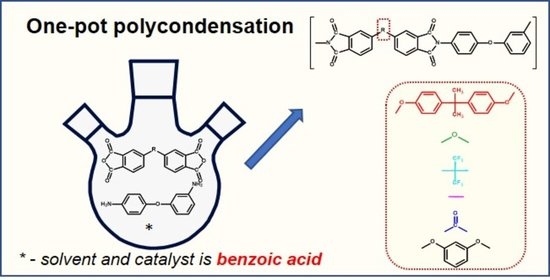
Synthesis of Aromatic Polyimides Based on 3,4′-Oxydianiline by One-Pot Polycondensation in Molten Benzoic Acid and Their Application as Membrane Materials for Pervaporation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Typical Synthesis of PI
2.3. Preparation of PI films
2.4. Methods
2.5. Study of PI Films Performance in Vacuum Pervaporation
3. Results
3.1. Synthesis and Chemical Structure
3.2. Molecular Weight Characteristics and Mechanical Properties of Soluble PIs
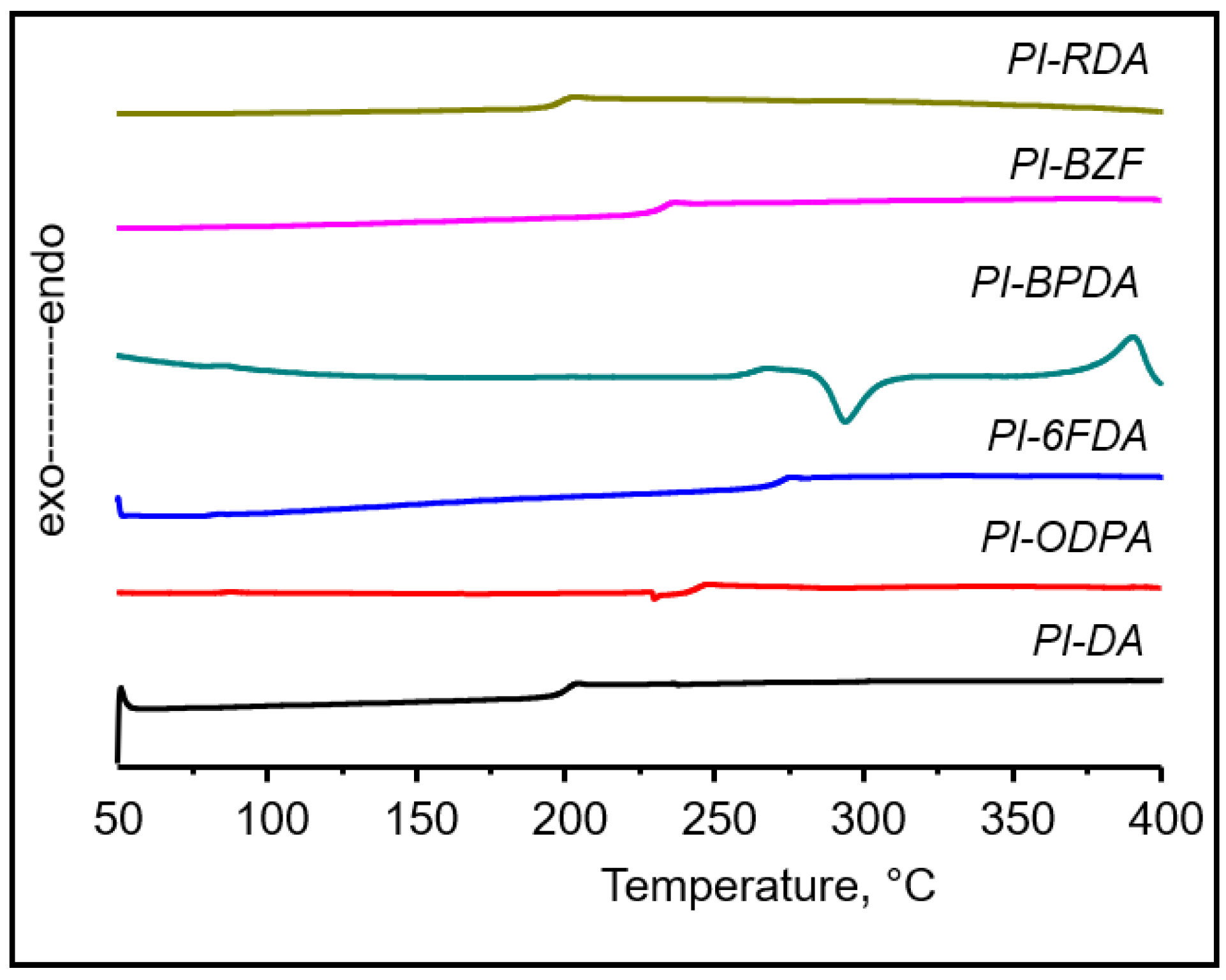
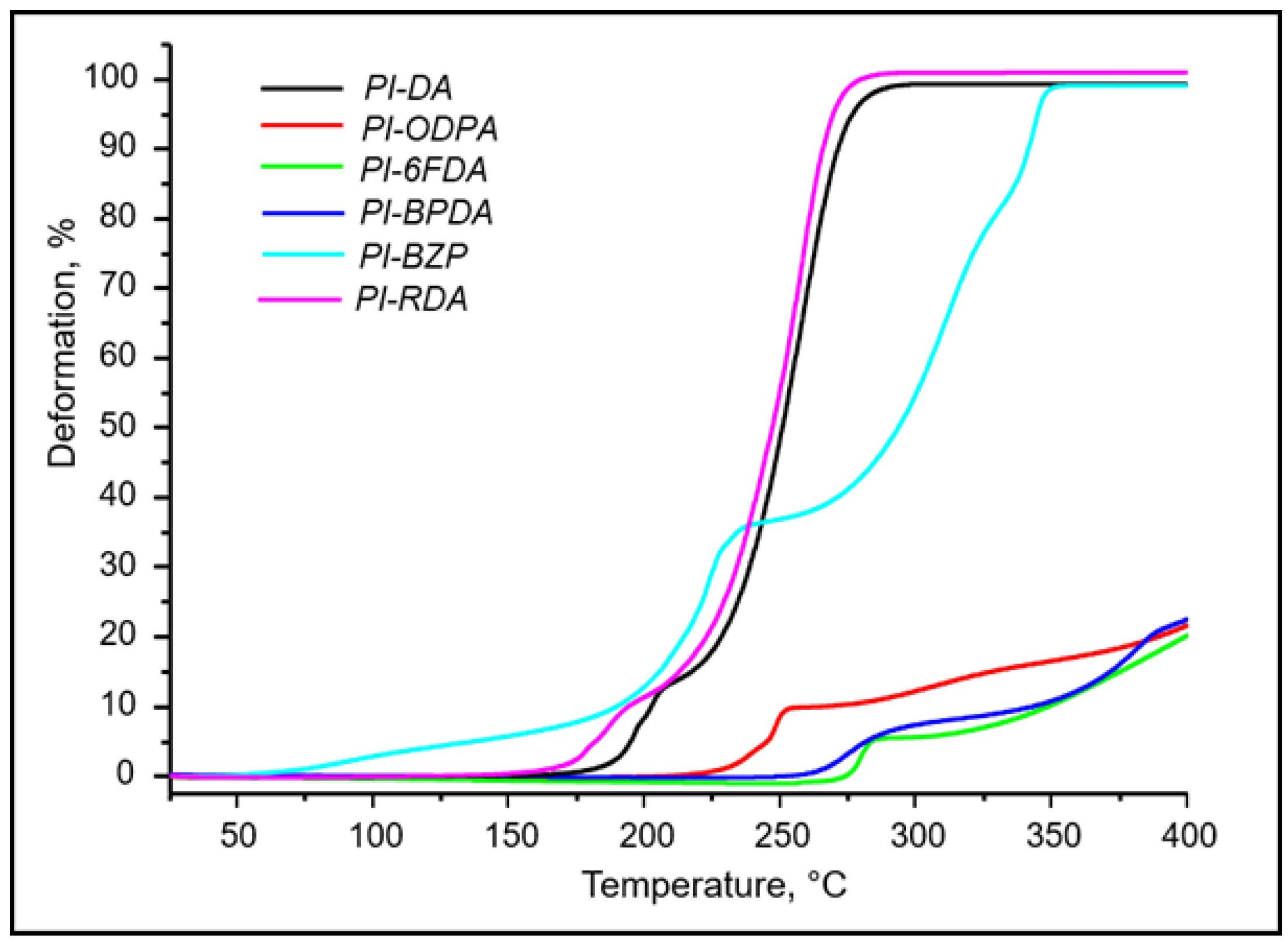
3.3. Thermal Properties
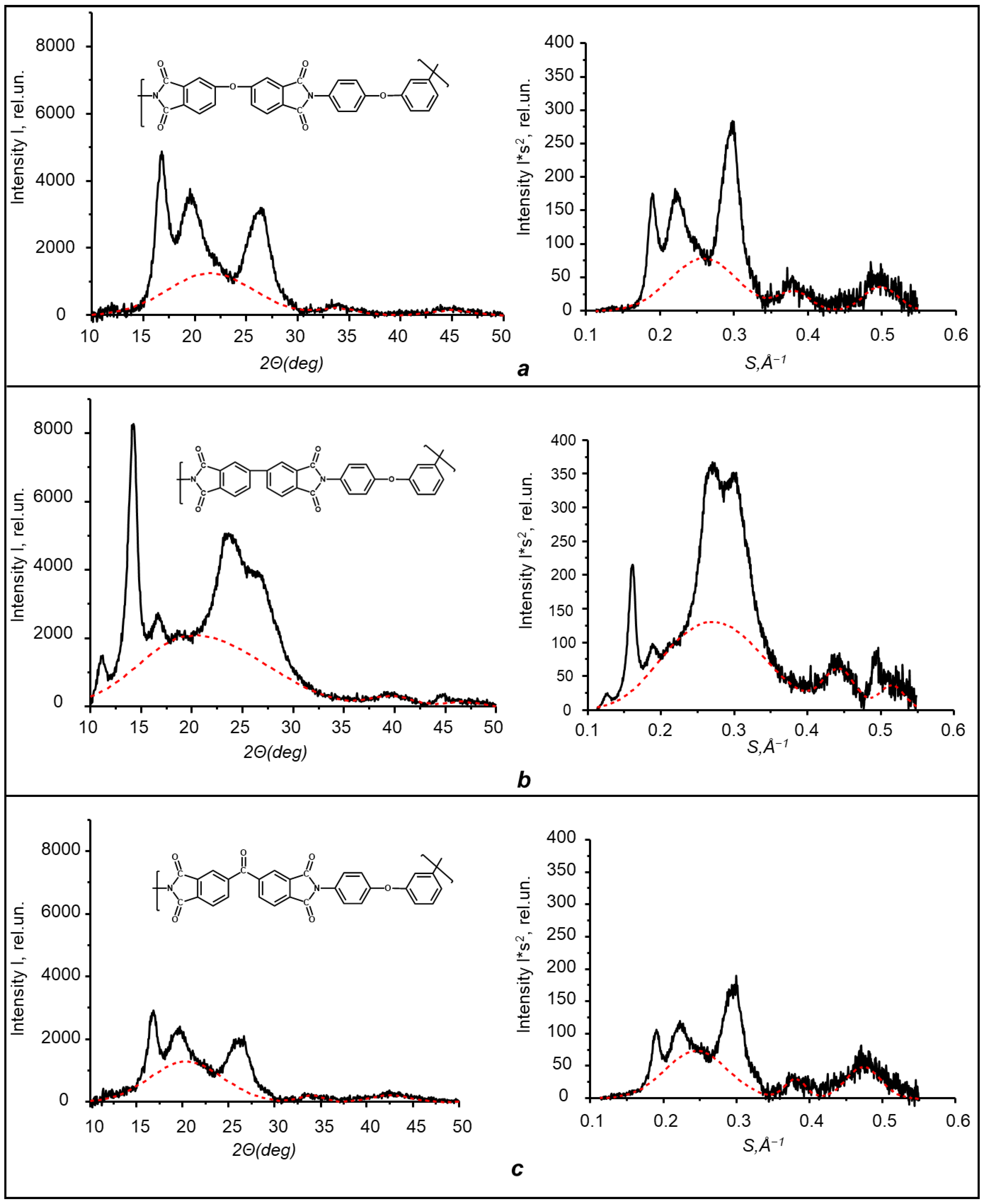
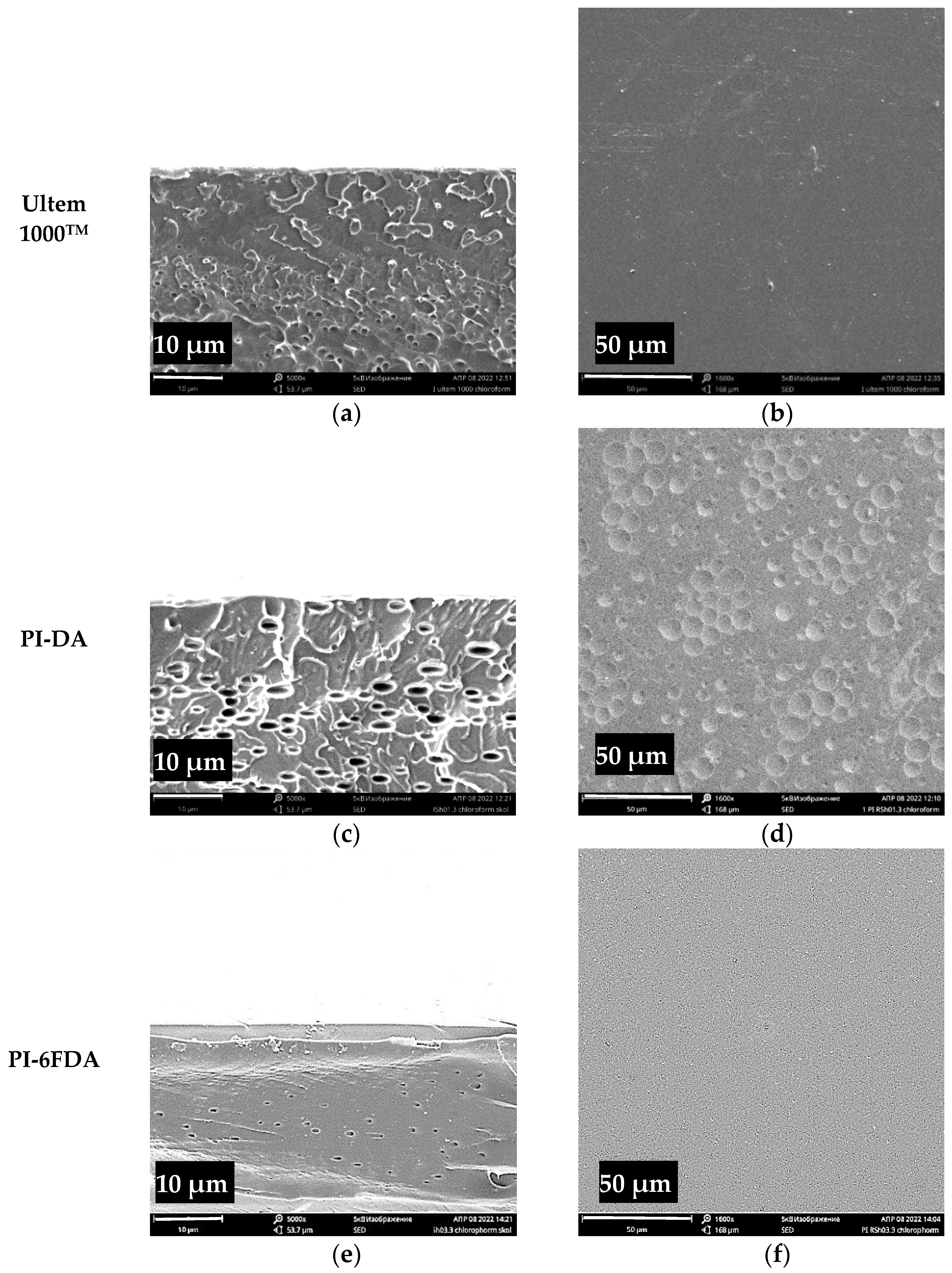
3.4. Morphology of Obtained Polyimides
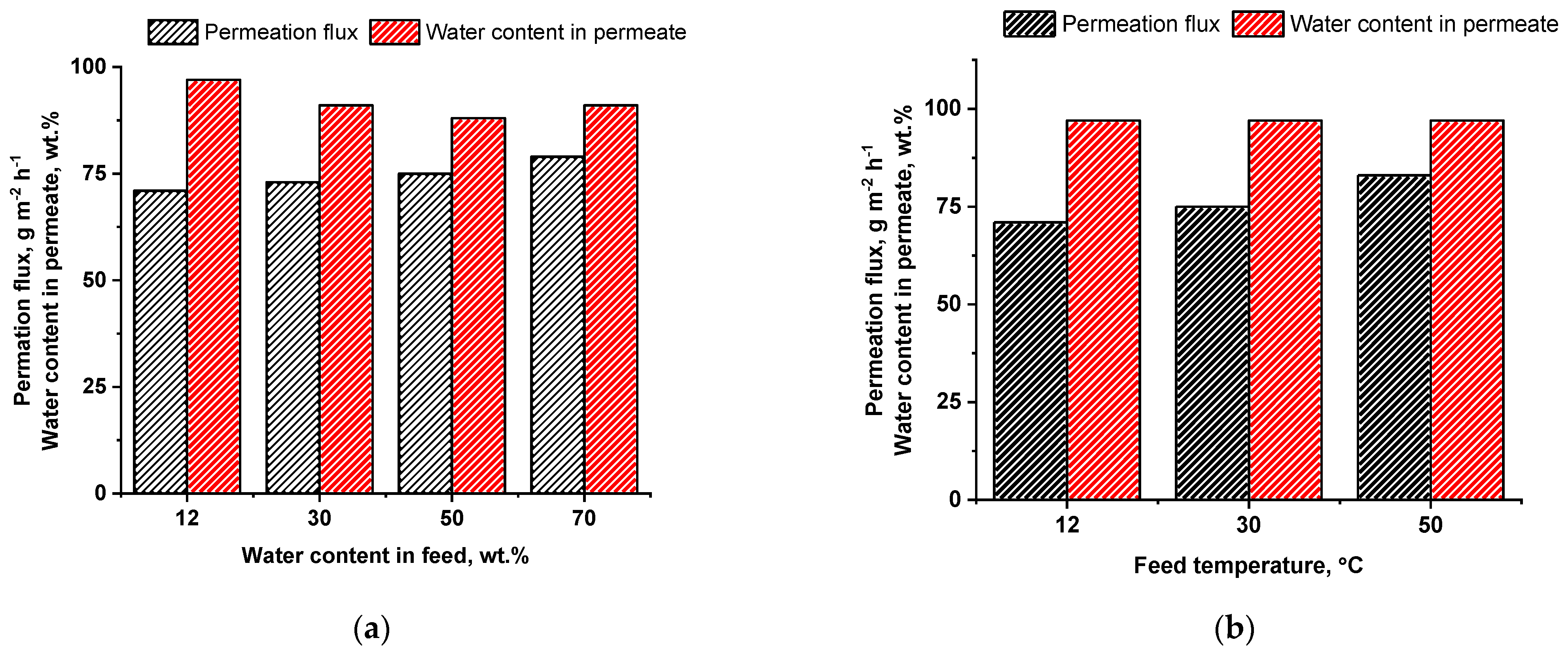
3.5. Study of Transport Properties PIs in Pervaporation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mittal, V. High Performance Polymers and Engineering Plastics; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Bessonov, M.I.; Koton, M.M.; Kudryavtsev, V.V. Polyimide; Springer: New York, NY, USA, 1987. [Google Scholar]
- Abajo, J.; de la Campa, J.G. Processable Aromatic Polyimides. In Progress in Polyimide Chemistry I; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Liaw, D.J.; Wang, K.L.; Huang, Y.C.; Lee, K.R.; Lai, J.Y.; Ha, C.S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Hou, T.H.; Chang, A.C.; Johnston, N.J.; St Clair, T.L. Processing and Properties of IM7/LARC [TM]-IAX2 Polyimide Composites. J. Adv. Mater. 1996, 27, 2. [Google Scholar]
