Synthesis, Characterization and Biomedical Application of Silver Nanoparticles
Abstract
:1. Introduction
2. General Synthesis Routes of AgNPs
2.1. Chemical Route of Synthesis of AgNPs
2.1.1. Chemical Reduction
2.1.2. Microemulsion Technique
2.1.3. Sonochemical Method
2.1.4. Microwave Assisted Synthesis
2.2. Physical Route of Synthesis of AgNPs
2.2.1. Evaporation–Condensation
2.2.2. Laser Ablation
2.2.3. Solvated Metal Atom Deposition (SMAD) Method
2.2.4. Ball Milling
2.2.5. Gamma Irradiation
2.3. Biological Route of Synthesis of AgNPs
2.3.1. Bacterial-Based Biosystems
2.3.2. Use of Fungi
2.3.3. Use of Plants
2.3.4. Use of Algae
2.4. Characterization of AgNPs
2.4.1. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.4.2. Dynamic Light Scattering (DLS)
3ηπd
2.4.3. X-ray Diffraction (XRD)
2.4.4. Transmission Electron Microscopy (TEM)
2.5. Application of AgNPs in Biomedical Applications
2.5.1. AgNPs for Antibacterial Activities
2.5.2. AgNPs for Antiviral Activities
2.5.3. AgNPs for Anticancer Therapy
| Organism | Source of AgNPs | Cancer Cell Line | IC50 Value | Size and Shape of AgNPs | References |
|---|---|---|---|---|---|
| Mixture of Curcuma longa and Zingiber officinale | Plant | HT-29 | 150 μg/mL | Spherical; 20–51 nm | [292] |
| Solanum trilobatum | Plant | MCF-7 | 30 μg/mL | Spherical; 12.5–41.9 nm | [293] |
| Aspergillus terreus ITCC 9932 | Fungus | MCF-7 | 25.24 ± 0.990 μg/mL | Spherical; 25 nm | [294] |
| Aspergillus niger Aspergillus michelle Aspergillus japonicus | Fungus | MCF-7 | 2.46 μg/mL 3.12 μg/mL 1.47 μg/mL | Varying in sizes Varying in sizes ~100 nm | [295] |
| Agaricus bisporus | Fungus | MCF-7 | 50 μg/mL | Spherical; 8–20 nm | [296] |
| Endophytic bacterium | Bacteria | MCF-7 | 50 μg/mL | Spherical; 83–176 nm | [297] |
| Dimocarpus longan | Plant | PC3 | 10 μg/mL | Cubical; 9–32 nm | [298] |
| Ginkgo biloba | Plant | HeLA, SiHa | Dose dependent | Spherical; 40 ± 1.2 nm | [299] |
| Punica granatum | Plant | A5449 | 5 μg/mL | Spherical; 6–45 nm | [300] |
| Detarium microcarpum | Plant | HeLa, PANC-1 | 31.5 μg/mL, 84 μg/mL | Spherical, circular, rectangular; 62–103 nm (TEM) | [301] |
| Punica granatum | Plant | HeLa | 100 μg/mL | 46.1 nm | [302] |
| Fagonia indica | Plant | MCF-7 | 12.35 μg/mL | Spherical;10–60 nm | [303] |
| Alternanthera sessilis | Plant | PC3 | 6.85 μg/mL | Spherical; 30–50 nm | [304] |
| Oscillatoria limnetica | Bacteria | MCF-7, HCT-116 | 6.147 μg/mL, 5.369 μg/mL | Quasi-spherical; 3.30–17.97 nm | [119] |
| Rhizopus stolonifer | Fungus | EAC, HT-29 | 2.15 μg/mL, 2 μg/mL | Spherical; 5–50 nm | [240] |
2.5.4. AgNPs for Bone Healing
2.5.5. AgNPs for Bone Cement
2.5.6. AgNPs for Dental Applications
2.5.7. AgNPs for Catheters
2.5.8. AgNPs for Wound Healing
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uthaman, A.; Lal, H.; Thomas, S. Fundamentals of Silver Nanoparticles and Their Toxicological Aspects. In Polymer Nanocomposites Based on Silver Nanoparticles. Engineering Materials; Lal, H.M., Thomas, S., Li, T., Maria, H.J., Eds.; Springer: Cham, Switzerland, 2021; pp. 1–24. [Google Scholar] [CrossRef]
- Dikshit, P.K.; Kumar, J.; Das, A.K.; Sadhu, S.; Sharma, S.; Singh, S.; Gupta, P.K.; Kim, B.S. Green synthesis of metallic nanoparticles: Applications and limitations. Catalysts 2021, 11, 902. [Google Scholar] [CrossRef]
- De Silva, C.; Mohammad Nawawi, N.; Abd Karim, M.M.; Abd Gani, S.; Masarudin, M.J.; Gunasekaran, B.; Ahmad, S.A. The mechanistic action of biosynthesised silver nanoparticles and its application in aquaculture and livestock, industries. Animals 2021, 11, 2097. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Bhagat, M. Silver nanoparticles (AgNPs): As nanopesticides and nanofertilizers. MOJ Biol. Med. 2019, 4, 19–20. [Google Scholar] [CrossRef]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size- and shape-dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Jun, B.H. Silver nanoparticles: Synthesis and application for nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, S.; Kalishwaralal, K.; Vaidyanathan, R.; Venkataraman, D.; Pandian, S.R.K.; Muniyandi, J.; Hariharan, N.; Eom, S.H. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids Surf. B. 2009, 74, 328–335. [Google Scholar] [CrossRef]
- Gurunathan, S.; Park, J.H.; Han, J.W.; Kim, J.H. Comparative assessment of the apoptotic potential of silver nanoparticles synthesized by Bacillus tequilensis and Calocybe indica in MDA-MB-231 human breast cancer cells: Targeting p53 for anticancer therapy. Int. J. Nanomed. 2015, 10, 4203–4223. [Google Scholar] [CrossRef] [Green Version]
- Hutchison, J.E. Greener nanoscience: A proactive approach to advancing applications and reducing implications of nanotechnology. ACS Nano 2008, 2, 395–402. [Google Scholar] [CrossRef]
- Saji, V.S.; Choe, H.C.; Yeung, K.W.K. Nanotechnology in biomedical applications: A review. Int. J. Nano Biomater. 2010, 3, 119–139. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef] [Green Version]
- Slawson, R.M.; Van Dyke, M.I.; Lee, H.; Trevors, J.T. Germanium and silver resistance, accumulation, and toxicity in microorganisms. Plasmid 1992, 27, 72–79. [Google Scholar] [CrossRef]
- Zhao, G.; Stevens, S.E., Jr. Multiple parameters for the comprehensive evaluation of the susceptibility of Escherichia coli to the silver ion. Biometals 1998, 11, 27–32. [Google Scholar] [CrossRef]
- Almatroudi, A. Silver nanoparticles: Synthesis, characterisation and biomedical applications. Open Life Sci. 2020, 15, 819–839. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.J.; Singh, H.; Wang, C.; Hwang, K.H.; Farh, M.E.A.; Yang, D.C. Biosynthesis, characterization, and antimicrobial applications of silver nanoparticles. Int. J. Nanomed. 2015, 10, 2567–2577. [Google Scholar] [CrossRef] [Green Version]
- Leung, T.C.Y.; Wong, C.K.; Xie, Y. Green synthesis of silver nanoparticles using biopolymers, carboxymethylated-curdlan and fucoidan. Mater. Chem. Phys. 2010, 121, 402–405. [Google Scholar] [CrossRef]
- Ahmed, K.B.A.; Kalla, D.; Uppuluri, K.B.; Anbazhagan, V. Green synthesis of silver and gold nanoparticles employing levan, a biopolymer from Acetobacter xylinum NCIM 2526, as a reducing agent and capping agent. Carbohydr. Polym. 2014, 112, 539–545. [Google Scholar] [CrossRef]
- Regiel-Futyra, A.; Kus-Liśkiewicz, M.; Sebastian, V. Development of noncytotoxic silver-chitosan nanocomposites for efficient control of biofilm forming microbes. RSC Adv. 2017, 7, 52398–52413. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.B.; Tay, M.Y.; Shameli, K.; Hussein, M.Z.; Lim, J.J. Green synthesis and characterization of silver/chitosan/polyethylene glycol nanocomposites without any reducing agent. Int. J. Mol. Sci. 2011, 12, 4872–4884. [Google Scholar] [CrossRef]
- Palem, R.R.; Ganesh, S.D.; Kronekova, Z.; Sláviková, M.; Saha, N.; Saha, P. Green synthesis of silver nanoparticles and biopolymer nanocomposites: A comparative study on physico-chemical, antimicrobial and anticancer activity. Bull. Mater. Sci. 2018, 41, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.S.; Lee, H.; Chen, Y.H.; Chae, Y. Bibliometric analysis of research assessing the use of acupuncture for pain treatment over the past 20 years. J. Pain Res. 2020, 13, 367–376. [Google Scholar] [CrossRef] [Green Version]
- De Moya-Anegón, F.; Chinchilla-Rodríguez, Z.; Vargas-Quesada, B.; Corera-Álvarez, E.; Muñoz-Fernández, F.J.; González-Molina, A.; Herrero-Solana, V. Coverage analysis of Scopus: A journal metric approach. Scientometrics 2007, 73, 53–78. [Google Scholar] [CrossRef] [Green Version]
- Schoenaker, D.A.J.M.; Mishra, G.D.; Callaway, L.K.; Soedamah-Muthu, S.S. The role of energy, nutrients, foods, and dietary patterns in the development of gestational diabetes mellitus: A systematic review of observational studies. Diabetes Care 2016, 39, 16–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuaib, W.; Khan, M.S.; Shahid, H.; Valdes, E.A.; Alweis, R. Bibliometric analysis of the top 100 cited cardiovascular articles. Am. J. Cardiol. 2015, 115, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.P.; Fleet, C.V. Knowledge into Action: Research and Evaluation in Library and Information Science; Libraries Unlimited: Santa Barbara, CA, USA, 2012; p. 39. [Google Scholar]
- De Souza, F.G. Bibliometric analysis of the hot theme “phytosynthesized nanoparticles”. Arch. Biomed. Eng. Biotechnol. 2020, 4, 1–5. [Google Scholar] [CrossRef]
- Feng, L.; Chen, Q. Bibliometric analysis of the synthesis of nanocatalyst (1999-2018). IOP Conf. Ser. Earth Environ. Sci. 2020, 558, 1–8. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2009, 84, 523–538. [Google Scholar] [CrossRef] [Green Version]
- Van Eck, N.J.; Waltman, L. Visualizing Bibliometric Networks. In Measuring Scholarly Impact; Springer International Publishing: New York, NY, USA, 2014; pp. 285–320. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. VOSviewer Manual: Manual for VOSviewer Version 1.6.7. 2018. Available online: https://www.vosviewer.com/documentation/Manual_VOSviewer_1.6.7.pdf (accessed on 25 July 2021).
- Fabregat-Aibar, L.; Barberà-Mariné, M.G.; Terceño, A.; Pié, L. A Bibliometric and visualization analysis of socially responsible funds. Sustainability 2019, 11, 2526. [Google Scholar] [CrossRef] [Green Version]
- Muritala, B.A.; Sánchez-Rebull, M.V.; Hernández-Lara, A.B. A Bibliometric analysis of online reviews research in tourism and hospitality. Sustainability 2020, 12, 9977. [Google Scholar] [CrossRef]
- Roberts, R.J. Bibliometrics: An obituary for the impact factor. Nature 2017, 546, 600. [Google Scholar] [CrossRef] [Green Version]
- Pulsiri, N.; Vatananan-Thesenvitz, R. Improving systematic literature review with automation and bibliometrics. In Proceedings of the PICMET 2018—Portland International Conference on Management of Engineering and Technology: Managing Technological Entrepreneurship: The Engine for Economic Growth, Honolulu, HI, USA, 19–23 August 2018. [Google Scholar] [CrossRef]
- Dos Santos, C.A.; Seckler, M.M.; Ingle, A.P.; Gupta, I.; Galdiero, S.; Galdiero, M.; Gade, A.; Rai, M. Silver nanoparticles: Therapeutical uses, toxicity, and safety issues. J. Pharm. Sci. 2014, 103, 1931–1944. [Google Scholar] [CrossRef]
- Raji, V.; Chakraborty, M.; Parikh, P.A. Synthesis of starch-stabilized silver nanoparticles and their antimicrobial activity. Part. Sci. Technol. 2012, 30, 565–577. [Google Scholar] [CrossRef]
- Mukherjee, S.; Patra, C.R. Biologically synthesized metal nanoparticles: Recent advancement and future perspectives in cancer theranostics. Future Sci. OA 2017, 3, FSO203. [Google Scholar] [CrossRef] [Green Version]
- Simões, M.F.; Ottoni, C.A.; Antunes, A. Biogenic metal nanoparticles: A new approach to detect life on mars? Life 2020, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Kowshik, M.; Ashtaputre, S.; Kharrazi, S.; Vogel, W.; Urban, J.; Kulkarni, S.K.; Paknikar, K.M. Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology 2003, 14, 95–100. [Google Scholar] [CrossRef]
- Gudikandula, K.; Charya Maringanti, S. Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. J. Exp. Nanosci. 2016, 11, 714–721. [Google Scholar] [CrossRef]
- Suriati, G.; Mariatti, M.; Azizan, A. Synthesis of silver nanoparticles by chemical reduction method: Effect of reducing agent and surfactant concentration. Int. J. Automot. Mech. Eng. 2014, 10, 1920–1927. [Google Scholar] [CrossRef]
- Güzel, R.; Erdal, G. Synthesis of Silver Nanoparticles. In Silver Nanoparticles; Maaz, K., Ed.; IntechOpen: London, UK, 2018; pp. 1–20. [Google Scholar] [CrossRef] [Green Version]
