Investigation on Carbonation and Permeability of Concrete with Rice Hush Ash and Shop Solution Addition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Used
2.2. Preparation of Sample and Test Procedure
2.3. Methods
2.3.1. Carbonation Test
2.3.2. Permeability Test
3. Result and Discussions
4. Conclusions
- i
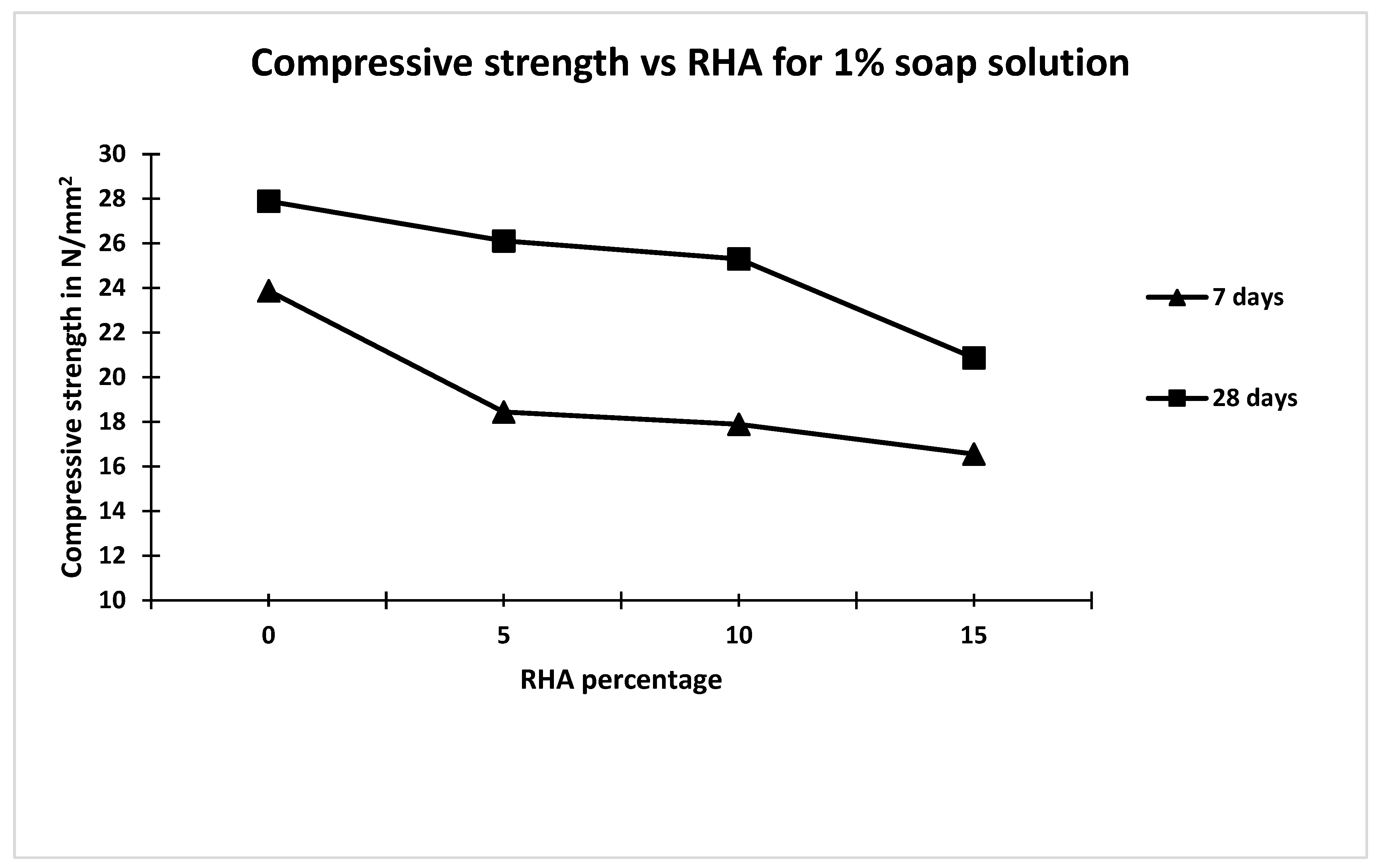
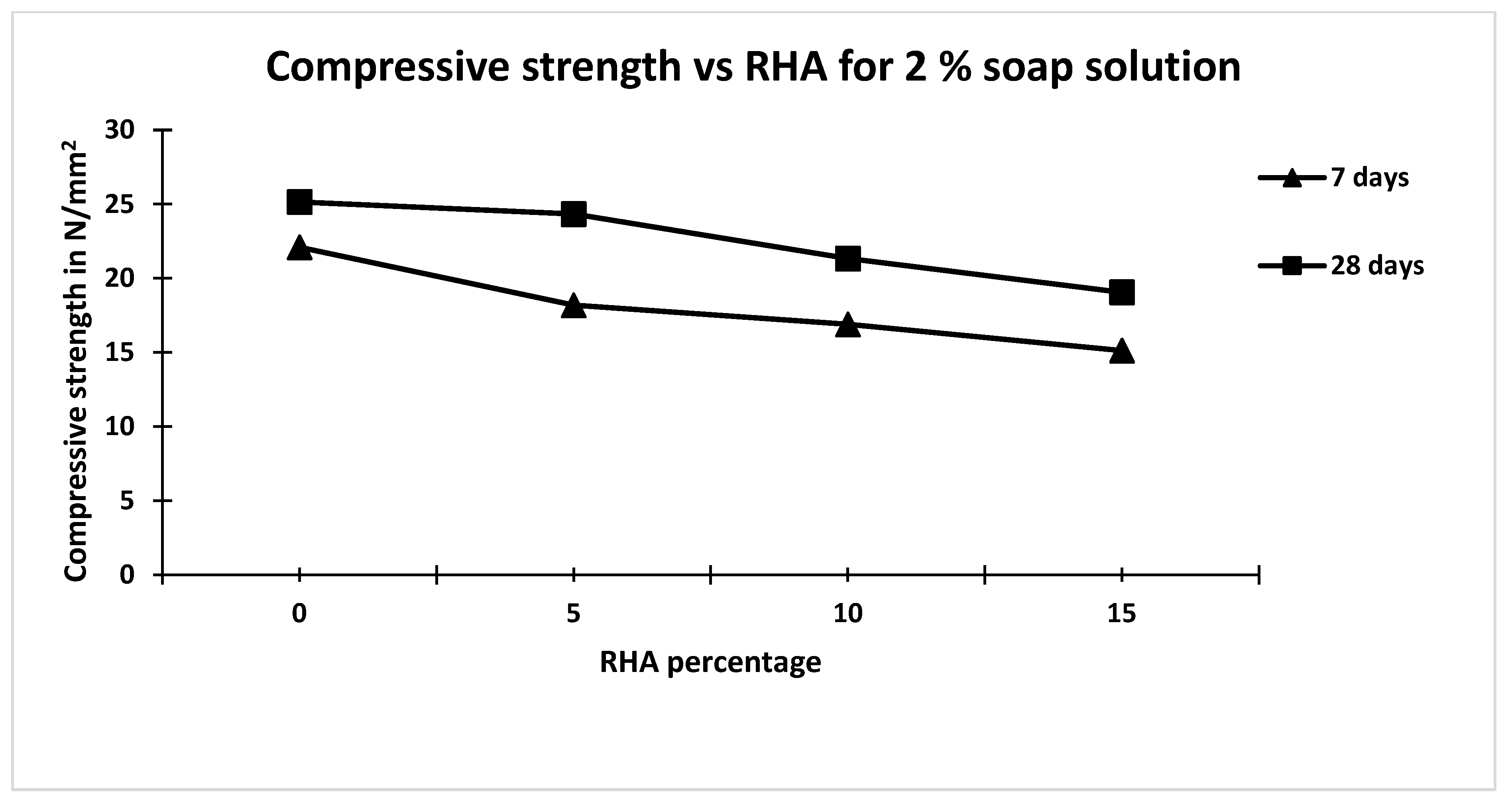
- The 28 days compressive strength of concrete containing 1% and 2% soap solution decreased by 8.4% and 15% compared to that made without soap solution and RHA.
