New Organic-Inorganic Hybrid Compounds Based on Sodium Peroxidomolybdates (VI) and Derivatives of Pyridine Acids: Structure Determination and Catalytic Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Syntheses
2.3. Methods
2.3.1. X-ray Powder/Single Crystal Diffraction Data Analysis
2.3.2. IR Measurements
2.3.3. X-ray Thermal Decomposition
2.3.4. Thermogravimetry-Differential Scanning Calorimetry (TG/DSC)
2.3.5. Brunauer, Emmett, Teller Methods (BET)
2.3.6. Biological Studies
Cell Cultures
Cytotoxicity
2.3.7. Catalytic Activity in the Baeyer–Villiger Oxidation
3. Results
3.1. Crystal Structure Data
3.2. IR Spectra
3.3. Thermal Decomposition—XRPD vs. Temperature
3.4. Thermal Decomposition-TG/DSC
- -
- In the range 75–150 °C, dehydration is observed (calc. weight loss 12%, obs. ~9%);
- -
- At 185 °C, a sharp exothermic maximum due to the peroxo groups decomposition is visible (O2 emission, calc. 8%, obs. 7%);
- -
- In the range 200–335 °C, decomposition of N-oxide and carboxylic groups occurs (2CO2 and ½O2 emission, calc. 24%);
- -
- Above 325 °C, fast exothermic loss of the remaining organic fragments is observed;
- -
- In the range of 75–175 °C, dehydration occurs (calc. 10%, obs. ~11%);
- -
- At 265 °C, a sharp exothermic maximum related to the decomposition of peroxo groups is observed (O2 emission, calc. 10%, obs. ~9%);
- -
- In the range 275–365 °C, decomposition of N-oxide and carboxylic groups takes place (CO2 and 1/2O2 emission, calc. 16%);
- -
- Above 365 °C, fast exothermic loss of the remaining organic fragments is visible.
3.5. BET Analysis: Specific Area and Porosity Determination
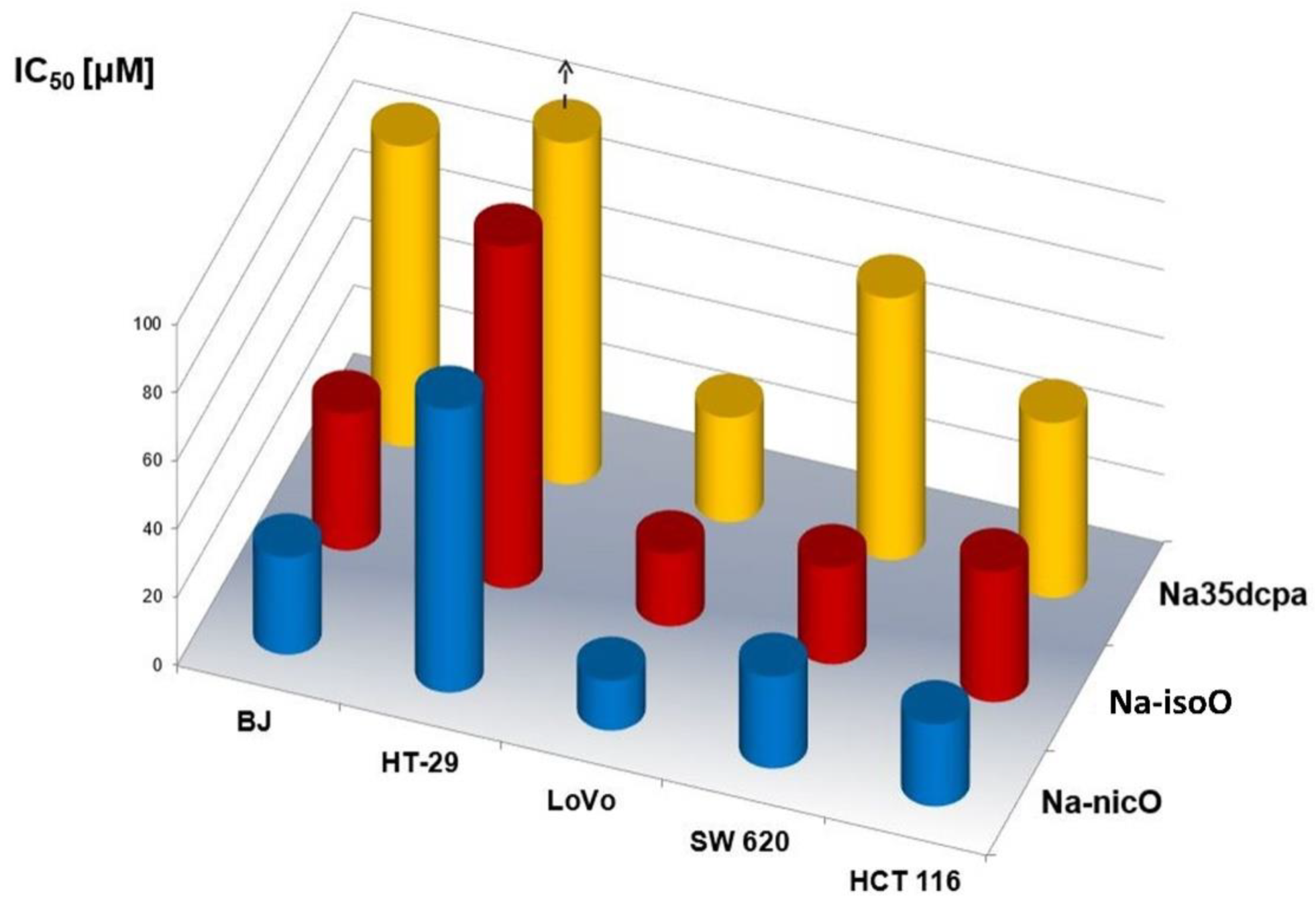
3.6. Biological Activity
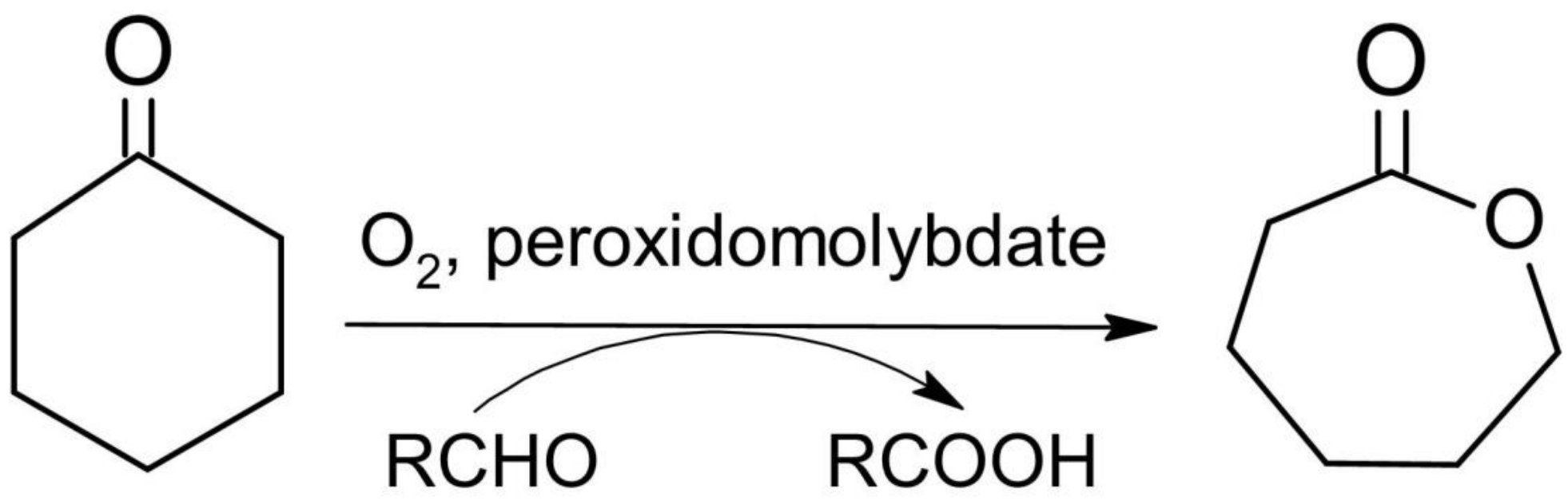
3.7. Catalytic Activity in the Baeyer–Villiger Oxidation
- -
- Na-35dcpa is weaker as a catalyst than K-35dcpa;
- -
- Na-isoO is definitely a much better catalyst than K-isoO;
- -
- Na-nicO and K-nicO have similar catalytic activity.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pope, M.T. Structural chemistry of actinide polyoxometalates. In Structural Chemistry of Inorganic Actinide Compounds, 1st ed.; Krivovichev, S., Burns, P., Tananaev, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 341–361. [Google Scholar]
- Dickman, M.H.; Pope, M.T. Peroxo and Superoxo Complexes of Chromium, Molybdenum, and Tungsten. Chem. Rev. 1994, 94, 569–584. [Google Scholar] [CrossRef]
- Grzywa, M.; Łasocha, W.; Rutkowska-Żbik, D. Structural investigation of tetraperoxo complexes of Mo(VI) and W(VI): X-ray and theoretical studies. J. Solid State Chem. 2009, 182, 973–982. [Google Scholar] [CrossRef]