- Barani, H.; Mahltig, B. Microwave-assisted synthesis of silver nanoparticles: Effect of reaction temperature and precursor concentration on fluorescent property. J. Clust. Sci. 2020. [Google Scholar] [CrossRef]
- Tsuji, M.; Gomi, S.; Maeda, Y.; Matsunaga, M.; Hikino, S.; Uto, K.; Tsuji, T.; Kawazumi, H. Rapid transformation from spherical nanoparticles, nanorods, cubes, or bipyramids to triangular prisms of silver with PVP, citrate, and H2O2. Langmuir 2012, 28, 8845–8861. [Google Scholar] [CrossRef]
- Kvítek, L.; Panáček, A.; Soukupová, J.; Kolář, M.; Večeřová, R.; Prucek, R.; Holecová, M.; Zbořil, R. Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (NPs). J. Phys. Chem. C 2008, 112, 5825–5834. [Google Scholar] [CrossRef]
- Sadeghi, B.; Garmaroudi, F.S.; Hashemi, M.; Nezhad, H.R.; Nasrollahi, A.; Ardalan, S.; Ardalan, S. Comparison of the anti-bacterial activity on the nanosilver shapes: Nanoparticles, nanorods and nanoplates. Adv. Powder Technol. 2012, 23, 22–26. [Google Scholar] [CrossRef]
- Dang, T.M.D.; Le, T.T.T.; Fribourg-Blanc, E.; Dang, M.C. Influence of surfactant on the preparation of silver nanoparticles by Polyol method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 1–4. [Google Scholar] [CrossRef]
- Ho, V.T.; Nga, D.T. Synthesis of silver nanoparticles via chemical reduction and its anti-bacterial activities in wastewater of shrimp pond. Int. J. Eng. Res. Technol. 2016, 5, 1–5. [Google Scholar]
- Nur, S.U.; Anung, P.; Enny, L.; Endang, S.; Hotman, L.; Triani, W.; Siska, F. Critical parameters of silver nanoparticles (AgNPs) synthesized by sodium borohydride reduction. Res. J. Chem. Environ. 2018, 22, 179–183. [Google Scholar]
- El-Nour, K.M.M.; Eftaiha, A.; Al-Warthan, A.; Ammar, R.A.A. Synthesis and applications of silver nanoparticles. Arab J. Chem. 2010, 3, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Reyes, P.Y.; López, R.G.; Espinoza, J.A.; Treviño, M.E.; Saade, H. Synthesis of silver nanoparticles by precipitation in bicontinuous microemulsions. J. Nanomater. 2010, 2010, 948941. [Google Scholar] [CrossRef]
- Das, M.; Patowary, K.; Vidya, R.; Malipeddi, H. Microemulsion synthesis of silver nanoparticles using biosurfactant extracted from Pseudomonas aeruginosa MKVIT3 strain and comparison of their antimicrobial and cytotoxic activities. IET Nanobiotechnol. 2016, 10, 411–418. [Google Scholar] [CrossRef]
- Chen, S.; Ju, Y.; Guo, Y.; Xiong, C.; Dong, L. In-site synthesis of monodisperse, oleylamine-capped Ag nanoparticles through microemulsion approach. J. Nanopart. Res. 2017, 19, 88. [Google Scholar] [CrossRef]
- Renganathan, S. Green Synthesis of Ecofriendly Nanoparticles and Their Medical Applications. In Environmental Sustainability Using Green Technologies, 1st ed.; Sivasubramaniam, V., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 79–102. [Google Scholar] [CrossRef]
- Gedanken, A. Sonochemistry and its application to nanochemistry. Curr. Sci. 2003, 85, 1720–1722. [Google Scholar]
- Jiang, L.P.; Xu, S.; Zhu, J.M.; Zhang, J.R.; Zhu, J.J.; Chen, H.Y. Ultrasonic-assisted synthesis of monodisperse single-crystalline silver nanoplates and gold nanorings. Inorg. Chem. 2004, 43, 5877–5883. [Google Scholar] [CrossRef]
- Suslick, K.S.; Hyeon, T.; Fang, M.; Cichowlas, A.A. Sonochemical preparation of nanostructured catalysts. Mater. Sci. End. Sci. 1995, 204, 197–212. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A.; Pathak, R.N. Sonochemical synthesis of silver nanoparticles using starch: A comparison. Bioinorg. Chem. Appl. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Elsupikhe, R.F.; Shameli, K.; Ahmad, M.B. Sonochemical method for the synthesis of silver nanoparticles in κ-carrageenan from silver salt at different concentrations. Res. Chem. Intermed. 2015, 41, 8515–8525. [Google Scholar] [CrossRef] [Green Version]
- Patil, H.A.; Shushilkumar, A.J.; More, V.B.; Kalas, D.S.; Pramod, S.P. Novel one step sonosynthesis and deposition technique to prepare silver nanoparticles coated cotton textile with antibacterial properties. Colloid J. 2019, 81, 720–727. [Google Scholar] [CrossRef]
- Kuntyi, O.; Shepida, M.; Sozanskyi, M.; Sukhatskiy, Y.; Mazur, A.; Kytsya, A.; Bazylyak, L. Sonoelectrochemical synthesis of silver nanoparticles in sodium polyacrylate solution. Biointerface Res. Appl. Chem. 2021, 11, 12202–12214. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Speth, T.F.; Varma, R.S. Microwave-assisted green synthesis of silver nanostructures. Acc. Chem. Res. 2011, 44, 469–478. [Google Scholar] [CrossRef]
- Seku, K.; Gangapuram, B.R.; Pejjai, B.; Kadimpati, K.K.; Golla, N. Microwave-assisted synthesis of silver nanoparticles and their application in catalytic, antibacterial and antioxidant activities. J. Nanostructure Chem. 2018, 8, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Renugadevi, K.; Aswini, V.; Raji, P. Microwave irradiation assisted synthesis of silver nanoparticle using leaf extract of Baliospermum montanum and evaluation of its antimicrobial, anticancer potential activity. Asian J. Pharm. Clin. Res. 2012, 5, 283–287. [Google Scholar]
- Joseph, S.; Mathew, B. Microwave assisted biosynthesis of silver nanoparticles using the rhizome extract of Alpinia galanga and evaluation of their catalytic and antimicrobial activities. J. Nanopart. Res. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Abboud, Y.; Eddahbi, A.; El Bouari, A.; Aitenneite, H.; Brouzi, K.; Mouslim, J. Microwave-assisted approach for rapid and green phytosynthesis of silver nanoparticles using aqueous onion (Allium cepa) extract and their antibacterial activity. J. Nanostructure Chem. 2013, 3, 84. [Google Scholar] [CrossRef]
- Navaladian, S.; Viswanathan, B.; Varadarajan, T.K.; Viswanath, R.P. Microwave-assisted rapid synthesis of anisotropic Ag nanoparticles by solid state transformation. Nanotechnology 2008, 19, 1–7. [Google Scholar] [CrossRef]
- Kumar, S.V.; Bafana, A.P.; Pawar, P.; Rahman, A.; Dahoumane, S.A.; Jeffryes, C.S. High conversion synthesis of <10 nm starch-stabilized silver nanoparticles using microwave technology. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Pal, A.; Shah, S.; Devi, S. Microwave-assisted synthesis of silver nanoparticles using ethanol as a reducing agent. Mater. Chem. Phys. 2009, 114, 530–532. [Google Scholar] [CrossRef]
- Chung, D.S.; Kim, H.; Ko, J.; Lee, J.; Hwang, B.; Chang, S.; Kim, B.; Chung, S.J. Microwave synthesis of silver nanoparticles using different pentose carbohydrates as reducing agents. J. Chem. Chem. Eng. 2018, 12, 1–10. [Google Scholar] [CrossRef] [Green Version]
- González-Pedroza, M.G.; Argueta-Figueroa, L.; García-Contreras, R.; Jiménez-Martínez, Y.; Martínez-Martínez, E.; Navarro-Marchal, S.A.; Marchal, J.A.; Morales-Luckie, R.A.; Boulaiz, H. Silver nanoparticles from annona muricata peel and leaf extracts as a potential potent, biocompatible and low cost antitumor tool. J. Nanomater. 2021, 11, 1273. [Google Scholar] [CrossRef]
- Singh, R.; Schmitt, J.J.; Knoedler, J.J.; Occhino, J.A. Management of a vesicovaginal fistula using holmium laser ablation. Int. Urogynecol. J. 2016, 27, 969–971. [Google Scholar] [CrossRef]
- Tola, O.H.; Oluwole, O.I.; Omotayo, A.B. Synthesis and characterization of silver nanoparticles from ecofriendly materials: A review. Int. J. Eng. Res. Technol. 2020, 9, 782–795. [Google Scholar]
- El-Batal, A.; Haroun, B.M.; Farrag, A.A.; Baraka, A.; El-Sayyad, G.S. Synthesis of silver nanoparticles and incorporation with certain antibiotic using gamma irradiation. Bri. J. Pharm. Res. 2014, 4, 1341–1363. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Mughal, S.; Shahzad, A.F.; Tahir, M.U.; Ayub, A.R. Role of silver nanoparticles in colorimetric detection of biomolecules. Biomed. Nurs. 2019, 5, 31–47. [Google Scholar] [CrossRef]
- Kruis, F.E.; Fissan, H.; Rellinghaus, B. Sintering and evaporation characteristics of gas-phase synthesis of size-selected PbS nanoparticles. Mater. Sci. Eng. B Solid. State. Mater. Adv. Technol. 2000, 69, 329–334. [Google Scholar] [CrossRef]
- Raffi, M.; Rumaiz, A.K.; Hasan, M.M.; Shah, S.I. Studies of the growth parameters for silver nanoparticle synthesis by inert gas condensation. J. Mater. Res. 2007, 22, 3378–3384. [Google Scholar] [CrossRef]
- Jung, J.H.; Cheol Oh, H.; Soo Noh, H.; Ji, J.H.; Soo Kim, S. Metal nanoparticle generation using a small ceramic heater with a local heating area. J. Aerosol Sci. 2006, 37, 1662–1670. [Google Scholar] [CrossRef]
- Harra, J.; Mäkitalo, J.; Siikanen, R.; Virkki, M.; Genty, G.; Kobayashi, T.; Kauranen, M.; Mäkelä, J.M. Size-controlled aerosol synthesis of silver nanoparticles for plasmonic materials. J. Nanopart. Res. 2012, 14, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Jayaprakash, N.; Suresh, R.; Rajalakshmi, S.; Raja, S.; Sundaravadivel, E. An assortment of synthesis methods of silver nanoparticles: A review. Asian J. Chem. 2019, 31, 1405–1412. [Google Scholar] [CrossRef]
- Sylvestre, J.P.; Kabashin, A.V.; Sacher, E.; Meunier, M.; Luong, J.H.T. Nanoparticle size reduction during laser ablation in aqueous solutions of cyclodextrins. In Proceedings of the Photon Processing in Microelectronics and Photonics III, San Jose, CA, USA, 25–29 January 2004. [Google Scholar] [CrossRef]
- Werner, D.; Hashimoto, S.; Tomita, T.; Matsuo, S.; Makita, Y. Examination of silver nanoparticle fabrication by pulsed-laser ablation of flakes in primary alcohols. J. Phy. Chem. C 2008, 112, 1321–1329. [Google Scholar] [CrossRef]
- Mukherjee, P.; Roy, M.; Mandal, B.P.; Dey, G.K.; Mukherjee, P.K.; Ghatak, J.; Tyagi, A.K.; Kale, S.P. Green synthesis of highly stabilized nanocrystalline silver particles by a non-pathogenic and agriculturally important fungus T. asperellum. Nanotechnology 2018, 19, 1–7. [Google Scholar] [CrossRef]
- Chen, Y.H.; Yeh, C.S. Laser ablation method: Use of surfactants to form the dispersed Ag nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2002, 197, 133–139. [Google Scholar] [CrossRef]
- Amendola, V.; Polizzi, S.; Meneghetti, M. Free silver nanoparticles synthesized by laser ablation in organic solvents and their easy functionalization. Langmuir 2007, 23, 6766–6770. [Google Scholar] [CrossRef]
- Tajdidzadeh, M.; Azmi, B.Z.; Yunus, W.M.M.; Talib, Z.A.; Sadrolhosseini, A.R.; Karimzadeh, K.; Gene, S.A.; Dorraj, M. Synthesis of silver nanoparticles dispersed in various aqueous media using laser ablation. Sci. World J. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Clemente, M.; Izzi, M.; Volpe, A.; Ancona, A.; Picca, R.A.; Palazzo, G.; Cioffi, N. Exceptionally stable silver nanoparticles synthesized by laser ablation in alcoholic organic solvent. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 148–158. [Google Scholar] [CrossRef]
- Alhamid, M.Z.; Hadi, B.S.; Khumaeni, A. Synthesis of silver nanoparticles using laser ablation method utilizing Nd:YAG laser. AIP Conf. Proc. 2019, 2202, 020013. [Google Scholar] [CrossRef]
- Menazea, A.A. Femtosecond laser ablation-assisted synthesis of silver nanoparticles in organic and inorganic liquids medium and their antibacterial efficiency. Radiat. Phys. Chem. 2020, 168, 1–13. [Google Scholar] [CrossRef]
- Rhim, J.; Wang, L.; Lee, Y.; Hong, S. Preparation and characterization of bio-nanocomposite films of agar and silver nanoparticles: Laser ablation method. Carbohydr. Polym. 2014, 103, 456–465. [Google Scholar] [CrossRef]
- Smetana, A.B.; Klabunde, K.J.; Sorensen, C.M. Synthesis of spherical silver nanoparticles by digestive ripening, stabilization with various agents, and their 3-D and 2-D superlattice formation. J. Colloid Interf. Sci. 2005, 284, 521–526. [Google Scholar] [CrossRef]
