Laboratory Experiments on the In Situ Upgrading of Heavy Crude Oil Using Catalytic Aquathermolysis by Acidic Ionic Liquid
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Characterization
2.2. Experimental Work on Catalytic Aquathermolysis of Heavy Crude Oil
3. Results and Discussion
3.1. Catalytic Aquathermolysis of Heavy Crude Oil
3.2. SARA Analysis of Heavy Oil
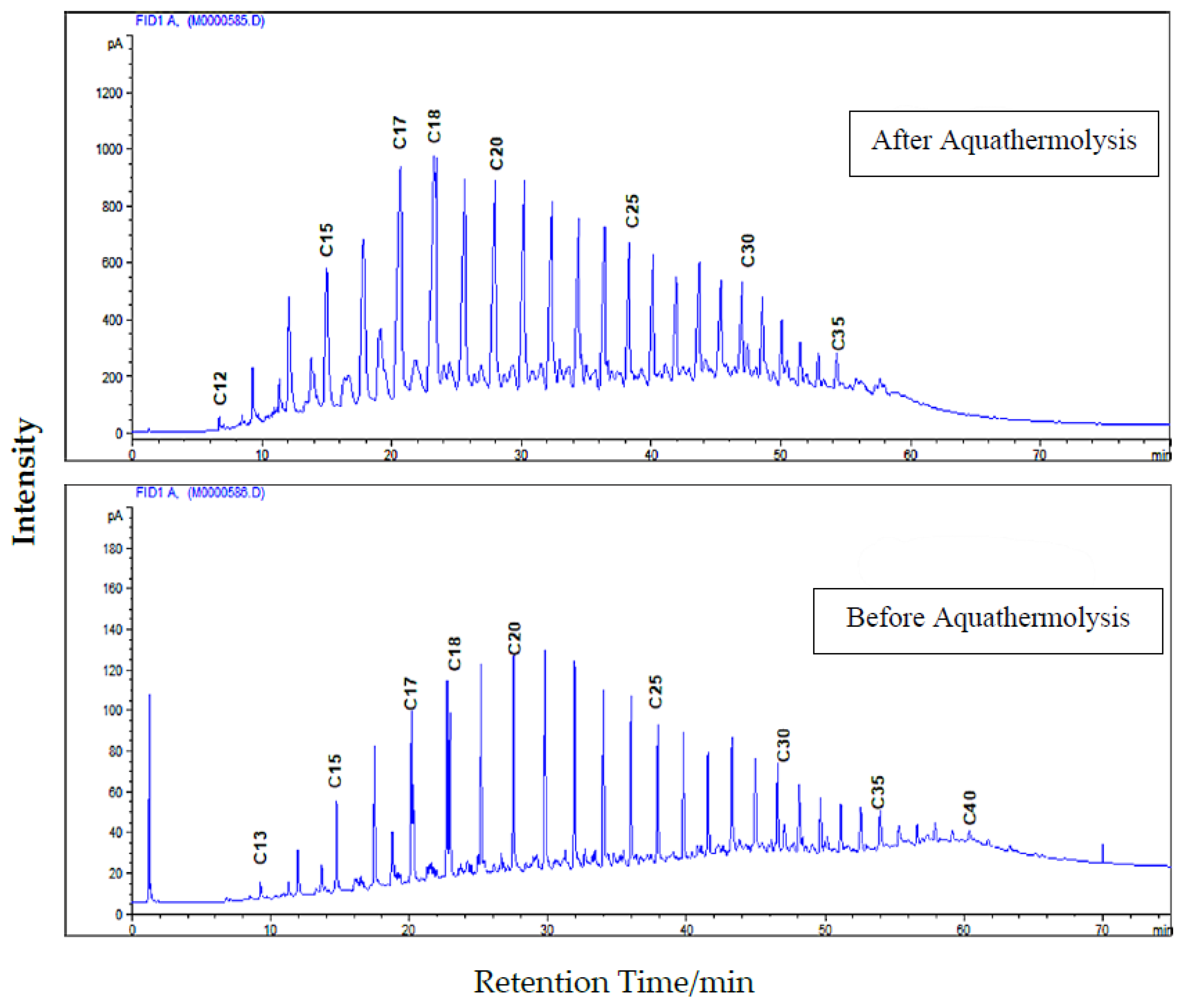
3.3. Effect of Catalytic Aquathermolysis on Compositional Analysis
3.4. Elemental Analysis of Asphaltenes and Resins
3.5. FT-IR of Asphaltene
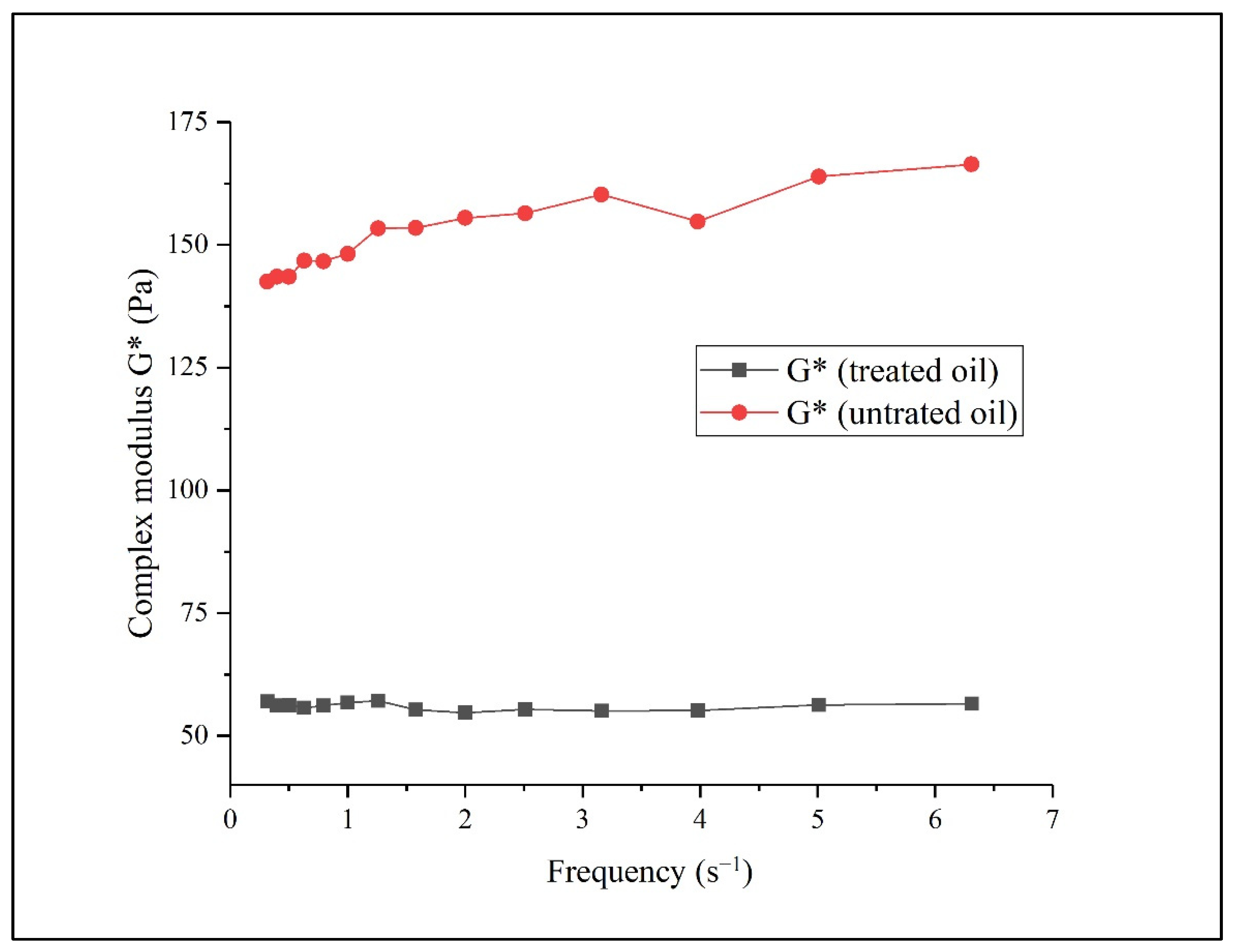
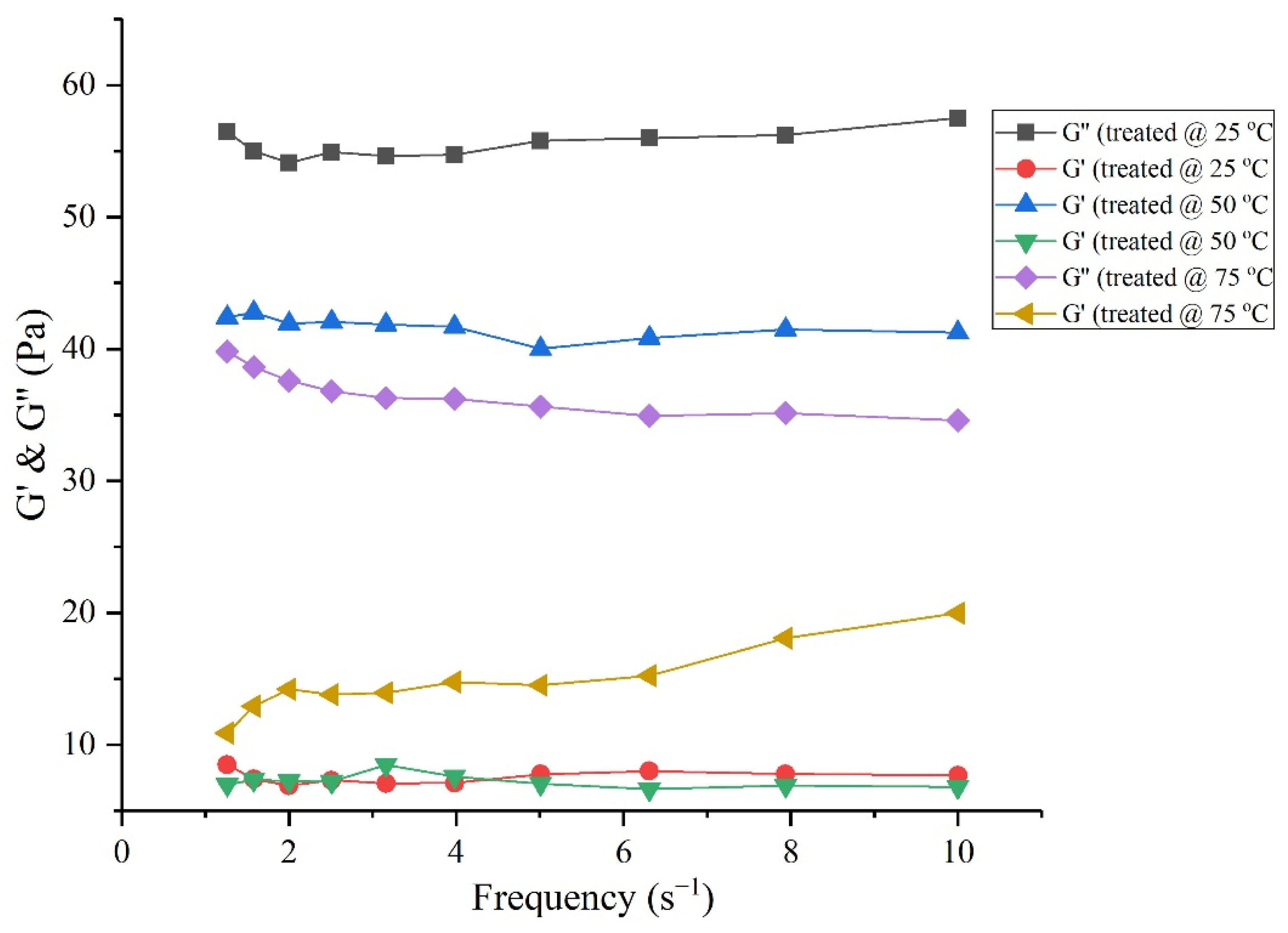
3.6. Rheological Behavior of Crude Oil
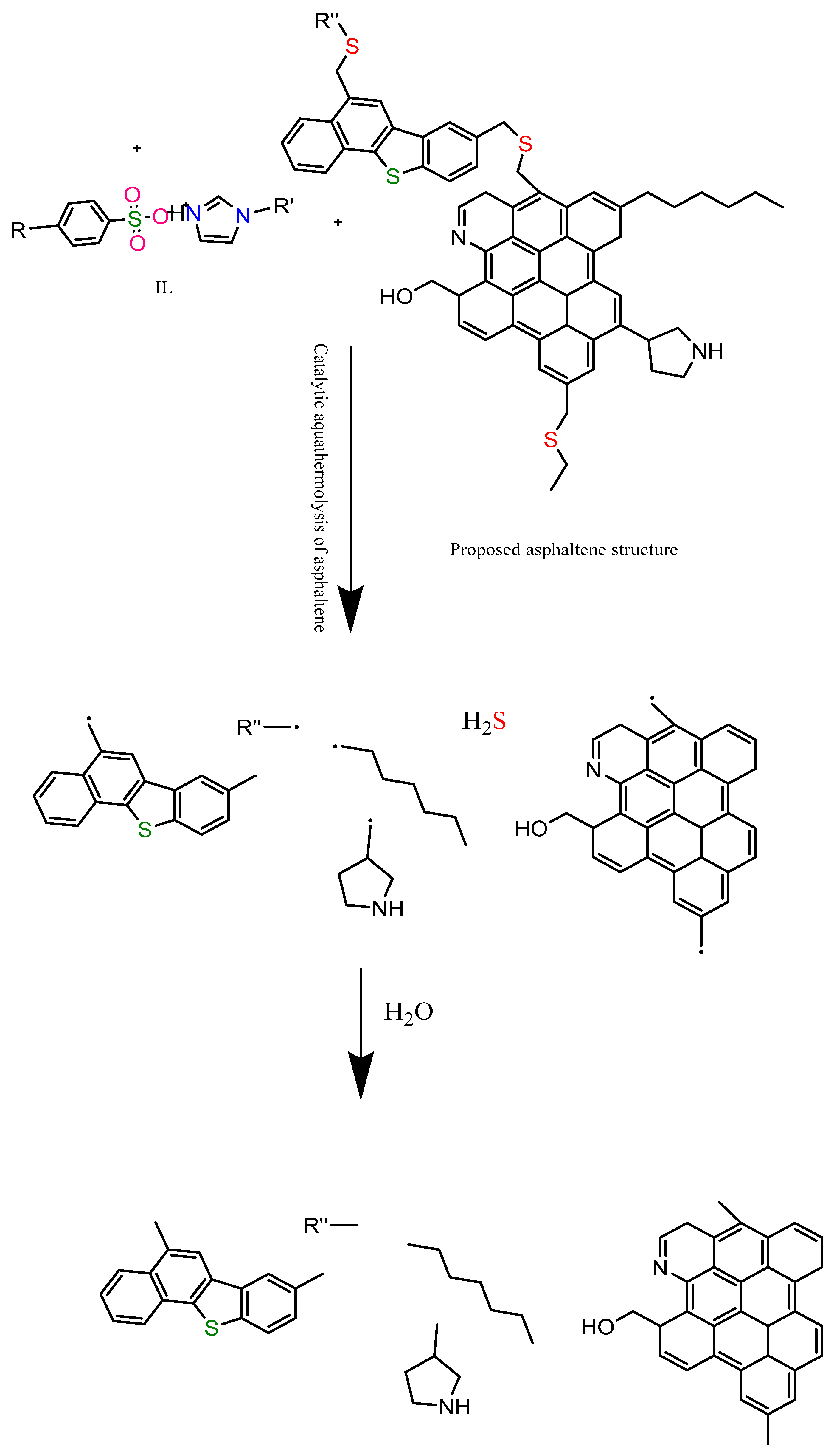
3.7. Proposed Mechanism of Catalytic Aquathermolysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huc, A.-Y. Heavy Crude Oils: From Geology to Upgrading: An Overview; Editions Technip: Paris, France, 2010. [Google Scholar]
- Li, Y.R.; Li, Q.Y.; Wang, X.D.; Yu, L.G.; Yang, J.J. Aquathermolysis of heavy crude oil with ferric oleate catalyst. Pet. Sci. 2018, 15, 613–624. [Google Scholar] [CrossRef]
- Premuzic, E.T.; Lin, M.S. Induced biochemical conversions of heavy crude oils. J. Pet. Sci. Eng. 1999, 22, 171–180. [Google Scholar] [CrossRef]
- Xia, T.; Greaves, M. Upgrading Athabasca tar sand using toe-to-heel air injection. J. Can. Pet. Technol. 2002, 41, 51–57. [Google Scholar] [CrossRef]
- Pineda-Perez, L.A.; Carbognani, L.; Spencer, R.J.; Maini, B.; Pereira-Almao, P. Hydrocarbon depletion of Athabasca core at near steam-assisted gravity drainage (SAGD) conditions. Energy Fuels 2010, 24, 5947–5954. [Google Scholar] [CrossRef]
- Bagci, S.; Kok, M.V. In-situ combustion laboratory studies of Turkish heavy oil reservoirs. Fuel Process. Technol. 2001, 74, 65–79. [Google Scholar] [CrossRef]
- Shokrlu, Y.H.; Maham, Y.; Tan, X.; Babadagli, T.; Gray, M. Enhancement of the efficiency of in situ combustion technique for heavy-oil recovery by application of nickel ions. Fuel 2013, 105, 397–407. [Google Scholar] [CrossRef]
- Desouky, S.; Betiha, M.; Badawi, A.; Ghanem, A.; Khalil, S. Catalytic aquathermolysis of Egyptian heavy crude oil. Int. J. Chem. Mol. Eng. 2013, 7, 638–643. [Google Scholar]
- Zhou, Z.; Slaný, M.; Kuzielová, E.; Zhang, W.; Ma, L.; Dong, S.; Zhang, J.; Chen, G. Influence of reservoir minerals and ethanol on catalytic aquathermolysis of heavy oil. Fuel 2022, 307, 121871. [Google Scholar] [CrossRef]
