Magnesium for Implants: A Review on the Effect of Alloying Elements on Biocompatibility and Properties
Abstract
1. Introduction
2. Requirements for Bio Application of Mg Alloys and Their Corrosion Strengths
3. Biotoxicity of Common Alloying Elements
3.1. Toxicology and Pathophysiology
3.1.1. Magnesium (Mg)
3.1.2. Iron (Fe)
3.1.3. Calcium (Ca)
3.1.4. Zinc (Zn)
3.1.5. Copper (Cu)
3.1.6. Silicon (Si)
3.1.7. Tin (Sn)
3.1.8. Manganese (Mn)
3.1.9. Aluminum (Al)
3.1.10. Lithium (Li)
3.1.11. Nickel (Ni)
3.1.12. Indium (In)
3.2. In Vitro Biotoxicity
3.3. In Vivo Biotoxicity
3.3.1. Clinical Trials: Animal Tests
3.3.2. Clinical Trials: Human Tests
| Alloy | Composition | Processing History | Comments on the Results | Animal | Location | Duration | Type of Implant | Implant Dimensions | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| ZEK100 | Mg-0.96 wt.% Zn-0.21 wt.% Zr-0.3 wt.% RE | Gravity die casting followed by direct extrusion. | ZEK100 did not show good biocompatibility. Pathological effects on the host tissue during complete degradation. This comes in spite of the favorable initial degradation and biocompatibility results. | Rabbit | Intramedullary tibia | 9 months, 12 months | Cylindrical implants | 2.5 mm dia, 25 mm length | [76] |
| ZX50 | Mg-5 wt.% Zn-0.25 wt.% Ca-0.15 wt.% Mn | Direct Chill Casted (DCC) followed by hot extrusion | Good tolerance is observed. | Rat (Sprague-Dawley) | Femoral bone | 24 weeks and 36 weeks 25 weeks and 36 weeks | Cylindrical pins | 1.6 mm dia, 8 mm length | [30] |
| WZ21 | Mg-1 wt.% Zn-2 wt.% Y-0.25 wt.% Ca-0.15 wt.% Mn | Direct Chill Casted (DCC) followed by hot extrusion | WZ21 encourages bone formation and gives evidence of osteoinductivity and osteoconductivity around magnesium. | ||||||
| LAE442 | Mg-3.7 wt.% Li-3.62 wt.% Al-0.73 wt.% Ce-0.38 wt.% La-0.16 wt.% Nd-0.03 wt.% Pr | Die casting followed by hot extrusion | Moderate gas formation and inflammatory reaction observed. Clinical tolerance deemed slightly lower than the austenitic stainless steel used as reference. Good regulation of the Mg levels by the body is observed, but Al and RE detected in the kidney, liver, and spleen. Conclusion: Prospective suitability of the alloy, although study was inconclusive on the final biocompatibility of the alloy. | Sheep | Right tibia | 24 weeks | Intramedullary Interlocked Nailing system (nails/screws) | 9 mm/3.5 mm dia, 130 mm/15–40 mm length | [3] |
| HP Mg | 99.99 wt.% Mg | Cast, hot extruded, Rolled and Heat Treated | Good osseointegration compared to commercial PLLA screw, resulting in fracture healing 8 weeks after operation with increased bone density and mineralization. | Rabbit | Left femoral condyle | 24 weeks | Screws | Major dia: 2.7 mm, Core dia: 2.1 mm, length: 27 mm, Pitch: 1 mm | [4] |
| Mg0.8Ca | Mg0.8 wt.% Ca | Machined from Extruded bar stock | Well-tolerated generally, although showed signs of slight reddening near the wound, which disappeared by 14 days after implantation. Mild to moderate amounts of gas accumulation was observed throughout the 8-week period. | Rabbit | Lateral cortex of tibia (both legs) | 2,4,6,8 weeks | Screws | Major dia: 4 mm, length: 6 mm. Thread length: 5 mm, Core dia: 3 mm, Pitch: 1 mm. | [77] |
| LAE442 | Mg-4.26 wt.% Li-3.30 Al-1.03 Ce-0.46 La-0.27 Nd-0.09 Pr | Cast and Extruded. | Clinically acceptable. No signs of deformity leading to lameness, swelling, pain, or gas formation was observed. Mg was well-degraded by 99.76%, but even after 3.5 years, the RE was not regulated or excreted out of the body, and Al, while present, had a decreased presence although Li was not detected. | Rabbit (New Zealand White Rabbits) | Intramedullary cavity of tibia | 9 months, 3.5 years | Cylindrical pins | Dia: 2.5 mm, Length: 25 mm. | [78,79] |
