Activated Sintering of Cr2O3-Based Composites by Hot Pressing
Abstract
:1. Introduction
2. Materials and Methods
3. Theoretical Background
4. Results and Discussion
4.1. Effect of Sintering Conditions on Relative Density
4.2. Effect of Solid Solution
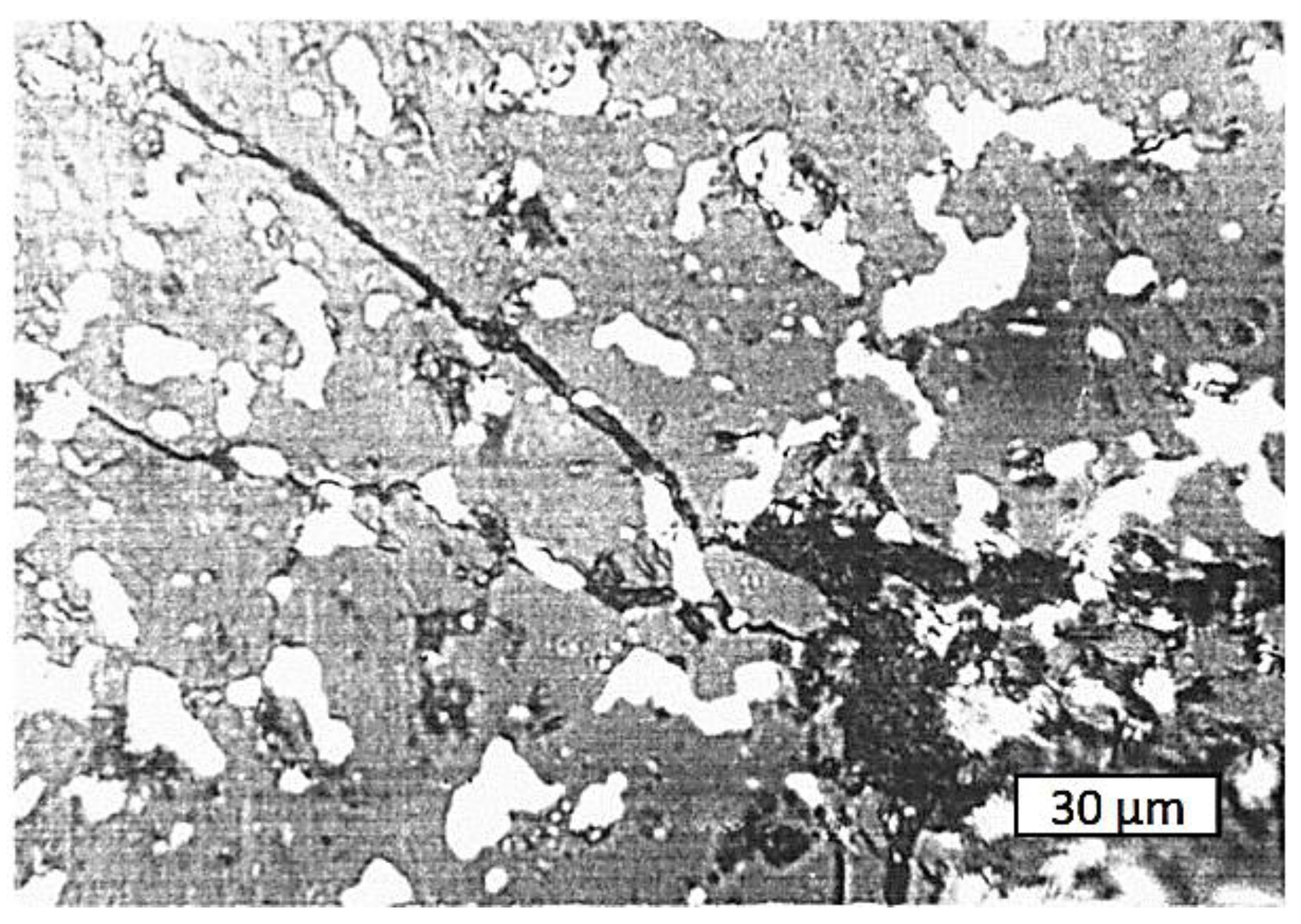
4.3. Composition of the Obtained Cermets
4.4. Strength Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eray, S. Application of metal oxides in composites. In Metal Oxides; Al-Douri, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 101–119. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, J.; Gan, Y.; Tang, X.; Gai, S.; Sun, X. Cutting performance and wear mechanisms of the graphene-reinforced Al2O3-WC-TiC composite ceramic tool in turning hardened 40Cr steel. Ceram. Int. 2022, 48, 13695–13705. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, J.; Cui, E.; Liu, H.; Dong, Y.; Sun, Z. Effects of sintering parameters on microstructure, graphene structure stability and mechanical properties of graphene reinforced Al2O3-based composite ceramic tool material. Ceram. Int. 2019, 45 Part B, 23384–23392. [Google Scholar] [CrossRef]
- Gevorkyan, E.; Mamalis, A.; Vovk, R.; Semiatkowski, Z.; Morozow, D.; Nerubatskyi, V.; Morozova, O. Special features of manufacturing cutting inserts from nanocomposite material Al2O3-SiC. J. Instrum. 2021, 16, P10015. [Google Scholar] [CrossRef]
- Hong, D.; Yin, Z.; Yan, S.; Xu, W. Fine grained Al2O3/SiC composite ceramic tool material prepared by two-step microwave sintering. Ceram. Int. 2019, 45, 11826–11832. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Liu, H. Mechanical properties and microstructure of spark plasma sintered Al2O3-SiCw-Si3N4 composite ceramic tool materials. Ceram. Int. 2022, 48, 5527–5534. [Google Scholar] [CrossRef]