- ii
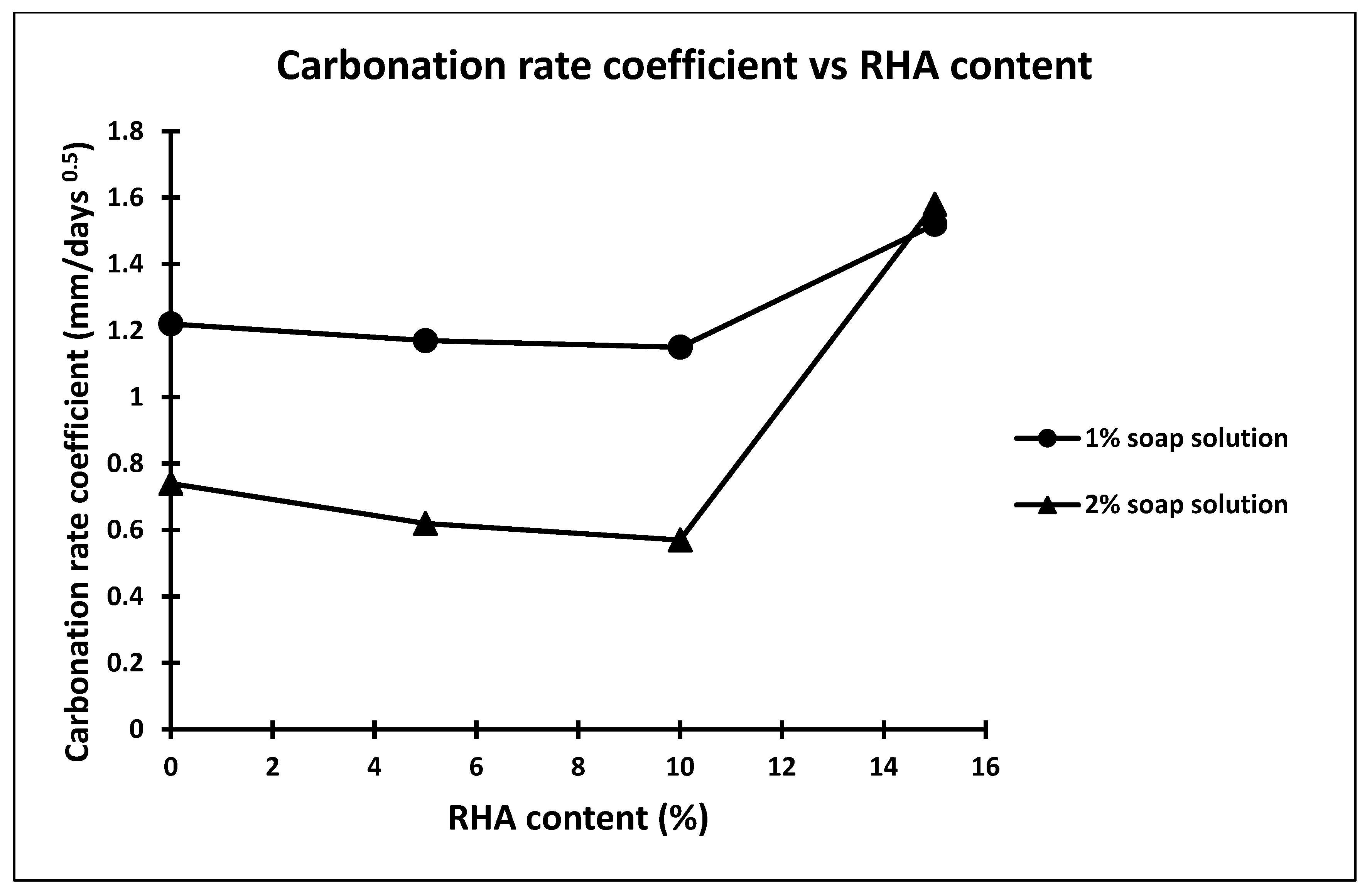
- The carbonation depth of concrete made with 1% and 2% soap solution concentration and without rice husk ash decreased 11.89% and 46.55%, respectively.
- iii
- The carbonation depth of concrete made with up to 10% replacement showed maximum carbonation resistance, while more than 10% replacement of cement showed higher carbonation depth.
- iv
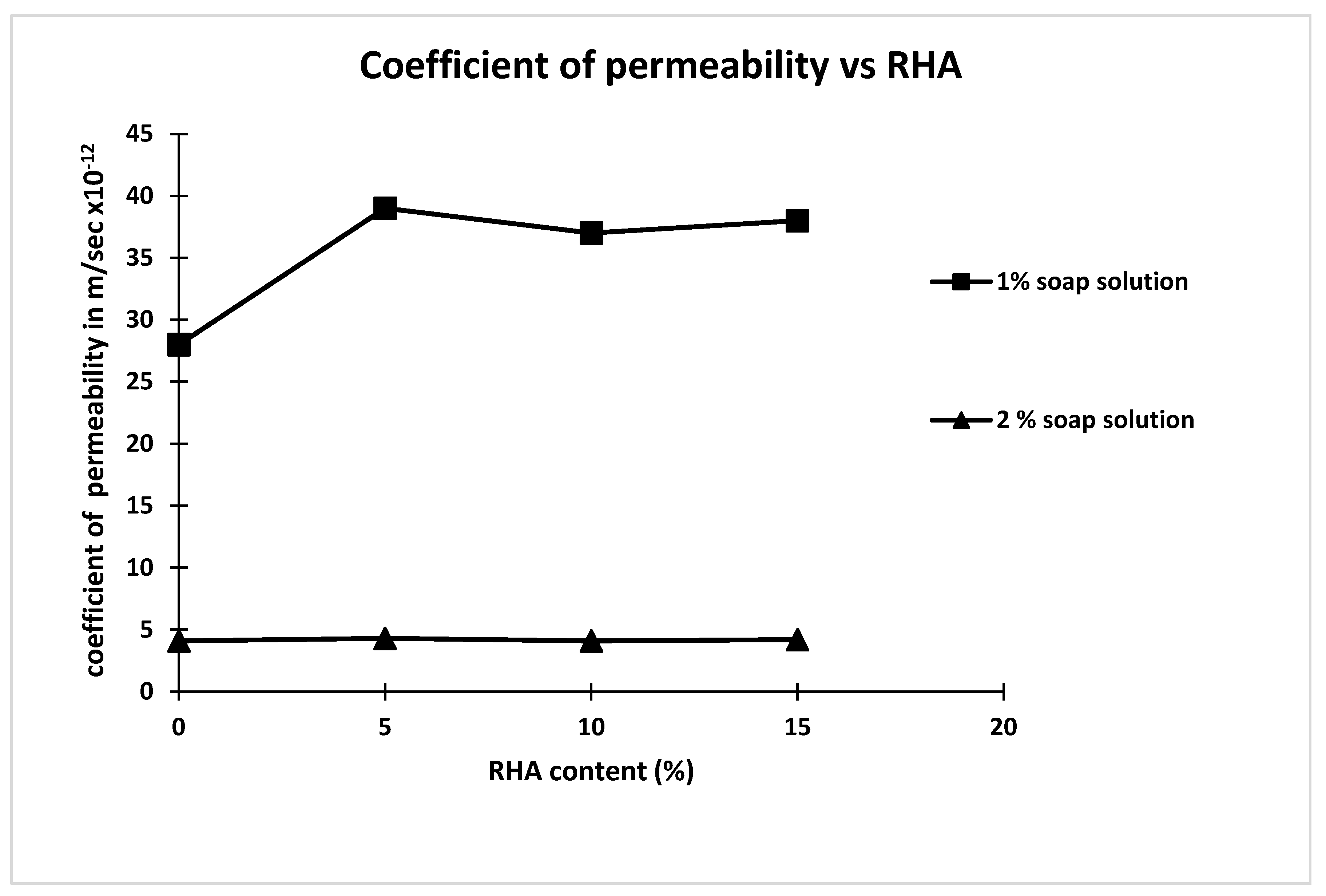
- The coefficient of permeability of concrete for 2% soap solution significantly decreased compared to that 1% soap solution and control mix.
