Evaluation of the Cathodic Electrodeposition Effectiveness of the Hydroxyapatite Layer Used in Surface Modification of Ti6Al4V-Based Biomaterials
Abstract
1. Introduction
2. Materials and Methods
2.1. The Synthesis of TiO2 and Titanate Nanocoatings
- (a)
- The anodic oxidation method allowed the production of T5 coatings on the surface of Ti6Al4V substrates. This method used the 0.3% HF solution as an electrolyte, t = 20 min, and the 5 V potential. The produced T5 coatings were rinsed with deionized water and in acetone for 10 min.
- (b)
- The TNF coatings were produced due to T sample surface chemical oxidation. The surfaces of the substrates were chemically etched in c.a. 5.8 M HCl. Afterwards, materials were heated in 30% H2O2 solution at 358 K under a reflux condenser (TNF6C) or in an incubator (TNF6S) for t = 6 h. The nanofibrous samples marked TNF72 were produced in a slightly different procedure. The surface etching of the Ti6Al4V substrate was applied (a) in 2M HF solution for 10 s (TNF72a) or (b) in a 1:4:5 mixture of HF:HNO3:H2O (TNF72b). Then, the samples were immersed in 30% H2O2 solution at 358 K for 72 h in an incubator.
- (c)
- The alkali-sodium treatment produced the T-S and T5-S samples. The Ti6Al4V sample (T) and nonporous one (T5) were immersed in a 7 M NaOH solution at 339 K for t = 48 h. In the next step, these materials were washed with distilled water and dried at 314 K for 24 h in an incubator.
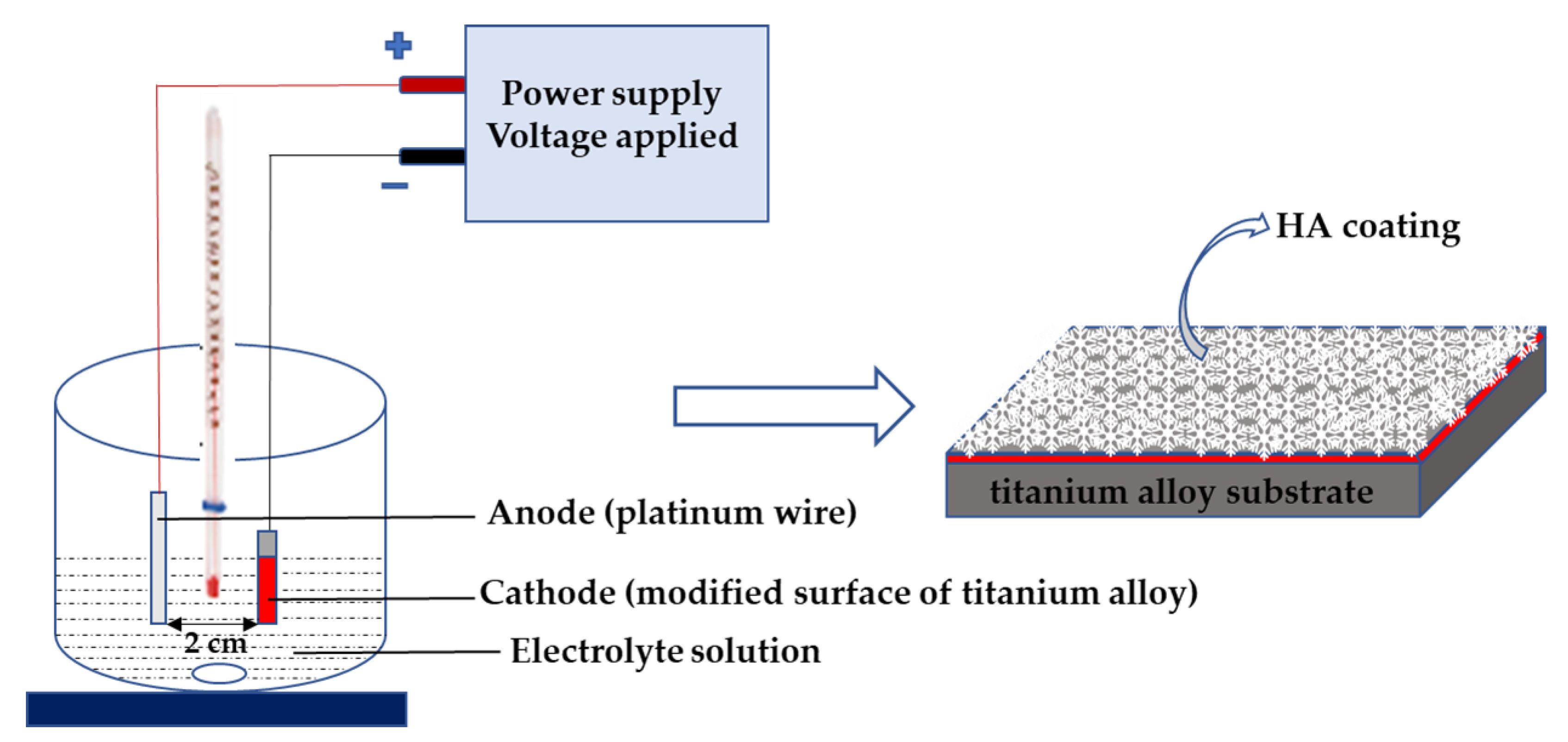
2.2. Synthesis of Hydroxyapatite on TiO2 and Titanate Nanocoatings
2.3. Structural and Morphological Characterization
2.4. Contact Angle
2.5. Nanomechanical Properties and Adhesion
3. Results
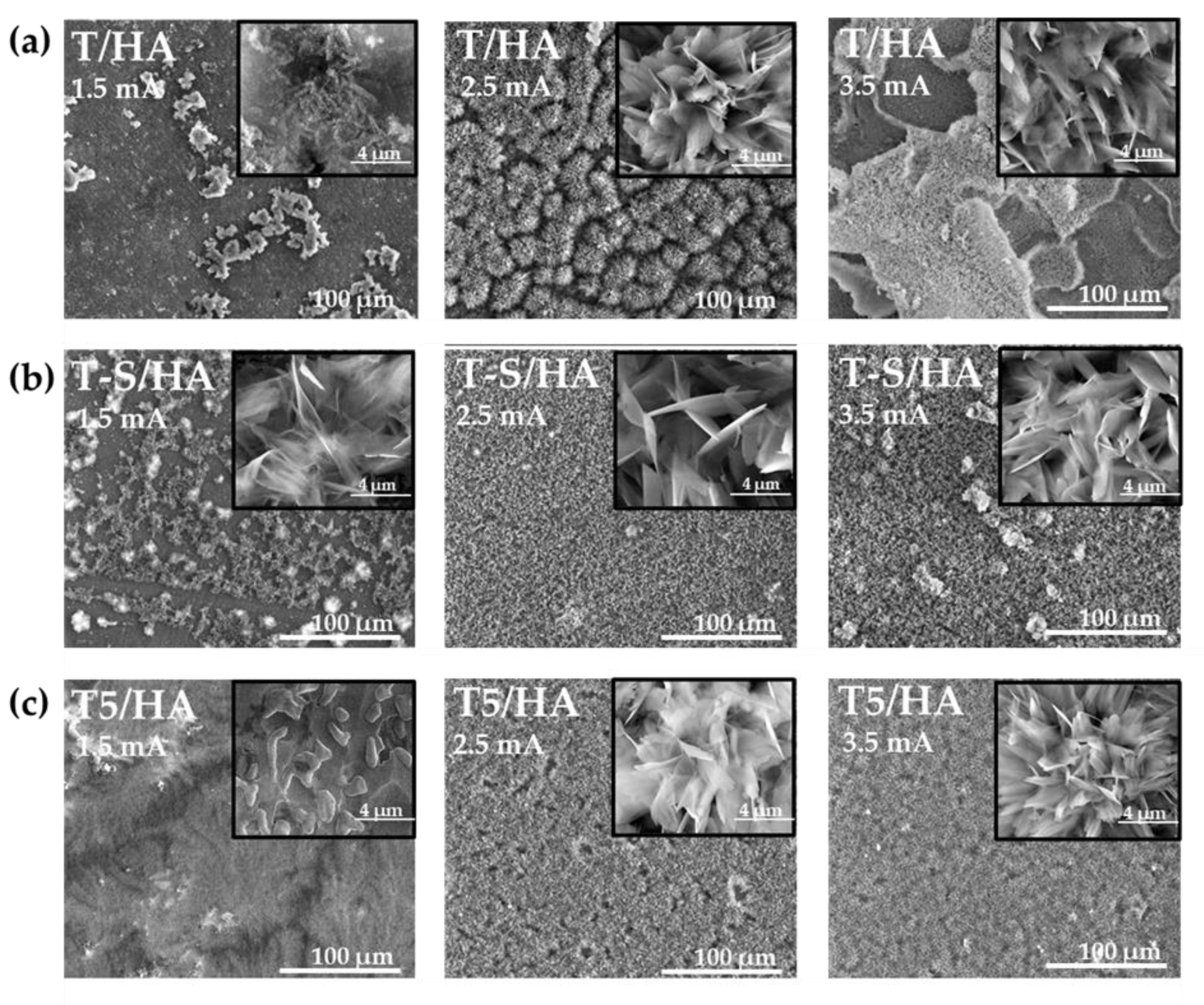
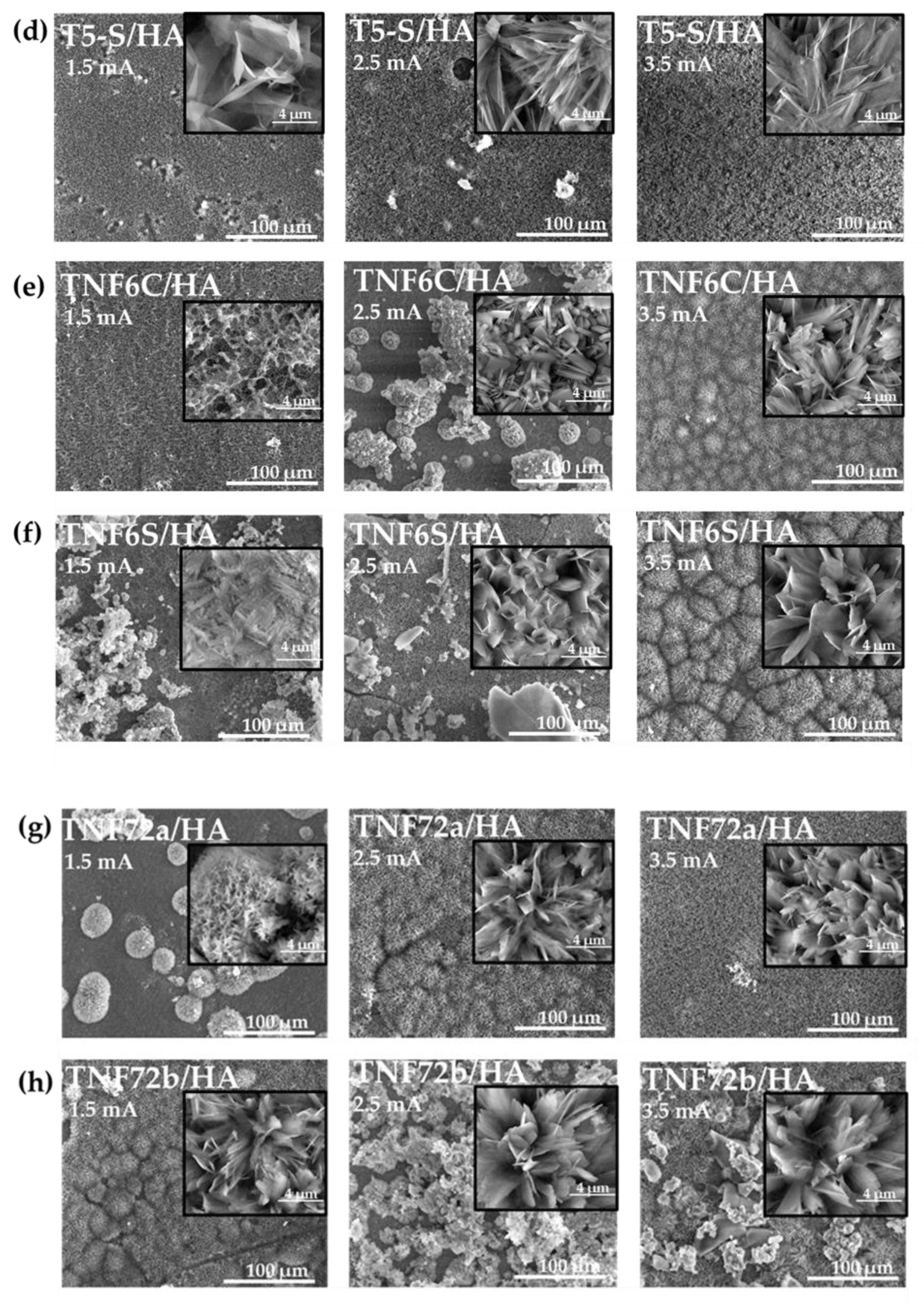
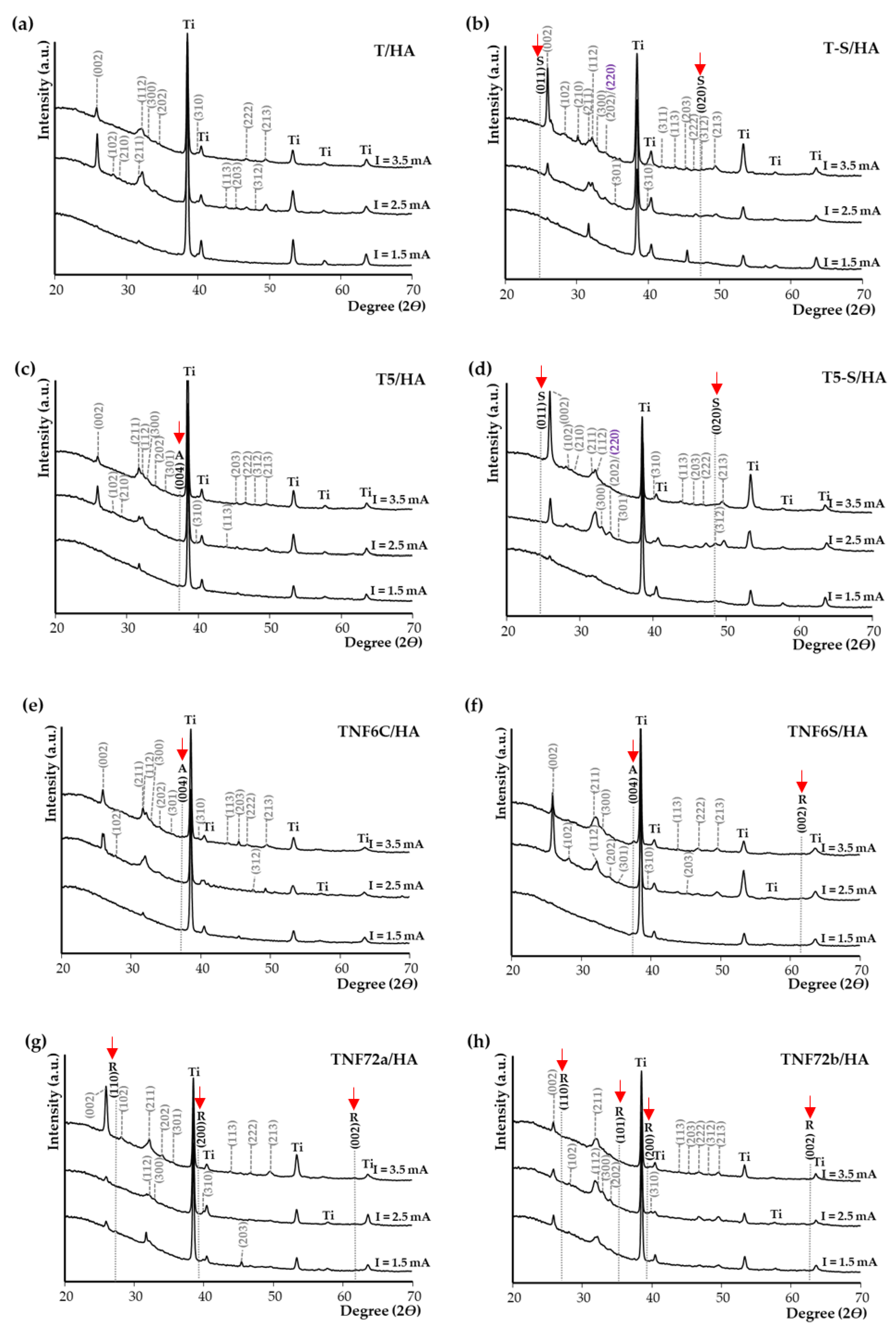
3.1. Structural and Morphological Characterization of TiO2 and Titanate Nanocoatings
3.2. Contact Angle
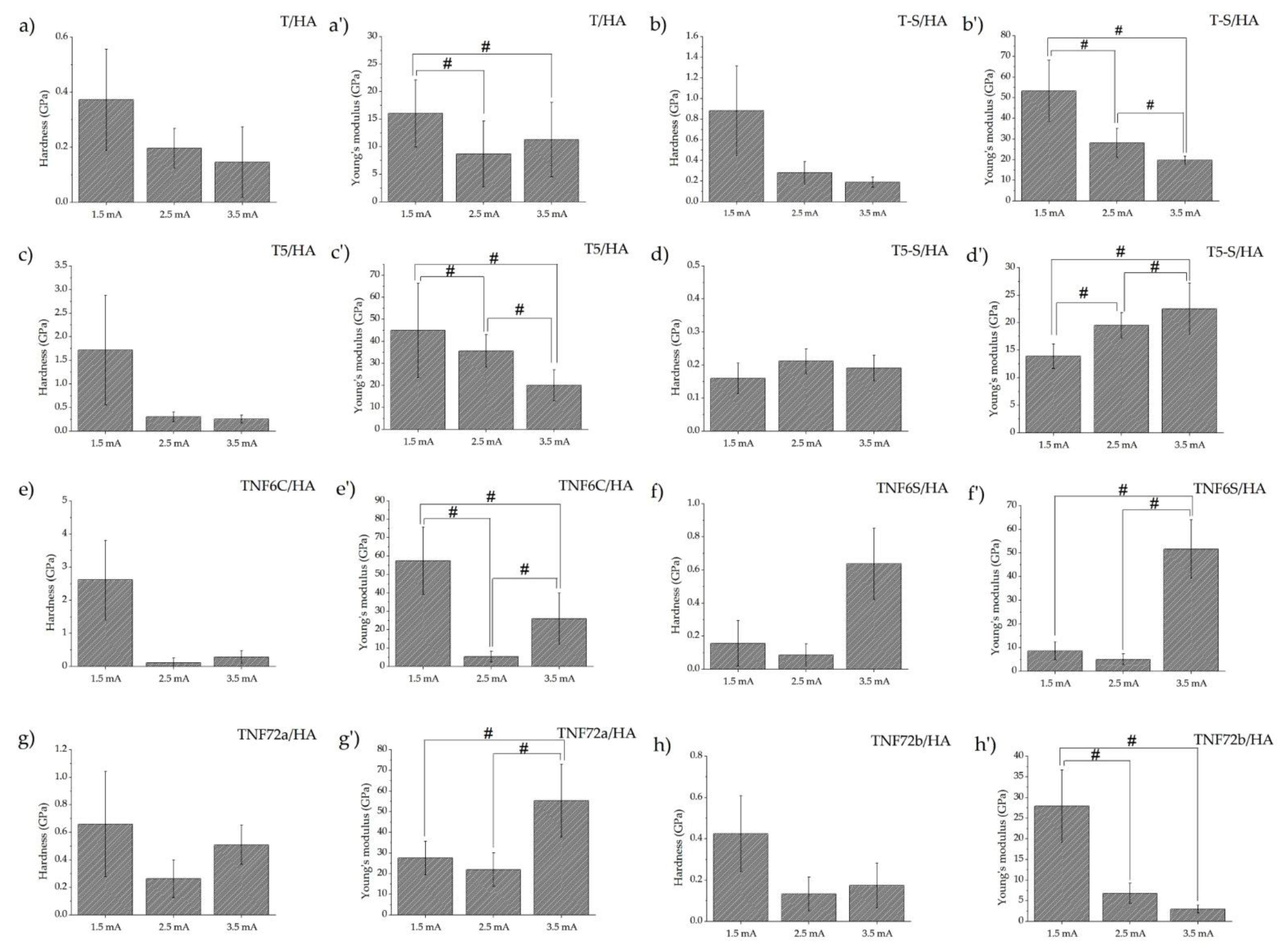
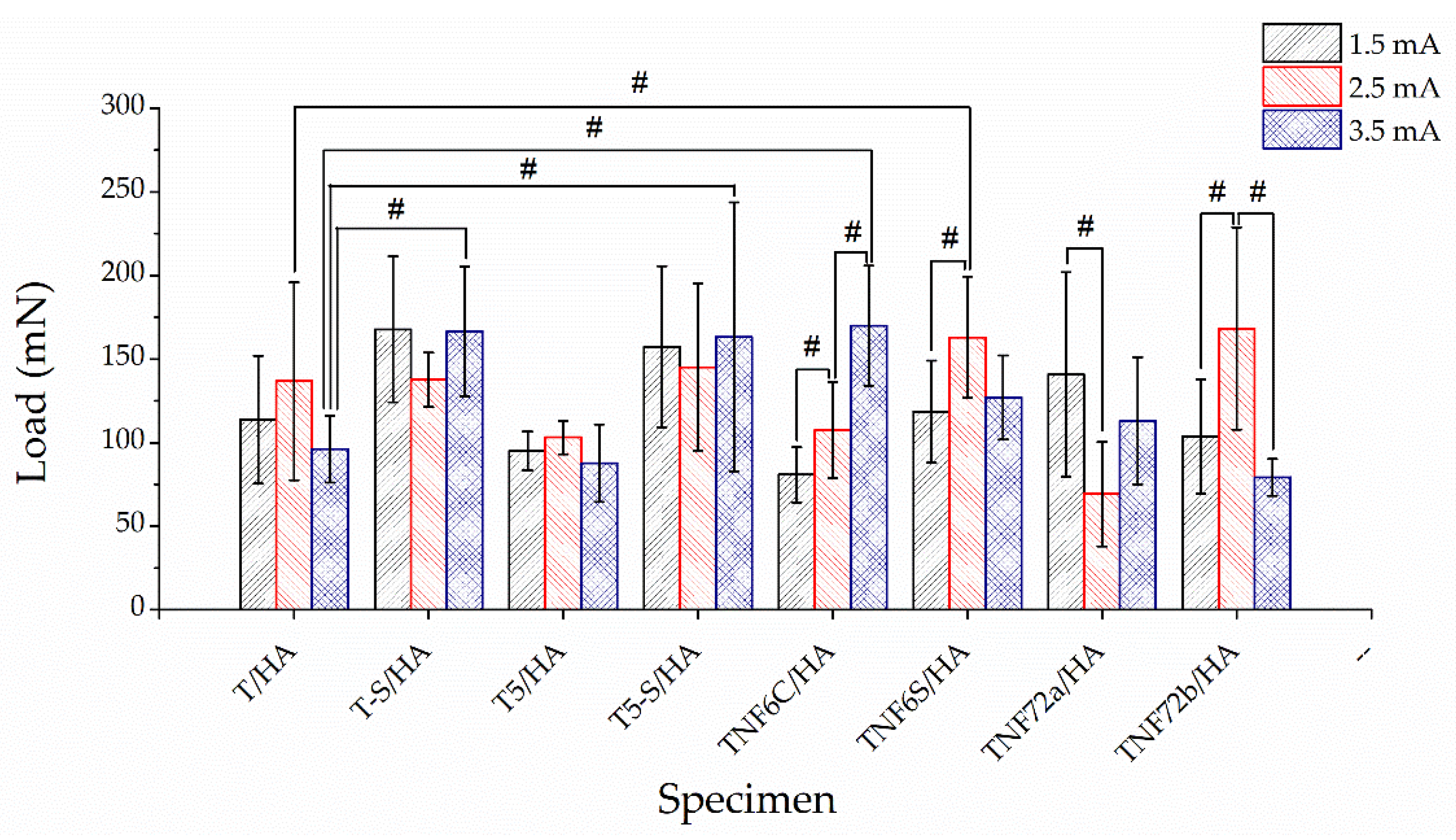
3.3. Mechanical Properties and Adhesion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti Based Biomaterials, the Ultimate Choice for Orthopaedic Implants—A Review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Attar, H.; Löber, L.; Funk, A.; Calin, M.; Zhang, L.C.; Prashanth, K.G.; Scudino, S.; Zhang, Y.S.; Eckert, J. Mechanical Behavior of Porous Commercially Pure Ti and Ti–TiB Composite Materials Manufactured by Selective Laser Melting. Mater. Sci. Eng. A 2015, 625, 350–356. [Google Scholar] [CrossRef]
- Gain, A.K.; Zhang, L.; Quadir, M.Z. Composites Matching the Properties of Human Cortical Bones: The Design of Porous Titanium-Zirconia (Ti-ZrO2) Nanocomposites Using Polymethyl Methacrylate Powders. Mater. Sci. Eng. A 2016, 662, 258–267. [Google Scholar] [CrossRef]
- Liu, S.; Shin, Y. Additive Manufacturing of Ti6Al4V Alloy: A Review. Mater. Des. 2018, 164, 107552. [Google Scholar] [CrossRef]
- Gain, A.K.; Zhang, L.; Lim, S. Tribological Behavior of Ti–6Al–4V Alloy: Subsurface Structure, Damage Mechanism and Mechanical Properties. Wear 2021, 464–465, 203551. [Google Scholar] [CrossRef]
- Sadeghi, M.; Kharaziha, M.; Salimijazi, H.R.; Tabesh, E. Role of Micro-Dimple Array Geometry on the Biological and Tribological Performance of Ti6Al4V for Biomedical Applications. Surf. Coat. Technol. 2019, 362, 282–292. [Google Scholar] [CrossRef]
- Sarker, A.; Tran, N.; Rifai, A.; Brandt, M.; Tran, P.A.; Leary, M.; Fox, K.; Williams, R. Rational Design of Additively Manufactured Ti6Al4V Implants to Control Staphylococcus Aureus Biofilm Formation. Materialia 2019, 5, 100250. [Google Scholar] [CrossRef]