| AZ31 | Mg-2.5–3.5 wt.% Al-0.6–1.4 wt.% Zn-0.2–1.0 wt.% Mn | Commercial bought and hot extruded. | ZJ41 > WKX41 > AZ31 in terms of both degradation rates and volume of H2 evolution. Histological study showed no significant toxic effects on kidney, spleen, liver, lung, intestine, skin, skull, heart, and brain within the period. | Athymic Nude Mouse | Subcutaneous pocket on the back. | 1 month | Disc | 5 mm dia, 1.4 mm thickness | [80] |
| ZJ41 | Mg-4 wt.% Zn-1 wt.% Sr-0.5 wt.% Zr | Cast and hot extruded. | |||||||
| WKX41 | Mg-4 wt.% Y-1 wt.% Zr-0.6 wt.% Ca | ||||||||
| JDBM | Mg-2.1 Nd-0.21 Zn-0.5 Zr (0.009Mn-0.006Si-0.005Cu-0.002Fe as impurities) | Alloy billet is machined, extruded, rolled, annealed, drawn and annealed | Study confirmed the safe metabolization of Mg and Zn. No sign of continuous accumulation of Nd and Zr in the organs (brain, lung, heart, liver, spleen, and kidney), although after the 1-month period, a sharp increase was detected in the liver and spleen. Lower aggregation of inflammatory cells compared to 316 L SS stent after 14 days. Endothelial cell recovery completed by 28 days. Ca concentration and degradation products decreased overtime without calcification of the vessel. | Rabbit (New Zealand White Rabbits) | Common carotid artery | 1,4,12 months. 20 months | Stent | 3 mm dia, 16 mm length, Stent strut thickness: 150 μm | [70,71] |
| Mg-Zn-Sr | Mg-6 wt.% Zn-0.5 wt.%Sr | Mold cast, Solution Treated and hot extruded | Increased peri-tunnel bone mass 16 weeks after ACL reconstruction surgery. Release of metal ions during degradation helps to heal. While the release of gases was expected to cause voids, no such large accumulation of gases was observed, which has been attributed to the excretion of the gas to local tissue via diffusion owing to the buffering role played by the knee-joint space. | Rabbit (New Zealand White Rabbits)—Male | ACL (Femur and tibia) | 16 weeks | Hollow interference screw | 3 mm outer dia, 8 mm length | [81] |
| AZ91 | Mg-9 wt.% Al-0.9 wt.% Zn-0.1 wt.% Si-0.2 wt.% Mn-0.002 wt.% Fe-0.0005 wt.% Ni | Extruded, T6 heat treated | The good in vitro antimicrobial property is not found in vivo, tested against A. baumanii | Long Evans Rats (male) | Humeral head | 7 days | Rods | 1.6 mm dia, 16 mm length | [82] |
| WE43 | Mg-4Y-3RE-Zr | Cast ingot hot extruded and machined. | No allergic or systemic reactions or complications during healing were observed. However, near the implant, foreign body reactions were observed. | Rabbit (New Zealand White Rabbits)—Male | Right tibia | 16 weeks (4-week intervals) | Screws (w/pads) | Screw head dia: 3 mm, thread dia: 1.5 mm, core dia: 1.1 mm, length: 3 mm, pad thickness: 1 mm. | [83] |
| Mg-Ag-Y | Mg-0.95 wt.% Ag-0.92 wt.% Y | Cast, Homogenized, Hot extruded, Wire Drawn w/annealing after every two passes. | No abnormal effects detected after 6 weeks in liver, heart, and lungs. More than double the bone volume compared with PMg was detected. | Rat (Sprague-Dawley) | Distal femoral metaphysis (perpendicular to axis) | 6 weeks | Rods (Intramedullary Nails) | 1 mm dia, | [61] |
4. Biodegradation
4.1. Different Aspects of Biocorrosion Tests
4.1.1. Electrochemical Measurements
4.1.2. Potentiodynamic Polarization (PDP)
4.1.3. Immersion Tests
4.1.4. Hydrogen Evolution Rates Measurement
4.1.5. Modes of Corrosion
4.1.6. Effect of pH
5. Effect of Individual Alloying Element on Mg Alloy Biodegradation
5.1. Magnesium
5.2. Iron
5.3. Calcium
5.4. Zinc
5.5. Copper
5.6. Silicon
5.7. Tin
5.8. Strontium
5.9. Manganese
5.10. Aluminum
5.11. Bismuth
5.12. Scandium
5.13. Gallium
6. Microstructure and Mechanical Properties Mg Alloys
6.1. Effect of the Alloying Elements
6.1.1. Mg
6.1.2. Zn
6.1.3. Ca
6.1.4. Cu
6.1.5. Si
6.1.6. Mn
6.1.7. Sn
6.1.8. Al
6.1.9. Sr