- v
- Increasing the percentage of rice hush ash content has a negligible effect on the coefficient of permeability of concrete made with 2% soap solution concentration.
- vi
- From these results, it is concluded that the reduction in coefficient of permeability is mainly because of the soap solution.
- vii
- The rice husk ash carbonation depth has been reduced but only up to 10%, and any further replacement increases the carbonation depth.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, M.; Malay, N.; Kujur, J. Study of natural carbonation of concrete incorporating marble dust. Proc. Inst. Civ. Eng.-Constr. Mater. 2018, 171, 85–92. [Google Scholar] [CrossRef]
- Kumar, M.; Sinha, A.K.; Kujur, J. Mechanical and durability studies on high volume fly-ash concrete. Struct. Concr. 2021, 22, 1036–1049. [Google Scholar] [CrossRef]
- Kumar, M.; Kujur, J.; Chatterjee, R.; Chattopadhyaya, S.; Sharma, S.; Dwivedi, S.P.; Saxena, A.; Rajkumar, S.; Anand, A. Corrosion Zones of Rebar in High-Volume Fly-Ash Concrete through Potentiodynamic Study in Concrete Powder Solution Extracts: A Sustainable Construction Approach. Adv. Civ. Eng. 2022, 2022, 5927819. [Google Scholar] [CrossRef]
- Dongmin, A.; Yupeng, G.; Yanchao, Z.; Zichen, W. A green route to preparation of silica powders with rice husk ash and waste gas. Chem. Eng. J. 2010, 162, 509–514. [Google Scholar]
- Devi, T.K.; Chanu, N.M. Contribution of rice husk ash to the properties of cement mortar and concrete. Int. J. Eng. Res. Technol. 2013, 2. [Google Scholar] [CrossRef]
- Habeeb, G.A.; Fayyadh, M.M. Rice Husk Ash Concrete: The Effect of RHA Average Particle Size on Mechanical Properties and Drying Shrinkage. Mater. Sci. Eng. 2009, 3, 1616–1622. [Google Scholar]
- Arredondo-Rea, S.P.; Corral-Higuera, R.; Gómez-Soberón, J.M.; Castorena-González, J.H.; Orozco-Carmona, V.; Almar-al-Sánchez, J.L. Carbonation rate and reinforcing steel corrosion of concretes with recycled concrete aggregates and supplemen-tary cementing materials. Int. J. Electrochem. Sci. 2012, 7, 1602–1610. [Google Scholar]
- Sulapha, P.; Wong, S.F.; Wee, T.H.; Swaddiwudhipong, S. Carbonation of Concrete Containing Mineral Admixtures. J. Mater. Civ. Eng. 2003, 15, 134–143. [Google Scholar] [CrossRef]
- Lombardo, M.C. Densifier and Waterproofing Agents for Mortar and Concrete and Method of Making same. United States Patents No. 569,574 9 Claims, 2 August 1966. [Google Scholar]
- Ozturk, E.; Ince, C.; Derogar, S.; Ball, R. Factors affecting the CO2 emissions, cost efficiency and eco-strength efficiency of con-crete containing rice husk ash: A database study. Constr. Build. Mater. 2022, 326, 126905. [Google Scholar] [CrossRef]
- Vishavkarma, A.; Harish, K.V. Effect of rice husk ash on permeation characteristic of cementitious mortar. Mater. Today Proc. 2022, 61, 406–412. [Google Scholar] [CrossRef]
- Abdila, S.R.; Abdullah, M.M.; Ahmad, R.; Nergis, D.D.B.; Rahim, S.Z.; Omar, M.F.; Sandu, A.V.; Vizureanu, P. Potential of soil stabilization using ground granulated blast furnace slag (GGBFS) and fly ash via geopolymerization method: A Review. Materials 2022, 15, 375. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.N.; Ahmad, W.; Khan, K.; Sayed, M.M. Mapping research knowledge on rice husk ash application in concrete: A sci-entometric review. Materials 2022, 15, 3431. [Google Scholar] [CrossRef] [PubMed]
- Thiedeitz, M.; Ostermaier, B.; Kränkel, T. Rice husk ash as an additive in mortar–Contribution to microstructural, strength and durability performance. Resour. Conserv. Recycl. 2022, 184, 106389. [Google Scholar] [CrossRef]
- Scripture, E.W., Jr. Waterproofing Composition for Concrete and Mortar. United States Patents 2305113, 15 December 1942. [Google Scholar]
- Khanna, P.N. Indian Practical Civil Engineers; Engineers Publishers: New Delhi, India, 2019. [Google Scholar]
- IS 8112; Specification for 43 Grade Ordinary Portland Cement. Bureau of Indian Standards: New Delhi, India, 2013.
- IS 383; Specification for Coarse and Fine Aggregates. Bureau of Indian Standards: New Delhi, India, 1970.
- IS 10262; Concrete Mix Design. Bureau of Indian Standards: New Delhi, India, 2009.
- IS 9103; Specification for Concrete Admixtures. Bureau of Indian Standards: New Delhi, India, 1999.
- IS 516; 1959 for Methods of Tests for Strength of Concrete. Bureau of Indian Standards: New Delhi, India, 1959.
- Draft v8 December 2008 Testing Hardened Concrete—Part XX: Determination of the Car-Bonation Resistance of Concrete: Accelerated Carbonation Method, prCEN/TS 12390-XXX:2008 (E). 2008. Available online: https://standards.iteh.ai/catalog/standards/sist/da305181-51c1-4f10-88e2-20424af56a72/sist-en-12390-12-2020 (accessed on 23 December 2021).
- Indian Standard Code IS 3085; 1965 for Coefficient of Permeability of Concrete. Bureau of Indian Standards: New Delhi, India, 1965.