- Chatzopoulos, G.S.; Wolff, L.F. Implant Failure and History of Failed Endodontic Treatment: A Retrospective Case-Control Study. J. Clin. Exp. Dent. 2017, 9, e1322–e1328. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Attarilar, S.; Wang, C.; Tamaddon, M.; Yang, C.; Xie, K.; Yao, J.; Wang, L.; Liu, C.; et al. Nano-Modified Titanium Implant Materials: A Way Toward Improved Antibacterial Properties. Front. Bioeng. Biotechnol. 2020, 8, 576969. [Google Scholar] [CrossRef]
- Ehlert, M.; Radtke, A.; Jędrzejewski, T.; Roszek, K.; Bartmański, M.; Piszczek, P. In Vitro Studies on Nanoporous, Nanotubular and Nanosponge-Like Titania Coatings, with the Use of Adipose-Derived Stem Cells. Materials 2020, 13, 1574. [Google Scholar] [CrossRef]
- Radtke, A.; Ehlert, M.; Jędrzejewski, T.; Bartmański, M. The Morphology, Structure, Mechanical Properties and Biocompatibility of Nanotubular Titania Coatings before and after Autoclaving Process. J. Clin. Med. 2019, 8, 272. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Monteiro, F.; Ferraz, M. Infection of Orthopedic Implants with Emphasis on Bacterial Adhesion Process and Techniques Used in Studying Bacterial-Material Interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Piszczek, P.; Radtke, A.; Ehlert, M.; Jędrzejewski, T.; Sznarkowska, A.; Sadowska, B.; Bartmański, M.; Erdoğan, Y.K.; Ercan, B.; Jędrzejczyk, W. Comprehensive Evaluation of the Biological Properties of Surface-Modified Titanium Alloy Implants. J. Clin. Med. 2020, 9, 342. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Grodzicka, M.; Ehlert, M.; Muzioł, T.M.; Szkodo, M.; Bartmański, M.; Piszczek, P. Studies on Silver Ions Releasing Processes and Mechanical Properties of Surface-Modified Titanium Alloy Implants. Int. J. Mol. Sci. 2018, 19, 3962. [Google Scholar] [CrossRef]
- Radtke, A.; Grodzicka, M.; Ehlert, M.; Jędrzejewski, T.; Wypij, M.; Golińska, P. “To Be Microbiocidal and Not to Be Cytotoxic at the Same Time…”-Silver Nanoparticles and Their Main Role on the Surface of Titanium Alloy Implants. J. Clin. Med. 2019, 8, 334. [Google Scholar] [CrossRef] [PubMed]
- Vranceanu, D.M.; Ungureanu, E.; Ionescu, I.C.; Parau, A.C.; Kiss, A.E.; Vladescu, A.; Cotrut, C.M. Electrochemical Surface Biofunctionalization of Titanium through Growth of TiO2 Nanotubes and Deposition of Zn Doped Hydroxyapatite. Coatings 2022, 12, 69. [Google Scholar] [CrossRef]
- Stocco, T.D.; Rodrigues, P.J.G.; Filho, M.A.d.A.; Lobo, A.O. Nanohydroxyapatite Electrodeposition onto Electrospun Nanofibers: Technique Overview and Tissue Engineering Applications. Bioengineering 2021, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Surmenev, R.A.; Surmeneva, M.A.; Ivanova, A.A. Significance of Calcium Phosphate Coatings for the Enhancement of New Bone Osteogenesis—A Review. Acta Biomater. 2014, 10, 557–579. [Google Scholar] [CrossRef]
- Brangule, A.; Gross, K.A. Importance of FTIR Spectra Deconvolution for the Analysis of Amorphous Calcium Phosphates. IOP Conf. Ser. Mater. Sci. Eng. 2015, 77, 012027. [Google Scholar] [CrossRef]
- Vecstaudza, J.; Gasik, M.; Locs, J. Amorphous Calcium Phosphate Materials: Formation, Structure and Thermal Behaviour. J. Eur. Ceram. Soc. 2019, 39, 1642–1649. [Google Scholar] [CrossRef]
- Safavi, M.S.; Walsh, F.C.; Surmeneva, M.A.; Surmenev, R.A.; Khalil-Allafi, J. Electrodeposited Hydroxyapatite-Based Biocoatings: Recent Progress and Future Challenges. Coatings 2021, 11, 110. [Google Scholar] [CrossRef]
