One-Stage Hydrothermal Growth and Characterization of Epitaxial LaMnO3 Films on SrTiO3 Substrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Bulk LaMnO3 Powders
2.2. Preparation of LaMnO3 thin Films on SrTiO3 Substrate
2.3. Material Characterization
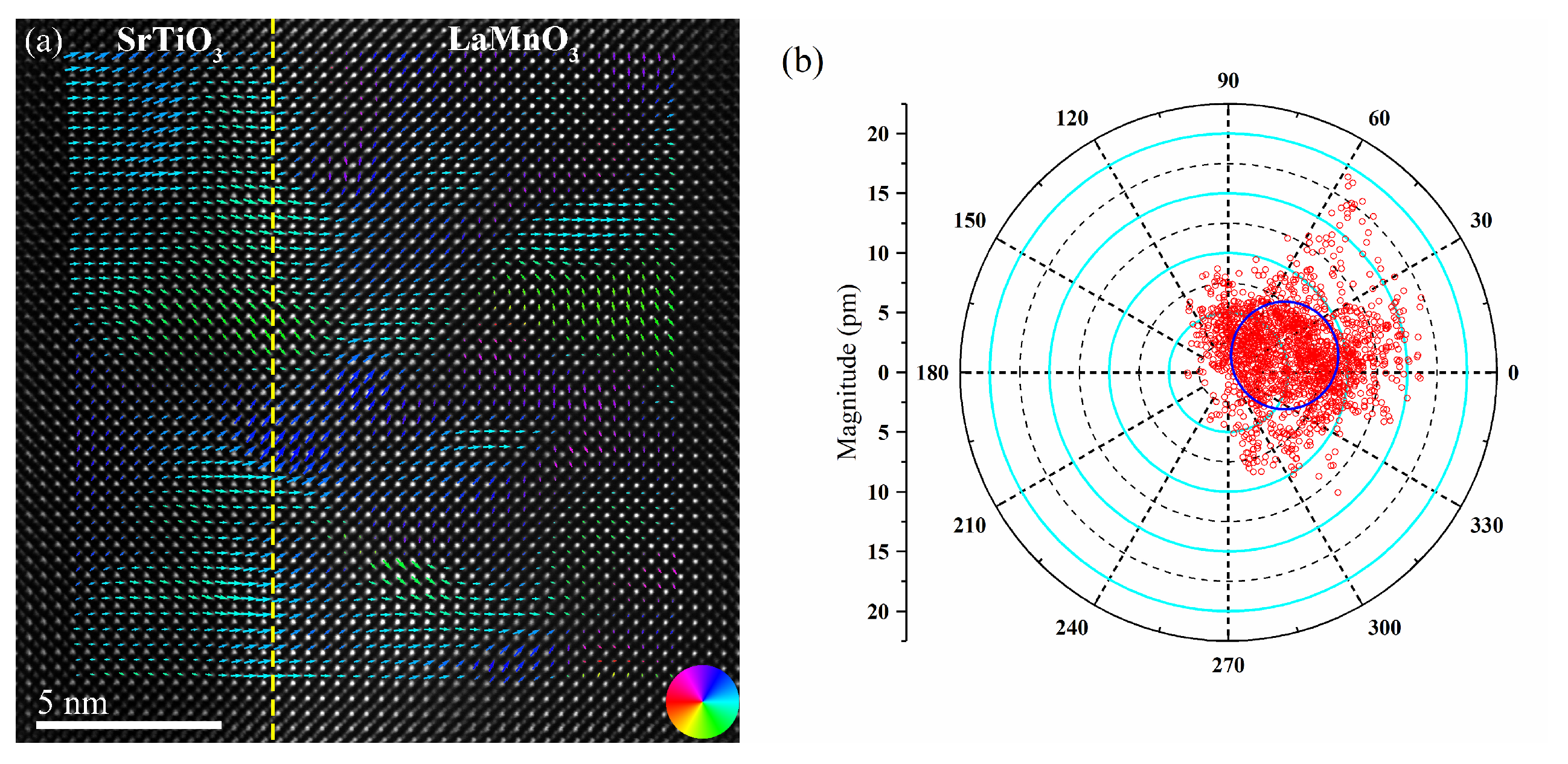
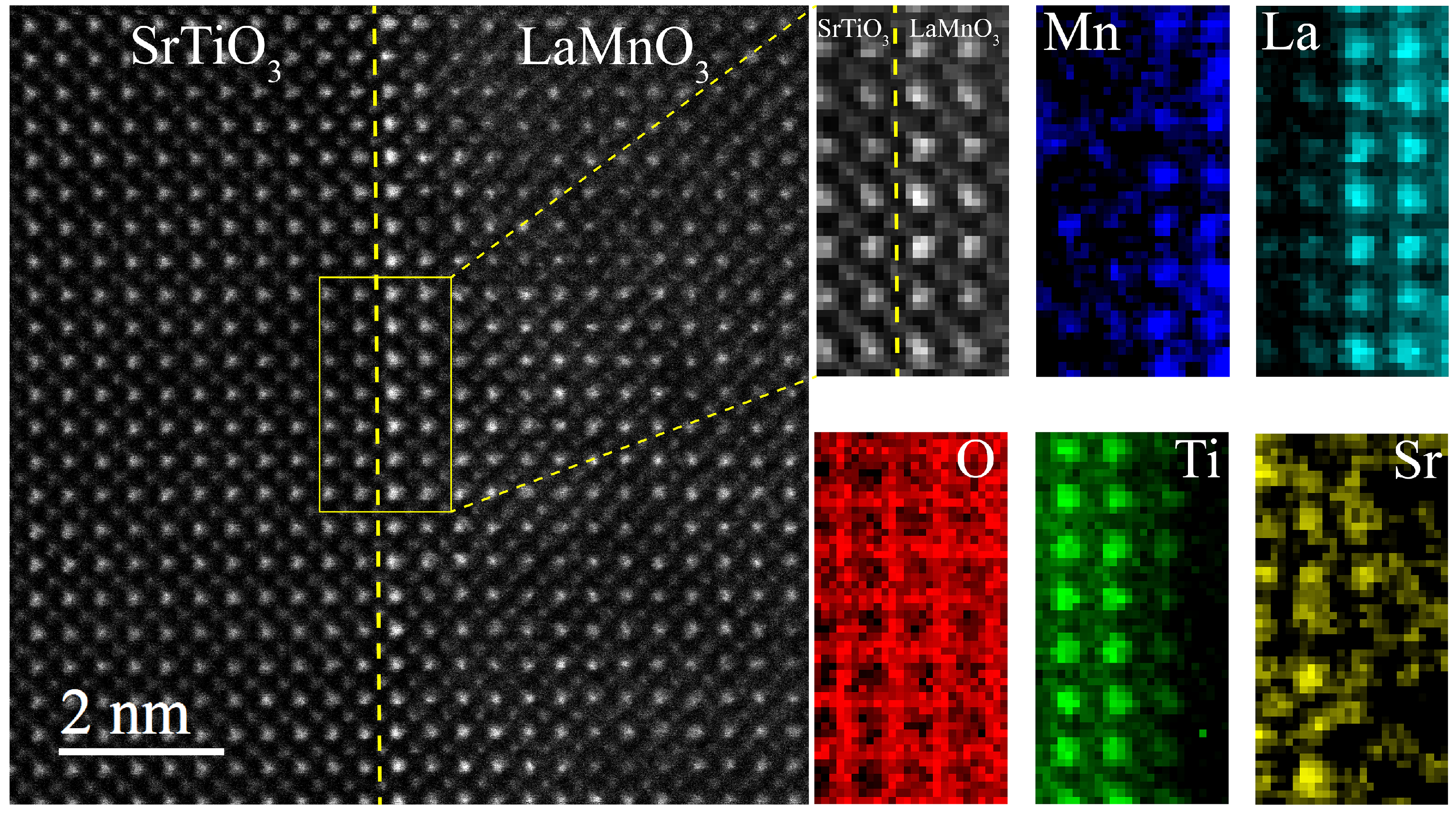
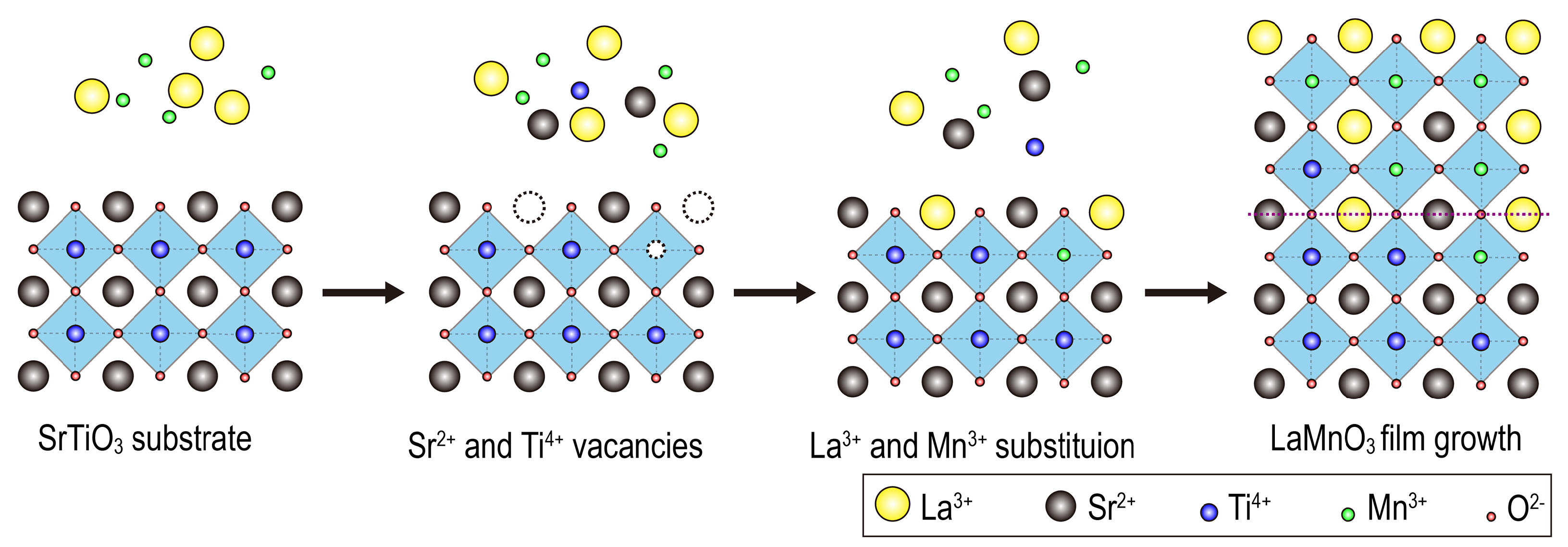
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Carda, M.; Budáč, D.; Paidar, M.; Bouzek, K. Current trends in the description of lanthanum strontium manganite oxygen electrode reaction mechanism in a high-temperature solid oxide cell. Curr. Opin. Electrochem. 2022, 31, 100852. [Google Scholar] [CrossRef]
- Park, B.-K.; Song, R.-H.; Lee, S.-B.; Lim, T.-H.; Park, S.-J.; Jung, W.; Lee, J.-W. Conformal bi-layered perovskite/spinel coating on a metallic wire network for solid oxide fuel cells via an electrodeposition-based route. J. Power Sources 2017, 348, 40–47. [Google Scholar] [CrossRef]
- Shimada, H.; Yamaguchi, T.; Sumi, H.; Nomura, K.; Yamaguchi, Y.; Fujishiro, Y. Extremely fine structured cathode for solid oxide fuel cells using Sr-doped LaMnO3 and Y2O3-stabilized ZrO2 nano-composite powder synthesized by spray pyrolysis. J. Power Sources 2017, 341, 280–284. [Google Scholar] [CrossRef]