- Conte, V.; Floris, B. Vanadium and molybdenum peroxides: Synthesis and catalytic activity in oxidation reactions. Dalton Trans. 2010, 40, 1419–1436. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, Q.; Shen, C.; He, L. Polyoxometalate-Based Catalysts for CO2 Conversion. Molecules 2019, 24, 2069. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Zhang, L.; Liu, C.; Pang, H. PBA @ POM Hybrids as Efficient Electrocatalysts for the Oxygen Evolution Reaction. Chem. Asian J. 2019, 14, 2790–2795. [Google Scholar]
- Bożek, B.; Neves, P.; Łasocha, W.; Valente, A.A. Ionic ammonium and anilinium based polymolybdate hybrid catalysts for olefin epoxidation. Appl. Catal. A Gen. 2018, 564, 13–25. [Google Scholar]
- Karcz, R.; Niemiec, P.; Pamin, K.; Połtowicz, J.; Kryściak-Czerwenka, J.; Napruszewska, B.D.; Michalik-Zym, A.; Witko, M.; Tokarz-Sobieraj, R.; Serwicka, E.M. Effect of cobalt location in Keggin-type heteropoly catalysts on aerobic oxidation of cyclooctane: Experimental and theoretical study. Appl. Catal. A Gen. 2017, 542, 317–326. [Google Scholar] [CrossRef]
- Szymańska, A.; Nitek, W.; Oszajca, M.; Łasocha, W.; Pamin, K.; Połtowicz, J. Molybdenum Complexes as Catalysts for the Oxidation of Cycloalkanes with Molecular Oxygen. Catal. Lett. 2016, 146, 998–1010. [Google Scholar] [CrossRef]
- Szymańska, A.; Nitek, W.; Mucha, D.; Karcz, R.; Pamin, K.; Połtowicz, J.; Łasocha, W. Structural studies and physico-chemical properties of new oxodiperoxomolybdenum complexes with nicotinic acid. Polyhedron 2013, 60, 39–46. [Google Scholar] [CrossRef]
- Sławińska, A.; Serda, P.; Pamin, K.; Połtowicz, J.; Łasocha, W. Synthesis, crystal structure and selected properties of a group of new peroxomolybdates. Polyhedron 2017, 121, 191–198. [Google Scholar] [CrossRef]
- Sławińska, A.; Serda, P.; Oszajca, M.; Pamin, K.; Połtowicz, J.; Łasocha, W. Synthesis, crystal structure and selected properties of two new peroxidomolybdates. Polyhedron 2020, 183, 114530. [Google Scholar] [CrossRef]
- Shen, Y.; Jiang, P.; Wai, P.; Gu, Q.; Zhang, W. Recent Progress in Application of Molybdenum-Based Catalysts for Epoxidation of Alkenes. Catalysts 2019, 9, 31. [Google Scholar] [CrossRef] [Green Version]
- Samaniyan, M.; Mirzaei, M.; Khajavian, R.; Eshtiagh-Hosseini, H.; Streb, C. Heterogeneous Catalysis by Polyoxometalates in Metal–Organic Frameworks. ACS Catal. 2019, 9, 10174–10191. [Google Scholar] [CrossRef]
- Pamin, K.; Połtowicz, J.; Prończuk, M.; Kryściak-Czerwenka, J.; Karcz, R.; Serwicka, E.M. Keggin-Type Heteropoly Salts as Bifunctional Catalysts in Aerobic Baeyer-Villiger Oxidation. Materials 2018, 11, 1208. [Google Scholar] [CrossRef]
