Calculation of Radiative Properties for [82%Ar-18%CO2]-Fe Plasmas in MAG Welding Arc
Abstract
:1. Introduction
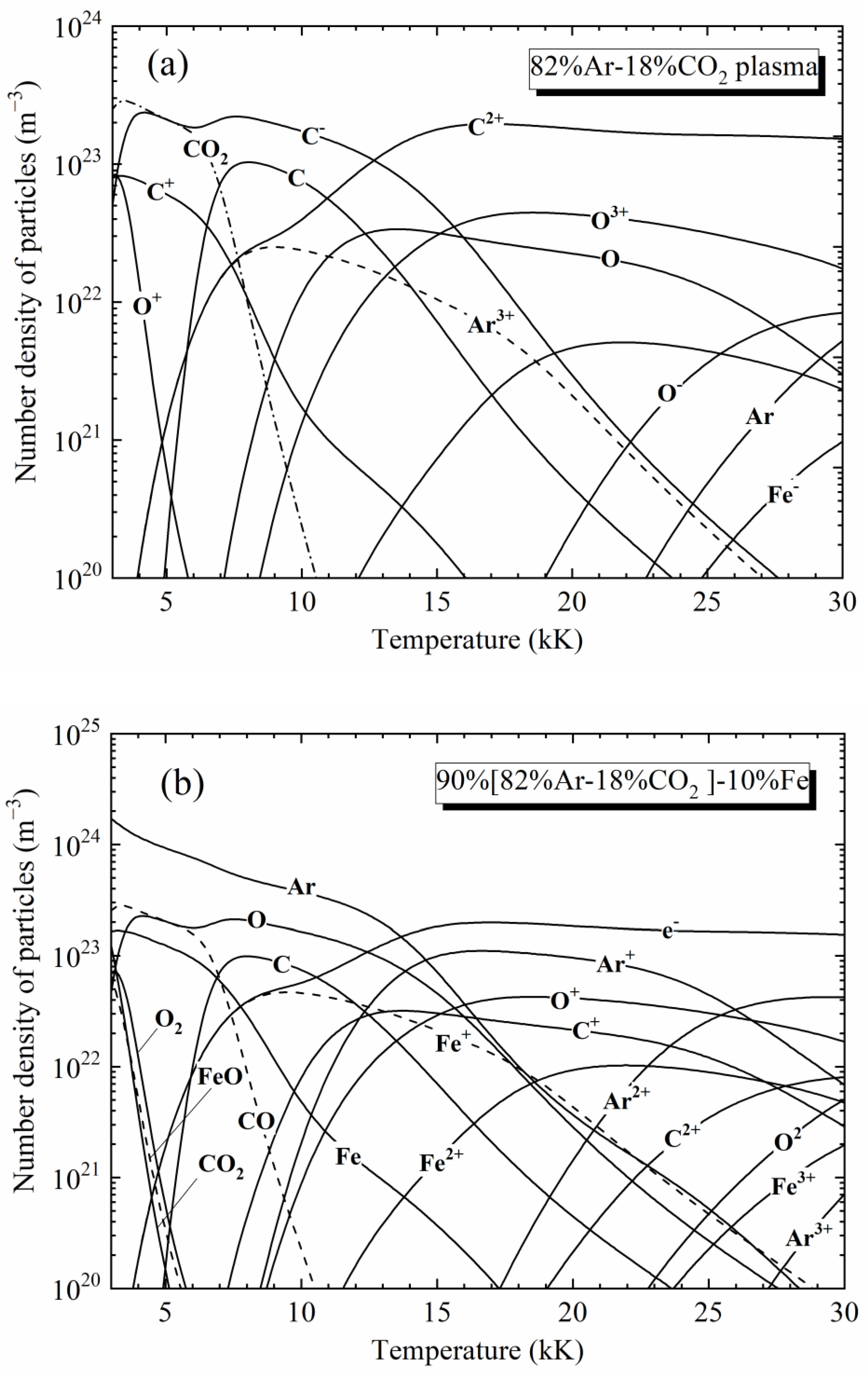
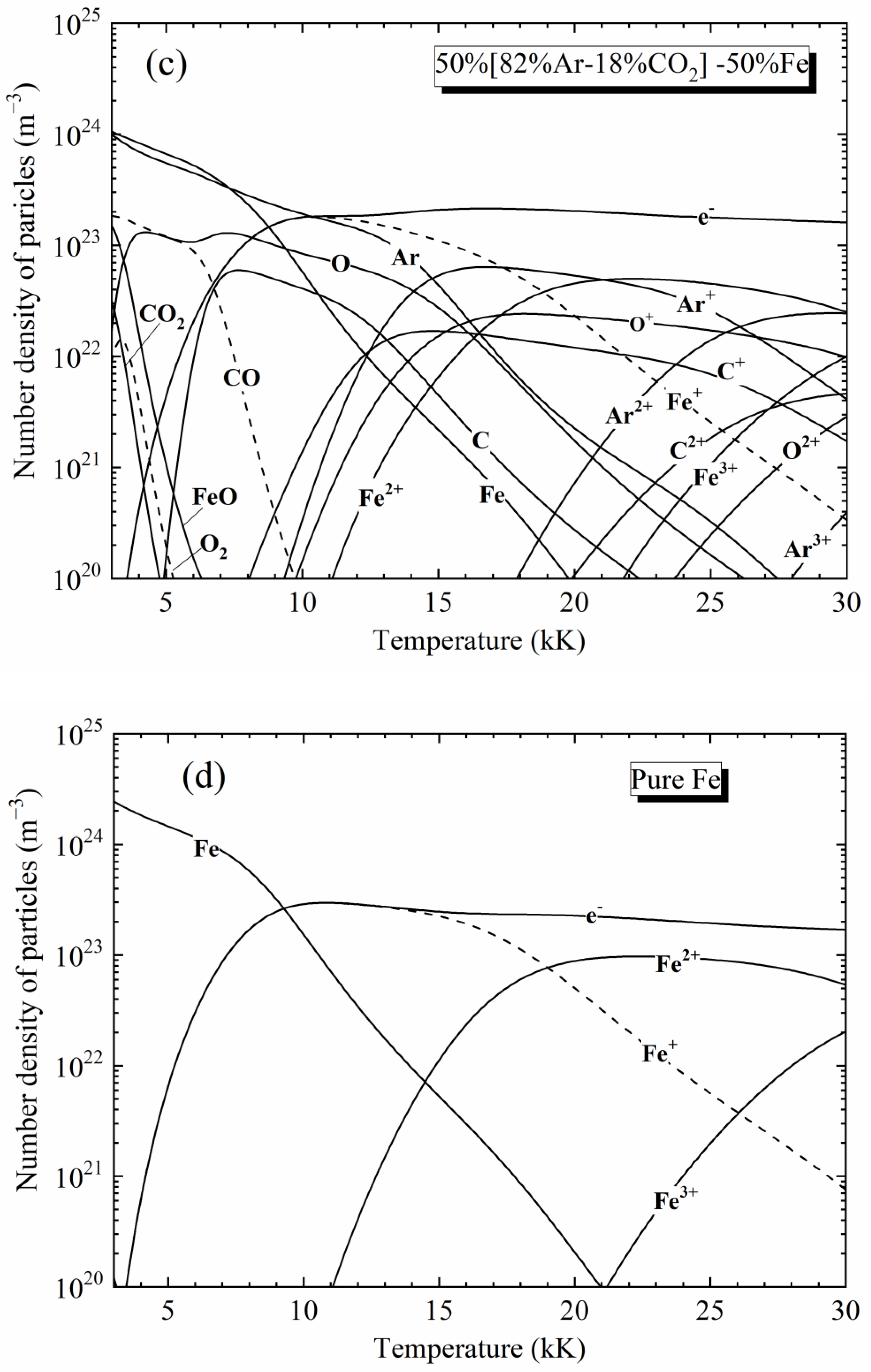
2. Plasma Composition
3. Radiative Properties
3.1. Radiative Mechanisms
3.1.1. Radiation from the Atomic Lines
3.1.2. Radiation from the Atomic Continuum
- Radiative attachment: even if the radiative attachment is often negligible, we nevertheless considered this mechanism due to the presence of negatively charged particles (C−, O−, Fe−) in the plasma mixtures. Knowing the electronic affinity for the different species (C = 121.9 kJ/mol, O = 141 kJ/mol, and Fe = 15 kJ/mol), the monochromatic emission coefficient was calculated according to [3]:where kB is the Boltzmann constant, is the population number density of the negatively charged particles, and is the photo-detachment cross-section taken from Yachkov for carbon [59], and Robinson and Geltman for oxygen [60]. We did not find data to take into account the attachment of iron.
