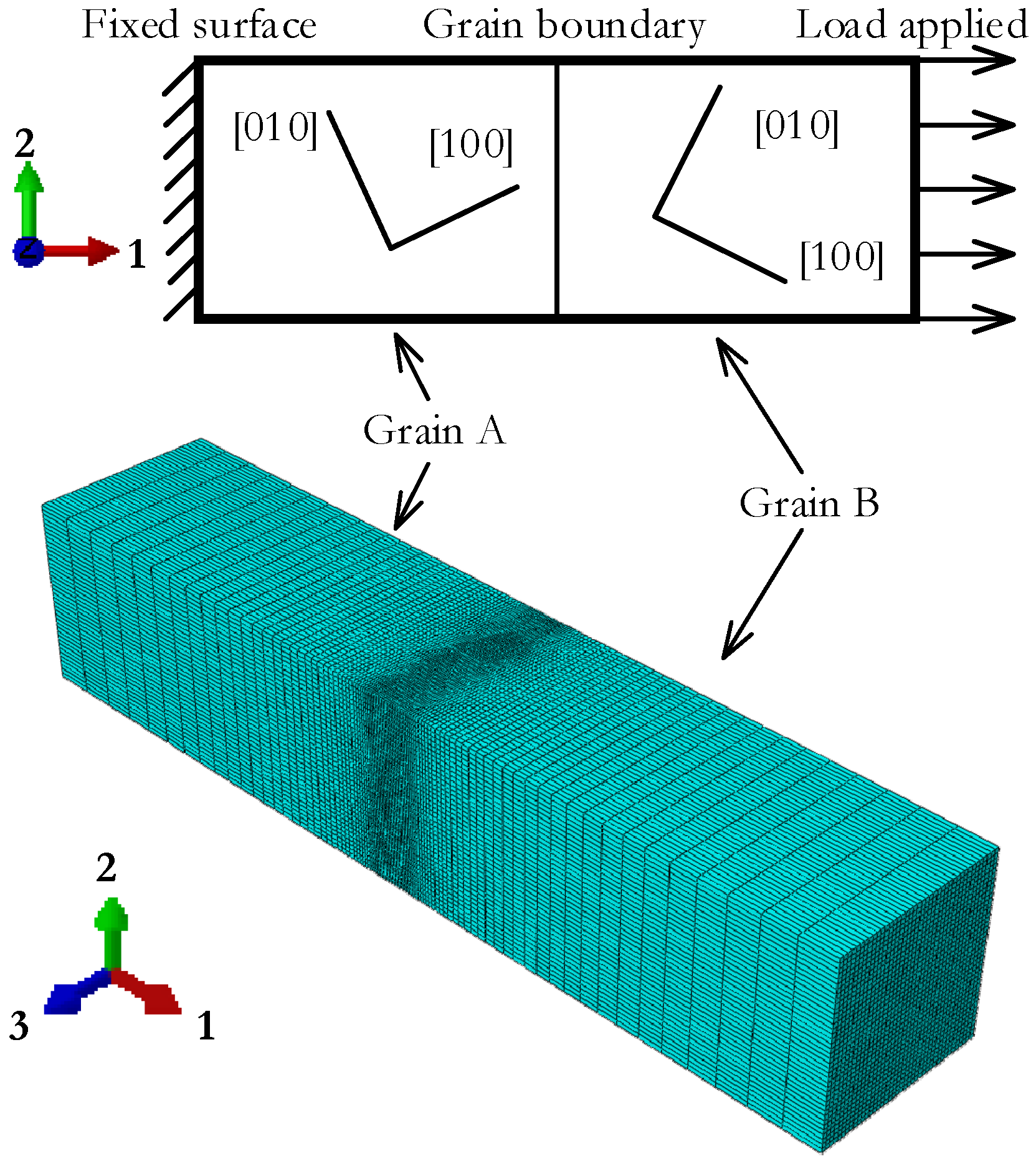
4.1. Geometry Model
4.2. Material Model
4.3. Boundary Conditions, Loading Process, and Meshing
5. Results and Discussion
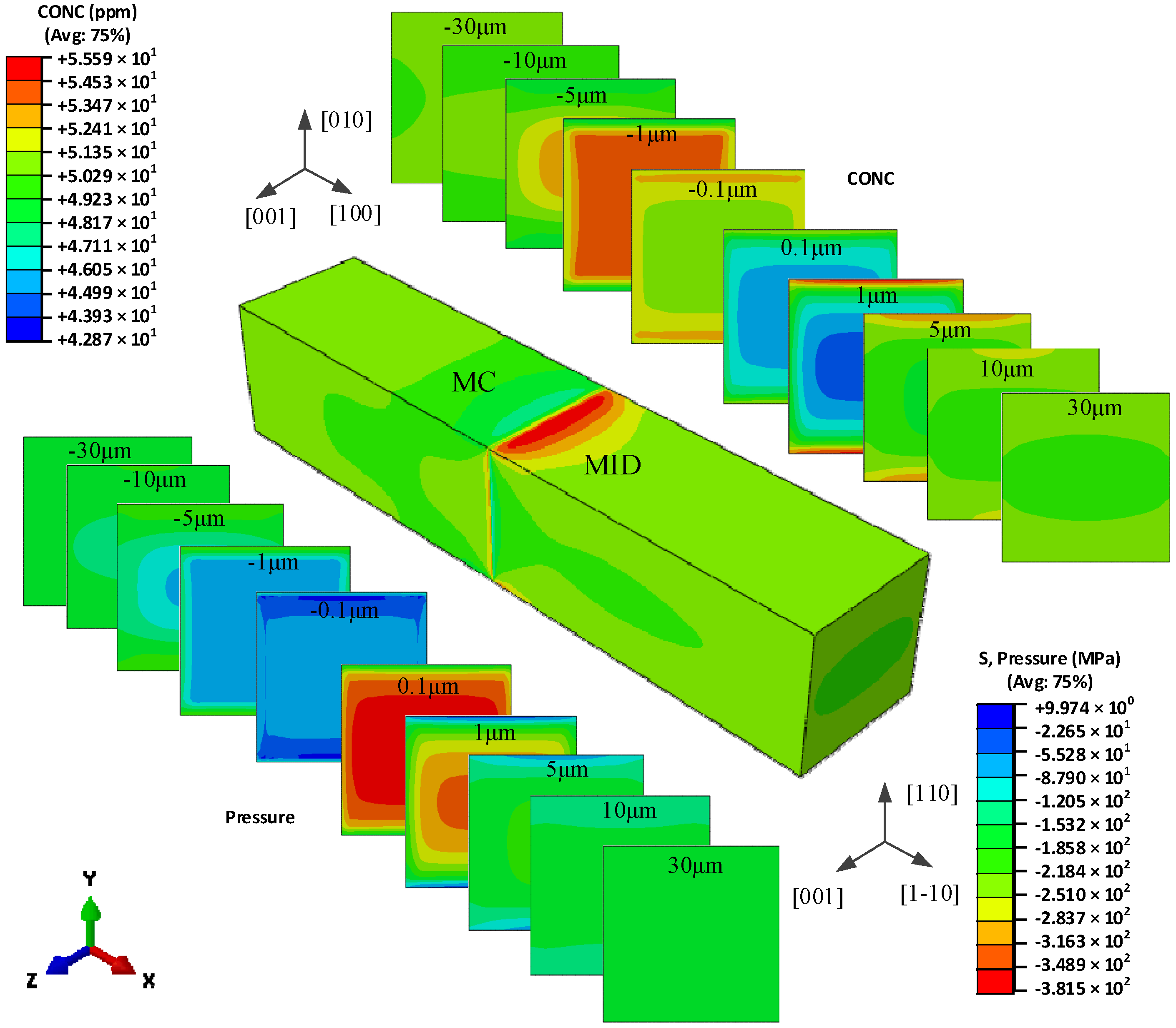
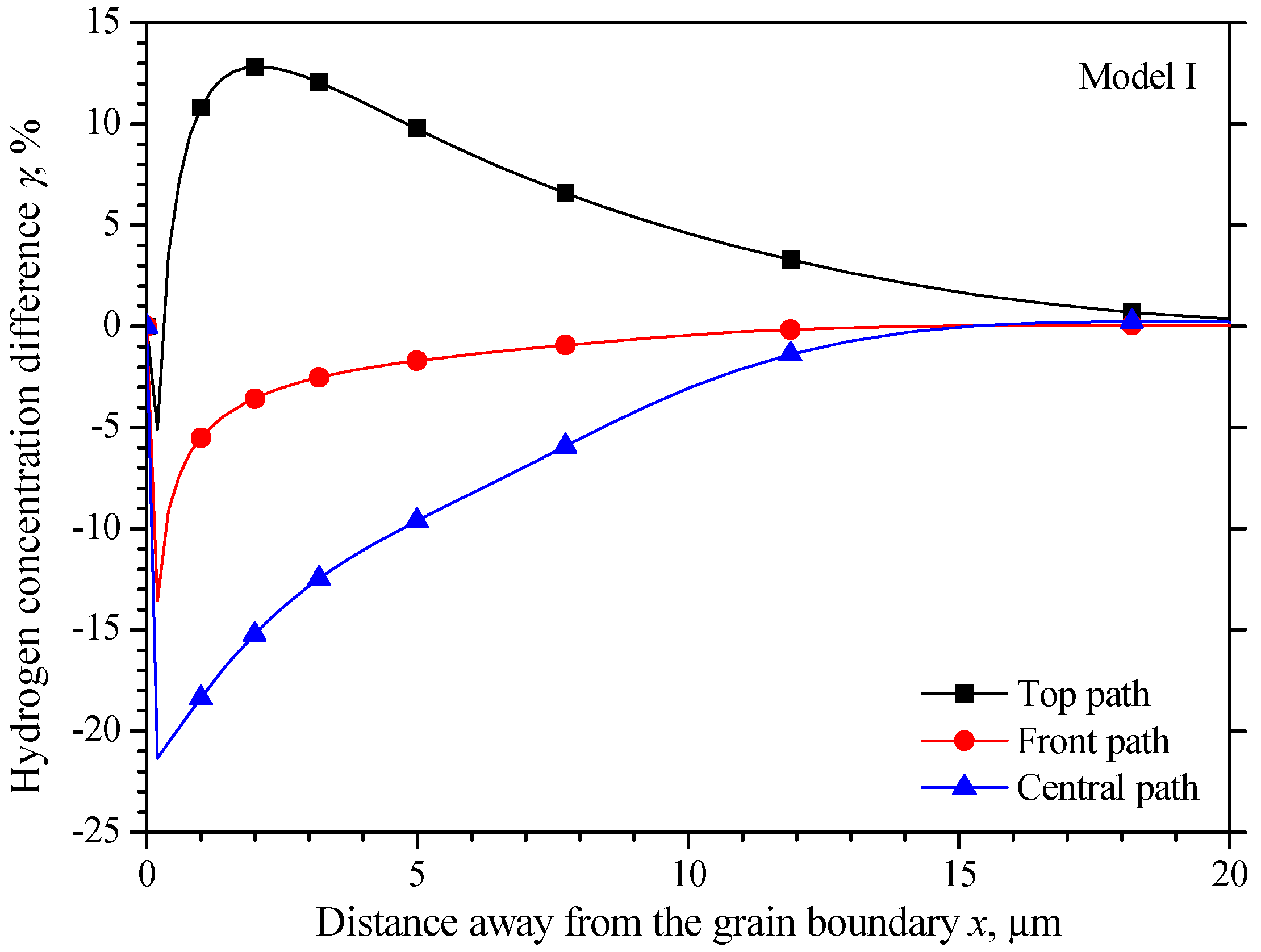
5.1. The Hydrogen Concentration Distribution in the Model I Crystal
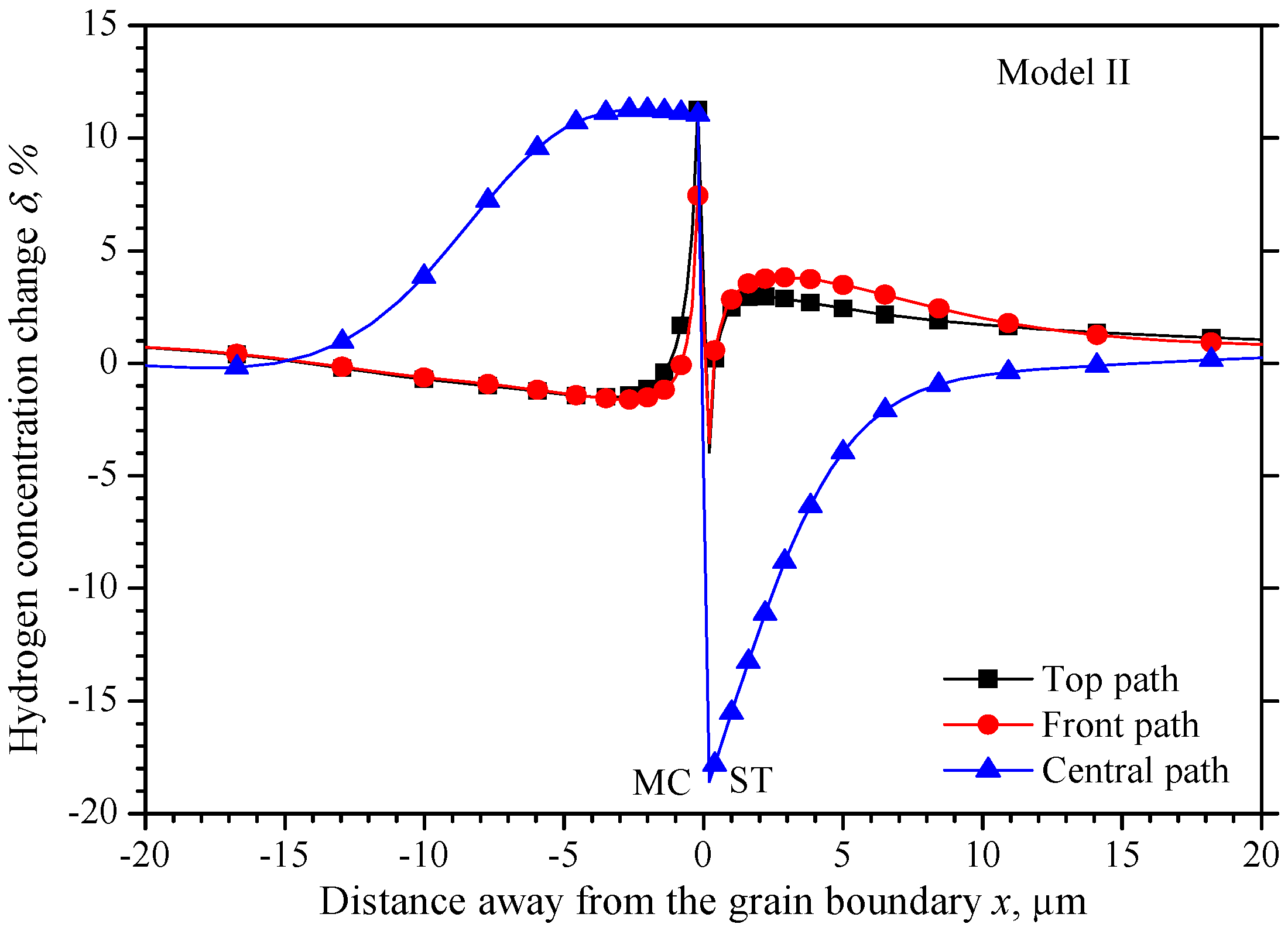
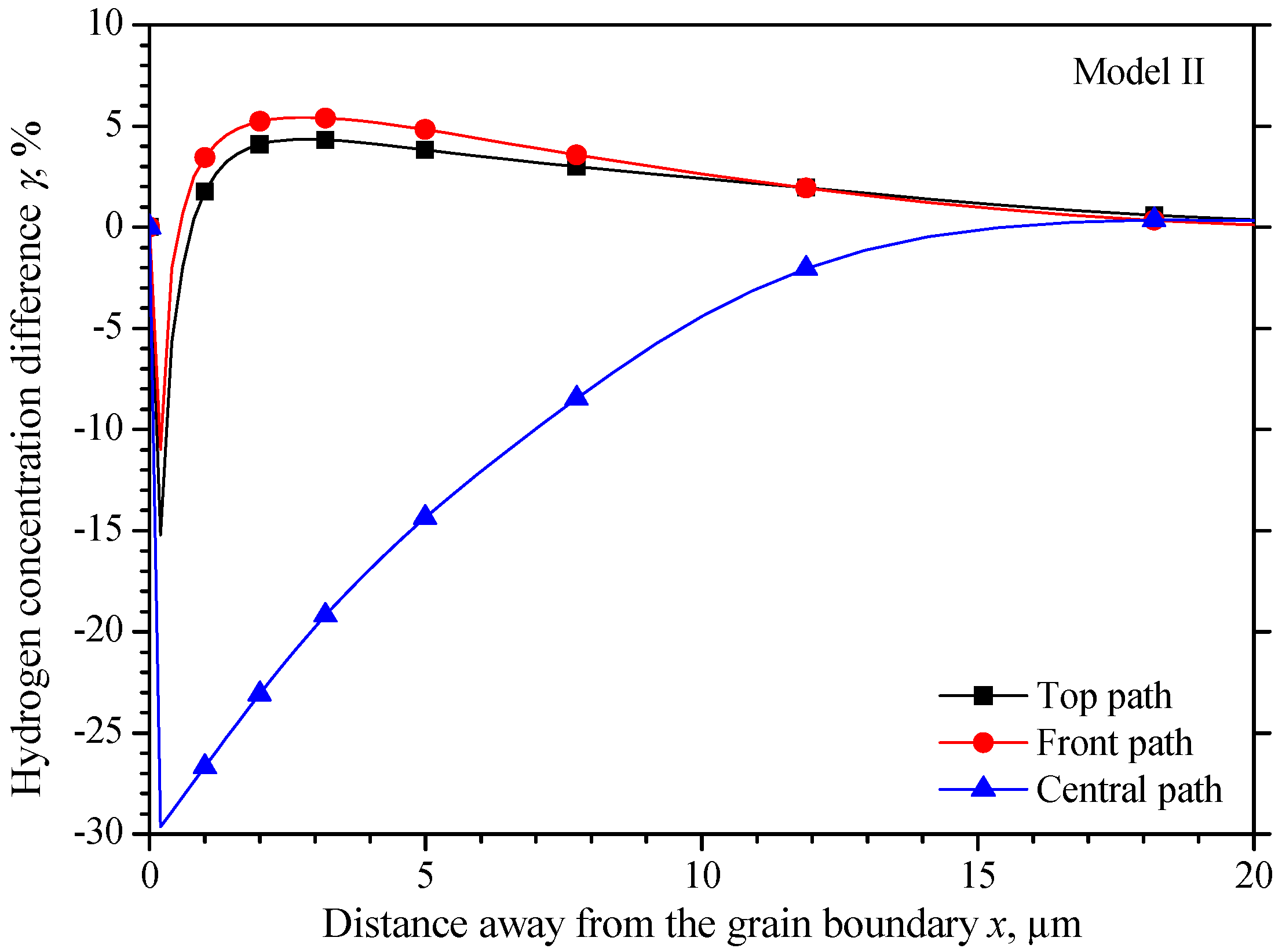
5.2. The Hydrogen Concentration Distribution in Model II Crystal
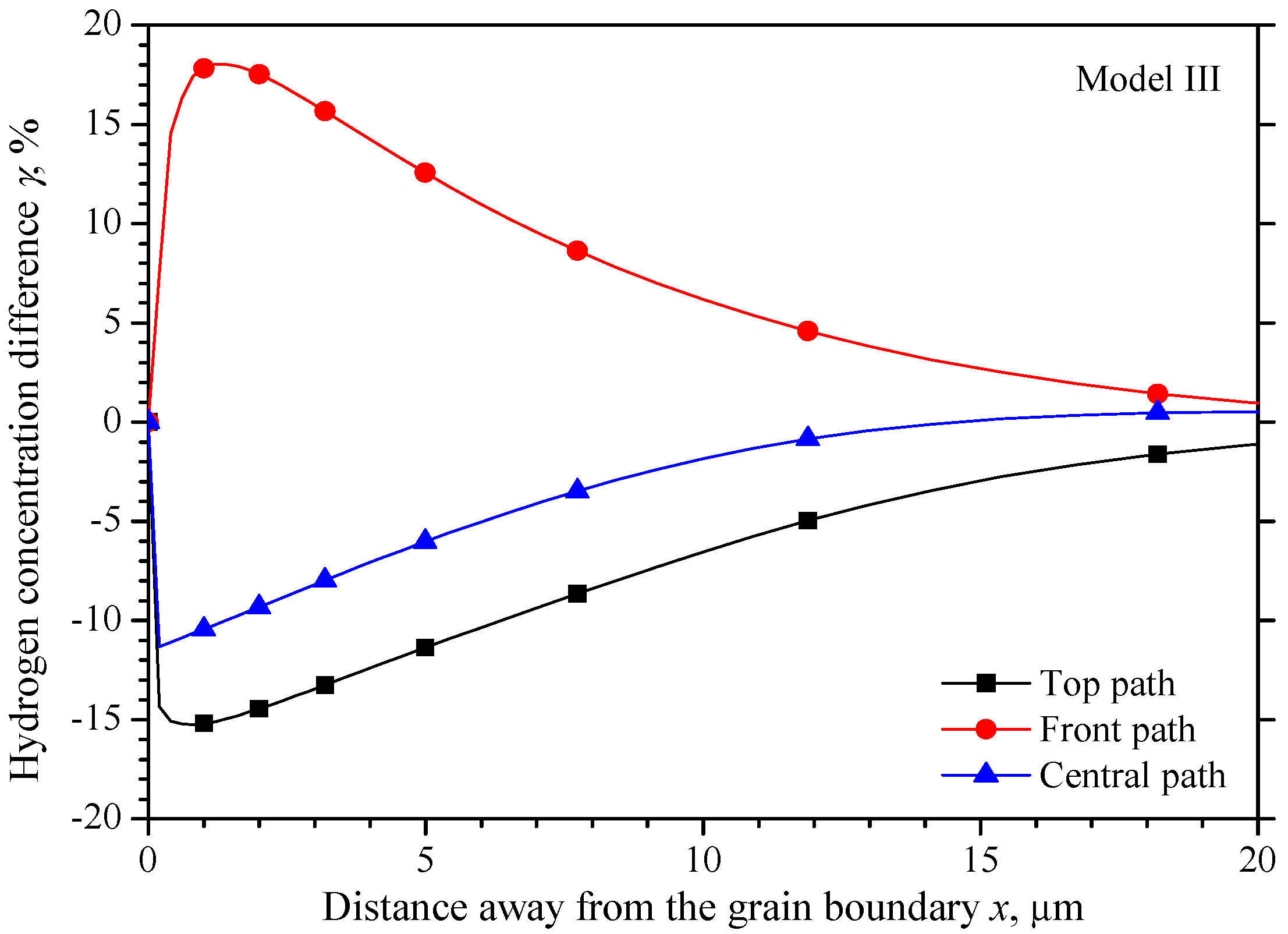
5.3. The Hydrogen Concentration Distribution in Model III Crystal
5.4. Comparison of the Three Models
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khanzhin, V.G.; Turilina, V.Y.; Rogachev, S.O.; Nikitin, A.V.; Belov, V.A. Effect of various factors on hydrogen embrittlement of structural steels. Met. Sci. Heat Treat. 2015, 57, 197–204. [Google Scholar] [CrossRef]
- Findley, K.O.; Lawrence, S.K.; O’Brien, M.K. Engineering challenges associated with hydrogen embrittlement in steels. Encycl. Mater. Met. Alloy. 2022, 2, 235–249. [Google Scholar]
- Gangloff, R.P. Hydrogen-assisted Cracking. Compr. Struct. Integr. 2003, 6, 31–101. [Google Scholar]
