Bright UV-C Phosphors with Excellent Thermal Stability—Y1−xScxPO4 Solid Solutions
Abstract
1. Introduction
2. Experimental Section
2.1. Synthesis of Samples
2.2. Structural Characterization
2.3. Luminescence Characterization
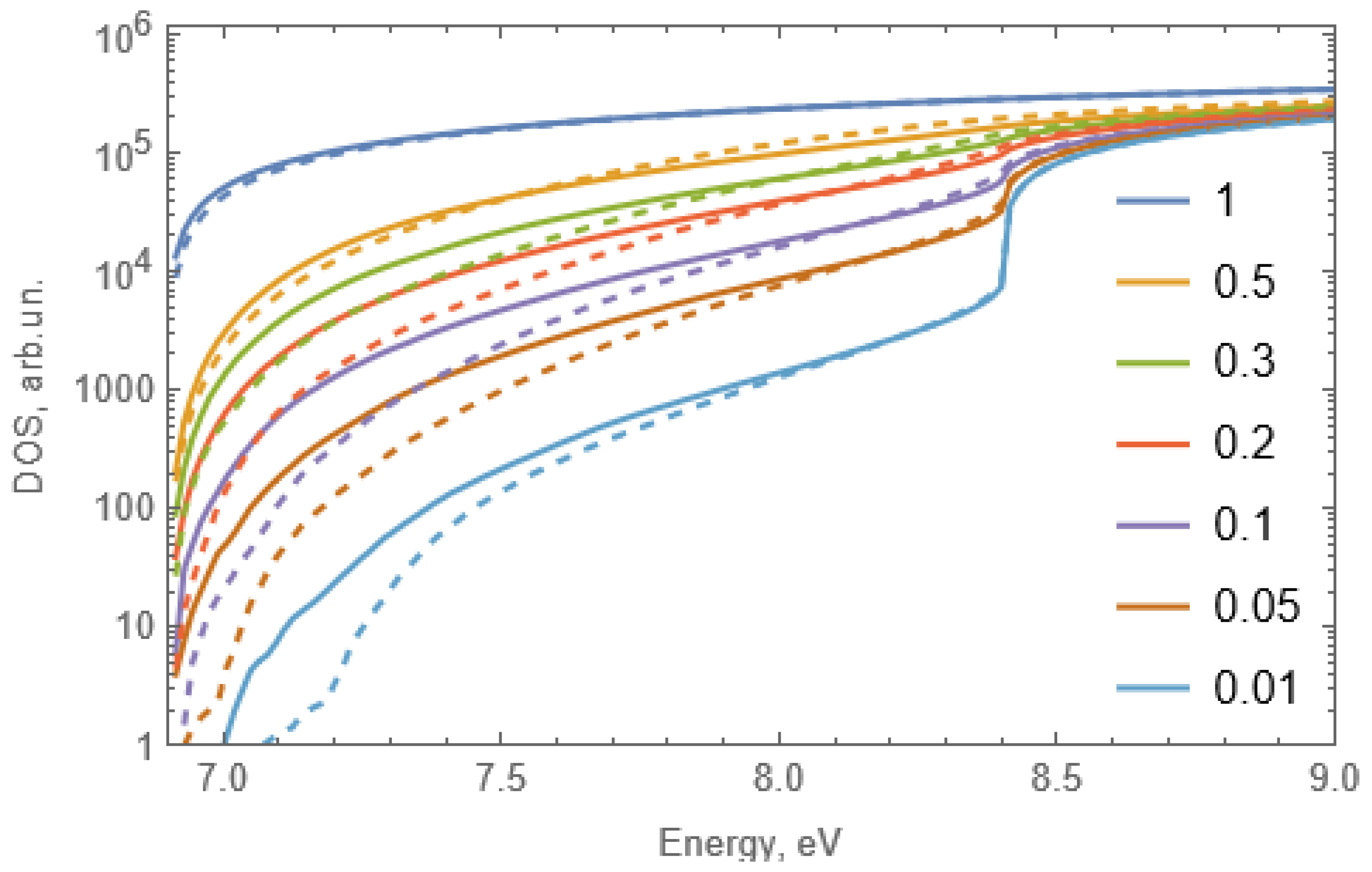
2.4. Numerical Simulation of the Density of States of Solid Solutions
2.5. SEM Investigation
3. Results and Discussion
3.1. Crystal Structure
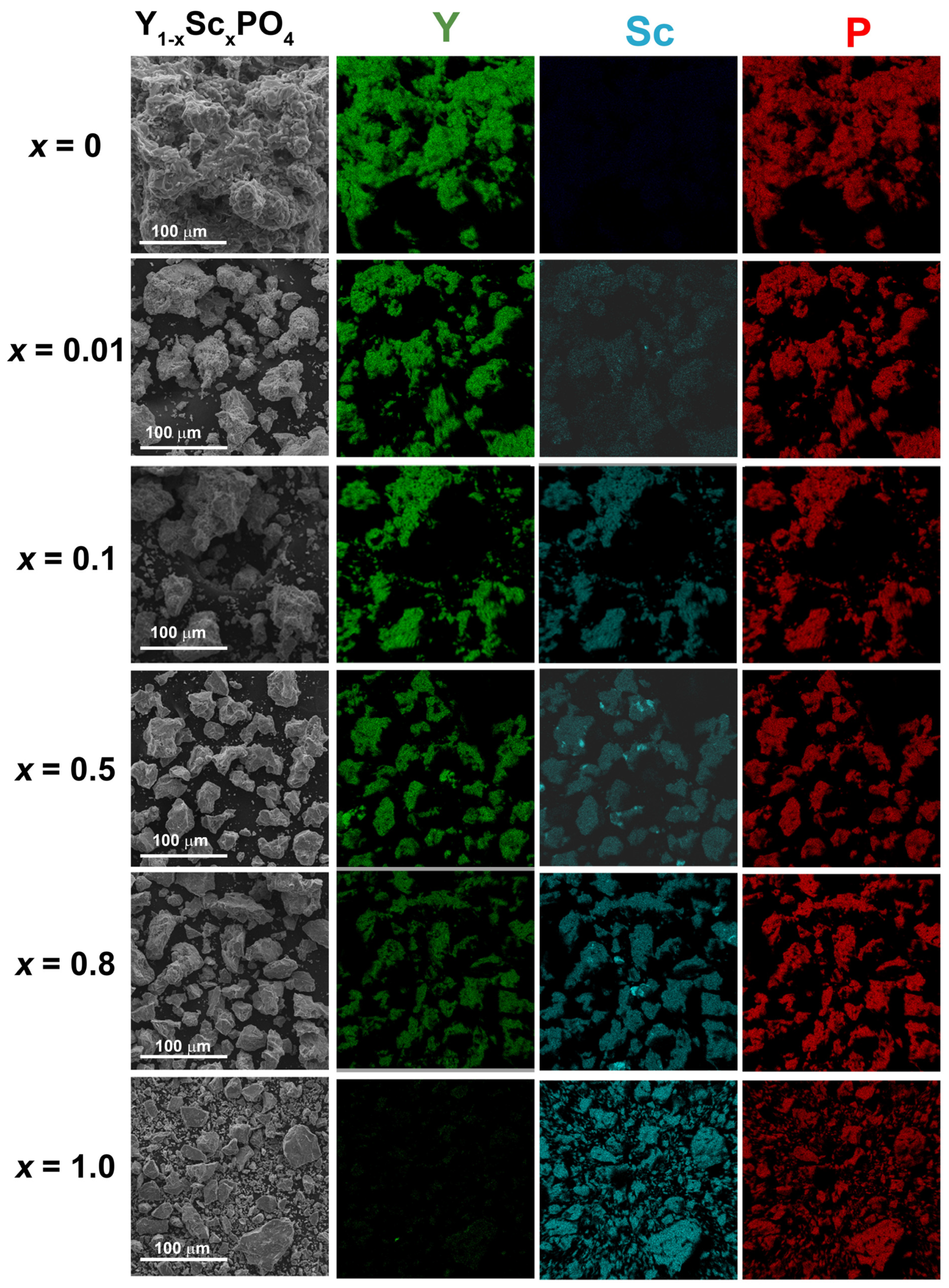
3.2. SEM Analysis
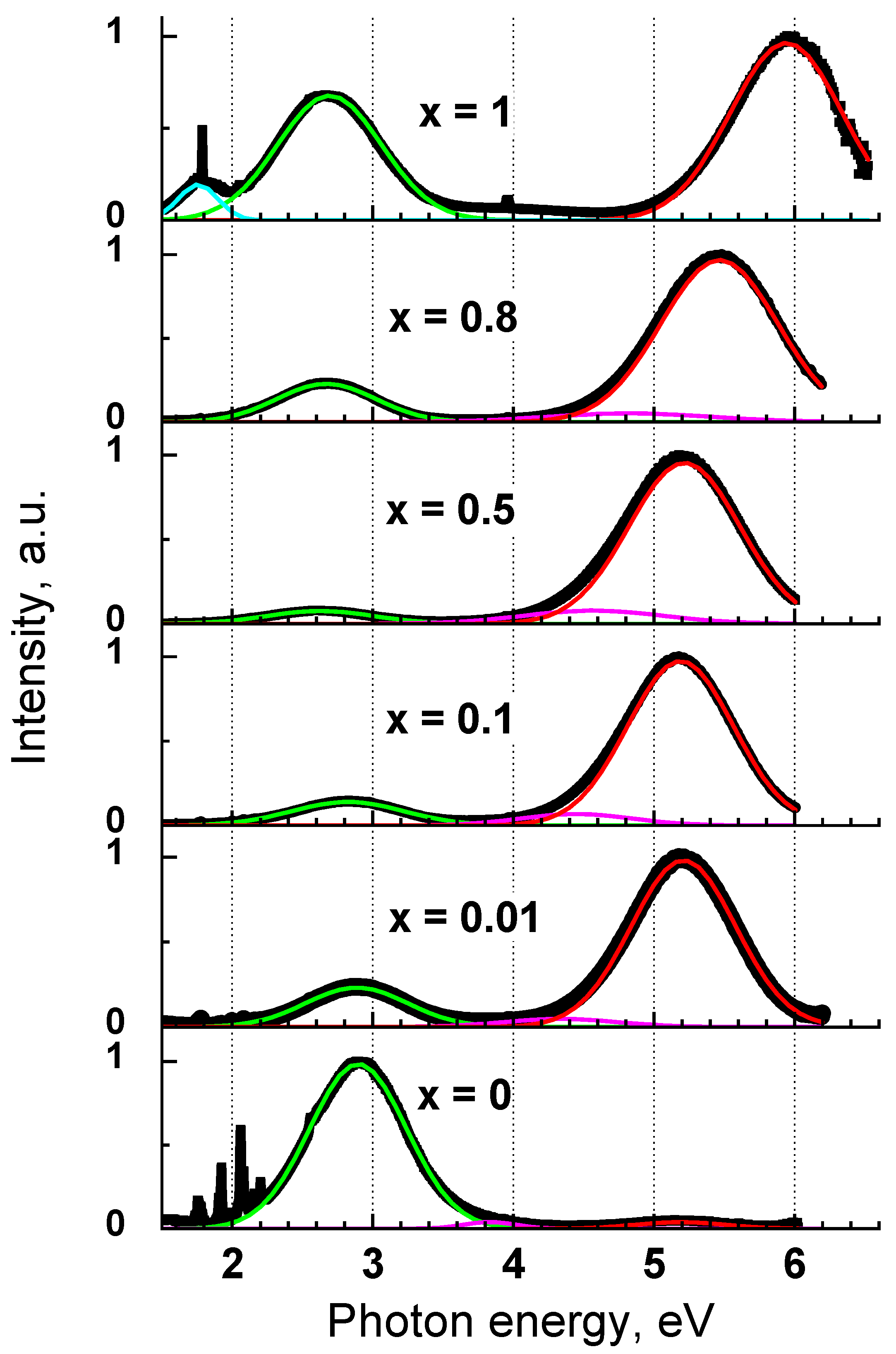
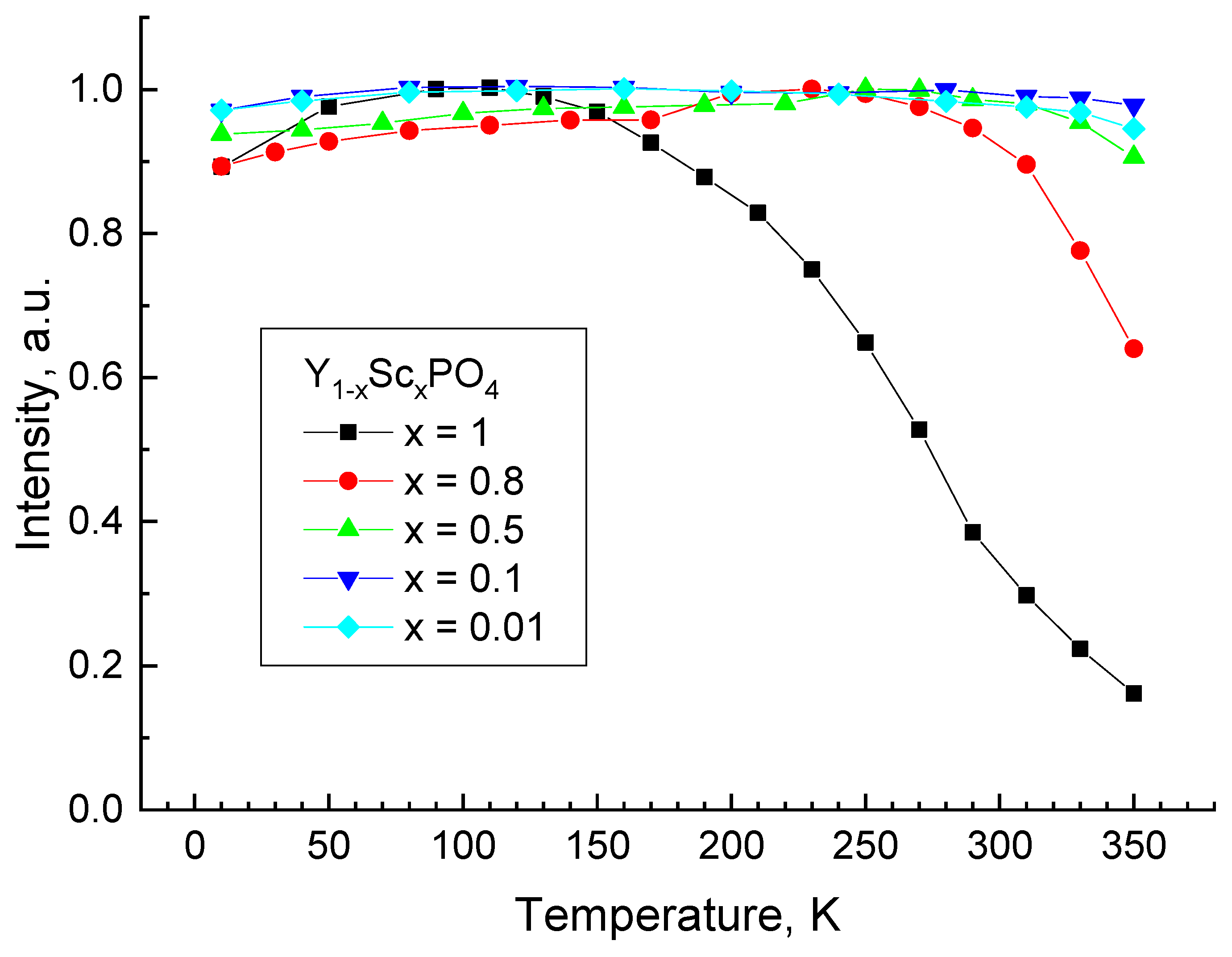
3.3. Luminescence Properties of Y1−xScxPO4
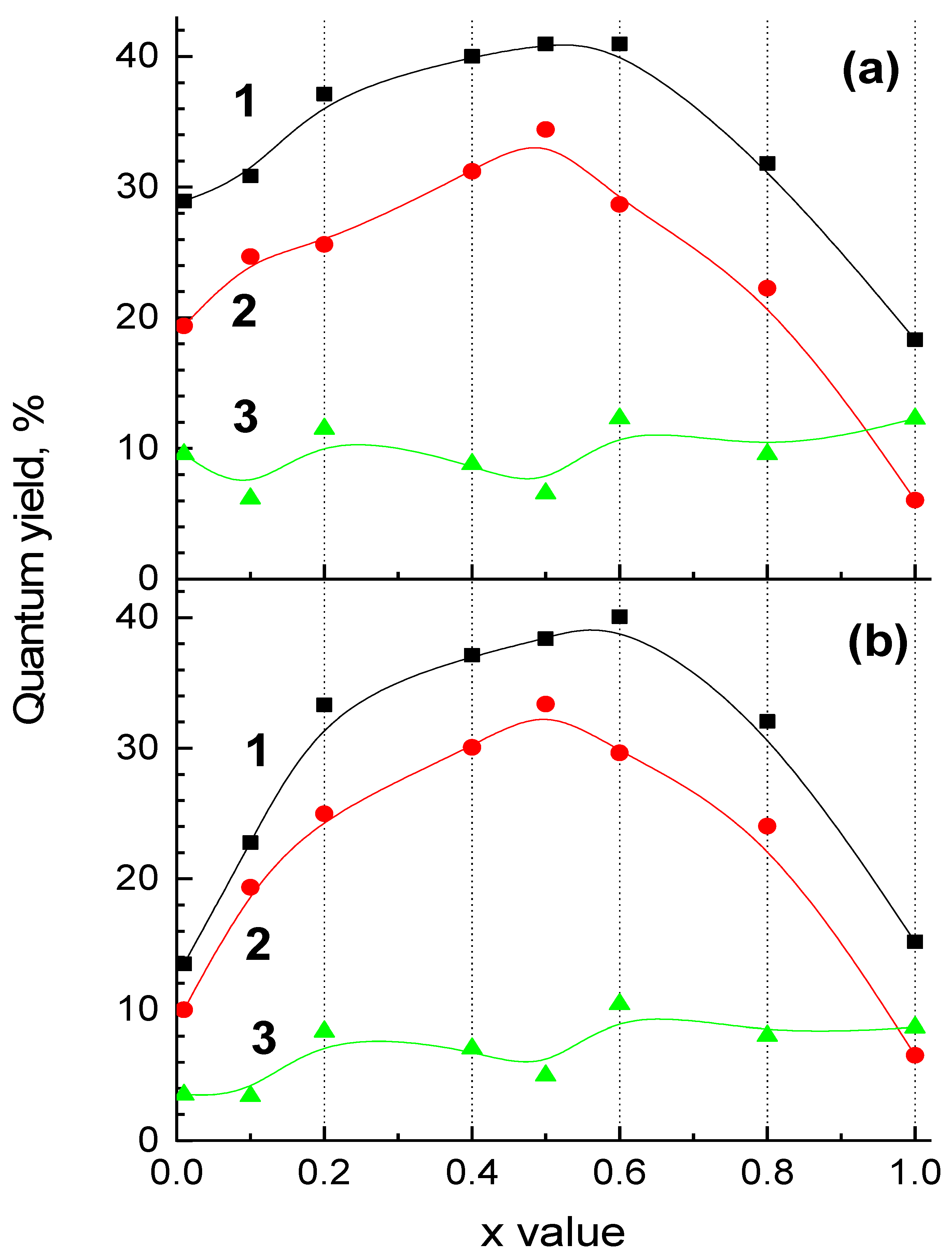
3.4. Quantum Yield of Y1−xScxPO4
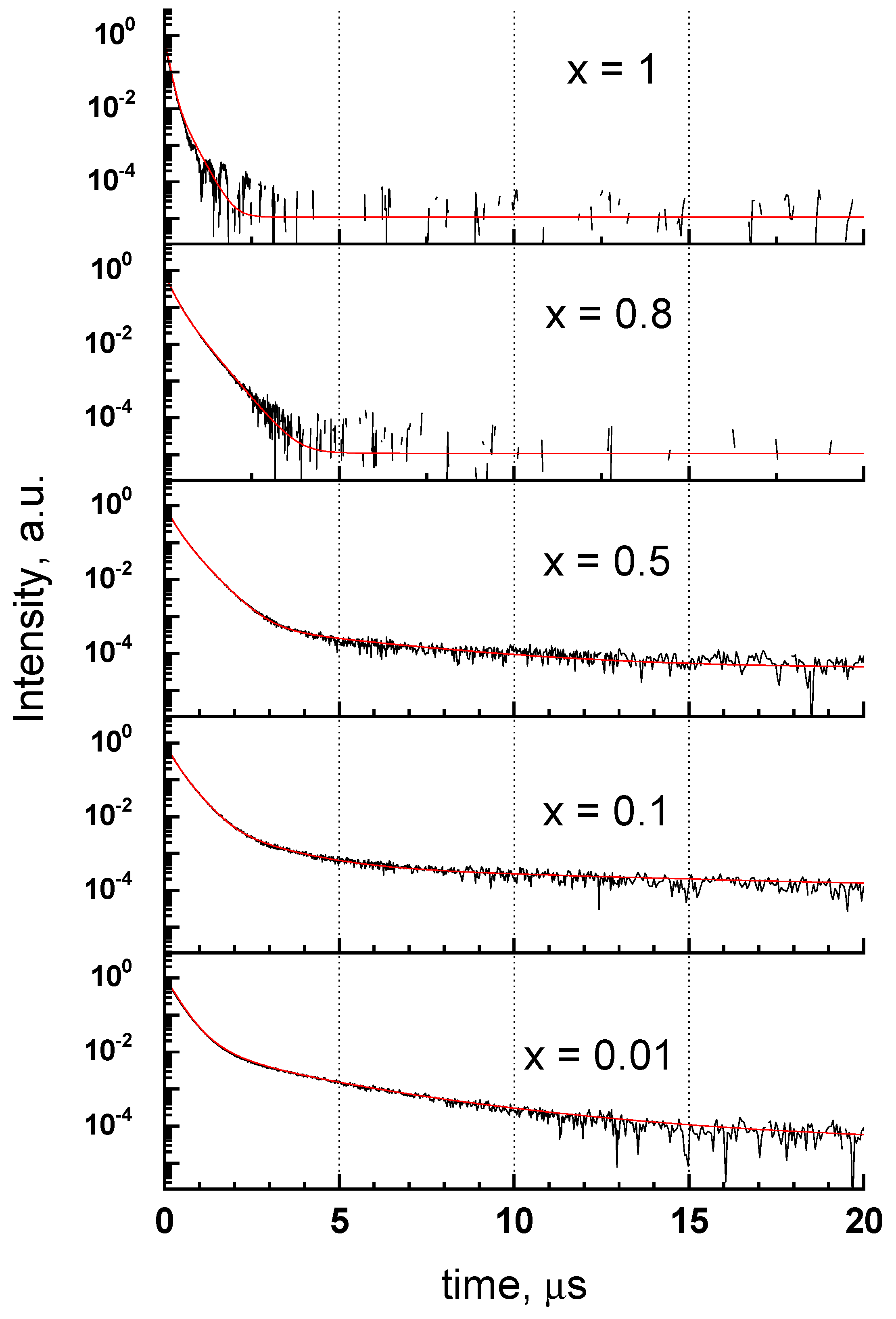
3.5. Cathodoluminescence Decay Characteristics of Y1−xScxPO4
3.6. Discussion
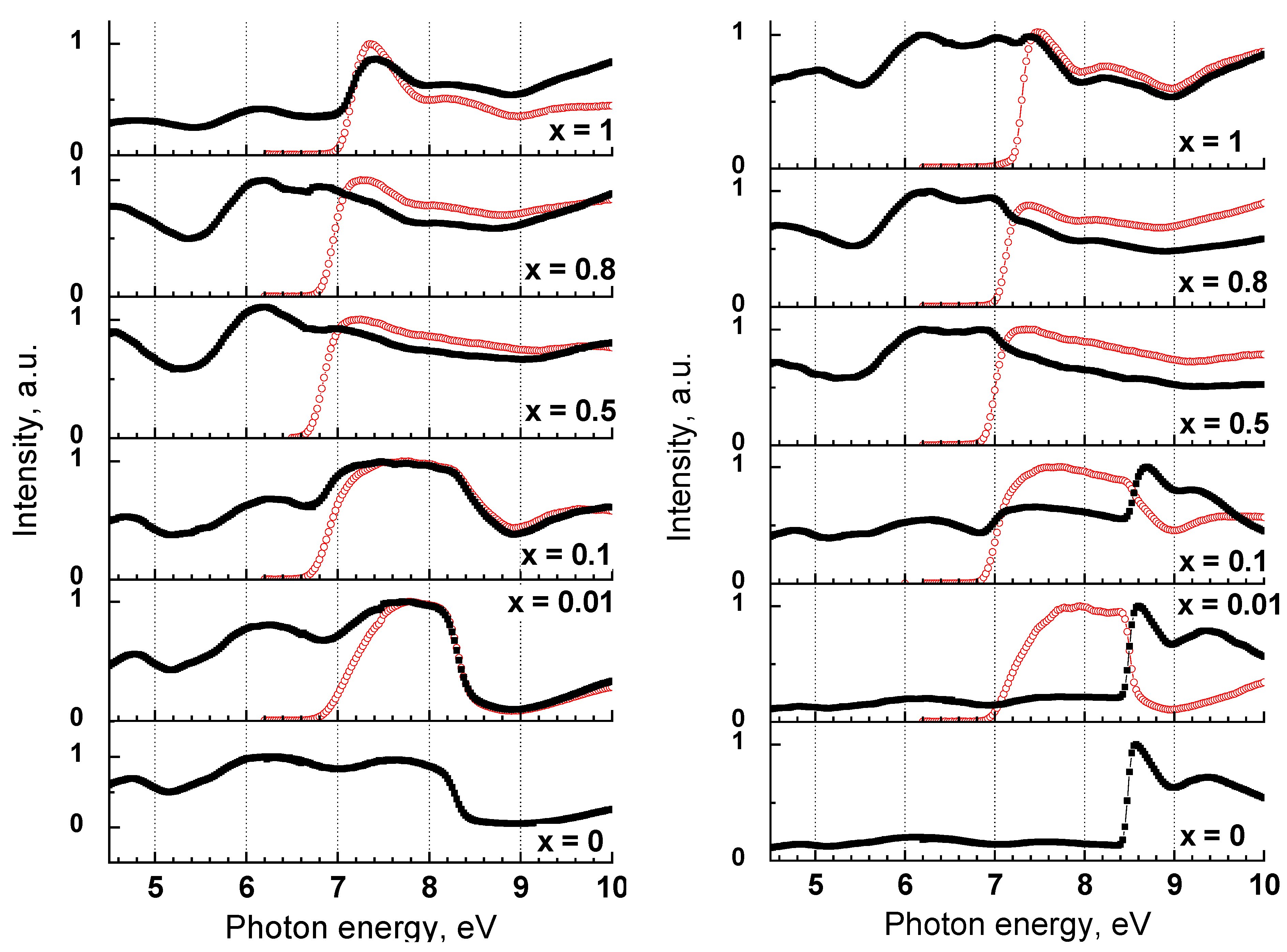
3.6.1. Origin of UV-C Emission Band
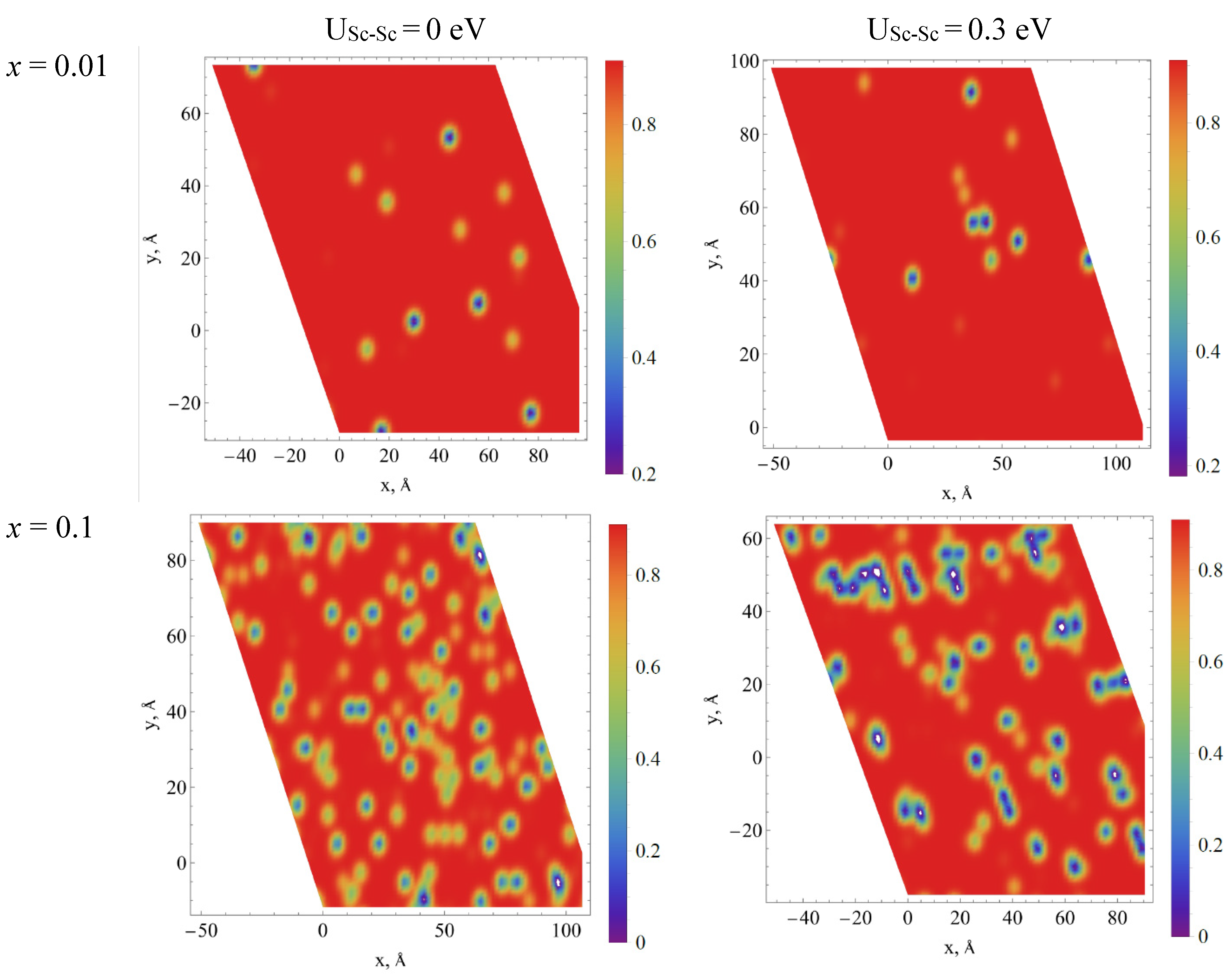
3.6.2. Influence of Solid Solution Clusterization on the Energy Transfer Processes in Y1−xScxPO4
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Chen, Y.; Liu, F.; Pan, Z. Solar-blind ultraviolet-C persistent luminescence phosphors. Nat. Commun. 2020, 11, 2040. [Google Scholar] [CrossRef]
- Zhou, Y.; Jia, D.; Lewis, L.; Feofilov, S.; Meltzer, R. Deep UV emitting scintillators for alpha and beta particle detection. Nucl. Instrum. Methods Phys. Res. A 2011, 633, 31–35. [Google Scholar] [CrossRef]
- Xiong, P.; Peng, M. Recent advances in ultraviolet persistent phosphors. Opt. Mater. X 2019, 2, 100022. [Google Scholar] [CrossRef]
- Caillier, B.; Caiut, J.M.A.; Muja, C.; Demoucron, J.; Mauricot, R.; Dexpert-Ghys, J.; Guillot, P. Decontamination Efficiency of a DBD Lamp Containing an UV-C Emitting Phosphor. Photochem. Photobiol. 2015, 91, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Belessiotis, G.V.; Falara, P.P.; Ibrahim, I.; Kontos, A.G. Magnetic Metal Oxide-Based Photocatalysts with Integrated Silver for Water Treatment. Materials 2022, 15, 4629. [Google Scholar] [CrossRef] [PubMed]