- Ilyas, R.A.; Zuhri, M.Y.M.; Norrrahim, M.N.F.; Misenan, M.S.M.; Jenol, M.A.; Samsudin, S.A.; Nurazzi, N.M.; Asyraf, M.R.M.; Supian, A.B.M.; Bangar, S.P.; et al. Natural Fiber-Reinforced Polycaprolactone Green and Hybrid Biocomposites for Various Advanced Applications. Polymers 2022, 14, 182. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Zuhri, M.Y.M.; Aisyah, H.A.; Asyraf, M.R.M.; Hassan, S.A.; Zainudin, E.S.; Sapuan, S.M.; Sharma, S.; Bangar, S.P.; Jumaidin, R.; et al. Natural Fiber-Reinforced Polylactic Acid, Polylactic Acid Blends and Their Composites for Advanced Applications. Polymers 2022, 14, 202. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Patyal, V.; Sudhakara, P.; Singh, J.; Petru, M.; Ilyas, R.A. Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques. Nanotechnol. Rev. 2021, 11, 65–85. [Google Scholar] [CrossRef]
- Chohan, J.S.; Mittal, N.; Kumar, R.; Singh, S.; Sharma, S.; Dwivedi, S.P.; Saxena, A.; Chattopadhyaya, S.; Ilyas, R.A.; Le, C.H.; et al. Optimization of FFF process parameters by naked mole-rat algorithms with en-hanced exploration and exploitation capabilities. Polymers 2021, 13, 1702. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Asyraf, M.R.M.; Dayana, D.A.Z.N.; Amelia, J.J.N.; Rani, M.S.A.; Norrrahim, M.N.F.; Nurazzi, N.M.; Aisyah, H.A.; Sharma, S.; et al. Polymer composites filled with metal derivatives: A review of flame retardants. Polymers 2021, 13, 1701. [Google Scholar] [CrossRef]
- Chohan, J.S.; Mittal, N.; Kumar, R.; Singh, S.; Sharma, S.; Singh, J.; Rao, K.V.; Mia, M.; Pimenov, D.Y.; Dwivedi, S.P. Mechanical Strength Enhancement of 3D Printed Acrylonitrile Butadiene Styrene Polymer Components Using Neural Network Optimization Algorithm. Polymers 2020, 12, 2250. [Google Scholar] [CrossRef]
- Singh, Y.; Singh, J.; Sharma, S.; Aggarwal, V.; Pruncu, C.I. Multi-objective optimization of kerf-taper and sur-face-roughness quality characteristics for cutting-operation on coir and carbon fibre reinforced epoxy hybrid polymeric com-posites during CO2-pulsed laser-cutting using RSM. Lasers Manuf. Mater. Process. 2021, 8, 157–182. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, J.; Kumar, H.; Sharma, A.; Aggarwal, V.; Gill, A.S.; Jayarambabu, N.; Kailasa, S.; Rao, K.V. Utilization of rapid prototyping technology for the fabrication of an orthopedic shoe inserts for foot pain reprieve using thermo-softening viscoelastic polymers: A novel experimental approach. Meas. Control 2020, 53, 519–530. [Google Scholar] [CrossRef]
- Singh, Y.; Singh, J.; Sharma, S.; Sharma, A.; Chohan, J.S. Process parameter optimization in laser cutting of coir fiber re-inforced epoxy composite—A review. Mater. Today Proc. 2022, 48, 1021–1027. [Google Scholar] [CrossRef]
- Chohan, J.S.; Kumar, R.; Singh, T.H.B.; Singh, S.; Sharma, S.; Singh, J.; Mia, M.; Pimenov, D.Y.; Chattopadhyaya, S.; Dwivedi, S.P.; et al. Taguchi S/N and TOPSIS Based Optimization of Fused Deposition Modelling and Vapor Finishing Process for Manufacturing of ABS Plastic Parts. Materials 2020, 13, 5176. [Google Scholar] [CrossRef]
- Prabhakaran, S.; Krishnaraj, V.; Sharma, S.; Senthilkumar, M.; Jegathishkumar, R.; Zitoune, R. Experimental study on thermal and morphological analyses of green composite sandwich made of flax and agglomerated cork. J. Therm. Anal. 2019, 139, 3003–3012. [Google Scholar] [CrossRef]