- Yin, Z.; Huang, C.; Zou, B.; Liu, H.; Zhu, H.; Wang, J. Dynamic fatigue behavior of Al2O3/TiC micro–nano-composite ceramic tool materials at ambient and high temperatures. Mater. Sci. Eng. A 2014, 593, 64–69. [Google Scholar] [CrossRef]
- Wang, D.; Xue, C.; Cao, Y.; Zhao, J. Microstructure design and preparation of Al2O3/TiC/TiN micro-nano-composite ceramic tool materials based on properties prediction with finite element method. Ceram. Int. 2018, 44, 5093–5101. [Google Scholar] [CrossRef]
- Gevorkyan, E.; Rucki, M.; Krzysiak, Z.; Chishkala, V.; Zurowski, W.; Kucharczyk, W.; Barsamyan, V.; Nerubatskyi, V.; Mazur, T.; Morozow, D.; et al. Analysis of the Electroconsolidation Process of Fine-Dispersed Structures Out of Hot Pressed Al2O3–WC Nanopowders. Materials 2021, 14, 6503. [Google Scholar] [CrossRef]
- Oguntuyi, S.D.; Johnson, O.T.; Shongwe, M.B. Spark plasma sintering of ceramic matrix composite of TiC: Microstructure, densification, and mechanical properties: A review. Int. J. Adv. Manuf. Technol. 2021, 116, 69–82. [Google Scholar] [CrossRef]
- Krenkel, W. Ceramic Matrix Composites; Viley-VCH: Berlin, Germany, 2008. [Google Scholar]
- Konopka, K. Particle-Reinforced Ceramic Matrix Composites—Selected Examples. J. Compos. Sci. 2022, 6, 178. [Google Scholar] [CrossRef]
- Ding, H.; Bao, X.; Jamili-Shirvan, Z.; Jin, J.; Deng, L.; Yao, K.; Gong, P.; Wang, X. Enhancing strength-ductility synergy in an ex situ Zr-based metallic glass composite via nanocrystal formation within high-entropy alloy particles. Mater. Des. 2021, 210, 110108. [Google Scholar] [CrossRef]