- Mo, H.; Nan, H.; Lang, X.; Liu, S.; Qiao, L.; Hu, X.; Tian, H. Influence of calcium doping on performance of LaMnO3 supercapacitors. Ceram. Int. 2018, 44, 9733–9741. [Google Scholar] [CrossRef]
- Hu, Q.; Yue, B.; Yang, F.; Shao, H.; Bao, M.; Wang, Y.; Liu, J. Electrochemical and magnetic properties of perovskite type RMnO3 (R = La, Nd, Sm, Eu) nanofibers. J. Alloys Compd. 2021, 872, 159727. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, W.; Li, X.; Zhu, Z.; Wang, Q.; Luo, S.; Xie, A. Honeycomb LaMnO3 Perovskite Synthesized by a Carbon Sphere as a Self-Sacrificing Template for Supercapacitors. Energy Fuels 2021, 35, 13457–13465. [Google Scholar] [CrossRef]
- Spinicci, R.; Faticanti, M.; Marini, P.; De Rossi, S.; Porta, P. Catalytic activity of LaMnO3 and LaCoO3 perovskites towards VOCs combustion. J. Mol. Catal. A Chem. 2003, 197, 147–155. [Google Scholar] [CrossRef]
- Musialik-Piotrowska, A.; Landmesser, H. Noble metal-doped perovskites for the oxidation of organic air pollutants. Catal. Today 2008, 137, 357–361. [Google Scholar] [CrossRef]