- Tirado, A.; Yuan, C.; Varfolomeev, M.A.; Ancheyta, J. Kinetic modeling of aquathermolysis for upgrading of heavy oils. Fuel 2022, 310, 122286. [Google Scholar] [CrossRef]
- Al-Ghefeili, H.M.A.; Devi, M.G.; Al-Abri, M.Z. Magnetite nanoparticle mediated catalytic aquathermolysis of Omani heavy crude oil. J. Indian Chem. Soc. 2022, 99, 100314. [Google Scholar] [CrossRef]
- Saeed, A.Q.; Al-Zaidi, B.Y.; Hamadi, A.S.; Majdi, H.S.; AbdulRazak, A.A. Upgrade of heavy crude oil via aquathermolysis over several types of catalysts. Mater. Express 2022, 12, 278–287. [Google Scholar] [CrossRef]
- Mukhamatdinov, I.I.; Salih, I.S.; Khelkhal, M.A.; Vakhin, A.V. Application of Aromatic and Industrial Solvents for Enhancing Heavy Oil Recovery from the Ashalcha Field. Energy Fuels 2020, 35, 374–385. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, C.; Wang, Y. Gemini catalyst for catalytic aquathermolysis of heavy oil. J. Anal. Appl. Pyrolysis 2010, 89, 159–165. [Google Scholar] [CrossRef]
- Sitnov, S.; Mukhamatdinov, I.; Aliev, F.; Khelkhal, M.A.; Slavkina, O.; Bugaev, K. Heavy oil aquathermolysis in the presence of rock-forming minerals and iron oxide (II, III) nanoparticles. Pet. Sci. Technol. 2020, 38, 574–579. [Google Scholar] [CrossRef]
- Fan, Z.-X.; Wang, T.-F.; He, Y.-H. Upgrading and viscosity reducing of heavy oils by [BMIM][AlCl4] ionic liquid. J. Fuel Chem. Technol. 2009, 37, 690–693. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, S.; Zhang, X.; Dong, S.; Yu, T.; Slaný, M.; Chen, G. Enhanced aquathermolysis of heavy oil catalysed by bentonite supported Fe (III) complex in the present of ethanol. J. Chem. Technol. Biotechnol. 2022, 97, 1128–1137. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, L.; Xu, Z.; Zhang, J.; Qu, C.; Zhang, Z. Heterogeneous degradation of oil field additives by Cu (II) complex-activated persulfate oxidation. Environ. Prog. Sustain. Energy 2021, 40, e13562. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Wu, C.; Xia, F. Laboratory experiments and field tests of an amphiphilic metallic chelate for catalytic aquathermolysis of heavy oil. Energy Fuels 2008, 22, 1502–1508. [Google Scholar] [CrossRef]
- Khalil, M.; Liu, N.; Lee, R.L. Catalytic aquathermolysis of heavy crude oil using surface-modified hematite nanoparticles. Ind. Eng. Chem. Res. 2017, 56, 4572–4579. [Google Scholar] [CrossRef]
- Maity, S.; Ancheyta, J.; Marroquín, G. Catalytic aquathermolysis used for viscosity reduction of heavy crude oils: A review. Energy Fuels 2010, 24, 2809–2816. [Google Scholar] [CrossRef]