- Miwa, S.; Yano, S.; Hiroshima, Y.; Tome, Y.; Uehara, F.; Mii, S.; Efimova, E.V.; Kimura, H.; Hayashi, K.; Tsuchiya, H.; et al. Imaging UVC-induced DNA damage response in models of minimal cancer. J. Cell. Biochem. 2013, 114, 2493–2499. [Google Scholar] [CrossRef]
- Kimura, H.; Lee, C.; Hayashi, K.; Yamauchi, K.; Yamamoto, N.; Tsuchiya, H.; Tomita, K.; Bouvet, M.; Hoffman, R.M. UV light killing efficacy of fluorescent protein-expressing cancer cells in vitro and in vivo. J. Cell. Biochem. 2010, 110, 1439–1446. [Google Scholar] [CrossRef]
- Caiut, J.; Lechevallier, S.; Dexpert-Ghys, J.; Caillier, B.; Guillot, P. UVC emitting phosphors obtained by spray pyrolysis. J. Lumin. 2010, 131, 628–632. [Google Scholar] [CrossRef]
- Broxtermann, M.; Funke, L.M.; Keil, J.-N.; Eckert, H.; Hansen, M.R.; Meijerink, A.; Yu, T.; Braun, N.; Jüstel, T. A detailed aging analysis of MPO4:X (M = Y3+, La3+, Lu3+; X = Bi3+, Pr3+, Gd3+) due to the Xe excimer discharge. J. Lumin. 2018, 202, 450–460. [Google Scholar] [CrossRef]
- Zhou, X.; Qiao, J.; Zhao, Y.; Han, K.; Xia, Z. Multi-responsive deep-ultraviolet emission in praseodymium-doped phosphors for microbial sterilization. Sci. China Mater. 2021, 65, 1103–1111. [Google Scholar] [CrossRef]
- Singh, N.; Kaur, S.; Jayasimhadri, M.; Pathak, M.; Watanabe, S.; Rao, T.G.; Lee, J.-K.; Singh, V. UV emitting Pb2+ doped SrZrO3 phosphors prepared by sol-gel procedure. Ceram. Int. 2018, 44, 17074–17078. [Google Scholar] [CrossRef]
- Singh, V.; Singh, N.; Pathak, M.; Watanabe, S.; Rao, T.G.; Singh, P.K.; Dubey, V. UV emission from Gd3+ ions in LaAl11O18 phosphors. Optik 2018, 157, 1391–1396. [Google Scholar] [CrossRef]
- Thakare, D.S.; Omanwar, S.; Muthal, P.L.; Dhopte, S.M.; Kondawar, V.K.; Moharil, S.V. UV-emitting phosphors: Synthesis, photoluminescence and applications. Phys. Status Solidi A 2004, 201, 574–581. [Google Scholar] [CrossRef]