- Shaterian, M.; Enhessari, M.; Rabbani, D.; Asghari, M.; Salavati-Niasari, M. Synthesis, characterization and photocatalytic activity of LaMnO3 nanoparticles. Appl. Surf. Sci. 2014, 318, 213–217. [Google Scholar] [CrossRef]
- Cossu, F.; Jilili, J.; Schwingenschlögl, U. 2D Electron Gas with 100% Spin-Polarization in the (LaMnO3)2/(SrTiO3)2 Superlattice under Uniaxial Strain. Adv. Mater. Interfaces 2014, 1, 1400057. [Google Scholar] [CrossRef]
- Tokura, Y.; Tomioka, Y. Colossal magnetoresistive manganites. J. Magn. Magn. Mater. 1999, 200, 1–23. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Tao, Y.J.; Jiao, P.J.; Wang, J.; Zhang, J.; Luo, Z.L.; Gu, Z.B.; Zhou, J.; Chen, Y.B.; Zhang, S.T. Robust ferromagnetic insulating and large exchange bias in LaMnO3:CoO composite thin films. J. Phys. D Appl. Phys. 2022, 55, 195003. [Google Scholar] [CrossRef]
- Chen, Y.F.; Wang, G.M.; Sun, Z.F.; Wang, S.G.; Mao, Y.W.; Deng, Q.R.; Yang, J.J. Magnetic characteristics of LaMnO3+δ thin films deposited by RF magnetron sputtering in an O2/Ar mixture gas. Mater. Res. Express 2021, 8, 016101. [Google Scholar]