- Félix, G.; Tirado, A.; Al-Muntaser, A.; Kwofie, M.; Varfolomeev, M.A.; Yuan, C.; Ancheyta, J. SARA-based kinetic model for non-catalytic aquathermolysis of heavy crude oil. J. Pet. Sci. Eng. 2022, 216, 110845. [Google Scholar] [CrossRef]
- Tirado, A.; Félix, G.; Kwofie, M.; Al-Muntaser, A.; Varfolomeev, M.A.; Yuan, C.; Ancheyta, J. Kinetics of heavy oil non-catalytic aquathermolysis with and without stoichiometric coefficients. Fuel 2022, 323, 124365–124376. [Google Scholar] [CrossRef]
- Félix, G.; Tirado, A.; Yuan, C.; Varfolomeev, M.A.; Ancheyta, J. Analysis of kinetic models for hydrocracking of heavy oils for In-situ and Ex-situ applications. Fuel 2022, 323, 124322–124342. [Google Scholar] [CrossRef]
- El-hoshoudy, A.; Ghanem, A.; Desouky, S. Imidazolium-based ionic liquids for asphaltene dispersion; experimental and computational studies. J. Mol. Liq. 2021, 324, 114698. [Google Scholar] [CrossRef]
- Ghanem, A.; Alharthy, R.D.; Desouky, S.M.; El-Nagar, R.A. Synthesis and Characterization of Imidazolium-Based Ionic Liquids and Evaluating Their Performance as Asphaltene Dispersants. Materials 2022, 15, 1600. [Google Scholar] [CrossRef]
- Yunus, N.M.M.; Dhevarajan, S.; Wilfred, C.D. Studies on the effect of sulfonate based ionic liquids on asphaltenes. J. Mol. Liq. 2022, 360, 119567. [Google Scholar] [CrossRef]
- Thulasiraman, S.; Yunus NM, M.; Kumar, P.; Kesuma, Z.R.; Norhakim, N.; Wilfred, C.D.; Burhanudin, Z.A. Effects of Ionic Liquid, 1-Ethyl-3-methylimidazolium Chloride ([EMIM] Cl), on the Material and Electrical Characteristics of Asphaltene Thin Films. Materials 2022, 15, 2818. [Google Scholar] [CrossRef]
- Atef, Y.; Ghanem, A. Ionic Liquids based on Different Chain Fatty Acids as Green Corrosion Inhibitors for C-steel in Produced Oilfield Water. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020. [Google Scholar]
- El-Nagar, R.A.; Ghanem, A.A.; Nessim, M.I. Capture of CO2 from Natural Gas Using Ionic Liquids. In Shale Gas—New Aspects and Technologies; IntechOpen: London, UK, 2018; Volume 2, pp. 83–99. [Google Scholar]
- El-Nagar, R.; Nessim, M.; Abd El-Wahab, A.; Ibrahim, R.; Faramawy, S. Investigating the efficiency of newly prepared imidazolium ionic liquids for carbon dioxide removal from natural gas. J. Mol. Liq. 2017, 237, 484–489. [Google Scholar] [CrossRef]
- Mukhamatdinov, I.I.; Khaidarova, A.R.; Zaripova, R.D.; Mukhamatdinova, R.E.; Sitnov, S.A.; Vakhin, A.V. The composition and structure of ultra-dispersed mixed oxide (Ii, iii) particles and their influence on in-situ conversion of heavy oil. Catalysts 2020, 10, 114. [Google Scholar] [CrossRef]