- Oriani, R.A. Hydrogen embrittlement of steels. Annu. Rev. Mater. Sci. 1978, 8, 327–357. [Google Scholar] [CrossRef]
- Birnbaum, H.K.; Sofronis, P. Hydrogen-enhanced localized plasticity—A mechanism for hydrogen-related fracture. Mater. Sci. Eng. A 1994, 176, 191–202. [Google Scholar] [CrossRef]
- Robertson, I.M. The effect of hydrogen on dislocation dynamics. Eng. Fract. Mech. 2001, 68, 671–692. [Google Scholar] [CrossRef]
- Lynch, S.P. Environmentally assisted cracking: Overview of evidence for an adsorption-induced localised-slip process. Acta Metall. 1988, 36, 2639–2661. [Google Scholar] [CrossRef]
- Merson, E.; Vinogradov, A.; Merson, D.L. Application of acoustic emission method for investigation of hydrogen embrittlement mechanism in the low-carbon steel. J. Alloys Compd. 2015, 645, S460–S463. [Google Scholar] [CrossRef]
- Oudriss, A.; Creus, J.; Bouhattate, J.; Savall, C.; Peraudeau, B.; Feaugas, X. The diffusion and trapping of hydrogen along the grain boundaries in polycrystalline nickel. Scr. Mater. 2012, 66, 37–40. [Google Scholar] [CrossRef]
- Tsuru, T.; Latanision, R.M. Grain boundary transport of hydrogen in nickel. Scr. Metall. 1982, 16, 575–578. [Google Scholar] [CrossRef]