- Niu, W.; Liu, W.Q.; Gu, M.; Chen, Y.D.; Zhang, X.Q.; Zhang, M.H.; Chen, Y.Q.; Wang, J.; Du, J.; Song, F.Q.; et al. Direct Demonstration of the Emergent Magnetism Resulting from the Multivalence Mn in a LaMnO3 Epitaxial Thin Film System. Adv. Electron. Mater. 2018, 4, 1800055. [Google Scholar] [CrossRef]
- Hibble, S.J.; Cooper, S.P.; Hannon, A.C.; Fawcett, I.D.; Greenblatt, M. Local distortions in the colossal magnetoresistive manganates La0.70Ca0.30MnO3, La0.80Ca0.20MnO3 and La0.70Sr0.30MnO3 revealed by total neutron diffraction. J. Phys. Condens. Mat. 1999, 11, 9221–9238. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Zhang, X.H.; Wappling, R. Crystal structure of La1-xSrxMnO3-2x+deltaF2x. J. Alloys Compd. 2000, 306, 133–140. [Google Scholar] [CrossRef]
- Wollan, E.O.; Koehler, W.C. Neutron Diffraction Study of the Magnetic Properties of the Series of Perovskite-Type Compounds [(1-x)La,xCa]MnO3. Phys. Rev. 1955, 100, 545–563. [Google Scholar] [CrossRef]
- Zener, C. Interaction between the d-Shells in the Transition Metals. II. Ferromagnetic Compounds of Manganese with Perovskite Structure. Phys. Rev. 1951, 82, 403–405. [Google Scholar] [CrossRef]