- Radtke, A.; Ehlert, M.; Jędrzejewski, T.; Sadowska, B.; Więckowska-Szakiel, M.; Holopainen, J.; Ritala, M.; Leskelä, M.; Bartmański, M.; Szkodo, M.; et al. Titania Nanotubes/Hydroxyapatite Nanocomposites Produced with the Use of the Atomic Layer Deposition Technique: Estimation of Bioactivity and Nanomechanical Properties. Nanomaterials 2019, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Cai, S.; Ling, L.; Zuo, Y.; Wang, Z.; Liu, P.; Bao, X.; Xu, G. Superhydrophilic Hydroxyapatite/Hydroxypropyltrimethyl Ammonium Chloride Chitosan Composite Coating for Enhancing the Antibacterial and Corrosion Resistance of Magnesium Alloy. Prog. Org. Coat. 2022, 165, 106745. [Google Scholar] [CrossRef]
- Panda, S.; Biswas, C.K.; Paul, S. A Comprehensive Review on the Preparation and Application of Calcium Hydroxyapatite: A Special Focus on Atomic Doping Methods for Bone Tissue Engineering. Ceram. Int. 2021, 47, 28122–28144. [Google Scholar] [CrossRef]
- Ahmadi, S.; Mohammadi, I.; Sadrnezhaad, S.K. Hydroxyapatite Based and Anodic Titania Nanotube Biocomposite Coatings: Fabrication, Characterization and Electrochemical Behavior. Surf. Coat. Technol. 2016, 287, 67–75. [Google Scholar] [CrossRef]
- Drevet, R.; Benhayoune, H. Electrodeposition of Calcium Phosphate Coatings on Metallic Substrates for Bone Implant Applications: A Review. Coatings 2022, 12, 539. [Google Scholar] [CrossRef]
- Taranu, B.-O.; Ianasi, P.; Rus, S.F.; Bucur, A.I. Simultaneous Precipitation and Electrodeposition of Hydroxyapatite Coatings at Different Temperatures on Various Metal Substrates. Coatings 2022, 12, 288. [Google Scholar] [CrossRef]
- Catauro, M.; Barrino, F.; Blanco, I.; Piccolella, S.; Pacifico, S. Use of the Sol–Gel Method for the Preparation of Coatings of Titanium Substrates with Hydroxyapatite for Biomedical Application. Coatings 2020, 10, 203. [Google Scholar] [CrossRef]
- Heimann, R.B. Structural Changes of Hydroxylapatite during Plasma Spraying: Raman and NMR Spectroscopy Results. Coatings 2021, 11, 987. [Google Scholar] [CrossRef]
- Mohseni, E.; Zalnezhad, E.; Bushroa, A.R. Comparative Investigation on the Adhesion of Hydroxyapatite Coating on Ti–6Al–4V Implant: A Review Paper. Int. J. Adhes. Adhes. 2014, 48, 238–257. [Google Scholar] [CrossRef]
- Vladescu, A.; Vranceanu, D.M.; Kulesza, S.; Ivanov, A.N.; Bramowicz, M.; Fedonnikov, A.S.; Braic, M.; Norkin, I.A.; Koptyug, A.; Kurtukova, M.O.; et al. Influence of the Electrolyte’s PH on the Properties of Electrochemically Deposited Hydroxyapatite Coating on Additively Manufactured Ti64 Alloy. Sci. Rep. 2017, 7, 16819. [Google Scholar] [CrossRef] [PubMed]
- Khlifi, K.; Dhiflaoui, H.; Ben Rhouma, A.; Faure, J.; Benhayoune, H.; Laarbi, A.B.C. Nanomechanical Behavior, Adhesion and Corrosion Resistance of Hydroxyapatite Coatings for Orthopedic Implant Applications. Coatings 2021, 11, 477. [Google Scholar] [CrossRef]
- Li, T.T.; Ling, L.; Lin, M.C.; Peng, H.K.; Ren, H.T.; Lou, C.W.; Lin, J.H. Recent Advances in Multifunctional Hydroxyapatite Coating by Electrochemical Deposition. J. Mater. Sci. 2020, 55, 6352–6374. [Google Scholar] [CrossRef]
- Nagentrau, M.; Tobi, A.L.M.; Jamian, S.; Otsuka, Y.; Hussin, R. Delamination-Fretting Wear Failure Evaluation at HAp-Ti-6Al–4V Interface of Uncemented Artificial Hip Implant. J. Mech. Behav. Biomed. Mater. 2021, 122, 104657. [Google Scholar] [CrossRef]
- Schönweger, F.; Sprecher, C.M.; Milz, S.; Dommann-Scherrer, C.; Meier, C.; Dommann, A.; Neels, A.; Wahl, P. New Insights into Osteointegration and Delamination from a Multidisciplinary Investigation of a Failed Hydroxyapatite-Coated Hip Joint Replacement. Materials 2020, 13, 4713. [Google Scholar] [CrossRef] [PubMed]
- Ammarullah, M.I.; Afif, I.Y.; Maula, M.I.; Winarni, T.I.; Tauviqirrahman, M.; Jamari, J. Tresca Stress Evaluation of Metal-on-UHMWPE Total Hip Arthroplasty during Peak Loading from Normal Walking Activity. Mater. Today Proc. 2022, 63, S143–S146. [Google Scholar] [CrossRef]