- Rao, C.N.P.J.T.; Kulkarni, G. Nanocrystals: Synthesis, properties and applications. Angewandte Chemie Int. Ed. 2009, 6, 951–952. [Google Scholar]
- Baudot, C.; Tan, C.M.; Kong, J.C. FTIR spectroscopy as a tool for nano-material characterization. Infrared Phys. Technol. 2010, 53, 434–438. [Google Scholar] [CrossRef]
- Benjamin, J.S. Dispersion strengthened superalloys by mechanical alloying. Metall. Trans. 1970, 1, 2943–2951. [Google Scholar] [CrossRef]
- Khayati, G.R.; Janghorban, K. The nanostructure evolution of Ag powder synthesized by high energy ball milling. Adv. Powder Technol. 2012, 23, 393–397. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Kumar, N.; Biswas, K.; Gupta, R.K. Green synthesis of Ag nanoparticles in large quantity by cryomilling. RSC Adv. 2016, 6, 111380–111388. [Google Scholar] [CrossRef] [Green Version]
- Rak, M.J.; Friščić, T.; Moores, A. One-step, solvent-free mechanosynthesis of silver nanoparticle-infused lignin composites for use as highly active multidrug resistant antibacterial filters. RSC Adv. 2016, 6, 58365–58370. [Google Scholar] [CrossRef] [Green Version]
- Pratiwi, N.I.; Handayani, W.; Imawan, C. Clean synthesis of silver nanoparticles by radiochemical methods for antimicrobial materials. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2020; p. 2314. [Google Scholar]
- Ali, I.; Meligi, G.A.; Akl, M.R.; Saleh, A. Influence of γ -ray irradiation doses on physicochemical properties of silver polystyrene polyvinyl pyrrolidone nanocomposites. Mater. Chem. Phys. 2019, 226, 250–256. [Google Scholar] [CrossRef]
- El-Batal, A.I.; Amin, M.A.; Shehata, M.M.K.; Hallol, M.M.A. Synthesis of silver nanoparticles by Bacillus stearothermophilus using gamma radiation and their antimicrobial activity. World Appl. Sci. J. 2013, 22, 1–16. [Google Scholar] [CrossRef]
- Rao, Y.N.; Banerjee, D.; Datta, A.; Das, S.K.; Guin, R.; Saha, A. Gamma irradiation route to synthesis of highly re-dispersible natural polymer capped silver nanoparticles. Radiat. Phys. Chem. 2010, 79, 1240–1246. [Google Scholar] [CrossRef]
- Leawhiran, N.; Pavasant, P.; Soontornvipart, K.; Supaphol, P. Gamma irradiation synthesis and characterization of AgNP/gelatin/PVA hydrogels for antibacterial wound dressings. J. Appl. Polym. Sci. 2014, 131, 1–11. [Google Scholar] [CrossRef]
- Eghbalifam, N.; Frounchi, M.; Dadbin, S. Antibacterial silver nanoparticles in polyvinyl alcohol/sodium alginate blend produced by gamma irradiation. Int. J. Biol. Macromol. 2015, 80, 170–176. [Google Scholar] [CrossRef]
- Madhukumar, R.; Byrappa, K.; Wang, Y.; Sangappa, Y. Effect of gamma irradiation on synthesis and characterization of bio-nanocomposite SF/Ag nanoparticles. Radiant. Eff. Defects S. 2018, 172, 915–921. [Google Scholar] [CrossRef]
- Kumar, A.; Mazumdar, R.S.; Dhewa, T. Biological synthesis of silver nanoparticles by using Viola serpens extract. Asian Pac. J. Trop. Dis. 2016, 6, 223–226. [Google Scholar] [CrossRef]
- Langopati, N.; Gotau, M.A.; Tsoukleris, G.; Pavlatau, E.A. Biogenic synthesis of silver nanoparticles with antimicrobial properties. Nanomed. Nanotechnol. 2020, 5, 1–12. [Google Scholar] [CrossRef]
- Singh, H.; Du, J.; Singh, P.; Yi, T.H. Extracellular synthesis of silver nanoparticles by Pseudomonas sp. THG-LS1.4 and their antimicrobial application. J. Pharm. Anal. 2018, 8, 258–264. [Google Scholar] [CrossRef]
- Thomas, R.; Janardhanan, A.; Varghese, R.T.; Soniya, E.V.; Mathew, J.; Radhakrishnan, E.K. Antibacterial properties of silver nanoparticles synthesized by marine Ochrobactrum sp. Braz. J. Microbiol. 2014, 45, 1221–1227. [Google Scholar] [CrossRef] [Green Version]
- Prakash, A.; Sharma, S.; Ahmad, N.; Ghosh, A.; Sinha, P. Synthesis of Agnps by Bacillus cereus bacteria and their antimicrobial potential. J. Biomater. Nanobiotechnol. 2011, 02, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Manivasagan, P.; Venkatesan, J.; Senthilkumar, K.; Sivakumar, K.; Kim, S.K. Biosynthesis, antimicrobial and cytotoxic effect of silver nanoparticles using a novel Nocardiopsis sp. MBRC-1. BioMed Res. Int. 2013, 2013, 287638. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, A.; Dolma, K.; Kaur, N.; Rathore, Y.S.; Mayilraj, S.; Choudhury, A.R. Biosynthesis of gold and silver nanoparticles using a novel marine strain of Stenotrophomonas. Bioresour. Technol. 2013, 142, 727–731. [Google Scholar] [CrossRef]
- Singh, R.; Wagh, P.; Wadhwani, S.; Gaidhani, S.; Kumbhar, A.; Bellare, J.; Chopade, B.A. Synthesis, optimization, and characterization of silver nanoparticles from Acinetobacter calcoaceticus and their enhanced antibacterial activity when combined with antibiotics. Int. J. Nanomed. 2013, 8, 4277–4290. [Google Scholar] [CrossRef] [Green Version]
- Kushwaha, A.; Singh, V.K.; Bhartariya, J.; Singh, P.; Yasmeen, K. Isolation and identification of E. coli bacteria for the synthesis of silver nanoparticles: Characterization of the particles and study of antibacterial activity. Eur. J. Exp. Biol. 2015, 5, 65–70. [Google Scholar]
- Saravanan, C.; Rajesh, R.; Kaviarasan, T.; Muthukumar, K.; Kavitake, D.; Shetty, P.H. Synthesis of silver nanoparticles using bacterial exopolysaccharide and its application for degradation of azo-dyes. Biotechnol. Rep. 2017, 15, 33–40. [Google Scholar] [CrossRef]
- Ajah, H.A.; Hassan, A.S.; Aja, H.A. Extracellular biosynthesis of silver nanoparticles using Fusarium graminearum and their antimicrobial activity. J. Glob. Pharma Technol. 2018, 10, 683–689. [Google Scholar]
- Hamouda, R.A.; Hussein, M.H.; Abo-elmagd, R.A.; Bawazir, S.S. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep. 2019, 9, 1–17. [Google Scholar] [CrossRef]
- Hamida, R.S.; Abdelmeguid, N.E.; Ali, M.A.; Bin-Meferij, M.M.; Khalil, M.I. Synthesis of silver nanoparticles using a novel cyanobacteria desertifilum sp. Extract: Their antibacterial and cytotoxicity effects. Int. J. Nanomed. 2020, 15, 49–63. [Google Scholar] [CrossRef] [Green Version]
- Akter, S.; Huq, M.A. Biologically rapid synthesis of silver nanoparticles by Sphingobium sp. MAH-11T and their antibacterial activity and mechanisms investigation against drug-resistant pathogenic microbes. Artif. Nanomed. Biotechnol. 2020, 48, 672–682. [Google Scholar] [CrossRef] [Green Version]
- Van Tan, L.; Tran, T.; Thi, V.D. Biosynthesis of silver nanoparticles from Bacillus licheniformis TT01 isolated from quail manure collected in Vietnam. Processes 2021, 9, 584. [Google Scholar] [CrossRef]
- Saleh, M.N.; Khoman Alwan, S. Bio-synthesis of silver nanoparticles from bacteria Klebsiella pneumonia: Their characterization and antibacterial studies. J. Phys. Conf. Ser. 2020, 1664, 012115. [Google Scholar] [CrossRef]
- Klaus, T.; Joerger, R.; Olsson, E. Microbially fabricated. Proc. Natl. Acad. Sci. USA 1999, 96, 13611–13614. [Google Scholar] [CrossRef] [Green Version]
- Samadi, N.; Golkaran, D.; Eslamifar, A.; Jamalifar, H.; Fazeli, M.R.; Mohseni, F.A. Intra/extracellular biosynthesis of silver nanoparticles by an autochthonous strain of Proteus mirabilis isolated from photographic waste. J. Biomed. Nanotechnol. 2009, 5, 247–253. [Google Scholar] [CrossRef]
- Huq, M.A. Biogenic silver nanoparticles synthesized by Lysinibacillus xylanilyticus MAHUQ-40 to control antibiotic-resistant human pathogens Vibrio parahaemolyticus and Salmonella Typhimurium. Front. Bioeng Biotechnol. 2020, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Deljou, A.; Goudarzi, S. Green extracellular synthesis of the silver nanoparticles using thermophilic Bacillus sp. AZ1 and its antimicrobial activity against several human pathogenetic bacteria. Iran. J. Biotechnol. 2016, 14, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S.; Balachandran, C.; Duraipandiyan, V.; Ramasamy, D.; Ignacimuthu, S.; Al-Dhabi, N.A. Extracellular biosynthesis of silver nanoparticle using Streptomyces sp. 09 PBT 005 and its antibacterial and cytotoxic properties. Appl. Nanosci. 2015, 5, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Tamboli, D.P.; Lee, D.S. Mechanistic antimicrobial approach of extracellularly synthesized silver nanoparticles against gram positive and gram negative bacteria. J. Hazard. Mater. 2013, 260, 878–884. [Google Scholar] [CrossRef]
- Mondal, A.H.; Yadav, D.; Mitra, S.; Mukhopadhyay, K. Biosynthesis of silver nanoparticles using culture supernatant of shewanella sp. ARY1 and their antibacterial activity. Int. J. Nanomed. 2020, 15, 8295–8310. [Google Scholar] [CrossRef]
- Matei, A.; Matei, S.; Matei, G.M.; Cogălniceanu, G.; Cornea, C.P. Biosynthesis of silver nanoparticles mediated by culture filtrate of lactic acid bacteria, characterization and antifungal activity. EuroBiotech. J. 2020, 4, 97–103. [Google Scholar] [CrossRef]
- Kumar, A.; Ghosh, A. Biosynthesis and characterization of silver nanoparticles with bacterial isolate from Gangetic-alluvial soil. Int. J. Biotechnol. Biochem. 2016, 12, 95–102. [Google Scholar]
- Wang, C.; Kim, Y.J.; Singh, P.; Mathiyalagan, R.; Jin, Y.; Yang, D.C. Green synthesis of silver nanoparticles by Bacillus methylotrophicus, and their antimicrobial activity. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1127–1132. [Google Scholar] [CrossRef]
- Karunakaran, G.; Jagathambal, M.; Gusev, A.; Torres, J.A.L.; Kolesnikov, E.; Kuznetsov, D. Rapid biosynthesis of AgNPs using soil bacterium Azotobacter vinelandii with promising antioxidant and antibacterial activities for biomedical applications. JOM 2017, 69, 1206–1212. [Google Scholar] [CrossRef]
- Otari, S.V.; Patil, R.M.; Ghosh, S.J.; Thorat, N.D.; Pawar, S.H. Intracellular synthesis of silver nanoparticle by actinobacteria and its antimicrobial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 136B, 1175–1180. [Google Scholar] [CrossRef]
- Ibrahim, E.; Fouad, H.; Zhang, M.; Zhang, Y.; Qiu, W.; Yan, C.; Li, B.; Mo, J.; Chen, J. Biosynthesis of silver nanoparticles using endophytic bacteria and their role in inhibition of rice pathogenic bacteria and plant growth promotion. RSC Adv. 2019, 9, 29293–29299. [Google Scholar] [CrossRef] [Green Version]
- Majeed, S.; Abdullah, M.S.; Nanda, A.; Ansari, M.T. In vitro study of the antibacterial and anticancer activities of silver nanoparticles synthesized from Penicillium brevicompactum (MTCC-1999). J. Taibah Univ. Sci. 2016, 10, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Wypij, M.; Jędrzejewski, T.; Trzcińska-Wencel, J.; Ostrowski, M.; Rai, M.; Golińska, P. Green synthesized silver nanoparticles: Antibacterial and anticancer activities, biocompatibility, and analyses of surface-attached proteins. Front. Microbiol. 2021, 12, 1–17. [Google Scholar] [CrossRef]
- Blackwell, M. The fungi: 1, 2, 3... 5.1 million species? Am. J. Bot. 2011, 98, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Berdy, J. Bioactive Microbial Metabolites. J. Antibiot. Res. 2005, 58, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, B.; Mukherjee, P.; Senapati, S.; Mandal, D.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B 2003, 28, 313–318. [Google Scholar] [CrossRef]
- Zomorodian, K.; Syedmohammad, P.; Arman, S.; Pouyan, M.; Keyvan, P.; Mohammad Javad, R.; Ali Arabi, M. Biosynthesis and characterization of silver nanoparticles by Pseudomonas pseudoalcaligenes. Res. J. Biotechnol. 2021, 16, 123–127. [Google Scholar]
- Guilger-Casagrande, M.; de Lima, R. Synthesis of Silver Nanoparticles Mediated by Fungi: A Review. Front. Bioeng. Biotechnol. 2019, 7, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Li, Y.; Wang, H.; Zu, Y. Differences in the activities of eight enzymes from ten soil fungi and their possible influences on the surface structure, functional groups, and element composition of soil colloids. PLoS ONE 2014, 9, 1–12. [Google Scholar] [CrossRef]