- Brass, A.M.; Chanfreau, A. Accelerated diffusion of hydrogen along grain boundaries in nickel. Acta Mater. 1996, 44, 3823–3831. [Google Scholar] [CrossRef]
- Oudriss, A.; Creus, J.; Bouhattate, J.; Conforto, E.; Berziou, C.; Savall, C.; Feaugas, X. Grain size and grain boundary effects on diffusion and trapping of hydrogen in pure nickel. Acta Mater. 2012, 60, 6814–6828. [Google Scholar] [CrossRef]
- Mine, Y.; Tachibana, K.; Horita, Z. Grain-boundary diffusion and precipitate trapping of hydrogen in ultrafine-grained austenitic stainless steels processed by high-pressure torsion. Mater. Sci. Eng. 2011, 528, 8100–8105. [Google Scholar] [CrossRef]
- Louthan, M.R.; Donovan, J.A.; Caskey, G.R. Hydrogen diffusion and trapping in nickel. Acta Metall. 1975, 23, 745–749. [Google Scholar] [CrossRef]
- Ladna, B.; Birnbaum, H.K. SIMS study of hydrogen at the surface and grain boundaries of nickel bicrystals. Acta Mater. 1987, 35, 2537–2542. [Google Scholar] [CrossRef]
- Birnbaum, H.; Ladna, B.; Kimura, A. Hydrogen segregation and diffusion at grain boundaries. J. Phys. Colloq. 1988, 49, c5-397–c5-401. [Google Scholar] [CrossRef]