- Chao, K.; Chen, Y.; Liu, H.; Zhang, X.; Li, J. Laboratory experiments and field test of a difunctional catalyst for catalytic aquathermolysis of heavy oil. Energy Fuels 2012, 26, 1152–1159. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Lu, J.; Wu, C. The viscosity reduction of nano-keggin-K3PMo12O40 in catalytic aquathermolysis of heavy oil. Fuel 2009, 88, 1426–1434. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, Y.; Lu, N.; Xu, T.; Zhu, G.; Wang, K. A review on upgrading and viscosity reduction of heavy oil and bitumen by underground catalytic cracking. Energy Rep. 2021, 7, 4249–4272. [Google Scholar] [CrossRef]
- Zou, C.; Liu, C.; Luo, P. Catalytic degradation of macromolecule constituents of asphaltic sand in ionic liquids. J. Chem. Ind. Eng. 2004, 55, 2095–2098. [Google Scholar]
- Fan, H.; Zhang, Y.; Lin, Y. The catalytic effects of minerals on aquathermolysis of heavy oils. Fuel 2004, 83, 2035–2039. [Google Scholar] [CrossRef]
- Hezave, A.Z.; Dorostkar, S.; Ayatollahi, S.; Nabipour, M.; Hemmateenejad, B. Dynamic interfacial tension behavior between heavy crude oil and ionic liquid solution (1-dodecyl-3-methylimidazolium chloride ([C12mim][Cl]+ distilled or saline water/heavy crude oil)) as a new surfactant. J. Mol. Liq. 2013, 187, 83–89. [Google Scholar] [CrossRef]
- Subramanian, D.; Wu, K.; Firoozabadi, A. Ionic liquids as viscosity modifiers for heavy and extra-heavy crude oils. Fuel 2015, 143, 519–526. [Google Scholar] [CrossRef]
- Saaid, I.; Mahat, S.Q.A.; Lal, B.; Mutalib, M.I.A.; Sabil, K.M. Experimental investigation on the effectiveness of 1-butyl-3-methylimidazolium perchlorate ionic liquid as a reducing agent for heavy oil upgrading. Ind. Eng. Chem. Res. 2014, 53, 8279–8284. [Google Scholar] [CrossRef]
- El Shafiee, C.E.; El-Nagar, R.; Nessim, M.I.; Khalil, M.M.H.; Shaban, M.E.; Alharthy, R.D.; Ismail, D.A.; Abdallah, R.I.; Moustafa, Y.M. Application of asymmetric dicationic ionic liquids for oil spill remediation in sea water. Arab. J. Chem. 2021, 14, 103123. [Google Scholar] [CrossRef]
- D4052; Standard Test Method for Density, Relative Density, and API Gravity of Liquids by Digital Density Meter. 2011 ASTM Annual Book of Standards. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM International. Standard Test Method for Sulfur in Petroleum and Petroleum Products by Energy Dispersive X-ray Fluorescence Spectrometry; ASTM international: West Conshohocken, PA, USA, 2010. [Google Scholar]