- Prokopiv, N.; Kharchenko, O.; Gevorkyan, E.; Gutsalenko, Y. Exploring the process to obtain a composite based on Cr2O3-AlN using a method of hot pressing. East.-Eur. J. Enterp. Technol. 2019, 3, 17–21. [Google Scholar] [CrossRef]
- Xia, J.F.; Nian, H.Q.; Liu, W.; Wang, X.G.; Jiang, D.Y. Effect of Cr2O3 derived from Cr(NO3)3·9H2O precursor on the densification and mechanical properties of zirconia-toughened alumina (ZTA) composites. Ceram. Int. 2016, 42, 9116–9124. [Google Scholar] [CrossRef]
- Corberán, V.C.; Rives, V.; Stathopoulos, V. Recent Applications of Nanometal Oxide Catalysts in Oxidation Reactions. In Advanced Nanomaterials for Catalysis and Energy; Sadykov, V.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 227–293. [Google Scholar] [CrossRef]
- Ratia, V.L.; Zhang, D.; Daure, J.L.; Shipway, P.H.; McCartney, D.G.; Stewart, D.A. Sliding wear of a self-mated thermally sprayed chromium oxide coating in a simulated PWR water environment. Wear 2019, 426–427 Pt B, 1466–1473. [Google Scholar] [CrossRef]
- Sun, H.; Yi, G.; Wan, S.; Kong, C.; Zhu, S.; Bai, L.; Yang, J. Effect of Cr2O3 addition on mechanical and tribological properties of atmospheric plasma-sprayed NiAl-Bi2O3 composite coatings. Surf. Coat. Technol. 2021, 427, 127818. [Google Scholar] [CrossRef]
- Das, B.; Gopinath, M.; Nath, A.K.; Bandyopadhyay, P.P. Online monitoring of thermo cycles during laser remelting of flame sprayed chromia coating in pulsed mode and coating properties. Optik 2021, 227, 166030. [Google Scholar] [CrossRef]
- Gerald, O.J.; Wenge, L.; Tao, Z.Y.; Long, L.C.; Qiang, L. Influence of plasma spraying current on the microstructural characteristics and tribological behaviour of plasma sprayed Cr2O3 coating. Bol. Soc. Esp. Ceram. Vidr. 2021, 60, 338–346. [Google Scholar] [CrossRef]