- Sánchez-Corzo, L.D.; Álvarez-Gutiérrez, P.E.; Meza-Gordillo, R.; Villalobos-Maldonado, J.J.; Enciso-Pinto, S.; Enciso-Sáenz, S. Lignocellulolytic enzyme production from wood rot fungi collected in chiapas, mexico, and their growth on lignocellulosic material. J. Fungi. 2021, 7, 450. [Google Scholar] [CrossRef]
- Vahabi, K.; Mansoori, G.A.; Karimi, S. Biosynthesis of silver nanoparticles by fungus Trichoderma Reesei (A route for large-scale production of AgNPs). Insciences J. 2011, 1, 65–79. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A.; Almoammar, H.; Rai, M.; Said-Galiev, E.; Abd-Elsalam, K.A. Myconanoparticles: Synthesis and their role in phytopathogens management. Biotechnol. Biotechnol. Equip. 2015, 29, 221–236. [Google Scholar] [CrossRef]
- Castro-Longoria, E.; Vilchis-Nestor, A.R.; Avalos-Borja, M. Biosynthesis of silver, gold and bimetallic nanoparticles using the filamentous fungus Neurospora crassa. Colloids Surf. B. 2011, 83, 42–48. [Google Scholar] [CrossRef]
- Mughal, B.; Zaidi, S.Z.J.; Zhang, X.; Hassan, S.U. Biogenic nanoparticles: Synthesis, characterisation and applications. Appl. Sci. 2021, 11, 2598. [Google Scholar] [CrossRef]
- Robinson, P.K. Enzymes: Principles and biotechnological applications. Essays Biochem. 2015, 59, 1–41. [Google Scholar] [CrossRef]
- Wang, D.; Xue, B.; Wang, L.; Zhang, Y.; Liu, L.; Zhou, Y. Fungus-mediated green synthesis of nano-silver using Aspergillus sydowii and its antifungal/antiproliferative activities. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Tyagi, S.; Tyagi, P.K.; Gola, D.; Chauhan, N.; Bharti, R.K. Extracellular synthesis of silver nanoparticles using entomopathogenic fungus: Characterization and antibacterial potential. SN Appl. Sci. 2019, 1, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Vivek, R.; Thangam, R.; Muthuchelian, K.; Gunasekaran, P.; Kaveri, K.; Kannan, S. Green biosynthesis of silver nanoparticles from Annona squamosa leaf extract and its in vitro cytotoxic effect on MCF-7 cells. Process. Biochem. 2012, 47, 2405–2410. [Google Scholar] [CrossRef]
- Husseiny, S.M.; Salah, T.A.; Anter, H.A. Biosynthesis of size-controlled silver nanoparticles by Fusarium oxysporum, their antibacterial and antitumor activities. Beni-Suef. Univ. J. Basic Appl. Sci. 2015, 4, 225–231. [Google Scholar] [CrossRef] [Green Version]
- El-aziz, A.; Mahmoud, M.A.; Metwaly, H.A. Biosynthesis of Silver Nanoparticles Using Fusarium Solani. Dig. J. Nanomater. Bios. 2015, 10, 655–662. [Google Scholar]
- Sundaravadivelan, C.; Padmanabhan, M.N. Effect of mycosynthesized silver nanoparticles from filtrate of Trichoderma harzianum against larvae and pupa of dengue vector Aedes aegypti L. Environ. Sci. Pollut. Res. 2014, 21, 4624–4633. [Google Scholar] [CrossRef]
- Elgorban, A.M.; Aref, S.M.; Seham, S.M.; Elhindi, K.M.; Bahkali, A.H.; Sayed, S.R.; Manal, M.A. Extracellular synthesis of silver nanoparticles using Aspergillus versicolor and evaluation of their activity on plant pathogenic fungi. Mycosphere 2016, 7, 844–852. [Google Scholar] [CrossRef]
- Bhainsa, K.C.; D’Souza, S.F. Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids Surf. B Biointerf. 2006, 47, 160–164. [Google Scholar] [CrossRef]
- Gajbhiye, M.; Kesharwani, J.; Ingle, A.; Gade, A.; Rai, M. Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Vigneshwaran, N.; Ashtaputre, N.M.; Varadarajan, P.V.; Nachane, R.P.; Paralikar, K.M.; Balasubramanya, R.H. Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Mater. Lett. 2007, 61, 1413–1418. [Google Scholar] [CrossRef]
- Akther, T.; Mathipi, V.; Kumar, N.S.; Davoodbasha, M.; Srinivasan, H. Fungal-mediated synthesis of pharmaceutically active silver nanoparticles and anticancer property against A549 cells through apoptosis. Environ. Sci. Pollut. Res. 2019, 26, 13649–13657. [Google Scholar] [CrossRef] [PubMed]
- Hulikere, M.M.; Joshi, C.G. Characterization, antioxidant and antimicrobial activity of silver nanoparticles synthesized using marine endophytic fungus- Cladosporium cladosporioides. Process. Biochem. 2019, 82, 199–204. [Google Scholar] [CrossRef]
- Kobashigawa, J.M.; Robles, C.A.; Martínez Ricci, M.L.; Carmarán, C.C. Influence of strong bases on the synthesis of silver nanoparticles (AgNPs) using the ligninolytic fungi Trametes trogii. Saudi J. Biol. Sci. 2019, 26, 1331–1337. [Google Scholar] [CrossRef]
- Prasad, R. Synthesis of silver nanoparticles in photosynthetic plants. J. Nanoparticles 2014, 2014, 963961. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef]
- Singhal, G.; Bhavesh, R.; Kasariya, K.; Sharma, A.R.; Singh, R.P. Biosynthesis of silver nanoparticles using Ocimum sanctum (Tulsi) leaf extract and screening its antimicrobial activity. J. Nanoparticle Res. 2011, 13, 2981–2988. [Google Scholar] [CrossRef]
- Huang, J.; Li, Q.; Sun, D.; Lu, Y.; Su, Y.; Yang, X.; Wang, H.; Wang, Y.; Shao, W.; He, N.; et al. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology 2007, 18, 105104. [Google Scholar] [CrossRef]
- Ansari, M.A.; Alzohairy, M.A. One-pot facile green synthesis of silver nanoparticles using seed extract of phoenix dactylifera and their bactericidal potential against MRSA. Evid. Based Complement. Alternat. Med. 2018, 2018, 1860280. [Google Scholar] [CrossRef] [Green Version]
- Rupiasih, N.N.; Aher, A.; Gosavi, S.; Vidyasagar, P.B. Green synthesis of silver nanoparticles using latex extract of Thevetia peruviana: A novel approach towards poisonous plant utilization. J. Phys. Conf. Ser. 2013, 423, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sigamoney, M.; Shaik, S.; Govender, P.; Krishna, S.B.N.; Sershen. African leafy vegetables as bio-factories for silver nanoparticles: A case study on Amaranthus dubius C Mart. Ex Thell. S. Afr. J. Bot. 2016, 103, 230–240. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Lallianrawna, S.; Biswas, A.; Selvaraj, M.; Changmai, B.; Rokhum, S.L. Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: A review of recent literature. RSC Adv. 2021, 11, 2804–2837. [Google Scholar] [CrossRef]
- Nalvolthula, R.; Merugu, R.; Pratap Rudra, M.P. Phytochemical analysis, synthesis, antitumor and antimicrobial activity of silver nanoparticles using flower extracts of Ixora coccinea. Int. J. Chem. Tech. Res. 2014, 7, 2374–2380. [Google Scholar]
- Kulkarni, N.; Muddapur, U. Biosynthesis of metal nanoparticles: A review. J. Nanotechnol. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Perera, K.M.K.G.; Kuruppu, K.A.S.S.; Chamara, A.M.R.; Thiripuranathar, G. Characterization of spherical Ag nanoparticles synthesized from the agricultural wastes of Garcinia mangostana and Nephelium lappaceum and their applications as a photo catalyzer and fluorescence quencher. SN Appl. Sci. 2020, 2, 1–24. [Google Scholar] [CrossRef]
- Akintelu, S.A.; Bo, Y.; Folorunso, A.S. A review on synthesis, optimization, mechanism, characterization, and antibacterial application of silver nanoparticles synthesized from plants. J. Chem. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Nadeem, M.; Abbasi, B.H.; Younas, M.; Ahmad, W.; Khan, T. A review of the green syntheses and anti-microbial applications of gold nanoparticles. Green Chem. Lett. Rev. 2017, 10, 216–227. [Google Scholar] [CrossRef] [Green Version]
- Bhaumik, J.; Thakur, N.S.; Aili, P.K.; Ghanghoriya, A.; Mittal, A.K.; Banerjee, U.C. Bioinspired nanotheranostic agents: Synthesis, surface functionalization, and antioxidant potential. ACS Biomater. Sci. Eng. 2015, 1, 382–392. [Google Scholar] [CrossRef]
- Sathishkumar, G.; Gobinath, C.; Karpagam, K.; Hemamalini, V.; Premkumar, K.; Sivaramakrishnan, S. Phyto-synthesis of silver nanoscale particles using Morinda citrifolia L. and its inhibitory activity against human pathogens. Colloids Surf. B. 2012, 95, 235–240. [Google Scholar] [CrossRef]
- Ortega-Arroyo, L.; Martin-Martinez, E.S.; Aguilar-Mendez, M.A.; Cruz-Orea, A.; Hernandez-Pérez, I.; Glorieux, C. Green synthesis method of silver nanoparticles using starch as capping agent applied the methodology of surface response. Starch/Staerke 2013, 65, 814–821. [Google Scholar] [CrossRef]
- Ashraf, S.; Abbasi, A.Z.; Pfeiffer, C.; Hussain, S.Z.; Khalid, Z.M.; Gil, P.R.; Parak, W.J.; Hussain, I. Protein-mediated synthesis, pH-induced reversible agglomeration, toxicity and cellular interaction of silver nanoparticles. Colloids Surf. B. 2013, 102, 511–518. [Google Scholar] [CrossRef]
- Philip, D. Honey mediated green synthesis of silver nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2010, 75, 1078–1081. [Google Scholar] [CrossRef]
- Bar, H.; Bhui, D.K.; Sahoo, G.P.; Sarkar, P.; De, S.P.; Misra, A. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf. A 2009, 339, 134–139. [Google Scholar] [CrossRef]
- Patil, R.S.; Kokate, M.R.; Jambhale, C.L.; Pawar, S.M.; Han, S.H.; Kolekar, S.S. One-pot synthesis of PVA-capped silver nanoparticles their characterization and biomedical application. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Anigol, L.B.; Charantimath, J.S.; Gurubasavaraj, P.M. Effect of Concentration and pH on the size of silver nanoparticles synthesized by green chemistry. Org. Med. Chem. Int. J. 2017, 3, 1–5. [Google Scholar] [CrossRef]
- Ferreira, S.J.; Sonnewald, U. The mode of sucrose degradation in potato tubers determines the fate of assimilate utilization. Front. Plant. Sci. 2012, 3, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Haverkamp, R.G.; Marshall, A.T. The mechanism of metal nanoparticle formation in plants: Limits on accumulation. J. Nanopart. Res. 2009, 11, 1453–1463. [Google Scholar] [CrossRef]
- Jha, A.K.; Prasad, K.; Prasad, K.; Kulkarni, A.R. Plant system: Nature’s nanofactory. Colloids Surf. B 2009, 73, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Alhag, S.K.; Ghramah, H.A.; Al-Keridis, L.A.; Al-Mekhlafi, F.A.; Ibrahim, E.H.; Khan, K.A. Biogenic synthesis of silver nanoparticles (AgNPs) using Solanum Indicum Linn. Indian J. Pharm. Educ. 2021, 55, S202–S208. [Google Scholar] [CrossRef]
- Krithiga, N.; Jayachitra, A.; Rajalakshmi, A.; Gopal, P. Study on antioxidant and antimicrobial activities of the selected medicinal plants. J. Ethnolbiol. Ethnomedicine 2014, 1, 1–12. [Google Scholar]
- Logeswari, P.; Silambarasan, S.; Abraham, J. Ecofriendly synthesis of silver nanoparticles from commercially available plant powders and their antibacterial properties. Sci. Iran. 2013, 20, 1049–1054. [Google Scholar] [CrossRef]
- Kolya, H.; Maiti, P.; Pandey, A.; Tripathy, T. Green synthesis of silver nanoparticles with antimicrobial and azo dye (Congo red) degradation properties using Amaranthus gangeticus Linn leaf extract. J. Anal. Sci. Technol. 2015, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Velmurugan, P.; Cho, M.; Lim, S.-S.; Seo, S.-K.; Myung, H.; Bang, K.-S.; Sivakumar, S.; Cho, K.-M.; Oh, B.-T. Phytosynthesis of silver nanoparticles by Prunus yedoensis leaf extract and their antimicrobial activity. Mater. Lett. 2015, 138, 272–275. [Google Scholar] [CrossRef]
- Saratale, R.G.; Shin, H.S.; Kumar, G.; Benelli, G.; Kim, D.S. Exploiting antidiabetic activity of silver nanoparticles synthesized using Punica granatum leaves and anticancer potential against human liver cancer cells (HepG2). Artif. Cells Nanomed. Biotechnol. 2018, 46, 211–222. [Google Scholar] [CrossRef] [Green Version]