- D4006-16e1; Standard Test Method for Water in Crude Oil by Distillation. ASTM Int 2016. American Society for Testing and Materials: West Conshohocken, PA, USA, 2016.
- Khormali, A.; Moghadasi, R.; Kazemzadeh, Y.; Struchkov, I. Development of a new chemical solvent package for increasing the asphaltene removal performance under static and dynamic conditions. J. Pet. Sci. Eng. 2021, 206, 109066. [Google Scholar] [CrossRef]
- Oguamah, I.A.; Oseh, J.O.; Yekeen, P.N. Effects of freezing point depression on molecular weight determination of hydrocarbon mixtures. Pac. J. Sci. Technol. 2014, 15, 240–244. [Google Scholar]
- Chen, G.; Yuan, W.; Wu, Y.; Zhang, J.; Song, H.; Jeje, A.; Song, S.; Qu, C. Catalytic aquathermolysis of heavy oil by coordination complex at relatively low temperature. Pet. Chem. 2017, 57, 881–884. [Google Scholar] [CrossRef]
- Aliev, F.A.; Mukhamatdinov, I.I.; Sitnov, S.A.; Ziganshina, M.R.; Onishchenko, Y.V.; Sharifullin, A.V.; Vakhin, A.V. In-situ heavy oil aquathermolysis in the presence of nanodispersed catalysts based on transition metals. Processes 2021, 9, 127. [Google Scholar] [CrossRef]
- Hyne, J. Aquathermolysis: A Synopsis of Work on the Chemical Reaction between Water (Steam) and Heavy Oil Sands during Simulated Steam Stimulation; Alberta Oil Sands Technology and Research Authority: Edmonton, AB, Canada, 1986. [Google Scholar]
- Buckley, J.S. Asphaltene deposition. Energy Fuels 2012, 26, 4086–4090. [Google Scholar] [CrossRef]
- Pillai, P.; Mandal, A. Synthesis and characterization of surface-active ionic liquids for their potential application in enhanced oil recovery. J. Mol. Liq. 2022, 345, 117900. [Google Scholar] [CrossRef]
- Hanamertani, A.; Pilus, R.M.; Irawan, S. ICIPEG 2016; Springer: Singapore, 2017. [Google Scholar]
- Ma, H.; Ke, H.; Wang, T.; Xiao, J.; Du, N.; Yu, L. Self-assembly of imidazolium-based surface active ionic liquids in aqueous solution: The role of different substituent group on aromatic counterions. J. Mol. Liq. 2017, 240, 556–563. [Google Scholar] [CrossRef]
- Xu, C.; Cheng, Z. Thermal stability of ionic liquids: Current status and prospects for future development. Processes 2021, 9, 337. [Google Scholar] [CrossRef]
- Khalil, R.; Chaabene, N.; Azar, M.; Malham, I.B.; Turmine, M. Effect of the chain lengthening on transport properties of imidazolium-based ionic liquids. Fluid Phase Equilibria 2020, 503, 112316. [Google Scholar] [CrossRef]