- Rondinella, A.; Furlani, E.; Magnan, M.; Scagnetto, F.; Driussi, S.; Marin, E.; Maschio, S. Synthesis, crystallographic characterization, and mechanical behavior of alumina chromia alloys. Ceram. Int. 2021, 47, 24494–24500. [Google Scholar] [CrossRef]
- Kisly, P.S.; Bodnaruk, N.I.; Borovikova, M.S. Cermets; Naukova Dumka: Kyiv, Ukraine, 1985. (In Russian) [Google Scholar]
- Turkevich, V.Z.; Shishkin, B.A. High temperature calorimeter up to 2050 K. In Proceedings of the 9th National Conference on Thermal Analysis, Kyiv, Ukraine; 1985; p. 31. [Google Scholar]
- Strizhalo, V.A.; Novogrudsky, L.S.; Vorobyov, E.V. Strength of the Alloys for Cryogenic Devices under Electromagnetic Influence; Naukova Dumka: Kyiv, Ukraine, 1990. (In Russian) [Google Scholar]
- Gevorkyan, E.; Nerubatskyi, V.; Chyshkala, V.; Morozova, O. Revealing specific features of structure formation in composites based on nanopowders of synthesized zirconium dioxide. East.-Eur. J. Enterp. Technol. 2021, 5, 6–19. [Google Scholar] [CrossRef]
- Gevorkyan, E.; Nerubatskyi, V.; Chyshkala, V.; Gutsalenko, Y.; Morozova, O. Determining the influence of ultra-dispersed aluminum nitride impurities on the structure and physical-mechanical properties of tool ceramics. East.-Eur. J. Enterp. Technol. 2021, 6, 40–52. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, H.; Gao, H.; Zhu, Y.; Yu, J.; Fan, R.; Zhao, H. Formation process and properties of aluminium–chromium slag. Ceram. Int. 2018, 44, 15730–15734. [Google Scholar] [CrossRef]
- Karapetyantz, M.H. Introduction to the Chemical Processes Theory; Higher School Press: Moscow, Russia, 1970. [Google Scholar]
- Vlasov, A.O. Ceramic composite materials. J. Sov. Chem. Soc. 1982, 27, 50–56. (In Russian) [Google Scholar]