- Dorsey, K.D.; Desai, P.; Abhiraman, A.S.; Hinkley, J.A.; St Clair, T.L. Structure and properties of melt-extruded laRC-IA (3, 4′-ODA 4, 4′-ODPA) polyimide fibers. J. Appl. Polym. Sci. 1999, 73, 7. [Google Scholar] [CrossRef]
- Fay, C.C.; Hinkley, J.A.; St Clair, T.L.; Working, D.C. Mechanical properties of LaRCTM-IA and ULTEM® melt-extruded fibers and melt-pressed films. Adv. Perform. Mater. 1998, 5, 193–200. [Google Scholar] [CrossRef]
- Hou, T.H.; St Clair, T.L. IM7/LaRCTM-IAX-3 polyimide composites. High Perform. Polym. 1998, 10, 193–206. [Google Scholar] [CrossRef]
- Wang, Q.; Bai, Y.; Chen, Y.; Ju, J.; Zheng, F.; Wang, T. High performance shape memory polyimides based on π–π interactions. J. Mater. Chem. A 2015, 3, 352–359. [Google Scholar] [CrossRef]
- Web Site Ube Corparation. Available online: https://www.ube.com/upilex/en/upilex.html (accessed on 15 June 1995).
- Tamai, S.; Kuroki, T.; Shibuya, A.; Yamaguchi, A. Synthesis and characterization of thermally stable semicrystalline polyimide based on 3, 4′-oxydianiline and 3, 3′, 4, 4′-biphenyltetracarboxylic dianhydride. Polymer 2001, 42, 2373–2378. [Google Scholar] [CrossRef]
- Bryant, R.G. LaRC ™-SI: A Soluble Aromatic Polyimide. High Perform. Polym. 1996, 8, 607–616. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, Q.; Xu, Y.; Xia, Q.; Chen, D. Homogenous one-pot synthesis of polyimides in polyphosphoric acid. Eur. Polym. J. 2009, 45, 2805–2811. [Google Scholar] [CrossRef]
- Seo, J.; Han, H.; Lee, A.; Han, J. Effect of isomeric oxydiphenylene diamine on the water sorption behavior of high temperature polyimide thin films. Polym. J. 1999, 31, 324–331. [Google Scholar] [CrossRef]
