Pulse Plasma Sintering of NiAl-Al2O3 Composite Powder Produced by Mechanical Alloying with Contribution of Nanometric Al2O3 Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples
2.3. Research Techniques
3. Results and Discussion
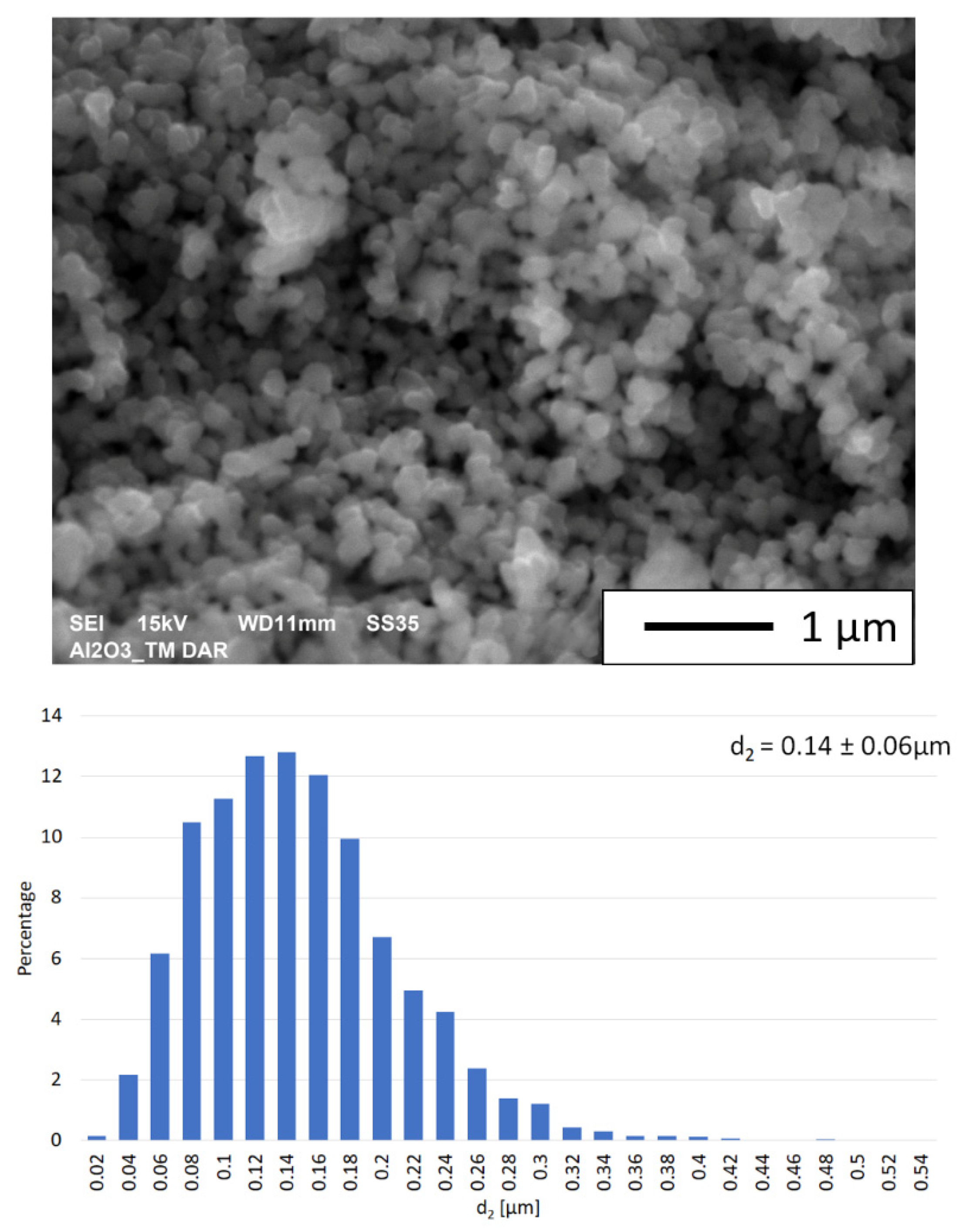
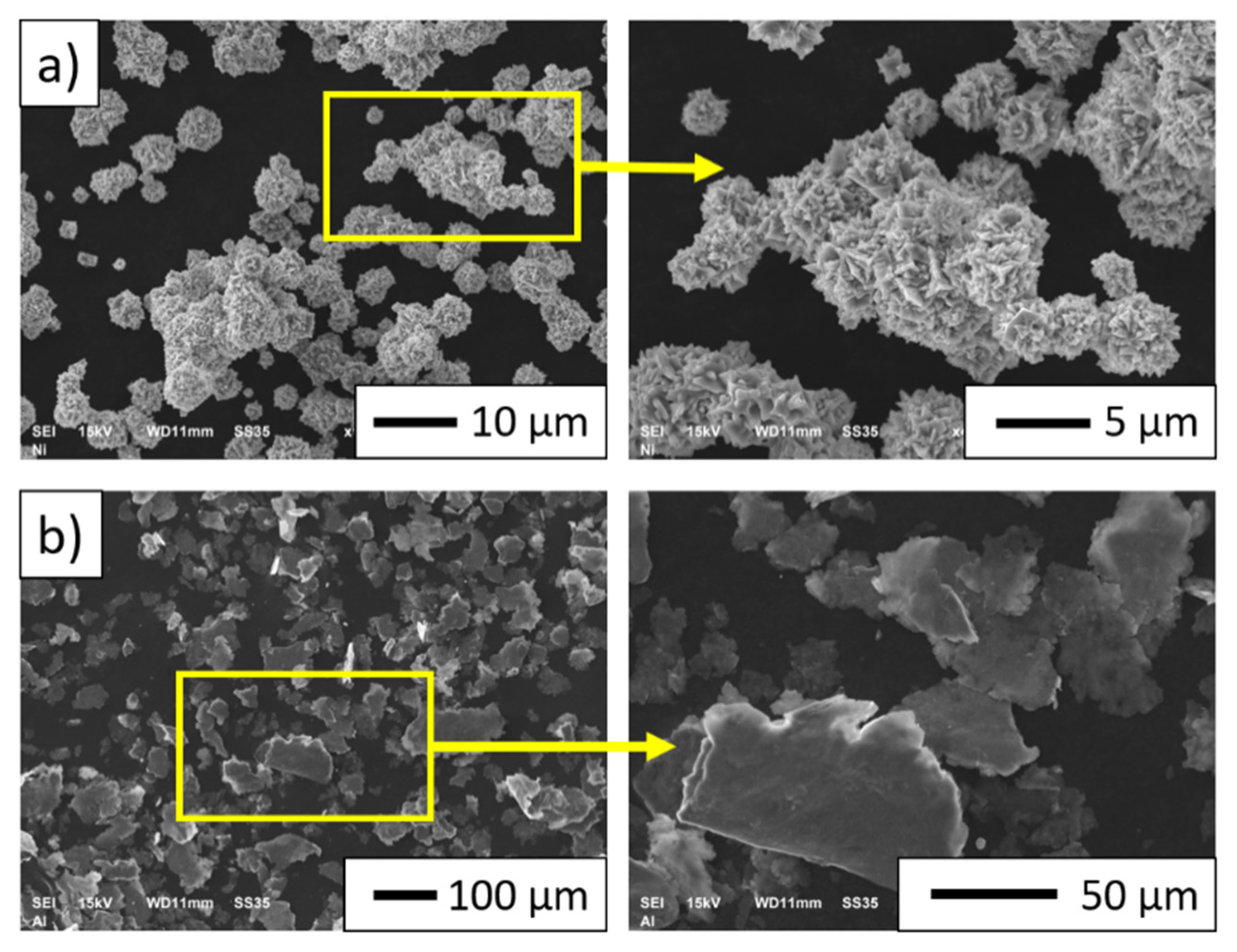
3.1. Description of the Initial Powders
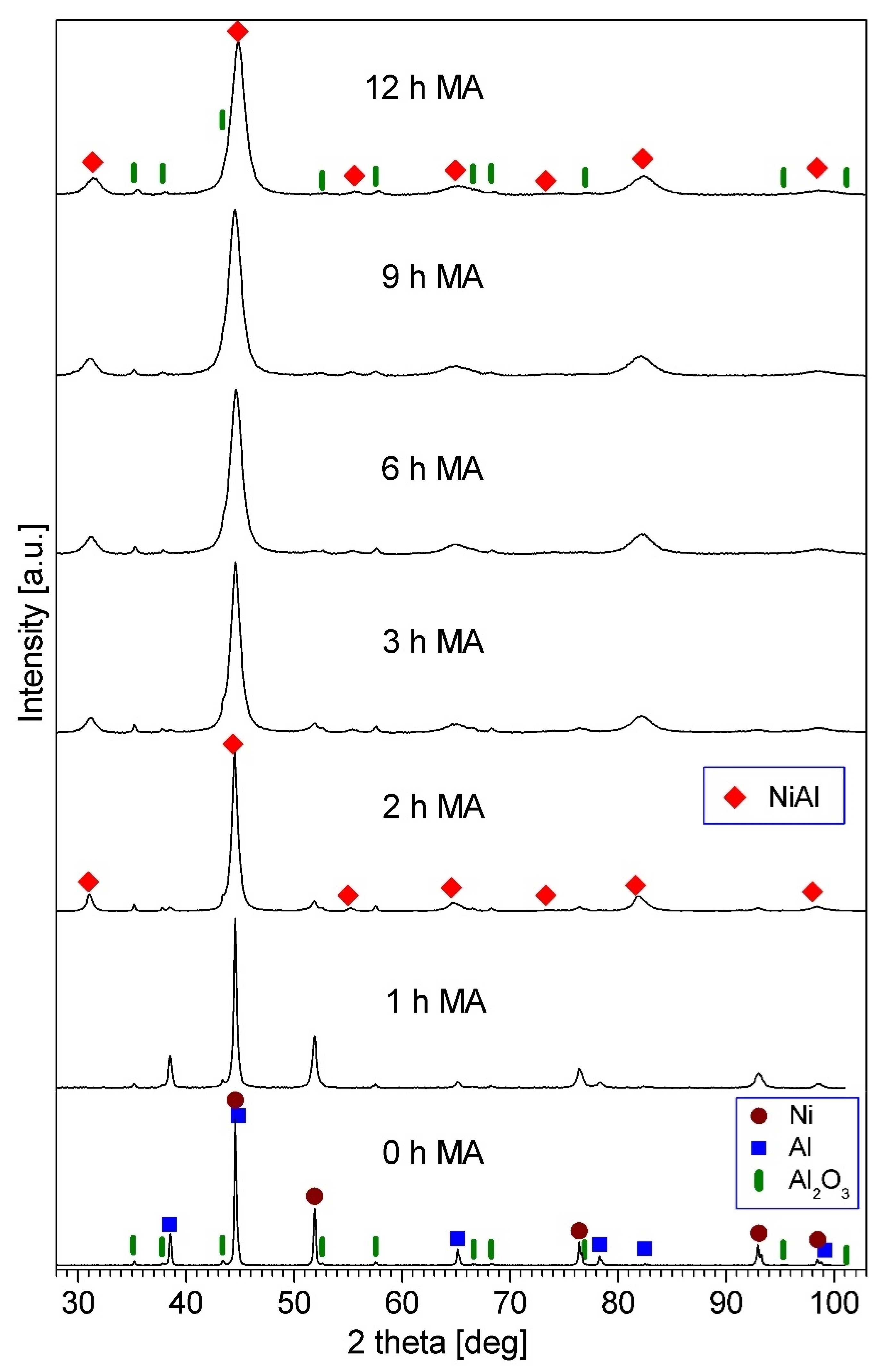
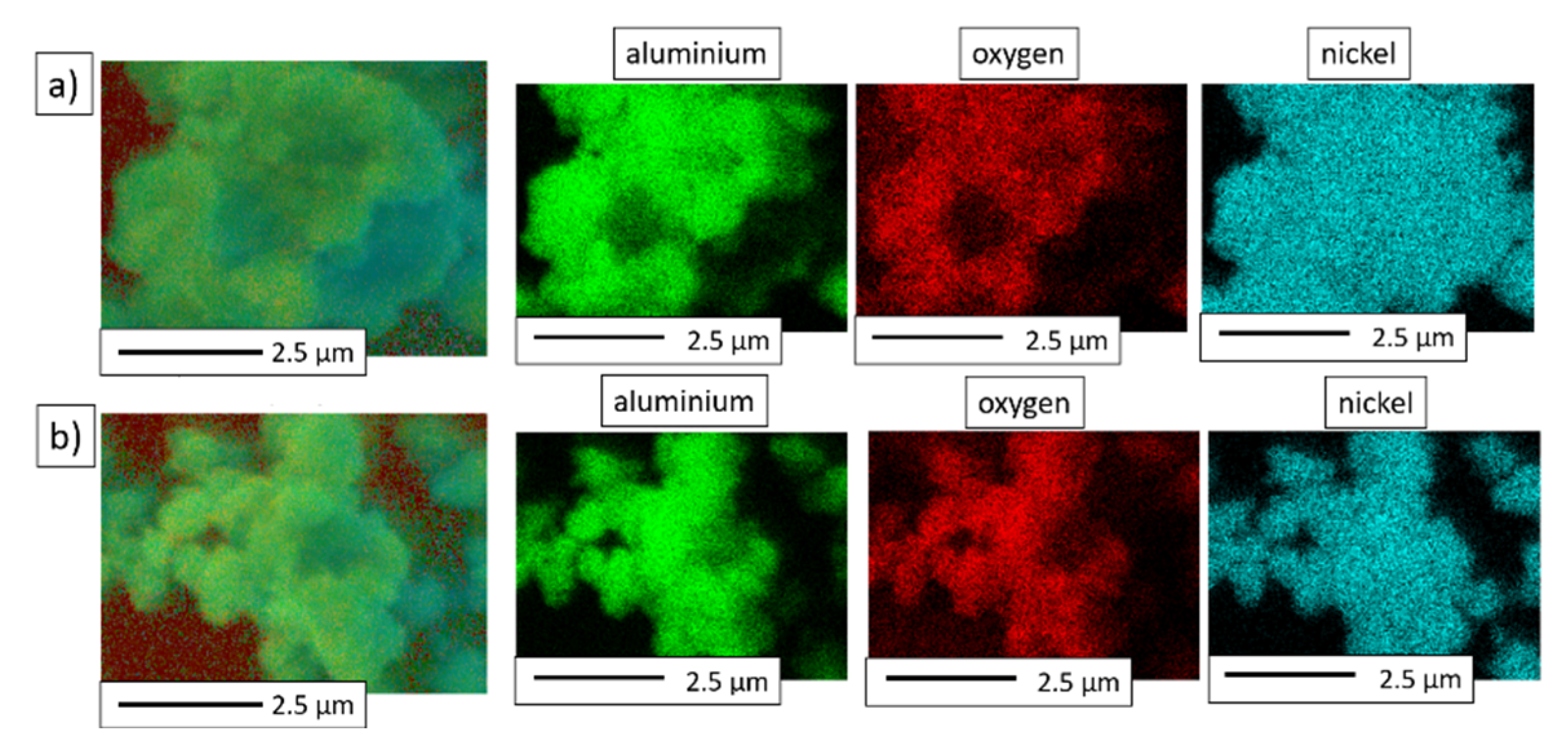
3.2. Description of NiAl-Al2O3 Composite Powders
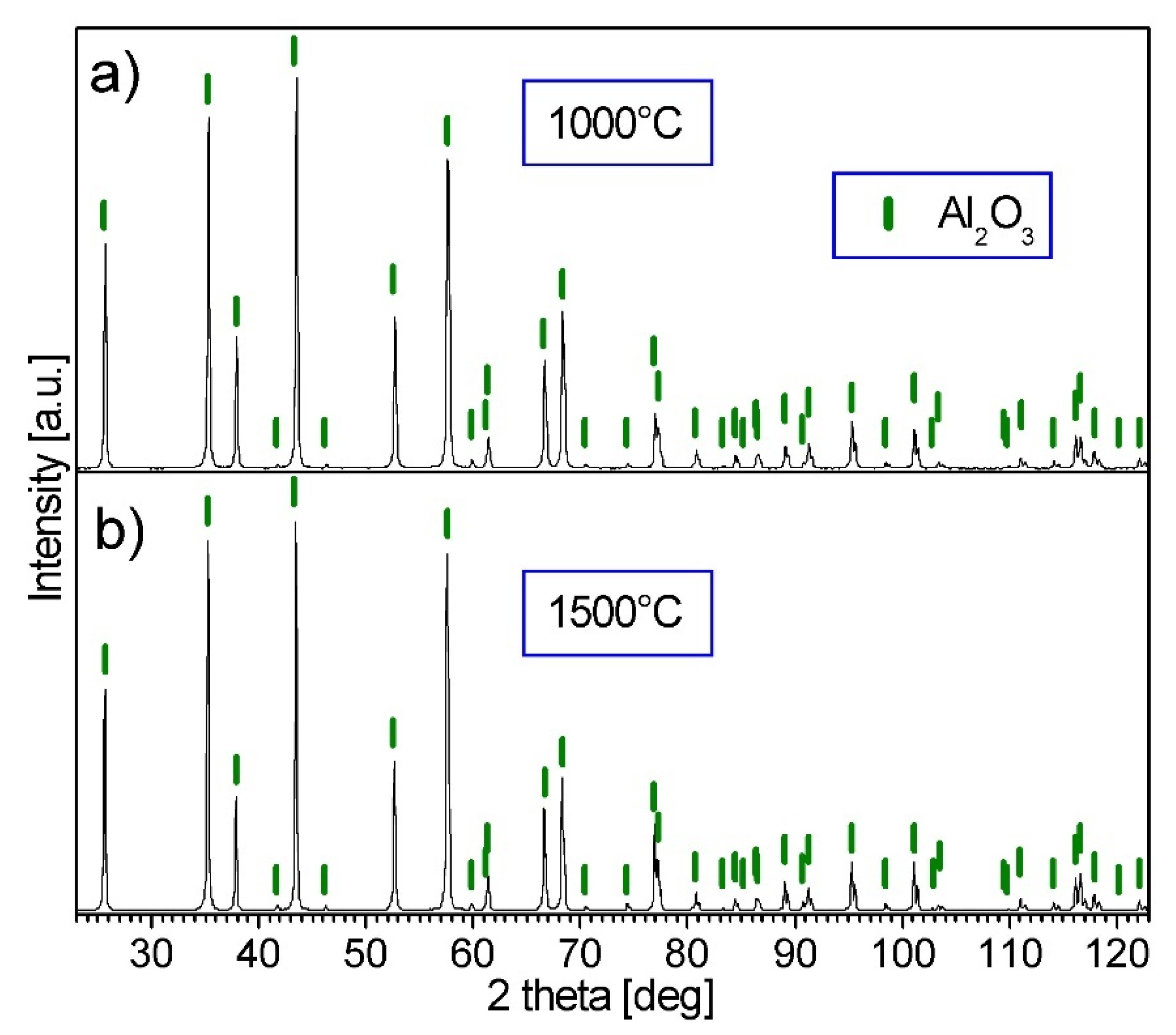
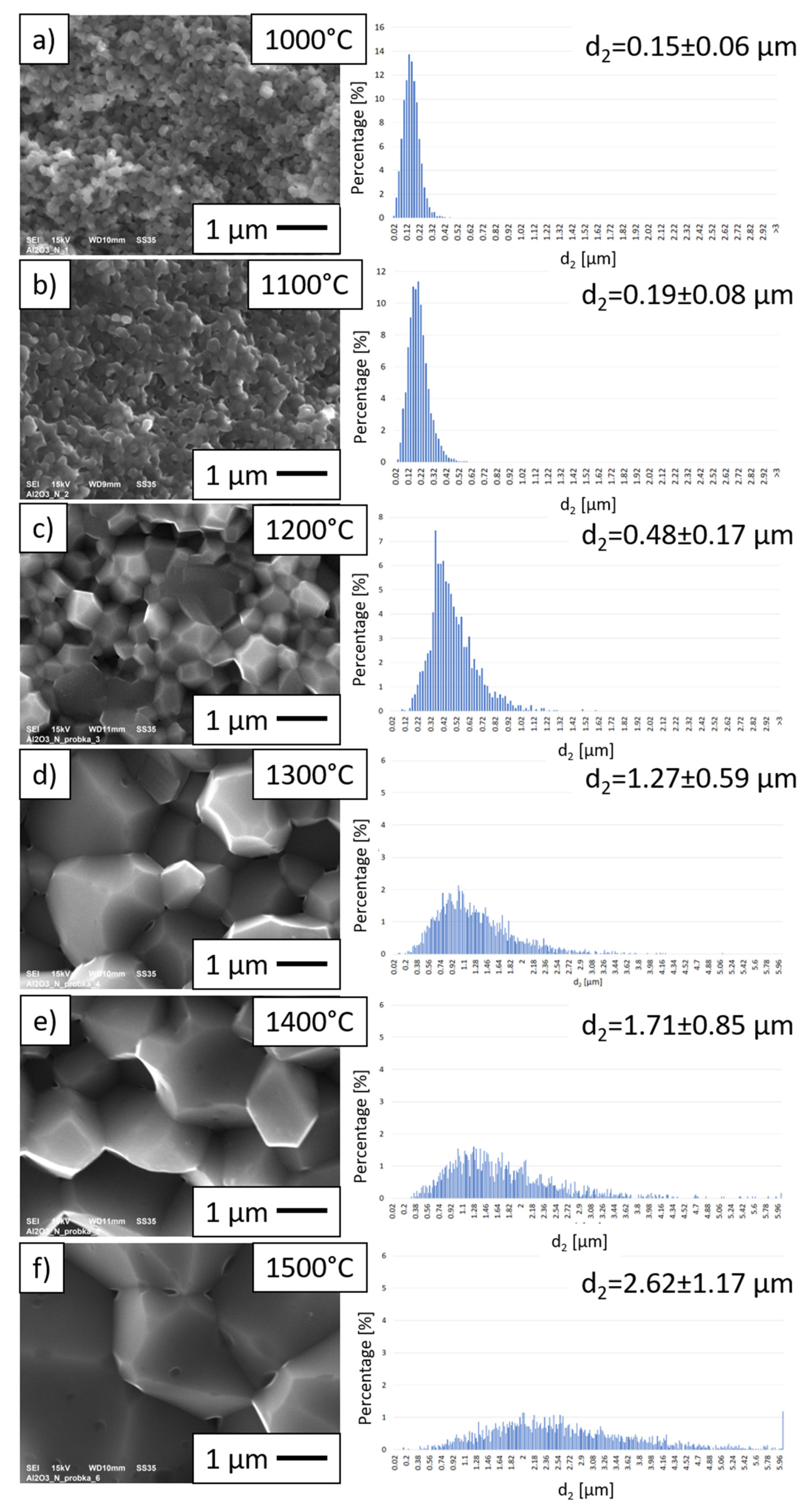
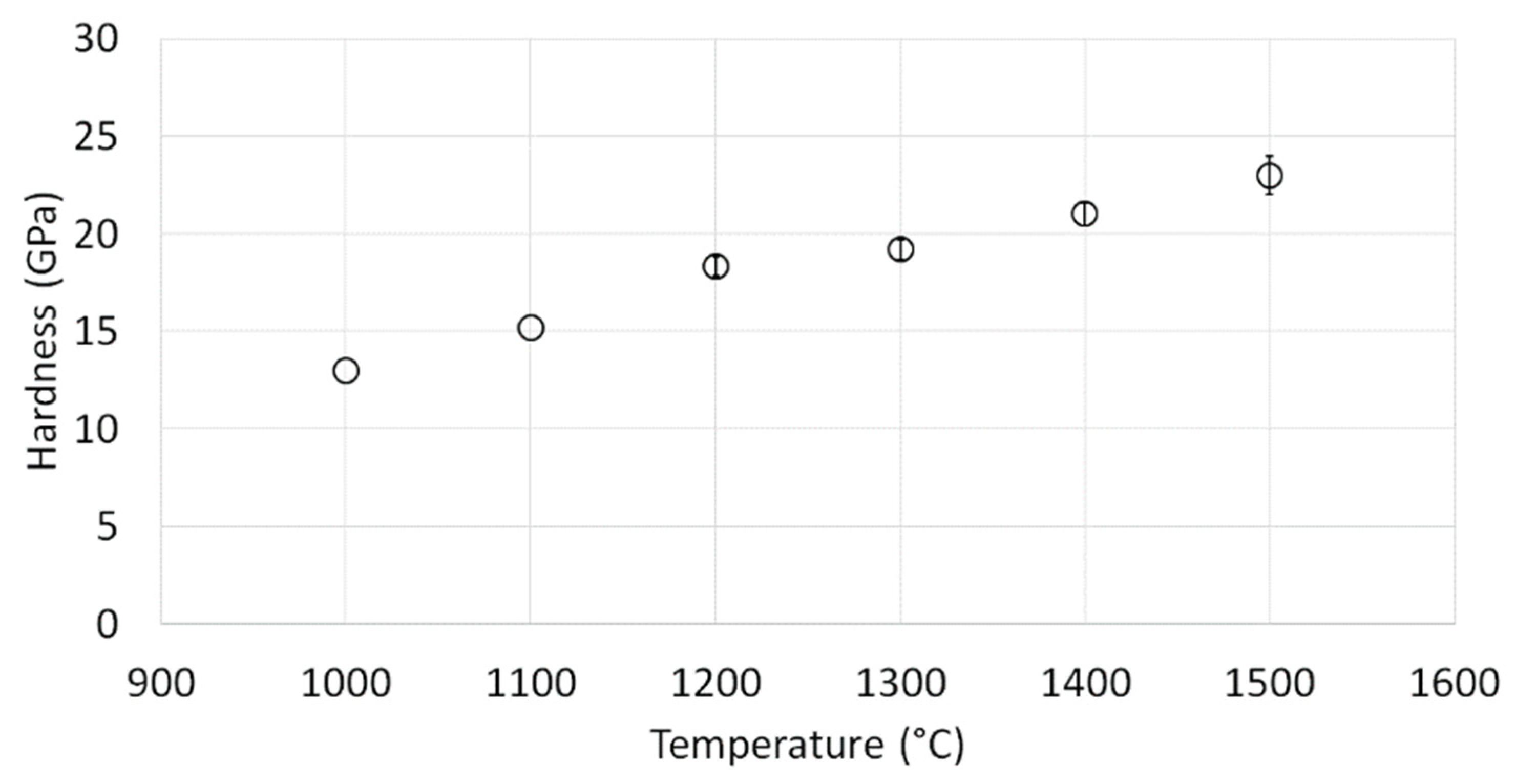
3.3. Characterization of Al2O3 Samples after PPS Consolidation
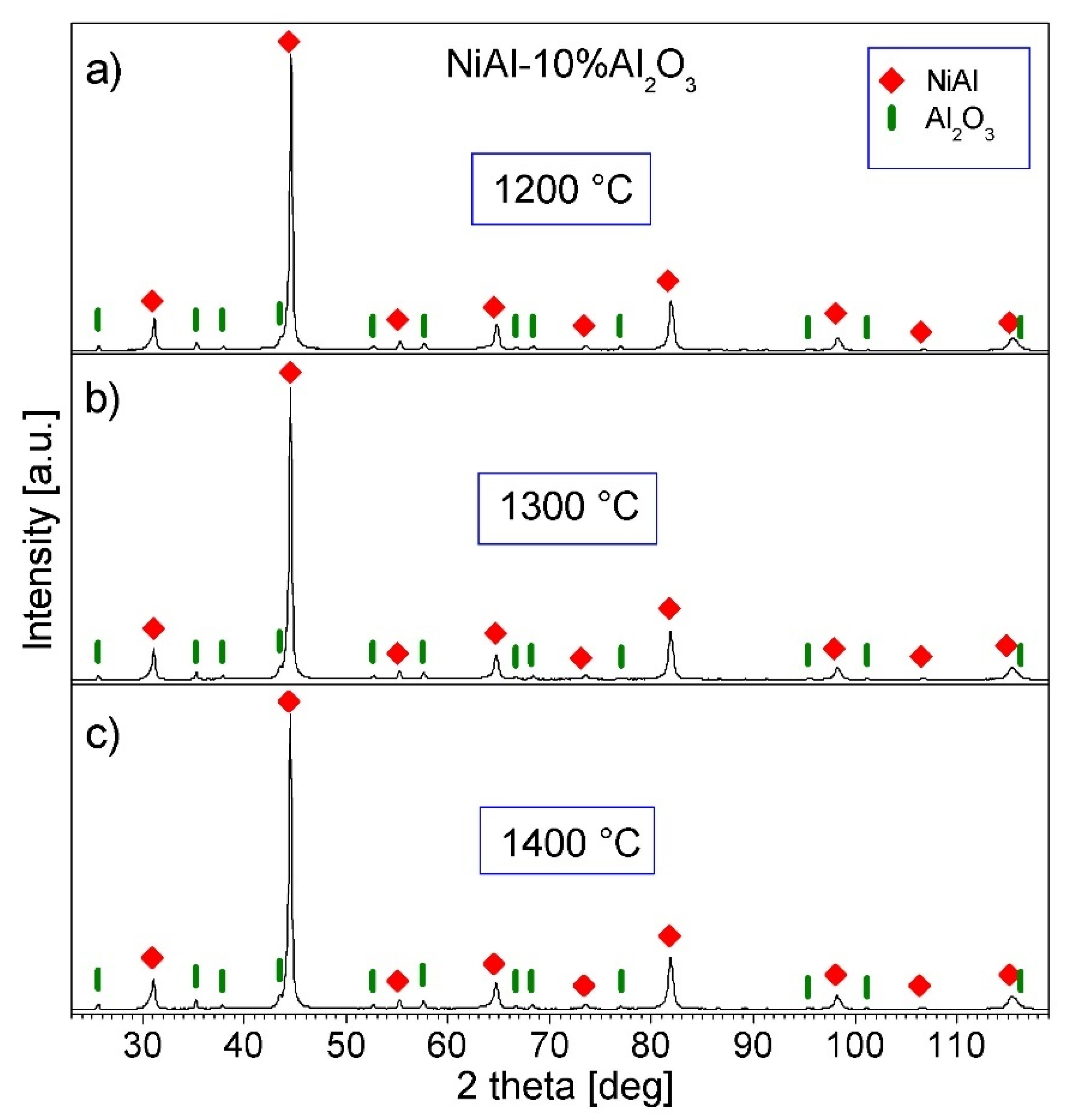
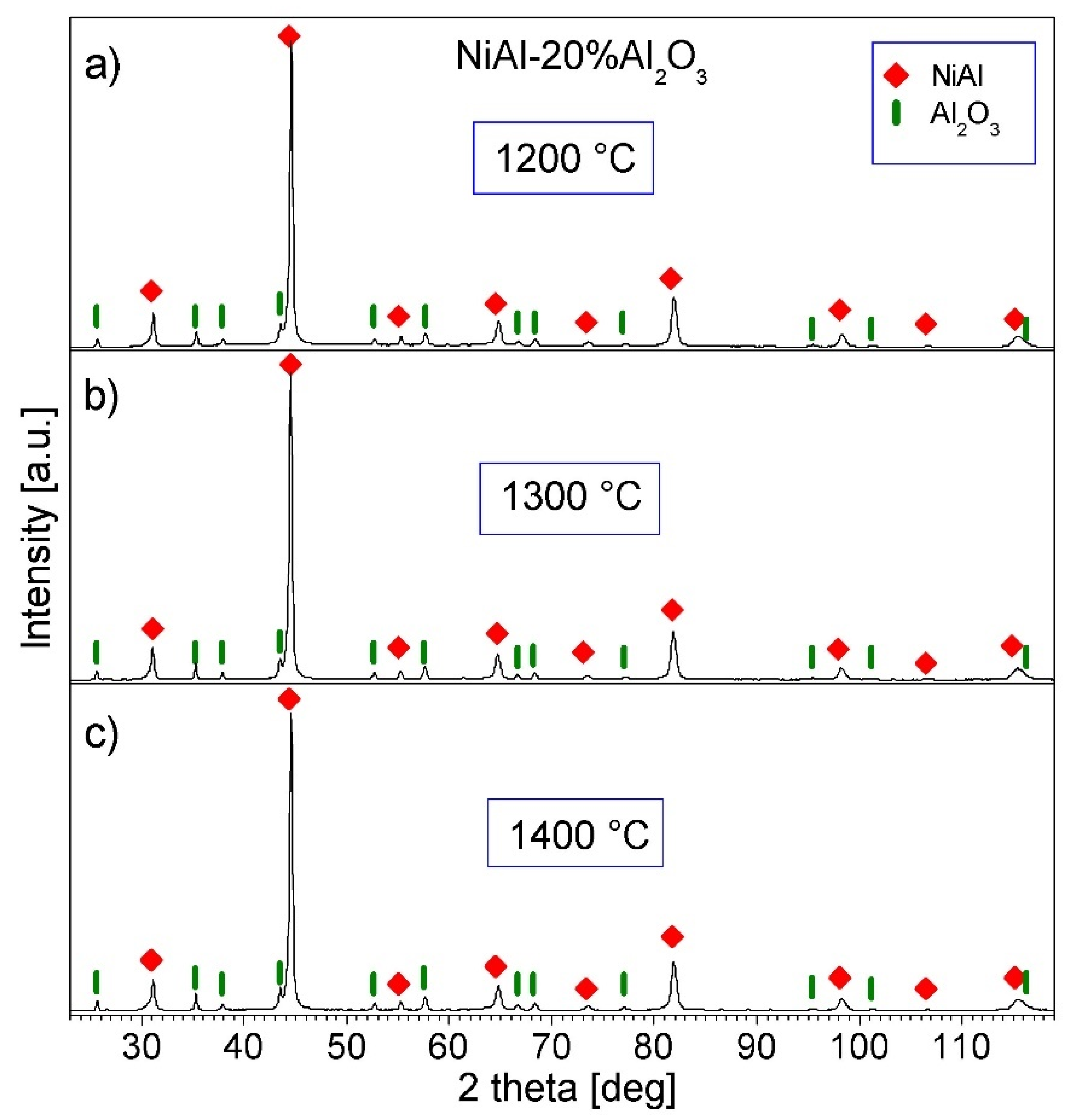
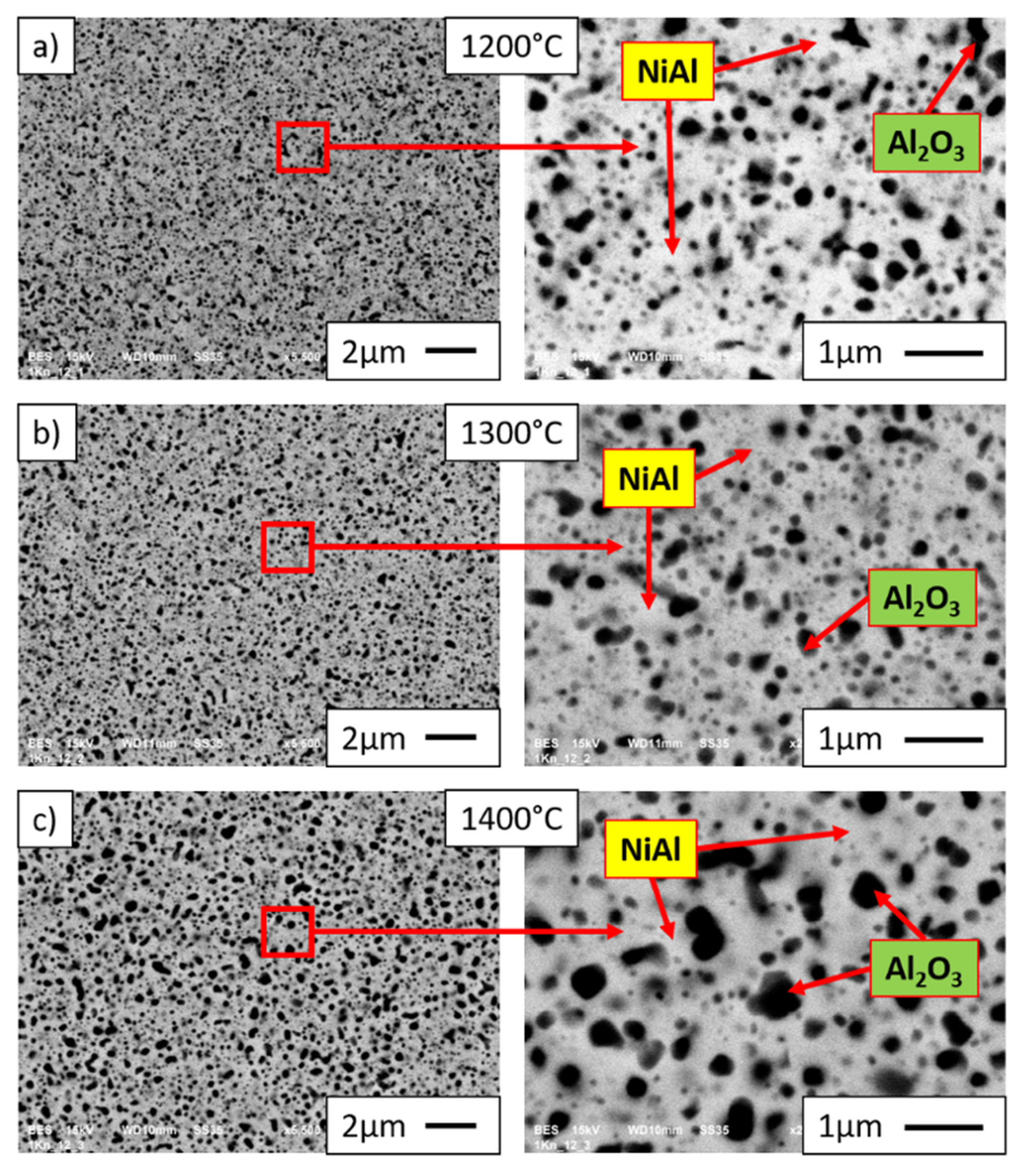
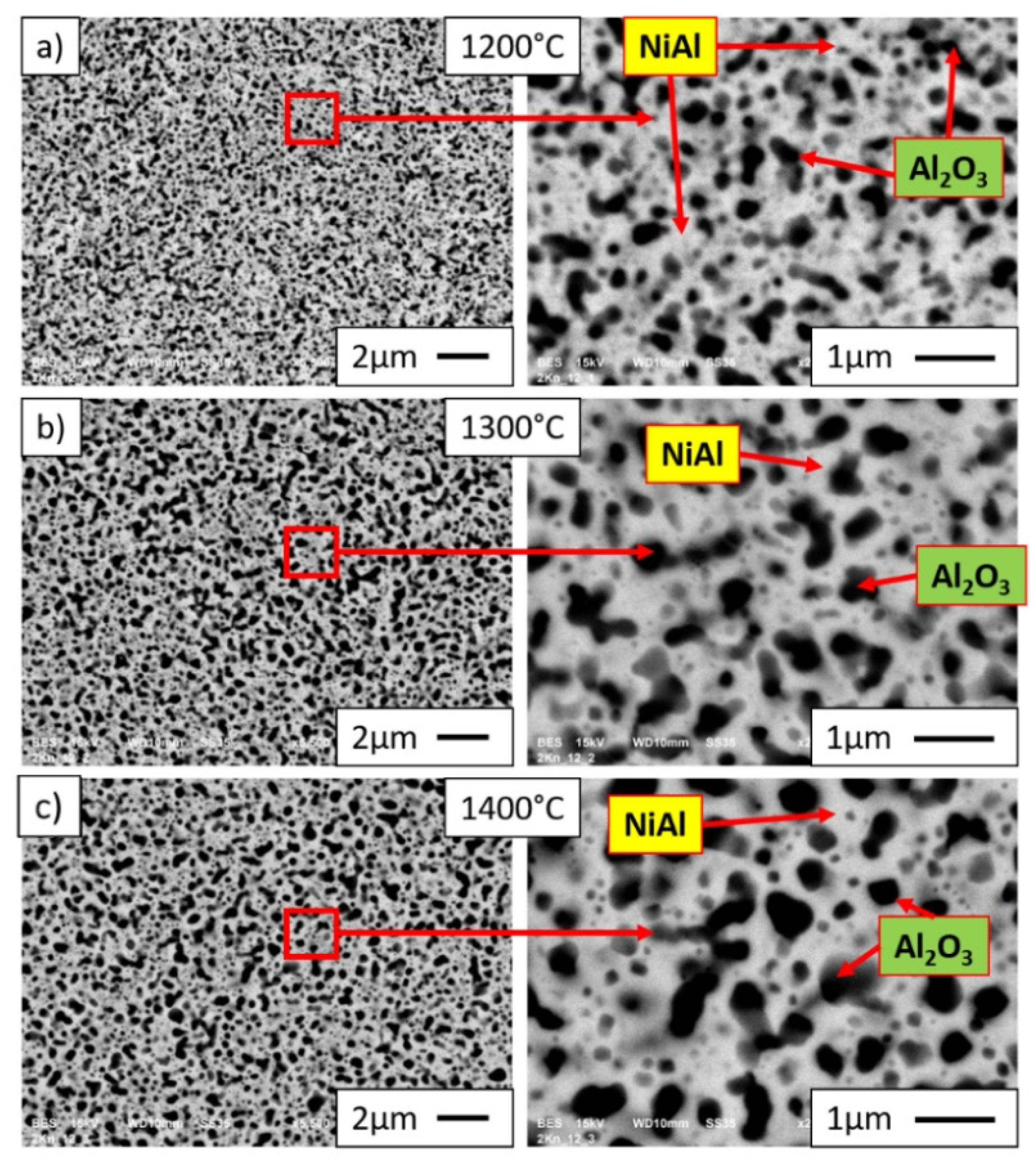
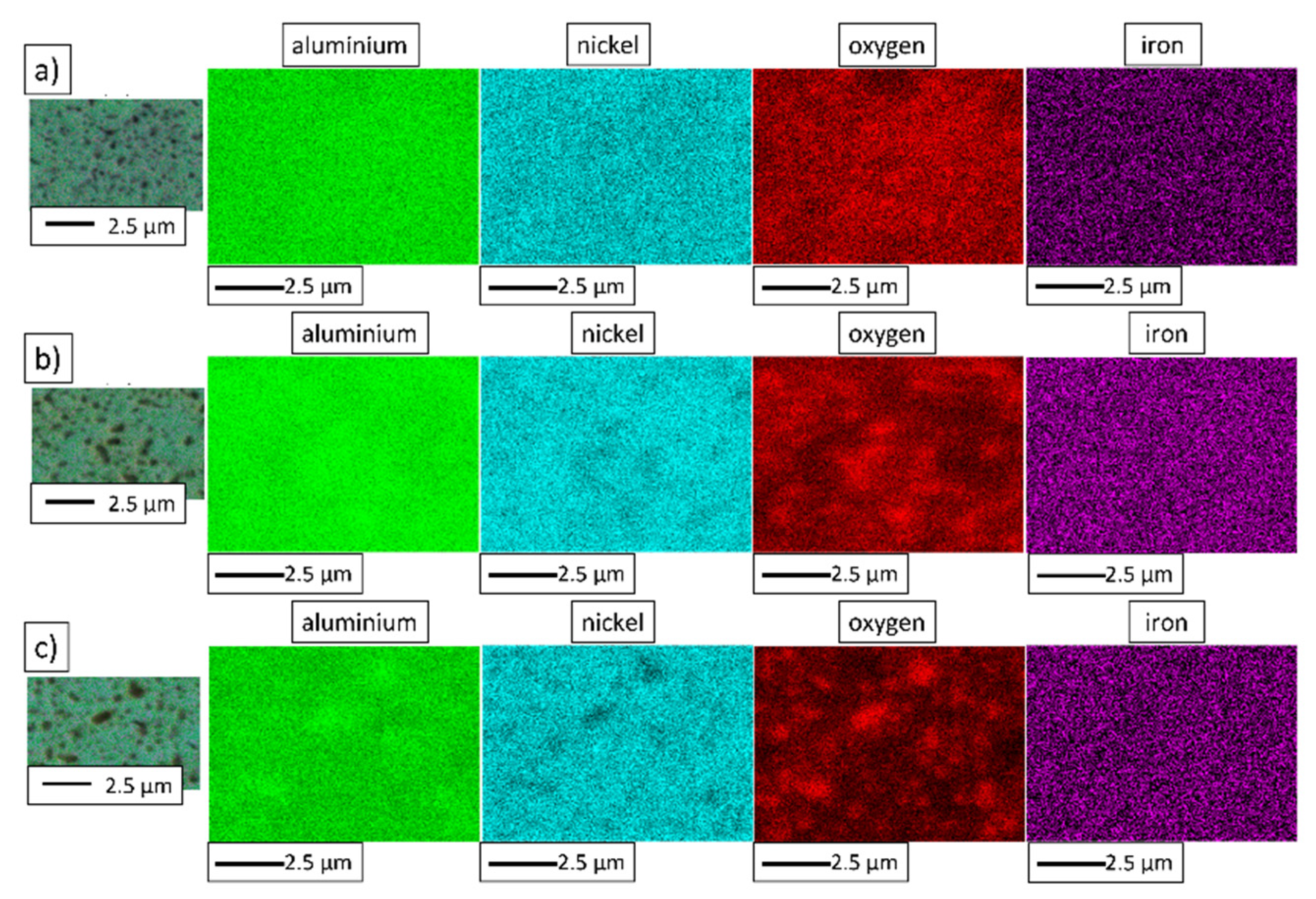
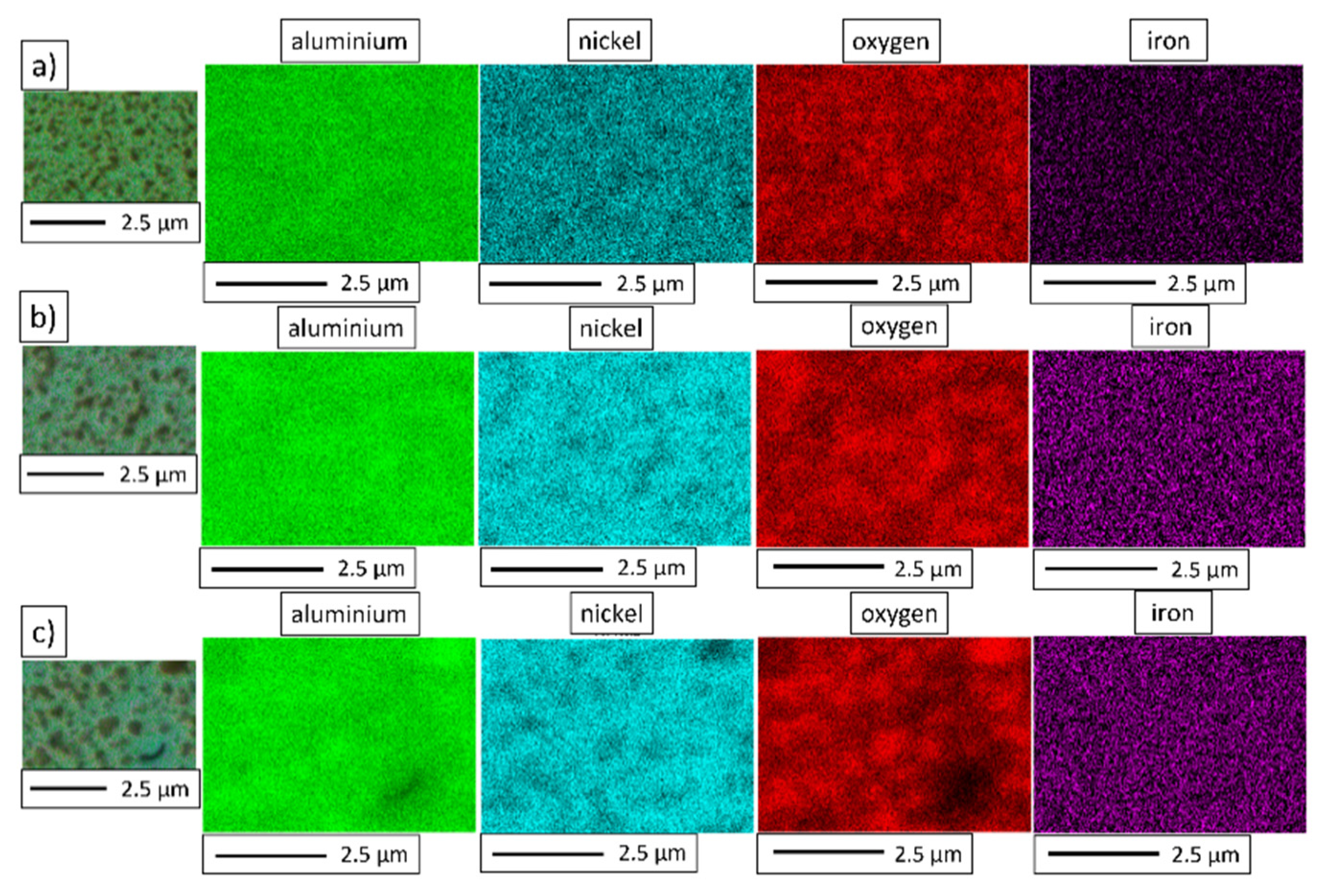