- Yao, J.; Cahoon, J.R. Experimental studies of grain boundary diffusion of hydrogen in metals. Acta Metall. Mater. 1991, 39, 119–126. [Google Scholar] [CrossRef]
- Ichimura, M.; Sasajima, Y.; Imabayashi, M. Grain Boundary Effect on Diffusion of Hydrogen in Pure Aluminum. Acta Mater. 1991, 32, 1109–1114. [Google Scholar] [CrossRef] [Green Version]
- Mütschele, T.; Kirchheim, R. Segregation and diffusion of hydrogen in grain boundaries of palladium. Scr. Metall. 1987, 21, 135–140. [Google Scholar] [CrossRef]
- Szpunar, B.; Lewis, L.J.; Swainson, I.; Erb, U. Thermal expansion and hydrogen diffusion in nanocrystalline nickel. Phys. Rev. B 1999, 60, 10107–10113. [Google Scholar] [CrossRef]
- Pedersen, A.; Jonsson, H. Simulations of hydrogen diffusion at grain boundaries in aluminum. Acta Mater. 2009, 57, 4036–4045. [Google Scholar] [CrossRef]
- Nagao, A.; Kuramoto, S.; Ichitani, K.; Kanno, M. Visualization of hydrogen transport in high strength steels affected by stress fields and hydrogen trapping. Scr. Mater. 2001, 45, 1227–1232. [Google Scholar] [CrossRef]
