Corrosion Performance and Mechanical Strength in Aluminum 6061 Joints by Pulsed Gas Metal Arc Welding
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Their Preparation
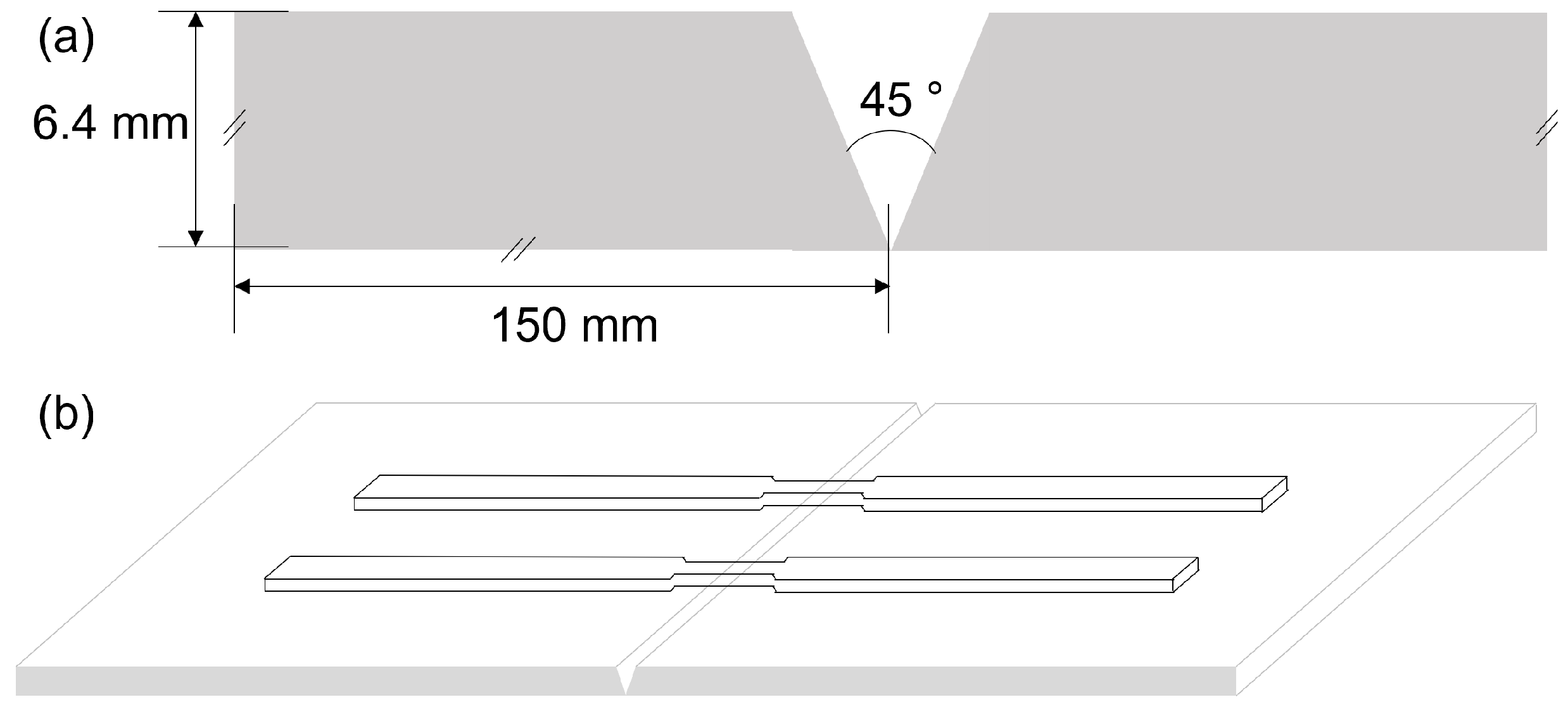
2.2. Welding Process
2.3. Mechanical Testing and Microstructural Study
2.4. Corrosion Tests
3. Results
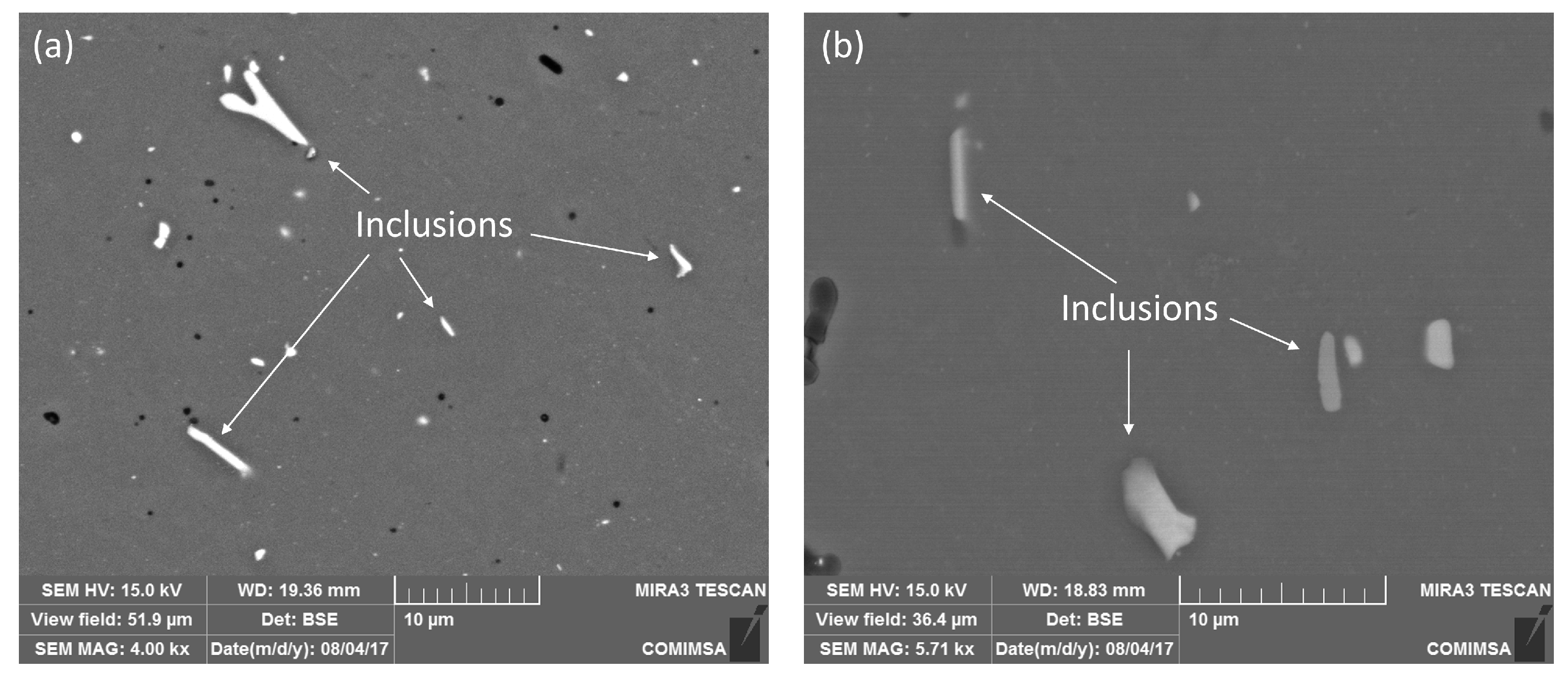
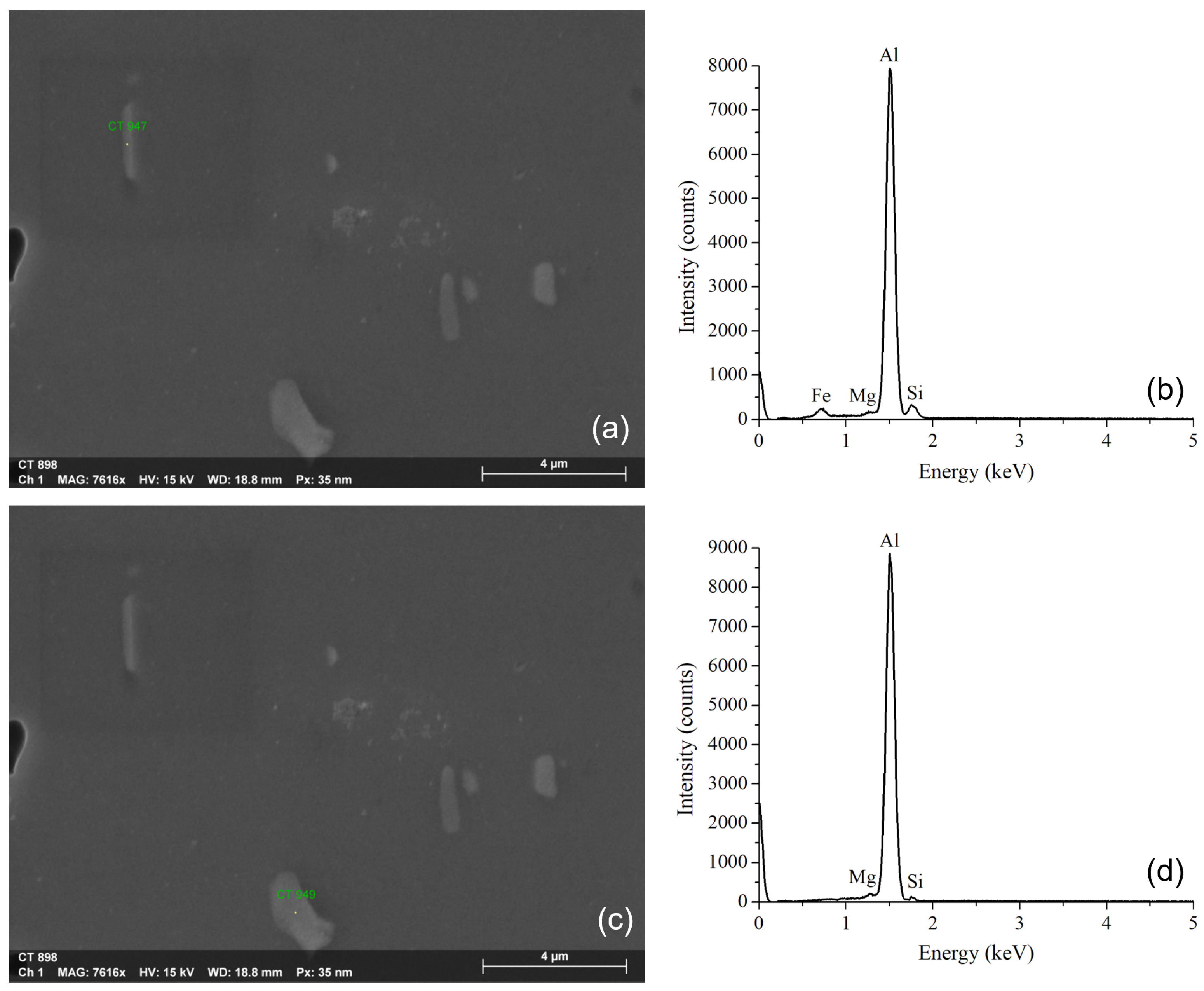
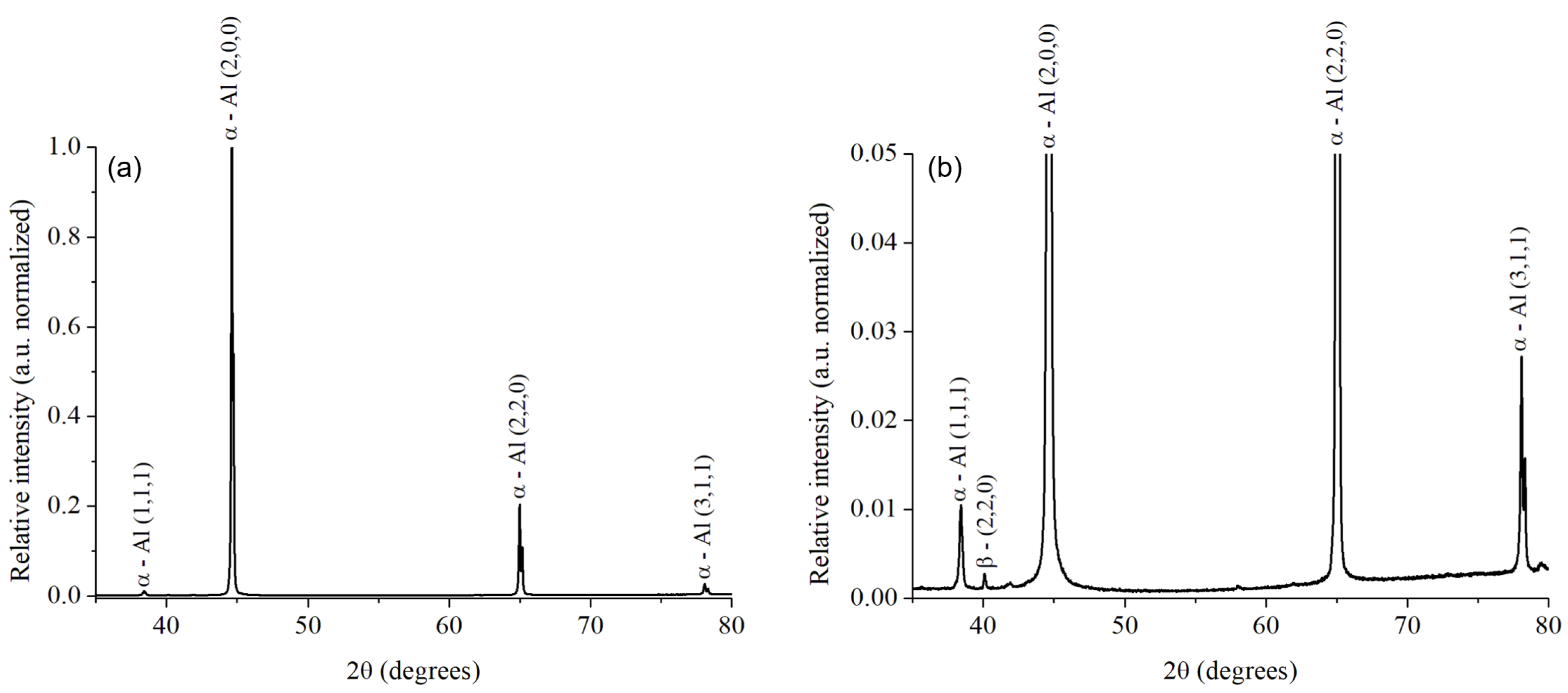
3.1. Microstructural Evaluation of the Alloy after the Solubilization Heat Treatment
3.2. Mechanical Testing
3.3. Macrostructural Study and Phase Composition of the Weld Junction
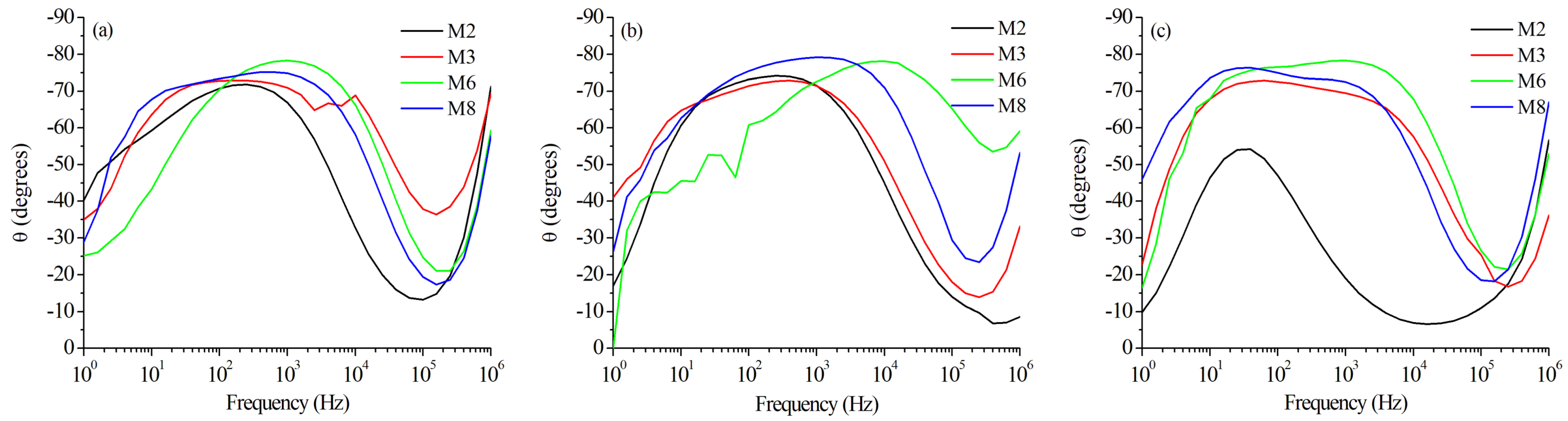
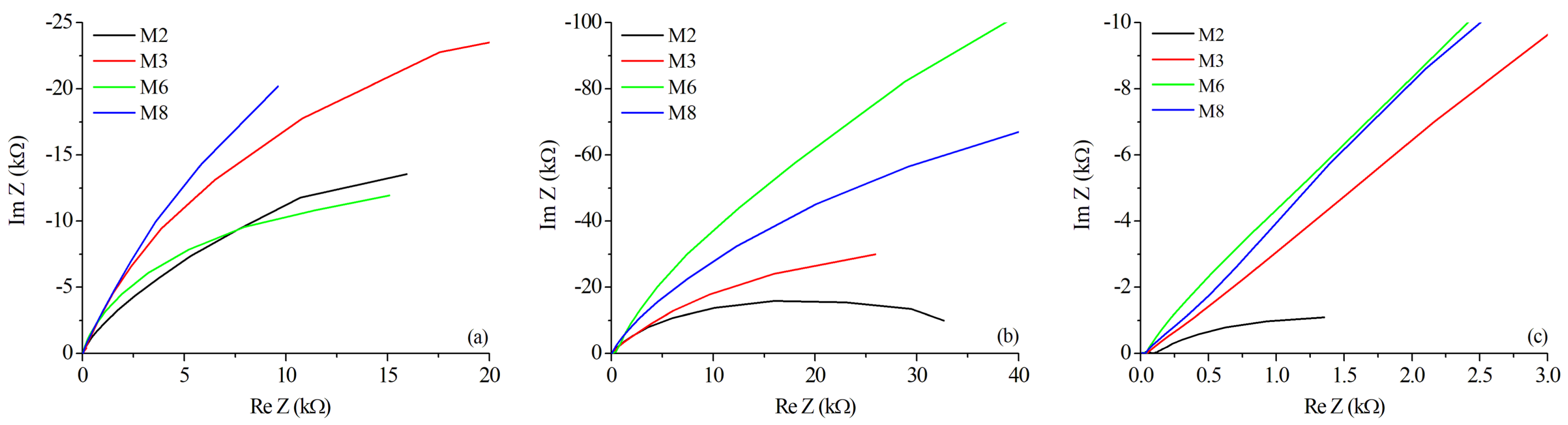
3.4. Corrosion Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BM | Base Metal (also identified as parent metal) |
| FSW | Friction Stir Welding |
| FZ | Fusion Zone |
| GMAW | Gas Metal Arc Welding |
| GMAW-P | Pulsed Gas Metal Arc Welding |
| GP | Guinier–Preston Zones |
| HAZ | Heat-Affected Zone |
| HV | Hardness Vickers |
| OCP | Open-Circuit Potential |
| SD | Standard Deviation |
| SEM | Scanning Electron Microscope, Scanning Electron Microscopy |
| TIG | Tungsten Inert Gas |
| WZ | Welding Zone |
| XRD | X-ray Diffraction, X-ray Diffractometer |
References
- Prillhofer, R.; Rank, G.; Berneder, J.; Antrekowitsch, H.; Uggowitzer, P.; Pogatscher, S. Property Criteria for Automotive Al-Mg-Si Sheet Alloys. Materials 2014, 7, 5047–5068. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, K.; Colas, K.; Ribis, J.; Lopez, A.; Garnier, J. Analysis of the metastable precipitates in peak-hardness aged Al-Mg-Si(-Cu) alloys with differing Si contents. Acta Mater. 2017, 132, 209–221. [Google Scholar] [CrossRef]
- Fallah, V.; Korinek, A.; Raeisinia, B.; Gallerneault, M.; Esmaeili, S. Early-Stage Precipitation Phenomena and Composition-Dependent Hardening in Al-Mg-Si-(Cu) Alloys. Mater. Sci. Forum 2014, 794–796, 933–938. [Google Scholar] [CrossRef]
- Hirsch, J.; Al-Samman, T. Superior light metals by texture engineering: Optimized aluminum and magnesium alloys for automotive applications. Acta Mater. 2013, 61, 818–843. [Google Scholar] [CrossRef]
- Ostermann, F. Anwendungstechnologie Aluminium, 3rd ed.; VDI-Buch Trimet; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Hakem, M.; Lebaili, S.; Mathieu, S.; Miroud, D.; Lebaili, A.; Cheniti, B. Effect of microstructure and precipitation phenomena on the mechanical behavior of AA6061-T6 aluminum alloy weld. Int. J. Adv. Manuf. Technol. 2019, 102, 2907–2918. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; Chen, J.; Wan, C.; Fang, L.; Liu, P.; Wu, C. Relationship Between the Strengthening Effect and the Morphology of Precipitates in Al–7.4Zn–1.7Mg–2.0Cu Alloy. Acta Metall. Sin. (Engl. Lett.) 2014, 27, 1070–1077. [Google Scholar] [CrossRef]