- Soldatova, A.E.; Tsegelskaya, A.Y.; Semenova, G.K.; Kurkin, T.S.; Dmitryakov, P.V.; Belousov, S.I.; Kuznetsov, A.A. One-pot synthesis of semicrystalline polyamide imide based on 4, 4’-diaminobenzanilide and 2, 2-propylidene-bis (1, 4-phenyleneoxy) diphthalic anhydride in molten benzoic acid. High Perform. Polym. 2019, 31, 63–71. [Google Scholar] [CrossRef]
- Koning, C.; Delmotte, A.; Larno, P.; Van Mele, B. Influence of polymerization conditions on melt crystallization of partially aliphatic polyimides. Polymer 1998, 39, 3697–3702. [Google Scholar] [CrossRef]
- Kuznetsov, A.A.; Tsegelskaya, A.Y. Synthesis of Polyimides in the Melt of Benzoic Acid. In Solvents, Ionic Liquids and Solvent Effects; IntechOpen: London, UK, 2019. [Google Scholar]
- Kuznetsov, A.A. One-pot polyimide synthesis in carboxylic acid medium. High Perform. Polym. 2000, 12, 445. [Google Scholar] [CrossRef]
- Tsegelskaya, A.; Dutov, M.; Serushkina, O.; Semenova, G.; Kuznetsov, A. The One-Stage Synthesis of Hyperbranched Polyimides by (A2+ B4) Scheme in Catalytic Solvent. Macromol. Symp. 2017, 375, 1600202. [Google Scholar] [CrossRef]
- Kuznetsov, A.A.; Soldatova, A.E.; Tokmashev, R.Y.; Tsegelskaya, A.Y.; Semenova, G.K.; Shakhnes, A.K.; Abramov, I.G. Synthesis of reactive three-arm star-shaped oligoimides with narrow molecular weight distribution. J. Polym. Sci. A Polym. Chem. 2018, 56, 2004–2009. [Google Scholar] [CrossRef]
- Batuashvili, M.R.; Tsegelskya, A.Y.; Perov, N.S.; Semenova, G.K.; Abramov, I.G.; Kuznetsov, A.A. Chain microstructure of soluble copolyimides containing moieties of aliphatic and aromatic diamines and aromatic dianhydrides prepared in molten benzoic acid. High Perform. Polym. 2014, 26, 470–476. [Google Scholar] [CrossRef]