- Veeramani, S.; Ernest Ravindran, R.; Preethi, R.; Cyrril, J.; Kirubanandan, S.; Renganathan, S. Silver nanoparticles—Green synthesis with Aq. Extract of stems Ipomoea Pes-Caprae, characterization, antimicrobial and anti-cancer potential. Int. J. Med. Nano Res. 2018, 5, 1–9. [Google Scholar] [CrossRef]
- Ansar, S.; Alkhudhayr, B.M.A.; Alsubki, R. Synthesis of biogenic silver nanoparticles from polyphenolic Brassica nigra and their potential antifungal, antibacterial, antioxidant, and anticancer activities. Int. Food Res. J. 2021, 28, 317–325. [Google Scholar]
- Kanmani, R.; Scleva, I.P. Antioxidant and antidiabetic activities of biologically synthesized silver nanoparticles using Linum usitatissimum Extract. Orient J. Chem. 2021, 37, 1235–1241. [Google Scholar] [CrossRef]
- Fawcett, D.; Verduin, J.J.; Shah, M.; Sharma, S.B.; Poinern, G.E.J. A Review of current research into the biogenic synthesis of metal and metal oxide nanoparticles via marine algae and seagrasses. Int. J. Nanosci. 2017, 2017, 1–15. [Google Scholar] [CrossRef]
- Patel, V.; Berthold, D.; Puranik, P.; Gantar, M. Screening of cyanobacteria and microalgae for their ability to synthesize silver nanoparticles with antibacterial activity. Biotechnol. Rep. 2015, 5, 112–119. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, P.C.; Kausar, F.; Lee, J.H.; Han, J.I. Facile fabrication of silver nanoparticle embedded CaCO3 microspheres via microalgae-templated CO2 biomineralization: Application in antimicrobial paint development. RSC Adv. 2014, 4, 32562–32569. [Google Scholar] [CrossRef]
- Khanna, P.; Kaur, A.; Goyal, D. Algae-based metallic nanoparticles: Synthesis, characterization and applications. J. Microbiol. Methods 2019, 163, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.R.R.; Stirk, W.A.; Van Staden, J. Synthesis of silver nanoparticles using the seaweed Codium capitatum P.C. Silva (Chlorophyceae). S. Afr. J. Bot. 2013, 86, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Vincy, W.; Mahathalana, T.J.; Sukumaran, S. Algae as a sources for synthesis of nanoparticles-A review. Int. J. Latest Trends Eng. Technol. 2017, 5. [Google Scholar]
- Prasad, T.N.V.K.V.; Kambala, V.S.R.; Naidu, R. Phyconanotechnology: Synthesis of silver nanoparticles using brown marine algae Cystophora moniliformis and their characterisation. J. Appl. Phycol. 2013, 25, 177–182. [Google Scholar] [CrossRef]
- Doshi, H.; Ray, A.; Kothari, I.L. Bioremediation potential of live and dead Spirulina: Spectroscopic, kinetics and SEM studies. Biotechnol. Bioeng. 2007, 96, 1051–1063. [Google Scholar] [CrossRef]
- Husain, S.; Sardar, M.; Fatma, T. Screening of cyanobacterial extracts for synthesis of silver nanoparticles. World J. Microbiol. Biotechnol. 2015, 31, 1279–1283. [Google Scholar] [CrossRef]
- Singh, Y.; Kaushal, S.; Sodhi, R.S. Biogenic synthesis of silver nanoparticles using cyanobacterium: Leptolyngbya sp. WUC 59 cell-free extract and their effects on bacterial growth and seed germination. Nanoscale Adv. 2020, 2, 3972–3982. [Google Scholar] [CrossRef]
- Sudha, S.S.; Rajamanickam, K.; Rengaramanujam, J. Microalgae mediated synthesis of silver nanoparticles and their antibacterial activity against pathogenic bacteria. Indian J. Exp. Biol. 2013, 51, 393–399. [Google Scholar]
- Chaudhary, R.; Nawaz, K.; Khan, A.K.; Hano, C.; Abbasi, B.H.; Anjum, S. An overview of the algae-mediated biosynthesis of nanoparticles and their biomedical applications. Biomolecules 2020, 10, 1498. [Google Scholar] [CrossRef]
- Rajkumar, R.; Ezhumalai, G.; Gnanadesigan, M. A green approach for the synthesis of silver nanoparticles by Chlorella vulgaris and its application in photocatalytic dye degradation activity. Environ. Technol. Innovat. 2021, 21, 101282. [Google Scholar] [CrossRef]
- Jena, J.; Pradhan, N.; Nayak, R.R.; Dash, B.P.; Sukla, L.B.; Panda, P.K.; Mishra, B.K. Microalga Scenedesmus sp.: A potential low-cost green machine for silver nanoparticle synthesis. J. Microbiol. Biotechnol. 2014, 24, 522–533. [Google Scholar] [CrossRef]
- El-Rafie, H.M.; El-Rafie, M.H.; Zahran, M.K. Green synthesis of silver nanoparticles using polysaccharides extracted from marine macro algae. Carbohydr. Polym. 2013, 96, 403–410. [Google Scholar] [CrossRef]
- Priyadharshini, R.I.; Prasannaraj, G.; Geetha, N.; Venkatachalam, P. Microwave-mediated extracellular synthesis of metallic silver and zinc oxide nanoparticles using macro-algae (Gracilaria edulis) extracts and its anticancer activity against human PC3 cell lines. Appl. Biochem. Biotechnol. 2014, 174, 2777–2790. [Google Scholar] [CrossRef]
- Bhimba, B.V.; Devi, J.S.; Nandhini, S.U. Green synthesis and cytotoxicity of silver nanoparticles from extracts of the marine macroalgae Gracilaria corticata. Indian J. Biotechnol. 2015, 14, 276–281. [Google Scholar]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, I.B.M.; Alharbi, R.M.; Alkhulaifi, M.M. Biosynthesis of silver nanoparticles by using of the marine brown alga Padina pavonia and their characterization. Saudi J. Biol. Sci. 2019, 26, 1207–1215. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.K.; Shim, M.S. Antimicrobial, antioxidant, and anticancer activities of biosynthesized silver nanoparticles using marine algae ecklonia cava. Nanomaterials 2016, 6, 235. [Google Scholar] [CrossRef]
- Supraja, N.; Dhivya, J.; Prasad, T.N.V.K.V.; David, E. Synthesis, characterization and dose dependent antimicrobial and anticancerous efficacy of phycogenic (Sargassum muticum) silver nanoparticles against breast cancer cells (MCF 7) cell line. Adv. Nano Res. 2018, 6, 183–200. [Google Scholar] [CrossRef]
- Kiran, M.V.; Murugesan, S. Biosynthesis and characterization of silver nanoparticles from marine macroscopic brown seaweed Colpomenia sinuosa (Mertens ex Roth) derbes and solier. J. Adv. Chem. Sci. 2020, 6, 663–666. [Google Scholar] [CrossRef]
- Devaraj, P.; Renganathan, A.; Prachi, K.; Chirom, A. Synthesis and characterization of silver nanoparticles using cannonball leaves and their cytotoxic activity against Mcf-7 cell line. J. Nanotechnol. 2013, 3, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Jyoti, K.; Baunthiyal, M.; Singh, A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J. Radiat. Res. Appl. Sci. 2016, 9, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Elamawi, R.M.; Al-Harbi, R.E.; Hendi, A.A. Biosynthesis and characterization of silver nanoparticles using Trichoderma longibrachiatum and their effect on phytopathogenic fungi. Egypt. J. Biol. Pest. Co. 2018, 28, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Raval, N.; Maheshwari, R.; Kalyane, D.; Youngren-Ortiz, S.R.; Chougule, M.B.; Tekade, R.K. Importance of physicochemical characterization of nanoparticles in pharmaceutical product development. In Basic Fundamentals of Drug Delivery, 1st ed.; Tekade, R.K., Ed.; Elsevier Inc: Amsterdam, The Netherlands, 2018; pp. 369–400. [Google Scholar] [CrossRef]
- Babick, F. Dynamic light scattering (DLS). In Characterization of Nanoparticles: Measurement Processes for Nanoparticles, 1st ed.; Hodoroaba, V., Unger, W.E.S., Shard, A.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 137–172. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Y.; Shen, J.; Liu, W.; Sun, X. The measurement system of nanoparticle size distribution from dynamic light scattering data. Opt. Lasers Eng. 2014, 56, 94–98. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, C.K. Methods for characterization of nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids, 1st ed.; Nimesh, S., Chandra, R., Gupta, Eds.; Woodhead Publishing: Sawston, UK, 2017; Volume 1, pp. 44–58. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Panda, S.K.; Bastia, A.K.; Mohanta, T.K. Biosynthesis of silver nanoparticles from Protium serratum and investigation of their potential impacts on food safety and control. Front. Microbiol. 2017, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Raj, S.; Chand Mali, S.; Trivedi, R. Green synthesis and characterization of silver nanoparticles using Enicostemma axillare (Lam.) leaf extract. Biochem. Biophys. Res. Commun. 2018, 503, 2814–2819. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [Green Version]
- Joshi, M.; Bhattacharyya, A.; Ali, S.W. Characterization techniques for nanotechnology applications in textiles. Indian J. Fibre Text. Res. 2008, 33, 304–317. [Google Scholar]
- Rather, M.; Pandian, J.; Sundarapandian, S.M.; Yogamoorthi, A. Biosynthesis and characterization of silver nanoparticles using leaf extract of Wedelia urticifolia (Blume) DC and evaluation of antibacterial efficacy. IOSR J. Pharm. Biol. Sci. 2017, 12, 14–23. [Google Scholar] [CrossRef]
- Jemal, K.; Sandeep, B.V.; Pola, S. Synthesis, characterization, and evaluation of the antibacterial activity of Allophylus serratus leaf and leaf derived callus extracts mediated silver nanoparticles. J. Nanomater. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Velavan, S.; Amargeetha, A. X-ray diffraction (XRD) and energy dispersive spectroscopy (EDS) analysis of silver nanoparticles synthesized from Erythrina Indica Flowers. Nanosci. Technol. 2018, 5, 1–5. [Google Scholar] [CrossRef]
- Soares, M.R.P.S.; Corrêa1, R.O.; Stroppa, P.H.F.; Marques, F.C.; Andrade, G.F.S.; Corrêa, C.C.; Brandão, M.A.F.; Raposo, N.R.B. Biosynthesis of silver nanoparticles using Caesalpinia ferrea (Tul.) Martius extract: Physicochemical characterization, antifungal activity and cytotoxicity. PeerJ 2018, 6, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.; Alajmi, M.F.; Khan, M.A.; Pervez, S.A.; Ahmed, F.; Amir, S.; Husain, F.M.; Khan, M.S.; Shaik, G.M.; Hassan, I. Biosynthesized silver nanoparticle (AgNP) from pandanus odorifer leaf extract exhibits anti-metastasis and anti-biofilm potentials. Front. Microbiol. 2019, 10, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Jebril, S.; Khanfir Ben Jenana, R.; Dridi, C. Green synthesis of silver nanoparticles using Melia azedarach leaf extract and their antifungal activities: In vitro and in vivo. Mater. Chem. Phys. 2020, 248, 1–25. [Google Scholar] [CrossRef]
- Manam, V.K.; Subbiah, M. Biological synthesis of silver nanoparticles from marine alga Colpomenia sinuosa and its in vitro anti-diabetic activity. Am. J. Bio-Pharmacol. Biochem. Life Sci. 2014, 3, 1–7. [Google Scholar] [CrossRef]
- Chouhan, N. Silver nanoparticles: Synthesis, characterization and applications. In Silver Nanoparticles—Fabrication, Characterization and Applications; Intech Open: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.B.; Carter, C.B. Transmission Electron. Microscopy: A Textbook for Materials Science; Springer: New York, NY, USA, 2009. [Google Scholar]
- Dawadi, S.; Katuwal, S.; Gupta, A. Current research on silver nanoparticles: Synthesis, characterization, and applications. J. Nanomater. 2021, 202, 6687290. [Google Scholar] [CrossRef]
- Banu, A.; Gousuddin, M.; Yahya, E.B. Green synthesized monodispersed silver nanoparticles’ characterization and their efficacy against cancer cells. Biomed. Res. Ther. 2021, 8, 4476–4482. [Google Scholar] [CrossRef]
- Kanipandian, N.; Li, D.; Kannan, S. Induction of intrinsic apoptotic signaling pathway in A549 lung cancer cells using silver nanoparticles from Gossypium hirsutum and evaluation of in vivo toxicity. Biotechnol. Rep. 2019, 23, e00339. [Google Scholar] [CrossRef]
- Yang, X.; Chung, E.; Johnston, I.; Ren, G.; Cheong, Y.K. Exploitation of antimicrobial nanoparticles and their applications in biomedical engineering. Appl. Sci. 2021, 11, 4520. [Google Scholar] [CrossRef]