- Massardier-Jourdan, V.; Epicier, T. Study of the Influence of a Low Copper Addition and of an Excess of Silicon on the Precipitation Kinetics and on the Precipitation Sequence of Al-Mg2Si Alloys. Mater. Sci. Forum 2002, 396–402, 851–856. [Google Scholar] [CrossRef]
- Andersen, S.; Zandbergen, H.; Jansen, J.; TrÆholt, C.; Tundal, U.; Reiso, O. The crystal structure of the β” phase in Al–Mg–Si alloys. Acta Mater. 1998, 46, 3283–3298. [Google Scholar] [CrossRef]
- Fang, X.; Song, M.; Li, K.; Du, Y. Precipitation sequence of an aged Al-Mg-Si alloy. J. Min. Metall. Sect. B Metall. 2010, 46, 171–180. [Google Scholar] [CrossRef]
- Murayama, M.; Hono, K. Pre-precipitate clusters and precipitation processes in Al–Mg–Si alloys. Acta Mater. 1999, 47, 1537–1548. [Google Scholar] [CrossRef]
- Menzemer, C.; Lam, P.; Srivatsan, T.; Wittel, C. An investigation of fusion zone microstructures of welded aluminum alloy joints. Mater. Lett. 1999, 41, 192–197. [Google Scholar] [CrossRef]
- Jia, Z.h.; Ding, L.P.; Weng, Y.y.; Wen, Z.; Liu, Q. Effects of high temperature pre-straining on natural aging and bake hardening response of Al–Mg–Si alloys. Trans. Nonferrous Met. Soc. China 2016, 26, 924–929. [Google Scholar] [CrossRef]
- Devaraj, A.; Perea, D.E.; Liu, J.; Gordon, L.M.; Prosa, T.J.; Parikh, P.; Diercks, D.R.; Meher, S.; Kolli, R.P.; Meng, Y.S.; et al. Three-dimensional nanoscale characterisation of materials by atom probe tomography. Int. Mater. Rev. 2018, 63, 68–101. [Google Scholar] [CrossRef]
- Safyari, M.; Moshtaghi, M.; Hojo, T.; Akiyama, E. Mechanisms of hydrogen embrittlement in high-strength aluminum alloys containing coherent or incoherent dispersoids. Corros. Sci. 2022, 194, 109895. [Google Scholar] [CrossRef]
- Safyari, M.; Moshtaghi, M.; Kuramoto, S. Environmental hydrogen embrittlement associated with decohesion and void formation at soluble coarse particles in a cold-rolled Al–Cu based alloy. Mater. Sci. Eng. A 2021, 799, 139850. [Google Scholar] [CrossRef]
- Isadare, D. Optimization of Mechanical Properties of Age-Hardened 6063 Aluminium Alloy Via Diffusion Annealing. Br. J. Appl. Sci. Technol. 2014, 4, 3360–3369. [Google Scholar] [CrossRef]
- Guzmán, I.; Granda, E.; Acevedo, J.; Martínez, A.; Dávila, Y.; Velázquez, R. Comparative in Mechanical Behavior of 6061 Aluminum Alloy Welded by Pulsed GMAW with Different Filler Metals and Heat Treatments. Materials 2019, 12, 4157. [Google Scholar] [CrossRef]
- Patel, A.; Prasad, B.; Singh, D.K. Effect of variable process parameter of MIG welding on aluminium alloy 6061-T6. Int. J. Adv. Res. Ideas Innov. Technol. 2018, 4, 49–52. [Google Scholar]