- Oriani, R.A. The diffusion and trapping of hydrogen in steel. Acta Metall. 1970, 18, 147–157. [Google Scholar] [CrossRef]
- Sofronis, P.; McMeeking, R.M. Numerical analysis of hydrogen transport near a blunting crack tip. J. Mech. Phys. Solids 1989, 37, 317–350. [Google Scholar] [CrossRef]
- Krom, A.H.M.; Koers, R.W.J.; Bakker, A. Hydrogen transport near a blunting crack tip. J. Mech. Phys. Solids 1999, 47, 971–992. [Google Scholar] [CrossRef]
- Yang, F.Q.; Zhang, W.J.; Yan, T.; Zhang, H.B.; Fang, X.R. Numerical analysis of the coupling between hydrogen diffusion and mechanical behavior near the crack tip of titanium. Math. Probl. Eng. 2020, 2020, 3618589. [Google Scholar] [CrossRef]
- Fallahi, A.; Ataeeb, A. Effects of crystal orientation on stress distribution near the triple junction in a tricrystal γ-TiAl. Mater. Sci. Eng. A 2010, 527, 4576–4581. [Google Scholar] [CrossRef]
- Yang, F.Q.; Xue, H.; Zhao, L.Y.; Fang, X.R. Effects of grain orientation on stress state near grain boundary of austenitic stainless steel bicrystals. Adv. Mater. Sci. Eng. 2018, 2018, 9409868. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.Q.; Xue, H.; Zhao, L.Y.; Fang, X.R.; Zhang, H.B. Effects of crystal orientation and grain boundary inclination on stress distribution in bicrystal interface of austenite stainless steel 316L. Adv. Mater. Sci. Eng. 2019, 2019, 2468487. [Google Scholar] [CrossRef] [Green Version]
- An, Y.H.; Jiang, H.Q. A finite element simulation on transient large deformation and mass diffusion in electrodes for lithium ion batteries. Model. Simul. Mater. Sci. Eng. 2013, 21, 074007. [Google Scholar] [CrossRef]
- Dassault Systèmes Simulia Corp. Abaqus Analysis User’s Manual 6.12; Simulia: Johnston, RI, USA, 2012. [Google Scholar]
- Meyers, M.A.; Chawla, K.K. Mechanical Behavior of Materials, 2nd ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 52–86. [Google Scholar]
- Xue, H.; Wang, Z.; Wang, S.; He, J.; Yang, H. Characterization of mechanical heterogeneity in dissimilar metal welded joints. Materials 2021, 14, 4145. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Kobayashi, R.; Watanabe, T. Control of grain boundary connectivity based on fractal analysis for improvement of intergranular corrosion resistance in SUS316L austenitic stainless steel random boundaries. Acta Mater. 2016, 2, 397–405. [Google Scholar] [CrossRef]