- Cai, H.; Song, Z.; Liu, Q. Infrared-photostimulable and long-persistent ultraviolet-emitting phosphor LiLuGeO4:Bi3+,Yb3+ for biophotonic applications. Mater. Chem. Front. 2020, 5, 1468–1476. [Google Scholar] [CrossRef]
- Broxtermann, M.; Jüstel, T. Photochemically induced deposition of protective alumina coatings onto UV emitting phosphors for Xe excimer discharge lamps. Mater. Res. Bull. 2016, 80, 249–255. [Google Scholar] [CrossRef]
- Broxtermann, M.; Dierkes, T.; Funke, L.M.; Salvermoser, M.; Laube, M.; Natemeyer, S.; Braun, N.; Hansen, M.R.; Jüstel, T. An UV-C/B emitting Xe excimer discharge lamp comprising BaZrSi3O9–A lamp performance and phosphor degradation analysis. J. Lumin. 2018, 200, 1–8. [Google Scholar] [CrossRef]
- Kominami, H.; Iguchi, T.; Nakanishi, Y.; Hara, K. Ultra-violet emission of aluminates phosphors prepared by solid phase synthesis. J. Adv. Res. Phys. 2011, 2, 3–5. [Google Scholar]
- Ishinaga, T.; Iguchi, T.; Kominami, H.; Hara, K.; Kitaura, M.; Ohnishi, A. Luminescent property and mechanism of ZnAl2O4 ultraviolet emitting phosphor. Phys. Status Solidi C Curr. Top. Solid State Phys. 2015, 12, 797–800. [Google Scholar] [CrossRef]
- Rojas-Hernandez, R.E.; Rubio-Marcos, F.; Romet, I.; del Campo, A.; Gorni, G.; Hussainova, I.; Fernandez, J.F.; Nagirnyi, V. Deep-Ultraviolet Emitter: Rare-Earth-Free ZnAl2O4 Nanofibers via a Simple Wet Chemical Route. Inorg. Chem. 2022, 61, 11886–11896. [Google Scholar] [CrossRef]
- Fasoli, M.; Vedda, A.; Nikl, M.; Jiang, C.; Uberuaga, B.P.; Andersson, D.A.; McClellan, K.J.; Stanek, C.R. Band-gap engineering for removing shallow traps in rare-earth Lu3Al5O12 garnet scintillators using Ga3+ doping. Phys. Rev. B 2011, 84, 081102. [Google Scholar] [CrossRef]