6.1.10. Zr
6.1.11. Bi
6.1.12. Sc
6.2. Effect of Processing
6.2.1. Liquid Metallurgy
6.2.2. Powder Metallurgy
6.2.3. Extrusion and Rolling
6.2.4. Equal Channel Angular Pressing/Extrusion (ECAP/ECAE)
6.3. Effect of Post-Processing Treatment
6.3.1. Effect of Heat Treatment
6.3.2. Surface Modifications
Surface Coating
Ion Implantation and Stress Impartation Methods
7. Effect of Implant Geometry on the Biodegradable Characteristics
8. Prospects of Some of the Commercially Popular Mg Alloys
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Witte, F. Reprint of: The history of biodegradable magnesium implants: A review. Acta Biomater. 2015, 23, S28–S40. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Gao, M.; Tan, L.; Ma, Z.; Ni, P.; Zhou, M.; Na, D. Application Potential of Mg–Zn–Nd Alloy as a Gas-trointestinal Anastomosis Nail Material. In Acta Metallurgica Sinica (English Letters); Springer: Berlin/Heidelberg, Germany, 2022; Volume 35, pp. 609–620. [Google Scholar]
- Chen, Y.T.; Hung, F.Y.; Lin, Y.L.; Lin, C.Y. Biodegradation ZK50 magnesium alloy compression screws: Mechanical properties, biodegradable characteristics and implant test. J. Ortho. Sci. 2020, 25, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Ghiotti, A.; Bruschi, S.; Bertolini, R.; Perzynski, K.; Madej, L. Forming of bioabsorbable clips using magnesium alloy strips with enhanced characteristics. CIRP Ann. 2020, 69, 257–260. [Google Scholar] [CrossRef]
- Rizvi, S.H.A.; Che, J.; Mehboob, A.; Zaheer, U.; Chang, S.-H. Experimental study on magnesium wire–polylactic acid biodegradable composite implants under in vitro material degradation and fatigue loading conditions. Compos. Struct. 2021, 272, 114267. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Shen, Z.; Miao, F.; Wang, J.; Sun, Y.; Zhu, S.; Zheng, Y.; Guan, S. A biodegradable magnesium alloy vascular stent structure: Design, optimisation and evaluation. Acta Biomater. 2022, 142, 402–412. [Google Scholar] [CrossRef]
- Callister, W.D.; Rethwisch, D.G. Materials Science and Engineering: An. Introduction, 7th ed.; Wiley: New York, NY, USA, 2007. [Google Scholar]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef]
- Nakahata, I.; Tsutsumi, Y.; Kobayashi, E. Mechanical Properties and Corrosion Resistance of Magnesium–Hydroxyapatite Composites Fabricated by Spark Plasma Sintering. Metals 2020, 10, 1314. [Google Scholar] [CrossRef]
- Durisin, M.; Reifenrath, J.; Weber, C.M.; Eifler, R.; Maier, H.J.; Lenarz, T.; Seitz, J.M. Biodegradable nasal stents (MgF2-coated Mg–2 wt %Nd alloy)—A long-term in vivo study. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2017, 105, 350–365. [Google Scholar] [CrossRef]
- Brown, A.; Zaky, S.; Ray, H.; Sfeir, C. Porous magnesium/PLGA composite scaffolds for enhanced bone regeneration following tooth extraction. Acta Biomater. 2015, 11, 543–553. [Google Scholar] [CrossRef]
- He, H.; Ren, H.; Ding, Z.; Ji, M.; Chen, H.; Yan, Y. Developing a novel magnesium calcium phosphate/sodium alginate composite cement with high strength and proper self-setting time for bone repair. J. Biomater. Appl. 2021, 36, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, F.; Wang, Z.; Liu, Y.; Zhao, X.; Fang, C.; Leung, F.; Yeung, K.W.K.; Wong, T.M. The Development of.a Magnesium-Releasing and Long-Term Mechanically Stable Calcium Phosphate Bone Cement Possessing Osteogenic and Immunomodulation Effects for Promoting Bone Fracture Regeneration. Front. Bioeng. Biotechnol. 2022, 9, 803723. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, S.; Brown, A.; Barchowsky, A.; Sfeir, C. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014, 10, 2834–2842. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-H.; Lee, S.-P.; Yang, C.-W.; Lo, C.-M. Surface Modification of Biodegradable Mg-Based Scaffolds for Human Mesenchymal Stem Cell Proliferation and Osteogenic Differentiation. Materials 2021, 14, 441. [Google Scholar] [CrossRef]