- Sharma, S.; Sudhakara, P.; Singh, J.; Ilyas, R.A.; Asyraf, M.R.M.; Razman, M.R. Critical Review of Biodegradable and Bioactive Polymer Composites for Bone Tissue Engineering and Drug Delivery Applications. Polymers 2021, 13, 2623. [Google Scholar] [CrossRef]
- Sharma, S.; Sudhakara, P.; Omran, A.A.B.; Singh, J.; Ilyas, R.A. Recent Trends and Developments in Conducting Polymer Nanocomposites for Multifunctional Applications. Polymers 2021, 13, 2898. [Google Scholar] [CrossRef]
- Jha, K.; Tyagi, Y.K.; Kumar, R.; Sharma, S.; Huzaifah, M.R.M.; Li, C.; Ilyas, R.A.; Dwivedi, S.P.; Saxena, A.; Pramanik, A. Assessment of Dimensional Stability, Biodegradability, and Fracture Energy of Bio-Composites Reinforced with Novel Pine Cone. Polymers 2021, 13, 3260. [Google Scholar] [CrossRef]
- Kadier, A.; Ilyas, R.A.; Huzaifah, M.R.M.; Harihastuti, N.; Sapuan, S.M.; Harussani, M.M.; Azlin, M.N.M.; Yuliasni, R.; Ibrahim, R.; Atikah, M.S.N.; et al. Use of industrial wastes as sustainable nutrient sources for bacterial cellu-lose (BC) production: Mechanism, advances, and future perspectives. Polymers 2021, 13, 3365. [Google Scholar] [CrossRef]
- Singh, Y.; Singh, J.; Sharma, S.; Lam, T.-D.; Nguyen, D.-N. Fabrication and characterization of coir/carbon-fiber rein-forced epoxy based hybrid composite for helmet shells and sports-good applications: Influence of fiber surface modifications on the mechanical, thermal and morphological properties. J. Mater. Res. Technol. 2020, 9, 15593–15603. [Google Scholar] [CrossRef]
- Suriani, M.J.; Ilyas, R.A.; Zuhri, M.Y.M.; Khalina, A.; Sultan, M.T.H.; Sapuan, S.M.; Ruzaidi, C.M.; Wan, F.N.; Zulkifli, F.; Harussani, M.M.; et al. Critical review of natural fiber reinforced hybrid composites: Processing, properties, applications and cost. Polymers 2021, 13, 3514. [Google Scholar] [CrossRef]
- 41. Kumar, R.; Ranjan, N.; Kumar, V.; Kumar, R.; Chohan, J.S.; Yadav, A.; Piyush; Sharma, S.; Prakash, C.; Singh, S.; et al. Characterization of Friction Stir-Welded Polylactic Acid/Aluminum Composite Primed through Fused Filament Fabrication. J. Mater. Eng. Perform. 2021, 31, 2391–2409. [Google Scholar] [CrossRef]
- Zhu, Z.; Wu, Y.; Liang, Z. Mining-Induced Stress and Ground Pressure Behavior Characteristics in Mining a Thick Coal Seam with Hard Roofs. Front. Earth Sci. 2022, 10, 843191. [Google Scholar] [CrossRef]
- Azlin, M.N.M.; Ilyas, R.A.; Zuhri, M.Y.M.; Sapuan, S.M.; Harussani, M.M.; Sharma, S.; Nordin, A.H.; Nurazzi, N.M.; Afiqah, A.N. 3D Printing and Shaping Polymers, Composites, and Nanocomposites: A Review. Polymers 2022, 14, 180. [Google Scholar] [CrossRef]
- Gu, M.; Mo, H.; Qiu, J.; Yuan, J.; Xia, Q. Behavior of Floating Stone Columns Reinforced with Geogrid Encasement in Model Tests. Front. Mater. 2022, 9, 980851. [Google Scholar] [CrossRef]
- Kumar, J.; Singh, D.; Kalsi, N.S.; Sharma, S.; Pruncu, C.I.; Pimenov, D.Y.; Rao, K.V.; Kapłonek, W. Comparative study on the mechanical, tribological, morphological and structural properties of vortex casting processed, Al-SiC-Cr hybrid metal matrix composites for high strength wear-resistant applications: Fabrication and characterizations. J. Mater. Res. Technol. 2020, 9, 13607–13615. [Google Scholar] [CrossRef]
- Dwivedi, S.P.; Saxena, A.; Sharma, S. Influence of Nano-CuO on Synthesis and Mechanical Behavior of Spent Alumina Catalyst and Grinding Sludge Reinforced Aluminum Based Composite. Int. J. Met. 2021, 16, 292–303. [Google Scholar] [CrossRef]