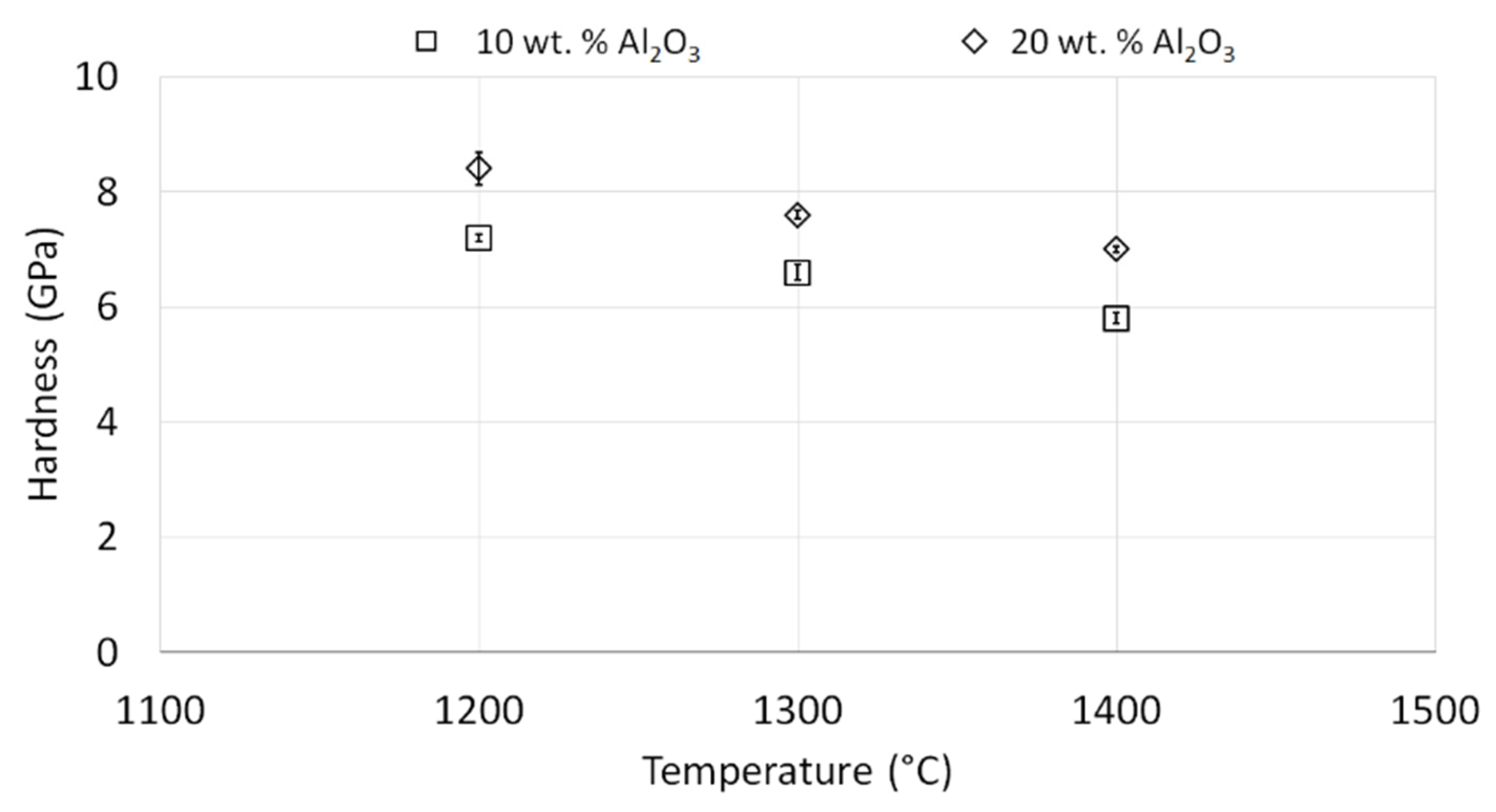
3.4. Characterization of NiAl-Al2O3 Samples after PPS Consolidation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qi, Y.; Chen, G.; Li, Z.; Chen, L.; Han, W.; Du, Z. A novel approach to fabricate ceramic/metal interpenetrating phase composites by ultrasonic-assisted spontaneous infiltration. Ceram. Int. 2021, 47, 2903–2907. [Google Scholar] [CrossRef]
- Okumus, S.C. Microstructural and mechanical characterization of plasma sprayed Al2O3–TiO2 composite ceramic coating on Mo/cast iron substrates. Mater. Lett. 2005, 59, 3214–3220. [Google Scholar] [CrossRef]
- Akter, N.; Becerril-Gonzalez, J.J.; Karabiyik, M.; Alam, F.; Pala, N.; Oskam, G.; Arés-Muzio, O. FDTD modeling of sputtered Mo–Al2O3 nanocomposites. Sol. Energy Mater. Sol. Cells 2021, 225, 111027. [Google Scholar] [CrossRef]