- Radiative recombination: radiative recombination occurs when an electron and an atomic ion can recombine. This mechanism is often important in the continuum radiation of thermal plasmas. Its calculation requires the knowledge of the Biberman–Schluter factors, which are performed by summing the photoionization cross sections for all the considered energy levels, assuming a Thomas–Fermi shielded potential [61]. For carbon, oxygen, and argon species, this factor has been calculated and tabulated by Hofsaess [62]. The corresponding monochromatic emission coefficient is defined by [3,63]:This is example 1 of an equation:where and are the population number densities of electron and ions, respectively, and is the charge of the ion Az+. and are the internal partition function and the ground level degeneracy of the ion Az+, respectively, me and e are the mass and the charge of the electron, respectively, is the permittivity of vacuum, and is the Biberman–Schluter factor issued from Hofsaess [62,64] for argon (Ar and Ar+), carbon (C and C+), and oxygen (O, O+, and O2+). As the Biberman–Schluter factor was not available for iron species and multi-charged species (Ar2+, Ar3+, C2+, C3+, and O3+), we used the hydrogen-like atoms approximation developed by Okuda et al. [65].
- Bremsstrahlung: the bremsstrahlung radiation is produced by the deceleration of an electron due to the deflection by an electric field. The monochromatic emission coefficients for electron-ion and electron-atom interactions are given, respectively, byis the neutral atom number density. For electron-ions interactions, the Gaunt factor is introduced to correct non-classical behavior using hydrogen-like approximation and is issued from the table calculated by Grant [66,67]. For electron-atoms interactions, the factor is homogeneous to a surface and depends on the elastic cross-section which is taken from Neynaber et al. [68] and Robinson and Geltman [60] for carbon and oxygen, and from Tanaka and Lowke [69] for argon.
3.1.3. Radiation from the Atomic Continuum
3.1.4. Radiation of the Molecular Bands
- Diatomic molecular systems: the emission coefficient of each molecular line between two rotational levels and is given by [33]:where σ is the wavenumber, is the population number density of the emitting level, and is the transition probability.
- Polyatomic molecular systems: the radiation of the polyatomic species can be very important in the Infrared region. The radiation coming from CO2 was calculated using the database CDSD-4000 of Tachkun and Perevelov [92] which seems to be the most complete in the literature with wavenumbers between 8310 and 226 cm−1, and temperatures up to 4000 K. Similar to Billoux et al. [33], we did not consider the radiation of CO2 for higher temperatures due to a lower contribution to the radiative spectrum. For the O3 molecule, we used the HITRAN 2012 database [93], even if these molecules are in small concentrations in the plasma. The broadenings were described with a Voigt profile and collisional effects