- Singh, G.; Singh, H.; Sidhu, B.S. Characterization and Corrosion Resistance of Plasma Sprayed HA and HA–SiO2 Coatings on Ti–6Al–4V. Surf. Coat. Technol. 2013, 228, 242–247. [Google Scholar] [CrossRef]
- Ehlert, M.; Radtke, A.; Roszek, K.; Jędrzejewski, T.; Piszczek, P. Assessment of Titanate Nanolayers in Terms of Their Physicochemical and Biological Properties. Materials 2021, 14, 806. [Google Scholar] [CrossRef]
- Ehlert, M.; Roszek, K.; Jędrzejewski, T.; Bartmański, M.; Radtke, A. Titania Nanofiber Scaffolds with Enhanced Biointegration Activity—Preliminary In Vitro Studies. Int. J. Mol. Sci. 2019, 20, 5642. [Google Scholar] [CrossRef]
- Ehlert, M.; Radtke, A.; Topolski, A.; Śmigiel, J.; Piszczek, P. The Photocatalytic Activity of Titania Coatings Produced by Electrochemical and Chemical Oxidation of Ti6Al4V Substrate, Estimated According to ISO 10678:2010. Materials 2020, 13, 2649. [Google Scholar] [CrossRef]
- Lin, D.-Y.; Wang, X.-X. Electrodeposition of Hydroxyapatite Coating on CoNiCrMo Substrate in Dilute Solution. Surf. Coat. Technol. 2010, 204, 3205–3213. [Google Scholar] [CrossRef]
- Huang, Y.; Hao, M.; Nian, X.; Qiao, H.; Zhang, X.; Zhang, X.; Song, G.; Guo, J.; Pang, X.; Zhang, H. Strontium and Copper Co-Substituted Hydroxyapatite-Based Coatings with Improved Antibacterial Activity and Cytocompatibility Fabricated by Electrodeposition. Ceram. Int. 2016, 42, 11876–11888. [Google Scholar] [CrossRef]
- Xiao, X.F.; Liu, R.F.; Zheng, Y.Z. Characterization of Hydroxyapatite/Titania Composite Coatings Codeposited by a Hydrothermal–Electrochemical Method on Titanium. Surf. Coat. Technol. 2006, 200, 4406–4413. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Zhang, H.; Qiao, H.; Zhang, X.; Jia, T.; Han, S.; Gao, Y.; Xiao, H.; Yang, H. Fabrication of Silver- and Strontium-Doped Hydroxyapatite/TiO2 Nanotube Bilayer Coatings for Enhancing Bactericidal Effect and Osteoinductivity. Ceram. Int. 2017, 43, 992–1007. [Google Scholar] [CrossRef]
- He, D.-H.; Wang, P.; Liu, P.; Liu, X.-K.; Ma, F.-C.; Zhao, J. HA Coating Fabricated by Electrochemical Deposition on Modified Ti6Al4V Alloy. Surf. Coat. Technol. 2016, 301, 6–12. [Google Scholar] [CrossRef]
- Zeng, H.; Lacefield, W.R. XPS, EDX and FTIR Analysis of Pulsed Laser Deposited Calcium Phosphate Bioceramic Coatings: The Effects of Various Process Parameters. Biomaterials 2000, 21, 23–30. [Google Scholar] [CrossRef]
- Rehman, I.; Bonfield, W. Characterization of Hydroxyapatite and Carbonated Apatite by Photo Acoustic FTIR Spectroscopy. J. Mater. Sci. Mater. Med. 1997, 8, 1–4. [Google Scholar] [CrossRef]
- Barinov, S.M.; Rau, J.V.; Cesaro, S.N.; Durisin, J.; Fadeeva, I.V.; Ferro, D.; Medvecky, L.; Trionfetti, G. Carbonate Release from Carbonated Hydroxyapatite in the Wide Temperature Rage. J. Mater. Sci. Mater. Med. 2006, 17, 597–604. [Google Scholar] [CrossRef]
- Berzina-Cimdina, L.; Borodajenko, N. Research of Calcium Phosphates Using Fourier Transform Infrared Spectroscopy. In Infrared Spectroscopy—Materials Science, Engineering and Technology; Theophanides, T., Ed.; InTech: London, UK, 2012; ISBN 978-953-51-0537-4. [Google Scholar]
- Wei, D.; Zhou, Y.; Jia, D.; Wang, Y. Biomimetic Apatite Deposited on Microarc Oxidized Anatase-Based Ceramic Coating. Ceram. Int. 2008, 34, 1139–1144. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Macak, J.M.; Müller, L.; Kunze, J.; Müller, F.; Greil, P.; Virtanen, S.; Schmuki, P. Hydroxyapatite Growth on Anodic TiO2 Nanotubes. J. Biomed. Mater. Res. A 2006, 77, 534–541. [Google Scholar] [CrossRef]