- Kuznetsov, A.A.; Tsegelskaya, A.Y.; Buzin, P.V.; Yablokova, M.Y.; Semenova, G.K. High Temperature Polyimide Synthesis in ‘Active’Medium: Reactivity Leveling of the High and the Low Basic Diamines. High Perform. Polym. 2007, 19, 711–721. [Google Scholar] [CrossRef]
- Ruland, W. X-ray determination of crystallinity and diffuse disorder scattering. Acta Cryst. 1961, 14, 1180–1185. [Google Scholar] [CrossRef]
- Burts, K.S.; Plisko, T.V.; Prozorovich, V.G.; Melnikova, G.B.; Ivanets, A.I.; Bildyukevich, A.V. Development and Study of PVA–SiO2/poly (AN-co-MA) Dynamic Nanocomposite Membranes for Ethanol Dehydration via Pervaporation. Membr. Membr. Technol. 2022, 4, 101. [Google Scholar] [CrossRef]
- Kujawski, J.; Rozicka, A.; Bryjak, M.; Kujawski, W. Pervaporative removal of acetone, butanol and ethanol from binary and multicomponent aqueous mixtures. Separ. Purif. Tech. 2014, 132, 422. [Google Scholar] [CrossRef]
- Lakshmy, K.S.; Lal, D.; Nair, A.; Babu, A.; Das, H.; Govind, N.; Dmitrenko, M.; Kuzminova, A.; Korniak, A.; Penkova, A.; et al. Pervaporation as a Successful Tool in the Treatment of Industrial Liquid Mixtures. Polymers 2022, 14, 1604. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, C.; Li, J. Experimental study on physical properties and pervaporation performances of polyimide membranes. Chem. Eng. Sci. 2007, 62, 2466. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Y. Novel thermally cross-linked polyimide membranes for ethanol dehydration via pervaporation. J. Membr. Sci. 2015, 496, 142. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W. Pervaporation membrane materials: Recent trends and perspectives. J. Membr. Sci. 2021, 636, 119557. [Google Scholar] [CrossRef]