- Hoffman, J.; Tung, I.C.; Nelson-Cheeseman, B.B.; Liu, M.; Freeland, J.W.; Bhattacharya, A. Charge transfer and interfacial magnetism in (LaNiO3) n/(LaMnO3)2 superlattices. Phys. Rev. B 2013, 88, 144411. [Google Scholar] [CrossRef]
- Zhai, X.F.; Mohapatra, C.S.; Shah, A.B.; Zuo, J.M.; Eckstein, J.N. Magnetic properties of the (LaMnO3)N/(SrTiO3)Natomic layer superlattices. J. Appl. Phys. 2013, 113, 173913. [Google Scholar] [CrossRef]
- Marton, Z.; Seo, S.S.A.; Egami, T.; Lee, H.N. Growth control of stoichiometry in LaMnO3 epitaxial thin films by pulsed laser deposition. J. Cryst. Growth 2010, 312, 2923–2927. [Google Scholar] [CrossRef]
- Ogugua, S.N.; Ntwaeaborwa, O.M.; Swart, H.C. Latest Development on Pulsed Laser Deposited Thin Films for Advanced Luminescence Applications. Coatings 2020, 10, 1078. [Google Scholar] [CrossRef]
- Li, T.; Ma, D.; Li, K.; Hu, Z. Self-biased magnetoelectric coupling effect in the layered La0.7Sr0.3MnO3/BaTiO3/La0.7Sr0.3MnO3 multiferroic heterostructure. J. Alloy. Compd. 2018, 747, 558–562. [Google Scholar] [CrossRef]
- Yu, T.; Chen, P.; Zhou, L.; Ning, X.; Deng, B.; Shi, X.; He, H. Cluster glass induced unconventional exchange bias in epitaxial LaMnO3/SrMnO3 bilayers. Mater. Lett. 2019, 242, 95–98. [Google Scholar] [CrossRef]