- Nalepka, K.; Pęcherski, R.B. The strength of the interfacial bond in the ceramic matrix composites Al2O3-Ni. Mech. Control 2010, 29, 132–137. [Google Scholar]
- Vuurman, M.A.; Wachs, I.E.; Stufkens, D.J.; Oskam, A. Characterization of chromium oxide supported on Al2O3, ZrO2, TiO2, and SiO2 under dehydrated conditions. J. Mol. Catal. 1993, 80, 209–227. [Google Scholar] [CrossRef]
- Fierro, J.L.G. Metal Oxides: Chemistry and Applications; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Bansal, N.P. Handbook of Ceramic Composites; Kluwer Academic Publishers: New York, NY, USA, 2005. [Google Scholar]
- Comyn, J. What are adhesives and sealants and how do they work? In Adhesive Bonding, 2nd ed.; Adams, R.D., Ed.; Woodhead Publishing: Cambridge, MA, USA, 2021; pp. 41–78. [Google Scholar] [CrossRef]
- Brogly, M. Forces Involved in Adhesion. In Handbook of Adhesion Technology; da Silva, L., Öchsner, A., Adams, R., Eds.; Springer: Cham, Switzerland, 2018; pp. 43–70. [Google Scholar] [CrossRef]
- Leite, F.L.; Bueno, C.C.; Da Róz, A.L.; Ziemath, E.C.; Oliveira, O.N. Theoretical Models for Surface Forces and Adhesion and Their Measurement Using Atomic Force Microscopy. Int. J. Mol. Sci. 2012, 13, 12773–12856. [Google Scholar] [CrossRef] [PubMed]
- Tahraoui, L.; Diafi, M.; Aidi, A.; Benhaoua, B. Chromium-rich Zn–Cr alloys: Electrochemical deposition, structure and corrosion resistance. Dig. J. Nanomater. Biostruct. 2021, 16, 191–196. [Google Scholar]
- Anger, G.; Halstenberg, J.; Hochgeschwender, K.; Scherhag, C.; Korallus, U.; Knopf, H.; Schmidt, P.; Ohlinger, M. Chromium Compounds. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Berlin, Germany, 2005. [Google Scholar] [CrossRef]