- Ni, S.; Xiong, X.; Ni, X. MgCl2 promotes mouse mesenchymal stem cell osteogenic differentiation by activating the p38/Osx/Runx2 signaling pathway. Mol. Med. Rep. 2020, 22, 3904–3910. [Google Scholar] [CrossRef]
- Kanter, B.; Vikman, A.; Brückner, T.; Schamel, M.; Gbureck, U.; Ignatius, A. Bone regeneration capacity of magnesium phosphate cements in a large animal model. Acta Biomater. 2018, 69, 352–361. [Google Scholar] [CrossRef]
- Nabiyouni, M.; Ren, Y.; Bhaduri, S.B. Magnesium substitution in the structure of orthopedic nanoparticles: A comparison between amorphous magnesium phosphates, calcium magnesium phosphates, and hydroxyapatites. Mater. Sci. Eng. C 2015, 52, 11–17. [Google Scholar] [CrossRef]
- Xu, C.; Wang, S.; Wang, H.; Liu, K.; Zhang, S.; Chen, B.; Liu, H.; Tong, F.; Peng, F.; Tu, Y.; et al. Magnesium-Based Micromotors as Hydrogen Generators for Precise Rheumatoid Arthritis Therapy. Nano Lett. 2021, 21, 1982–1991. [Google Scholar] [CrossRef]
- Fanelli, A.; Ghezzi, D. Transient electronics: New opportunities for implantable neurotechnology. Curr. Opin. Biotechnol. 2021, 72, 22–28. [Google Scholar] [CrossRef]
- Yu, Y.; Jin, G.; Xue, Y.; Wang, D.; Liu, X.; Sun, J. Multifunctions of dual Zn/Mg ion co-implanted titanium on oste-ogenesis, angiogenesis and bacteria inhibition for dental implants. Acta Biomater. 2017, 49, 590–603. [Google Scholar] [CrossRef]
- Wang, G.; Li, J.; Zhang, W.; Xu, L.; Pan, H.; Wen, J.; Wu, Q.; She, W.; Jiao, T.; Liu, X.; et al. Magnesium ion implantation on a micro/nanostructured titanium surface promotes its bioactivity and osteogenic differentiation function. Int. J. Nanomed. 2014, 2387, 2387–2398. [Google Scholar]
- Wang, J.; Witte, F.; Xi, T.; Zheng, Y.; Yang, K.; Yang, Y.; Zhao, D.; Meng, J.; Li, Y.; Li, W.; et al. Recommendation for modifying current cytotoxicity testing standards for biodegradable magnesium-based materials. Acta Biomater. 2015, 21, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Pröfrock, D.; Hort, N.; Willumeit, R.; Feyerabend, F. Reprint of: Improved cytotoxicity testing of magnesium materials. Mater. Sci. Eng. B 2011, 176, 1773–1777. [Google Scholar] [CrossRef]
- Li, Z.; Shang, Z.; Wei, X.; Zhao, Q. Corrosion resistance and cytotoxicity of AZ31 magnesium alloy with N+ ion im-plantation. Mater. Technol. 2019, 34, 730–736. [Google Scholar] [CrossRef]
- Scheideler, L.; Füger, C.; Schille, C.; Rupp, F.; Wendel, H.-P.; Hort, N.; Reichel, H.; Geis-Gerstorfer, J. Comparison of different in vitro tests for biocompatibility screening of Mg alloys. Acta Biomater. 2013, 9, 8740–8745. [Google Scholar] [CrossRef]
- Liu, X.; Xi, T.; Zheng, Y. Influence of the extraction parameters on the cytotoxicity test results of Mg materials. Prog. Nat. Sci. 2014, 24, 507–515. [Google Scholar] [CrossRef]
- Jung, O.; Smeets, R.; Porchetta, D.; Kopp, A.; Ptock, C.; Müller, U.; Heiland, M.; Schwade, M.; Behr, B.; Kröger, N.; et al. Optimized in vitro procedure for assessing the cytocompatibility of magnesium-based biomaterials. Acta Biomater. 2015, 23, 354–363. [Google Scholar] [CrossRef]
- Kraus, T.; Fischerauer, S.F.; Hänzi, A.C.; Uggowitzer, P.J.; Löffler, J.F.; Weinberg, A.M. Magnesium alloys for temporary implants in osteosynthesis: In vivo studies of their degradation and interaction with bone. Acta Biomater. 2012, 8, 1230–1238. [Google Scholar] [CrossRef]
- World Health Organization. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization: Geneva, Switzerland, 2004; pp. 340–342. [Google Scholar]