- Lin, D.; Zhu, H.; Wu, Y.; Lu, T.; Liu, Y.; Chen, X.; Peng, C.; Yang, C.; Feng, X. Morphological insights into the catalytic aquathermolysis of crude oil with an easily prepared high-efficiency Fe3O4-containing catalyst. Fuel 2019, 245, 420–428. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Liu, H.; Wang, P.; Liu, F. Influences on the aquathermolysis of heavy oil catalyzed by two different catalytic ions: Cu2+ and Fe3+. Energy Fuels 2013, 27, 2555–2562. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, Z.; Liu, X.; Liu, L.; Peng, J. Studies on viscosity reduction and structural change of crude oil treated with acoustic cavitation. Fuel 2020, 263, 116638–116644. [Google Scholar] [CrossRef]
- Asemani, M.; Rabbani, A.R. Detailed FTIR spectroscopy characterization of crude oil extracted asphaltenes: Curve resolve of overlapping bands. J. Pet. Sci. Eng. 2020, 185, 106618. [Google Scholar] [CrossRef]
- Ghannam, M.T.; Hasan, S.W.; Abu-Jdayil, B.; Esmail, N. Rheological properties of heavy & light crude oil mixtures for improving flowability. J. Pet. Sci. Eng. 2012, 81, 122–128. [Google Scholar]
- Kumar, R.; Banerjee, S.; Kumar, N.; Mandal, A.; Kumar Naiya, T. Comparative studies on synthetic and naturally extracted surfactant for improving rheology of heavy crude oil. Pet. Sci. Technol. 2015, 33, 1101–1109. [Google Scholar] [CrossRef]
- Sakthivel, S.; Velusamy, S. Eco-efficient rheological improvement of heavy crude oil using lactam based ionic liquids at high temperature high pressure condition. Fuel 2020, 276, 118027. [Google Scholar] [CrossRef]
- Fan, Y.; Simon, S.; Sjöblom, J. Interfacial shear rheology of asphaltenes at oil–water interface and its relation to emulsion stability: Influence of concentration, solvent aromaticity and nonionic surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2010, 366, 120–128. [Google Scholar] [CrossRef]
- Taborda, E.A.; Alvarado, V.; Franco, C.A.; Cortés, F.B. Rheological demonstration of alteration in the heavy crude oil fluid structure upon addition of nanoparticles. Fuel 2017, 189, 322–333. [Google Scholar] [CrossRef]
- Montes, D.; Henao, J.; Taborda, E.A.; Gallego, J.; Cortés, F.B.; Franco, C.A. Effect of textural properties and surface chemical nature of silica nanoparticles from different silicon sources on the viscosity reduction of heavy crude oil. ACS Omega 2020, 5, 5085–5097. [Google Scholar] [CrossRef]
- Leon, V.; Kumar, M. Biological upgrading of heavy crude oil. Biotechnol. Bioprocess Eng. 2005, 10, 471–481. [Google Scholar] [CrossRef]