- Jõgiaas, T.; Tarre, A.; Mändar, H.; Kozlova, J.; Tamm, A. Nanoindentation of Chromium Oxide Possessing Superior Hardness among Atomic-Layer-Deposited Oxides. Nanomaterials 2022, 12, 82. [Google Scholar] [CrossRef]
- Ćurković, L.; Bakić, A.; Kodvanj, J.; Haramina, T. Flexural strength of alumina ceramics: Weibull analysis. Trans. FAMENA 2010, 34, 13–19. [Google Scholar]
- Gómez-Rodríguez, C.; Antonio-Zárate, Y.; Revuelta-Acosta, J.; Verdeja, L.F.; Fernández-González, D.; López-Perales, J.F.; Rodríguez-Castellanos, E.A.; García-Quiñonez, L.V.; Castillo-Rodríguez, G.A. Research and Development of Novel Refractory of MgO Doped with ZrO2 Nanoparticles for Copper Slag Resistance. Materials 2021, 14, 2277. [Google Scholar] [CrossRef]
- Lamon, J. Properties and Characteristics of SiC and SiC/SiC Composites. In Comprehensive Nuclear Materials, 2nd ed.; Konings, R.J.M., Stoller, R.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 400–418. [Google Scholar] [CrossRef]
- Ovid’ko, I.A.; Sheinerman, A.G. Toughening due to crack deflection in ceramic- and metal-graphene nanocomposites. Rev. Adv. Mater. Sci. 2015, 43, 52–60. [Google Scholar]
- Luiz, A.F.; Tomaz, Í.V.; Oliveira, M.P.; Simão, L.; Monteiro, S.N. Development and evaluation of TiB2–AlN ceramic composites sintered by spark plasma. Ceram. Int. 2016, 42, 18718–18723. [Google Scholar] [CrossRef]
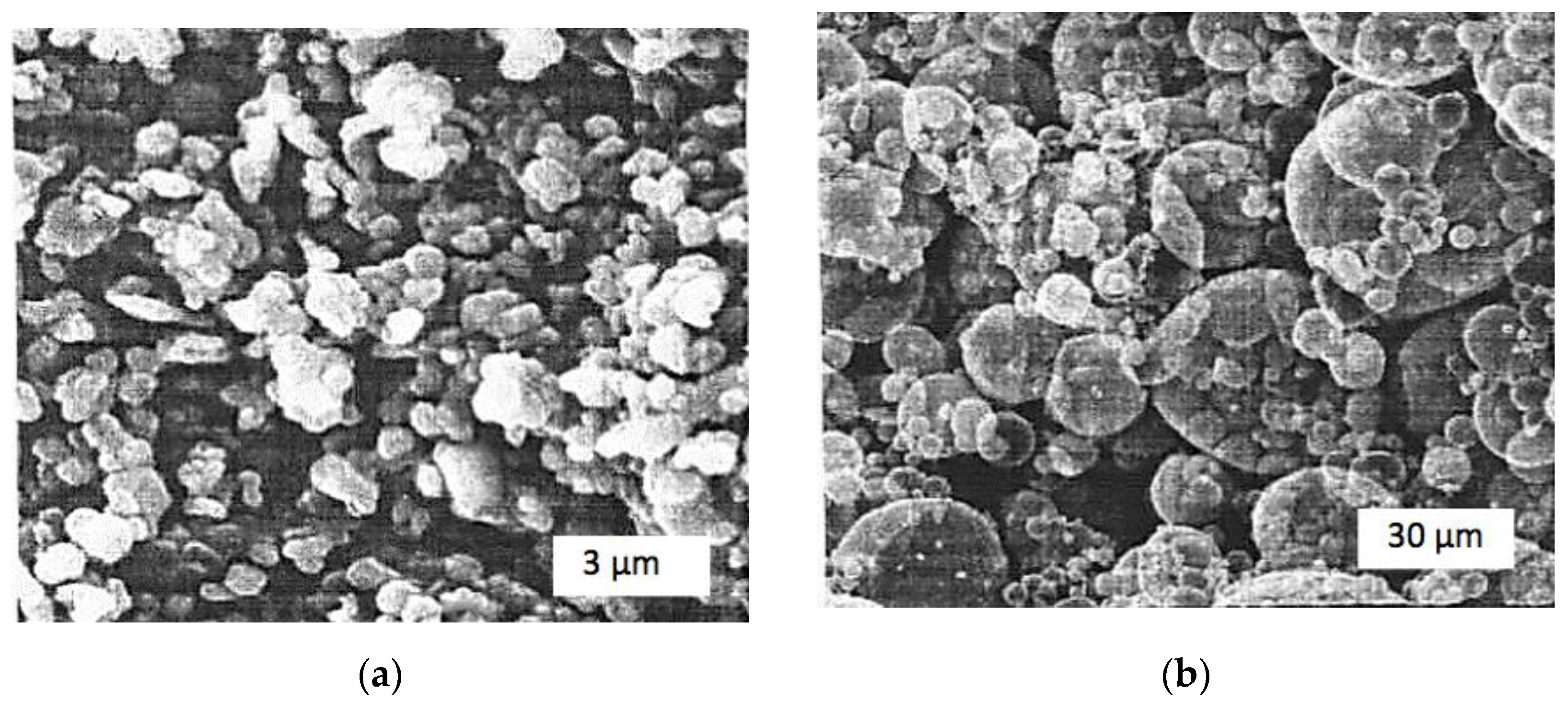
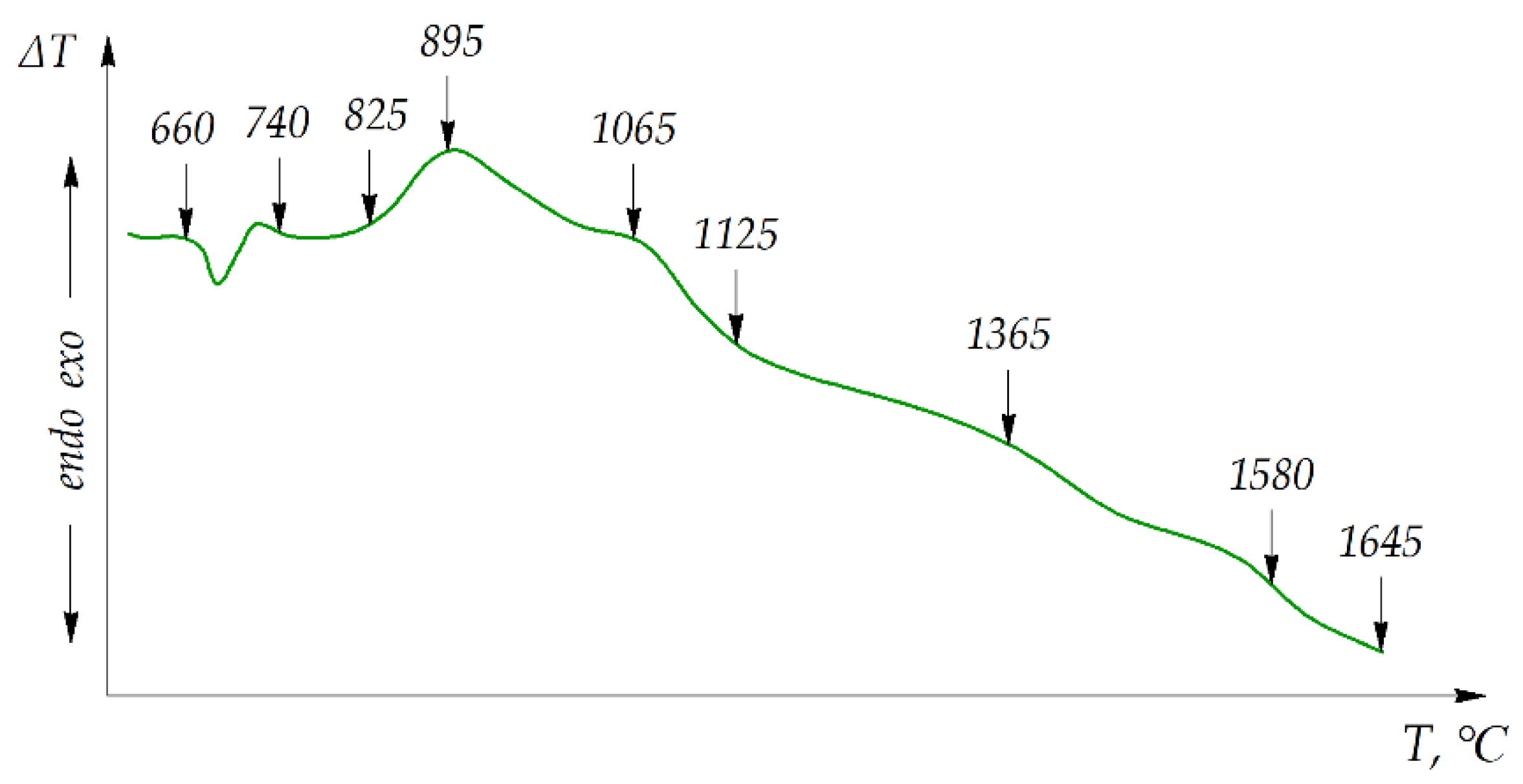
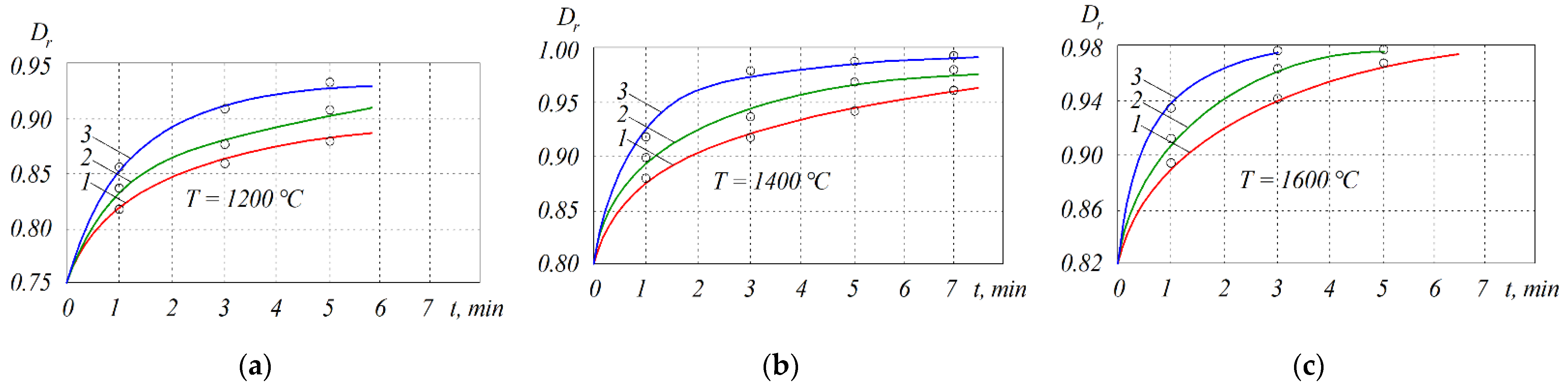
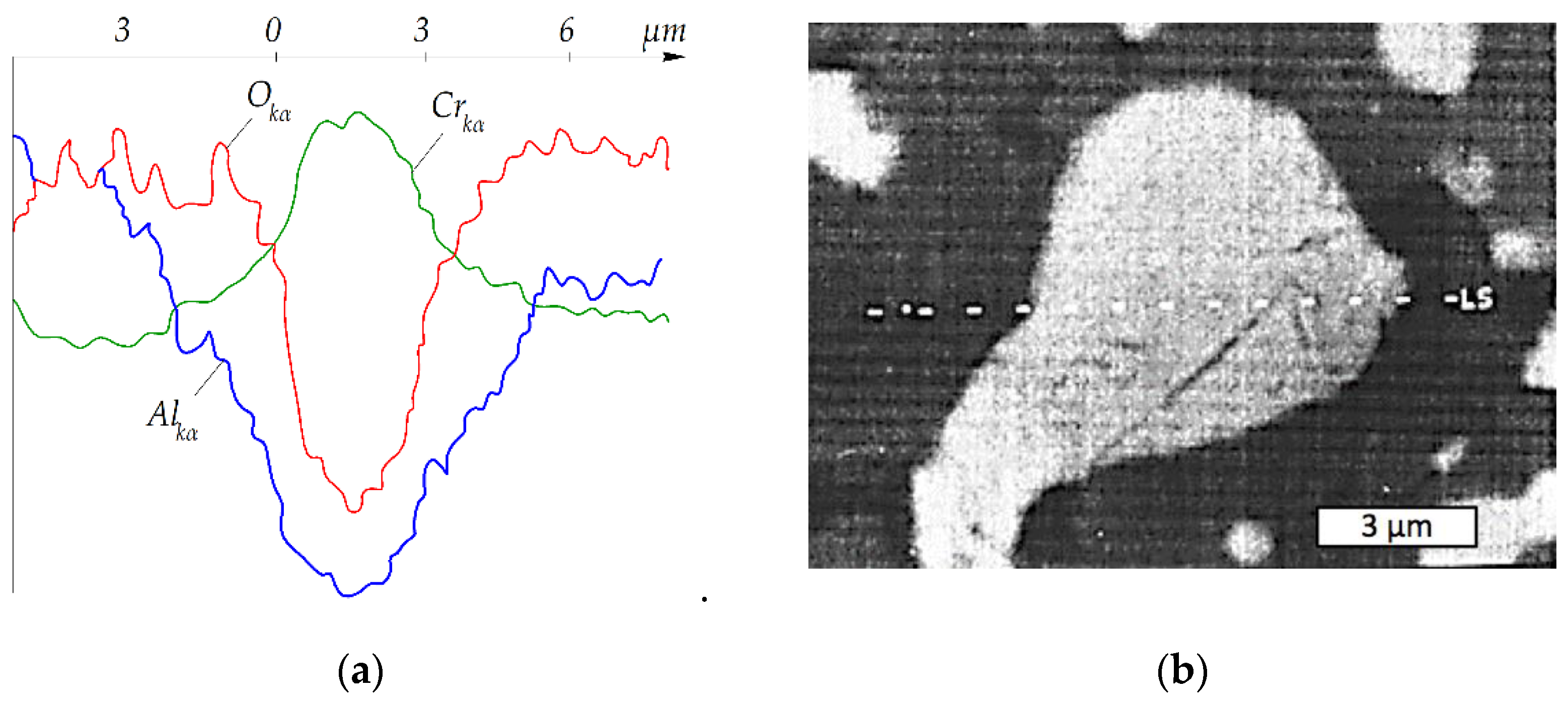
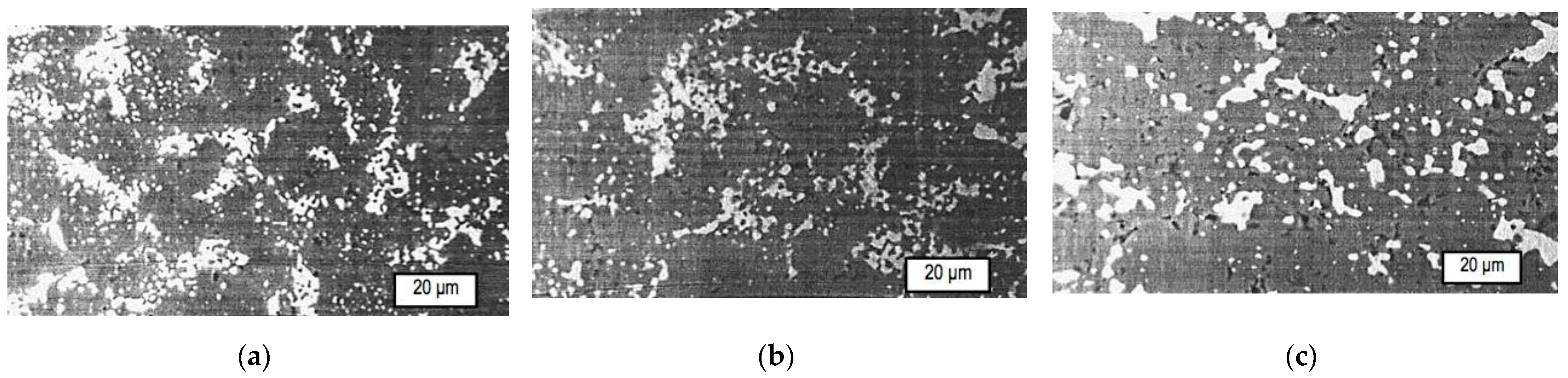
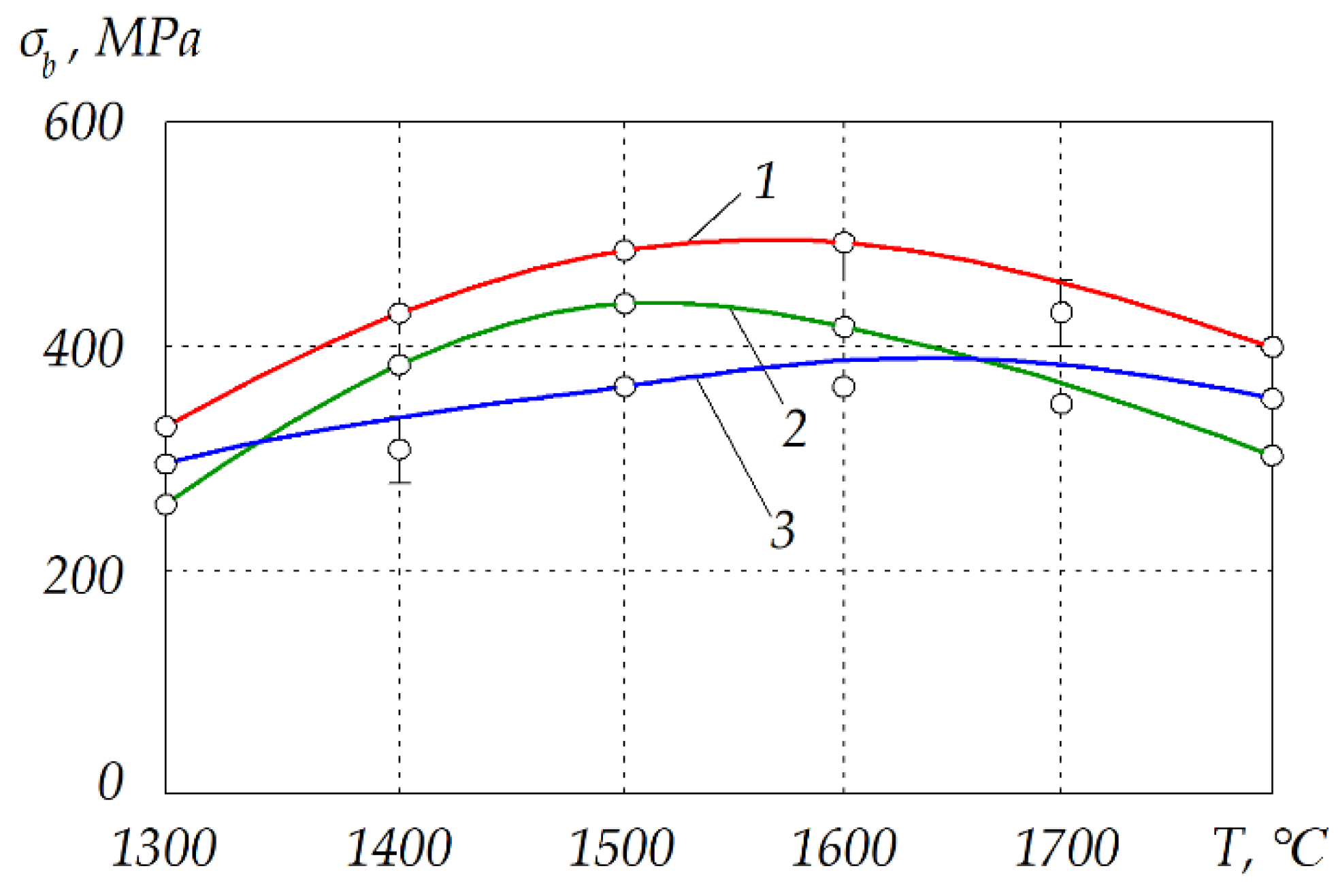
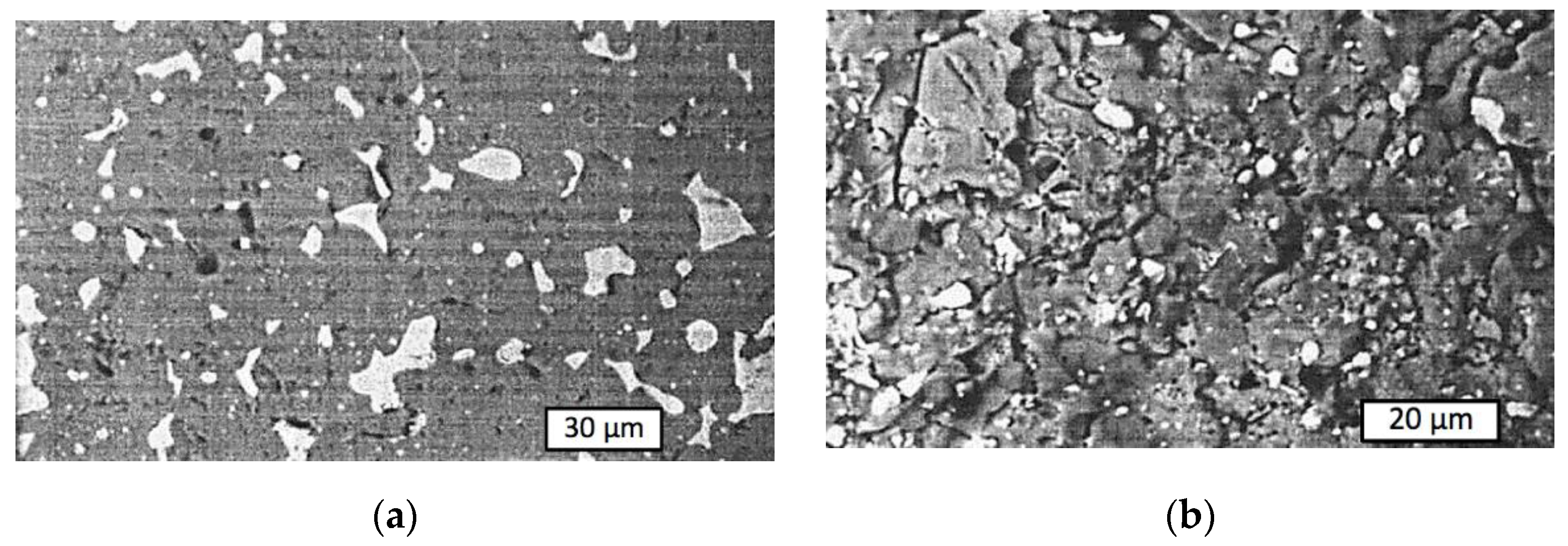
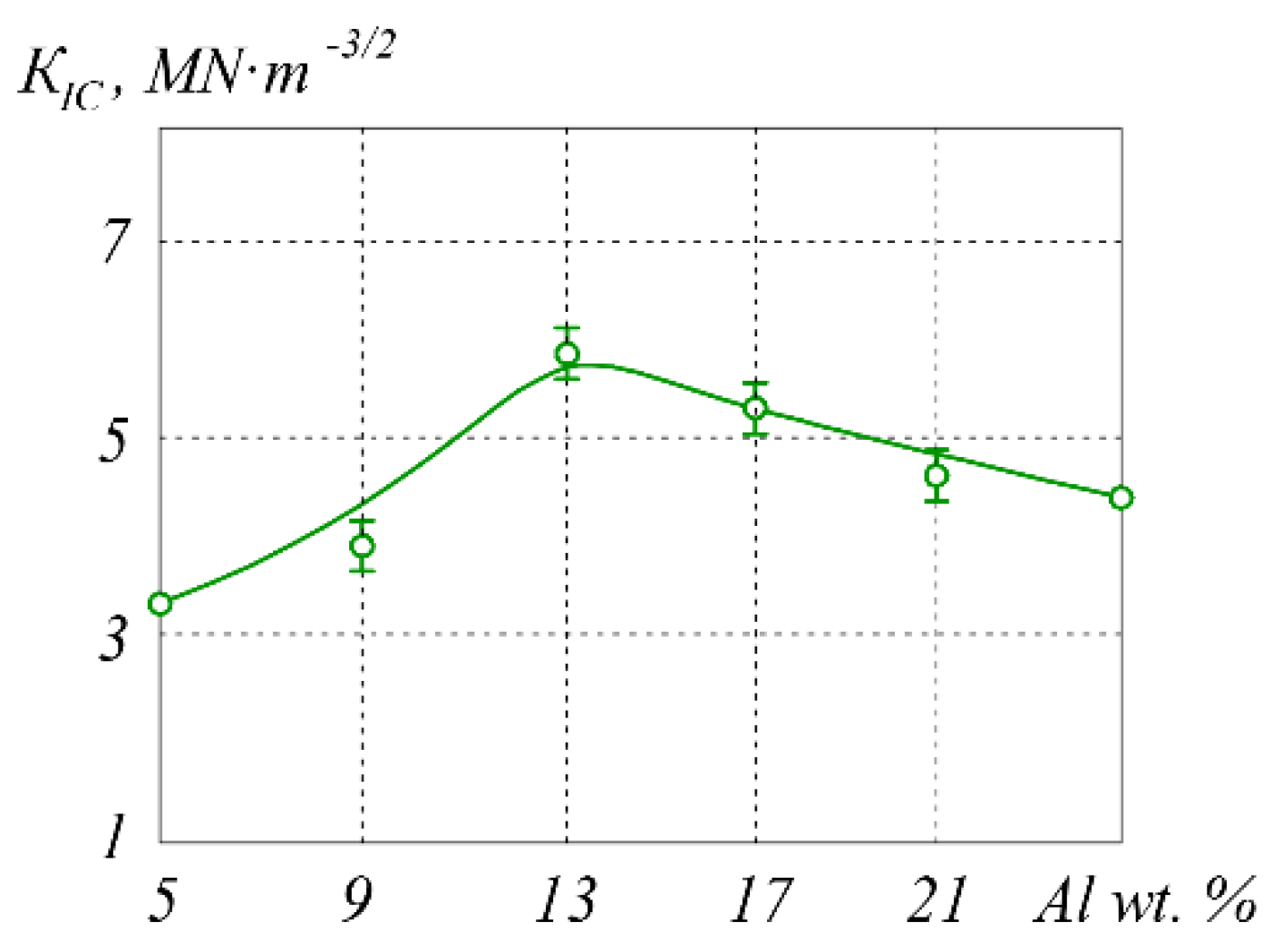
| T, °C | Components | P = 30 MPa | P = 0.1 MPa | P = 0.01 MPa |
|---|---|---|---|---|
| 700 | Cr2O3 | 1.217 | 1.055 | 1.055 |
| Al2O3 | 3.917 | 0.407 | 4.077 | |