- Gao, D.; Trentin, I.; Schwiedrzik, L.; González, L.; Streb, C. The Reactivity and Stability of Polyoxometalate Water Oxidation Electrocatalysts. Molecules 2020, 25, 157. [Google Scholar] [CrossRef]
- Ma, Q.G.; Zhao, J.R.; Xing, W.Z.; Peng, X.H. Baeyer-Villiger Oxidation of Cyclic Ketones Using Aqueous Hydrogen Peroxide Catalyzed by Heteropolyacids in Solvent-free System. J. Adv. Oxid. Technol. 2014, 17, 212–216. [Google Scholar]
- Balbinot, L.; Schuchardt, U.; Vera, C.; Sepúlveda, J. Oxidation of cyclohexanol to epsilon-caprolactone with aqueous hydrogen peroxide on H3PW12O40 and Cs2.5H0.5PW12O40. Catal. Commun. 2008, 9, 1878–1881. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, X.; Li, T.; Zhang, N.; Zhao, X.; Chen, W.; Liang, X.; He, X.; Ma, H. Preparation and Catalytic Property of Multi-walled Carbon Nanotubes Supported Keggin-Typed Tungstosilicic Acid for the Baeyer–Villiger Oxidation of Ketones. Catal. Lett. 2015, 145, 1955–1960. [Google Scholar] [CrossRef]
- Pamin, K.; Prończuk, M.; Basąg, S.; Kubiak, W.; Sojka, Z.; Połtowicz, J. A new hybrid porphyrin-heteropolyacid material: Synthesis, characterization and investigation as catalyst in Baeyer-Villiger oxidation. Synergistic effect. Inorg. Chem. Commun. 2015, 59, 13–16. [Google Scholar]
- Authier, T. Applications of Polyoxometalates in Medicine and their Putative Mechanisms of Action. Bachelor Thesis, Universidade do Algavre, Faro, Portugal, 2015. [Google Scholar]
- Ma, T.; Yang, P.; Dammann, I.; Lin, Z.; Mougharbel, A.S.; Li, M.-X.; Adǎscǎliţei, F.; Mitea, R.; Silvestru, C.; Thorstenson, C.; et al. Tetra-(p-tolyl)antimony(III)-Containing Heteropolytungstates, [{(p-tolyl)SbIII}4(A-α-XW9O34)2]n− (X = P, As, or Ge): Synthesis, Structure, and Study of Antibacterial and Antitumor Activity. Inorg. Chem. 2020, 59, 2978–2987. [Google Scholar] [CrossRef]
- Ong, B.C.; Lim, H.K.; Tay, C.Y.; Lim, T.-T.; Dong, Z. Polyoxometalates for bifunctional applications: Catalytic dye degradation and anticancer activity. Chemosphere 2021, 286, 131869. [Google Scholar] [CrossRef] [PubMed]
- Boulmier, A.; Feng, X.; Oms, O.; Mialane, P.; Rivière, E.; Shin, C.J.; Yao, J.; Kubo, T.; Furuta, T.; Oldfield, E.; et al. Anticancer Activity of Polyoxometalate-Bisphosphonate Complexes: Synthesis, Characterization, In Vitro and In Vivo Results. Inorg. Chem. 2017, 56, 7558–7565. [Google Scholar] [CrossRef] [PubMed]
- Bijelic, A.; Aureliano, M.; Rompel, A. Polyoxometalates as Potential Next-Generation Metallodrugs in the Combat Against Cancer. Angew. Chem. Int. Ed. 2019, 58, 2980–2999. [Google Scholar] [CrossRef]