- Ramesh, S.T.; Rameshbabu, N.; Gandhimathi, R.; Nidheesh, P.V.; Kumar, M.S. Kinetics and Equilibrium Studies for the Removal of Heavy Metals in Both Single and Binary Systems Using Hydroxyapatite. Appl. Water Sci. 2012, 2, 187–197. [Google Scholar] [CrossRef]
- Elliott, J.C. Structure and Chemistry of the Apatites and Other Calcium Orthophosphates, 1st ed.; Studies in Inorganic Chemistry; Elsevier: London, UK, 1994; Volume 18, ISBN 978-1-4832-9031-7. [Google Scholar]
- El Boujaady, H.; Mouraber, M.; El Rhilassi, A.; Bennani-Ziatni, M.; El Hamri, R.; Tajtai, A. Adsorption of a Textile Dye on Synthesized Calcium Deficient Hydroxyapatite (CDHAp): Kinetic and Thermodynamic Studies. J. Mater. Env. Sci. 2016, 7, 4049–4063. [Google Scholar]
- Ronan, K.; Kannan, M.B. Novel Sustainable Route for Synthesis of Hydroxyapatite Biomaterial from Biowastes. ACS Sustain. Chem. Eng. 2017, 5, 2237–2245. [Google Scholar] [CrossRef]
- Tripathy, A.; Sharma, P.; Sahoo, N.; Pramanik, S.; Abu Osman, N.A. Moisture Sensitive Inimitable Armalcolite/PDMS Flexible Sensor: A New Entry. Sens. Actuators B Chem. 2018, 262, 211–220. [Google Scholar] [CrossRef]
- Bohre, A.; Avasthi, K.; Singh, B.; Shrivastava, O.P. Crystallographic Evaluation of Titanate Ceramics as a Host Structure for Immobilization of Samarium. Radiochemistry 2014, 56, 92–97. [Google Scholar] [CrossRef]
- Cotrut, C.M.; Vladescu, A.; Dinu, M.; Vranceanu, D.M. Influence of Deposition Temperature on the Properties of Hydroxyapatite Obtained by Electrochemical Assisted Deposition. Ceram. Int. 2018, 44, 669–677. [Google Scholar] [CrossRef]
- Du, J.; Wang, G.; Song, D.; Jiang, J.; Jiang, H.; Gao, J. In-Vitro Degradation Behavior and Biocompatibility of Superhydrophilic Hydroxyapatite Coating on Mg–2Zn–Mn–Ca–Ce Alloy. J. Mater. Res. Technol. 2022, 17, 2742–2754. [Google Scholar] [CrossRef]
- Yang, Y.; Ao, H.; Wang, Y.; Lin, W.; Yang, S.; Zhang, S.; Yu, Z.; Tang, T. Cytocompatibility with Osteogenic Cells and Enhanced in Vivo Anti-Infection Potential of Quaternized Chitosan-Loaded Titania Nanotubes. Bone Res. 2016, 4, 16027. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, H.; Shao, Y.; Zhang, H.; Zhu, J. Recent Progresses of Superhydrophobic Coatings in Different Application Fields: An Overview. Coatings 2021, 11, 116. [Google Scholar] [CrossRef]
- Li, W.; Zhan, Y.; Yu, S. Applications of Superhydrophobic Coatings in Anti-Icing: Theory, Mechanisms, Impact Factors, Challenges and Perspectives. Prog. Org. Coat. 2021, 152, 106117. [Google Scholar] [CrossRef]
- Gu, C.G.; Fu, Q.-G.; Li, H.; Lu, J.H.; Zhang, L.L. Study on Special Morphology Hydroxyapatite Bioactive Coating by Electrochemical Deposition. Key Eng. Mater. 2013, 537, 256–260. [Google Scholar] [CrossRef]
- Chakraborty, R.; Sengupta, S.; Saha, P.; Das, S. Synthesis of Biocompatible Calcium Hydrogen Phosphate and Hydroxyapatite Coating on SS316 Substrate through Pulsed Electrodeposition. Mater. Sci. Eng. C 2016, 69, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Fu, T.; Lu, J.; Xu, K. Characterization and Stability of Hydroxyapatite Coatings Prepared by an Electrodeposition and Alkaline-Treatment Process. J. Biomed. Mater. Res. 2001, 54, 96–101. [Google Scholar] [CrossRef]
- Gopi, D.; Indira, J.; Kavitha, L. A Comparative Study on the Direct and Pulsed Current Electrodeposition of Hydroxyapatite Coatings on Surgical Grade Stainless Steel. Surf. Coat. Technol. 2012, 206, 2859–2869. [Google Scholar] [CrossRef]
- Marashi-Najafi, F.; Khalil-Allafi, J.; Etminanfar, M.R.; Faezi-Alivand, R. Corrosion Resistance and in Vitro Evaluation of the Pulsed Current Electrodeposited Hydroxyapatite Coatings on Nitinol Shape Memory Alloy. Mater. Corros. 2017, 68, 1237–1245. [Google Scholar] [CrossRef]