- Jeong, C.Y.; Kim, K.J.; Hong, H.U.; Nam, S.W. Effects of aging temperature and grain size on the formation of serrated grain boundaries in an AISI 316 stainless steel. Mater. Chem. Phys. 2013, 139, 27–33. [Google Scholar] [CrossRef]
- Ledbetter, H.M. Monocrystal-polycrystal elastic constants of a stainless steel. Appl. Mater. Sci. 2010, 85, 89–96. [Google Scholar]
- Siciliano, B.; Sciavicco, L.; Villani, L.; Oriol, G. Robotics Modelling, Planning and Control, 1st ed.; Springer: London, UK, 2009; pp. 303–361. [Google Scholar]
- Fujii, T.; Hazama, T.; Nakajima, H.; Horita, R. A safety analysis on overlay disbonding of pressure vessels for hydrogen service. In Proceedings of the First International Conference on Current Solutions to Hydrogen Problems in Steels, Washington, DC, USA, 1–5 November 1982; pp. 361–368. [Google Scholar]
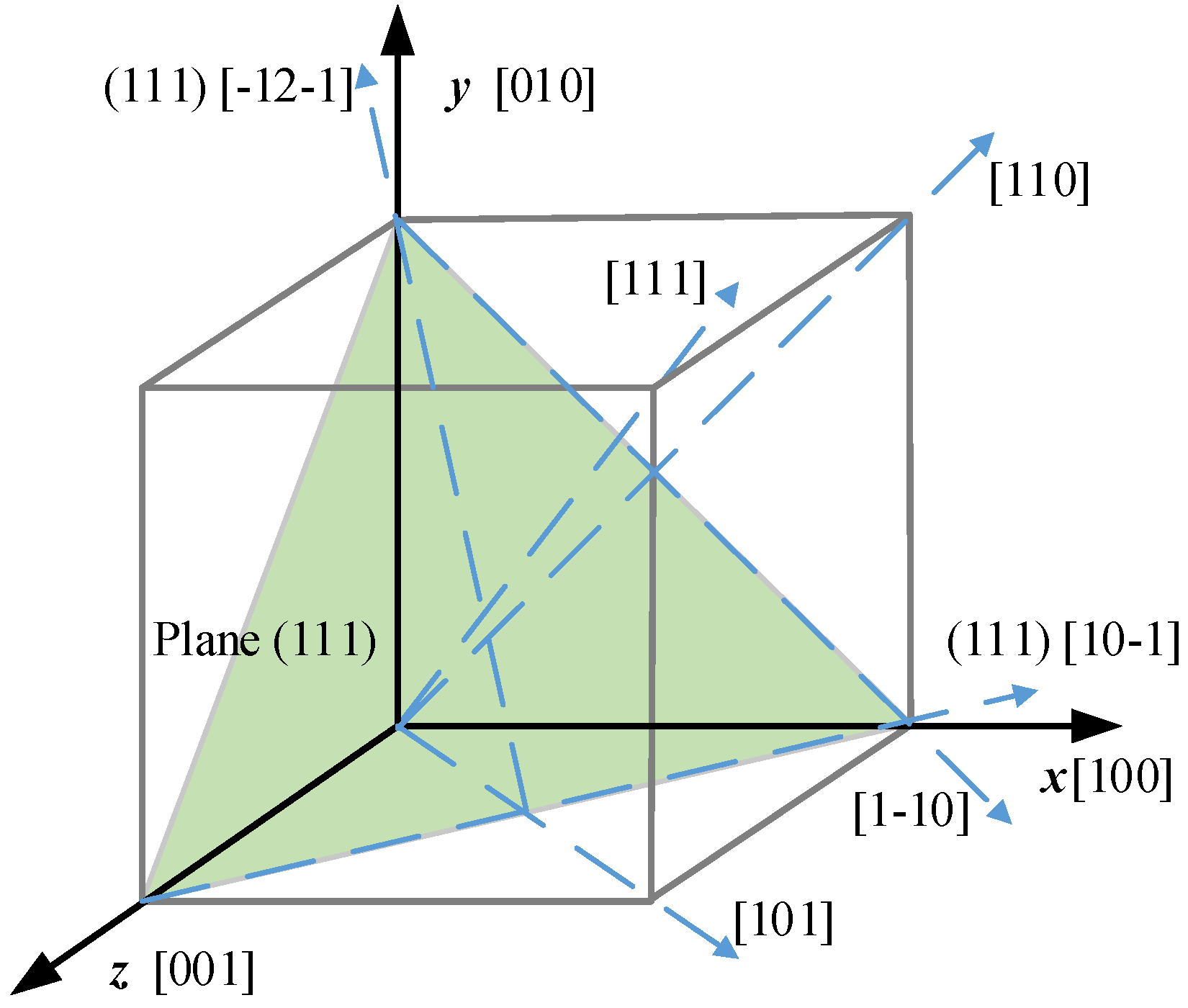



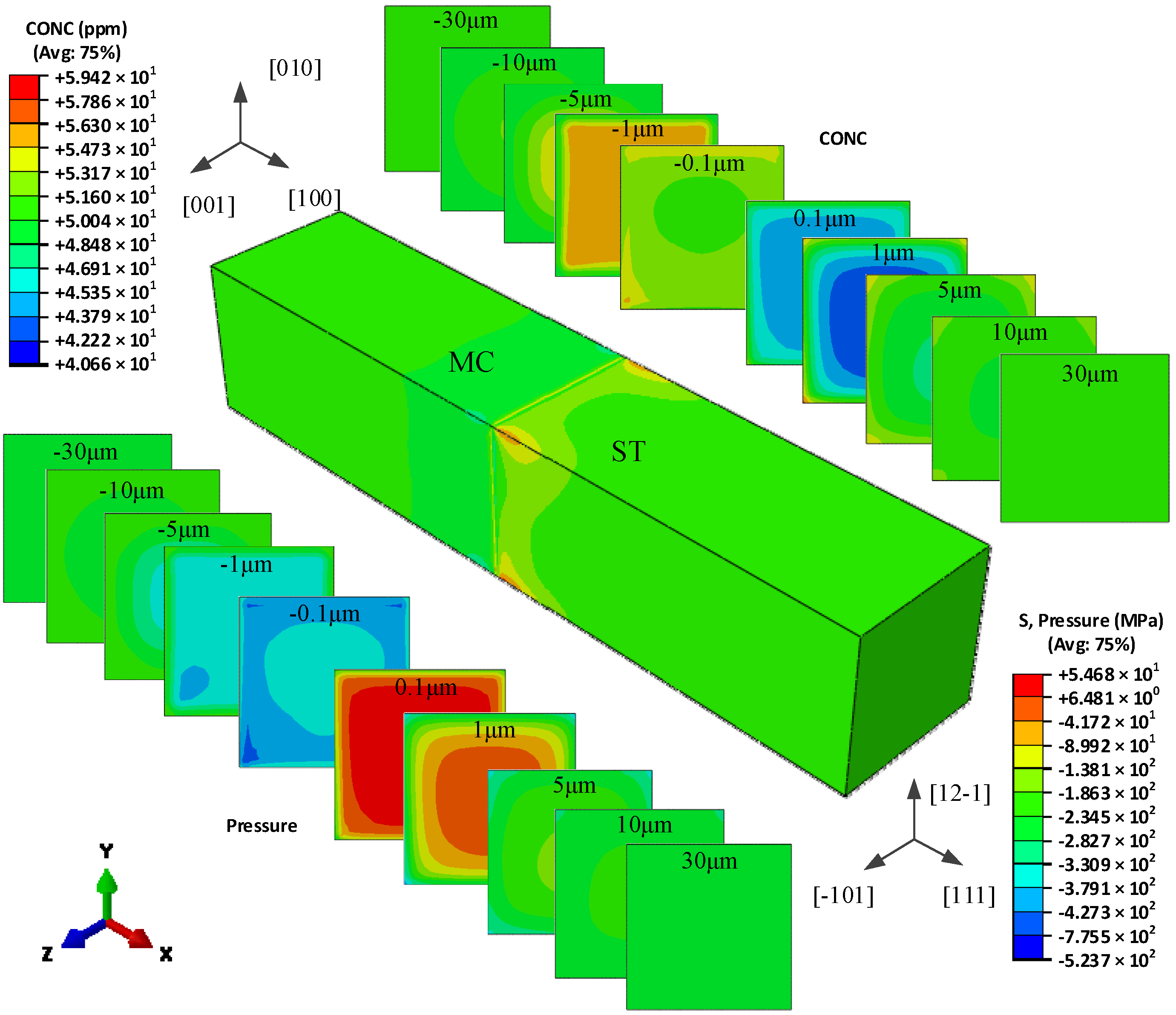
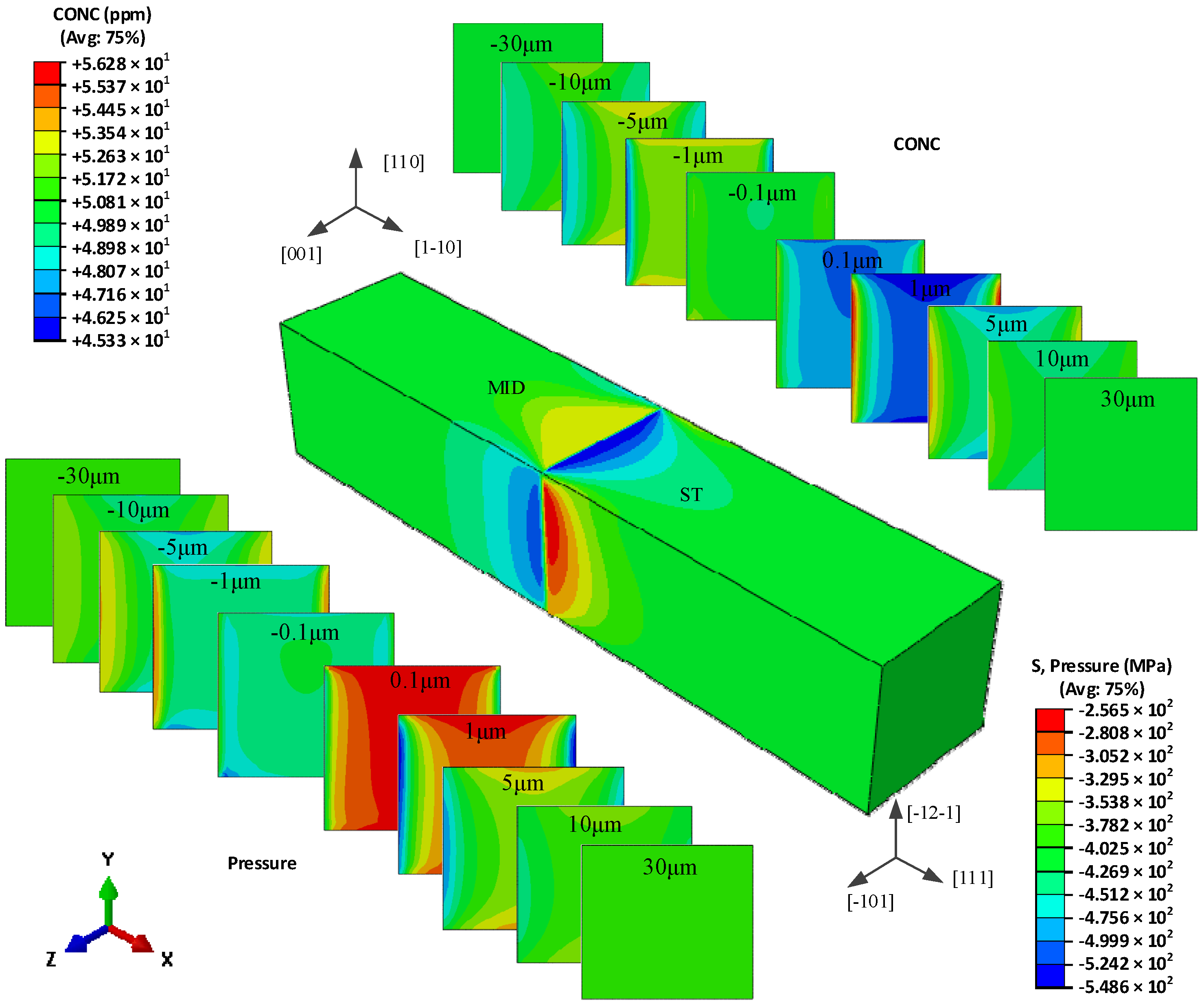

| Grain Type | x′ | y′ | z′ |
|---|---|---|---|
| Most compliant (MC) | [100] | [010] | [001] |
| Middle (MID) | [1-10] | [110] | [001] |
| Stiffness (ST) | [111] | [-12-1] | [-101] |