- Hofmann, F.B.; Board, E.; Beavo, J.a.; Busch, a.; Ganten, D.; Michel, M.C.; Page, C.P.; Rosenthal, W. Handbook of Experimental Pharmacology: Methylxanthines; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Hashimoto, Y.; Kim, D.J.; Adams, J.C. The roles of fascins in health and disease. J. Pathol. 2011, 224, 289–300. [Google Scholar] [CrossRef]
- Barceloux, D.G.; Barceloux, D. Copper. J. Toxicol. Clin. Toxicol. 1999, 37, 217–230. [Google Scholar] [CrossRef]
- Ellingsen, D.G.; Møller, L.B.; Aaseth, J. Handbook on the Toxicology of Metals, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 1, pp. 765–786. [Google Scholar]
- Cuatrecasas, P.; Arbor, A.; Ganten, D.; Herken, H.; Melman, K.L. Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Nielsen, F.H. Importance of making dietary recommendations for elements designated as nutritionally beneficial, pharmacologically beneficial, or conditionally essential. J. Trace Elem. Exp. Med. 2000, 13, 113–129. [Google Scholar] [CrossRef]
- Pavan, C.; Delle Piane, M.; Gullo, M.; Filippi, F.; Fubini, B.; Hoet, P.; Turci, F. The puzzling issue of silica toxicity: Are silanols bridging the gaps between surface states and pathogenicity? Part. Fibre Tox. 2019, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, D. Siliconosis: A spectrum of illness. Semin. Art. Rheum. 1994, 24, 1–7. [Google Scholar] [CrossRef]
- Kossovsky, N.; Stassi, J. A pathophysiological examination of the biophysics and bioreactivity of silicone breast implants. Semin. Art. Rheum. 1994, 24, 18–21. [Google Scholar] [CrossRef]
- Onnekink, C.; Kappel, R.M.; Boelens, W.C.; Pruijn, G.J.M. Low molecular weight silicones induce cell death in cultured cells. Sci. Rep. 2020, 10, 9558. [Google Scholar] [CrossRef]
- Ivanov, S.; Zhuravsky, S.; Yukina, G.; Tomson, V.; Korolev, D.; Galagudza, M. In Vivo Toxicity of Intravenously Administered Silica and Silicon Nanoparticles. Materials 2012, 5, 1873–1889. [Google Scholar] [CrossRef]
- Ostrakhovitch, E.A. Tin. In Handbook on the Toxicology of Metals, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 2, pp. 1241–1285. [Google Scholar]
- Lucchini, R.G.; Aschner, M.; Kim, Y.; Šarić, M. Manganese. In Handbook on the Toxicology of Metals, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 1, pp. 975–1011. [Google Scholar]
- Sjögren, B.; Iregren, A.; Montelius, J.; Yokel, R.A. Aluminum, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Bernard, A. Lithium. In Handbook on the Toxicology of Metals, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 2, pp. 969–974. [Google Scholar]
- Fowler, B.A.; Maples-Reynolds, N. Indium. In Handbook on the Toxicology of Metals, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 2, pp. 845–853. [Google Scholar]
- Cesbron, A.; Saussereau, E.; Mahieu, L.; Couland, I.; Guerbet, M.; Goullé, J.-P. Metallic Profile of Whole Blood and Plasma in a Series of 106 Healthy Volunteers. J. Anal. Toxicol. 2013, 37, 401–405. [Google Scholar] [CrossRef]
- Schultze, B.; Lind, P.M.; Larsson, A.; Lind, L. Whole blood and serum concentrations of metals in a Swedish population-based sample. Scand. J. Clin. Lab. Investig. 2013, 74, 143–148. [Google Scholar] [CrossRef]
- Goullé, J.-P.; Le Roux, P.; Castanet, M.; Mahieu, L.; Guyet-Job, S.; Guerbet, M. Metallic Profile of Whole Blood and Plasma in a Series of 99 Healthy Children. J. Anal. Toxicol. 2015, 39, 707–713. [Google Scholar] [CrossRef]
- Ernst, W. Overview of naturally occurring Earth materials and human health concerns. J. Southeast Asian Earth Sci. 2012, 59, 108–126. [Google Scholar] [CrossRef]
- European Food Safety Authority, Tolerable Upper Intake Levels Scientific Committee on Food Scientific Panel on Dietetic Products, Nutrition and Allergies. February 2006. Available online: https://www.efsa.europa.eu/sites/default/files/efsa_rep/blobserver_assets/ndatolerableuil.pdf (accessed on 20 August 2021).