- Neihaya, H.Z.; Zaman, H.H. Investigating the effect of biosynthesized silver nanoparticles as antibiofilm on bacterial clinical isolates. Microb. Pathog. 2018, 116, 200–208. [Google Scholar] [CrossRef]
- Rodríguez-Serrano, C.; Guzmán-Moreno, J.; Ángeles-Chávez, C. Biosynthesis of silver nanoparticles by Fusarium scirpi and its potential as antimicrobial agent against uropathogenic Escherichia coli biofilms. PLoS ONE 2020, 15, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LewisOscar, F.; Vismaya, S.; Arunkumar, M.; Thajuddin, N.; Dhanasekaran, D.; Nithya, C. Algal Nanoparticles: Synthesis and Biotechnological Potentials. In Algae—Organisms for Imminent Biotechnology; Thajuddin, N., Dhanasekaran, D., Eds.; IntechOpen: London, UK, 2016; pp. 158–182. [Google Scholar] [CrossRef] [Green Version]
- Okeke, I.N.; Edelman, R. Dissemination of antibiotic-resistant bacteria across geographic borders. Clin. Infect. Dis. 2001, 33, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Webb, G.F.; D’Agata, E.M.C.; Magal, P.; Ruan, S. A model of antibiotic-resistant bacterial epidemics in hospitals. Proc. Natl. Acad. Sci. USA 2005, 102, 13343–13348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, J.K.; Swarnkar, R.K.; Soumya, K.K. Silver nanoparticles synthesized by pulsed laser ablation: As a potent antibacterial agent for human enteropathogenic Gram-positive and Gram-negative bacterial strains. Appl. Biochem. Biotechnol. 2014, 174, 1021–1031. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Cavassin, E.D.; de Figueiredo, L.F.P.; Otoch, J.P. Comparison of methods to detect the in vitro activity of silver nanoparticles (AgNP) against multidrug resistant bacteria. J. Nanobiotechnology 2015, 13, 1–16. [Google Scholar] [CrossRef]
- Khanna, K.; Panja, S.; Choudhuri, I.; Chakraborty, A.; Bhattacharyya, N. Evaluation of antibacterial activity and cytotoxicity of green synthesized silver nanoparticles using Scoparia dulcis. Nano Biomed. Eng. 2015, 7, 128–133. [Google Scholar] [CrossRef] [Green Version]
- Huq, M.A.; Akter, S. Biosynthesis, characterization and antibacterial application of novel silver nanoparticles against drug resistant pathogenic klebsiella pneumoniae and salmonella enteritidis. Molecules 2021, 26, 5996. [Google Scholar] [CrossRef]
- Huq, M.A. Green synthesis of silver nanoparticles using pseudoduganella eburnea MAHUQ-39 and their antimicrobial mechanisms investigation against drug resistant human pathogens. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef] [Green Version]
- Nanda, A.; Saravanan, M. Biosynthesis of silver nanoparticles from Staphylococcus aureus and its antimicrobial activity against MRSA and MRSE. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 452–456. [Google Scholar] [CrossRef]
- Du, J.; Singh, H.; Yi, T.H. Biosynthesis of silver nanoparticles by Novosphingobium sp. THG-C3 and their antimicrobial potential. Artif. Cells Nanomed. Biotechnol. 2017, 45, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, R.; Ganesan, N.; Balu, S.K.; Alagar, S.; Thandavamoorthy, P.; Thiruvengadam, D. Green synthesis, characterization, antimicrobial and cytotoxic effects of silver nanoparticles using Origanum Heracleoticum L. leaf extract. Int. J. Pharm.Sci. 2015, 7, 288–293. [Google Scholar]
- Singh, P.; Singh, H.; Kim, Y.J.; Mathiyalagan, R.; Wang, C.; Yang, D.C. Extracellular synthesis of silver and gold nanoparticles by Sporosarcina koreensis DC4 and their biological applications. Enzyme Microb. Technol. 2016, 86, 75–83. [Google Scholar] [CrossRef]
- Daniel, S.C.G.; Vinothini, G.; Subramanian, N.; Nehru, K.; Sivakumar, M. Biosynthesis of Cu, ZVI, and Ag nanoparticles using Dodonaea viscosa extract for antibacterial activity against human pathogens. J. Nanoparticle Res. 2013, 15, 1–10. [Google Scholar] [CrossRef]
- Hassan, S.W.M.; Abd El-latif, H.H. Characterization and applications of the biosynthesized silver nanoparticles by marine Pseudomonas sp. H64. J. Pure Appl. Microbiol. 2018, 12, 1289–1299. [Google Scholar] [CrossRef]
- Kumar, S.S.D.; Houreld, N.N.; Kroukamp, E.M.; Abrahamse, H. Cellular imaging and bactericidal mechanism of green-synthesized silver nanoparticles against human pathogenic bacteria. J. Photochem. Photobiol. B Biol. 2018, 178, 259–269. [Google Scholar] [CrossRef]
- Sudhakar, C.; Selvam, K.; Govarthanan, M. Acorus calamus rhizome extract mediated biosynthesis of silver nanoparticles and their bactericidal activity against human pathogens. J. Genet. Eng. Biotechnol. 2015, 13, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, S.; Sadat Shandiz, S.A.; Ghanbar, F. Phytosynthesis of silver nanoparticles using artemisia marschalliana sprengel aerial part extract and assessment of their antioxidant, anticancer, and antibacterial properties. Int. J. Nanomed. 2016, 11, 1835–1846. [Google Scholar] [CrossRef] [Green Version]
- Bindhu, M.R.; Umadevi, M.; Esmail, G.A.; Al-Dhabi, N.A.; Arasu, M.V. Green synthesis and characterization of silver nanoparticles from Moringa oleifera flower and assessment of antimicrobial and sensing properties. J. Photochem. Photobiol. B Biol. 2020, 205, 111836. [Google Scholar] [CrossRef]
- Kathiraven, T.; Sundaramanickam, A.; Shanmugam, N.; Balasubramanian, T. Green synthesis of silver nanoparticles using marine algae Caulerpa racemosa and their antibacterial activity against some human pathogens. Appl. Nanosci. 2015, 5, 499–504. [Google Scholar] [CrossRef] [Green Version]
- Zada, S.; Ahmad, A.; Khan, S. Biogenic synthesis of silver nanoparticles using extracts of Leptolyngbya JSC-1 that induce apoptosis in HeLa cell line and exterminate pathogenic bacteria. Artif. Cells Nanomed. Biotechnol. 2018, 46, S471–S480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seetharaman, P.K.; Chandrasekaran, R.; Periakaruppan, R. Functional attributes of myco-synthesized silver nanoparticles from endophytic fungi: A new implication in biomedical applications. Biology 2021, 10, 473. [Google Scholar] [CrossRef] [PubMed]
- Sagar, G.; Ashok, B. Green synthesis of silver nanoparticles using Aspergillus niger and its efficacy against human pathogens. Library 2012, 2, 1654–1658. [Google Scholar]
- Murugesan, S.; Bhuvaneswari, S.; Sivamurugan, V. Green Synthesis, Characterization of silver nanoparticles of a marine red alga Spyridia Fusiformis and their antibacterial activity. Int. J. Pharm. Sci. 2017, 9, 192. [Google Scholar] [CrossRef] [Green Version]
- Khaled, J.M.; Alharbi, N.S.; Kadaikunnan, S. Green synthesis of Ag nanoparticles with anti-bacterial activity using the leaf extract of an African medicinal plant, Ipomoea asarifolia (Convolvulaceae). J. Clust. Sci. 2017, 28, 3009–3019. [Google Scholar] [CrossRef]
- Kumar, S.D.; Singaravelu, G.; Ajithkumar, S.; Murugan, K.; Nicoletti, M.; Benelli, G. Mangrove-mediated green synthesis of silver nanoparticles with high HIV-1 reverse transcriptase inhibitory potential. J. Clust. Sci. 2017, 28, 359–367. [Google Scholar] [CrossRef]
- Dhanasezhian, A.; Srivan, S.; Govindaraju, K.; Parija, P.; Sasikala, S.; Ramesh Kumar, M.R. Anti-herpes simplex virus (HSV-1 and HSV-2) activity of biogenic gold and silver nanoparticles using seaweed Sargassum wightii. Indian J. Geo-Marine Sci. 2019, 48, 1252–1257. [Google Scholar]
- Sujitha, V.; Murugan, K.; Paulpandi, M. Green-synthesized silver nanoparticles as a novel control tool against dengue virus (DEN-2) and its primary vector Aedes aegypti. Parasitol. Res. 2015, 114, 3315–3325. [Google Scholar] [CrossRef]
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, B.M. The potential of silver nanoparticles for antiviral and antibacterial applications: A mechanism of action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef]
- Gaikwad, S.; Ingle, A.; Gade, A. Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int. J. Nanomed. 2013, 8, 4303–4314. [Google Scholar] [CrossRef] [Green Version]
- Aziz, A.T.; Alshehri, M.A.; Alanazi, N.A. Phytochemical analysis of Rhazya stricta extract and its use in fabrication of silver nanoparticles effective against mosquito vectors and microbial pathogens. Sci. Total Environ. 2020, 700, 1–11. [Google Scholar] [CrossRef]
- Murugan, K.; Dinesh, D.; Paulpandi, M. Nanoparticles in the fight against mosquito-borne diseases: Bioactivity of Bruguiera cylindrica-synthesized nanoparticles against dengue virus DEN-2 (in vitro) and its mosquito vector Aedes aegypti (Diptera: Culicidae). Parasitol. Res. 2015, 114, 4349–4361. [Google Scholar] [CrossRef]
- Bere, A.W.; Mulati, O.; Kimotho, J.; Ng’Ong’, A.F. Carica papaya leaf extract silver synthesized nanoparticles inhibit dengue type 2 viral replication in vitro. Pharmaceuticals 2021, 14, 718. [Google Scholar] [CrossRef]
- Elumalai, D.; Hemavathi, M.; Deepaa, C.V.; Kaleena, P.K. Evaluation of phytosynthesised silver nanoparticles from leaf extracts of Leucas aspera and Hyptis suaveolens and their larvicidal activity against malaria, dengue and filariasis vectors. Parasite Epidemiol. Control. 2017, 2, 15–26. [Google Scholar] [CrossRef]
- Haggag, E.G.; Elshamy, A.M.; Rabeh, M.A. Antiviral potential of green synthesized silver nanoparticles of lampranthus coccineus and malephora lutea. Int. J. Nanomed. 2019, 14, 6217–6229. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.X.; Li, C.M.; Huang, C.H. Curcumin modified silver nanoparticles for highly efficient inhibition of respiratory syncytial virus infection. Nanoscale 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Sharma, V.; Kaushik, S.; Pandit, P.; Dhull, D.; Yadav, J.P.; Kaushik, S. Green synthesis of silver nanoparticles from medicinal plants and evaluation of their antiviral potential against chikungunya virus. Appl. Microbiol. Biotechnol. 2019, 103, 881–891. [Google Scholar] [CrossRef]
- Sreekanth, T.V.M.; Nagajyothi, P.C.; Muthuraman. Ultra-sonication-assisted silver nanoparticles using Panax ginseng root extract and their anti-cancer and antiviral activities. J. Photochem. Photobiol. B Biol. 2018, 188, 6–11. [Google Scholar] [CrossRef]
- Khan, M.S.; Tabrez, S.; Al-Okail, M.S.; Shaik, G.M.; Bhat, S.A.; Rehman, M.T.; Husain, F.M.; AlAjmi, M.F. Non-enzymatic glycation of protein induces cancer proliferation and its inhibition by quercetin; Spectroscopic, cytotoxicity and molecular docking studies. J. Biomol. Struct. Dyn. 2021, 9, 777–786. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Q.; Feng, L.; Liu, Z. Nanomedicine for tumor microenvironment modulation and cancer treatment enhancement. Nano Today 2018, 21, 55–73. [Google Scholar] [CrossRef]
- Park, Y.H.; Hwang, C.; Kim, Y.; Lee, Y.; Jeong, D.; Cho, M. Antimicrobial effects of silver nanoparticles . Nanomedicine 2007, 3, 95–101. [Google Scholar]
- Nguyen, V.P.; Le Trung, H.; Nguyen, T.H.; Hoang, D.; Tran, T.H. Synthesis of biogenic silver nanoparticles with eco-friendly processes using Ganoderma lucidum extract and evaluation of their theranostic applications. J. Nanomater. 2021, 2021, 6135920. [Google Scholar] [CrossRef]
- Narasimha, V.R.; Latha, T.S.; Pallu, R.; Panati, K.; Narala, V.R. Anticancer activities of biogenic silver nanoparticles targeting apoptosis and inflammatory pathways in colon cancer cells. J. Clust. Sci. 2021, 1–17. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Muthukumaran, P.; Ramachandran, R.; Balakumaran, M.D.; Kalaichelvan, P.T. Acalypha indica Linn.: Biogenic synthesis of silver and gold nanoparticles and their cytotoxic effects against MDA-MB-231, human breast cancer cells. Biotechnol. Rep. 2014, 4, 42–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deeb, N.; Abo-Eleneen, M.; Awad, O.; Aboshady, A. Arthrospira platensis mediated biosynthesis of silver nanoparticles as breast cancer proliferation and differentiation controlling agent: In-vitro and in-vivo safety approach; Research Square (Nano Express): Durham, NC, USA, 5 April 2021; pp. 1–25. [Google Scholar] [CrossRef]