- Kim, J.-H.; Chang, B.-J.; Lee, S.-B.; Kim, S.Y. Incorporation effect of fluorinated side groups into polyimide membranes on their pervaporation properties. J. Membr. Sci. 2000, 169, 185. [Google Scholar] [CrossRef]
- Qiao, X.; Chung, T.-S.; Rajagopalan, R. Zeolite filled P84 co-polyimide membranes for dehydration of isopropanol through pervaporation process. Chem. Eng. Sci. 2006, 61, 6816–6825. [Google Scholar] [CrossRef]
- Jiang, L.Y.; Chung, T.S. Homogeneous polyimide/cyclodextrin composite membranes for pervaporation dehydration of isopropanol. J. Membr. Sci. 2010, 346, 45–58. [Google Scholar] [CrossRef]
- Mosleh, S.; Khosravi, T.; Bakhtiari, O.; Mohammadi, T. Zeolite filled polyimide membranes for dehydration of isopropanol through pervaporation process. Chem. Eng. Res. Design. 2012, 90, 433–441. [Google Scholar] [CrossRef]
- Ong, Y.K.; Wang, H.; Chung, T.S. A prospective study on the application of thermally rearranged acetate-containing polyimide membranes in dehydration of biofuels via pervaporation. Chem. Eng. Sci. 2012, 9, 41–53. [Google Scholar] [CrossRef]
- Jiang, L.Y.; Chung, T.S.; Rajagopalan, R. Matrimid®/MgO mixed matrix membranes for pervaporation. AIChE J. 2007, 53, 1745–1757. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, L.Y.; Matsuura, T.; Chung, T.S.; Goh, S.H. Investigation of the fundamental differences between polyamide-imide (PAI) and polyetherimide (PEI) membranes for isopropanol dehydration via pervaporation. J. Membr. Sci. 2008, 318, 217–226. [Google Scholar] [CrossRef]