- Querido, W.; Rossi, A.L.; Farina, M. The effects of strontium on bone mineral: A review on current knowledge and microanalytical approaches. Micron 2016, 80, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Rim, K.T.; Koo, K.H.; Park, J.S. Toxicological Evaluations of Rare Earths and Their Health Impacts to Workers: A Literature Review. Saf. Health Work 2013, 4, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Slikkerveer, A.; de Wolff, F.A. Pharmacokinetics and Toxicity of Bismuth Compounds. Med. Toxicol. Adverse Drug Exp. 1989, 4, 303–323. [Google Scholar] [CrossRef] [PubMed]
- Holler, J.S.; Fowler, B.A.; Nordberg, F.G. Nordberg, Silver. In Handbook on the Toxicology of Metals, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 2, pp. 1209–1216. [Google Scholar]
- Ostrakhovitch, E.A.; Cherian, M.G. Tin. In Handbook on the Toxicology of Metals, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 2, pp. 839–860. [Google Scholar]
- Blaschke, C.; Volz, U. Soft and hard tissue response to zirconium dioxide dental implants: A clinical study in man. Neuro Endocrinol Lett. 2006, 27 (Suppl. S1), 69–72. [Google Scholar]
- Greiter, M.B.; Giussani, A.; Höllriegl, V.; Li, W.B.; Oeh, U. Human biokinetic data and a new compartmental model of zirconium—A tracer study with enriched stable isotopes. Sci. Total Environ. 2011, 409, 3701–3710. [Google Scholar] [CrossRef]
- Li, N.; Zheng, Y. Novel Magnesium Alloys Developed for Biomedical Application: A Review. J. Mater. Sci. Technol. 2013, 29, 489–502. [Google Scholar] [CrossRef]
- Yu, K.; Dai, Y.; Luo, Z.; Long, H.; Zeng, M.; Li, Z.; Zhu, J.; Cheng, L.; Zhang, Y.; Liu, H.; et al. In vitro and in vivo evaluation of novel biodegradable Mg-Ag-Y alloys for use as resorbable bone fixation implant. J. Biomed. Mater. Res. Part A 2018, 106, 2059–2069. [Google Scholar] [CrossRef]
- Gu, X.; Zheng, Y.; Cheng, Y.; Zhong, S.; Xi, T. Biomaterials In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials 2009, 30, 484–498. [Google Scholar] [CrossRef]
- Kubásek, J.; Vojtěch, D.; Lipov, J.; Ruml, T. Structure, mechanical properties, corrosion behavior and cytotoxicity of biodegradable Mg-X (X = Sn, Ga, In) alloys. Mater. Sci. Eng. C 2013, 33, 2421–2432. [Google Scholar] [CrossRef]
- Li, Z.; Gu, X.; Lou, S.; Zheng, Y. The development of binary Mg–Ca alloys for use as biodegradable materials within bone. Biomaterials 2008, 29, 1329–1344. [Google Scholar] [CrossRef]
- Liu, C.; Fu, X.; Pan, H.; Wan, P.; Wang, L.; Tan, L.; Wang, K. Biodegradable Mg-Cu alloys with enhanced osteogenesis, angiogenesis, and long-lasting antibacterial effects. Nat. Publ. Gr. 2016, 6, 27374. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Huang, N.; Xu, L.; Zhang, Y.; Liu, H.; Sun, H.; Leng, Y. Biocompatibility of pure iron: In vitro assessment of degradation kinetics and cytotoxicity on endothelial cells. Mater. Sci. Eng. C 2009, 29, 1589–1592. [Google Scholar] [CrossRef]
- Zhao, C.; Pan, F.; Zhao, S.; Pan, H.; Song, K.; Tang, A. Microstructure, corrosion behavior and cytotoxicity of bio-degradable Mg-Sn implant alloys prepared by sub-rapid solidification. Mater. Sci. Eng. C 2015, 54, 245–251. [Google Scholar] [CrossRef]
- Sanchez, A.H.M.; Feyerabend, F.; Laipple, D.; Willumeit, R.; Weinberg, A.; Luthringer, B. Chondrogenic differentiation of ATDC5-cells under the influence of Mg and Mg alloy degradation. Mater. Sci. Eng. C 2017, 72, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Willbold, E.; Gu, X.; Albert, D.; Kalla, K.; Bobe, K.; Brauneis, M.; Janning, C.; Nellesen, J.; Czayka, W.; Tillmann, W.; et al. Effect of the addition of low rare earth elements (lanthanum, neodymium, cerium) on the biodegradation and biocompatibility of magnesium. Acta Biomater. 2014, 11, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, H.; Wang, W.; Huang, H.; Pei, J.; Qu, H.; Yuan, G.; Li, Y. Acta Biomaterialia The degradation and transport mechanism of a Mg-Nd-Zn-Zr stent in rabbit common carotid artery: A 20-month study. Acta Biomater. 2018, 69, 372–384. [Google Scholar] [CrossRef]
- Liu, F.; Chen, C.; Niu, J.; Pei, J.; Zhang, H.; Huang, H.; Yuan, G. The processing of Mg alloy micro-tubes for biodegradable vascular stents. Mater. Sci. Eng. C 2014, 48, 400–407. [Google Scholar] [CrossRef]