- Li, X.-H.; Chen, W.-L.; Wei, M.; Liu, J.; Di, Y.; Liu, L.; Li, Y.-G.; Wang, E.-B. Polyoxometalates nanoparticles improve anti-tumor activity by maximal cellular uptake. Inorg. Chim. Acta 2019, 486, 104–112. [Google Scholar] [CrossRef]
- Hu, X.; Wang, H.; Huang, B.; Li, N.; Hu, K.; Wu, B.; Xiao, Z.; Wei, Y.; Wu, P. A new scheme for rational design and synthesis of polyoxovanadate hybrids with high antitumor activities. J. Inorg. Biochem. 2019, 193, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Wang, M.; Li, N.; Tang, B. Polyoxometalate-Based Nanomaterials Toward Efficient Cancer Diagnosis and Therapy. Chem. Eur. J. 2020, 27, 6422–6434. [Google Scholar] [CrossRef]
- Chaudhary, H.; Iashchishyn, I.A.; Romanova, N.V.; Rambaran, M.A.; Musteikyte, G.; Smirnovas, V.; Holmboe, M.; Ohlin, C.A.; Svedružić, M.; Morozova-Roche, L.A. Polyoxometalates as Effective Nano-inhibitors of Amyloid Aggregation of Pro-inflammatory S100A9 Protein Involved in Neurodegenerative Diseases. ACS Appl. Mater. Interfaces 2021, 13, 26721–26734. [Google Scholar] [CrossRef]
- Van Rompuy, L.S.; Parac-Vogt, T.N. Interactions between polyoxometalates and biological systems: From drug design to artificial enzymes. Curr. Opin. Biotechnol. 2019, 58, 92–99. [Google Scholar] [CrossRef]
- Sławińska, A.; Tyszka-Czochara, M.; Serda, P.; Oszajca, M.; Ruggiero-Mikołajczyk, M.; Pamin, K.; Karcz, R.; Łasocha, W. Newly-Obtained Two Organic-Inorganic Hybrid Compounds Based on Potassium Peroxidomolybdate and Dicarboxypyridinic Acid: Structure Determination, Catalytic Properties, and Cytotoxic Effects of Eight Peroxidomolybdates in Colon and Hepatic Cancer Cells. Materials 2021, 15, 241. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Cryst. 2008, 64, 112–122. [Google Scholar]
- Altomare, A.; Cuocci, A.; Giacovazzo, C.; Moliterni, C.; Rizzi, A.; Corriero, R.; Falcicchio, N.A. EXPO2013: A kit of tools for phasing crystal structures from powder data. J. Appl. Cryst. 2013, 46, 1231–1235. [Google Scholar]
- Petricek, V.; Dusek, M.; Palatinus, L.Z. Crystallographic Computing System JANA2006: General features. Z. Für Krist. Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Favre-Nicolin, V.; Černý, R. FOX, free objects for crystallography’: A modular approach to ab initio structure determination from powder diffraction. Appl. Cryst. 2002, 35, 734–743. [Google Scholar]
- Brandenburg, K. Diamond, Version 3.2g; Crystal Impact GbR: Bonn, Germany, 1997–2001; Available online: https://www.crystalimpact.com/diamond (accessed on 22 December 2020).