- Gopi, D.; Prakash, V.C.A.; Kavitha, L.; Kannan, S.; Bhalaji, P.R.; Shinyjoy, E.; Ferreira, J.M.F. A Facile Electrodeposition of Hydroxyapatite onto Borate Passivated Surgical Grade Stainless Steel. Corros. Sci. 2011, 53, 2328–2334. [Google Scholar] [CrossRef]
- Zhao, D.; Sun, R.; Chen, K. Influence of Different Chelating Agents on Corrosion Performance of Microstructured Hydroxyapatite Coatings on AZ91D Magnesium Alloy. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2017, 32, 179–185. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, M.; Wang, T.; Guo, S. Fabrication of Porous Micro-Nanostructured Networks on Ti6Al4V for Faster Hydroxyapatite Deposition in SBF. Mater. Lett. 2019, 256, 126571. [Google Scholar] [CrossRef]
- Mazumder, S.; Falkinham, J.; Dietrich, A.; Puri, I. Role of Hydrophobicity in Bacterial Adherence to Carbon Nanostructures and Biofilm Formation. Biofouling 2010, 26, 333–339. [Google Scholar] [CrossRef]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A Review on the Wettability of Dental Implant Surfaces II: Biological and Clinical Aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef]
- Tsai, W.L.; Hsu, P.C.; Hwu, Y.; Chen, C.H.; Chang, L.W.; Je, J.H.; Lin, H.M.; Groso, A. Electrochemistry—Building on Bubbles in Metal Electrodeposition. Nature 2002, 417, 139. [Google Scholar] [CrossRef] [PubMed]
- Dumelié, N.; Benhayoune, H.; Rousse, C.; Bouthors, S.; Perchet, A.; Wortham, L.; Douglade, J.; Laurent-Maquin, D.; Balossier, G. Characterization of Electrodeposited Calcium Phosphate Coatings by Complementary Scanning Electron Microscopy and Scanning-Transmission Electron Microscopy Associated to X-Ray Microanalysis. Thin Solid Films 2005, 492, 131–139. [Google Scholar] [CrossRef]
- Aminzare, M.; Eskandari, A.; Baroonian, M.H.; Berenov, A.; Hesabi, Z.R.; Taheri, M.; Sadrnezhaad, S.K. Hydroxyapatite Nanocomposites: Synthesis, Sintering and Mechanical Properties. Ceram. Int. 2013, 39, 2197–2206. [Google Scholar] [CrossRef]
- Zhang, Z.; Dunn, M.F.; Xiao, T.D.; Tomsia, A.P.; Saiz, E. Nanostructured Hydroxyapatite Coatings for Improved Adhesion and Corrosion Resistance for Medical Implants. MRS Online Proc. Libr. 2011, 703, 75. [Google Scholar] [CrossRef]
- Zhang, E.; Zou, C.; Zeng, S. Preparation and Characterization of Silicon-Substituted Hydroxyapatite Coating by a Biomimetic Process on Titanium Substrate. Surf. Coat. Technol. 2009, 203, 1075–1080. [Google Scholar] [CrossRef]
- Brizuela, A.; Herrero-Climent, M.; Rios-Carrasco, E.; Rios-Santos, J.; Pérez, R.; Manero, J.; Gil Mur, J. Influence of the Elastic Modulus on the Osseointegration of Dental Implants. Materials 2019, 12, 980. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y.; Tanimoto, Y.; Maruyama, N.; Nagakura, M. A Review of Improved Fixation Methods for Dental Implants. Part II: Biomechanical Integrity at Bone–Implant Interface. J. Prosthodont. Res. 2015, 59, 84–95. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, J.; Wang, L.; He, P.; Wang, T. HA Coating on Titanium with Nanotubular Anodized TiO2 Intermediate Layer via Electrochemical Deposition. Trans. Nonferrous Met. Soc. China 2008, 18, 631–635. [Google Scholar] [CrossRef]
- Wang, H.; Chen, C.; Wang, D. Development of Hydroxyapatite Coating Prepared by Sol–Gel Technique. Surf. Rev. Lett. 2006, 13, 737–745. [Google Scholar] [CrossRef]
- Brammer, K.S.; Frandsen, C.J.; Jin, S. TiO2 Nanotubes for Bone Regeneration. Trends Biotechnol. 2012, 30, 315. [Google Scholar] [CrossRef]
