Effect of Cooling Rate on the Microstructure Evolution and Mechanical Properties of Iron-Rich Al–Si Alloy
Abstract
:1. Introduction
2. Materials and Methods
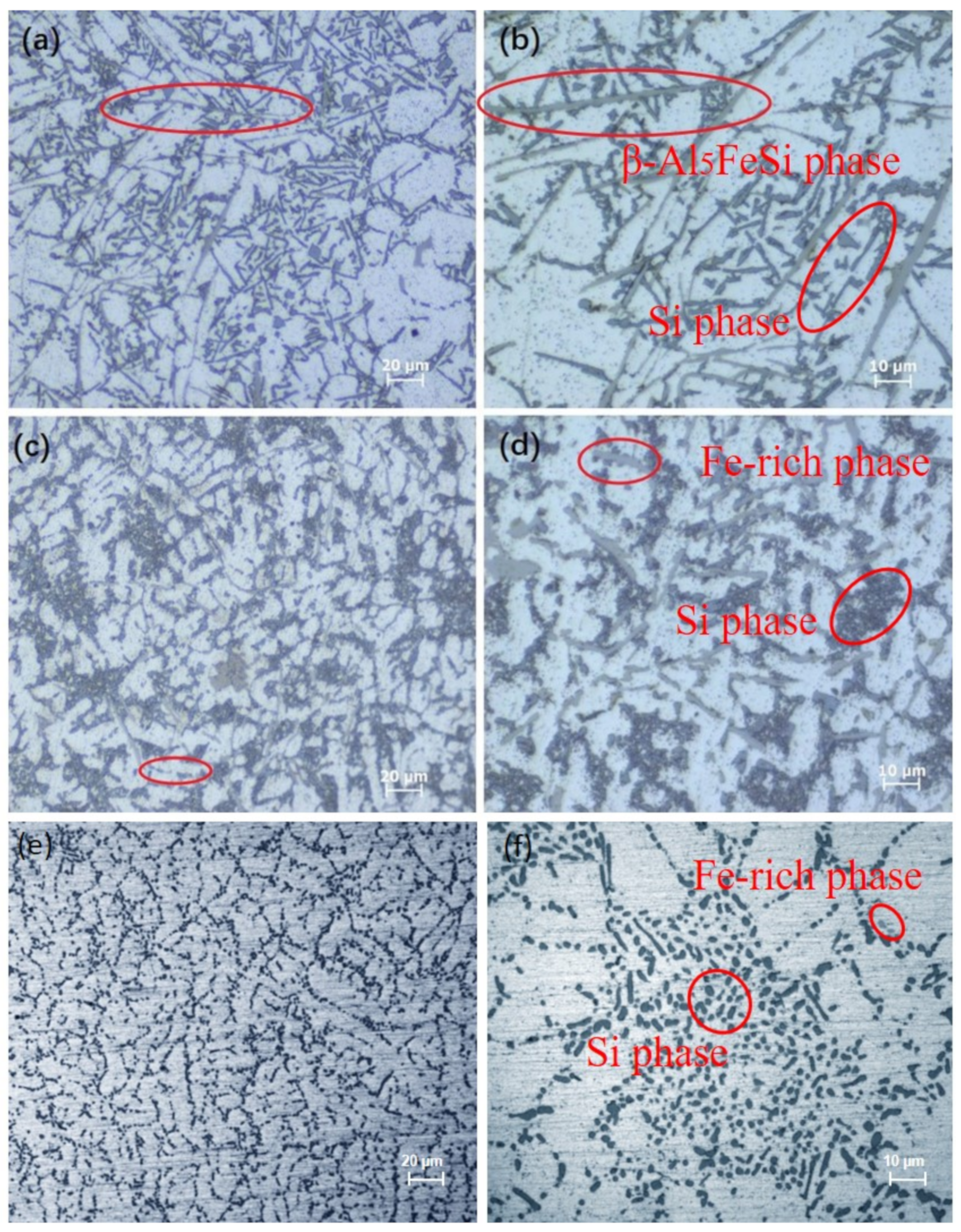
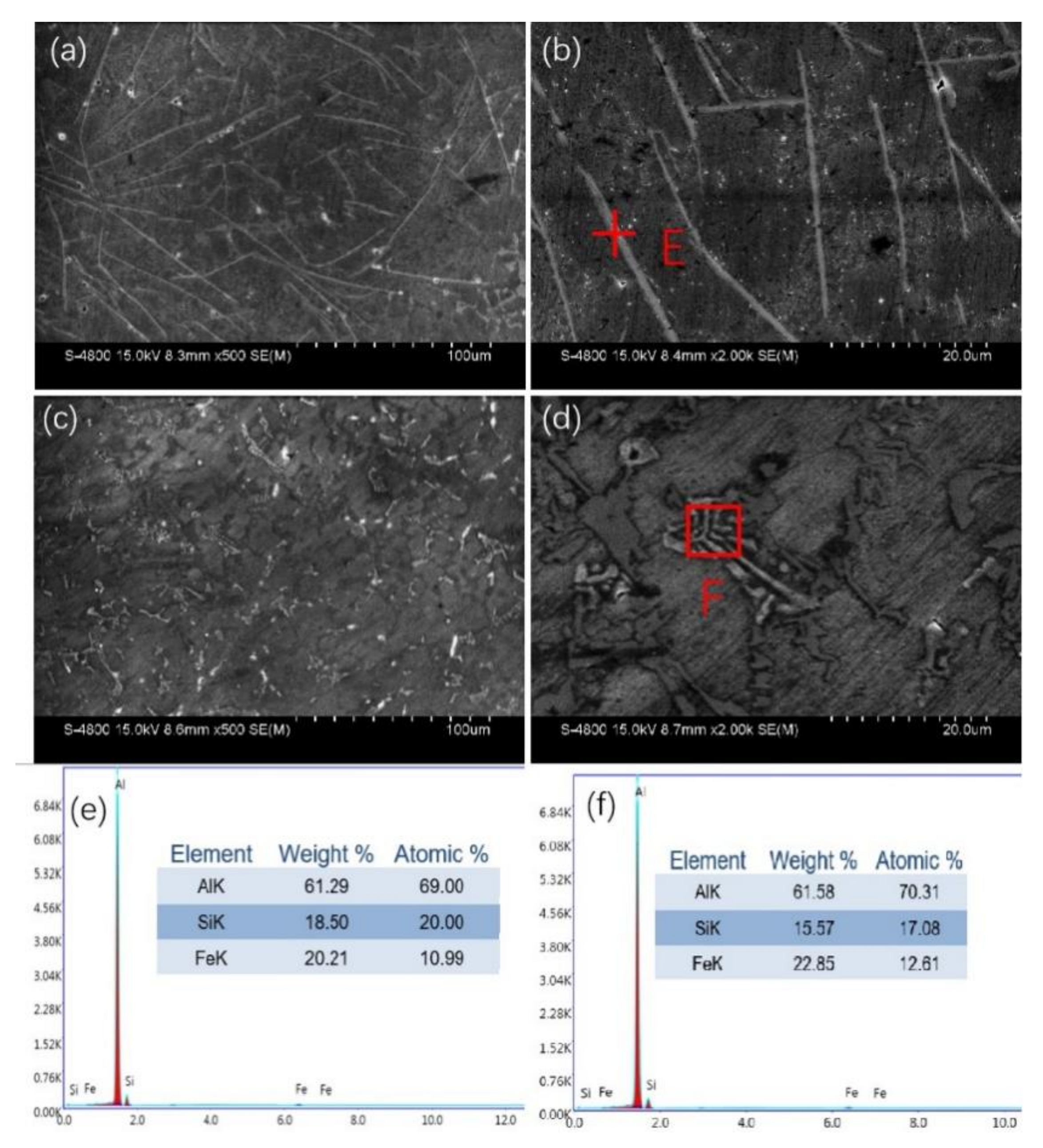
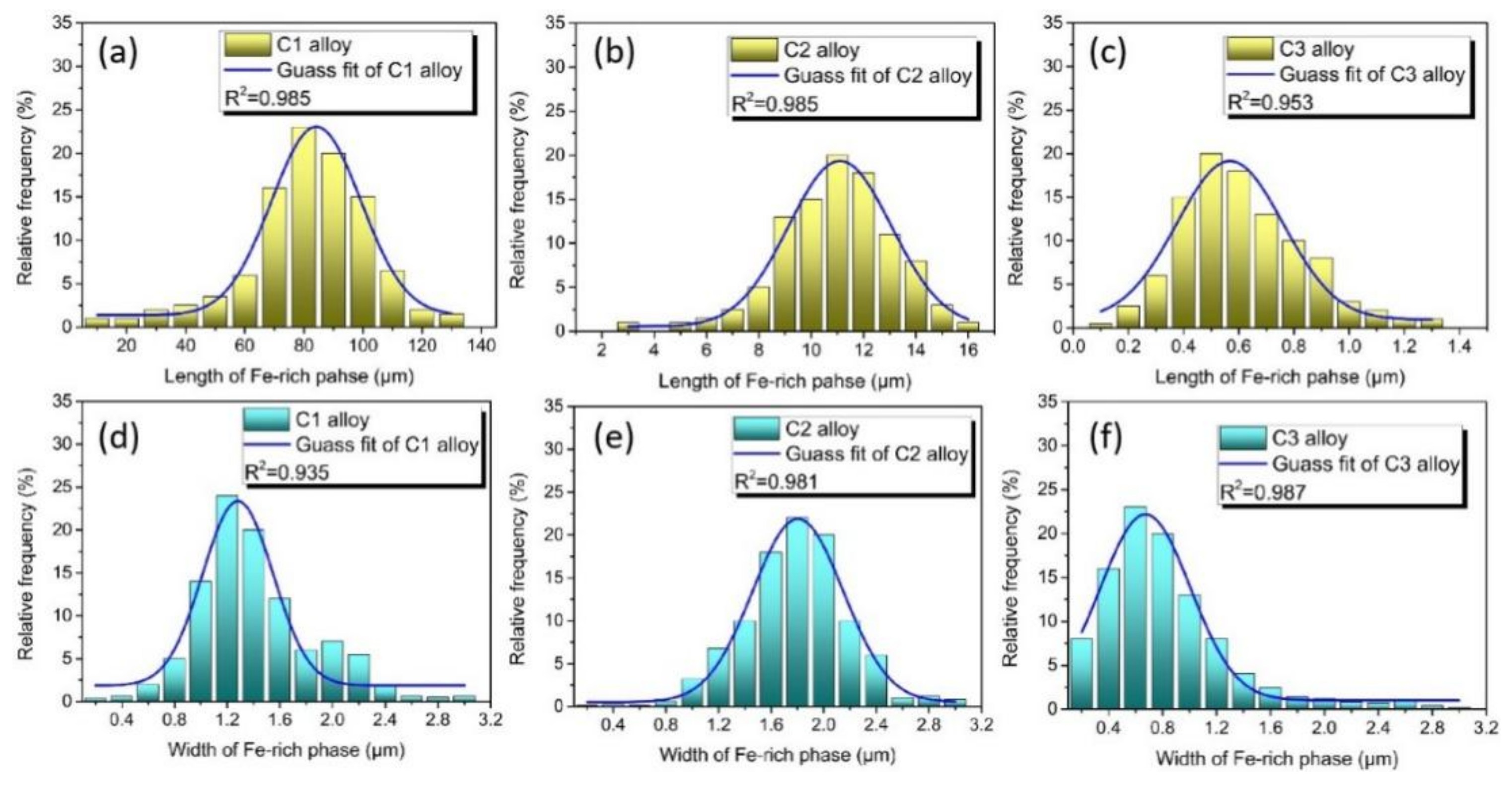
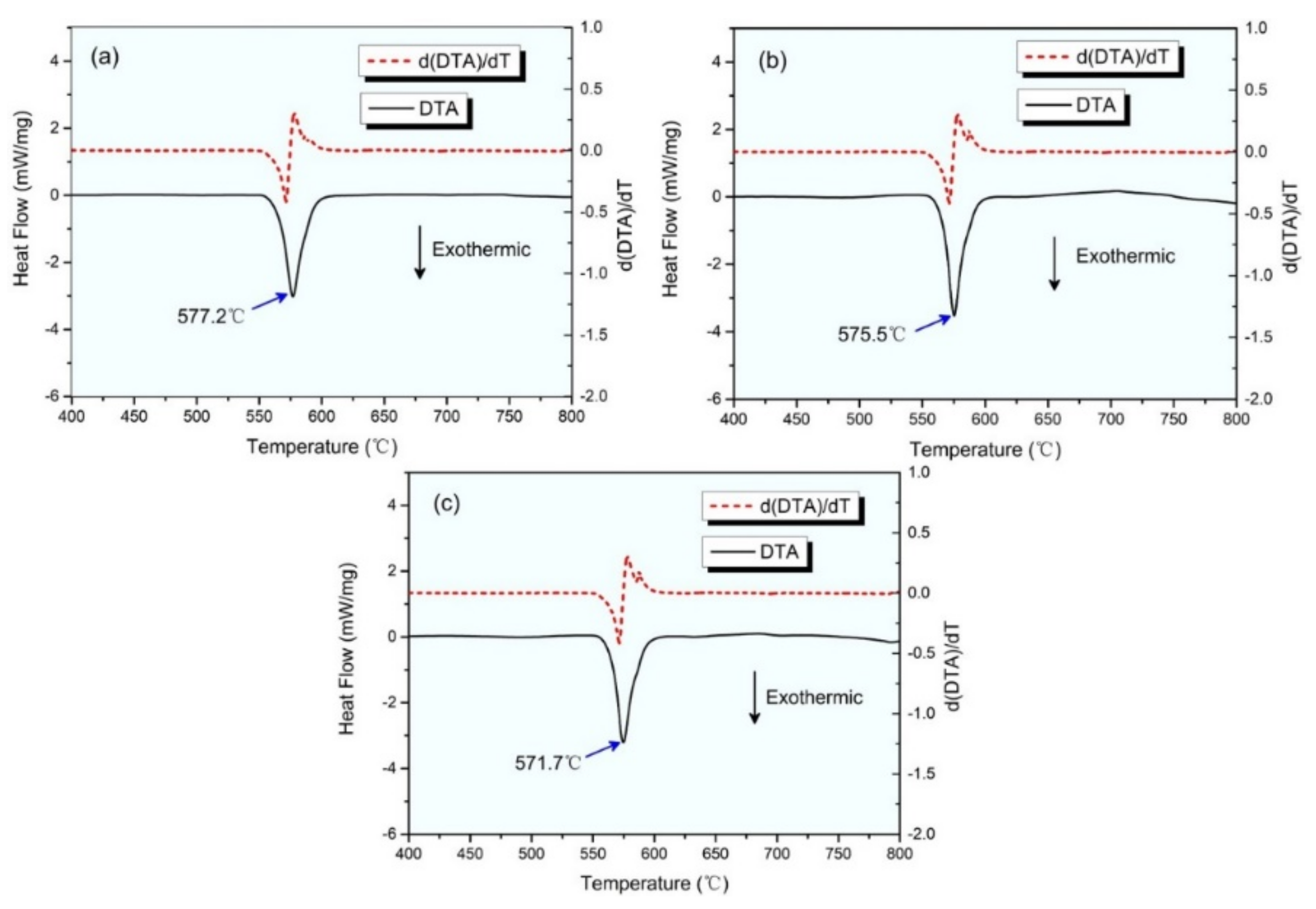
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, X.; Blake, P.; Dou, K.; Ji, S. Strengthening die-cast Al-Mg and Al-Mg-Mn alloys with Fe as a beneficial element. Mater. Sci. Eng. A 2018, 732, 240–250. [Google Scholar] [CrossRef]
- Puncreobutr, C.; Lee, P.D.; Karech, K.M.; Connolley, T.; Fife, J.L.; Phillion, A.B. Influence of Fe-rich intermetallics on solidification defects in Al-Si-Cu alloys. Acta Mater. 2014, 68, 42–51. [Google Scholar] [CrossRef]
- Cameron, M.D.; John, A.T.; Arne, K.D. As-cast morphology of iron-intermetallics in Al-Si foundry alloys. Scripta Mater. 2005, 53, 955–958. [Google Scholar]