- Yadav, S.K.; Uberuaga, B.P.; Nikl, M.; Jiang, C.; Stanek, C.R. Band-Gap and Band-Edge Engineering of Multicomponent Garnet Scintillators from First Principles. Phys. Rev. Appl. 2015, 4, 054012. [Google Scholar] [CrossRef]
- Gektin, A.V.; Belsky, A.N.; Vasil’Ev, A.N. Scintillation Efficiency Improvement by Mixed Crystal Use. IEEE Trans. Nucl. Sci. 2013, 61, 262–270. [Google Scholar] [CrossRef]
- Sidletskiy, O.; Gektin, A.; Belsky, A. Light-yield improvement trends in mixed scintillation crystals. Phys. Status Solidi A 2014, 211, 2384–2387. [Google Scholar] [CrossRef]
- Belsky, A.; Auffray, E.; Lecoq, P.; Dujardin, C.; Garnier, N.; Canibano, H.; Pedrini, C.; Petrosyan, A. Progress in the development of LuAlO3-based scintillators. IEEE Trans. Nucl. Sci. 2001, 48, 1095–1100. [Google Scholar] [CrossRef]
- Spassky, D.; Omelkov, S.; Mägi, H.; Mikhailin, V.; Vasil’Ev, A.; Krutyak, N.; Tupitsyna, I.; Dubovik, A.; Yakubovskaya, A.; Belsky, A. Energy transfer in solid solutions ZnxMg1−xWO4. Opt. Mater. 2014, 36, 1660–1664. [Google Scholar] [CrossRef]
- Levushkina, V.; Spassky, D.; Brik, M.; Zych, E.; Madej, A.; Belsky, A.; Bartosiewicz, A.; Nikl, M. Mixed vanadates: Optimization of optical properties by varying chemical composition. J. Lumin. 2017, 189, 140–147. [Google Scholar] [CrossRef]
- Spassky, D.; Vasil’Ev, A.; Vielhauer, S.; Sidletskiy, O.; Voloshyna, O.; Belsky, A. Composition effect in luminescence properties of Y(NbxTa1−x)O4 mixed crystals. Opt. Mater. 2018, 80, 247–252. [Google Scholar] [CrossRef]
- Dorenbos, P. The electronic level structure of lanthanide impurities in REPO4, REBO3, REAlO3, and RE2O3(RE = La, Gd, Y, Lu, Sc) compounds. J. Physics: Condens. Matter 2013, 25, 225501. [Google Scholar] [CrossRef] [PubMed]
- Spassky, D.; Voznyak-Levushkina, V.; Arapova, A.; Zadneprovski, B.; Chernenko, K.; Nagirnyi, V. Enhancement of Light Output in ScxY1−xPO4:Eu3+ Solid Solutions. Symmetry 2020, 12, 946. [Google Scholar] [CrossRef]