were defined by the relation (14). This last database gave us two kinds of broadenings: the broadenings parameters due to the perturbation of the species existing in air and the broadenings parameters corresponding to the perturbation of the molecular energy levels by species of the same type (self-broadenings). As this work is not focused on air plasmas, we considered all the broadening phenomena of a molecular line due to molecules of the same type with a total self-broadening. Under this consideration, we made two strong assumptions that we suppose not to be far from reality: (1) the molecular species is important in the plasma’s composition and the perturbation due to the other species can be neglected; (2) if the previous condition is not respected, all the perturbations caused by the other molecular species can be considered identically even if they are similar (or not) to the perturbed species. The second assumption can be debated but we supposed that low temperatures tend to increase the molecular species and reduce the impact on the broadening coefficients.
3.1.5. Discretization and Integration of the Monochromatic Emission Coefficient (Equation (1))
3.2. Net Emission Coefficient
4. Results
4.1. Spectra of [82%Ar-18%CO2]-Fe Plasmas
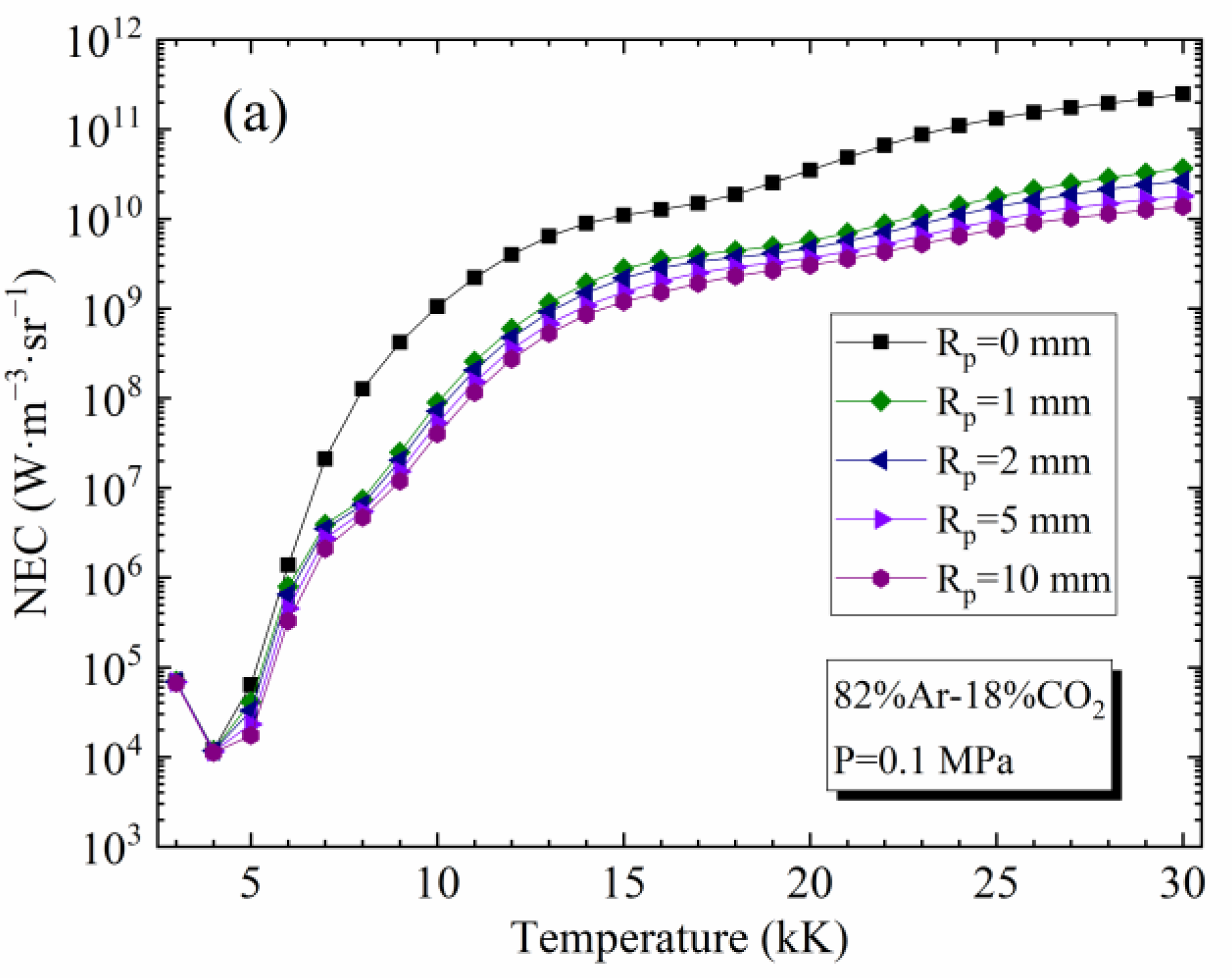
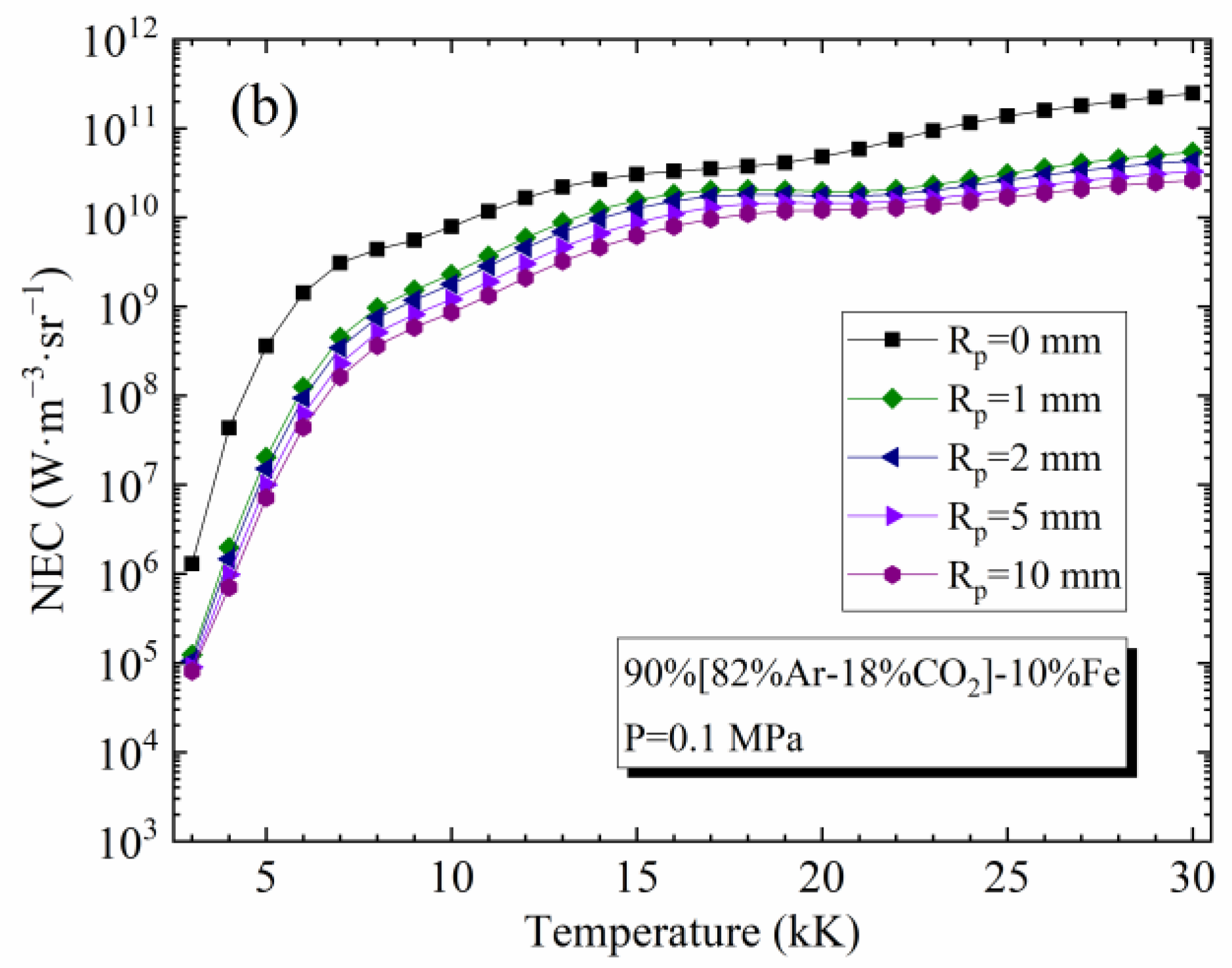
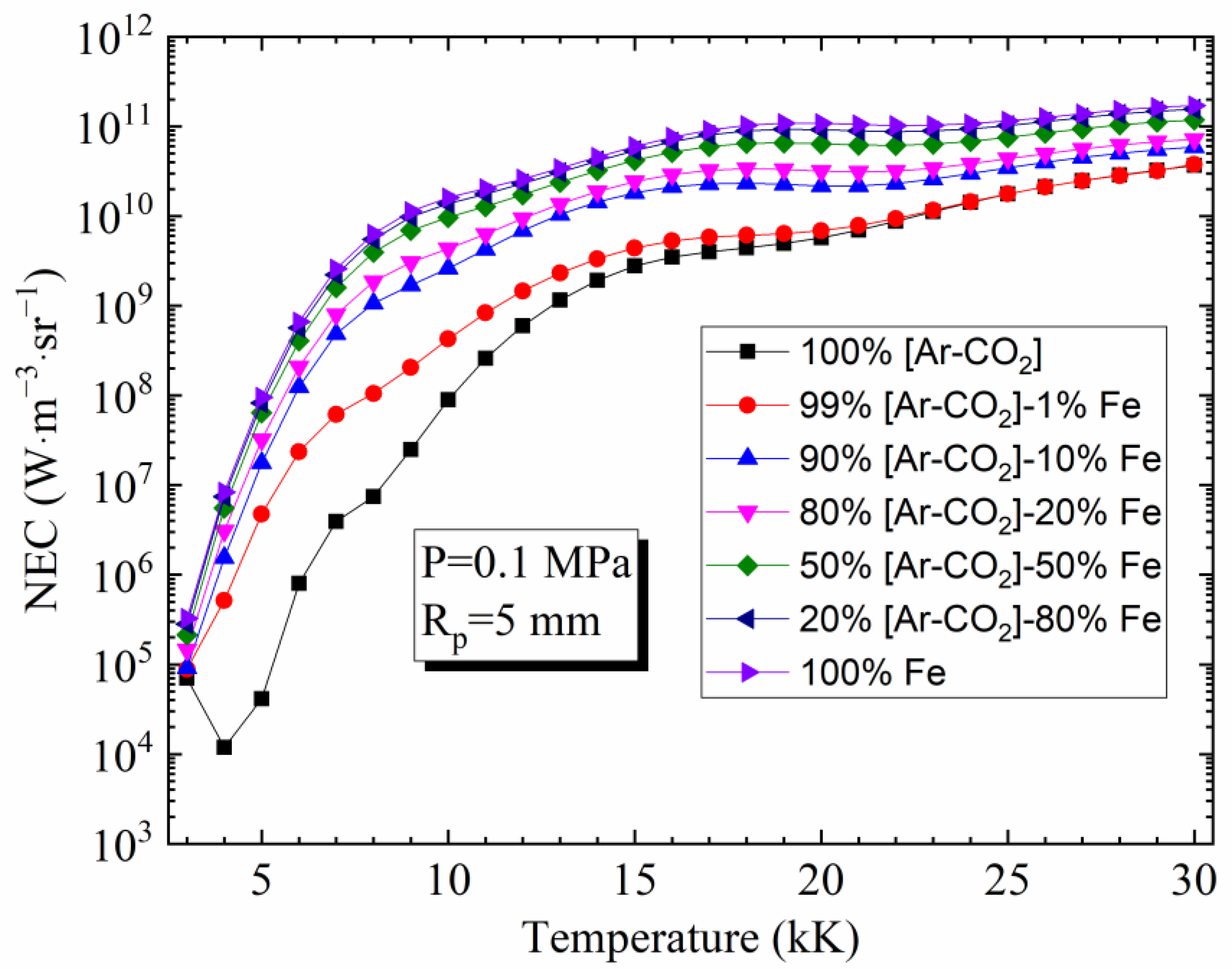
4.2. Influence of Temperature and Rp on the NEC
4.3. Influence of Vapor Concentration on the NEC
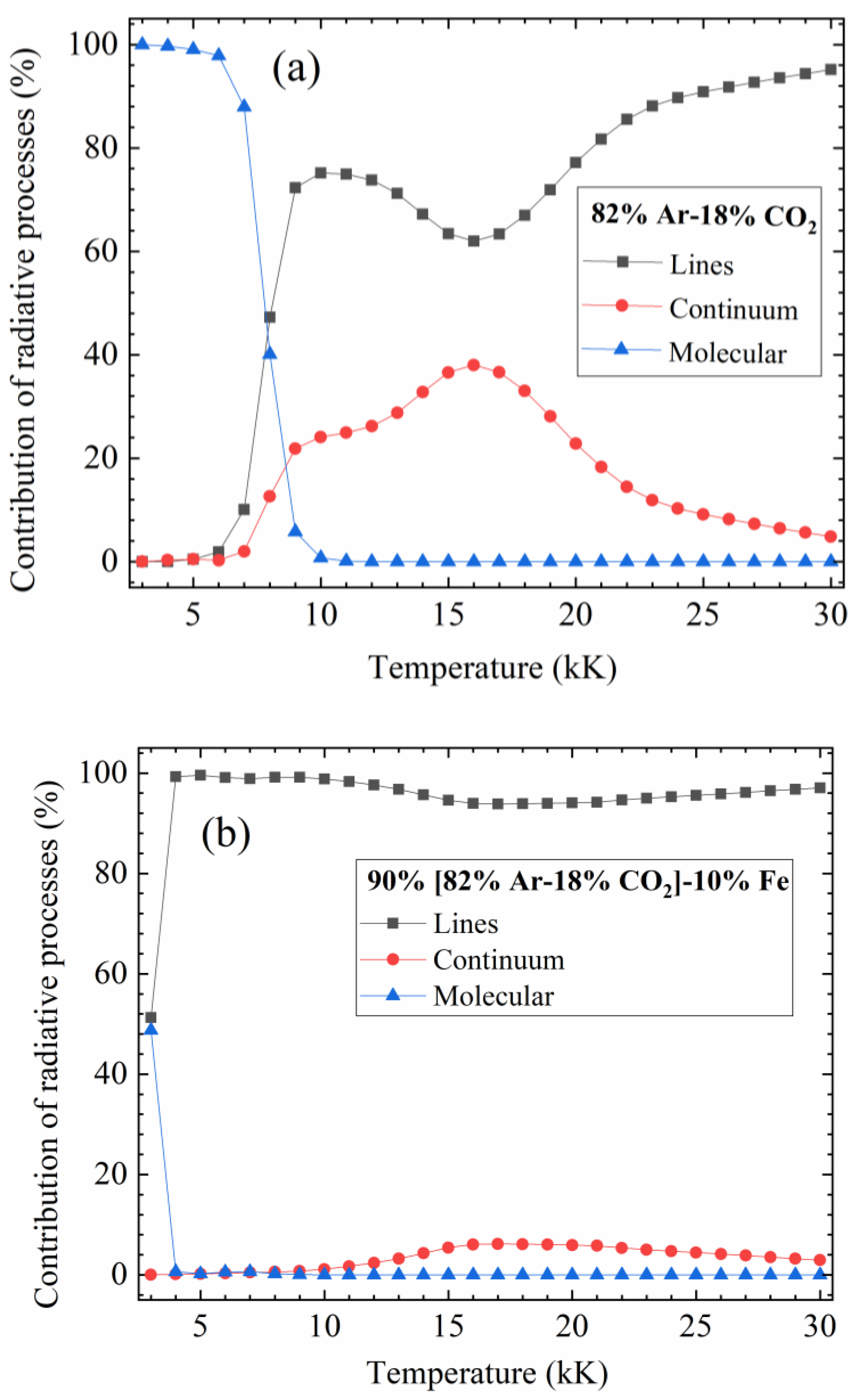
4.4. Influence of the Different Contributions to the Total Radiation
4.5. NECs for Certain Spectral Intervals
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walker, J.R. Arc Welding: Basic Fundamentals, 5th ed.; Goodheart-Willcox Pub: Tinley Park, IL, USA, 1993; ISBN 978-0870060168. [Google Scholar]
- Murphy, A.B. The effects of metal vapour in arc welding. J. Phys. D Appl. Phys. 2010, 43, 434001. [Google Scholar] [CrossRef]
- Cressault, Y.; Gleizes, A. Thermal plasma properties for Ar–Al, Ar–Fe and Ar–Cu mixtures used in welding plasmas processes: I. Net emission coefficients at atmospheric pressure. J. Phys. D Appl. Phys. 2013, 46, 415206. [Google Scholar] [CrossRef]
- Cressault, Y.; Murphy, A.B.; Teulet, P.; Gleizes, A.; Schnick, M. Thermal plasma properties for Ar–Cu, Ar–Fe and Ar–Al mixtures used in welding plasmas processes: II. Transport coefficients at atmospheric pressure. J. Phys. D Appl. Phys. 2013, 46, 415207. [Google Scholar] [CrossRef]
- Cressault, Y. Basic knowledge on radiative and transport properties to begin in thermal plasmas modelling. AIP Adv. 2015, 5, 57112. [Google Scholar] [CrossRef]
- Murphy, A.B. A self-consistent three-dimensional model of the arc, electrode and weld pool in gas–metal arc welding. J. Phys. D Appl. Phys. 2011, 44, 4009. [Google Scholar] [CrossRef]
- Haidar, J. The dynamic effects of metal vapour in gas metal arc welding. J. Phys. D Appl. Phys. 2010, 43, 165204. [Google Scholar] [CrossRef]