- Tan, A.W.; Pingguan-Murphy, B.; Ahmad, R.; Akbar, S.A. Review of Titania Nanotubes: Fabrication and Cellular Response. Ceram. Int. 2012, 38, 4421–4435. [Google Scholar] [CrossRef]
- Rakngarm, A.; Miyashita, Y.; Mutoh, Y. Formation of Hydroxyapatite Layer on Bioactive Ti and Ti–6Al–4V by Simple Chemical Technique. J. Mater. Sci. Mater. Med. 2008, 19, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Hamada, K.; Kon, M.; Hanawa, T.; Yokoyama, K.; Miyamoto, Y.; Asaoka, K. Hydrothermal Modification of Titanium Surface in Calcium Solutions. Biomaterials 2002, 23, 2265–2272. [Google Scholar] [CrossRef]
- Zavgorodniy, A.V.; Borrero-López, O.; Hoffman, M.; LeGeros, R.Z.; Rohanizadeh, R. Mechanical Stability of Two-Step Chemically Deposited Hydroxyapatite Coating on Ti Substrate: Effects of Various Surface Pretreatments. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 99, 58–69. [Google Scholar] [CrossRef]
- Yılmaz, E.; Çakıroğlu, B.; Gökçe, A.; Findik, F.; Gülsoy, N.; Özacar, M. Novel Hydroxyapatite/Graphene Oxide/Collagen Bioactive Composite Coating on Ti16Nb Alloys by Electrodeposition. Mater. Sci. Eng. C 2019, 101, 292–305. [Google Scholar] [CrossRef]
- Blackwood, D.J.; Seah, K.H.W. Electrochemical Cathodic Deposition of Hydroxyapatite: Improvements in Adhesion and Crystallinity. Mater. Sci. Eng. C 2009, 29, 1233–1238. [Google Scholar] [CrossRef]
- Sarraf, M.; Razak, A.; Crum, R.; Gamez, C.; Ramirez, B.; Hayaty, A.K.N.; Nasiri-Tabrizi, B.; Gupta, V.; Sukiman, N.; Basirun, W. Adhesion Measurement of Highly-Ordered TiO2 Nanotubes on Ti-6Al-4V Alloy. Process. Appl. Ceram. 2017, 11, 311–321. [Google Scholar] [CrossRef]
- Jaafar, A.; Hecker, C.; Árki, P.; Joseph, Y. Sol-Gel Derived Hydroxyapatite Coatings for Titanium Implants: A Review. Bioengineering 2020, 7, 127. [Google Scholar] [CrossRef]
- Isa, N.N.C.; Mohd, Y.; Yury, N. Electrochemical Deposition and Characterization of Hydroxyapatite (HAp) on Titanium Substrate. APCBEE Procedia 2012, 3, 46–52. [Google Scholar] [CrossRef]
- Schmidt, R.; Hoffmann, V.; Helth, A.; Gostin, P.F.; Calin, M.; Eckert, J.; Gebert, A. Electrochemical Deposition of Hydroxyapatite on Beta-Ti-40Nb. Surf. Coat. Technol. 2016, 294, 186–193. [Google Scholar] [CrossRef]
- Hayakawa, T.; Kawashita, M.; Takaoaka, G. Coating of Hydroxyapatite Films on Titanium Substrates by Electrodeposition under Pulse Current. J. Ceram. Soc. Jpn. 2008, 116, 68–73. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Mao, H.; Li, T.; Zhao, R.; Yan, Y.; Pang, X. Osteoblastic Cell Responses and Antibacterial Efficacy of Cu/Zn Co-Substituted Hydroxyapatite Coatings on Pure Titanium Using Electrodeposition Method. RSC Adv. 2015, 5, 17076–17086. [Google Scholar] [CrossRef]
- Eliaz, N.; Ritman-Hertz, O.; Aronov, D.; Weinberg, E.; Shenhar, Y.; Rosenman, G.; Weinreb, M.; Ron, E. The Effect of Surface Treatments on the Adhesion of Electrochemically Deposited Hydroxyapatite Coating to Titanium and on Its Interaction with Cells and Bacteria. J. Mater. Sci. Mater. Med. 2011, 22, 1741–1752. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.F.; Bandyopadhyay, A.; Bose, S. Titania Nanotube Interface to Increase Adhesion Strength of Hydroxyapatite Sol-Gel Coatings on Ti-6Al-4V for Orthopedic Applications. Surf. Coat. Technol. 2019, 372, 140–147. [Google Scholar] [CrossRef]
- Mohammad, N.F.; Ahmad, R.N.; Rosli, N.L.M.; Manan, M.S.A.; Marzuki, M.; Wahi, A. Sol Gel Deposited Hydroxyapatite-Based Coating Technique on Porous Titanium Niobium for Biomedical Applications: A Mini Review. Mater. Today Proc. 2021, 41, 127–135. [Google Scholar] [CrossRef]
- Tranquillo, E.; Bollino, F. Surface Modifications for Implants Lifetime Extension: An Overview of Sol-Gel Coatings. Coatings 2020, 10, 589. [Google Scholar] [CrossRef]