- Terzi, S.; Taylor, J.A.; Cho, Y.H.; Salvo, L.; Suery, M.; Boller, E.; Dahle, A.K. In situ study of nucleation and growth of the irregular α-Al/β-AlFeSi eutectic by 3D synchrotron X-ray microtomography. Acta Mater. 2010, 58, 5370–5380. [Google Scholar] [CrossRef]
- Feng, S.K.; Liotti, E.; Lui, A.; Matthew, D.; Connolley, T.; Mathiesen, R.; Patrick, S. In-situ X-ray radiography of primary Fe-rich intermetallic compound formation. Acta Mater. 2020, 196, 759–769. [Google Scholar] [CrossRef]
- Que, Z.P.; Wang, Y.; Zhang, F. Formation of the Fe-containing intermetallic compounds during solidification of Al-5Mg-2Si-0.7Mn-1.1Fe alloy. Metall. Trans. A. 2018, 49, 2173–2181. [Google Scholar] [CrossRef] [Green Version]
- Gao, T.; Li, Z.Q.; Zhang, Y.X.; Qin, J.Y.; Liu, X.F. Evolution of Fe-rich phases in Mg melt and a novel method for separating Al and Fe from Al-Si-Fe alloys. Mater. Des. 2017, 134, 71–80. [Google Scholar] [CrossRef]
- Li, X.F.; Xia, C.J.; Wu, Y.; Chen, D.; Wang, M.L.; Ma, N.H.; Wang, H.W. Effect of Er addition on the high temperature strength of Al-Si-Cu-Ni-Mg-Fe piston alloys by T5 and T6 heat treatment. Mater. Sci. 2019, 25, 376–382. [Google Scholar]