- Schnick, M.; Fuessel, U.; Hertel, M.; Haessler, M.; Spille-Kohoff, A.; Murphy, A.B. Modelling of gas–metal arc welding taking into account metal vapour. J. Phys. D Appl. Phys. 2010, 43, 434008. [Google Scholar] [CrossRef]
- Murphy, A.B.; Farmer, A.J.D.; Haidar, J. Laser-scattering measurement of temperature profiles of a free-burning arc. Appl. Phys. Lett. 1992, 60, 1304–1306. [Google Scholar] [CrossRef]
- Dzierzega, K.; Zawadzki, W.; Pokrzywka, B.; Pellerin, S. Experimental investigations of plasma perturbation in Thomson scattering applied to thermal plasma diagnostics. Phys. Rev. E 2006, 74, 26404. [Google Scholar] [CrossRef]
- Snyder, S.C.; Reynolds, L.D.; Fincke, J.R.; Lassahn, G.D.; Grandy, J.D.; Repetti, T.E. Electron-temperature and electron-density profiles in an atmospheric-pressure argon plasma jet. Phys. Rev. E 1994, 50, 519–525. [Google Scholar] [CrossRef]
- Meiering, E.M.; Serrano, L.; Fersht, A.R. Analysis of the enthalpy probe technique for thermal plasma diagnostics. Rev. Sci. Inst. 1995, 66, 3424–3431. [Google Scholar]
- Swank, W.D.; Fincke, J.R.; Haggard, D.C. Modular enthalpy probe and gas analyzer for thermal plasma measurements. Rev. Sci. Inst. 1993, 64, 56–62. [Google Scholar] [CrossRef]
- Akbar, S.; Etemadi, K. Impact of copper vapor contamination on argon arcs. Plasma Chem. Plasma Process. 1997, 17, 251–262. [Google Scholar] [CrossRef]
- Etemadi, K.; Pfender, E. Impact of anode evaporation on the anode region of a high-intensity argon arc. Plasma Chem. Plasma P. 1985, 5, 175–182. [Google Scholar] [CrossRef]
- Gonzalez, J.J.; Bouaziz, M.; Razafinimanana, M.; Gleizes, A. The influence of iron vapour on an argon transferred arc. Plasma Sources Sci. Technol. 1997, 6, 20–28. [Google Scholar] [CrossRef]
- Razafinimanana, M.; Hamidi, L.E.; Gleizes, A.; Vacquie, S. Experimental study of the influence of anode ablation on the characteristics of an argon transferred arc. Plasma Sources Sci. Technol. 1998, 4, 501–510. [Google Scholar] [CrossRef]
- Rouffet, M.; Wendt, M.; Goett, G.; Kozakov, R.; Schoepp, H.; Weltmann, K.; Uhrlandt, D. Spectroscopic investigation of the high-current phase of a pulsed GMAW process. J. Phys. D Appl. Phys. 2010, 43, 434003. [Google Scholar] [CrossRef]
- Valensi, F.; Pellerin, S.; Boutaghane, A.; Dzierzega, K.; Zielinska, S.; Pellerin, N.; Briand, F. Plasma diagnostics in gas metal arc welding by optical emission spectroscopy. J. Phys. D Appl. Phys. 2010, 43, 434002. [Google Scholar] [CrossRef]
- Valensi, F.; Pellerin, S.; Castillon, Q.; Boutaghane, A.; Dzierzega, K.; Zielinska, S.; Pellerin, N.; Briand, F. Study of the spray to globular transition in gas metal arc welding: A spectroscopic investigation. J. Phys. D Appl. Phys. 2013, 46, 224005. [Google Scholar] [CrossRef]
- Wilhelm, G.; Gött, G.; Schöpp, H.; Uhrlandt, D. Study of the welding gas influence on a controlled short-arc GMAW process by optical emission spectroscopy. J. Phys. D Appl. Phys. 2010, 43, 434004. [Google Scholar] [CrossRef]