- Xiao, S.; Feng, X.S.; Huang, R.Y.M. 2,2-Bis[4-(3,4-dicarboxyphenoxy) phenyl] propane dianhydride (BPADA)-based polyimide membranes for pervaporation dehydration of isopropanol: Ccharacterization and comparison with 4,4′-(hexafluoroisopropylidene) diphthalic anhydride (6FDA)-based polyimide membranes. J. Appl. Polym. Sci. 2008, 110, 283–296. [Google Scholar] [CrossRef]
- Hua, D.; Ong, Y.K.; Wang, Y.; Yang, T.X.; Chung, T.S. ZIF-90/P84 mixed matrix membranes for pervaporation dehydration of isopropanol, J. Membr. Sci. 2014, 53, 155–167. [Google Scholar] [CrossRef]
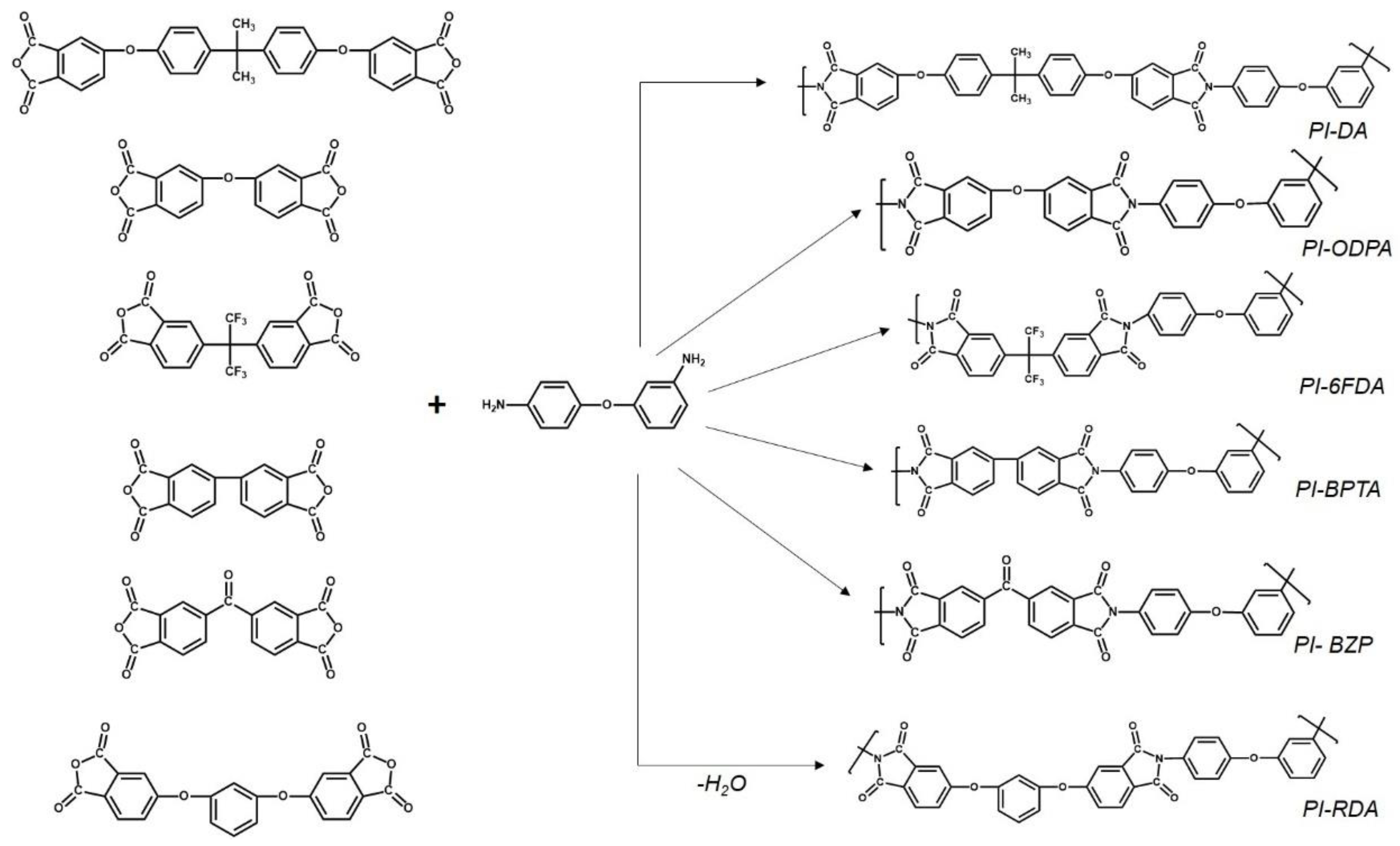

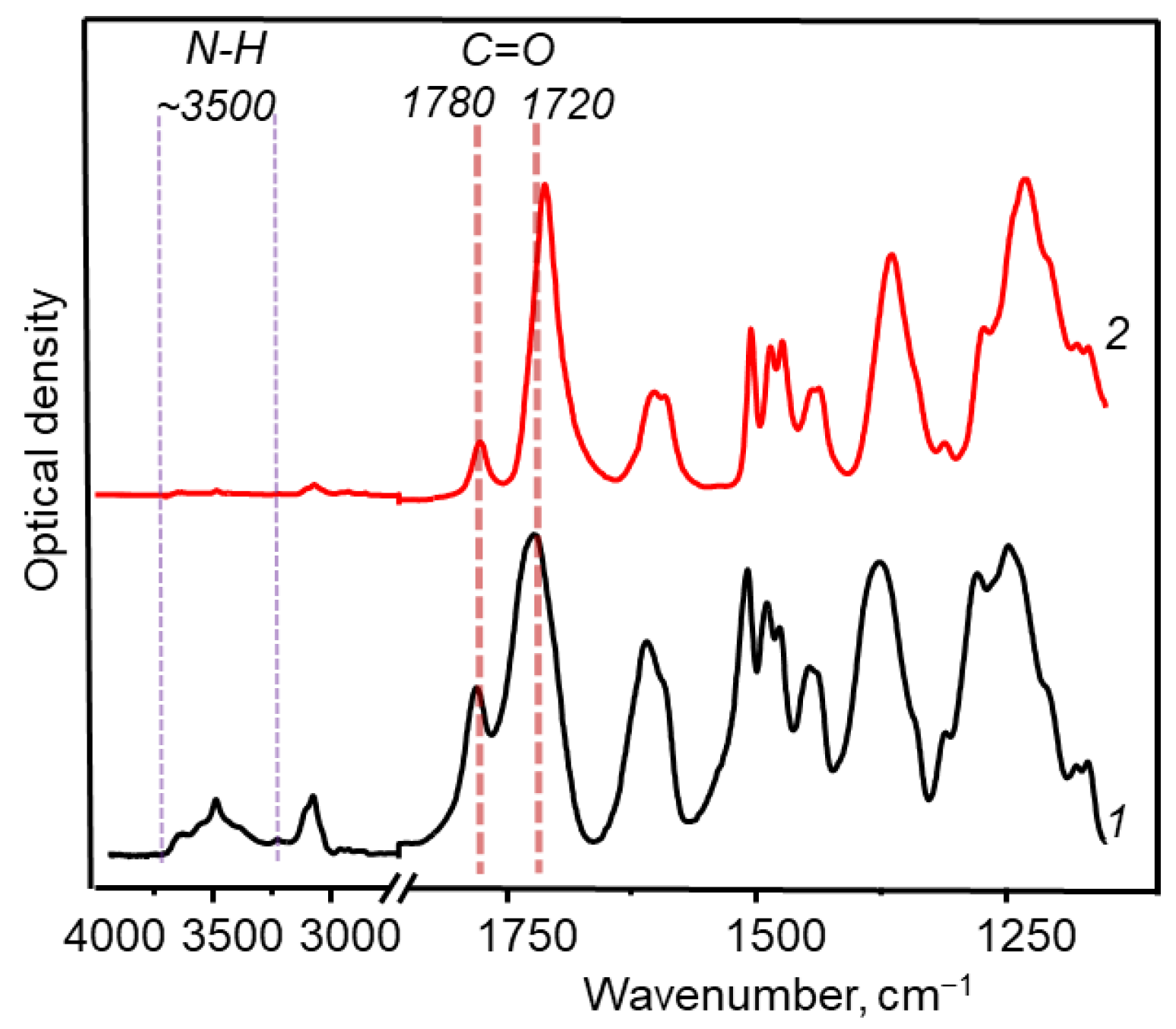
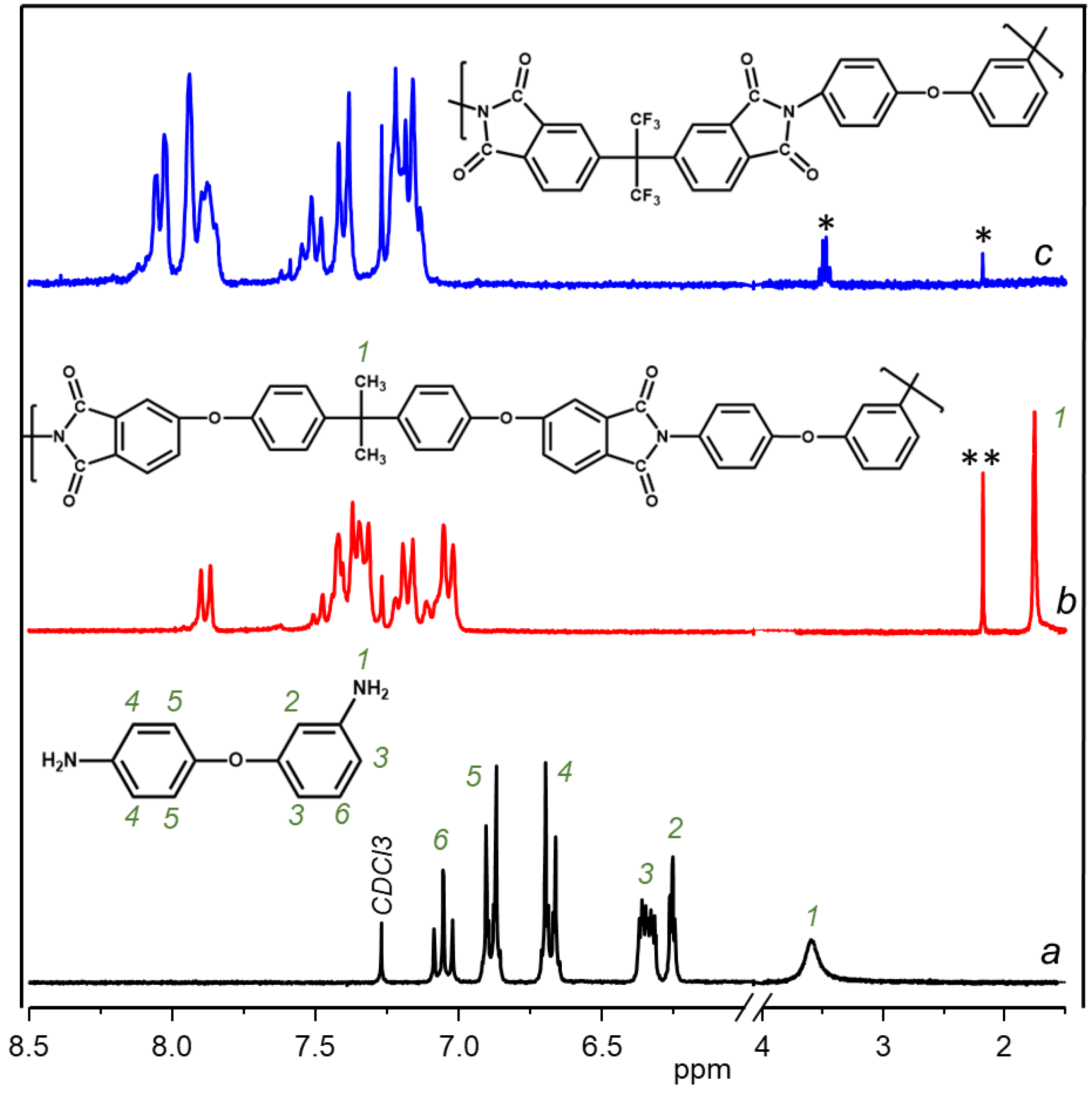




| Sample | Dianhydride | Solubility | |||||
|---|---|---|---|---|---|---|---|
| BA | THF | CHCl3 | NMP | DMAA | DMSO | ||
| PI-DA | 4,4′-(4,4′-Isopropylidenediphenoxy)bis(phthalic anhydride) | + | + | + | + | + | + * |
| PI-ODPA | 4,4′-Oxydiphthalic anhydride | - | - | - | - | - | - |
| PI-6FDA | 4,4′-(Hexafluoroisopropylidene) diphthalic anhydride | + | + | + | + | + | + |
| PI-BPTA | 3,3′,4,4′-Biphenyltetracarboxylic dianhydride | - | - | - | - | - | - |
| PI-BZP | Benzophenone-3,3′,4,4′-tetracarboxylic dianhydride | - | - | - | - | - | - |
| PI-RDA | 1,4-Bis(3,4-Dicarboxyphenoxy)Benzene dianhydride | + | - | +- | + | + | + * |
| Sample | Mn | Mw | Mw/Mn | η, dL/g |
|---|---|---|---|---|
| PI-DA | 44300 | 78200 | 1.8 | 0.39 |
| PI-ODPA | - | - | - | 0.86/ND * |
| PI-6FDA | 108200 | 118500 | 1.1 | 1.04 |
| PI-BPDA | - | - | - | 0.71/1.28 * |