- Dwivedi, S.P.; Saxena, A.; Sharma, S.; Srivastava, A.K.; Maurya, N.K. Influence of SAC and Eggshell addition in the Physical, Me-chanical and Thermal Behaviour of Cr reinforced Aluminium Based Composite. Int. J. Cast Met. Res. 2021, 34, 43–55. [Google Scholar] [CrossRef]
- Saxena, A.; Dwivedi, S.; Dixit, A.; Sharma, S.; Srivastava, A.; Maurya, N. Computational and experimental investigation on mechanical behavior of zirconia toughened alumina and nickel powder reinforced EN31 based composite material. Mater. Werkst. 2021, 52, 548–560. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, J.; Gupta, M.K.; Mia, M.; Dwivedi, S.P.; Saxena, A.; Chattopadhyaya, S.; Singh, R.; Pimenov, D.Y.; Korkmaz, M.E. Investigation on mechanical, tribological and microstruc-tural properties of Al-Mg-Si-T6/SiC/muscovite-hybrid metal-matrix composites for high strength applications. J. Mate-Rials Res. Technol. 2021, 12, 1564–1581. [Google Scholar] [CrossRef]
- Dwivedi, S.P.; Agrawal, R.; Sharma, S. Effect of Friction Stir Process Parameters on Mechanical Properties of Chrome Containing Leather Waste Reinforced Aluminium Based Composite. Int. J. Precis. Eng. Manuf. Technol. 2021, 8, 935–943. [Google Scholar] [CrossRef]
- Kumar, J.; Singh, D.; Kalsi, N.S.; Sharma, S.; Mia, M.; Singh, J.; Rahman, M.A.; Khan, A.M.; Rao, K.V. Investigation on the mechanical, tribological, morphological and machinability behavior of stir-casted Al/SiC/Mo reinforced MMCs. J. Mater. Res. Technol. 2021, 12, 930–946. [Google Scholar] [CrossRef]
- Islam, S.; Dwivedi, S.P.; Dwivedi, V.K.; Sharma, S.; Kozak, D. Development of Marble Dust/Waste PET Based Polymer Composite Ma-terial for Environmental Sustainability: Fabrication and Characterizations. J. Mater. Perform. Charact. 2021, 10, 538–552. [Google Scholar]
- Guo, Y.; Yang, Y.; Kong, Z.; He, J.; Wu, H. Development of Similar Materials for Liquid-Solid Coupling and Its Application in Water Outburst and Mud Outburst Model Test of Deep Tunnel. Geofluids 2022, 2022, 8784398. [Google Scholar] [CrossRef]
- Sharma, S.; Sudhakara, P. Fabrication and optimization of hybrid AA-6082-T6 alloy/8%Al2O3(Alumina)/2%Grp metal matrix composites using novel Box-Behnken methodology processed by wire-sinking electric discharge machining. Mater. Res. Express 2019, 6, 116594. [Google Scholar] [CrossRef]
- Dwivedi, S.P.; Saxena, A.; Sharma, S.; Singh, G.; Singh, J.; Mia, M.; Chattopadhyaya, S.; Pramanik, A.; Pimenov, D.Y.; Wojciechowski, S. Effect of ball-milling process parameters on mechanical properties of Al/Al2O3/collagen powder composite using statistical approach. J. Mater. Res. Technol. 2021, 15, 2918–2932. [Google Scholar] [CrossRef]
- Khare, J.M.; Dahiya, S.; Gangil, B.; Ranakoti, L.; Sharma, S.; Huzaifah, M.R.M.; Ilyas, R.A.; Dwivedi, S.P.; Chattopadhyaya, S.; Kilinc, H.C.; et al. Comparative Analysis of Erosive Wear Behaviour of Epoxy, Polyester and Vinyl Esters Based Thermosetting Polymer Composites for Human Prosthetic Applications Using Taguchi Design. Polymers 2021, 13, 3607. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.P.; Maurya, M.; Sharma, S. Study of CCLW, Alumina and the Mixture of Alumina- and CCLW-Reinforced Aluminum-Based Composite Material with and Without Mechanical Alloying. J. Inst. Eng. Ser. D 2021, 103, 319–331. [Google Scholar] [CrossRef]
- Dwivedi, S.P.; Sahu, R.; Saxena, A.; Dwivedi, V.K.; Srinivas, K.; Sharma, S. Recovery of Cr from chrome-containing leather waste and its utilization as reinforcement along with waste spent alumina catalyst and grinding sludge in AA 5052-based metal matrix composites. Proc. Inst. Mech. Eng. Part E J. Process. Mech. Eng. 2021, 236, 160–170. [Google Scholar] [CrossRef]