- Origin(Pro), Version 2019b; OriginLab Corporation: Northampton, MA, USA, 2019.
- Adach, A.; Daszkiewicz, M.; Tyszka-Czochara, M. A family of complexes with: N -scorpionate-type and other N -donor ligands obtained in situ from pyrazole derivative and zerovalent cobalt. Physicochemical and cytotoxicity studies. RSC Adv. 2016, 6, 44070–44079. [Google Scholar]
- Makowska-Wąs, J.; Galanty, A.; Gdula-Argasińska, J.; Tyszka-Czochara, M.; Szewczyk, A.; Nunes, R.; Carvalho, I.S.; Michalik, M.; Paśko, P. Identification of Predominant Phytochemical Compounds and Cytotoxic Activity of Wild Olive Leaves (Olea europaea L. ssp. sylvestris) Harvested in South Portugal. Chem. Biodiversity 2017, 14, e160033110. [Google Scholar] [CrossRef] [PubMed]
- Adach, A.; Daszkiewicz, M.; Tyszka-Czochara, M.; Barszcz, B. New oxovanadium(iv) complexes with pincer ligand obtained in situ: Experimental and theoretical studies on the structure, spectroscopic properties and antitumour activity. RSC Adv. 2015, 5, 85470–85479. [Google Scholar] [CrossRef]
- Adach, A.; Daszkiewicz, M.; Tyszka-Czochara, M. Comparative X-ray, vibrational, theoretical and biological studies of new in situ formed [CoLSX]2[CdX4] halogeno-cadmate(II) complexes containing N-scorpionate ligand. Polyhedron 2020, 175, 114229. [Google Scholar] [CrossRef]
- Tyszka-Czochara, M.; Adach, A.; Grabowski, T.; Konieczny, P.; Pasko, P.; Ortyl, J.; Świergosz, T.; Majka, M. Selective Cytotoxicity of Complexes with N,N,N-Donor Dipodal Ligand in Tumor Cells. Int. J. Mol. Sci. 2021, 22, 1802. [Google Scholar] [CrossRef]
- Kee, C.W. Assignment of O–O and Mo = O Stretching Frequencies of Molybdenum /Tungsten Complexes Revisited. J. Chem. 2015, 2015, 439270. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies:Tables and Charts, 3rd ed.; John and Wiley and Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Bart, J.C.J.; Ragaini, V. Molybdenum–nitrogen bond-strength bond-length relationships. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1980, 36, 1351–1354. [Google Scholar] [CrossRef]
- IR Spectrum Table & Chart. Available online: https://www.sigmaaldrich.com/PL/pl/technical-documents/technical-article/analytical-chemistry/photometry-and-reflectometry/ir-spectrum-table (accessed on 28 August 2022).
- Gumerova, N.I.; Al-Sayed, E.; Krivosudský, L.; Čipčić-Paljetak, H.; Verbanac, D.; Rompel, A. Antibacterial Activity of Polyoxometalates Against Moraxella catarrhalis. Front. Chem. 2018, 6, 336. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Li, N.; Wang, N.; Hu, K.; Xiao, Z.; Wu, P.; Wei, Y. Synthesis, structure and antitumor studies of a novel decavanadate complex with a wavelike two-dimensional network. Polyhedron 2018, 155, 313–319. [Google Scholar] [CrossRef]
- Aureliano, M. The Future Is Bright for Polyoxometalates. BioChem 2022, 2, 8–26. [Google Scholar] [CrossRef]
- ATCC human cells are invaluable for your public health research. Available online: https://www.atcc.org/cell-products/human-cells#t=productTab&numberOfResults=24 (accessed on 22 August 2022).