- Gajendran, B.; Chinnasamy, A.; Durai, P.; Raman, J.; Ramar, M. Biosynthesis and characterization of silver nanoparticles from Datura inoxia and its apoptotic effect on human breast cancer cell line MCF7. Mater. Lett. 2014, 122, 98–102. [Google Scholar] [CrossRef]
- Sathishkumar, G.; Gobinath, C.; Wilson, A.; Sivaramakrishnan, S. Dendrophthoe falcata (L.f) Ettingsh (Neem mistletoe): A potent bioresource to fabricate silver nanoparticles for anticancer effect against human breast cancer cells (MCF-7). Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2014, 128, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Venkatadri, B.; Shanparvish, E.; Rameshkumar, M.R. Green synthesis of silver nanoparticles using aqueous rhizome extract of Zingiber officinale and Curcuma longa: In-vitro anti-cancer potential on human colon carcinoma HT-29 cells. Saudi J. Biol. Sci. 2020, 27, 2980–2986. [Google Scholar] [CrossRef] [PubMed]
- Ramar, M.; Manikandan, B.; Marimuthu, P.N. Synthesis of silver nanoparticles using Solanum trilobatum fruits extract and its antibacterial, cytotoxic activity against human breast cancer cell line MCF 7. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2015, 140, 223–228. [Google Scholar] [CrossRef]
- Kumari, R.M.; Kumar, V.; Kumar, M.; Pareek, N.; Nimesh, S. Assessment of antibacterial and anticancer capability of silver nanoparticles extracellularly biosynthesized using Aspergillus terreus. Nano Express 2020, 1, 030011. [Google Scholar] [CrossRef]
- Rajeswari, P.; Samuel, P.; Vijayakumar, J.; Selvarathinam, T.; Sudarmani, D.N.P.; Amirtharaj, K.; Deenathayalan, R. Green synthesis of silver nanoparticles by Aspergillus consortium and evaluating its anticancer activity against Human breast adenocarcinoma cell line (MCF7). Pharm. Biol. Eval. 2017, 4, 28. [Google Scholar] [CrossRef] [Green Version]
- El-Sonbaty, S.M. Fungus-mediated synthesis of silver nanoparticles and evaluation of antitumor activity. Cancer Nanotechnol. 2013, 4, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.S.; Soundhararajan, R.; Akther, T. Biogenic AgNPs synthesized via endophytic bacteria and its biological applications. Environ. Sci. Pollut. Res. 2019, 26, 26939–26946. [Google Scholar] [CrossRef]
- He, Y.; Du, Z.; Ma, S. Biosynthesis, antibacterial activity and anticancer effects against prostate cancer (PC-3) cells of silver nanoparticles using Dimocarpus Longan Lour. Peel Extract. Nanoscale Res. Lett. 2016, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Feng, Q.; Wang, M.; Zhao, H.; Lin, Y.; Zhou, S. Green biosynthesized silver nanoparticles with aqueous extracts of Ginkgo Biloba induce apoptosis via mitochondrial pathway in cervical cancer cells. Front. Oncol. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Annu, M.; Ahmed, S.; Kaur, G.; Sharma, P.; Singh, S.; Ikram, S. Evaluation of the antioxidant, antibacterial and anticancer (lung cancer cell line A549) activity of: Punica granatum mediated silver nanoparticles. Toxicol. Res. 2018, 7, 923–930. [Google Scholar] [CrossRef] [Green Version]
- Adebayo, I.A.; Arsad, H.; Gagman, H.A.; Ismail, N.Z.; Samian, M.R. Inhibitory effect of eco-friendly naturally synthesized silver nanoparticles from the leaf extract of medicinal Detarium microcarpum plant on pancreatic and cervical cancer cells. Asian Pacific J. Cancer Prev. 2020, 21, 1247–1252. [Google Scholar] [CrossRef]
- Sarkar, S.; Kotteeswaran, V. Green synthesis of silver nanoparticles from aqueous leaf extract of Pomegranate (Punica granatum) and their anticancer activity on human cervical cancer cells. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Ullah, I.; Khalil, A.T.; Ali, M. Green-synthesized silver nanoparticles induced apoptotic cell death in MCF-7 breast cancer cells by generating reactive oxygen species and activating Caspase 3 and 9 enzyme activities. Oxid. Med. Cell Longev. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Firdhouse, M.J.; Lalitha, P. Biogenic silver nanoparticles—Synthesis, characterization and its potential against cancer inducing bacteria. J. Mol. Liq. 2016, 222, 1041–1050. [Google Scholar] [CrossRef]
- Ferreira, L.A.B.; Garcia-Fossa, F.; Radaic, A.; Durán, N.; Fávaro, W.J.; de Jesus, M.B. Biogenic silver nanoparticles: In vitro and in vivo antitumor activity in bladder cancer. Eur. J. Pharm. Biopharm. 2020, 151, 162–170. [Google Scholar] [CrossRef]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [Green Version]
- Cochis, A.; Azzimonti, B.; Della Valle, C.; De Giglio, E.; Bloise, N.; Visai, L.; Cometa, S.; Rimondini, L.; Chiesa, R. The effect of silver or gallium doped titanium against the multidrug resistant Acinetobacter baumannii. Biomaterials 2016, 80, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Hang, R.; Huang, X.; Zhao, L.; Zhang, X.; Wang, L.; Tang, B.; Ma, S.; Chu, P.K. The effects of titania nanotubes with embedded silver oxide nanoparticles on bacteria and osteoblasts. Biomaterials 2014, 35, 4223–4235. [Google Scholar] [CrossRef] [PubMed]
- Angelini, A.; Drago, G.; Trovarelli, G.; Calabrò, T.; Ruggieri, P. Infection after surgical resection for pelvic bone tumors: An analysis of 270 patients from one institution tumor. Clin. Orthop. Relat. Res. 2014, 472, 349–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tande, A.J.; Patel, R. Prosthetic joint infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan, S.A.; Fhoghlú, C.N.; DeVitt, B.M.; O’Mahony, F.J.; Brabazon, D.; Walsh, A. Instructional review: General orthopaedics silver nanoparticles and their orthopaedic applications. Bone Joint J. 2015, 97, 582–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soucacos, P.N.; Johnson, E.O.; Babis, G. An update on recent advances in bone regeneration. Injury 2008, 39, 1–4. [Google Scholar] [CrossRef]
- Marsich, E.; Bellomo, F.; Turco, G.; Travan, A.; Donati, I.; Paoletti, S. Nano-composite scaffolds for bone tissue engineering containing silver nanoparticles: Preparation, characterization and biological properties. J. Mater. Sci. Mater. Med. 2013, 24, 1799–1807. [Google Scholar] [CrossRef]
- Felix, W.P.; Muthu, P. Bioscaffolds impregnated with Ormocarpum cochinchinense mediated Ag nanoparticles. In Proceedings of the International Conference on Electrical, Electronics, and Optimization Techniques (ICEEOT), Chennai, India, 3–5 March 2016. [Google Scholar]
- Prokopovich, P.; Köbrick, M.; Brousseau, E.; Perni, S. Potent antimicrobial activity of bone cement encapsulating silver nanoparticles capped with oleic acid. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2015, 103, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Tyllianakis, M.E.; Karageorgos, A.C.; Marangos, M.N.; Saridis, A.G.; Lambiris, E.E. Antibiotic prophylaxis in primary hip and knee arthroplasty. Comparison between cefuroxime and two specific antistaphylococcal agents. J. Arthroplasty 2010, 25, 1078–1082. [Google Scholar] [CrossRef]
- Buckley, J.J.; Lee, A.F.; Olivi, L.; Wilson, K. Hydroxyapatite supported antibacterial Ag3PO4 nanoparticles. J. Mater. Chem. 2010, 20, 8056–8063. [Google Scholar] [CrossRef] [Green Version]
- Ewald, A.; Hösel, D.; Patel, S.; Grover, L.M.; Barralet, J.E.; Gbureck, U. Silver-doped calcium phosphate cements with antimicrobial activity. Acta Biomater. 2011, 7, 4064–4070. [Google Scholar] [CrossRef]
- Allaker, R.P. The use of nanoparticles to control oral biofilm formation. J. Dent. Res. 2010, 89, 1175–1186. [Google Scholar] [CrossRef]
- Sivolella, S.; Stellini, E.; Brunello, G.; Gardin, C.; Ferroni, L.; Bressan, E.; Zavan, B. Silver nanoparticles in alveolar bone surgery devices. J. Nanomater. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Zitzmann, N.U.; Berglundh, T. Definition and prevalence of peri-implant diseases. J. Clin. Periodontol. 2008, 35, 286–291. [Google Scholar] [CrossRef]
- Lang, N.P.; Berglundh, T. Periimplant diseases: Where are we now?—Consensus of the Seventh European Workshop on Periodontology. J. Clin. Periodontol. 2011, 38, 178–181. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.; Snigdha, S.; Bhavitha, K.B.; Babu, S.; Ajith, A.; Radhakrishnan, E.K. Biofabricated silver nanoparticles incorporated polymethyl methacrylate as a dental adhesive material with antibacterial and antibiofilm activity against Streptococcus mutans. 3 Biotech 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Campos, V.; DeAlba-Montero, I.; Ruiz, F.; Butrón-Téllez Girón, C.; García-García, E.; Loredo-Tovías, M. Simple and rapid method for silver nanoparticles incorporation in polymethyl methacrylate (PMMA) substrates. Superf. y Vacío 2017, 30, 51–55. [Google Scholar] [CrossRef]
- Acosta-Torres, L.S.; Mendieta, I.; Nuñez-Anita, R.E.; Cajero-Juárez, M.; Castaño, V.M. Cytocompatible antifungal acrylic resin containing silver nanoparticles for dentures. Int. J. Nanomed. 2012, 7, 4777–4786. [Google Scholar] [CrossRef] [Green Version]
- Al-Nerabieah, Z.; Arrag, E.A.; Comisi, J.C.; Rajab, A. Effectiveness of a novel nano-silver fluoride with green tea extract compared with silver diamine fluoride: A randomized, controlled, non-inferiority trial. Int. J. Dent. Oral Sci. 2020, 7, 753–761. [Google Scholar] [CrossRef]
- Torabinejad, M.; Watson, T.F.; Pitt Ford, T.R. Sealing ability of a mineral trioxide aggregate when used as a root end filling material. J. Endod. 1993, 19, 591–595. [Google Scholar] [CrossRef]
- Bahador, A.; Esmaeili, D.; Khaledi, A.; Ghorbanzadeh, R.J. An in vitro assessment of the antibacterial properties of nanosilver Iranian MTA against Porphyromonas gingivalis. J. Chem. Pharm. Res. 2013, 5, 65–71. [Google Scholar]
- Sundeep, D.; Vijaya Kumar, T.; Rao, P.S.S.; Ravikumar, R.V.S.S.N.; Gopala Krishna, A. Green synthesis and characterization of Ag nanoparticles from Mangifera indica leaves for dental restoration and antibacterial applications. Prog. Biomater. 2017, 6, 57–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, R.P.; Roy, A.; Aafreen, M.; Shanmugam, R. Antibacterial activity of white pepper oleoresin mediated silver nanoparticles against oral pathogens. J. Evol. Med. Dent. Sci. 2020, 9, 2352–2355. [Google Scholar] [CrossRef]
- Umai, D.; Vikranth, A.; Meenambiga, S.S. A study on the green synthesis of silver nanoparticles from Olea europaea and its activity against oral pathogens. Mater. Today Proc. 2021, 44, 3647–3651. [Google Scholar] [CrossRef]
- Halkai, K.R.; Mudda, J.A.; Shivanna, V.; Rathod, V.; Halkai, R. Evaluation of antibacterial efficacy of fungal-derived silver nanoparticles against Enterococcus faecalis. Contemp. Clin. Dent. 2018, 9, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Dai, A.P.; Shi, Y.; Liu, Z.J.; Gong, M.F.; Yin, X.B. Effectiveness of silver-impregnated central venous catheters for preventing catheter-related blood stream infections: A meta-analysis. Int. J. Infect. Dis. 2014, 29, e279–e286. [Google Scholar] [CrossRef] [Green Version]
- Sette, P.; Dorizzi, R.M.; Azzini, A.M. Vascular access: An historical perspective from Sir William Harvey to the 1956 Nobel prize to André F. Cournand, Werner Forssmann, and Dickinson W. Richards. J. Vasc. Access 2012, 13, 137–144. [Google Scholar] [CrossRef]