- Get’Man, E.I.; Radio, S.V.; Ardanova, L.I. Predicting the Phase Stability of Sc1–xLnxPO4 (Ln = Gd–Lu, Y) Zircon-Structured Solid Solutions. Inorg. Mater. 2018, 54, 596–600. [Google Scholar] [CrossRef]
- Le Bail, A.; Duroy, H.; Fourquet, J. Ab-initio structure determination of LiSbWO6 by X-ray powder diffraction. Mater. Res. Bull. 1988, 23, 447–452. [Google Scholar] [CrossRef]
- Petricek, V.; Dusek, M.; Palatinus, L. Jana2006. Structure Determination Software Programs; Institute of Physics: Praha, Czech Republic, 2006. [Google Scholar]
- Kristianpoller, N. Absolute Quantum Yield of Sodium Salicylate*. J. Opt. Soc. Am. 1964, 54, 1285–1286. [Google Scholar] [CrossRef]
- Omelkov, S.; Nagirnyi, V.; Vasil’ev, A.; Kirm, M. New features of hot intraband luminescence for fast timing. J. Lumin. 2016, 176, 309–317. [Google Scholar] [CrossRef]
- Belsky, A.; Gektin, A.; Vasil’Ev, A.N. Influence of Disorder in Scintillating Solid Solutions on Thermalization and Recombination of Electronic Excitations. Phys. Status Solidi B 2019, 257, 1900535. [Google Scholar] [CrossRef]
- Milligan, W.; Mullica, D.; Beall, G.; Boatner, L. Structural investigations of YPO4, ScPO4, and LuPO4. Inorg. Chim. Acta 1982, 60, 39–43. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–766. [Google Scholar] [CrossRef]
- Vegard, L. Die Konstitution der Mischkristalle und die Raumfüllung der Atome. Z. Physik 1921, 5, 17–26. [Google Scholar] [CrossRef]
- Voloshyna, O.; Sidletskiy, O.; Spassky, D.; Gerasymov, I.; Romet, I.; Belsky, A. Nonlinear behavior of structural and luminescent properties in Gd(NbxTa1−x)O4 mixed crystals. Opt. Mater. 2018, 76, 382–387. [Google Scholar] [CrossRef]
- Melnikov, P.; Massabni, A.M.; Malta, O. Luminescent Properties of Some Rare Earth Phosphates. Phosphorus Sulfur Silicon Relat. Elem. 1996, 111, 1. [Google Scholar] [CrossRef]