- Wilhelm, G.; Kozakov, R.; Gött, G.; Schöpp, H.; Uhrlandt, D. Behaviour of the iron vapour core in the arc of a controlled short-arc GMAW process with different shielding gases. J. Phys. D Appl. Phys. 2012, 45, 85202. [Google Scholar] [CrossRef]
- Zielińska, S.; Musioł, K.; Dzierżęga, K.; Pellerin, S.; Valensi, F.; de Izarra, C.; Briand, F. Investigations of GMAW plasma by optical emission spectroscopy. Plasma Sources Sci. Technol. 2007, 16, 832. [Google Scholar] [CrossRef]
- Goecke, S.F. Auswirkungen von Aktivgaszumischungen im vpm-Bereich zu Argon auf das MIG-Impulsschweißen von Aluminium. PhD Thesis, Technical University, Berlin, Germany, 2004. [Google Scholar]
- Goecke, S.F.; Metzke, E.; Spille-Kohoff, A.; Langula, M. ChopArc. MSG-Lichtbogenschweißen für den Ultraleichtbau; Fraunhofer IRB Verlag: Stuttgart, Germany, 2005; ISBN 978-3816767664. [Google Scholar]
- Bachmann, B.; Kozakov, R.; Gött, G.; Ekkert, K.; Bachmann, J.; Marques, J.; Schöpp, H.; Uhrlandt, D.; Schein, J. High-speed three-dimensional plasma temperature determination of axially symmetric free-burning arcs. J. Phys. D Appl. Phys. 2013, 46, 125203. [Google Scholar] [CrossRef]
- Ogawa, Y. High speed imaging technique Part 1–high speed imaging of arc welding phenomena. Sci. Technol. Weld. Join. 2013, 16, 33–43. [Google Scholar] [CrossRef]
- Rouffet, M.E.; Cressault, Y.; Gleizes, A.; Hlina, J. Thermal plasma diagnostic methods based on the analysis of large spectral regions of plasma radiation. J. Phys. D Appl. Phys. 2008, 41, 125204. [Google Scholar] [CrossRef]
- Hlína, J.; Šonský, J.; Gruber, J.; Cressault, Y. Fast tomographic measurements of temperature in an air plasma cutting torch. J. Phys. D Appl. Phys. 2016, 49, 105202. [Google Scholar] [CrossRef]
- Billoux, T.; Cressault, Y.; Gleizes, A. Tables of radiative transition probabilities for the main diatomic molecular systems of OH, CH, CH+, CO and CO+ occurring in CO–H2 syngas-type plasma. J. Quant. Spectrosc. Radiat. Transf. 2014, 133, 434–444. [Google Scholar] [CrossRef]
- Mościcki, T.; Hoffman, J.; Szymański, Z. Emission coefficients of low temperature thermal iron plasma. Czech. J. Phys. 2004, 54, C677–C682. [Google Scholar] [CrossRef]
- Billoux, T.; Cressault, Y.; Boretskij, V.; Veklich, A.; Gleizes, A. Net emission coefficient of CO2-Cu thermal plasmas: Role of copper and molecules. J. Phys. Conf. Ser. 2012, 406, 12027. [Google Scholar] [CrossRef]
- Essoltani, A.; Proulx, P.; Boulos, M.I.; Gleizes, A. Effect of the presence of iron vapors on the volumetric emission of Ar/Fe and Ar/Fe/H2 plasmas. Plasma Chem. Plasma Process. 1994, 14, 301–315. [Google Scholar] [CrossRef]
- Zhong, L.L.; Wang, X.H.; Rong, M.Z.; Cressault, Y. Effects of copper vapour on thermophysical properties of CO2-N2 plasma. Eur. Phys. J. Appl. Phys. 2016, 70, 233. [Google Scholar] [CrossRef]
- Drawin, H.W.; Emard, F. Optical escape factors for bound-bound and free-bound radiation from plasmas. I. constant source function. Beit. Plasmaphys. 1973, 13, 143–168. [Google Scholar] [CrossRef]
- Menart, J.; Heberlein, J.; Pfender, E. Line-by-line method of calculating emission coefficients for thermal plasmas consisting of monatomic species. J. Quant. Spectrosc. Radiat. Transf. 1996, 56, 377–398. [Google Scholar] [CrossRef]