- Chikhale, S.R.; Kolhe, K.P.; Kumar, P. Prediction of Mechanical properties of Al Alloy 6061-T6 by using GMAW. Int. J. Curr. Eng. Technol. 2016, 5, 300–306. [Google Scholar] [CrossRef]
- Mathivanan, A.; Devakumaran, K.; Kumar, A.S. Comparative Study on Mechanical and Metallurgical Properties of AA6061 Aluminum Alloy Sheet Weld by Pulsed Current and Dual Pulse Gas Metal Arc Welding Processes. Mater. Manuf. Process. 2014, 29, 941–947. [Google Scholar] [CrossRef]
- Chen, C.; Fan, C.; Cai, X.; Lin, S.; Yang, C. Analysis of droplet transfer, weld formation and microstructure in Al-Cu alloy bead welding joint with pulsed ultrasonic-GMAW method. J. Mater. Process. Technol. 2019, 271, 144–151. [Google Scholar] [CrossRef]
- Fahimpour, V.; Sadrnezhaad, S.; Karimzadeh, F. Corrosion behavior of aluminum 6061 alloy joined by friction stir welding and gas tungsten arc welding methods. Mater. Des. 2012, 39, 329–333. [Google Scholar] [CrossRef]
- Zaid, B.; Maddache, N.; Saidi, D.; Souami, N.; Bacha, N.; Si Ahmed, A. Electrochemical evaluation of sodium metabisulfite as environmentally friendly inhibitor for corrosion of aluminum alloy 6061 in a chloride solution. J. Alloys Compd. 2015, 629, 188–196. [Google Scholar] [CrossRef]
- Abbass, M.K.; Hassan, K.S.; Alwan, A.S. Study of Corrosion Resistance of Aluminum Alloy 6061/SiC Composites in 3.5% NaCl Solution. Int. J. Mater. Mech. Manuf. 2015, 3, 31–35. [Google Scholar] [CrossRef]
- Atkins, G.; Thiessen, N.; Nissley, N.; Adonyi, Y. Welding Process Effects in Weldability Testing of Steels. Weld. J. 2002, 81, 61S–68S. [Google Scholar]
- International Organization for Standardization. Part 1: General guidance for arc welding. In Welding—Recommendations for Welding of Metallic Materials; International Organization for Standardization: Geneva, Switzerland, 2002; Volume ISO/TR 17671-1:2002. [Google Scholar]
- Jang, K.; Lee, D.; Kuk, J.; Kim, I. Welding and environmental test condition effect in weldability and strength of Al alloy. J. Mater. Process. Technol. 2005, 164–165, 1038–1045. [Google Scholar] [CrossRef]
- American Welding Society. AWS D1.2/D1.2M Structural Welding Code—Aluminum; American National Standard AWS D1.2/D1.2M; American Welding Society: Miami, FL, USA, 2013. [Google Scholar]
- ASTM International. ASTM International. ASTM E8/E8M—16a Standard Test Methods for Tension Testing of Metallic Materials. In ASTM Volume 03.01 Metals—Mechanical Testing; Elevated and Low-Temperature Tests; Metallography; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- ASTM International. ASTM International. ASTM E3-11 (2017) Standard Guide for Preparation of Metallographic Specimens. In ASTM Volume 03.01 