- Panjan, P.; Drnovsek, A.; Gselman, P.; Cekada, M.; Panjan, M. Review of Growth Defects in Thin Films Prepared by PVD Techniques. Coatings 2020, 10, 447. [Google Scholar] [CrossRef]
- Dunnill, C.W.; Kafizas, A.; Parkin, I.P. CVD Production of Doped Titanium Dioxide Thin Films. Chem. Vapor Depos. 2012, 18, 89–101. [Google Scholar] [CrossRef]
- Saha, I.; Tomohiro, Y.; Kanazawa, K.; Nitani, H.; Kuroda, S. Structural and Magnetic Properties of Nitrogen Acceptor Co-doped (Zn,Fe)Te Thin Films Grown in Zn-Rich Condition by Molecular Beam Epitaxy (MBE). J. Electron. Mater. 2020, 49, 5739–5749. [Google Scholar] [CrossRef]
- Wang, T.; Thoutam, L.R.; Prakash, A.; Nunn, W.; Haugstad, G.; Jalan, B. Defect-driven localization crossovers in MBE-grown La-doped SrSnO3 films. Phys. Rev. Mater. 2017, 1, 06160. [Google Scholar] [CrossRef]
- Morita, T. Piezoelectric Materials Synthesized by the Hydrothermal Method and Their Applications. Materials 2010, 3, 5236. [Google Scholar] [CrossRef]
- Ngida, R.E.A.; Zawrah, M.F.; Khattab, R.M.; Heikal, E. Hydrothermal synthesis, sintering and characterization of nano La-manganite perovskite doped with Ca or Sr. Ceram. Int. 2019, 45, 4894–4901. [Google Scholar] [CrossRef]