| PI-BZP | - | - | - | 0.55/0.66 * |
| PI-RDA | 46700 | 87300 | 1.9 | 0.39 |
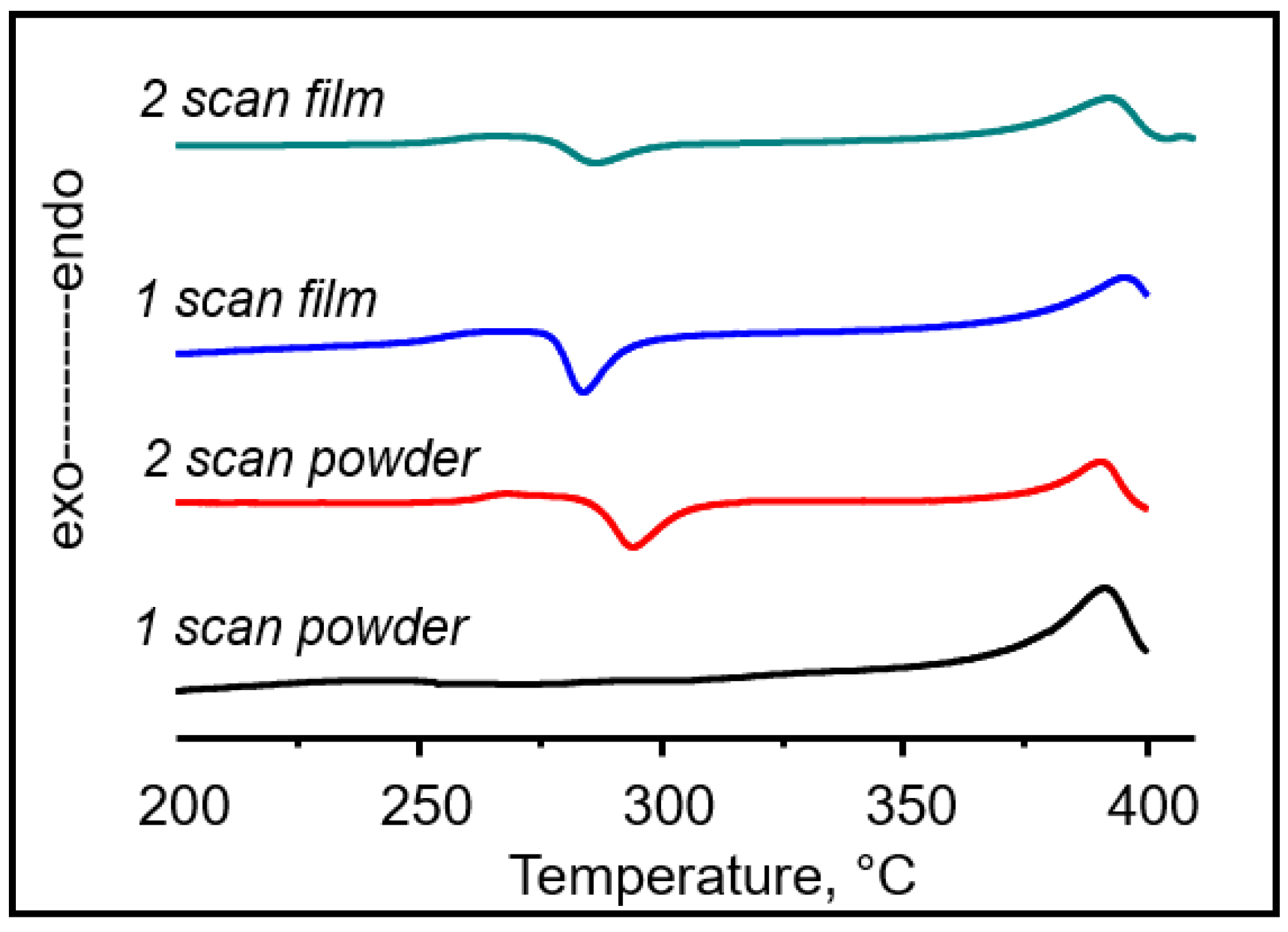
| DSC-Mode | State | Tc, °C | ∆Hc, J/g | Tm, °C | ∆Hm, J/g |
|---|---|---|---|---|---|
| 1 scan | powder | - | - | 388 | 9.88 |
| 2 scan | 294 | 13.77 | 390 | 14.21 | |
| 1 scan | film | 284 | 14.6 | 395 | 5.81 |
| 2 scan | 286 | 7.53 | 392 | 17.77 |
| Name of Sample | Tg, °C | Tm, °C | Td5%, °C | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 Scan | 2 Scan | Film | 1 Scan | 2 Scan | Film | Powder | Film | |||
| Air | Argon | Air | Argon | |||||||
| PI-DA | 197 | 200 | 200 | - | 517 | 522 | 520 | 520 | ||
| PI-ODPA | ND | 244 | 244 | 302 | - | - | 535 | 570 | 551 | 554 |
| PI-6FDA | 268 | 270 | 270 | - | 512 | 530 | 525 | 515 | ||
| PI-BPTA | ND | 264 | 262 | 390 | 390 | 390 | 494 | 573 | 548 | 560 |
| PI-BZP | ND | 230 | 233 | 306 | - | - | 443 | 565 | 512 | 520 |
| PI-RDA | 187 | 198 | 198 | - | 515 | 522 | 511 | 520 | ||
| PI | J 1, kg m−2 h−1 | L 2, µm | JN 3, kg µm m−2 h−1 | Water Content in Permeate, wt.% | β 4 | PSIN 5, kg µm m−2 h−1 | Water Contact Angle, ° |
|---|---|---|---|---|---|---|---|
| Ultem 1000™ | 0.032 | 36 | 1.15 | 74 | 21 | 0.63 | 87 |
| PI-DA | 0.071 | 39 | 2.77 | 97 | 264 | 18.65 | 87 |
| PI-DA | 0.045 | 61 | 2.77 | 97 | 264 | 18.65 | 87 |
| PI-6FDA | 0.015 | 28 | 0.42 | 62 | 12 | 0.17 | 92 |
| Membrane Designation | Eapp (Water), J mol−1 | Eapp (Ethanol), J mol−1 |
|---|---|---|
| PI-DA | 6.08 | 14.35 |