- Feng, T.; Zheng, W.; Chen, W.; Shi, Y.; Fu, Y.Q. Enhanced interfacial wettability and mechanical properties of Ni@Al2O3/Cu ceramic matrix composites using spark plasma sintering of Ni coated Al2O3 powders. Vacuum 2021, 184, 109938. [Google Scholar] [CrossRef]
- Das, S.; Das, S. Properties for Polymer, Metal and Ceramic Based Composite Materials. In Encyclopedia of Materials: Composites; Brabazon, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 20, pp. 815–821. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, J.; Cui, E.; Sun, Z.; Yu, H. Grain growth kinetics and grain refinement mechanism in Al2O3/WC/TiC/graphene ceramic composite. J. Eur. Ceram. Soc. 2021, 41, 1391–1398. [Google Scholar] [CrossRef]
- Gong, F.; Zhao, J.; Liu, G.; Ni, X. Design and fabrication of TiB2–TiC–Al2O3 gradient composite ceramic tool materials reinforced by VC/Cr3C2 additives. Ceram. Int. 2021, 47, 20341–20351. [Google Scholar] [CrossRef]
- Troncy, R.; Bonnet, G.; Pedraza, F. Microstructural characterization of NiAl–Al2O3 composite materials obtained by in situ aluminothermic reduction of NiO for potential coating applications. Mater. Chem. Phys. 2020, 251, 123124. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, C.C.; Chen, H.; He, J. Pinning effect of reactive elements on structural stability and adhesive strength of environmental sulfur segregation on Al2O3/NiAl interface. Scr. Mater. 2020, 188, 174–178. [Google Scholar] [CrossRef]
- Sglavo, V.M.; Marino, F.; Zhang, B.R.; Gialanella, S. Ni3Al intermetallic compound as second phase in Al2O3 ceramic composites. Mater. Sci. Eng. A 1997, 239–240, 665–671. [Google Scholar] [CrossRef]
- Smirnov, A.; Peretyagin, P.; Bartolomé, J.F. Processing and mechanical properties of new hierarchical metal-graphene flakes reinforced ceramic matrix composites. J. Eur. Ceram. Soc. 2019, 39, 3491–3497. [Google Scholar] [CrossRef]
- Rebillat, F. Ceramic Matrix Fiber Composites. In Encyclopedia of Materials: Technical Ceramics and Glasses; Pomeroy, M., Ed.; Elsevier: Oxford, UK, 2021; pp. 317–339. [Google Scholar]