| Cr | 7.835 | 8.153 | 8.158 | |
| Al | 0.001 | – | – | |
| 800 | Cr2O3 | 2.385 | 1.055 | 0.087 |
| Al2O3 | 0.052 | 4.077 | 4.843 | |
| Cr | 8.104 | 8.153 | 9.687 | |
| Al | 0.049 | – | – | |
| 900 | Cr2O3 | 1.055 | 1.055 | 1.055 |
| Al2O3 | 4.077 | 4.077 | 4.077 | |
| Cr | 8.153 | 8.153 | 8.153 | |
| Al | – | – | – | |
| 1000 | Cr2O3 | 1.055 | 1.055 | 1.055 |
| Al2O3 | 4.077 | 4.077 | 4.077 | |
| Cr | 8.153 | 8.153 | 8.158 | |
| Al | – | – | – | |
| 1100 | Cr2O3 | 0.721 | 0.621 | 0.621 |
| Al2O3 | 1.368 | 0.319 | 0.319 | |
| Cr | 4.346 | 6.214 | 6.214 | |
| Al | 1.030 | – | – | |
| 1200 | Cr2O3 | 0.965 | 0.621 | 0.621 |
| Al2O3 | 1.063 | 0.319 | 0.319 | |
| Cr | 5.605 | 6.214 | 6.214 | |
| Al | 0.065 | – | – | |
| 1300 | Cr2O3 | 1.217 | 0.422 | 0.422 |
| Al2O3 | 0.698 | 0.915 | 0.915 | |
| Cr | 6.713 | 7.215 | 7.215 | |
| Al | – | – | – | |
| 1400 | Cr2O3 | 1.217 | 0.422 | 0.422 |
| Al2O3 | 0.698 | 0.915 | 0.915 | |
| Cr | 6.713 | 7.215 | 7.215 | |
| Al | – | – | – |
| No. | Initial Powder Content | a1, Å | a2, Å | a3, Å | c1, Å | c2, Å | c3, Å |
|---|---|---|---|---|---|---|---|
| 1 | Cr2O3 + 9 wt.% Al | 4.947 | 4.936 | 4.922 | 13.302 | 13.366 | 13.433 |
| 2 | Cr2O3 + 13 wt.% Al | 4.941 | 4.936 | 4.922 | 13.366 | 13.410 | 13.433 |
| 3 | Cr2O3 + 17 wt.% Al | 4.936 | 4.926 | 4.922 | 13.410 | 13.428 | 13.433 |
| Compound | t = 5 min | t = 10 min | t = 15 min |
|---|---|---|---|
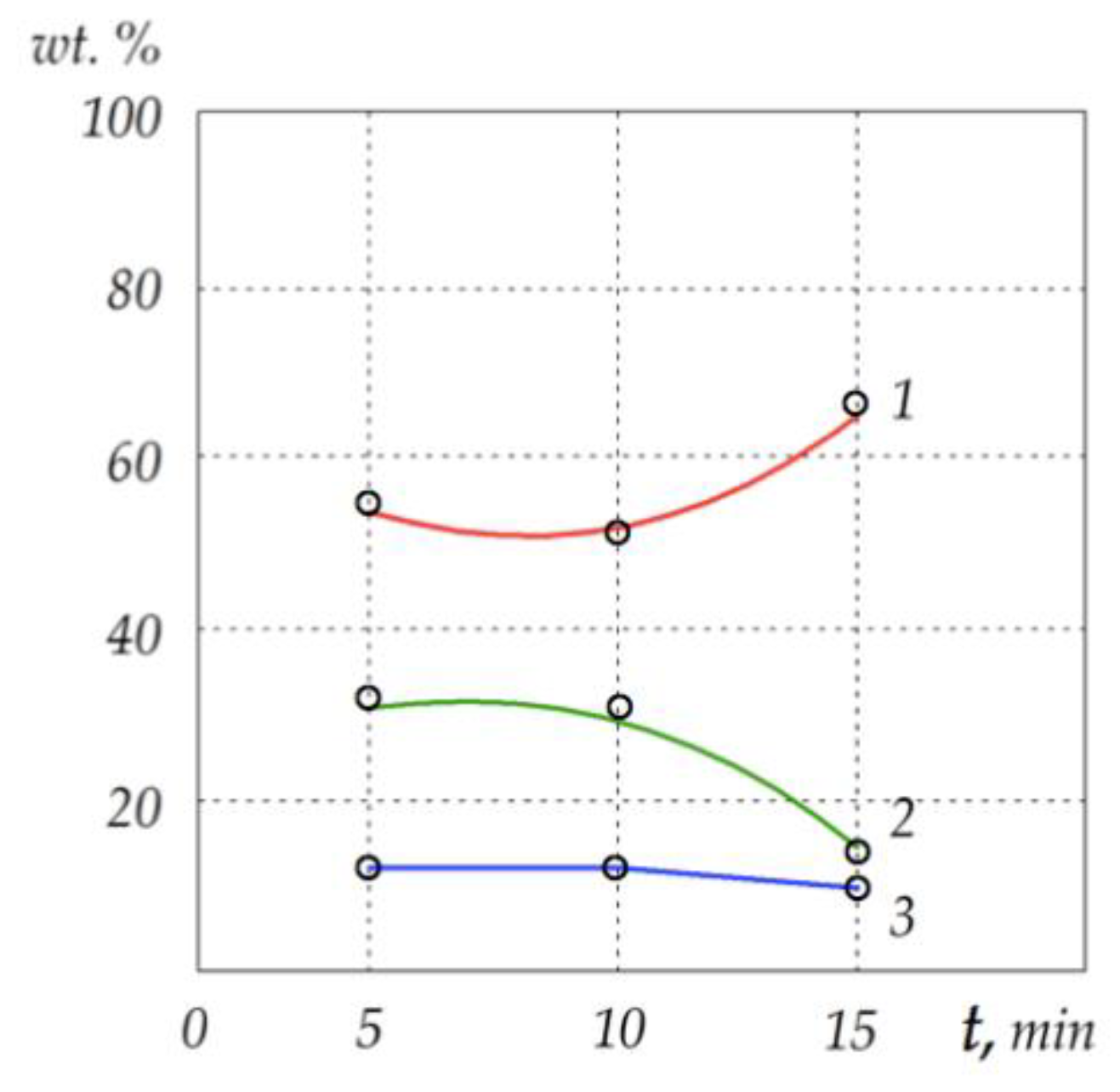
| Aluminum | 40.5 | 27 | 13.5 |
| Chromium | 26 | 52 | 78 |
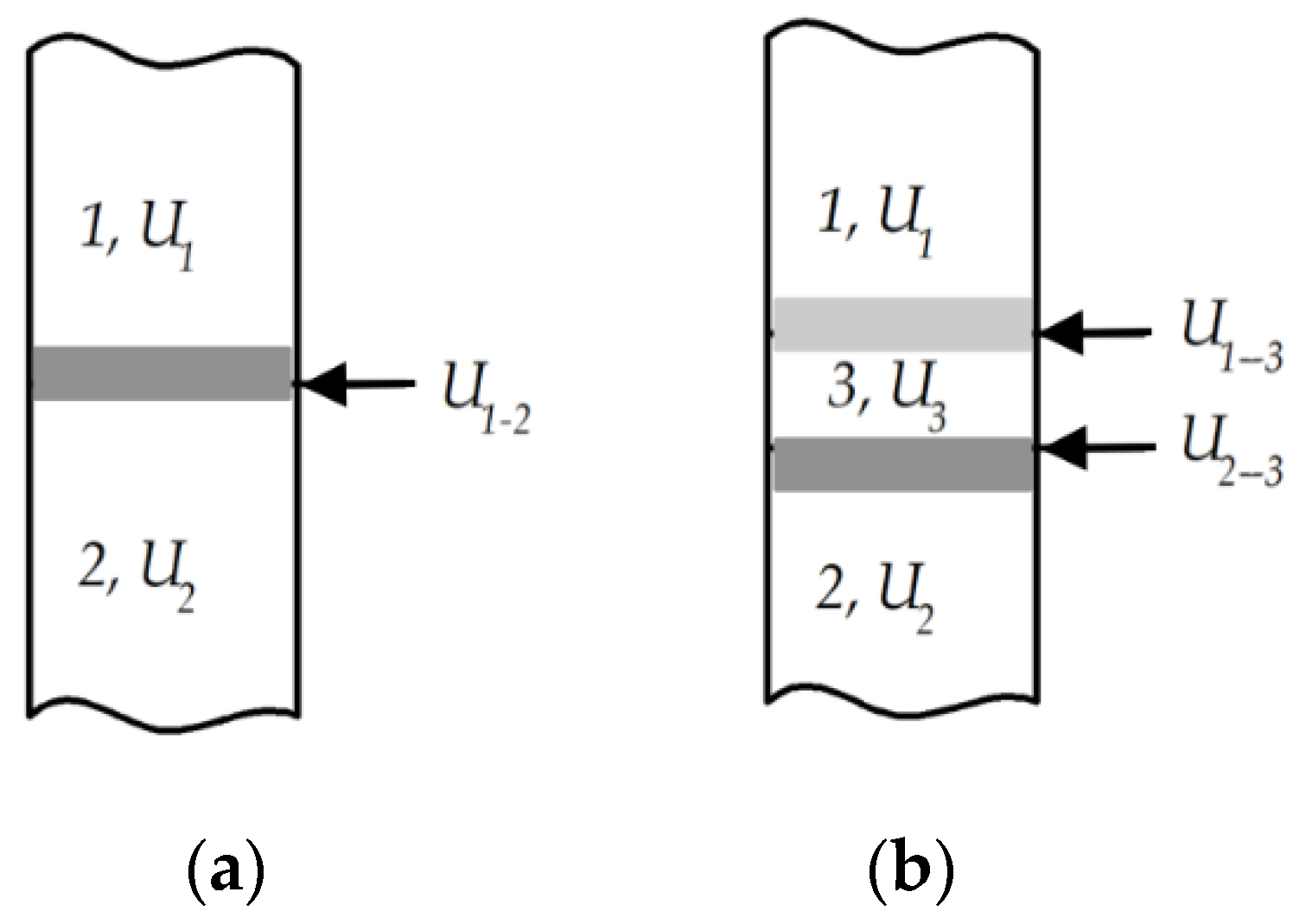
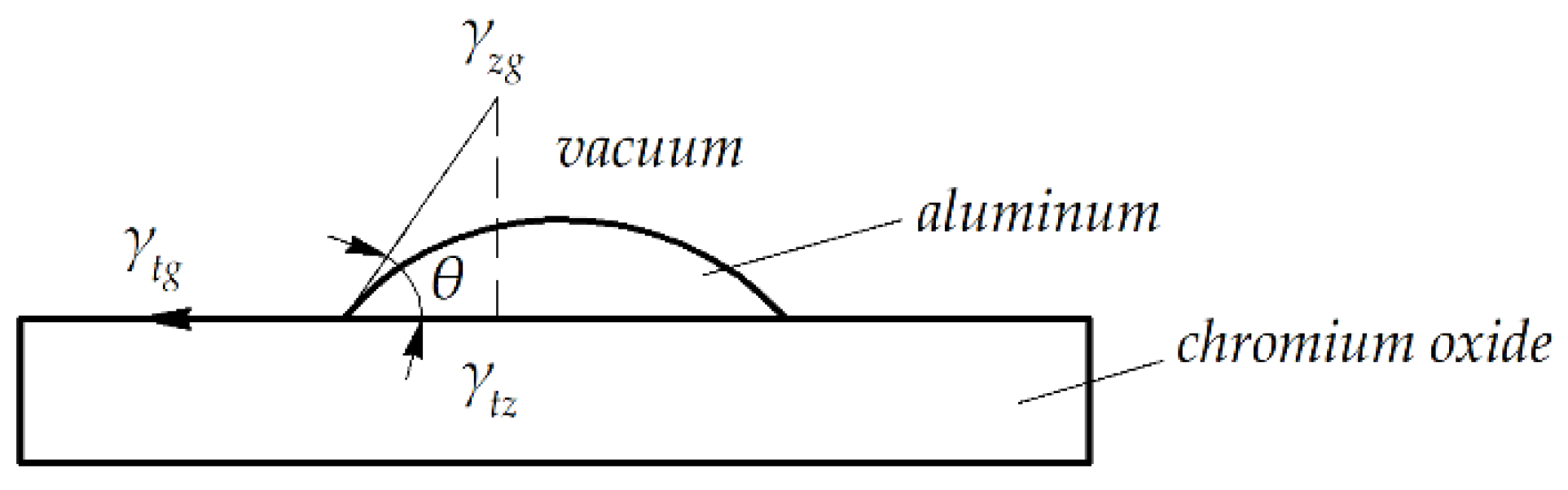
| System | T, K | Adhesion Work Wa and Its Components, MJ/m2 | θ, ° | ||||
|---|---|---|---|---|---|---|---|
| Wch | Wb | Calculated | Experimental | Calculated | Experimental | ||