- Wu, X.Y.; Zhang, H.R.; Zhang, F.X.; Ma, Z.; Yang, B.; Tao, T.X.; Zhang, H. Effect of cooling rate and Co content on the formation of Fe-rich intermetallics in hypoeutectic Al7Si0.3Mg alloy with 0.5%Fe. Mater. Charact. 2018, 139, 116–124. [Google Scholar] [CrossRef]
- Tang, Q.; Zhao, J.H.; Chen, J.; He, K. The effects of neodymium addition on the intermetallic microstructure and mechanical properties of Al-7Si-0.3Mg-0.3Fe alloys. J. Alloys Compd. 2018, 741, 161–173. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Feng, J.; Zuo, L.J.; Ye, B.; Kong, X.Y.; Jiang, H.Y.; Ding, W.J. Effect of Sc microalloying addition on microstructure and mechanical properties of as-cast Al-12Si alloy. Mater. Sci. Eng. A 2019, 24, 138343. [Google Scholar] [CrossRef]
- Khan, M.H.; Das, A.; Li, Z.; Kotadia, H.R. Effects of Fe, Mn, chemical grain refinement and cooling rate on the evolution of Fe intermetallics in a model 6082 Al-alloy. Intermetallics 2021, 132, 107132. [Google Scholar] [CrossRef]