- Krasnowski, M.; Kulik, T. Nanocrystalline FeAl matrix composites reinforced with TiC obtained by hot-pressing consolidation of mechanically alloyed powders. Intermetallics 2007, 15, 1377–1383. [Google Scholar] [CrossRef]
- Meyers, M.A.; Mishra, A.; Benson, D.J. Mechanical properties of nanocrystalline materials. Progr. Mater. Sci. 2006, 51, 427–556. [Google Scholar] [CrossRef]
- Jimba, Y.; Kondo, S.; Yu, H.; Wang, H.; Okuno, Y.; Kasada, R. Effect of mechanically alloyed sintering aid on sinterability of TiB2. Ceram. Int. 2021, 47, 21660–21667. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Krasnowski, M.; Gierlotka, S.; Kulik, T. NiAl-B composites with nanocrystalline intermetallic matrix produced by mechanical alloying and consolidation. Adv. Powder Technol. 2019, 30, 2742–2750. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, F.; Lin, C.; Yuan, D.; Huang, H.; Wang, E.; Wang, Z.; Guo, C. Microstructure and mechanical properties of continuous ceramic SiC and shape memory alloy NiTi hybrid fibers reinforced Ti-Al metal-intermetallic laminated composite. J. Alloys Compd. 2017, 729, 1145–1155. [Google Scholar] [CrossRef]
- Cymerman, K.; Oleszak, D.; Rosinski, M.; Michalski, A. Structure and mechanical properties of TiB2/TiC–Ni composites fabricated by pulse plasma sintering method. Adv. Powder Technol. 2018, 29, 1795–1803. [Google Scholar] [CrossRef]
- Zygmuntowicz, J.; Falkowski, P.; Wachowski, M.; Cymerman, K.; Piotrkiewicz, P.; Kaszuwara, W. Effect of the sintering temperature on microstructure and properties of Al2 O3 –Cu–Ni hybrid composites obtained by PPS. Int. J. Appl. Ceram. Technol. 2020, 17, 1731–1741. [Google Scholar] [CrossRef]
- Konopka, K.; Krasnowski, M.; Zygmuntowicz, J.; Cymerman, K.; Wachowski, M.; Piotrkiewicz, P. Characterization of Al2O3 Samples and NiAl–Al2O3 Composite Consolidated by Pulse Plasma Sintering. Materials 2021, 14, 3398. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Murashkin, M.Y.; Semenova, I.P. Grain boundaries and mechanical properties of ultrafine-grained metals. Metall. Mater. Trans. 2010, 41, 816–822. [Google Scholar] [CrossRef]
- Groza, J.R.; Zavaliangos, A. Nanostructured bulk solids by field activated sintering. Rev. Adv. Mater. Sci. 2003, 5, 24–33. [Google Scholar]
- Michalski, A.; Jaroszewicz, J.; Rosiński, M. The synthesis of NiAl using the Pulse Plasma Method with the Participation of the SHS Reaction. Int. J. Self-Propagating High-Temp. 2003, 12, 237–246. [Google Scholar]
- ANSI/ISA-S88.01-1995; Batch Control, Part 1: Models and Terminology. PN-EN 61512-1:2002(U) Regulacja Procesów Wsadowych. Scientific Publisher of the Institute of Sustainable Technologies—National Research Institute: Radom, Poland, 1995.