- Han, P.; Cheng, P.; Zhao, C.; Zhang, S.; Zhou, R.; Zhang, X.; Chai, Y. Comparative Study about Degradation of High-purity Magnesium Screw in Intact Femoral Intracondyle and in Fixation of Femoral Intracondylar Fracture. J. Mater. Sci. Technol. 2017, 33, 305–310. [Google Scholar] [CrossRef]
- Bai, H.; He, X.; Ding, P.; Liu, D.; Chen, M. Fabrication, microstructure, and properties of a biodegradable Mg-Zn-Ca clip. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1741–1749. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, S.; Lu, F.; Wang, B.; Yang, L.; Qin, L.; Yang, K.; Li, Y.; Li, W.; Wang, W.; et al. Vascularized bone grafting fixed by biodegradable magnesium screw for treating oste-onecrosis of the femoral head. Biomaterials 2016, 81, 84–92. [Google Scholar] [CrossRef]
- Windhagen, H.; Radtke, K.; Weizbauer, A.; Diekmann, J.; Noll, Y.; Kreimeyer, U.; Schavan, R.; Stukenborg-Colsman, C.; Waizy, H. Biodegradable magnesium-based screw clinically equivalent to titanium screw in hallux valgus surgery: Short term results of the first prospective, randomized, controlled clinical pilot study. Biomed. Eng. Online 2013, 12, 62. [Google Scholar] [CrossRef] [PubMed]
- Dziuba, D.; Meyer-Lindenberg, A.; Seitz, J.M.; Waizy, H.; Angrisani, N.; Reifenrath, J. Long-term in vivo degradation behaviour and biocompatibility of the magnesium alloy ZEK100 for use as a biodegradable bone implant. Acta Biomater. 2013, 9, 8548–8560. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, N.; Angrisani, N.; Reifenrath, J.; Lucas, A.; Thorey, F.; Bormann, D.; Meyer-Lindenberg, A. Biomechanical testing and degradation analysis of MgCa0.8 alloy screws: A comparative in vivo study in rabbits. Acta Biomater. 2011, 7, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Krause, A.; Von Der Höh, N.; Bormann, D.; Krause, C.; Bach, F.-W.; Windhagen, H.; Meyer-Lindenberg, A. Degradation behaviour and mechanical properties of magnesium implants in rabbit tibiae. J. Mater. Sci. 2010, 45, 624–632. [Google Scholar] [CrossRef]
- Angrisani, N.; Reifenrath, J.; Zimmermann, F.; Eifler, R.; Meyer-Lindenberg, A.; Vano-Herrera, K.; Vogt, C. Bio-compatibility and degradation of LAE442-based magnesium alloys after implantation of up to 3.5 years in a rabbit model. Acta Biomater. 2016, 44, 355–365. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, T.; Nahan, K.; Guo, X.; Zhang, Z.; Dong, Z.; Chen, S.; Chou, D.T.; Hong, D.; Kumta, P.N.; et al. In vivo characterization of magnesium alloy biodegradation using electrochemical H2monitoring, ICP-MS, and XPS. Acta Biomater. 2017, 50, 556–565. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Li, H.; Liu, Y.; Bai, X.; Chau, W.; Zheng, Y.; Qin, L. Magnesium alloy based interference screw developed for ACL reconstruction attenuates peri-tunnel bone loss in rabbits. Biomaterials 2018, 157, 86–97. [Google Scholar] [CrossRef]
- Brooks, E.K.; Ahn, R.; Tobias, M.E.; Hansen, L.A.; Luke-Marshall, N.R.; Wild, L.; Campagnari, A.A.; Ehrensberger, M.T. Magnesium alloy AZ91 exhibits antimicrobial properties in vitro but not in vivo. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 106, 221–227. [Google Scholar] [CrossRef]
- Levorova, J.; Duskova, J.; Drahos, M.; Vrbová, R.; Vojtech, D.; Kubásek, J.; Bartoš, M.; Dugova, L.; Ulmann, D.; Foltan, R. In vivo study on biodegradable magnesium alloys: Bone healing around WE43 screws. J. Biomater. Appl. 2017, 32, 886–895. [Google Scholar] [CrossRef]
- Zhang, B.; Hou, Y.; Wang, X.; Wang, Y.; Geng, L. Mechanical properties, degradation performance and cytotoxicity of Mg-Zn-Ca biomedical alloys with different compositions. Mater. Sci. Eng. C 2011, 31, 1667–1673. [Google Scholar] [CrossRef]
- Gil-Santos, A.; Marco, I.; Moelans, N.; Hort, N.; Van der Biest, O. Microstructure and degradation performance of biodegradable Mg-Si-Sr implant alloys. Mater. Sci. Eng. C 2017, 71, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Rad, H.R.B.; Idris, M.H.; Kadir, M.R.A.; Farahany, S. Microstructure analysis and corrosion behavior of biode-gradable Mg-Ca implant alloys. Mater. Des. 2012, 33, 88–97. [Google Scholar] [CrossRef]
- Pourbaix, M.; Staehle, R.W. Lectures on Electrochemical Corrosion; PWN: Warszawa, Poland, 1978. [Google Scholar]