- Blasse, G.; Bril, A. Luminescence of Phosphors Based on Host Lattices ABO4 (A is Sc, In; B is P, V, Nb). J. Chem. Phys. 1969, 50, 2974–2980. [Google Scholar] [CrossRef]
- Trukhin, A.; Boatner, L. Electronic Structure of ScPO4 Single Crystals: Optical and Photoelectric Properties. Mater. Sci. Forum 1997, 239–241, 573–576. [Google Scholar] [CrossRef]
- Trukhin, A.N.; Boatner, L.A. Luminescence properties of ScPO4 single crystals. In Proceedings of the 5th International Conference on Inorganic Scintillators and Their Applications, Moscow, Russia, 16–20 August 1999; pp. 697–702. [Google Scholar]
- Trukhin, A.N.; Shmits, K.; Jansons, J.L.; A Boatner, L. Ultraviolet luminescence of ScPO4, AlPO4 and GaPO4 crystals. J. Phys. Condens. Matter 2013, 25, 385502. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Guo, C.; Ci, Z.; Wang, C.; Wang, Y.; Huang, Y. Morphology-controllable synthesis, electronic structure and multicolor-tunable luminescence properties of multifunctional ScPO4 based nano/micro-phosphor. Chem. Eng. J. 2017, 312, 204–219. [Google Scholar] [CrossRef]
- AFLOW. Available online: https://aflow.org/material/?id=aflow:0d48f6442e93b38d (accessed on 15 July 2022).
- Kirm, M.; Zimmerer, G.; Feldbach, E.; Lushchik, A.; Lushchik, C.; Savikhin, F. Self-trapping and multiplication of electronic excitations in Al2O3 and Al2O3:Sc crystals. Phys. Rev. B 1999, 60, 502–510. [Google Scholar] [CrossRef]
- Kučera, M.; Nikl, M.; Průša, P.; Mareš, J.; Nitsch, K.; Hanuš, M.; Onderišinová, Z.; Kučerková, R. Growth and emission properties of Sc, Pr, and Ce co-doped Lu3Al5O12 epitaxial layers for scintillators. J. Cryst. Growth 2010, 318, 813–819. [Google Scholar] [CrossRef]
- Zorenko, Y.; Gorbenko, V.; Zorenko, T.; Vasylkiv, Y. Luminescent properties of the Sc3+ doped single crystalline films of (Y,Lu,La)3(Al,Ga)5O12 multi-component garnets. Opt. Mater. 2014, 36, 1760–1764. [Google Scholar] [CrossRef]