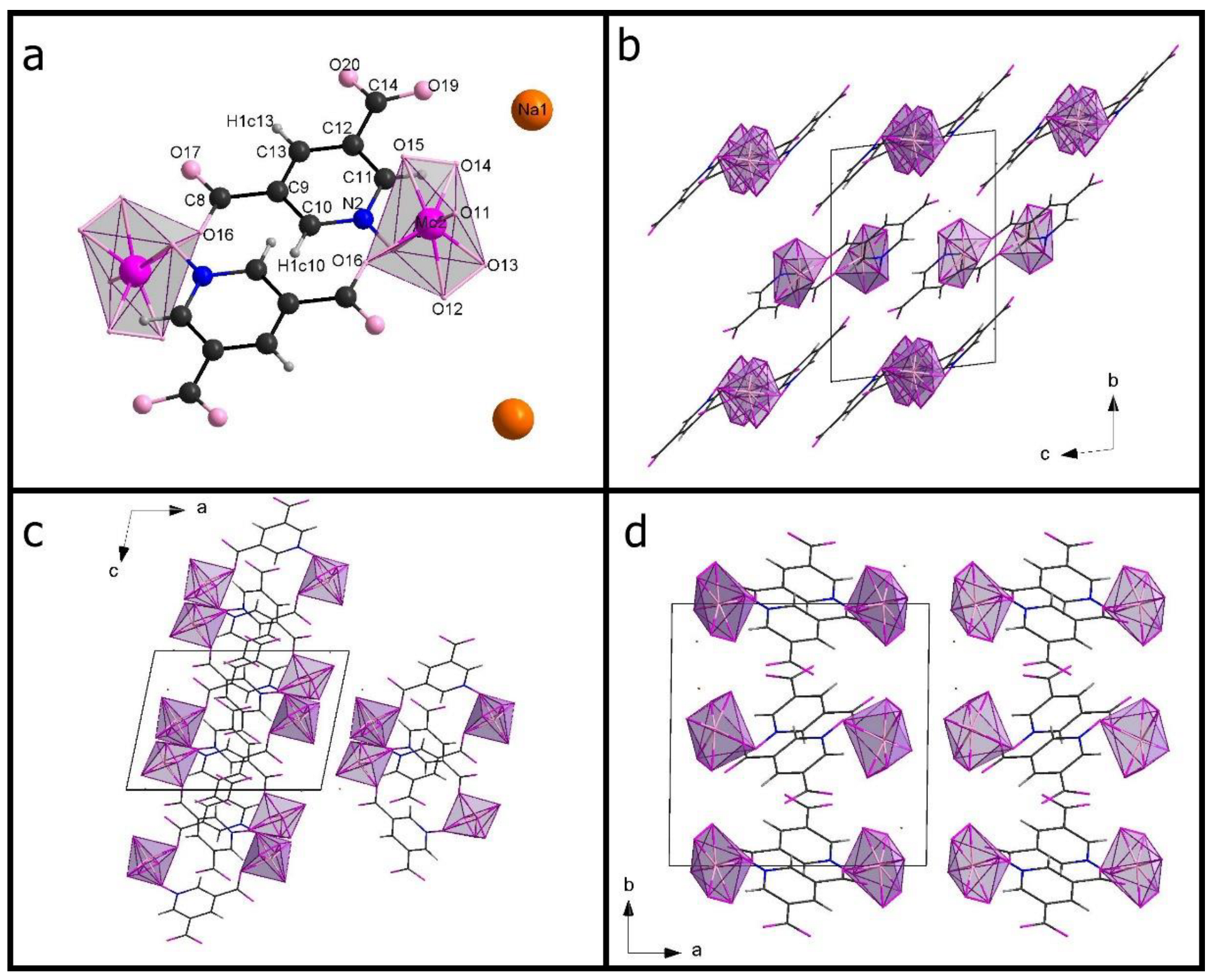
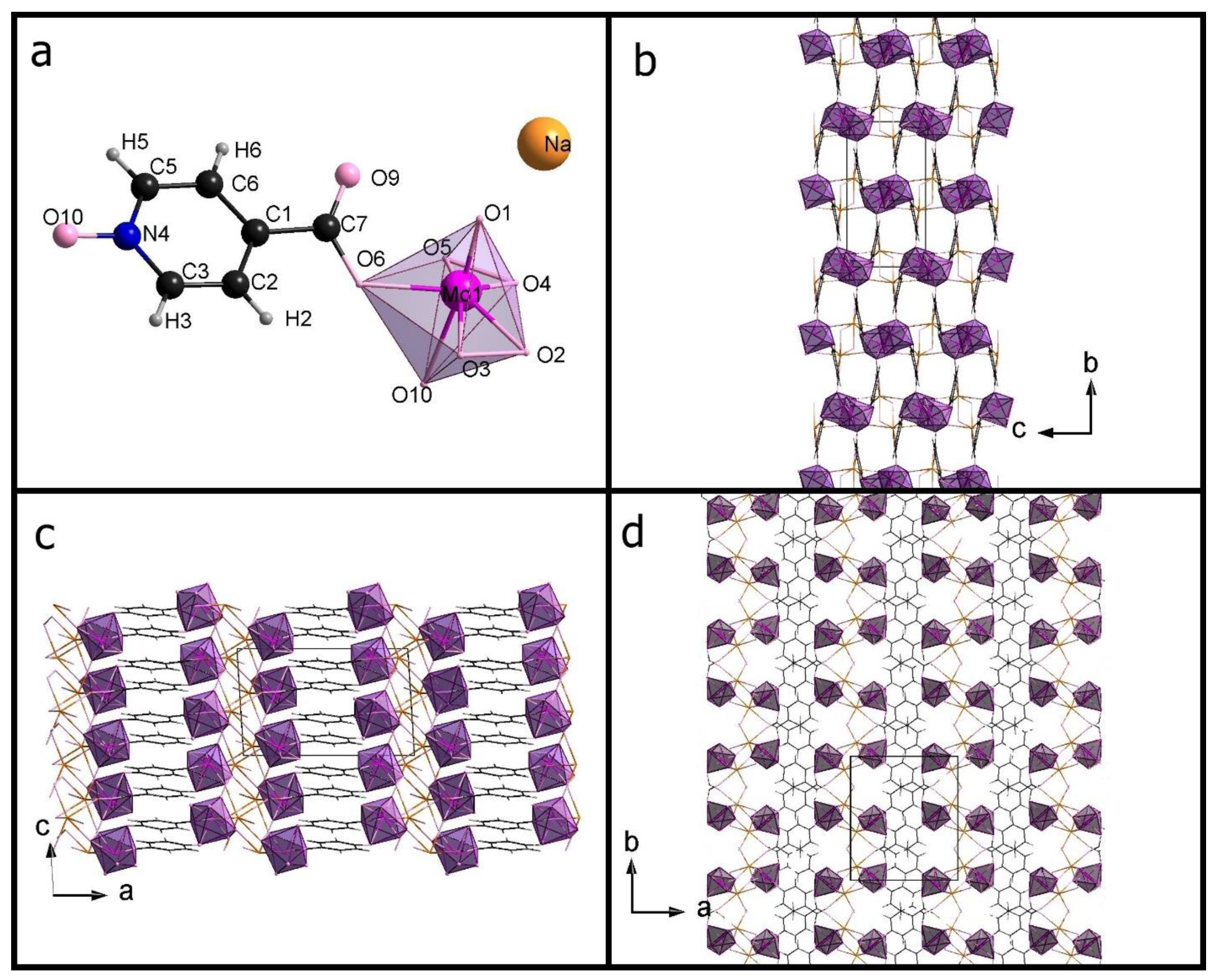



| Compound Code, (XRD Technique) | Na-35dcpa, Powder | Na-isoO, Single Crystal (s.c.) |
|---|---|---|
| Chemical formula | C7 H10 Mo N Na O13 | C6 H8 Mo N Na O10 |
| Chemical formula, structural | Na[MoO(O2)2C5H3NO(COO) (COOH)]·3H2O | Na[MoO(O2)2C6H4NO (COO)]·2H2O |
| MW (g/mol) | 435.09 | 373.06 |
| T(K) | 293 | −173 |
| Wavelength λ [Å] | CuKα: 1.54187 | CuKα: 1.54184 |
| Crystal system, SG | triclinic, P-1 | monoclinic, P2(1)/c |
| Cell parameters: | ||
| a [Å] | 12.2307 (7) | 11.6753 (2) |
| b [Å] | 12.2653 (7) | 13.4514 (3) |
| c [Å] | 8.9485 (6) | 7.2919 (2) |
| α [°] | 97.167 (4) | 90.0 |
| β [°] | 101.840 (3) | 93.602 (2) |
| γ [°] | 87.893 (5) | 90.0 |
| V(Å3) | 1303.48 (14) | 1142.92 (4) |
| Z, calculated density (g/cm3) | 4, 2.21 | 4, 2.156 |
| Absorption coefficient (mm−1) | 9.079 | 10.323 |
| F(000) | 740 | 728 |
| Theta range [°] | 5.007–79.992 | 3.793–80.579 |
| Limiting indices | −10≤h≤9; −10≤k≤10; 0≤l≤7 | −14≤h≤14; −17≤k≤16; −8≤l≤9 |
| completeness to theta | 100% (powder sample) | 80.579, 98.2% |
| Absorption correction | Capillary, calc. for cylindrical sample | Multi-scan |
| refinement method | Rietveld | F2 (Fsqd) |
| Data/restraints/parame-ters | 5712/83/86 | 2462/0/176 |
| goodness of fit (on F2 in s.c.) | 4.45 | 1.113 |
| Final R indices (I > 2σ(I) in s.c.) | Rp = 0.0434, Rwp = 0.0632 | R1 = 0.0575, wR2 = 0.1306 |
| R indices (all data) | RF = 0.0644 | R1 = 0.0526, wR2 = 0.1281 |