- Li, D.F.; Cui, C.X.; Wang, X.; Wang, Q.Z.; Chen, C.; Liu, S.Q. Microstructure evolution and enhanced mechanical properties of eutectic Al-Si die cast alloy by combined alloying Mg and La. Mater. Des. 2016, 90, 820–828. [Google Scholar] [CrossRef]
- Easton, M.A.; StJohn, D.H. Improved prediction of the grain size of aluminum alloys that includes the effect of cooling rate. Mater. Sci. Eng. A 2008, 486, 8–13. [Google Scholar] [CrossRef]
- Verma, A.; Kumar, S.; Grant, P.S.; O’Reilly, K.A.Q. Influence of cooling rate on the Fe intermetallic formation in an AA6063 Al alloy. J. Alloys Compd. 2013, 555, 274–282. [Google Scholar] [CrossRef]
- Kilicaslan, M.F.; Altaib, S.S.; Vurdu, C.D. Effect of Ni Addition on the Morphology and Microstructure of Both Conventional Cast and Melt-Spun of Al-Si-Fe-Nb (at wt%) Alloy. Met. Mater. Int. 2019, 25, 1457–1466. [Google Scholar] [CrossRef]
- Rajabi, M.; Simchia, A.; Davamia, P. Microstructure and mechanical properties of Al-20Si-5Fe-2X (X=Cu, Ni, Cr) alloys produced by melt-spinning. Mater. Sci. Eng. A 2008, 492, 443–449. [Google Scholar] [CrossRef]