Metals—Mechanical Testing; Elevated and Low-Temperature Tests; Metallography; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM International. ASTM International. ASTM E407-07 (2015)e1 Standard Practice for Microetching Metals and Alloys. In ASTM Volume 03.01 Metals—Mechanical Testing; Elevated and Low-Temperature Tests; Metallography; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- ASTM International. ASTM International. ASTM E92-17 Standard Test Methods for Vickers Hardness and Knoop Hardness of Metallic Materials. In ASTM Volume 03.01 Metals—Mechanical Testing; Elevated and Low-Temperature Tests; Metallography; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Styles, M.; Hutchinson, C.; Chen, Y.; Deschamps, A.; Bastow, T. The coexistence of two S (Al2CuMg) phases in Al–Cu–Mg alloys. Acta Mater. 2012, 60, 6940–6951. [Google Scholar] [CrossRef]
- ASTM International. ASTM International. ASTM G-102 Standard practice for calculation of corrosion rates and related information from electrochemical measurements. In ASTM Volume 03.02—Corrosion of Metals; Wear and Erosion; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Zhou, H.; Fu, F.; Dai, Z.; Qiao, Y.; Chen, J.; Liu, W. Effect of Laser Power on Microstructure and Micro-Galvanic Corrosion Behavior of a 6061-T6 Aluminum Alloy Welding Joints. Metals 2020, 11, 3. [Google Scholar] [CrossRef]
- Zhu, M.; Zhao, B.Z.; Yuan, Y.F.; Guo, S.Y.; Pan, J. Effect of Solution Temperature on the Corrosion Behavior of 6061-T6 Aluminum Alloy in NaCl Solution. J. Mater. Eng. Perform. 2020, 29, 4725–4732. [Google Scholar] [CrossRef]
- Zhou, H.; Fu, F.; Dai, Z.; Qiao, Y.; Chen, J.; Yang, L.; Liu, W. Effect of Laser Power on Hybrid Laser-Gas Metal Arc Welding (GMAW) of a 6061 Aluminum Alloy. J. Korean Phys. Soc. 2020, 77, 991–996. [Google Scholar] [CrossRef]
- Chen, M.A.; Ou, Y.C.; Fu, Y.H.; Li, Z.H.; Li, J.M.; Liu, S.D. Effect of friction stirred Al-Fe-Si particles in 6061 aluminum alloy on structure and corrosion performance of MAO coating. Surf. Coat. Technol. 2016, 304, 85–97. [Google Scholar] [CrossRef]
- Jacobs, M.H. The structure of the metastable precipitates formed during ageing of an Al-Mg-Si alloy. Philos. Mag. 1972, 26, 1–13. [Google Scholar] [CrossRef]
- Marioara, C.D.; Andersen, S.J.; Stene, T.N.; Hasting, H.; Walmsley, J.; Van Helvoort, A.T.J.; Holmestad, R. The effect of Cu on precipitation in Al–Mg–Si alloys. Philos. Mag. 2007, 87, 3385–3413. [Google Scholar] [CrossRef]
- Sasabe, S. Effect of Mn on welding liquation micro-cracking in heat affected zone of 6082 aluminum alloy. J. Jpn. Inst. Light Met. 2010, 60, 213–219. [Google Scholar] [CrossRef][Green Version]