- Aubrecht, V.; Bartlova, M. Net emission coefficients of radiation in air and SF6 thermal plasmas. Plasma Chem. Plasma Process. 2009, 29, 131–147. [Google Scholar] [CrossRef]
- Naghizadeh-Kashani, Y.; Cressault, Y.; Gleizes, A. Net emission coefficient of air thermal plasmas. J. Phys. D Appl. Phys. 2002, 35, 2925. [Google Scholar] [CrossRef]
- Randrianandraina, H.Z.; Cressault, Y.; Gleizes, A. Improvements of radiative transfer calculation for SF6 thermal plasmas. J. Phys. D Appl. Phys. 2011, 44, 252–260. [Google Scholar] [CrossRef]
- Godin, D.; Trépanier, J.Y. A robust and efficient method for the computation of equilibrium composition in gaseous mixtures. Plasma Chem. Plasma Process. 2004, 24, 447–473. [Google Scholar] [CrossRef]
- Hirschfelder, J.O.; Curtis, C.F.; Bird, R.B. Molecular Theory of Gases and Liquids, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1965; ISBN 978-0-471-40065-3. [Google Scholar]
- Boulos, M.I.; Fauchais, P.; Pfender, E. Thermal Plasmas: Fundamentals and Applications, 1st ed.; Plenum Press: New York, NY, USA, 1994; ISBN 978-0-306-44607-8. [Google Scholar]
- Wang, F.; Li, H.; Teulet, P.; Cressault, Y.; Yang, K. Computation of equilibrium compositions of GMAW arc plasmas and its applications in spectroscopy. Spectrosc Spect Anal. 2018, 38, 1998–2003. [Google Scholar] [CrossRef]
- Kramida, A.E.; Ralchenko, Y.; Reader, J.; Team, N.A. 2012 NIST Atomic Spectra Database (version 5.0). Available online: http://physics.nist.gov/asd (accessed on 12 September 2022).
- Drawin, H.W.; Felenbok, P. Data for Plasmas in Local Thermodynamic Equilibrium; Gauthier-Villars: Paris, France, 1965. [Google Scholar]
- Herzberg, G.; Mrozowski, S. Molecular Spectra and Molecular Structure. I. Spectra of Diatomic Molecules; Van-Nostrand Reinhold: New York, NY, USA, 1955. [Google Scholar] [CrossRef]
- Drellishak, K.S.; Aeschliman, D.P.; Cambel, A.B. Partition functions and thermodynamic properties of nitrogen and oxygen. Phys. Fluids 1965, 8, 1590–1600. [Google Scholar] [CrossRef]
- Herzberg, G. Molecular Spectra and Molecular Structure II. Infrared and Raman Spectra, 2nd ed.; Van-Nostrand Reinhold: New York, NY, USA, 1945; ISBN 978-0442033866. [Google Scholar]
- Huber, K.P.; Herzberg, G. Molecular Spectra and Molecular Structure: IV. Constants of Diatomic Molecules; Van-Nostrand Reinhold: New York, NY, USA, 1978; ISBN 9780442233945. [Google Scholar]
- Chase, M.W.J.; Davies, C.A.; Downey, J.R.J.; Frurip, D.J.; McDonald, R.A.; Syverud, A.N. JANAF thermochemical tables. J. Phys. Chem. Ref. Data 1985, 14, 3. [Google Scholar]
- Moore, C.E. 1949–1958 Atomic Energy Levels, Vols I-III; U.S Government Printing Office: Washington, DC, USA, 1949. [Google Scholar]
- Smith, P.L.; Heise, C.; Esmond, J.R.; Robert, L.K. Atomic Spectral Line Database from CD-ROM 23 of R. L. Kurucz. Available online: http://cfa-www.harvard.edu/amp/ampdata/kurucz23/sekur.html (accessed on 12 September 2022).