| Largest difference peak and hole (eA−3) | 0.57, −0.47 | 0.775, −1.479 |
| CCDC | 2170626 | 2171243 |
| Compound | ν (Mo=O) | νsym (O-O) | νasym (Mo-(O)2) | νsym (Mo-(O)2) | (N-Oxide) Vibrations |
|---|---|---|---|---|---|
| Na-35dcpa | 956 vs, 969 s | 861 vs | 580 s | 523 m | 493 w, 797 w |
| Na-isoO | 953 s | 867 vs, 586 vs | 582 s | 544 m | 814 w |
| Compound | SSA (m2/g) | Pore Size BJHdes (Å) | Pore Volume BJHdes (cm3/g) |
|---|---|---|---|
| Na-35dcpa | ≤1 | 33, 62, 260 | 0.026 |
| Na-isoO | ≤1 | 53, 157 | 0.068 |
| Na-nicO | 2.48 | 33, 62, 115 | 0.028 |
| Compound | Normal Cells | Human Colon Tumor Cells | |||
|---|---|---|---|---|---|
| Primary Tumor Cells | Tumor Cells Derived from Metastatic Sites | ||||
| Fibroblasts | HT-29 | LoVo | SW 620 | HCT 116 | |
| Na-isoO | 40 ± 4.9 | 105 ± 9.1 | 21.1 ± 4.3 | 28.2 ± 1.0 | 38.1 ± 9.6 |
| Na-35dcpa | 87.9 ± 0 | 131.2 ± 6.9 | 30.6 ± 0.8 | 76.7 ± 1.7 | 51.3 ± 8.7 |
| Na-nicO | 28.5 ± 0.4 | 82.9 ± 11.8 | 14.6 ± 2.0 | 26.9 ± 3.7 | 24.1 ± 2.7 |
| Catalyst Number and Code | Conversion [%] | Yield [%] | Selectivity [%] | TON |
|---|---|---|---|---|
| Na-35dcpa | 45.4 | 41.5 | 91.4 | 190.9 |
| Na-isoO | 50.0 | 48.8 | 97.6 | 224.5 |
| Na-nicO | 49.1 | 32.3 | 65.8 | 148.58 |
| Selected examples from previous studies [31] | ||||
| K-35dcpa | 90.9 | 89.7 | 98.7 | 412.62 |
| K-isoO | 37.6 | 6.8 | 18.1 | 31.28 |