- Sathish, T.; Dinesh Kumar, S.; Muthukumar, K.; Karthick, S. Natural inspiration technique for the parameter optimization of A-GTAW welding of naval steel. Mater. Today Proc. 2020, 21, 843–846. [Google Scholar] [CrossRef]
- Huang, Y.; Yuan, Y.; Yang, L.; Wu, D.; Chen, S. Real-time monitoring and control of porosity defects during arc welding of aluminum alloys. J. Mater. Process. Technol. 2020, 286, 116832. [Google Scholar] [CrossRef]
- Han, X.; Yang, Z.; Ma, Y.; Shi, C.; Xin, Z. Porosity distribution and mechanical response of laser-MIG hybrid butt welded 6082-T6 aluminum alloy joint. Opt. Laser Technol. 2020, 132, 106511. [Google Scholar] [CrossRef]
- Kah, P.; Rajan, R.; Martikainen, J.; Suoranta, R. Investigation of weld defects in friction-stir welding and fusion welding of aluminium alloys. Int. J. Mech. Mater. Eng. 2015, 10, 26. [Google Scholar] [CrossRef]
- Ardika, R.D.; Triyono, T.; Muhayat, N.; Triyono. A review porosity in aluminum welding. Procedia Struct. Integr. 2021, 33, 171–180. [Google Scholar] [CrossRef]
- Sevim, I.; Hayat, F.; Kaya, Y.; Kahraman, N.; Şahin, S. The study of MIG weldability of heat-treated aluminum alloys. Int. J. Adv. Manuf. Technol. 2013, 66, 1825–1834. [Google Scholar] [CrossRef]
- Dawood, H.I.; Mohammed, K.S.; Rajab, M.Y. Advantages of the Green Solid State FSW over the Conventional GMAW Process. Adv. Mater. Sci. Eng. 2014, 2014, 105713. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Z.; Gordon, R.A.; Takarabe, K.; Reid, J.; Tse, J.S. Pressure-induced phase transition and electrical properties of thermoelectric Al-doped Mg2 Si. J. Appl. Phys. 2015, 118, 145902. [Google Scholar] [CrossRef]
- Vissers, R.; van Huis, M.; Jansen, J.; Zandbergen, H.; Marioara, C.; Andersen, S. The crystal structure of the β’ phase in Al–Mg–Si alloys. Acta Mater. 2007, 55, 3815–3823. [Google Scholar] [CrossRef]
- Ayieko, C.O.; Musembi, R.J.; Ogacho, A.A.; Aduda, B.O.; Muthoka, B.M.; Jain, P.K. Controlled Texturing of Aluminum Sheet for Solar Energy Applications. Adv. Mater. Phys. Chem. 2015, 05, 458–466. [Google Scholar] [CrossRef]
- Zhang, W.; He, H.; Shan, Y.; Sun, C.; Li, L. Diffusion assisted hardness recovering and related microstructural characteristics in fusion welded Al–Mg–Si alloy butt. Mater. Res. Express 2019, 6, 066549. [Google Scholar] [CrossRef]
- Vargas, J.A.; Torres, J.E.; Pacheco, J.A.; Hernandez, R.J. Analysis of heat input effect on the mechanical properties of Al-6061-T6 alloy weld joints. Mater. Des. 2013, 52, 556–564. [Google Scholar] [CrossRef]
- Liu, S.; Li, K.; Lu, J.; Sha, G.; Wang, J.; Yang, M.; Ji, G.; Song, M.; Wang, J.; Du, Y. On the atomic model of Guinier-Preston zones in Al-Mg-Si-Cu alloys. J. Alloys Compd. 2018, 745, 644–650. [Google Scholar] [CrossRef]