- Morita, T.; Cho, Y. Epitaxial PbTiO3 thin films on SrTiO3(100) and SrRuO3/SrTiO3(100) substrates deposited by a hydrothermal method. Jpn. J. Appl. Phys. 2004, 43, 6535–6538. [Google Scholar] [CrossRef]
- Rout, D.; Han, S.H.; Moon, K.S.; Kim, H.G.; Cheon, C.I.; Kang, S.J.L. Low temperature hydrothermal epitaxy and Raman study of heteroepitaxial BiFeO3 film. Appl. Phys. Lett. 2009, 95, 122509. [Google Scholar] [CrossRef]
- Huang, A.; Handoko, A.D.; Goh, G.K.L.; Shannigrahi, S.R.; Tan, C.K. Hydrothermal epitaxy of BiFeO3 films on SrTiO3 substrates. Prog. Cryst. Growth Charact. Mater. 2011, 57, 109–116. [Google Scholar] [CrossRef]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Mahendiran, R.; Mahesh, R.; Raychaudhuri, A.K.; Rao, C.N.R. Giant magnetoresistance in bulk samples of LaMnO3 with varying Mn4+ content. PRAMANA-J. Phys. 1995, 44, 393–396. [Google Scholar] [CrossRef]
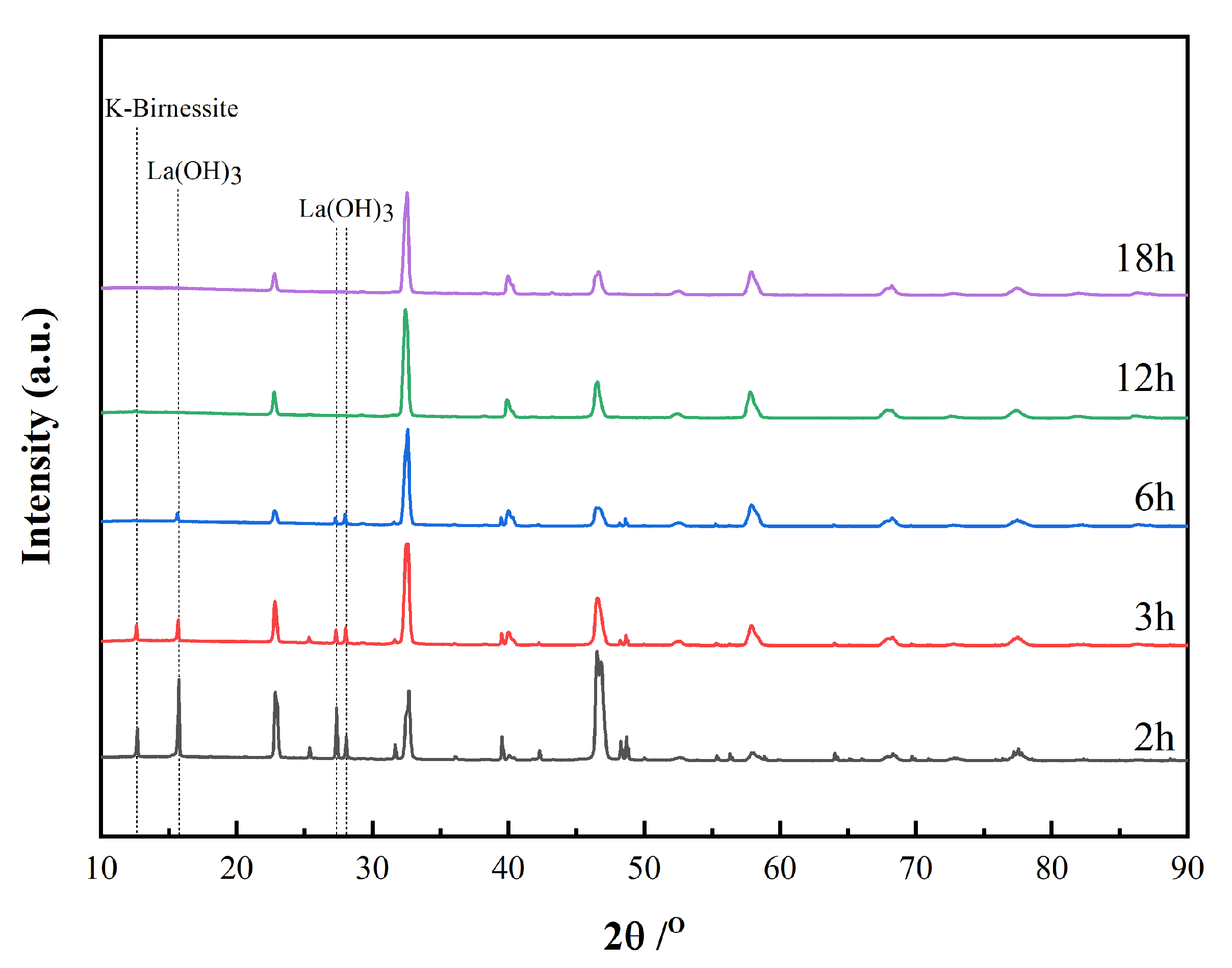
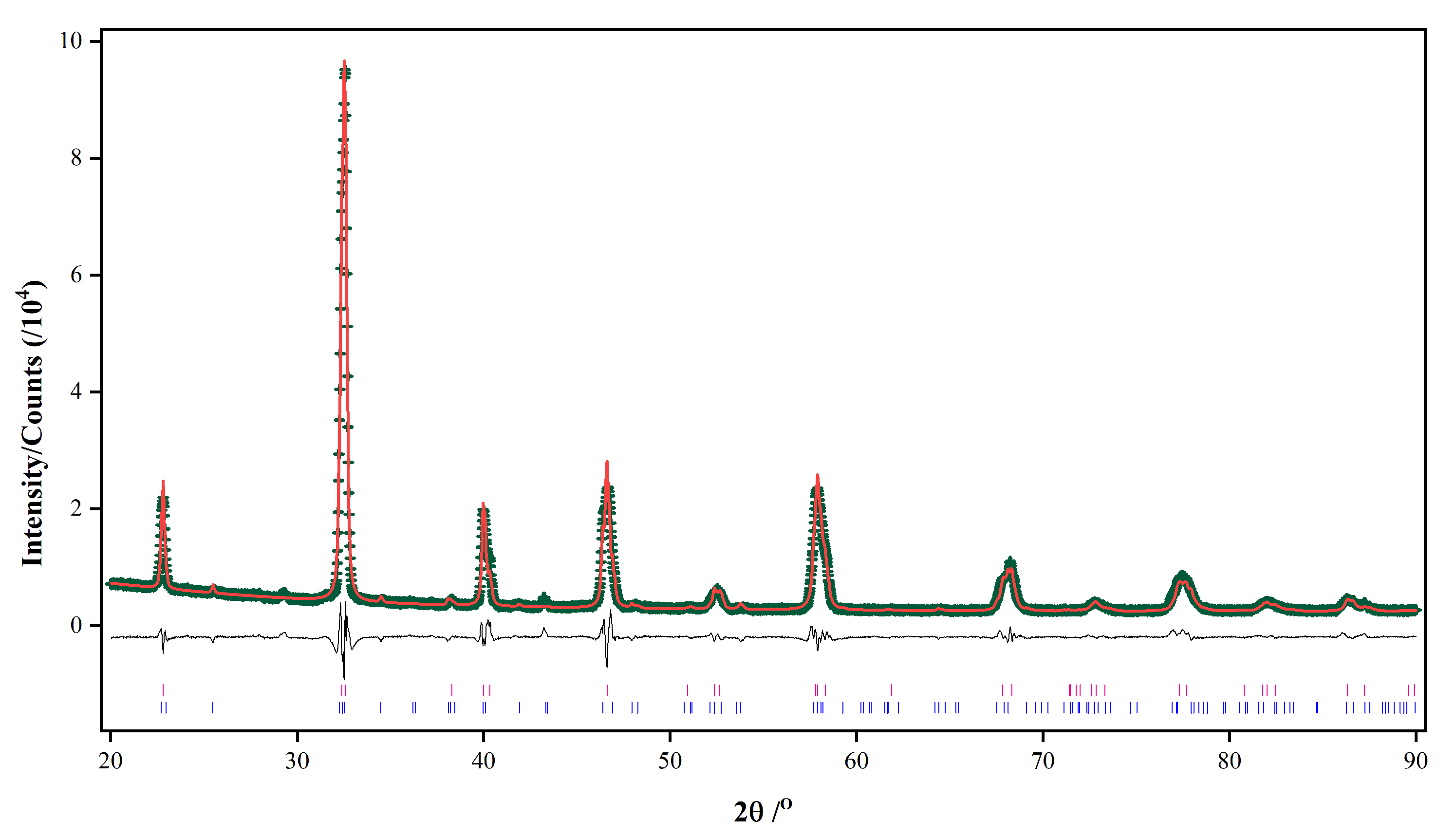

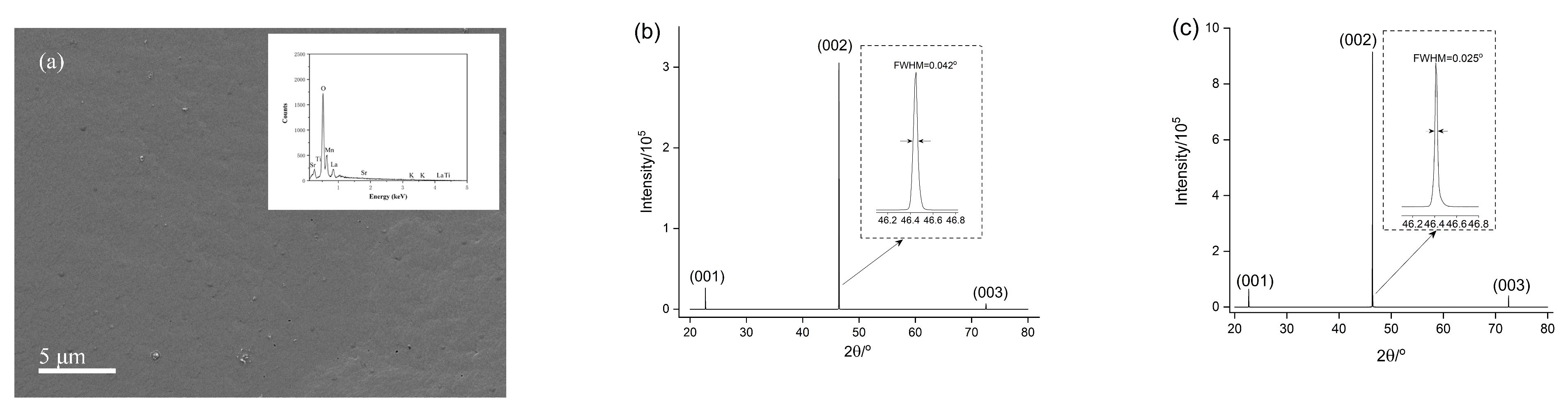

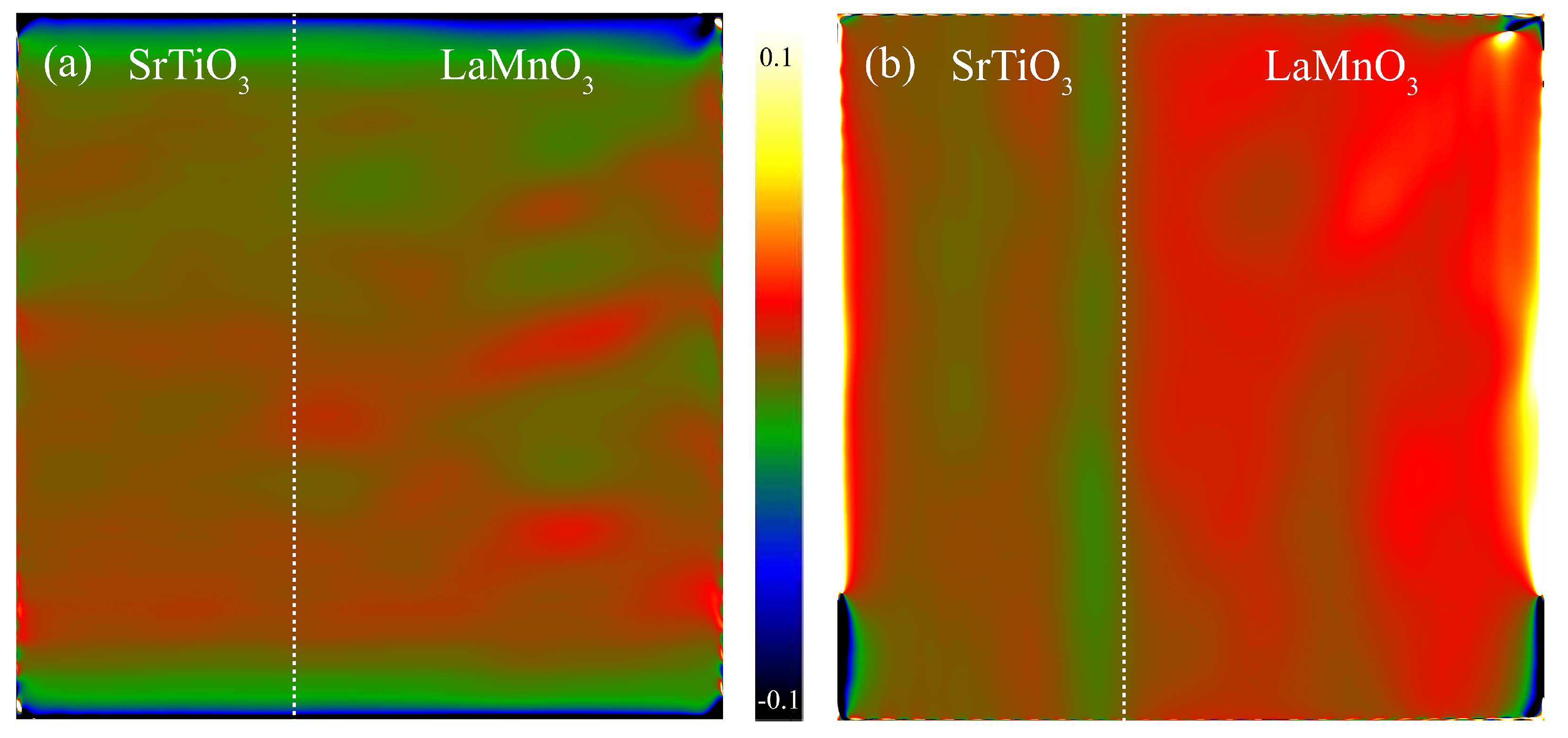