- Laux, C.O. Optical Diagnostics and Radiative Emission of Air Plasmas. Ph.D. Thesis, Stanford University, Standford, CA, USA, 1993. [Google Scholar]
- Traving, G. Interpretation of Line Broadening and Line Shift Plasma Diagnostics; Lochte-Holtgreven, W., Ed.; North-Holland Publishing Company: Amsterdam, The Netherlands, 1968; pp. 66–134. [Google Scholar]
- Ali, A.W.; Griem, H.R. Theory of resonance broadening of spectral lines by atom-atom impacts. Phys. Rev. 1966, 144, 366–466. [Google Scholar] [CrossRef]
- Walkup, R.; Stewart, B.; Pritchard, D.E. Collisional line broadening due to van der Waals potentials. Phys. Rev. A 1984, 29, 169–173. [Google Scholar] [CrossRef]
- Griem, H.R. Semiempirical formulas for the electron-impact widths and shifts of isolated ion lines in plasmas. Phys. Rev. 1968, 165, 258–266. [Google Scholar] [CrossRef]
- Whiting, E.E. An empirical approximation to the Voigt profile. J. Quant. Spectrosc. Radiat. Transf. 1968, 8, 1379–1384. [Google Scholar] [CrossRef]
- D’Yachkov, L.G.; Golubev, O.A. Studies of continuum radiation from nitrogen, oxygen and carbon dioxide plasmas in the vacuum ultraviolet region. J. Quant. Spectrosc. Radiat. Transf. 1978, 20, 175–189. [Google Scholar] [CrossRef]
- Robinson, E.J.; Geltman, S. Single- and double-quantum photodetachment of negative ions. Phys. Rev. 1967, 153, 4–8. [Google Scholar] [CrossRef]
- Stewart, J.C.; Rotenberg, M. Wave functions and transition probabilities in scaled Thomas-Fermi ion potentials. Phys. Rev. 1967, 156, A1508–A1519. [Google Scholar] [CrossRef]
- Hofsaess, D. Emission continua of rare gas plasmas. J. Quant. Spectrosc. Radiat. Transf. 1978, 19, 339–352. [Google Scholar] [CrossRef]
- Gleizes, A.; Gongassian, M.; Rahmani, B. Continuum absorption coefficient in SF6 and SF6-N2 mixture plasmas. J. Phys. D Appl. Phys. 2000, 22, 83–89. [Google Scholar] [CrossRef]
- Hofsaess, D. Photoionization cross sections calculated by the scaled Thomas-Fermi method (hv ≤ 50 eV). At. Data Nucl. Data Tables 1979, 24, 285–321. [Google Scholar] [CrossRef]
- Okuda, S.; Ueda, Y.; Murai, Y.; Miyamoto, T.; Doi, Y.; Uenosono, C. Spectroscopic approach to the analysis of high current arcs in SF6. IEEE Trans. Plasma Sci. 1980, 8, 395–399. [Google Scholar] [CrossRef]
- Cabannes, F.; Chapelle, J. Reactions Under Plasma Conditions. In Spectroscopic Plasma Diagnostics; Venugopalan, M., Ed.; John Wiley & Sons: New York, NY, USA, 1971; Volume 2, p. 367. ISBN 0471906115. [Google Scholar]
- Grant, I.P. Calculation of Gaunt factors for free-free transitions near positive ions. Mon. Not. R. Astron. Soc. 1958, 118, 241–257. [Google Scholar] [CrossRef]
- Neynaber, R.H.; Marino, L.L.; Rothe, E.W.; Trujillo, S.M. Scattering of low-energy electrons by atomic hydrogen. Phys. Rev. 1961, 124, 135–136. [Google Scholar] [CrossRef]
- Tanaka, M.; Lowke, J.J. Predictions of weld pool profiles using plasma physics. J. Phys. D Appl. Phys. 2006, 40, R1–R23. [Google Scholar] [CrossRef]
- Jan, C.; Cressault, Y.; Gleizes, A.; Bousoltane, K. Calculation of radiative properties of SF6-C2F4 thermal plasmas-application to radiative transfer in high-voltage circuit breakers modelling. J. Phys. D Appl. Phys. 2014, 47, 15204. [Google Scholar] [CrossRef]
- Pouilly, B.; Robbe, J.M.; Schamps, J.; Roueff, E. Photodissociation and radiative processes in interstellar C2. J. Phys. B At. Mol. Phys. 1983, 16, 437–448. [Google Scholar] [CrossRef]
- Padial, N.T.; Collins, L.A.; Schneider, B.I. Photoionization of ground-state molecular carbon C2. Astrophys. J. 1985, 298, 369–374. [Google Scholar] [CrossRef]
- Cole, B.E.; Dexter, R.N. Photoabsorption and photoionisation measurements on some atmospheric gases in the wavelength region 50–340 A. J. Phys. B At. Mol. Opt. Phys. 1978, 11, 1011–1023. [Google Scholar] [CrossRef]
- Reilhac, L.D.; Damany, N. Photoabsorption cross-section measurements of some gases, from 10 to 50 nm. J. Quant. Spectrosc. Radiat. Transf. 1977, 18, 121–131. [Google Scholar] [CrossRef]
- Cook, G.R.; Metzger, P.H.; Ogawa, M. Photoionization and absorption coefficients of CO in the 600 to 1000 Å region. Can. J. Phys. 1965, 43, 1706–1722. [Google Scholar] [CrossRef]
- Moortgat, G.K.; Warneck, P. Relative O/1D/ quantum yields in the near UV photolysis of ozone at 298 K. Z. NATURFORSCH. A 1975, 30, 835–844. [Google Scholar] [CrossRef]
- Fiocco, G. Mesospheric Models and Related Experiments. In Proceedings of the Fourth Esrin-Eslab Symposium Held, Frascati, Italy, 6–10 July 1970; Springer Netherlands: Berlin, Germany, 1971. [Google Scholar]
- Griggs, M. Absorption coefficients of ozone in the ultraviolet and visible regions. J. Chem. Phys. 1968, 49, 857–859. [Google Scholar] [CrossRef]
- Tanaka, Y.; Inn, E.C.Y.; Watanabe, K. Absorption coefficients of gases in the vacuum ultraviolet. Part IV. Ozone. J. Chem. Phys. 1953, 21, 1651–1653. [Google Scholar] [CrossRef]
- Fennelly, J.A.; Torr, D.G. Photoionization and photoabsorption cross sections of O, N2, O2, and N for aeronomic calculations. At. Data Nucl. Data Tables 1992, 51, 321–363. [Google Scholar] [CrossRef]
- Lee, L.C.; Carlson, R.W.; Judge, D.L.; Ogawa, M. The absorption cross sections of N2, O2, CO, NO, CO2, N2O, CH4, C2H4, C2H6 and C4H10 from 180 to 700 Å. J. Quant. Spectrosc. Radiat. Transf. 1973, 13, 1023–1031. [Google Scholar] [CrossRef]
- Hudson, R.D. Absorption Cross Sections of Stratospheric Molecules Can. J. Chem. 1974, 52, 1–9. [Google Scholar]
- Babou, Y.; Rivière, P.; Perrin, M.Y.; Soufiani, A. Spectroscopic data for the prediction of radiative transfer in CO2–N2 plasmas. J. Quant. Spectrosc. Radiat. Transf. 2009, 110, 89–108. [Google Scholar] [CrossRef]
- Chauveau, S. Constitution de bases de données spectroscopiques relatives à un plasma d’air: Application au calcul du transfert radiatif. Ph.D. Thesis, Ecole Centrale, Paris, France, 2001. [Google Scholar]
- Lino, D.S.M.; Dudeck, M. Arrays of radiative transition probabilities for CO2–N2 plasmas. J. Quant. Spectrosc. Radiat. Transf. 2006, 102, 348–386. [Google Scholar] [CrossRef]