- Lakshminarayanan, A.K.; Balasubramanian, V.; Elangovan, K. Effect of welding processes on tensile properties of AA6061 aluminium alloy joints. Int. J. Adv. Manuf. Technol. 2009, 40, 286–296. [Google Scholar] [CrossRef]
- Zhang, W.; He, H.; Xu, C.; Yu, W.; Li, L. Precipitates Dissolution, Phase Transformation, and Re-precipitation-Induced Hardness Variation in 6082-T6 Alloy during MIG Welding and Subsequent Baking. JOM 2019, 71, 2711–2720. [Google Scholar] [CrossRef]
- Li, P.; Nie, F.; Dong, H.; Li, S.; Yang, G.; Zhang, H. Pulse MIG Welding of 6061-T6/A356-T6 Aluminum Alloy Dissimilar T-joint. J. Mater. Eng. Perform. 2018, 27, 4760–4769. [Google Scholar] [CrossRef]
- Gou, G.; Zhang, M.; Chen, H.; Chen, J.; Li, P.; Yang, Y. Effect of humidity on porosity, microstructure, and fatigue strength of A7N01S-T5 aluminum alloy welded joints in high-speed trains. Mater. Des. 2015, 85, 309–317. [Google Scholar] [CrossRef]
- Gharavi, F.; Matori, K.A.; Yunus, R.; Othman, N.K.; Fadaeifard, F. Corrosion behavior of Al6061 alloy weldment produced by friction stir welding process. J. Mater. Res. Technol. 2015, 4, 314–322. [Google Scholar] [CrossRef]
- Venkatasubramanian, G. Corrosion Behavior Of Aluminium Alloy Aa2219–T87 Welded Plates in Sea Water. Indian J. Sci. Technol. 2012, 5, 1–6. [Google Scholar] [CrossRef]
- Reena Kumari, P.; Nayak, J.; Nityananda Shetty, A. Corrosion behavior of 6061/Al-15 vol. pct. SiC(p) composite and the base alloy in sodium hydroxide solution. Arab. J. Chem. 2016, 9, S1144–S1154. [Google Scholar] [CrossRef]
- Zhou, B.; Yang, L.; Yang, S.; Bai, D.; Olugbade, O.; Huang, G. Stress corrosion behavior of 6082 aluminum alloy. Mater. Corros. 2020, 71, 1194–1205. [Google Scholar] [CrossRef]
- Al-Kharafi, F.M.; Badawy, W.A. Inhibition of Corrosion of Al 6061, Aluminum, and an Aluminum-Copper Alloy in Chloride-Free Aqueous Media: Part 2—Behavior in Basic Solutions. Corrosion 1998, 54, 377–385. [Google Scholar] [CrossRef]
- Tang, C.; Cheng, F.; Man, H. Effect of laser surface melting on the corrosion and cavitation erosion behaviors of a manganese–nickel–aluminium bronze. Mater. Sci. Eng. A 2004, 373, 195–203. [Google Scholar] [CrossRef]
- Kaushal, C.; Sharma, L. To Determine Effects of Gas Metal Arc Welding (GMAW) Parameters on Mechanical Properties of Aluminium Alloys. Int. J. Innov. Res. Sci. Eng. Technol. 2015, 4, 4564–4572. [Google Scholar] [CrossRef]
- Polmear, I.; Stjohn, D.; Nie, J.F.; Qian, M. Light Alloys: Metallurgy of the Light Metals, 5th ed.; Elsevier Science and Technology Books, Inc.: Oxford, UK, 2017. [Google Scholar]
- Norman, A.; Drazhner, V.; Prangnell, P. Effect of welding parameters on the solidification microstructure of autogenous TIG welds in an Al–Cu–Mg–Mn alloy. Mater. Sci. Eng. A 1999, 259, 53–64. [Google Scholar] [CrossRef]