| Al2O3—Al | 1500 | 510 | 243 | 753 | 1260 ± 50 | 98 | 60 |
| Al2O3—Cr | 2173 | 0 | 285 | 285 | 2260 ± 50 | 145 | 65 |
| Cr2O3—Al | 1573 | 730 | 235 | 955 | 1400 ± 50 | 118 | 60 |
| Cr2O3—Cr | 1573 | 605 | 260 | 865 | 1360 ± 50 | 120 | 65 |
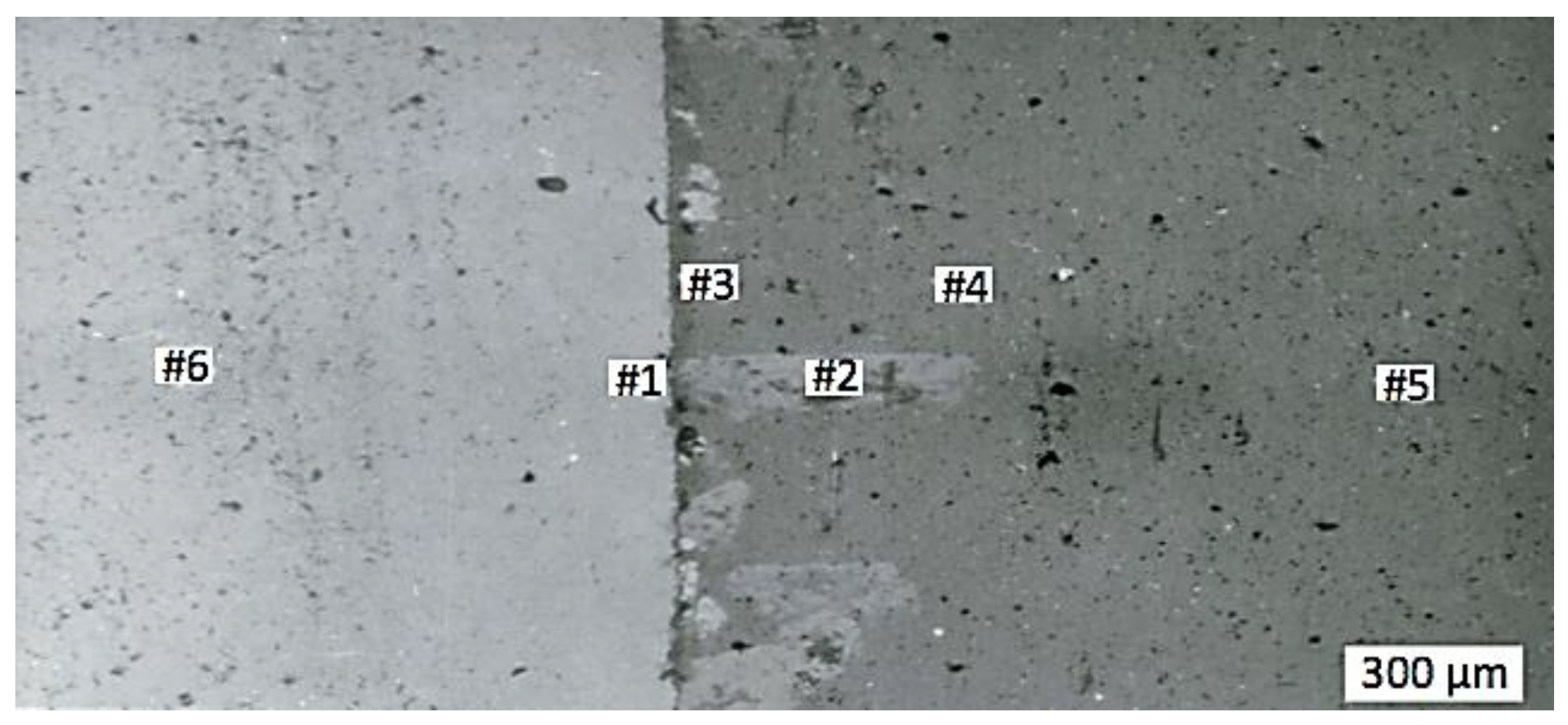
| Content, wt.% | |||||
|---|---|---|---|---|---|
| #1 | #2 | #3 | #4 | #5 | #6 |
| Cr/Al | Cr/Al | Cr/Al | Cr/Al | Cr/Al | Cr/Al |
| 99.161/0.423 | 45.946/53.706 | 13.707/85.856 | 7.197/92.475 | 6.027/93.761 | 98.917/0.339 |
| Content, wt.% | |||||
|---|---|---|---|---|---|
| #6 | #5 | ||||
| Cr | Al | O | Cr | Al | O |
| 2.940 | 95.776 | 1.329 | 96.195 | 0.186 | 3.414 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gevorkyan, E.; Cepova, L.; Rucki, M.; Nerubatskyi, V.; Morozow, D.; Zurowski, W.; Barsamyan, V.; Kouril, K. Activated Sintering of Cr2O3-Based Composites by Hot Pressing. Materials 2022, 15, 5960. https://doi.org/10.3390/ma15175960
Gevorkyan E, Cepova L, Rucki M, Nerubatskyi V, Morozow D, Zurowski W, Barsamyan V, Kouril K. Activated Sintering of Cr2O3-Based Composites by Hot Pressing. Materials. 2022; 15(17):5960. https://doi.org/10.3390/ma15175960
Chicago/Turabian StyleGevorkyan, Edwin, Lenka Cepova, Mirosław Rucki, Volodymyr Nerubatskyi, Dmitrij Morozow, Wojciech Zurowski, Voskan Barsamyan, and Karel Kouril. 2022. "Activated Sintering of Cr2O3-Based Composites by Hot Pressing" Materials 15, no. 17: 5960. https://doi.org/10.3390/ma15175960
APA StyleGevorkyan, E., Cepova, L., Rucki, M., Nerubatskyi, V., Morozow, D., Zurowski, W., Barsamyan, V., & Kouril, K. (2022). Activated Sintering of Cr2O3-Based Composites by Hot Pressing. Materials, 15(17), 5960. https://doi.org/10.3390/ma15175960