- Liu, Z.T.; Wang, B.Y.; Wang, C.; Zha, M.; Liu, G.J.; Yang, Z.Z.; Wang, J.G.; Li, J.H.; Wang, H.Y. Microstructure and mechanical properties of Al-Mg-Si alloy fabricated by a short process based on sub-rapid solidification. J. Mater. Sci. Technol. 2020, 41, 178–186. [Google Scholar] [CrossRef]
- Liu, S.Q.; Wang, X.; Cui, C.X.; Zhao, L.C.; Liu, S.J.; Chen, C. Fabrication, microstructure and refining mechanism of in situ CeB6/Al inoculant in aluminum. Mater. Des. 2015, 65, 432–437. [Google Scholar] [CrossRef]
- Liu, S.Q.; Wang, X.; Cui, C.X.; Zhao, L.C. Enhanced grain refinement of in situ CeB6/Al composite inoculant on pure aluminum by microstructure control. J. Alloys Compd. 2017, 701, 926–934. [Google Scholar] [CrossRef]
- Liu, S.Q.; Wang, X.; Cui, C.X.; Han, X.; Cui, C. Significantly improved particle strengthening of Al-Sc alloy by high Sc composition design and rapid solidification. Mater. Sci. Eng. A 2021, 800, 140304. [Google Scholar] [CrossRef]