| Incompatibility Model | Grain A | Grain B |
|---|---|---|
| Model I | MC | MID |
| Model II | MC | ST |
| Model III | MID | ST |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Yan, T.; Zhang, W.; Zhang, H.; Zhao, L. Modeling the Hydrogen Redistribution at the Grain Boundary of Misoriented Bicrystals in Austenite Stainless Steel. Materials 2022, 15, 479. https://doi.org/10.3390/ma15020479
Yang F, Yan T, Zhang W, Zhang H, Zhao L. Modeling the Hydrogen Redistribution at the Grain Boundary of Misoriented Bicrystals in Austenite Stainless Steel. Materials. 2022; 15(2):479. https://doi.org/10.3390/ma15020479
Chicago/Turabian StyleYang, Fuqiang, Tao Yan, Wenjuan Zhang, Haibing Zhang, and Lingyan Zhao. 2022. "Modeling the Hydrogen Redistribution at the Grain Boundary of Misoriented Bicrystals in Austenite Stainless Steel" Materials 15, no. 2: 479. https://doi.org/10.3390/ma15020479
APA StyleYang, F., Yan, T., Zhang, W., Zhang, H., & Zhao, L. (2022). Modeling the Hydrogen Redistribution at the Grain Boundary of Misoriented Bicrystals in Austenite Stainless Steel. Materials, 15(2), 479. https://doi.org/10.3390/ma15020479