| Time | Trigonal LaMnO3/% | Orthorhombic LaMnO3/% | K-Rich Birnessite/% | La(OH)3/% | Trigonal/ Orthorhombic |
|---|---|---|---|---|---|
| 3 h | 62.5 | 23.9 | 7.1 | 6.4 | 2.61 |
| 6 h | 61.4 | 33.1 | - | 5.5 | 1.85 |
| 12 h | 63.7 | 36.3 | - | - | 1.75 |
| 18 h | 61.7 | 38.3 | - | - | 1.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, K.; Tao, Y.; Liu, Y.; Lyu, Y.; Pan, Z. One-Stage Hydrothermal Growth and Characterization of Epitaxial LaMnO3 Films on SrTiO3 Substrate. Materials 2022, 15, 5928. https://doi.org/10.3390/ma15175928
Guo K, Tao Y, Liu Y, Lyu Y, Pan Z. One-Stage Hydrothermal Growth and Characterization of Epitaxial LaMnO3 Films on SrTiO3 Substrate. Materials. 2022; 15(17):5928. https://doi.org/10.3390/ma15175928
Chicago/Turabian StyleGuo, Keyu, Yaqiu Tao, Yunfei Liu, Yinong Lyu, and Zhigang Pan. 2022. "One-Stage Hydrothermal Growth and Characterization of Epitaxial LaMnO3 Films on SrTiO3 Substrate" Materials 15, no. 17: 5928. https://doi.org/10.3390/ma15175928
APA StyleGuo, K., Tao, Y., Liu, Y., Lyu, Y., & Pan, Z. (2022). One-Stage Hydrothermal Growth and Characterization of Epitaxial LaMnO3 Films on SrTiO3 Substrate. Materials, 15(17), 5928. https://doi.org/10.3390/ma15175928