- Whiting, E.E.; Schadee, A.; Tatum, J.B.; Hougen, J.T.; Nicholls, R.W. Recommended conventions for defining transition moments and intensity factors in diatomic molecular spectra. J. Mol. Spectrosc. 1980, 80, 249–256. [Google Scholar] [CrossRef]
- Jr, B.R. Spectral line broadening in air molecule systems. Appl. Opt. 1967, 6, 141–147. [Google Scholar] [CrossRef]
- Sick, V.; Decker, M.; Heinze, J.; Stricker, W. Collisional processes in the O2 B 3Sigma-u state. Chem. Phys. Lett. 1996, 249, 335–340. [Google Scholar] [CrossRef]
- Lewis, B.R.; Berzins, L.; Dedman, C.J.; Scholz, T.T.; Carver, J.H. Pressure-broadening in the Schumann-Runge bands of molecular oxygen. J. Quant. Spectrosc. Radiat. Transf. 1988, 39, 271–282. [Google Scholar] [CrossRef]
- Rothman, L.S.; Gordon, I.E.; Barbe, A.; Benner, D.C.; Bernath, P.F.; Birk, M.; Boudon, V.; Brown, L.R.; Campargue, A.; Champion, J.-P.; et al. The HITRAN 2008 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2009, 110, 533–572. [Google Scholar] [CrossRef]
- Sung, K.; Varanasi, P. Intensities, collision-broadened half-widths, and collision-induced line shifts in the second overtone band of ja:math. J. Quant. Spectrosc. Radiat. Transf. 2004, 83, 445–458. [Google Scholar] [CrossRef]
- Tashkun, S.A.; Perevalov, V.I. CDSD-4000: High-resolution, high-temperature carbon dioxide spectroscopic databank. J. Quant. Spectrosc. Radiat. Transf. 2011, 112, 1403–1410. [Google Scholar] [CrossRef]
- Rothman, L.S.; Gordon, I.E.; Babikov, Y.; Barbe, A.; Benner, D.C.; Bernath, P.F.; Birk, M.; Bizzocchi, L.; Boudon, V.; Brown, L.R. The HITRAN2012 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2013, 130, 4–50. [Google Scholar] [CrossRef]
- Drayson, S.R. Rapid computation of the Voigt profile. J. Quant. Spectrosc. Radiat. Transf. 1976, 16, 611–614. [Google Scholar] [CrossRef]
- Lowke, J.J. Predictions of arc temperature profiles using approximate emission coefficients for radiation losses. J. Quant. Spectrosc. Radiat. Transf. 1974, 14, 111–122. [Google Scholar] [CrossRef]
- Aubrecht, V.; Lowke, J.J. Calculations of radiation transfer in SF6 plasmas using the method of partial characteristics. J. Phys. D Appl. Phys. 1994, 27, 2066–2073. [Google Scholar] [CrossRef]
- Menart, J. Radiative transport in a two-dimensional axisymmetric thermal plasma using the S–N discrete ordinates method on a line-by-line basis. J. Quant. Spectrosc. Radiat. Transf. 2000, 67, 273–291. [Google Scholar] [CrossRef]
- Nordborg, H.; Iordanidis, A.A. Self-consistent radiation based modelling of electric arcs: I. Efficient radiation approximations. J. Phys. D Appl. Phys. 2008, 41, 135205. [Google Scholar] [CrossRef]
- Larsen, E.W.; Thömmes, G.; Klar, A.; Seai, D.M.; Götz, T. Simplified PN approximations to the equations of radiative heat transfer and applications. J. Comput. Phys. 2002, 183, 652–675. [Google Scholar] [CrossRef]
- Soucasse, L.; Rivière, P.; Soufiani, A.; Xin, S.; Quéré, P.L. Transitional regimes of natural convection in a differentially heated cubical cavity under the effects of wall and molecular gas radiation. Phys. Fluids 2014, 26, 1–23. [Google Scholar] [CrossRef]
- Siegel, R.; Howell, J.R. Thermal Radiation Heat Transfer, 3rd ed.; Hemisphere Publishing: London, UK, 1992; ISBN 0-89116-271-2. [Google Scholar]
- Modest, M.F. Radiative Heat Transfer, 2nd ed.; Academic Press: New York, NY, USA, 2003; ISBN 978-0-12-503163-9. [Google Scholar]
- Gleizes, A.; Gonzalez, J.J.; Razafinimanana, M.; Robert, T. Influence of radiation on temperature field calculation in SF6 arcs. Plasma Sources Sci. Technol. 1992, 1, 135–140. [Google Scholar] [CrossRef]
- Lowke, J.J.; Liebermann, R.W. Predicted arc properties in sulfur hexafluoride. J. Appl. Phys. 1971, 42, 3532–3539. [Google Scholar] [CrossRef]
- Wang, F.; Cressault, Y.; Teulet, P.H.; Li, H.; Yang, K.; Yu, F. Use of radiative properties to determine temperature and vapours concentration in MAG welding arc (Ar–CO2–Fe plasmas). J. Appl. Phys. 2018, 51, 415202. [Google Scholar] [CrossRef]



| Atoms, Atomic Ions, and Electron | Diatomic Molecules and Ions | Polyatomic Molecules and Ions |
|---|---|---|
| Ar, Ar+, Ar2+, Ar3+, C, C−, C+, C2+, C3+, O, O−, O+, O2+, O3+, Fe, Fe−, Fe+, Fe2+, Fe3+, and e− | C2, C2+, C2−, O2, O2+, O2−, CO, CO+, FeO, and Fe2 | C3, C3−, CO2, CO2−, C2O, O3, C4, C3O2, and FeC5O5 |
| Molecule | References |
|---|---|
| C2 | [71,72] |
| CO | [73,74,75] |
| O3 | [76,77,78,79] |
| O2 | [80] |
| CO2 | [74,81,82] |
| Molecule | Electronic System | Electronic Transition | ||
|---|---|---|---|---|
| O2 | Schumann–Runge | 49,358 | ||
| CO | Infrared | - | ||
| Fourth Positive | 64,748 | |||
| Hopfield–Birge | 86,916 | |||
| Angström | 22,171 | |||
| Third Positive | 35,358 | |||
| CO+ | Comet-tail | 20,408 | ||
| First negative | 45,633 | |||
| Baldet–Johnson | 25,226 | |||
| C2 | Phillips | 8268 | ||
| Mulliken | 43,668 | |||
| Deslandres–D’az. | 25,969 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Liu, H.; Liu, X.; Zhang, L.; Yang, P.; Zhang, T.; Yu, Z.; Li, H.; Cressault, Y. Calculation of Radiative Properties for [82%Ar-18%CO2]-Fe Plasmas in MAG Welding Arc. Materials 2022, 15, 6415. https://doi.org/10.3390/ma15186415
Wang F, Liu H, Liu X, Zhang L, Yang P, Zhang T, Yu Z, Li H, Cressault Y. Calculation of Radiative Properties for [82%Ar-18%CO2]-Fe Plasmas in MAG Welding Arc. Materials. 2022; 15(18):6415. https://doi.org/10.3390/ma15186415
Chicago/Turabian StyleWang, Fei, Hongbing Liu, Xiaoli Liu, Lingfeng Zhang, Po Yang, Tianli Zhang, Zhishui Yu, Huan Li, and Yann Cressault. 2022. "Calculation of Radiative Properties for [82%Ar-18%CO2]-Fe Plasmas in MAG Welding Arc" Materials 15, no. 18: 6415. https://doi.org/10.3390/ma15186415
APA StyleWang, F., Liu, H., Liu, X., Zhang, L., Yang, P., Zhang, T., Yu, Z., Li, H., & Cressault, Y. (2022). Calculation of Radiative Properties for [82%Ar-18%CO2]-Fe Plasmas in MAG Welding Arc. Materials, 15(18), 6415. https://doi.org/10.3390/ma15186415