| K-nicO | 44.7 | 35.6 | 79.6 | 163.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sławińska, A.; Tyszka-Czochara, M.; Serda, P.; Oszajca, M.; Ruggiero-Mikołajczyk, M.; Pamin, K.; Napruszewska, B.D.; Prochownik, E.; Łasocha, W. New Organic-Inorganic Hybrid Compounds Based on Sodium Peroxidomolybdates (VI) and Derivatives of Pyridine Acids: Structure Determination and Catalytic Properties. Materials 2022, 15, 5976. https://doi.org/10.3390/ma15175976
Sławińska A, Tyszka-Czochara M, Serda P, Oszajca M, Ruggiero-Mikołajczyk M, Pamin K, Napruszewska BD, Prochownik E, Łasocha W. New Organic-Inorganic Hybrid Compounds Based on Sodium Peroxidomolybdates (VI) and Derivatives of Pyridine Acids: Structure Determination and Catalytic Properties. Materials. 2022; 15(17):5976. https://doi.org/10.3390/ma15175976
Chicago/Turabian StyleSławińska, Adrianna, Malgorzata Tyszka-Czochara, Paweł Serda, Marcin Oszajca, Małgorzata Ruggiero-Mikołajczyk, Katarzyna Pamin, Bogna D. Napruszewska, Ewelina Prochownik, and Wiesław Łasocha. 2022. "New Organic-Inorganic Hybrid Compounds Based on Sodium Peroxidomolybdates (VI) and Derivatives of Pyridine Acids: Structure Determination and Catalytic Properties" Materials 15, no. 17: 5976. https://doi.org/10.3390/ma15175976
APA StyleSławińska, A., Tyszka-Czochara, M., Serda, P., Oszajca, M., Ruggiero-Mikołajczyk, M., Pamin, K., Napruszewska, B. D., Prochownik, E., & Łasocha, W. (2022). New Organic-Inorganic Hybrid Compounds Based on Sodium Peroxidomolybdates (VI) and Derivatives of Pyridine Acids: Structure Determination and Catalytic Properties. Materials, 15(17), 5976. https://doi.org/10.3390/ma15175976