- ASTM D3766-08(2018); Standard Terminology Relating to Catalysts and Catalysis. ASTM International: West Conshohocken, PA, USA, 2018.
- EN 623–2; Advanced Technical Ceramics–Determination of Density and Porosity. British Standards Institute Staff: London, UK, 1993.
- Wejrzanowski, T.; Spychalski, W.; Rożniatowski, K.; Kurzydłowski, K. Image Based Analysis of Complex Microstructures of Engineering Materials. Int. J. Appl. Math. Comput. Sci. 2008, 18, 33–39. [Google Scholar] [CrossRef]
- Michalski, J.; Wejrzanowski, T.; Pielaszek, R.; Konopka, K.; Łojkowski, W.; Kurzydłowski, K.J. Application of image analysis for characterization of powders. Mater. Sci. Pol. 2005, 23, 79–86. [Google Scholar]
- Zygmuntowicz, J.; Piotrkiewicz, P.; Gizowska, M.; Tomaszewska, J.; Suchecki, P.; Wachowski, M.; Torzewski, J.; Żurowski, R. The Potential of Al2O3–ZrO2-Based Composites, Formed via CSC Method, in Linear Infrastructure Applications Based on Their Mechanical, Thermal and Environmental performance. Met. Mater. Trans. A 2021, 1–16. [Google Scholar] [CrossRef]
- Krasnowski, M.; Gierlotka, S.; Ciołek, S.; Kulik, T. Nanocrystalline NiAl intermetallic alloy with high hardness produced by mechanical alloying and hot-pressing consolidation. Adv. Powder Technol. 2019, 30, 1312–1318. [Google Scholar] [CrossRef]
- Suryanarajana, C.; Grant Norton, M. X-ray Diffraction. In A Practical Approach; Springer Science + Business Media: New York, NY, USA, 1998. [Google Scholar]
- Yuan, Y.; Fan, J.; Li, J.; Liu, J.; Zhao, K.; Liu, D.; An, L. Oscillatory pressure sintering of Al2O3 ceramics. Ceram. Int. 2020, 46, 15670–15673. [Google Scholar] [CrossRef]
- Sun, Z.; Li, B.; Hu, P.; Ding, F.; Yuan, F. Alumina ceramics with uniform grains prepared from Al2O3 nanospheres. J. Alloys Compd. 2016, 688, 933–938. [Google Scholar] [CrossRef]
- Albiter, A.; Salazar, M.; Bedolla, E.; Drew, R.A.L.; Perez, R. Improvement of the mechanical properties in a nanocrystalline NiAl intermetallic alloy with Fe, Ga and Mo additions. Mater. Sci. Eng. A 2003, 347, 154–164. [Google Scholar] [CrossRef]
- Biranvand, K.; Vaezi, M.-R.; Razavi, M. Mechanical Properties of Mechanical Alloyed and Spark Plasma Sintered NiAl-Based Intermetallic Composites. J. Mater. Eng. Perform. 2020, 30, 535–545. [Google Scholar] [CrossRef]
- Michalczyk, J. Inaccuracy in self-synchronisation of vibrators of two-drive vibratory machines caused by insufficient stiffness of vibrators mounting. Arch. Met. Mater. 2012, 57, 823–828. [Google Scholar] [CrossRef][Green Version]
- Zhu, X.; Zhang, T.; Morris, V.; Marchant, D. Combustion synthesis of NiAl/Al2O3 composites by induction heating. Intermetallics 2010, 18, 1197–1204. [Google Scholar] [CrossRef]
- Udhayabanu, V.; Ravi, K.R.; Murty, B.S. Development of in situ NiAl–Al2O3 nanocomposite by reactive milling and spark plasma sintering. J. Alloys Compd. 2011, 509, S223–S228. [Google Scholar] [CrossRef]

| PPS Process Parameter | Voltage (kV) | Stored Energy (kJ) | Electro-Pulse Repetition (s) | Sintering Temperature (°C) | Heating Rate (°C/min) | Sintering Time (s) | Load (MPa) |
|---|---|---|---|---|---|---|---|
| Al2O3 samples | 5.2–5.8 | 4.06–5.05 | 1–1.3 | 1000, 1100, 1200, 1300, 1400, 1500 | 250 | 180 | 20–80 |
| NiAl-Al2O3 composite | 4.3 | 2.77 | 1.3 | 1200, 1300, 1400 |
| Sintering Temperatures of Al2O3 Samples | 1000 °C | 1100 °C | 1200 °C | 1300 °C | 1400 °C | 1500 °C |
|---|---|---|---|---|---|---|
| Apparent density (g/cm3) | 2.95 ± 0.78 | 3.56 ± 0.43 | 3.97 ± 0.02 | 3.98 ± 0.01 | 3.98 ± 0.01 | 3.98 ± 0.01 |
| Relative density (%) | 74.39 ± 2.45 | 89.64 ± 1.35 | 99.92 ± 0.01 | 99.98 ± 0.01 | 99.98 ± 0.01 | 99.98 ± 0.01 |
| Open porosity (%) | 32.23 ± 1.03 | 10.67 ± 0.86 | - | - | - | - |
| Soaking (%) | 11.06 ± 0.89 | 3.62 ± 0.45 | - | - | - | - |
| Sintering Temperatures of Al2O3 Samples | Convexity | Curvature of Grain Boundaries | Elongation |
|---|---|---|---|
| 1000 °C | 1.08 ± 0.01 | 1.26 ± 0.03 | 1.38 ± 0.02 |
| 1100 °C | 1.09 ± 0.01 | 1.27 ± 0.01 | 1.38 ± 0.02 |
| 1200 °C | 1.06 ± 0.01 | 1.19 ± 0.01 | 1.30 ± 0.01 |
| 1300 °C | 1.06 ± 0.01 | 1.19 ± 0.01 | 1.30 ± 0.01 |
| 1400 °C | 1.07 ± 0.01 | 1.22 ± 0.02 | 1.22 ± 0.02 |
| 1500 °C | 1.06 ± 0.01 | 1.20 ± 0.02 | 1.30 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konopka, K.; Zygmuntowicz, J.; Krasnowski, M.; Cymerman, K.; Wachowski, M.; Piotrkiewicz, P. Pulse Plasma Sintering of NiAl-Al2O3 Composite Powder Produced by Mechanical Alloying with Contribution of Nanometric Al2O3 Powder. Materials 2022, 15, 407. https://doi.org/10.3390/ma15020407
Konopka K, Zygmuntowicz J, Krasnowski M, Cymerman K, Wachowski M, Piotrkiewicz P. Pulse Plasma Sintering of NiAl-Al2O3 Composite Powder Produced by Mechanical Alloying with Contribution of Nanometric Al2O3 Powder. Materials. 2022; 15(2):407. https://doi.org/10.3390/ma15020407
Chicago/Turabian StyleKonopka, Katarzyna, Justyna Zygmuntowicz, Marek Krasnowski, Konrad Cymerman, Marcin Wachowski, and Paulina Piotrkiewicz. 2022. "Pulse Plasma Sintering of NiAl-Al2O3 Composite Powder Produced by Mechanical Alloying with Contribution of Nanometric Al2O3 Powder" Materials 15, no. 2: 407. https://doi.org/10.3390/ma15020407
APA StyleKonopka, K., Zygmuntowicz, J., Krasnowski, M., Cymerman, K., Wachowski, M., & Piotrkiewicz, P. (2022). Pulse Plasma Sintering of NiAl-Al2O3 Composite Powder Produced by Mechanical Alloying with Contribution of Nanometric Al2O3 Powder. Materials, 15(2), 407. https://doi.org/10.3390/ma15020407