- Feofilov, S.P.; Zhou, Y.; Seo, H.J.; Jeong, J.Y.; Keszler, D.A.; Meltzer, R.S. Host sensitization of Gd3+ ions in yttrium and scandium borates and phosphates: Application to quantum cutting. Phys. Rev. B 2006, 74, 085101. [Google Scholar] [CrossRef]
- Voznayk-Levushkina, V.; Arapova, A.; Spassky, D.A.; Nikiforov, I.V.; Zadneprovskii, B.I. Luminescent Properties of the Solid Solutions of Yttrium-Scandium Phosphates Doped with Europium Ions. Phys. Solid State. 2022, 64, 1925. (In Russian) [Google Scholar] [CrossRef]
- Nagirnyi, V.; Spassky, D.; Laguta, V.; Buryi, M.; Kirm, M.; Omelkov, S.; Romet, I.; Tupitsyna, I.; Dorenbos, P. Energy transfer to luminescent impurity by thermally quenching excitons in CdWO4:Sm. J. Lumin. 2020, 228, 117609. [Google Scholar] [CrossRef]
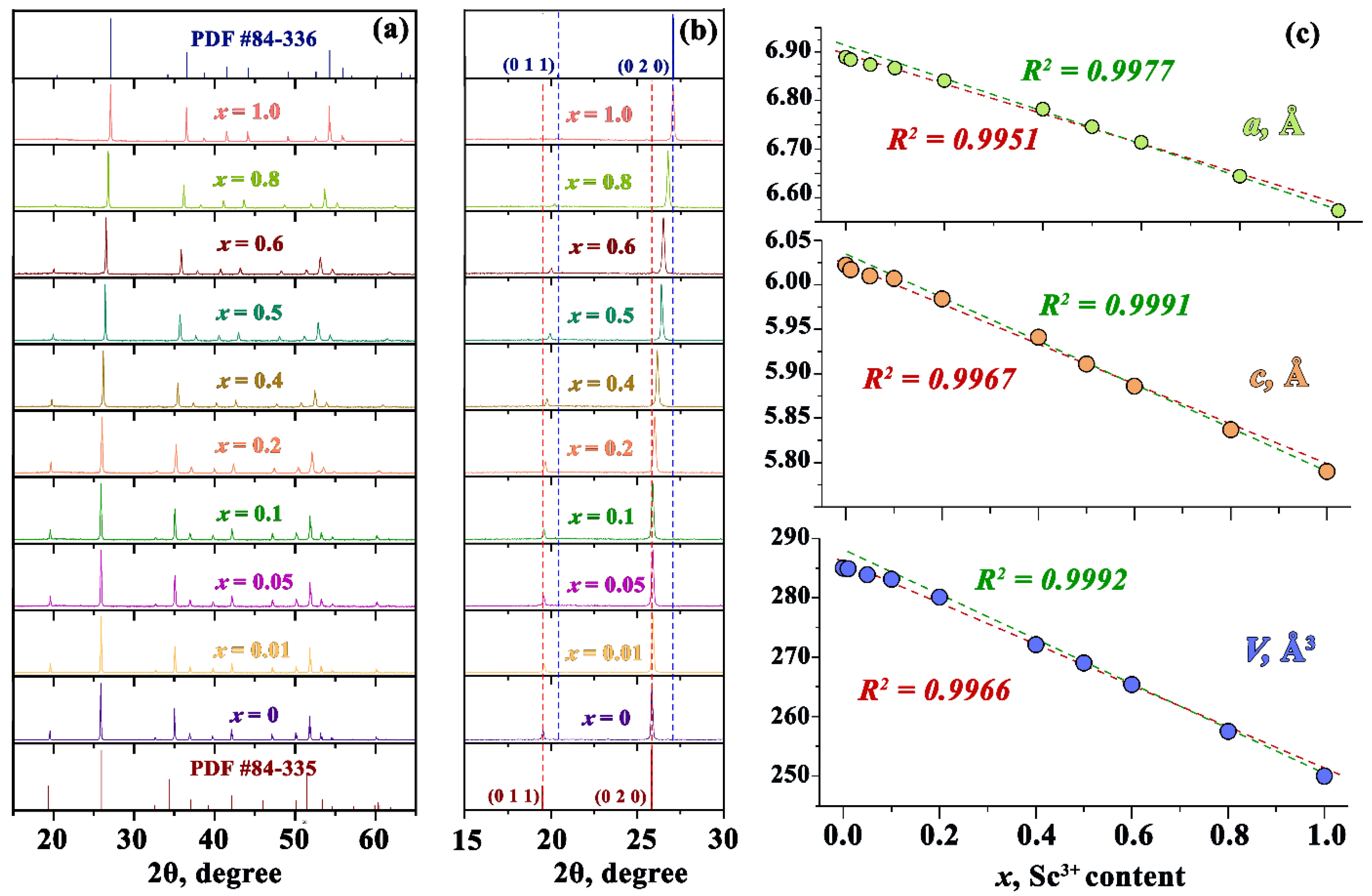

| X | a, Å | c, Å | V, Å3 |
|---|---|---|---|
| 0 | 6.880(3) | 6.019(5) | 284.9(7) |
| 0.01 | 6.879(1) | 6.017(2) | 284.8(1) |
| 0.05 | 6.872(1) | 6.010(4) | 283.8(2) |
| 0.1 | 6.867(2) | 6.007(1) | 283.2(5) |
| 0.2 | 6.844(1) | 5.987(3) | 280.4(5) |
| 0.4 | 6.794(3) | 5.947(1) | 274.4(7) |
| 0.5 | 6.746(4) | 5.911(1) | 269.0(4) |
| 0.6 | 6.714(3) | 5.886(6) | 265.4(1) |
| 0.8 | 6.642(4) | 5.836(5) | 257.5(1) |
| 1 | 6.571(4) | 5.789(1) | 249.9(3) |
| Ratio | |||
|---|---|---|---|
| X | Y | Sc | P |
| 0 | 1.01 ± 0.03 | 0 | 1.00 ± 0.03 |
| 0.01 | 0.98 ± 0.03 | 0.01 ± 0.01 | 1.00 ± 0.02 |
| 0.05 | 0.93 ± 0.02 | 0.07 ± 0.04 | 1.00 ± 0.03 |
| 0.1 | 0.86 ± 0.04 | 0.08 ± 0.02 | 1.00 ± 0.03 |
| 0.2 | 0.76 ± 0.11 | 0.23 ± 0.11 | 1.00 ± 0.11 |
| 0.4 | 0.61 ± 0.09 | 0.35 ± 0.11 | 1.00 ± 0.06 |
| 0.5 | 0.48 ± 0.06 | 0.50 ± 0.08 | 1.00 ± 0.04 |
| 0.6 | 0.35 ± 0.02 | 0.63 ± 0.09 | 1.00 ± 0.08 |
| 0.8 | 0.18 ± 0.05 | 0.84 ± 0.04 | 1.00 ± 0.09 |
| 1.0 | 0 | 1.02 ± 0.03 | 1.00 ± 0.07 |
| x Value | UV-C Band | Defect 1 | Defect 2 |
|---|---|---|---|
| 0 | 5.19 (0.74) * | 3.82 (0.36) | 2.89 (0.69) |
| 0.01 | 5.21 (0.74) | 4.34 (0.81) | 2.88 (0.72) |