- Liu, S.Q.; Cui, C.X.; Wang, X.; Li, N.; Cui, S. Effect of cooling rate on microstructure and grain refining behavior of in situ CeB6/Al composite inoculant in aluminum. Metals 2017, 7, 204. [Google Scholar] [CrossRef] [Green Version]
- Han, B.H.; Liu, S.Q.; Wang, X.; Cui, C.X. Simultaneously improving strength and ductility of hybrid Al-Si matrix composite with polyphasic and multi-scale ceramic particles reinforced. Mater. Sci. Eng. A 2021, 804, 140517. [Google Scholar] [CrossRef]
- Wu, X.F.; Wang, Z.C.; Wang, K.Y.; Zhao, R.D.; Wu, F.F. Microstructural refinement and tensile properties enhancement of Al-10Mg2Si cast alloys by copper addition. J. Alloys Compd. 2022, 896, 163058. [Google Scholar] [CrossRef]
- Azimi, H.; Nourouzi, S.; Jamatti, R. Effects of Ti particles and T6 heat treatment on the microstructure and mechanical properties of A356 alloy fabricated by compocasting. Mater. Sci. Eng. A 2021, 818, 141443. [Google Scholar] [CrossRef]
- Liu, T.; Pei, Z.R.; Barton, D.; Brewer, L.N. Characterization of nanostructures in a high pressure die cast Al-Si-Cu alloy. Acta Mater. 2022, 224, 117500. [Google Scholar] [CrossRef]