- Dwivedi, S.P.; Maurya, M.; Saxena, A.; Sharma, S. Synthesis and characterization of spent alumina catalyst and grinding sludge reinforced aluminium-based composite material. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2021, 236, 5523–5534. [Google Scholar] [CrossRef]
- Dwivedi, S.P.; Maurya, M.; Sharma, S. Synthesis and characterisation of chromium, eggshell and grinding sludge-reinforced aluminium metal matrix composite: An experimental approach. Green Mater. 2022, 1–10. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Aisyah, H.A.; Nordin, A.H.; Ngadi, N.; Zuhri, M.Y.M.; Asyraf, M.R.M.; Sapuan, S.M.; Zainudin, E.S.; Sharma, S.; Abral, H.; et al. Natural-Fiber-Reinforced Chitosan, Chitosan Blends and Their Nanocomposites for Various Advanced Applications. Polymers 2022, 14, 874. [Google Scholar] [CrossRef] [PubMed]
- Asyraf, M.R.M.; Syamsir, A.; Zahari, N.M.; Supian, A.B.M.; Ishak, M.R.; Sapuan, S.M.; Sharma, S.; Rashedi, A.; Razman, M.R.; Zakaria, S.Z.S.; et al. Product Development of Natural Fibre-Composites for Various Applications: Design for Sustainability. Polymers 2022, 14, 920. [Google Scholar] [CrossRef] [PubMed]
- Chandel, P.S.; Tyagi, Y.K.; Jha, K.; Kumar, R.; Sharma, S.; Singh, J.; Ilyas, R.A. Study of mode II interlaminar fracture toughness of laminated composites of glass and jute fibres in epoxy for structural applications. Funct. Compos. Struct. 2021, 3, 044002. [Google Scholar] [CrossRef]
- Yeswanth, I.; Jha, K.; Bhowmik, S.; Kumar, R.; Sharma, S.; Rushdan, A.I. Recent developments in RAM based MWCNT composite materials: A short review. Funct. Compos. Struct. 2022, 4, 024001. [Google Scholar] [CrossRef]
- Virk, G.S.; Singh, B.; Singh, Y.; Sharma, S.; Ilyas, R.A.; Patyal, V. Abrasive water jet machining of coir fiber reinforced epoxy composites: A review. Funct. Compos. Struct. 2022, 4, 014001. [Google Scholar] [CrossRef]
- Juneja, S.; Chohan, J.S.; Kumar, R.; Sharma, S.; Ilyas, R.A.; Asyraf, M.R.M.; Razman, M.R. Impact of Process Variables of Acetone Vapor Jet Drilling on Surface Roughness and Circularity of 3D-Printed ABS Parts: Fabrication and Studies on Thermal, Morphological, and Chemical Characterizations. Polymers 2022, 14, 1367. [Google Scholar] [CrossRef]
- Singh, S.; Khairandish, M.I.; Razahi, M.M.; Kumar, R.; Chohan, J.S.; Tiwary, A.; Sharma, S.; Li, C.; Ilyas, R.A.; Asyraf, M.R.M.; et al. Preference Index of Sustainable Natural Fibers in Stone Matrix Asphalt Mixture Using Waste Marble. Materials 2022, 15, 2729. [Google Scholar] [CrossRef]
- Tiwary, A.K.; Singh, S.; Chohan, J.S.; Kumar, R.; Sharma, S.; Chattopadhyaya, S.; Abed, F.; Stepinac, M. Behavior of RC Beam–Column Joints Strengthened with Modified Reinforcement Techniques. Sustainability 2022, 14, 1918. [Google Scholar] [CrossRef]
- Tiwary, A.K.; Bhatia, S.; Singh, S.; Chohan, J.S.; Kumar, R.; Sharma, S.; Chattopadhyaya, S.; Rajkumar, S. Performance Comparison and Critical Finite Element Based Experimental Analysis of Various Forms of Reinforcement Retaining Structural System. Math. Probl. Eng. 2022, 2022, 4434679. [Google Scholar] [CrossRef]
- Ranakoti, L.; Gangil, B.; Mishra, S.K.; Singh, T.; Sharma, S.; Ilyas, R.; El-Khatib, S. Critical Review on Polylactic Acid: Properties, Structure, Processing, Biocomposites, and Nanocomposites. Materials 2022, 15, 4312. [Google Scholar] [CrossRef]