- Heimann, R.B. Structure, Properties, and Biomedical Performance of Osteoconductive Bioceramic Coatings. Surf. Coat. Technol. 2013, 233, 27–38. [Google Scholar] [CrossRef]
- Carradò, A.; Perrin-Schmitt, F.; Le, Q.V.; Giraudel, M.; Fischer, C.; Koenig, G.; Jacomine, L.; Behr, L.; Chalom, A.; Fiette, L.; et al. Nanoporous Hydroxyapatite/Sodium Titanate Bilayer on Titanium Implants for Improved Osteointegration. Dent. Mater. 2017, 33, 321–332. [Google Scholar] [CrossRef]
- Fomin, A.; Fomina, M.; Koshuro, V.; Rodionov, I.; Zakharevich, A.; Skaptsov, A. Structure and Mechanical Properties of Hydroxyapatite Coatings Produced on Titanium Using Plasma Spraying with Induction Preheating. Ceram. Int. 2017, 43, 11189–11196. [Google Scholar] [CrossRef]
- Ritwik, A.; Saju, K.K.; Vengellur, A.; Saipriya, P.P. Development of Thin-Film Hydroxyapatite Coatings with an Intermediate Shellac Layer Produced by Dip-Coating Process on Ti6Al4V Implant Materials. J. Coat. Technol. Res. 2022, 19, 597–605. [Google Scholar] [CrossRef]

| Ca/P (Mole Ratio) of HA Layer at Different Currents | |||
|---|---|---|---|
| Sample | I = 1.5 mA | I = 2.5 mA | I = 3.5 mA |
| T/HA | 1.83 | 1.60 | 1.54 |
| T-S/HA | 1.45 | 1.69 | 1.73 |
| T5/HA | 0.82 | 1.58 | 1.82 |
| T5-S/HA | 1.39 | 1.62 | 1.92 |
| TNF6c/HA | 1.13 | 1.45 | 1.76 |
| TNF6s/HA | 1.66 | 1.54 | 1.58 |
| TNF72a/HA | 1.70 | 1.96 | 1.75 |
| TNF72b/HA | 1.58 | 1.63 | 1.65 |
| Functional Groups | Frequencies (Experimental) (cm−1) | Frequencies (Reference) (cm−1) | Reference |
|---|---|---|---|
| ν3(PO43−) | 1188–1006 | 1200–960 | [43,44,45,46,47,48,49,50] |
| ν1(PO43−) | 960–956 | 963–960 | [43,44,47,48,49,51] |
| ν4(PO43−) | 601–531 | 660–520 | [19,43,44,45,46,47,48,50,51,52,53] |
| ν1(CO32−) | 1460–1398 | 1500–1400 | [43,44,45,46,47,48,49,51,52] |
| ν3 or ν4(CO32−) | 875–867 | 880–865 | [43,44,45,49,50,54] |
| ν3(CO32−) | 1323 | 1300, 1321 | [47,48] |
| ν(OH) | 3456–3000 | 3571–3420 | [41,42,43,44,45,46,49,50,51,55] |
| σ(OH) | 1653–1594 | 1650–1631 | [43,44,45,50,51,55] |
| I = 2.5 mA | I = 3.5 mA | |
|---|---|---|
| Sample | Roughness (Ra) (µm) | |
| T/HA | 0.57 ± 0.07 | 0.33 ± 0.00 |
| T-S/HA | 0.21 ± 0.03 | 0.29 ± 0.04 |
| T5/HA | 0.21 ± 0.00 | 0.31 ± 0.02 |
| T5-S/HA | 0.20 ± 0.05 | 0.38 ± 0.05 |
| TNF6C/HA | 0.20 ± 0.01 | 0.61 ± 0.03 |
| TNF6S/HA | 0.15 ± 0.00 | 0.43 ± 0.03 |
| TNF72a/HA | 0.13 ± 0.01 | 0.32 ± 0.02 |
| TNF72b/HA | 0.11 ± 0.01 | 0.13 ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehlert, M.; Radtke, A.; Bartmański, M.; Piszczek, P. Evaluation of the Cathodic Electrodeposition Effectiveness of the Hydroxyapatite Layer Used in Surface Modification of Ti6Al4V-Based Biomaterials. Materials 2022, 15, 6925. https://doi.org/10.3390/ma15196925
Ehlert M, Radtke A, Bartmański M, Piszczek P. Evaluation of the Cathodic Electrodeposition Effectiveness of the Hydroxyapatite Layer Used in Surface Modification of Ti6Al4V-Based Biomaterials. Materials. 2022; 15(19):6925. https://doi.org/10.3390/ma15196925
Chicago/Turabian StyleEhlert, Michalina, Aleksandra Radtke, Michał Bartmański, and Piotr Piszczek. 2022. "Evaluation of the Cathodic Electrodeposition Effectiveness of the Hydroxyapatite Layer Used in Surface Modification of Ti6Al4V-Based Biomaterials" Materials 15, no. 19: 6925. https://doi.org/10.3390/ma15196925
APA StyleEhlert, M., Radtke, A., Bartmański, M., & Piszczek, P. (2022). Evaluation of the Cathodic Electrodeposition Effectiveness of the Hydroxyapatite Layer Used in Surface Modification of Ti6Al4V-Based Biomaterials. Materials, 15(19), 6925. https://doi.org/10.3390/ma15196925