- Bogdanoff, T.; Lattanzi, L.; Merlin, M.; Ghassemali, E.; Jarfors, A.E.W.; Senfeddine, S. The complex interaction between microstructural features and crack evolution during cyclic testing in heat-treated Al–Si–Mg–Cu cast alloys. Mater. Sci. Eng. A 2021, 825, 141930. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.H.; Jeon, J.Y.; Kim, Y.W.; Kobayashi, E. Local elongation of a high Fe-containing Al-Si-Cu-Mg alloy by a deformation-semisolid extrusion process. Mater. Lett. 2021, 309, 131337. [Google Scholar] [CrossRef]
- Que, Z.P.; Mendis, C.L. Heterogeneous nucleation and phase transformation of Fe-rich intermetallic compounds in Al–Mg–Si alloys. J. Alloys Compd. 2020, 836, 155515. [Google Scholar] [CrossRef]


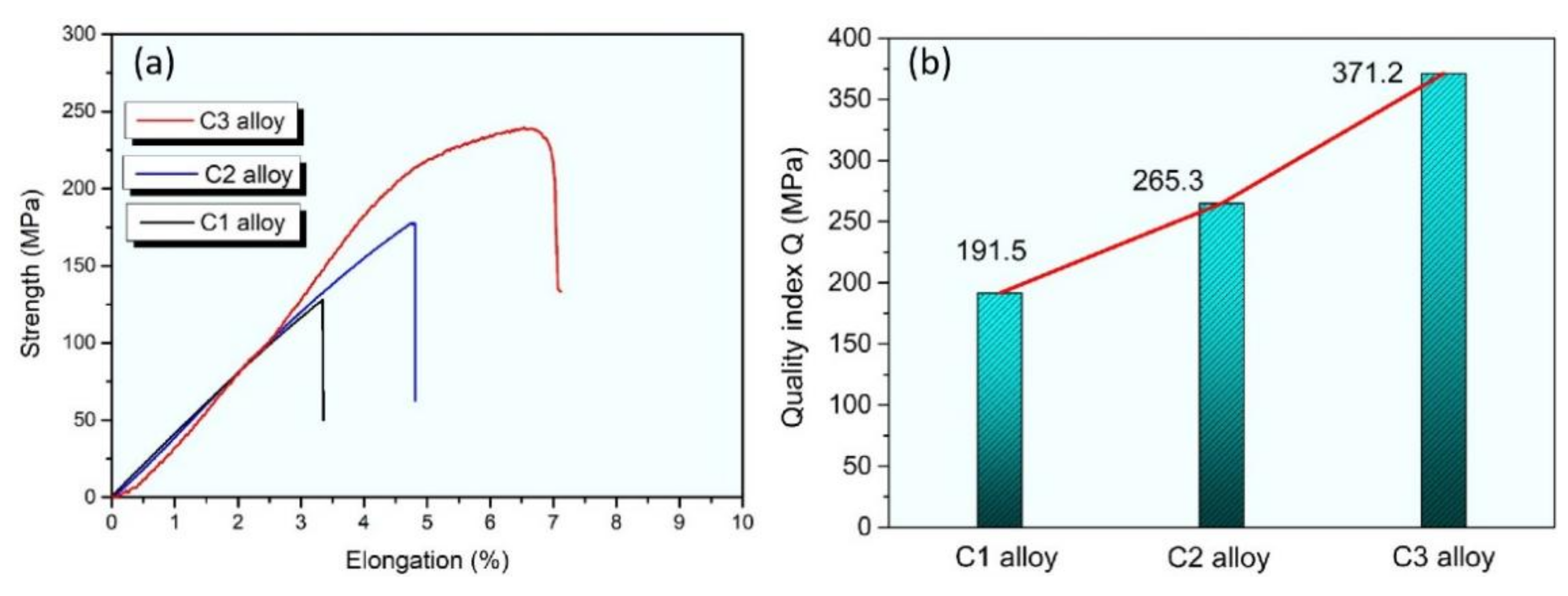
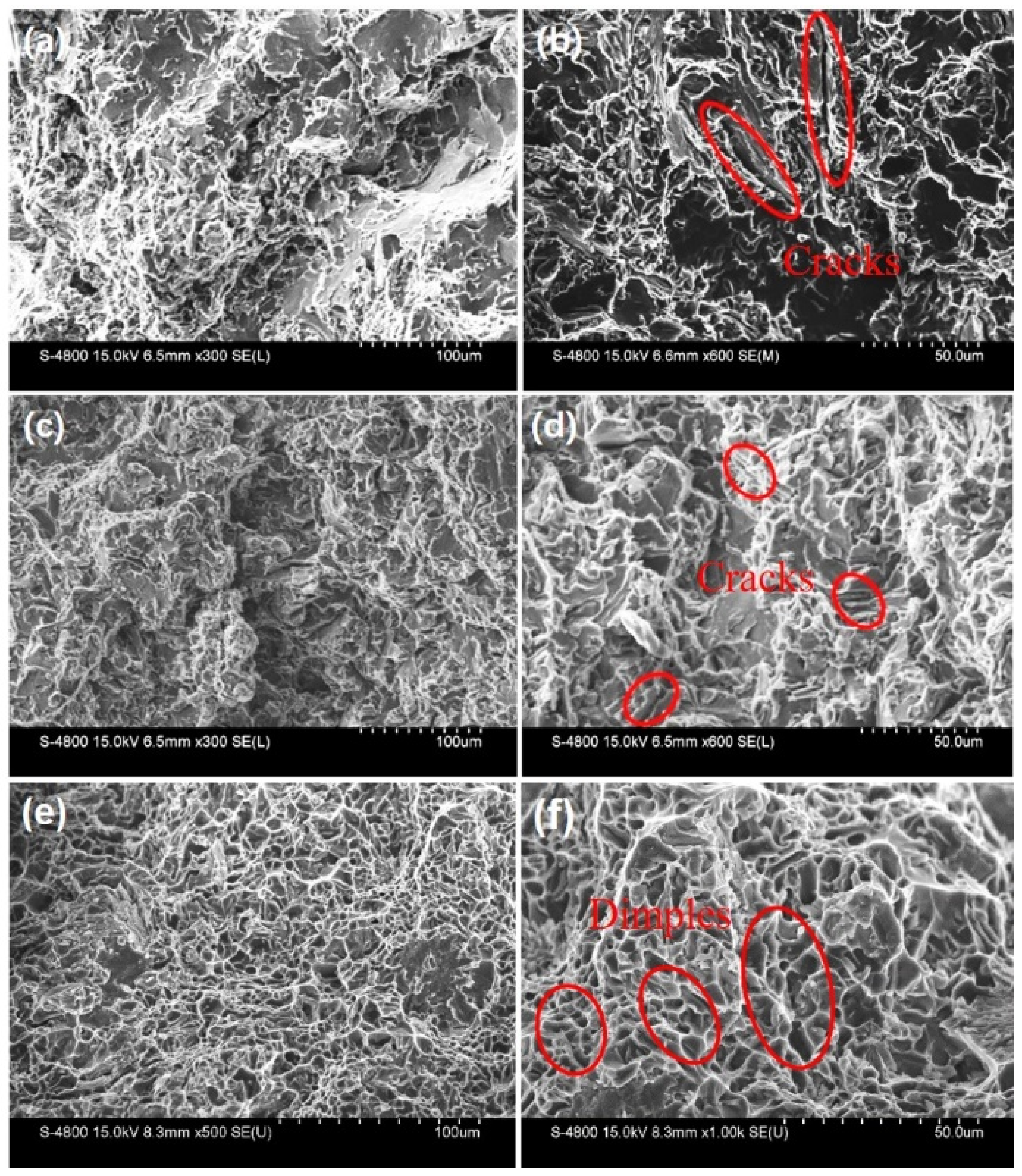
| Element | Al | Si | Fe | Mg | Cu | Zn |
|---|---|---|---|---|---|---|