- Norfarhana, A.; Ilyas, R.; Ngadi, N.; Sharma, S.; Sayed, M.M.; El-Shafay, A.; Nordin, A. Natural Fiber-Reinforced Thermoplastic ENR/PVC Composites as Potential Membrane Technology in Industrial Wastewater Treatment: A Review. Polymers 2022, 14, 2432. [Google Scholar] [CrossRef] [PubMed]
- Tiwary, A.K.; Singh, S.; Kumar, R.; Chohan, J.S.; Sharma, S.; Singh, J.; Li, C.; Ilyas, R.A.; Asyraf, M.R.M.; Malik, M.A. Effects of Elevated Temperature on the Residual Behavior of Concrete Containing Marble Dust and Foundry Sand. Materials 2022, 15, 3632. [Google Scholar] [CrossRef] [PubMed]
- Robert, J.B.; Prabhavathy, R.A.; Joanna, P.S.; Singh, S.C.E.; Murugan, S.; Rajkumar, S.; Sharma, S. Flexural Behaviour of RC Beams with a Circular Opening at the Flexural Zone and Shear Zone Strengthened Using Steel Plates. Adv. Civ. Eng. 2021, 2021, 6733402. [Google Scholar] [CrossRef]
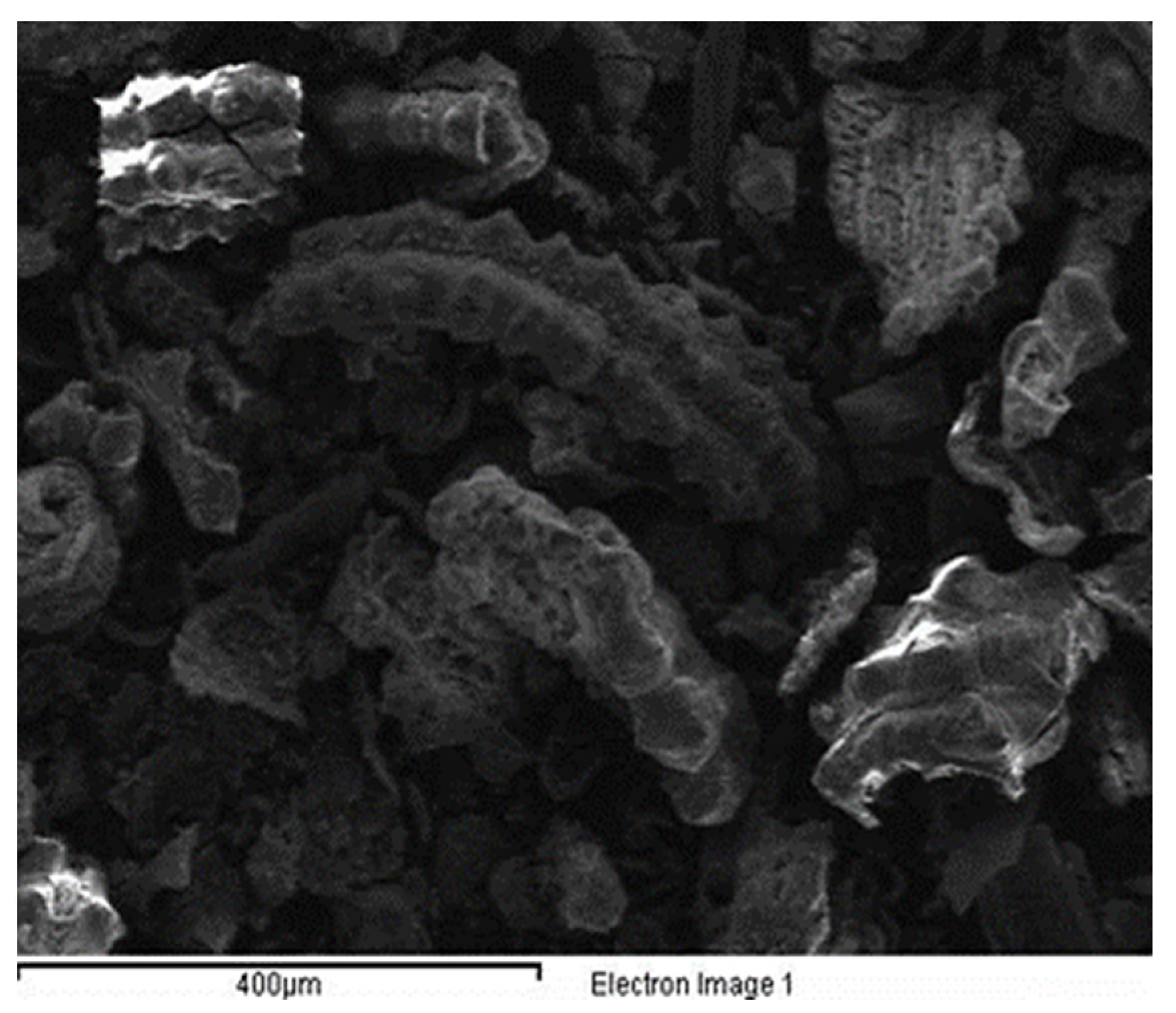

| PH | Moisture | Free Fatty Acid as Oleic Acid (C18H34O2) | Chlorides | Alcohol Insoluble | Total Alkalinity as NaOH |
|---|---|---|---|---|---|
| 11.5 | 9.3% | 37.2% | 0.3% | 37% | 7.2% |
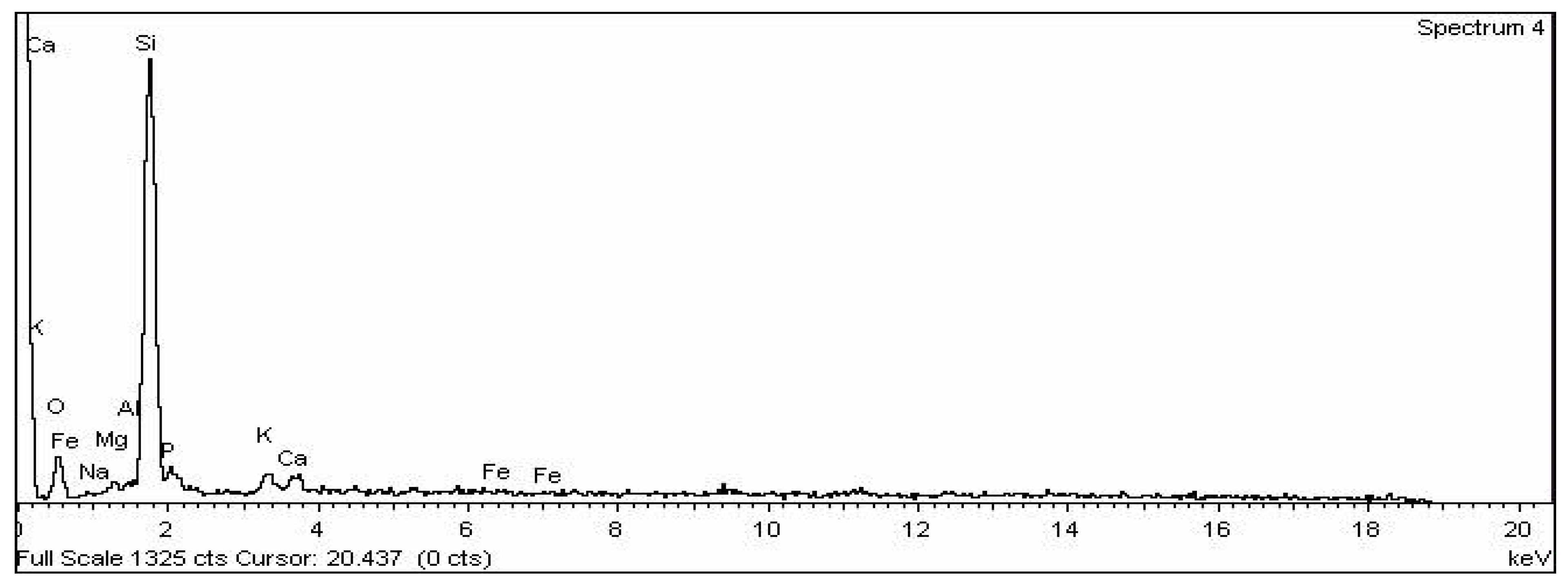
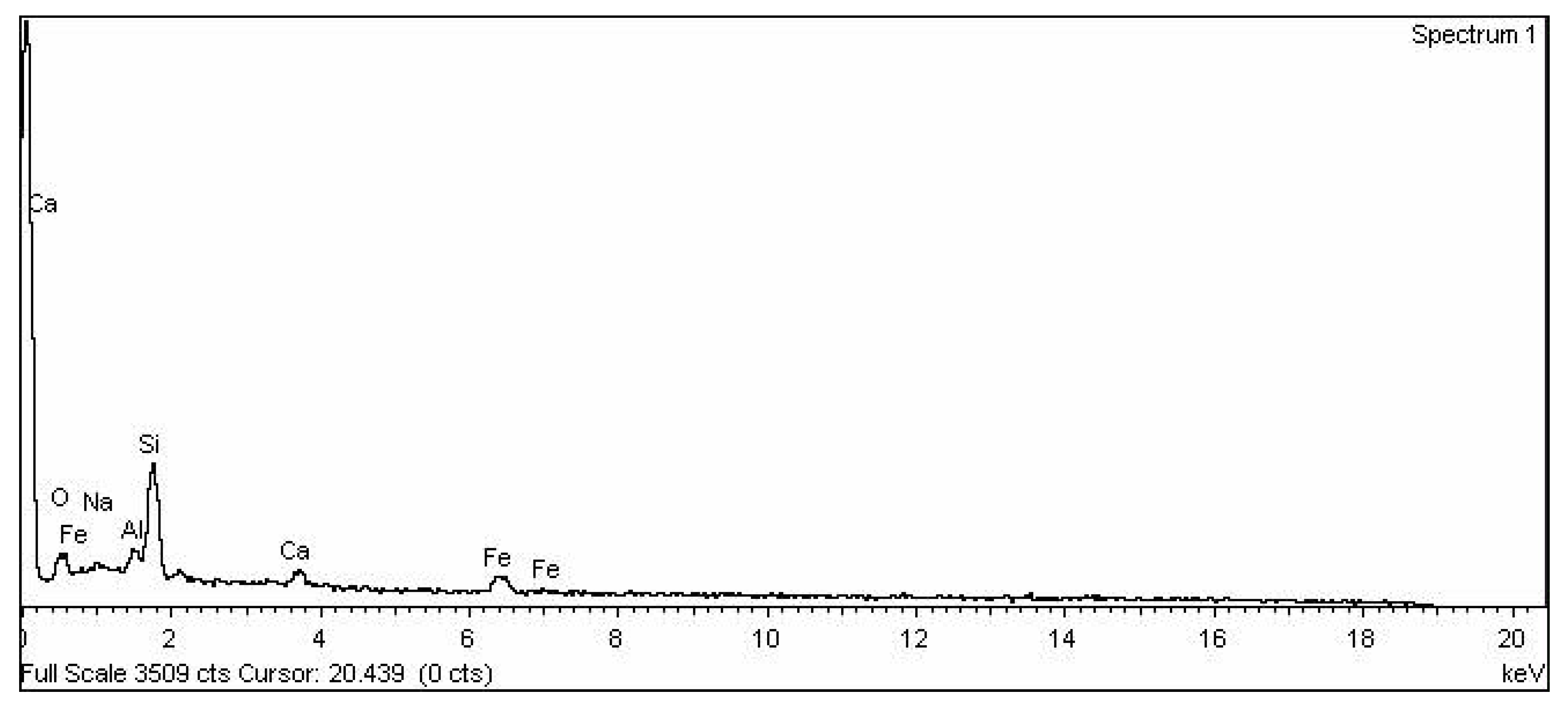
| Element | Weight% | Atomic% |
|---|---|---|
| O | 34.07 | 48.47 |
| Na | 0.13 | 0.13 |
| Mg | 1.39 | 1.30 |
| Al | 1.21 | 1.02 |
| Si | 51.61 | 41.82 |
| P | 4.27 | 3.14 |
| K | 3.70 | 2.15 |
| Ca | 3.09 | 1.76 |
| Fe | 0.53 | 0.22 |
| Concrete Mix | Soap Solution Concentration | RHA Percentage |
|---|---|---|
| M1 | 0 | 0 |
| M2 | 1 | 0 |
| M3 | 1 | 5 |
| M4 | 1 | 10 |
| M5 | 1 | 15 |
| M6 | 2 | 0 |
| M7 | 2 | 5 |
| M8 | 2 | 10 |
| M9 | 2 | 15 |
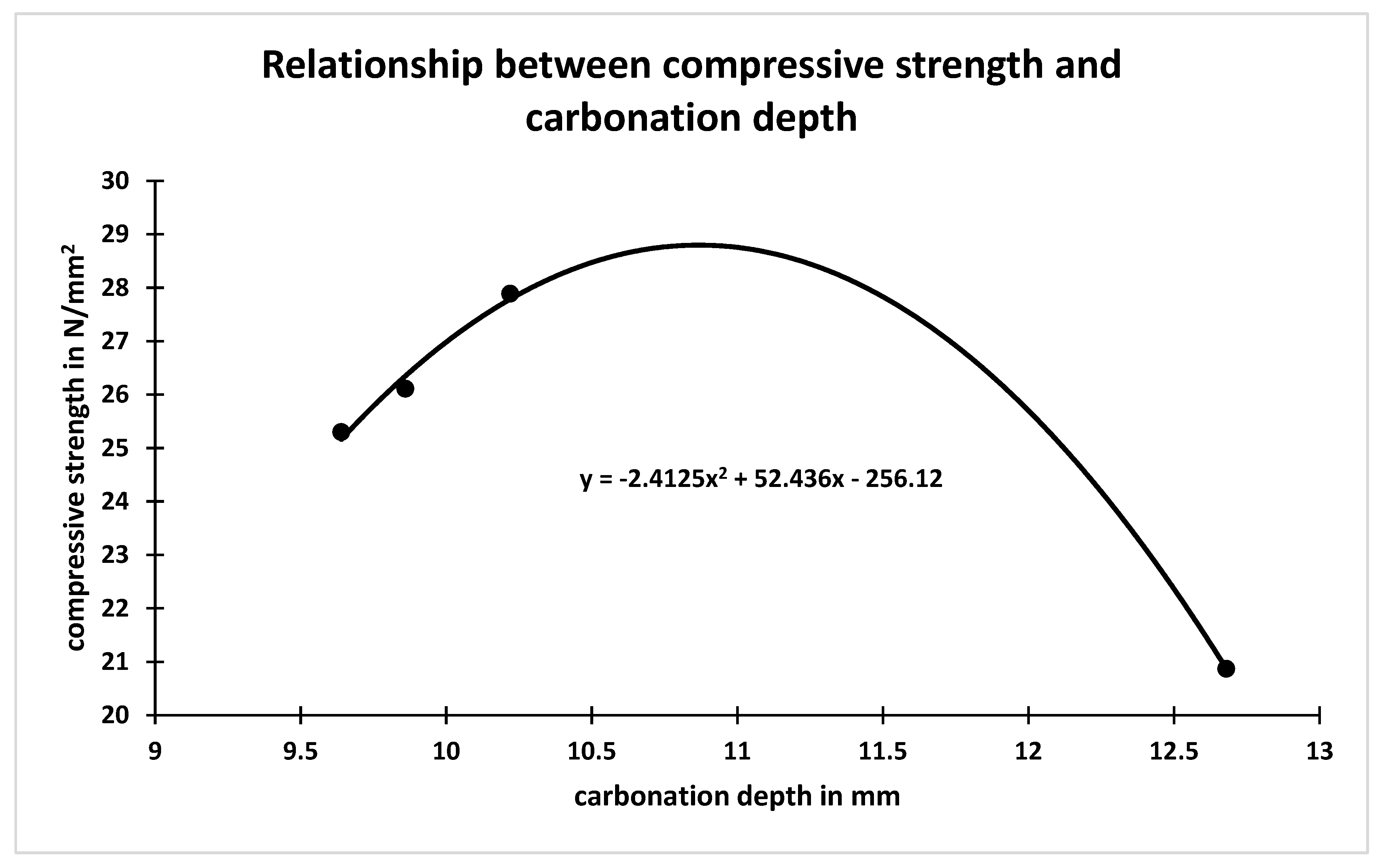
| Concrete Mix | Soap Solution Concentration | RHA Percentage | Carbonation Depth mm | Carbonation Rate Coefficient (K) mm/days0.5 | Coefficient of Permeability m/s |
|---|---|---|---|---|---|
| M1 | 0 | 0 | 11.6 | 1.38 | 2.9 × 10−11 |
| M2 | 1 | 0 | 10.22 | 1.22 | 2.8 × 10−11 |
| M3 | 1 | 5 | 09.86 | 1.17 | 3.9 × 10−11 |
| M4 | 1 | 10 | 09.64 | 1.15 | 3.7 × 10−11 |
| M5 | 1 | 15 | 12.68 | 1.52 | 3.8 × 10−11 |
| M6 | 2 | 0 | 6.2 | 0.74 | 4.1 × 10−12 |
| M7 | 2 | 5 | 5.18 | 0.62 | 4.3 × 10−12 |
| M8 | 2 | 10 | 4.78 | 0.57 | 4.1 × 10−12 |
| M9 | 2 | 15 | 13.25 | 1.58 | 4.2 × 10−12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, M.; Anand, A.; Chatterjee, R.; Sharma, S.; Maiti, T.K.; Dwivedi, S.P.; Saxena, A.; Li, C.; Eldin, E.M.T. Investigation on Carbonation and Permeability of Concrete with Rice Hush Ash and Shop Solution Addition. Materials 2022, 15, 6149. https://doi.org/10.3390/ma15176149
Kumar M, Anand A, Chatterjee R, Sharma S, Maiti TK, Dwivedi SP, Saxena A, Li C, Eldin EMT. Investigation on Carbonation and Permeability of Concrete with Rice Hush Ash and Shop Solution Addition. Materials. 2022; 15(17):6149. https://doi.org/10.3390/ma15176149
Chicago/Turabian StyleKumar, Manish, Ashutosh Anand, Rajeshwari Chatterjee, Shubham Sharma, Tushar Kanti Maiti, Shashi Prakash Dwivedi, Ambuj Saxena, Changhe Li, and Elsayed Mohamed Tag Eldin. 2022. "Investigation on Carbonation and Permeability of Concrete with Rice Hush Ash and Shop Solution Addition" Materials 15, no. 17: 6149. https://doi.org/10.3390/ma15176149
APA StyleKumar, M., Anand, A., Chatterjee, R., Sharma, S., Maiti, T. K., Dwivedi, S. P., Saxena, A., Li, C., & Eldin, E. M. T. (2022). Investigation on Carbonation and Permeability of Concrete with Rice Hush Ash and Shop Solution Addition. Materials, 15(17), 6149. https://doi.org/10.3390/ma15176149