- Shah, H.; Bosch, W.; Hellinger, W.C.; Thompson, K.M. Intravascular Catheter-related bloodstream infection. Neurohospitalist 2013, 3, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Lemaster, C.H.; Schuur, J.D.; Pandya, D.; Pallin, D.J.; Silvia, J.; Yokoe, D.; Agrawal, A.; Hou, P.C. Infection and natural history of emergency department placed central venous catheters. Ann. Emerg. Med. 2010, 56, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Yang, Y.; Zhang, Y.; Deng, J.; Lin, C. Antimicrobial activity and cytocompatibility of silver nanoparticles coated catheters via a biomimetic surface functionalization strategy. Int. J. Nanomed. 2015, 10, 7241–7252. [Google Scholar] [CrossRef] [Green Version]
- Kumar, C.G.; Sujitha, P. Green synthesis of Kocuran-functionalized silver glyconanoparticles for use as antibiofilm coatings on silicone urethral catheters. Nanotechnology 2014, 25, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tong, H.; Liu, H.; Wang, Y.; Wang, R.; Gao, H.; Yu, P.; Lv, Y.; Chen, S.; Wang, G.; et al. Effectiveness of antimicrobial-coated central venous catheters for preventing catheter-related blood-stream infections with the implementation of bundles: A systematic review and network meta-analysis. Ann. Intensive Care 2018, 15, 71. [Google Scholar] [CrossRef]
- Fufă, O.; Andronescu, E.; Grumezescu, V.; Holban, A.; Mogoanta, L.; Mogoșanu, G.; Socol, G.; Lordache, F.; Chifiriuc, M.; Grumezescu, A. Silver nanostructurated surfaces prepared by MAPLE for biofilm prevention. Biointerface Res. Appl. Chem. 2015, 5, 1011–1017. [Google Scholar]
- Mala, R.; Aglin, A.A.; Celsia, A.S.R.; Geerthika, S.; Kiruthika, N.; VazagaPriya, C.; Kumar, K.S. Foley catheters functionalised with a synergistic combination of antibiotics and silver nanoparticles resist biofilm formation. IET Nanobiotechnol. 2017, 11, 612–620. [Google Scholar] [CrossRef]
- LewisOscar, F.; Nithya, C.; Dhamodharan, B.; Kumar, A.; Alharbi, N.; Thajuddin, N. Biofilm Inhibitory Effect of Spirulina platensis extracts on bacteria of clinical significance. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015, 87, 1–9. [Google Scholar] [CrossRef]
- Rahuman, H.B.H.; Dhandapani, R.; Palanivel, V. Bioengineered phytomolecules-capped silver nanoparticles using Carissa carandas leaf extract to embed on to urinary catheter to combat UTI pathogens. PLoS ONE 2021, 16, 1–27. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration; Mechanism, signaling and translation. Sci. Transl. Med. 2014, 6, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Hamdan, S.; Pastar, I.; Drakulich, S. Nanotechnology-driven therapeutic interventions in wound healing: Potential uses and applications. ACS Cent. Sci. 2017, 3, 163–175. [Google Scholar] [CrossRef]
- Krishnan, N.; Velramar, B.; Ramatchandirin, B. Effect of biogenic silver nanocubes on matrix metalloproteinases 2 and 9 expressions in hyperglycemic skin injury and its impact in early wound healing in streptozotocin-induced diabetic mice. Mater. Sci. Eng. C 2018, 91, 146–152. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Biswas, K.; Panda, S.K.; Bandyopadhyay, J.; De, D.; Jayabalan, R.; Bastia, A.K.; Mohanta, T.P. Phyto-assisted synthesis of bio-functionalised silver nanoparticles and their potential anti-oxidant, anti-microbial and wound healing activities. IET Nanobiotechnol. 2017, 11, 1027–1034. [Google Scholar] [CrossRef]
- Garg, S.; Chandra, A.; Mazumder, A.; Mazumder, R. Green synthesis of silver nanoparticles using Arnebia nobilis root extract and wound healing potential of its hydrogel. Asian J. Pharm. 2014, 8, 95–101. [Google Scholar] [CrossRef]
- Dhapte, V.; Kadam, S.; Moghe, A.; Pokharkar, V. Probing the wound healing potential of biogenic silver nanoparticles. J. Wound Care 2014, 23, 431–441. [Google Scholar] [CrossRef]
- Al-Shmgani, H.S.A.; Mohammed, W.H.; Sulaiman, G.M.; Saadoon, A.H. Biosynthesis of silver nanoparticles from Catharanthus roseus leaf extract and assessing their antioxidant, antimicrobial, and wound-healing activities. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1234–1240. [Google Scholar] [CrossRef]
- Pigłowski, M. Pathogenic and non-pathogenic microorganisms in the rapid alert system for food and feed. Int. J. Environ. Res. Public Health 2019, 16, 477. [Google Scholar] [CrossRef] [Green Version]
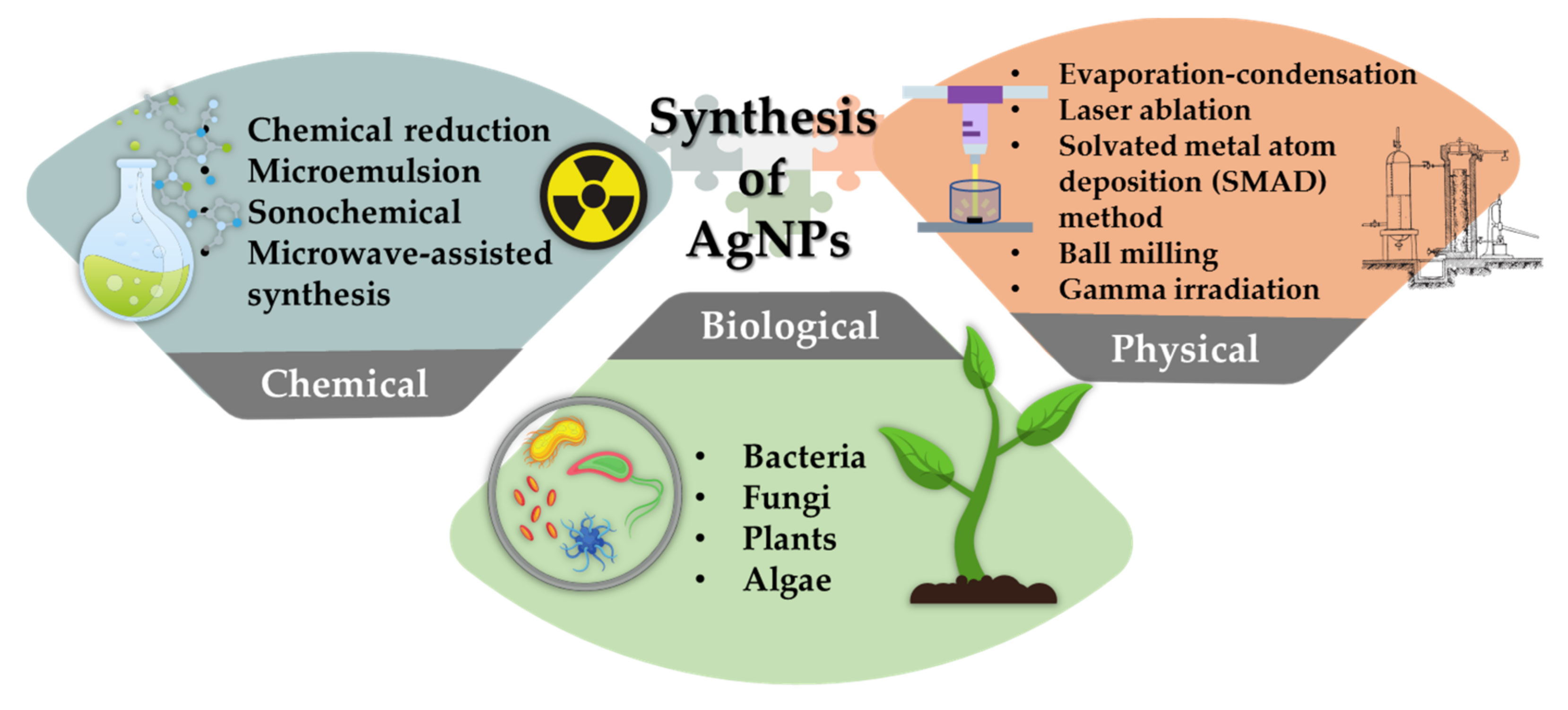
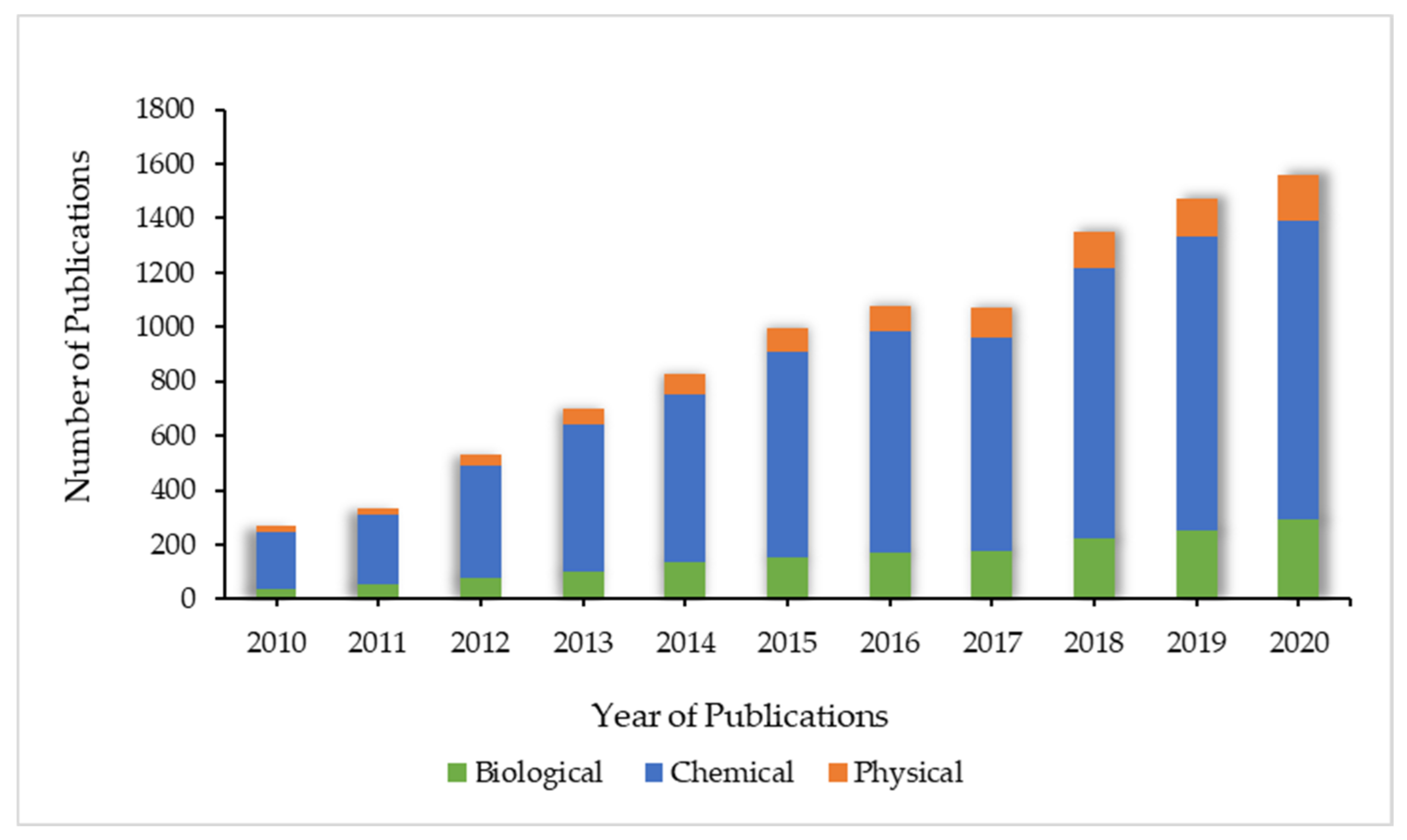
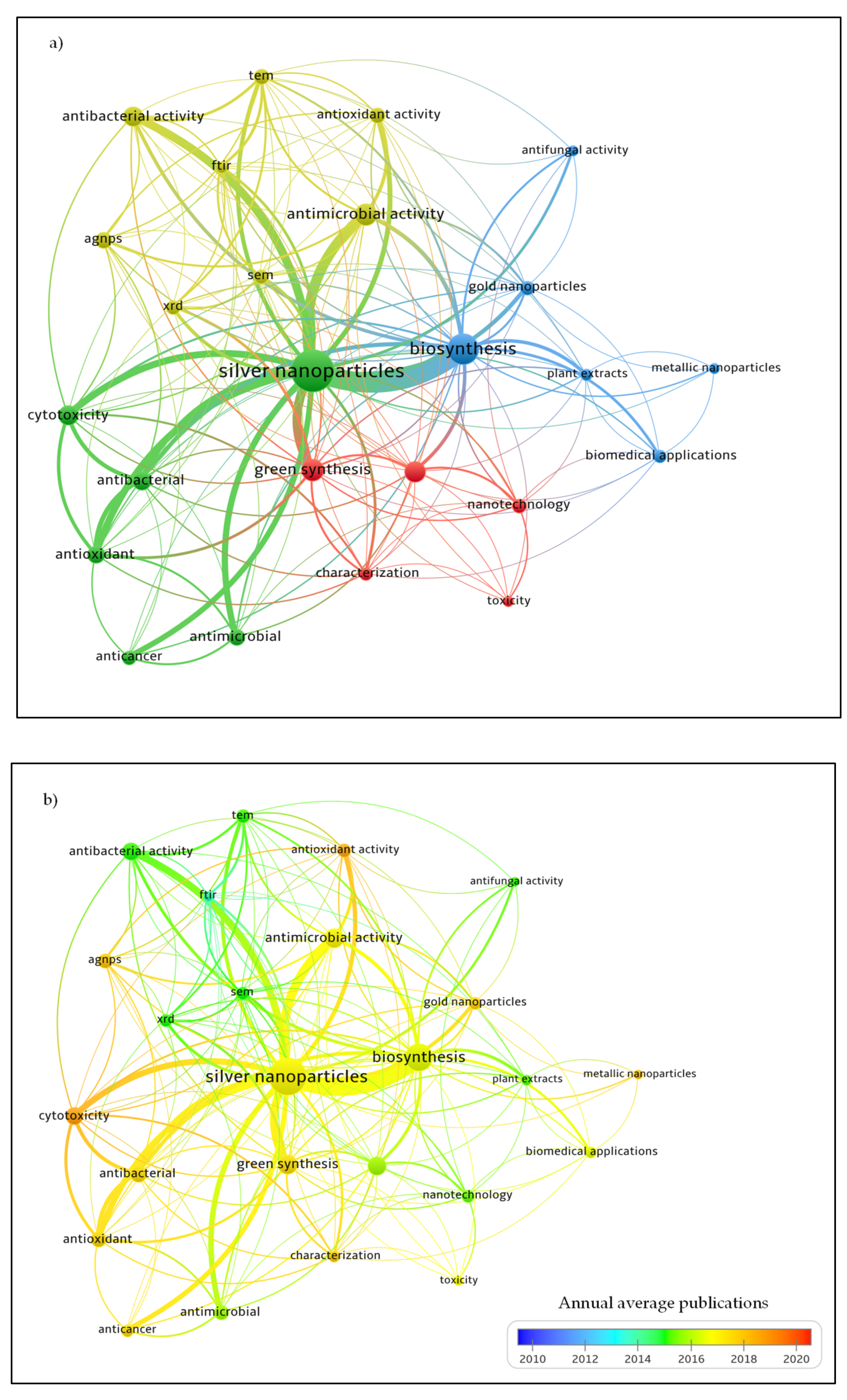
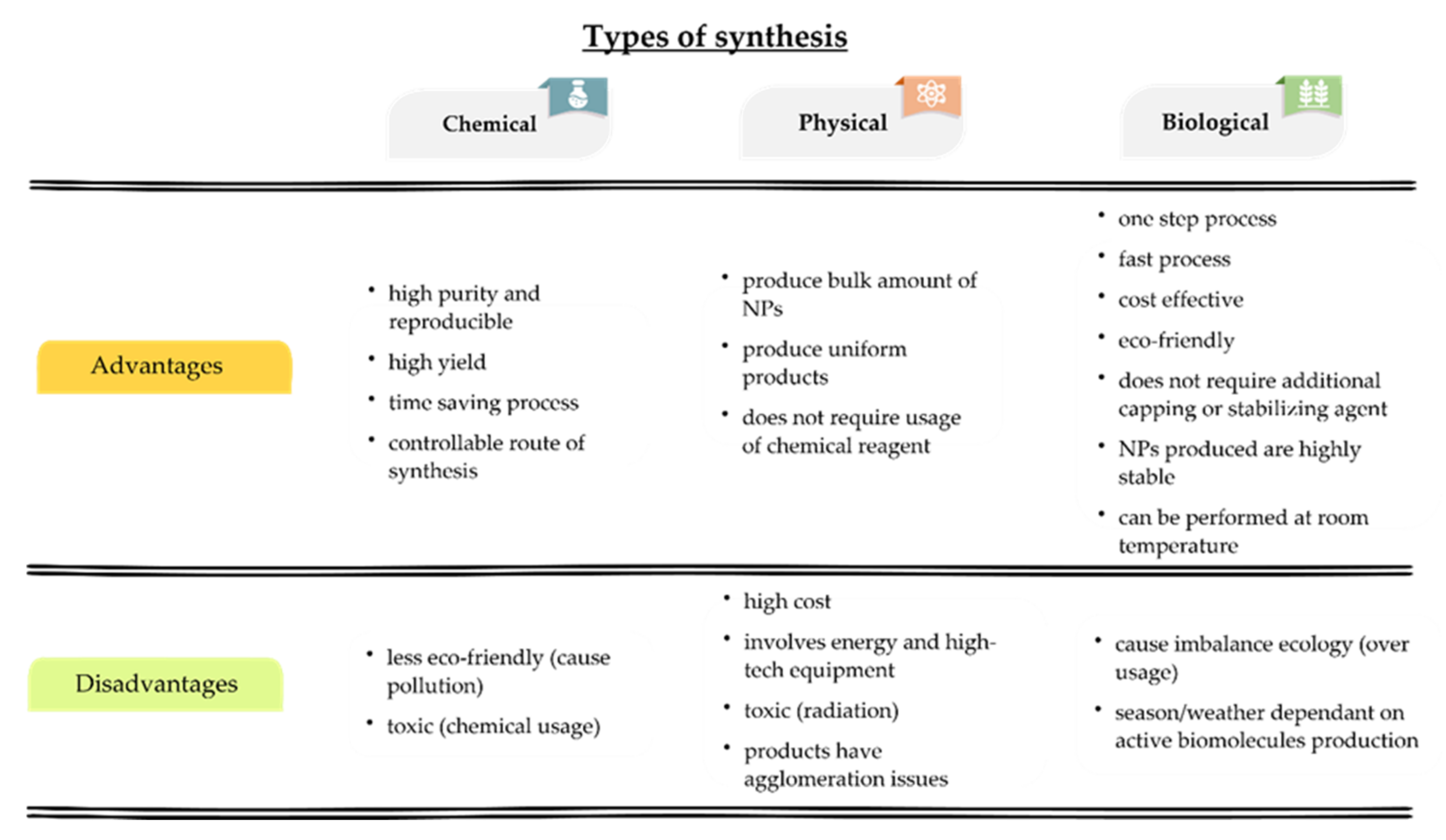
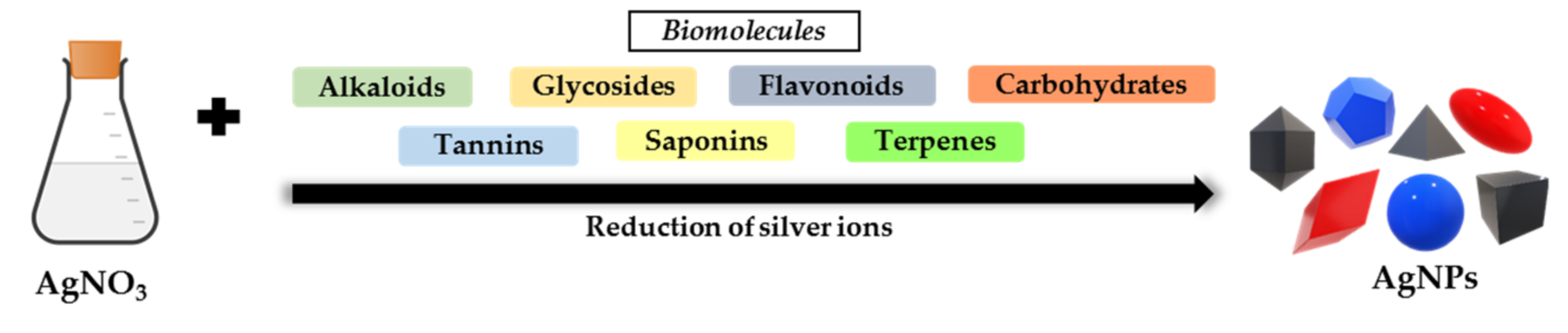

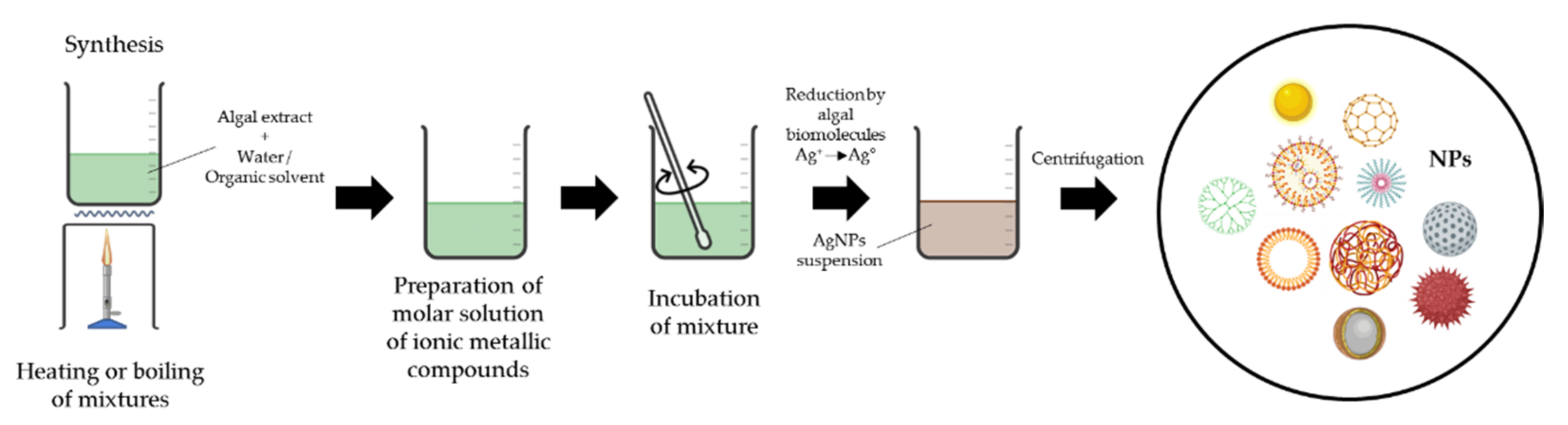

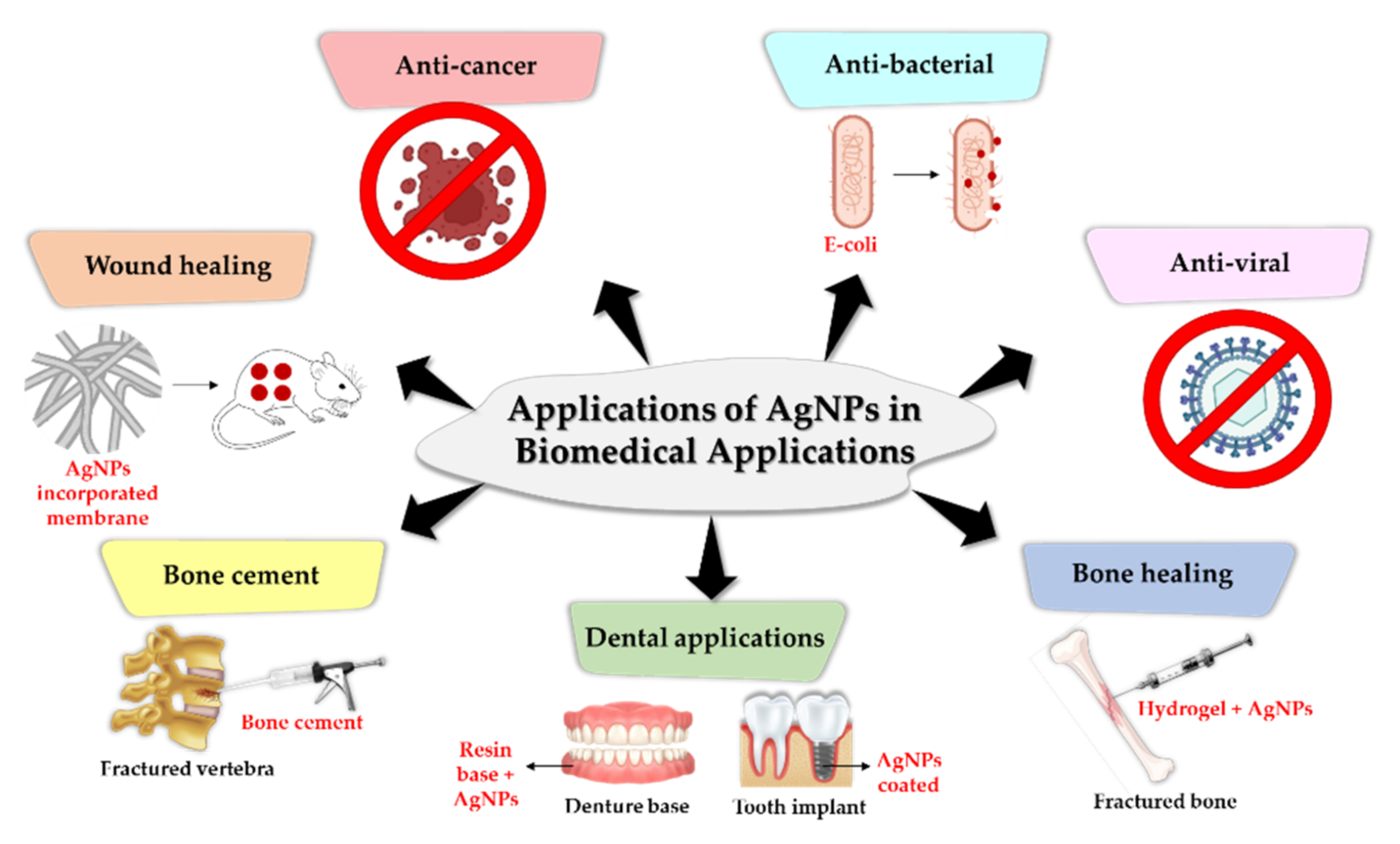
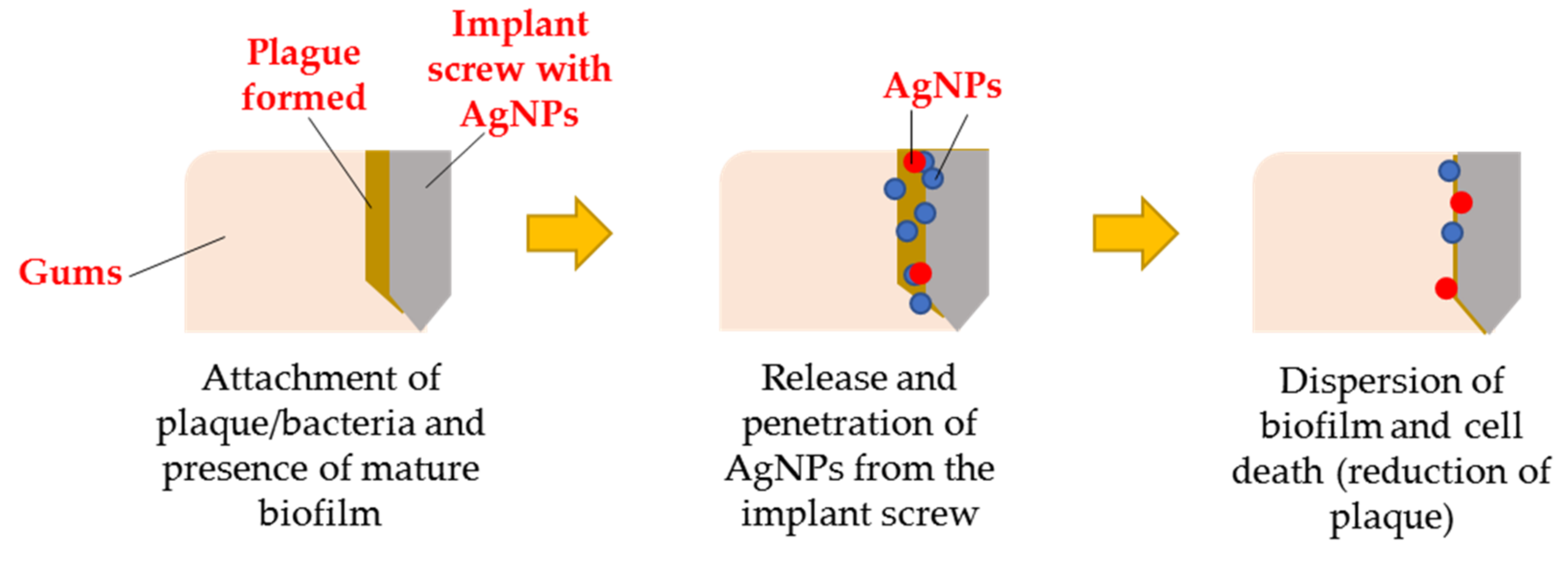

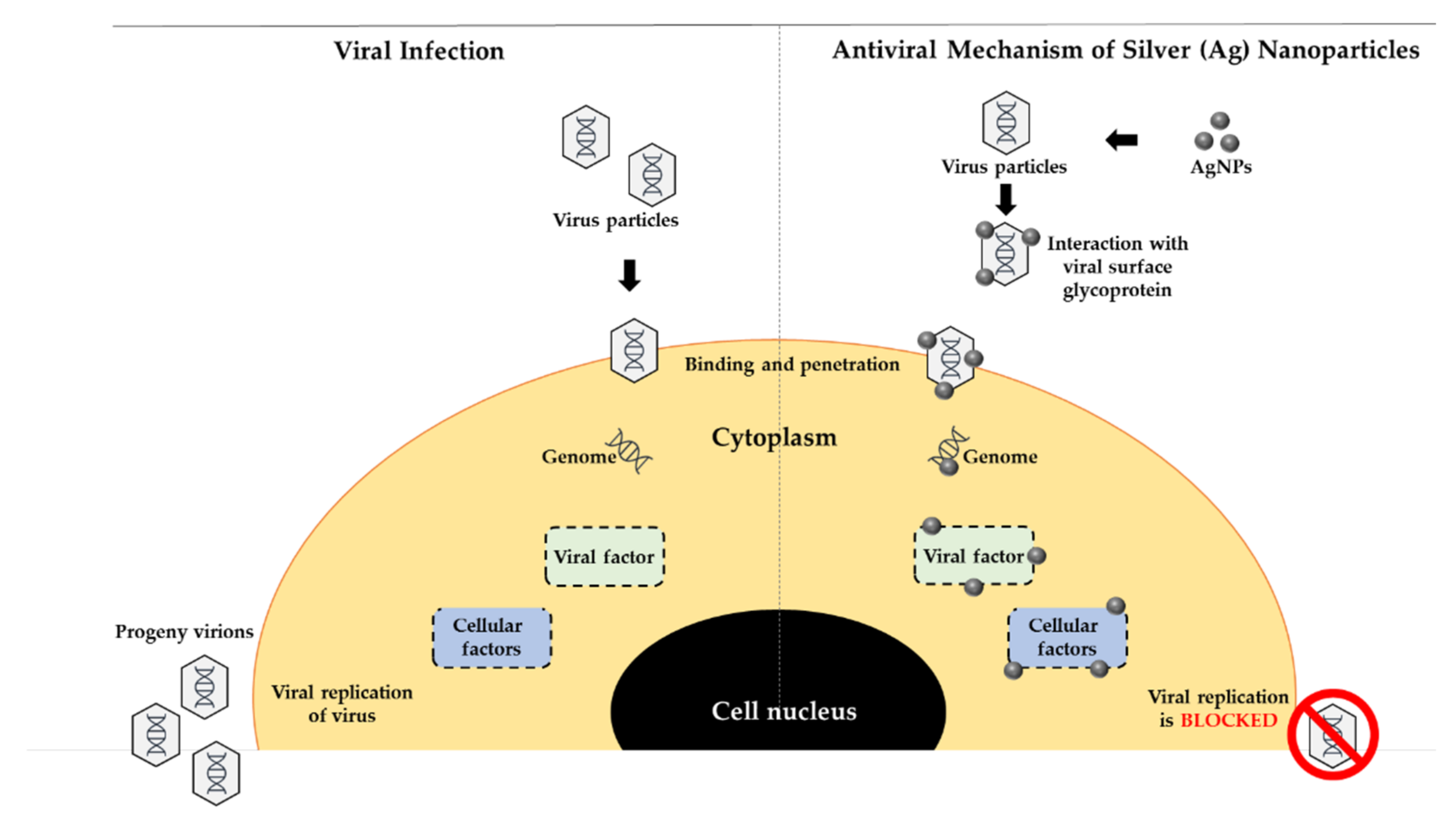
| Name of Bacteria | Size (nm) | Shape | References |
|---|---|---|---|
| Bacillus cereus | 10–30 | spherical | [112] |
| Nocardiopsis sp. MBRC-1 | 45 ± 0.15 | spherical | [113] |
| Stenotrophomonas | 40–60 | multi-shaped | [114] |
| Acinetobacter calcoaceticus | 8–12 | spherical | [115] |
| Escherichia coli | 20–50 | spherical | [116] |
| Leuconostoc lactis | 35 | spherical | [117] |
| Haemophilus influenzae | 80–101 | spherical | [118] |
| Cyanobacterium Oscillatoria limnetica | 3.30–17.97 | quasi-spherical | [119] |
| Cyanobacteria De sertifilum sp. | 5–26 | spherical | [120] |
| Sphingobium sp. MAH 11 | 7–22 | spherical | [121] |
| Bacillus licheniformis | 7–22 | spherical | [122] |
| Klebsiella pneumonia | 26.84–44.42 | spherical | [123] |
| Pseudomonas stutzeri AG259 | 200 | Triangle, hexagon and spherical | [124] |
| Proteus mirabilis PTCC 1710 | 10–20 | spherical | [125] |
| Lysinibacillus xylanilyticus strain MAHUQ-40 | 8–30 | spherical | [126] |
| Bacillus sp. AZ1 | 7–31 | spherical | [127] |
| Streptomyces sp. 09 PBT 005 | 198–595 | spherical | [128] |
| Exiguobacterium sp. KNU1 | 4.4 | spherical | [129] |
| Shewanella sp. ARY1 | 38 | spherical | [130] |
| Lactobacillus sp. strain LCM5 | 13.84 ± 4.56 | spherical | [131] |
| Bacillus sp-Brevibacillus borstelensis_MTCC10642 | 5–15 | cubical | [132] |
| Plants/Bacteria/Algae | Average Crystallite Size (nm) | AgNPs Peaks at 2θ Angles | References |
|---|---|---|---|
| Nocardiopsis sp. MBRC-1 | 45 ± 0.15 nm | 38.44°, 44.38°, 56.77°, 64.38° and 77.50° | [113] |
| Urtica dioica (Linn.) leaf extract | 20–30 nm | 38.45°, 46.35°, 64.75° and 78.05° | [219] |
| Wedelia urticifolia (Blume) DC | 179.3 nm, 90.38 nm and 80.28 nm | 38.22°, 44.42°, 64.56° and 77.50° | [230] |
| Allophylus serratus Leaf and Leaf Derived Callus Extracts | 42 nm (leaf) and 44 nm (extract) | 32.5°, 38.3°, 44.4°, 64.6°, and 76.8° and 32.4°, 38.3°, 44.5°, 64.5°, and 76.7° | [231] |
| Erythrina indica leaf extract | ~28.19 nm | 24.934°, 37.0359° and 43.8572° | [232] |
| Caesalpinia ferrea seed extract | 30–50 nm | 38.15°, 44.25°, 64.47°, 77.38° and 81.64° | [233] |
| Pandanus odorifer leaf extract | 10–50 nm | 37.2°, 43.4°, 63.5° and 76.6° | [234] |
| M. azedarach leaf extract | 21 nm | 37.88°, 44.31°, 64.34° and 77.39° | [235] |
| Brown seaweed Colpomenia sinuosa | 54–85 nm | 11.58°, 32.04°, 37.89° and 46.96° | [236] |
| Organism | Source | Size and Shape | Test Organism | References |
|---|---|---|---|---|
| Origanum heracleoticum L. | Bacteria | Spherical; 30–40 nm | E. coli, P. aeruginosa, S. aureus, S. pneumoniae, K. pneumoniae | [256] |
| Sporosarcina koreensis DC4 | Bacteria | Spherical; 102 nm | V. parahaemolyticus, E. coli, S. enterica, B. anthracis, B. cereus and S. aureus | [257] |
| Dodonaea viscosa extract | Plant | Spherical; 16 nm | E. coli, K. pneumoniae, P. fluorescens, S. aureus, B. subtilis | [258] |
| Marine Pseudomonas sp. H64 | Bacteria | Spherical; 3–22 nm | B. subtilis, S. faecalis, S. aureus, E. coli, A. hydrophila, V. parahaemolyticus | [259] |
| Aloe arborescens | Plant | Spherical; 38 ± 2 nm | P. aeruginosa, S. aureus | [260] |
| Acorus calamus | Plant | Spherical; 20–35 nm | S. aureus, E. coli | [261] |
| Fusarium scirpi | Fungus | Quasi-spherical; 2–20 nm | E. coli | [244] |
| Artemisia marschalliana extract | Plant | Spherical; 5–50 nm | S. aureus, B. cereus, A. baumannii | [262] |
| Moringa oleifera | Plant | Spherical; 8 nm | K. pneumoniae, S. aureus | [263] |
| Caulerpa racemose | Algae | Spherical and few triangular; 5–25 nm | S. aureus, P. mirabilis | [264] |
| Leptolyngbya strain JSC-1 | Algae | Spherical; 5–50 nm | S. aureus, E. coli | [265] |
| Penicillium oxalicum strain LA-1 | Fungus | Spherical; 52.26 nm | K. pneumoniae, V. cholerae, E. coli, M. luteus, M. smegmatis, B. subtilis | [266] |
| Aspergillus niger | Fungus | Spherical; 1–10 nm | C. krusei, C. parapsilosis, C. tropicalis, A. flavus, A. fumigates, S. aureus, P. aeruginosa, E. coli | [267] |
| Alga Syridia fusiformis | Algae | Spherical, Triangle, Pseudo-spherical, rectangle; 5–50 nm | K. pneumoniae, S. aureus | [268] |
| Ipomoea asarifolia | Plant | Spherical; 20–60 nm | B. subtilis, S. aureus, E. coli, K. pneumoniae | [269] |
| Virus | Family | Source of AgNPs | Size of AgNPs | Mechanism | References |
|---|---|---|---|---|---|
| Herpes simplex virus type 1 and type 2 (HSV-1 & HSV-2) | Hepesviridae | Dryopteris species, Musa paradisiaca, Catharanthus roseus, Selaginella bryopteris, Syzygium cumini | 4–31 nm | Block interaction of viral cells | [274] |
| Human parainfluenza virus type 3 (HPIV3) | Paramyxoviridae | ||||
| Zika virus | Flaviviridae | Rhazya stricta | 20–40 nm | Penetrate the infectious agent | [275] |
| Dengue virus (DEN-2) | Flaviviridae | Bruguiera cylindrica | 30–70 nm | Inhibitory effect on viral RNA synthesis | [276] |
| Carica papaya leaf | 10–35 nm | Inhibition of viral replication | [277] | ||
| Leucas aspera and Hyptis suaveolens | 7–22 nm | Capping facilitated surface activity makes these AgNPs a tool for vector control | [278] | ||
| HSV-1, HAV-10 and CoxB4 virus | Herpesviridae | Lampranthus coccineus | 10.12–27.89 nm | Interact with herpes simplex thymidine kinase, hepatitis A 3c proteinase and Coxsackie virus B4 3c protease | [279] |
| Malephora lutea F. Aizoaceae | 8.91–14.48 nm | Interact with herpes simplex thymidine kinase, hepatitis A 3c proteinase and Coxsackie virus B4 3c protease | [279] | ||
| Respiratory Syncytial Virus (RSV) | Pneumoviridae | Curcuma longa | 0.23 nm | Prevent the virus from entering cells and inhibition of viral replication | [280] |
| HIV-1 | Retroviridae | Rhizophora lamaeckii | 12–28 nm | HIV-1 reverse transcriptase inhibitory activity | [270] |
| Herpes Simplex Virus (HSV-I,II) | Herpesviridae | Sargassum withtii | - | Prevent the virus from entering cells | [271] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naganthran, A.; Verasoundarapandian, G.; Khalid, F.E.; Masarudin, M.J.; Zulkharnain, A.; Nawawi, N.M.; Karim, M.; Che Abdullah, C.A.; Ahmad, S.A. Synthesis, Characterization and Biomedical Application of Silver Nanoparticles. Materials 2022, 15, 427. https://doi.org/10.3390/ma15020427
Naganthran A, Verasoundarapandian G, Khalid FE, Masarudin MJ, Zulkharnain A, Nawawi NM, Karim M, Che Abdullah CA, Ahmad SA. Synthesis, Characterization and Biomedical Application of Silver Nanoparticles. Materials. 2022; 15(2):427. https://doi.org/10.3390/ma15020427
Chicago/Turabian StyleNaganthran, Ashwini, Gayathiri Verasoundarapandian, Farah Eryssa Khalid, Mas Jaffri Masarudin, Azham Zulkharnain, Norazah Mohammad Nawawi, Murni Karim, Che Azurahanim Che Abdullah, and Siti Aqlima Ahmad. 2022. "Synthesis, Characterization and Biomedical Application of Silver Nanoparticles" Materials 15, no. 2: 427. https://doi.org/10.3390/ma15020427
APA StyleNaganthran, A., Verasoundarapandian, G., Khalid, F. E., Masarudin, M. J., Zulkharnain, A., Nawawi, N. M., Karim, M., Che Abdullah, C. A., & Ahmad, S. A. (2022). Synthesis, Characterization and Biomedical Application of Silver Nanoparticles. Materials, 15(2), 427. https://doi.org/10.3390/ma15020427