- Osório, W.R.; Freire, C.M.; Garcia, A. The effect of the dendritic microstructure on the corrosion resistance of Zn–Al alloys. J. Alloys Compd. 2005, 397, 179–191. [Google Scholar] [CrossRef]
- Song, D.; Ma, A.; Jiang, J.; Lin, P.; Yang, D.; Fan, J. Corrosion behavior of equal-channel-angular-pressed pure magnesium in NaCl aqueous solution. Corros. Sci. 2010, 52, 481–490. [Google Scholar] [CrossRef]


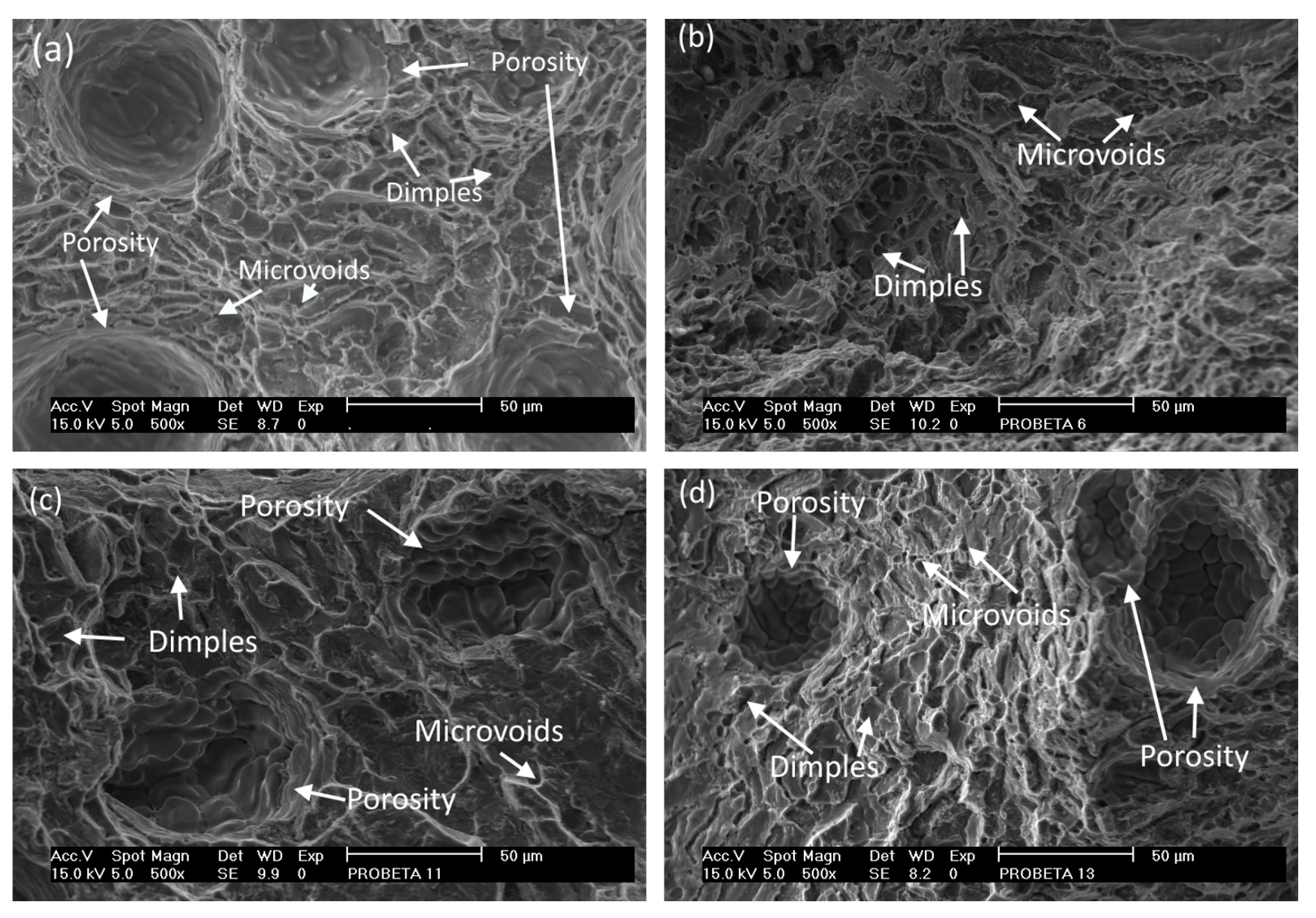

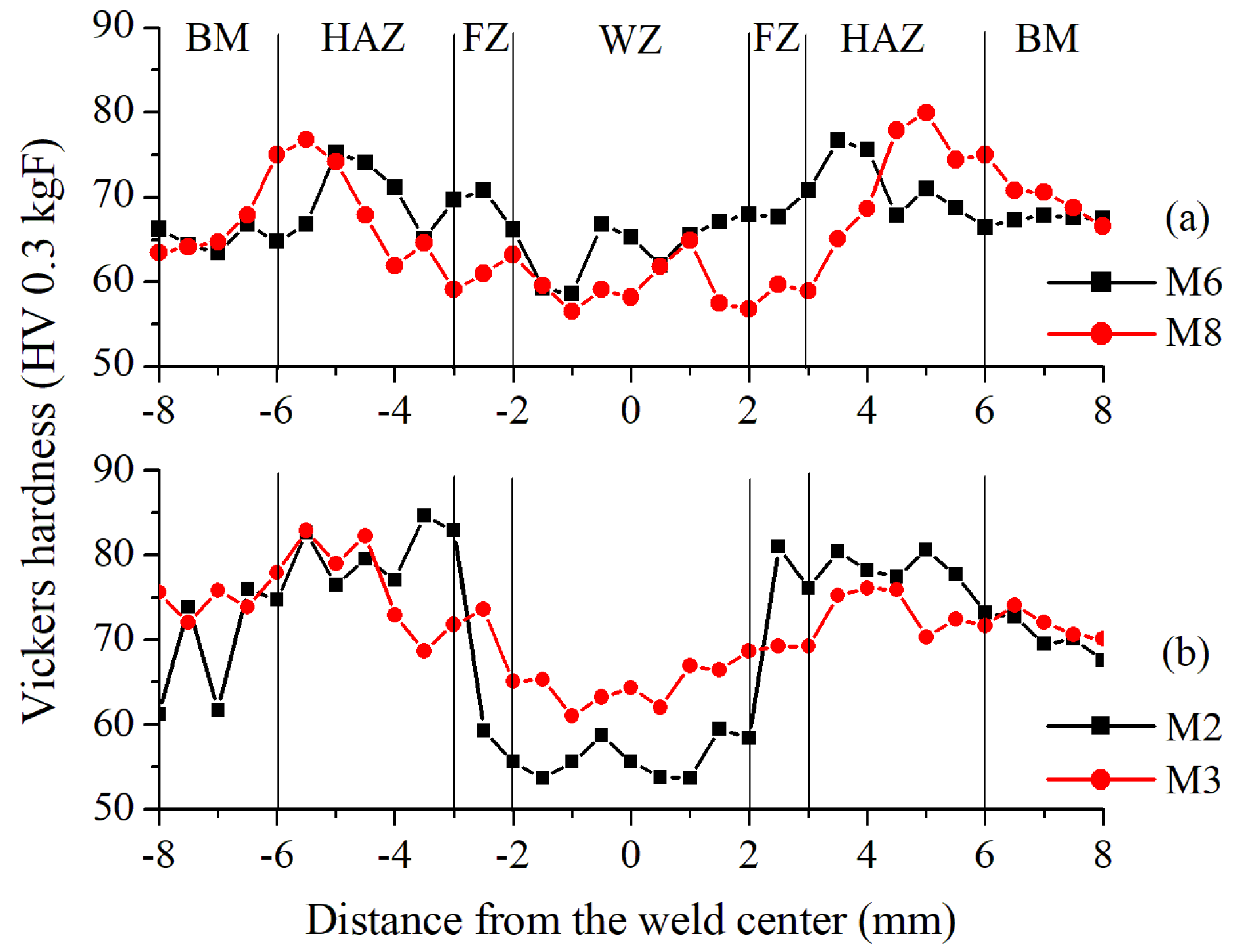
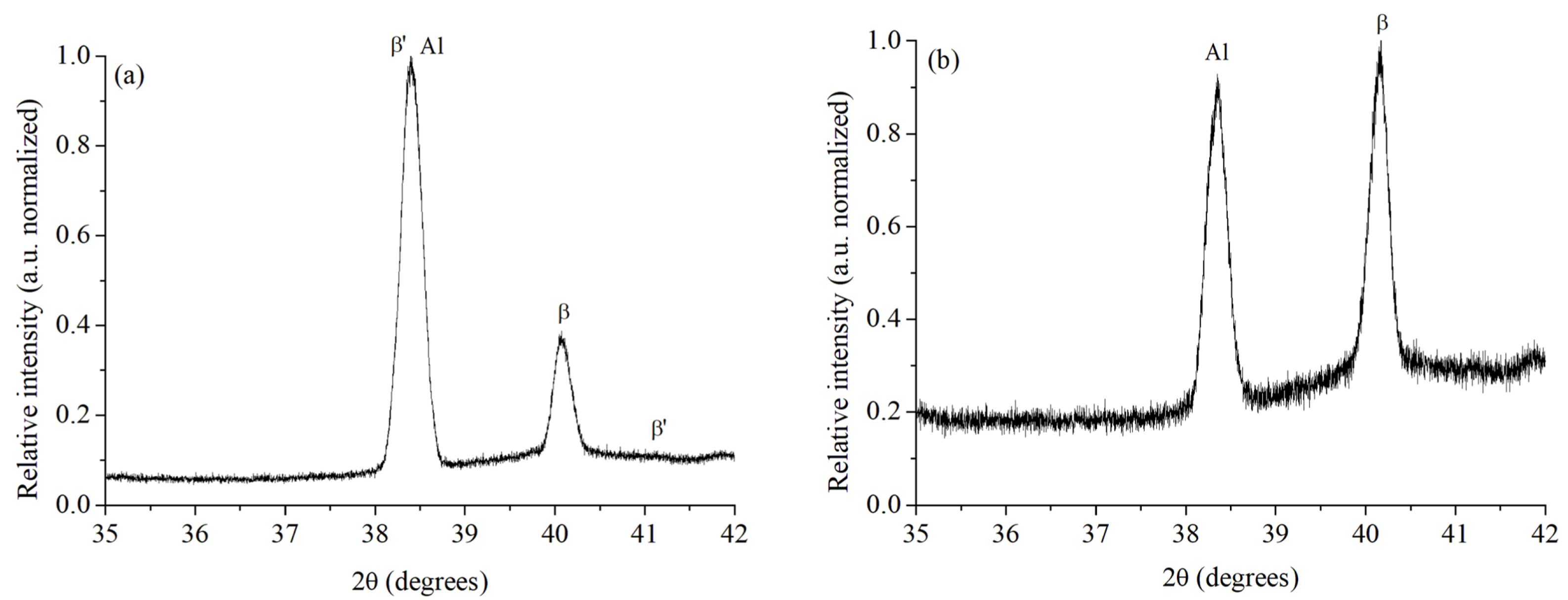
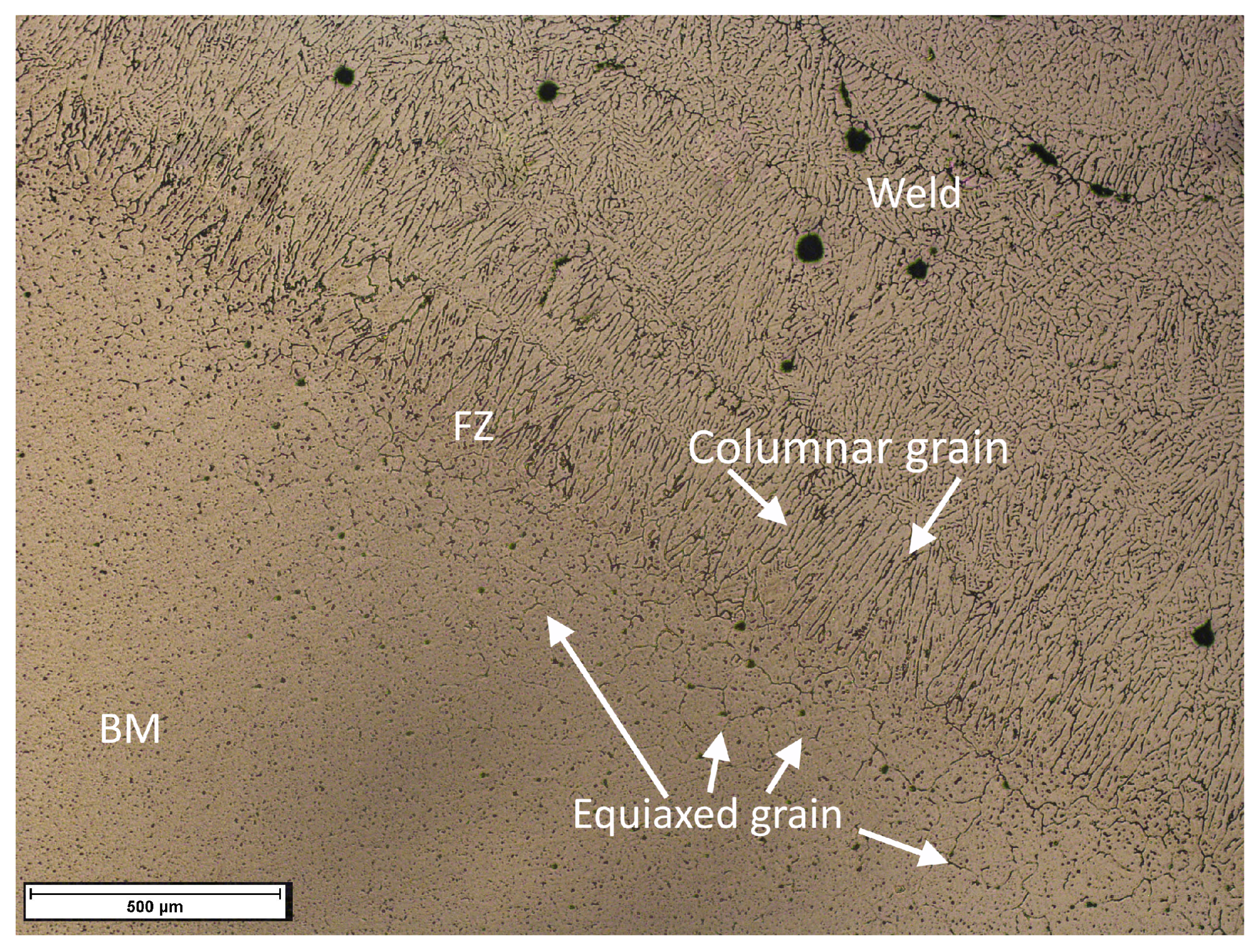


| Alloy | UNS No. | ISO No. | Composition wt. % (Al, Balance) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Others | ||||
| Indiv. | Total | |||||||||||
| 4043–ER | A94043 | AlSi5 | 6.00 | 0.80 | 0.30 | 0.05 | 0.05 | 0.10 | 0.20 | 0.05 | 0.15 | |
| AA6061–T6 | A06061 | AlMgSiCu | 0.80 | 0.70 | 0.40 | 0.15 | 1.20 | 0.35 | 0.25 | 0.15 | 0.05 | 0.15 |
| ID | HT | I (Amperes) | V (Volts) | (mm/min) | (J/mm) |
|---|---|---|---|---|---|
| M1 | T6 | 250 | 25 | 318 | 1014 |
| M2 | T6 | 175 | 25 | 174 | 1297 |
| M3 | T6 | 212 | 25 | 234 | 1169 |
| M4 | T6 | 280 | 25 | 366 | 987 |
| M5 | T4 | 212 | 25 | 216 | 1266 |
| M6 | T4 | 250 | 25 | 294 | 1097 |
| M7 | T4 | 280 | 25 | 336 | 1075 |
| M8 | T4 | 175 | 25 | 192 | 1176 |
| Yield | Ultimate | Rupture | Young | ||
|---|---|---|---|---|---|
| Strength | Strength | Load | Modulus | Elongation | |
| ID | (MPa) | (MPa) | (N) | (MPa) | (%) |
| M1 | 22 (2.4) | 74 (11.8) | 5449 (544) | 2925 (139) | 14 (9.9) |
| M2 | 40 (3.2) | 104 (11.0) | 7139 (211) | 1146 (3.53) | 40 (1.4) |
| M3 | 30 (4.4) | 54 (2.1) | 4048 (2.12) | 1316 (75.7) | 15 (0.7) |
| M4 | 36 (2.1) | 65 (0.6) | 4906 (149) | 654 (106) | 35 (2.1) |
| M5 | 33 (1.3) | 83 (5.0) | 6128 (19.8) | 813 (47.3) | 25 (1.4) |
| M6 | 79 (0.9) | 117 (4.5) | 8793 (83.4) | 568 (82.7) | 43 (26.9) |
| M7 | 40 (1.1) | 105 (0.5) | 7802 (240) | 850 (35.1) | 39 (2.1) |
| M8 | 30 (0.8) | 80 (3.8) | 6577 (40.3) | 2972 (242) | 24 (24.0) |
| Weld Crown | Weld Penetration | Porosity | |
|---|---|---|---|
| ID | (mm) | (mm) | (pores/mm2) |
| M1 | 1.1 | 6.6 | 0.0065 |
| M2 | 1.8 | 6.7 | 0.0044 |
| M3 | 1.4 | 6.0 | 0.0011 |
| M4 | 0.7 | 6.5 | 0.0023 |
| M5 | 2.1 | 6.6 | 0.0017 |
| M6 | 1.3 | 9.0 | 0.0068 |
| M7 | 0.8 | 8.1 | 0.0067 |
| M8 | 1.7 | 6.1 | 0.0048 |
| Position | Height | FWHM | d-Spacing | Rel. Int. | Orientation | Phase |
|---|---|---|---|---|---|---|
| (° 2) | (cps) | (° 2) | (Angstrom) | (%) | ||
| 36.0746 | 28.59 | 0.0864 | 2.48775 | 1.80 | 2 0 3 | ’ |
| 38.2650 | 18.72 | 0.0858 | 2.35023 | 0.88 | 1 2 0 | ’ |