| 0.1 | 5.19 (0.75) | 4.44 (0.79) | 2.82 (0.79) |
| 0.5 | 5.21 (0.79) | 4.57 (0.99) | 2.62 (0.75) |
| 0.8 | 5.46 (0.83) | 4.83 (1.16) | 2.67 (0.7) |
| 1 | 5.94 (0.79) | 2.69 (0.73) |
| x Value | τdec, ns | τ1/e, ns |
|---|---|---|
| 0.01 | 254 (73%) 535 (18%) 1790 (6%) 4240 (3%) | 294 |
| 0.05 | 134 (16%) 333 (77%) 1580 (6%) 7780 (1%) | 259 |
| 0.1 | 166 (23%) 345 (68%) 1120 (7%) 11,370 (2%) | 265 |
| 0.2 | 197 (42%) 378 (48%) 802 (8%) 11,580 (2%) | 276 |
| 0.4 | 196 (53%) 433 (35%) 628 (11%) 6360 (1%) | 256 |
| 0.5 | 34 (1%) 192 (42 %) 430 (56%) 3201 (1%) | 253 |
| 0.6 | 32 (1%) 192 (44 %) 424 (54%) 3690 (1%) | 250 |
| 0.8 | 26 (2%) 158 (44%) 357 (54%) | 162 |
| 1 | 10 (4%) 92 (92%) 413 (4%) | 56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spassky, D.; Vasil’ev, A.; Nagirnyi, V.; Kudryavtseva, I.; Deyneko, D.; Nikiforov, I.; Kondratyev, I.; Zadneprovski, B. Bright UV-C Phosphors with Excellent Thermal Stability—Y1−xScxPO4 Solid Solutions. Materials 2022, 15, 6844. https://doi.org/10.3390/ma15196844
Spassky D, Vasil’ev A, Nagirnyi V, Kudryavtseva I, Deyneko D, Nikiforov I, Kondratyev I, Zadneprovski B. Bright UV-C Phosphors with Excellent Thermal Stability—Y1−xScxPO4 Solid Solutions. Materials. 2022; 15(19):6844. https://doi.org/10.3390/ma15196844
Chicago/Turabian StyleSpassky, Dmitry, Andrey Vasil’ev, Vitali Nagirnyi, Irina Kudryavtseva, Dina Deyneko, Ivan Nikiforov, Ildar Kondratyev, and Boris Zadneprovski. 2022. "Bright UV-C Phosphors with Excellent Thermal Stability—Y1−xScxPO4 Solid Solutions" Materials 15, no. 19: 6844. https://doi.org/10.3390/ma15196844
APA StyleSpassky, D., Vasil’ev, A., Nagirnyi, V., Kudryavtseva, I., Deyneko, D., Nikiforov, I., Kondratyev, I., & Zadneprovski, B. (2022). Bright UV-C Phosphors with Excellent Thermal Stability—Y1−xScxPO4 Solid Solutions. Materials, 15(19), 6844. https://doi.org/10.3390/ma15196844