- Kapadia, P.R.; Kallos, M.S.; Gates, I.D. A new reaction model for aquathermolysis of Athabasca bitumen. Can. J. Chem. Eng. 2013, 91, 475–482. [Google Scholar] [CrossRef]
- Hyne, J.; Clark, P.D.; Clarke, R.A.; Koo, J.; Greidanus, J.W. Aquathermolysis of heavy oils. Rev. Tec. Intevep. 1982, 2, 87–94. [Google Scholar]
- Ogunlaja, A.S.; Hosten, E.; Tshentu, Z.R. Dispersion of asphaltenes in petroleum with ionic liquids: Evaluation of molecular interactions in the binary mixture. Ind. Eng. Chem. Res. 2014, 53, 18390–18401. [Google Scholar] [CrossRef]




| Chemical Composition, wt.% | |||
|---|---|---|---|
| Asphaltenes | Maltene, wt.% | ||
| Resins | Saturates | Aromatics | |
| 19.83 | 28.524 | 14.432 | 37.214 |
| Item | Temperature (°C) | Catalyst (wt %) | Water Concentration (v/v %) | Time (h) |
|---|---|---|---|---|
| 1 | 125 | 0.03 | 20 | 12 |
| 2 | 150 | 0.06 | 30 | 24 |
| 3 | 175 | 0.09 | 40 | 36 |
| 4 | 200 | 0.12 | 50 | 48 |
| No. | Temperature (°C) | Catalyst (wt %) | Water Concentration (v/v %) | Time (h) |
|---|---|---|---|---|
| 1 | 150 | 0.03 | 30 | 24 |
| 2 | 200 | 0.09 | 30 | 36 |
| 3 | 125 | 0.03 | 20 | 12 |
| 4 | 175 | 0.12 | 50 | 12 |
| 5 | 125 | 0.09 | 50 | 24 |
| 6 | 175 | 0.09 | 30 | 36 |
| 7 | 150 | 0.12 | 20 | 48 |
| 8 | 125 | 0.06 | 40 | 48 |
| 9 | 200 | 0.03 | 50 | 48 |
| 10 | 150 | 0.09 | 40 | 12 |
| 11 | 175 | 0.06 | 20 | 24 |
| 12 | 200 | 0.06 | 20 | 12 |
| 13 | 200 | 0.12 | 40 | 24 |
| 14 | 150 | 0.06 | 50 | 36 |
| 15 | 125 | 0.12 | 30 | 36 |
| 16 | 175 | 0.03 | 40 | 48 |
| Samples | Viscosity Reduction, % | Sulfur Content, ppm | Content, wt.% | |||
|---|---|---|---|---|---|---|
| Asphaltenes | Resins | Saturates | Aromatics | |||
| Crude oil | … | 23,520 | 19.83 | 28.52 | 14.43 | 37.21 |
| Aquathermolysis | 28.5 | 22,980 | 18.5 | 27.3 | 16.6 | 37.6 |
| Aquathermolysis + IL-4 | 89.8 | 18,675 | 14.9 | 26.8 | 18.43 | 39.21 |
| Aquathermolysis + IL-10 | 94.3 | 14,580 | 12.8 | 25.6 | 21 | 40.6 |
| Aquathermolysis + IL-16 | 93.7 | 15,120 | 12.3 | 26.4 | 21.5 | 39.8 |
| Used Catalyst | Catalyst, wt.% | Sulfur Reduction, % | Crude Oil Origin | Viscosity Reduction, % | Asphaltene Content Reduction, % | Ref. |
|---|---|---|---|---|---|---|
| Fe3O4 | 5 | 4.8 | Shengli Oilfield | 85 | 21 | [56] |
| Gemini catalyst | 0.1 | 28 | Karamay Oilfield | 92 | 6.6 | [14] |
| Fe(C7H7O3S)3 | 0.2 | 5.2 | Y913 | 90.48 | 23.6 | [57] |
| Cu(C7H7O3S)2 | 0.15 | 5.2 | Y913 | 92.19 | 30 | [57] |
| IL-10 | 0.09 | 38 | GPC-heavy oil | 94.3 | 35 | This work |
| Treated (After Aquathermolysis) | Untreated (Before Aquathermolysis) | ||