| 38.4093 | 2139.19 | 0.1939 | 2.34174 | 100 | 1 1 1 | -Al |
| 40.0805 | 781.91 | 0.1981 | 2.24786 | 36.55 | 2 2 0 | |
| 41.1628 | 13.10 | 0.0845 | 2.15826 | 0.25 | 1 2 2 | ’ |
| Position | Height | FWHM | d-Spacing | Rel. Int. | Orientation | Phase |
|---|---|---|---|---|---|---|
| (° 2) | (cps) | (° 2) | (Angstrom) | (%) | ||
| 38.3134 | 603.63 | 0.2113 | 2.34838 | 100 | 1 1 1 | -Al |
| 40.1158 | 597.2 | 0.2078 | 2.24597 | 98.94 | 2 2 0 |
| OCP | Ecorr | Icorr | Corr. Rate | Rs | Rp | Cdl | Area | |
|---|---|---|---|---|---|---|---|---|
| Zone | (mV) | (mV) | (A/cm2) | (mmPY) | (/cm2) | (k/cm2) | (F/cm2) | (cm2) |
| M2 BM | −790 | −776 | 3.41 | 0.037 | 26.33 | 16.89 | 1.710 | 0.47 |
| M2 HAZ | −746 | −754 | 1.28 | 0.014 | 95.99 | 2.15 | 7.710 | 0.08 |
| M2 WZ | −765 | −741 | 1.61 | 0.018 | 30.18 | 29.43 | 0.702 | 0.13 |
| M3 BM | −766 | −770 | 1.58 | 0.017 | 29.63 | 12.56 | 0.554 | 0.13 |
| M3 HAZ | −774 | −721 | 1.09 | 0.012 | 49.33 | 90.71 | 0.238 | 0.05 |
| M3 WZ | −746 | −723 | 1.79 | 0.020 | 29.38 | 44.49 | 0.556 | 0.12 |
| M6 BM | −765 | −762 | 2.48 | 0.027 | 18.88 | 17.57 | 0.382 | 0.35 |
| M6 HAZ | −786 | −806 | 4.59 | 0.050 | 40.17 | 54.50 | 0.151 | 0.13 |
| M6 WZ | −767 | −750 | 3.15 | 0.034 | 406.90 | 239.17 | 0.0018 | 0.15 |
| M8 BM | −765 | −767 | 3.50 | 0.038 | 21.78 | 28.78 | 0.552 | 0.30 |
| M8 HAZ | −763 | −764 | 4.38 | 0.048 | 34.45 | 48.34 | 0.480 | 0.14 |
| M8 WZ | −757 | −743 | 3.53 | 0.041 | 42.87 | 129.60 | 0.122 | 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán, I.; Granda, E.; Cruz, C.; Martínez, D.; Vargas, B.; Acevedo, J.; Cruz, G.; Avila, Y.; Velazquez, R.; Flores, L. Corrosion Performance and Mechanical Strength in Aluminum 6061 Joints by Pulsed Gas Metal Arc Welding. Materials 2022, 15, 6226. https://doi.org/10.3390/ma15186226
Guzmán I, Granda E, Cruz C, Martínez D, Vargas B, Acevedo J, Cruz G, Avila Y, Velazquez R, Flores L. Corrosion Performance and Mechanical Strength in Aluminum 6061 Joints by Pulsed Gas Metal Arc Welding. Materials. 2022; 15(18):6226. https://doi.org/10.3390/ma15186226
Chicago/Turabian StyleGuzmán, Isidro, Everardo Granda, Celso Cruz, Dora Martínez, Benjamín Vargas, Jorge Acevedo, Gilberto Cruz, Yuliana Avila, Ruben Velazquez, and Leonardo Flores. 2022. "Corrosion Performance and Mechanical Strength in Aluminum 6061 Joints by Pulsed Gas Metal Arc Welding" Materials 15, no. 18: 6226. https://doi.org/10.3390/ma15186226
APA StyleGuzmán, I., Granda, E., Cruz, C., Martínez, D., Vargas, B., Acevedo, J., Cruz, G., Avila, Y., Velazquez, R., & Flores, L. (2022). Corrosion Performance and Mechanical Strength in Aluminum 6061 Joints by Pulsed Gas Metal Arc Welding. Materials, 15(18), 6226. https://doi.org/10.3390/ma15186226