| Content | Balance | 9.96 | 1.52 | 0.33 | 0.09 | 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, X.; Liu, S.; Wang, X.; Cui, C.; Gong, P.; Zhao, L.; Han, X.; Li, Z. Effect of Cooling Rate on the Microstructure Evolution and Mechanical Properties of Iron-Rich Al–Si Alloy. Materials 2022, 15, 411. https://doi.org/10.3390/ma15020411
Shen X, Liu S, Wang X, Cui C, Gong P, Zhao L, Han X, Li Z. Effect of Cooling Rate on the Microstructure Evolution and Mechanical Properties of Iron-Rich Al–Si Alloy. Materials. 2022; 15(2):411. https://doi.org/10.3390/ma15020411
Chicago/Turabian StyleShen, Xiao, Shuiqing Liu, Xin Wang, Chunxiang Cui, Pan Gong, Lichen Zhao, Xu Han, and Zirui Li. 2022. "Effect of Cooling Rate on the Microstructure Evolution and Mechanical Properties of Iron-Rich Al–Si Alloy" Materials 15, no. 2: 411. https://doi.org/10.3390/ma15020411
APA StyleShen, X., Liu, S., Wang, X., Cui, C., Gong, P., Zhao, L., Han, X., & Li, Z. (2022). Effect of Cooling Rate on the Microstructure Evolution and Mechanical Properties of Iron-Rich Al–Si Alloy. Materials, 15(2), 411. https://doi.org/10.3390/ma15020411