|---|---|---|---|
| Components | Mol. % | Mol. % | |
| Dodecanes | C12 | 0.18 | 0.00 |
| Tridecanes | C13 | 0.86 | 0.77 |
| Tetradecanes | C14 | 4.14 | 2.43 |
| Pentadecanes | C15 | 7.56 | 4.12 |
| Hexadecanes | C16 | 7.356 | 4.89 |
| Heptadecanes | C17 | 13.81 | 10.97 |
| Octadecanes | C18 | 13.44 | 12.93 |
| Nonadecanes | C19 | 8.93 | 6.62 |
| Icosanes | C20 | 7.45 | 6.46 |
| Eneicosanes | C21 | 6.44 | 7.40 |
| Dodeicosanes | C22 | 4.95 | 6.69 |
| Tricosanes | C23 | 4.14 | 5.42 |
| Tetraicosanes | C24 | 3.56 | 4.83 |
| Petaicosanes | C25 | 2.86 | 3.80 |
| Hexaicosanes | C26 | 2.66 | 3.21 |
| Heptaicosanes | C27 | 1.95 | 2.78 |
| Octaicosanes | C28 | 2.10 | 3.21 |
| Nonaicosanes | C29 | 1.89 | 2.66 |
| Tricontanes | C30 | 1.80 | 2.50 |
| Entricontanes | C31 | 1.28 | 2.12 |
| Dodetricontanes | C32 | 0.77 | 1.52 |
| Tritricontanes | C33 | 0.62 | 1.32 |
| Tetratricontanes | C34 | 0.53 | 1.11 |
| Pentatricontanes | C35 | 0.44 | 0.93 |
| Hexatricontanes | C36 | 0.29 | 0.43 |
| Hepatricontanes | C37 | 0.00 | 0.32 |
| Octatricontanes | C38 | 0.00 | 0.26 |
| Nonatricontanes | C39 | 0.00 | 0.20 |
| Tetracontanes | C40 | 0.00 | 0.12 |
| Total | 100.00 | 100.00 | |
| Mol. Wt. | 284.27 | 307.04 | |
| C % | H % | N % | S % | O % | |
|---|---|---|---|---|---|
| Asphaltene, before | 85.34 | 9.1 | 0.89 | 1.77 | 2.9 |
| Resin, before | 81 | 11.5 | 1.47 | 1.06 | 4.97 |
| Asphaltene, after | 83.1 | 14.13 | 0.46 | 1.21 | 1.1 |
| Resin, after | 79.39 | 14.8 | 0.89 | 0.82 | 4.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D. Alharthy, R.; El-Nagar, R.A.; Ghanem, A. Laboratory Experiments on the In Situ Upgrading of Heavy Crude Oil Using Catalytic Aquathermolysis by Acidic Ionic Liquid. Materials 2022, 15, 5959. https://doi.org/10.3390/ma15175959
D. Alharthy R, El-Nagar RA, Ghanem A. Laboratory Experiments on the In Situ Upgrading of Heavy Crude Oil Using Catalytic Aquathermolysis by Acidic Ionic Liquid. Materials. 2022; 15(17):5959. https://doi.org/10.3390/ma15175959
Chicago/Turabian StyleD. Alharthy, Rima, Raghda A. El-Nagar, and Alaa Ghanem. 2022. "Laboratory Experiments on the In Situ Upgrading of Heavy Crude Oil Using Catalytic Aquathermolysis by Acidic Ionic Liquid" Materials 15, no. 17: 5959. https://doi.org/10.3390/ma15175959
APA StyleD. Alharthy, R., El-Nagar, R. A., & Ghanem, A. (2022). Laboratory Experiments on the In Situ Upgrading of Heavy Crude Oil Using Catalytic Aquathermolysis by Acidic Ionic Liquid. Materials, 15(17), 5959. https://doi.org/10.3390/ma15175959