| PI-6FDA | 42.26 | 52.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soldatova, A.E.; Shamsutdinova, R.N.; Plisko, T.V.; Burts, K.S.; Tsegelskaya, A.Y.; Khanin, D.A.; Monakhova, K.Z.; Kurkin, T.S.; Bildyukevich, A.V.; Kuznetsov, A.A. Synthesis of Aromatic Polyimides Based on 3,4′-Oxydianiline by One-Pot Polycondensation in Molten Benzoic Acid and Their Application as Membrane Materials for Pervaporation. Materials 2022, 15, 6845. https://doi.org/10.3390/ma15196845
Soldatova AE, Shamsutdinova RN, Plisko TV, Burts KS, Tsegelskaya AY, Khanin DA, Monakhova KZ, Kurkin TS, Bildyukevich AV, Kuznetsov AA. Synthesis of Aromatic Polyimides Based on 3,4′-Oxydianiline by One-Pot Polycondensation in Molten Benzoic Acid and Their Application as Membrane Materials for Pervaporation. Materials. 2022; 15(19):6845. https://doi.org/10.3390/ma15196845
Chicago/Turabian StyleSoldatova, Anastasiia E., Regina N. Shamsutdinova, Tatiana V. Plisko, Katsiaryna S. Burts, Anna Yu. Tsegelskaya, Dmitry A. Khanin, Kristina Z. Monakhova, Tikhon S. Kurkin, Alexandr V. Bildyukevich, and Alexander A. Kuznetsov. 2022. "Synthesis of Aromatic Polyimides Based on 3,4′-Oxydianiline by One-Pot Polycondensation in Molten Benzoic Acid and Their Application as Membrane Materials for Pervaporation" Materials 15, no. 19: 6845. https://doi.org/10.3390/ma15196845
APA StyleSoldatova, A. E., Shamsutdinova, R. N., Plisko, T. V., Burts, K. S., Tsegelskaya, A. Y., Khanin, D. A., Monakhova, K. Z., Kurkin, T. S., Bildyukevich, A. V., & Kuznetsov, A. A. (2022). Synthesis of Aromatic Polyimides Based on 3,4′-Oxydianiline by One-Pot Polycondensation in Molten Benzoic Acid and Their Application as Membrane Materials for Pervaporation. Materials, 15(19), 6845. https://doi.org/10.3390/ma15196845