- Gu, X.; Xie, X.; Li, N.; Zheng, Y.; Qin, L. In vitro and in vivo studies on a Mg–Sr binary alloy system developed as a new kind of biodegradable metal. Acta Biomater. 2012, 8, 2360–2374. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.I.; Kuroda, K.; Okido, M. Temperature-dependent corrosion behaviour of flame-resistant, Ca-containing AZX911 and AMX602 Mg alloys. Corros. Sci. 2016, 103, 181–188. [Google Scholar] [CrossRef]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef]
- Liu, D.; Guo, C.; Chai, L.; Sherman, V.R.; Qin, X.; Ding, Y.; Meyers, M.A. Mechanical properties and corrosion resistance of hot extruded Mg-2.5Zn-1Ca alloy. Mater. Sci. Eng. B 2015, 195, 50–58. [Google Scholar] [CrossRef]
- Witecka, A.; Yamamoto, A.; Święszkowski, W. Influence of SaOS-2 cells on corrosion behavior of cast Mg-2.0Zn0.98Mn magnesium alloy. Colloids Surf. B Biointerfaces 2017, 150, 288–296. [Google Scholar] [CrossRef]
- Hofstetter, J.; Martinelli, E.; Weinberg, A.M.; Becker, M.; Mingler, B.; Uggowitzer, P.J.; Löffler, J.F. Assessing the degradation performance of ultrahigh-purity magnesium in vitro and in vivo. Corros. Sci. 2015, 91, 29–36. [Google Scholar] [CrossRef]
- Xie, G.; Takada, H.; Kanetaka, H. Development of high performance MgFe alloy as potential biodegradable ma-terials. Mater. Sci. Eng. A 2016, 671, 48–53. [Google Scholar] [CrossRef]
- Harandi, S.E.; Mirshahi, M.; Koleini, S.; Idris, M.H.; Jafari, H.; Kadir, M.R.A. Effect of calcium content on the microstructure, hardness and in-vitro corrosion behavior of biodegradable Mg-Ca binary alloy. Mater. Res. 2012, 16, 11–18. [Google Scholar] [CrossRef]
- Zeng, R.; Qi, W.; Cui, H.; Zhang, F.; Li, S.; Han, E. In vitro corrosion of as-extruded Mg-Ca alloys-The influence of Ca concentration. Corros. Sci. 2015, 96, 23–31. [Google Scholar] [CrossRef]
- Seong, J.; Kim, W. Development of biodegradable Mg–Ca alloy sheets with enhanced strength and corrosion properties through the refinement and uniform dispersion of the Mg2Ca phase by high-ratio differential speed rolling. Acta Biomater. 2015, 11, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, X.; Zhao, C.; Li, J.; Song, Y.; Xie, C. Acta Biomaterialia Research on an Mg–Zn alloy as a degradable biomaterial. Acta Biomater. 2010, 6, 626–640. [Google Scholar] [CrossRef]
- Salleh, E.M.; Zuhailawati, H.; Noor, S.N.F.M.; Othman, N.K. In Vitro Biodegradation and Mechanical Properties of Mg-Zn Alloy and Mg-Zn-Hydroxyapatite Composite Produced by Mechanical Alloying for Potential Application in Bone Repair. Met. Mater. Trans. A 2018, 49, 5888–5903. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, X.; Qiao, Y.; Wang, X.; Lin, Q. Preparation of medical Mg–Zn alloys and the effect of different zinc contents on the alloy. J. Mater. Sci. Mater. Med. 2022, 33, 9. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Han, E.H.; Shan, D.; Yim, C.D.; You, B.S. The effect of Zn concentration on the corrosion behavior of Mg-xZn alloys. Corros. Sci. 2012, 65, 322–330. [Google Scholar] [CrossRef]
- Yan, Y.; Cao, H.; Kang, Y.; Yu, K.; Xiao, T.; Luo, J.; Deng, Y.; Fang, H.; Xiong, H.; Dai, Y. Effects of Zn concentration and heat treatment on the microstructure, mechanical properties and corrosion behavior of as-extruded Mg-Zn alloys produced by powder metallurgy. J. Alloys Compd. 2016, 693, 1277–1289. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, T.; Wu, S.; Lü, S.; Yang, X.; Guo, W. Effects of Si Content and Ca Addition on Thermal Conductivity of As-Cast Mg–Si Alloys. Materials 2018, 11, 2376. [Google Scholar] [CrossRef]
- Zhang, E.; Yang, L.; Xu, J.; Chen, H. Microstructure, mechanical properties and bio-corrosion properties of Mg-Si(-Ca, Zn) alloy for biomedical application. Acta Biomater. 2010, 6, 1756–1762. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Kang, J.-Y.; Kim, S.G.; Kim, B.; Park, S.S.; Yim, C.D.; You, B.S. Influences of metallurgical factors on the corrosion behaviour of extruded binary Mg–Sn alloys. Corros. Sci. 2014, 82, 369–379. [Google Scholar] [CrossRef]
- Bornapour, M.; Celikin, M.; Pekguleryuz, M. Thermal exposure effects on the in vitro degradation and mechanical properties of Mg–Sr and Mg–Ca–Sr biodegradable implant alloys and the role of the microstructure. Mater. Sci. Eng. C 2015, 46, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Bin, Y.; Zou, W.; Wang, X.; Li, W. The influence of Sr on the microstructure, degradation and stress corrosion cracking of the Mg alloys—ZK40xSr. J. Mech. Behav. Biomed. Mater. 2017, 66, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Pan, F.; Zhang, L.; Pan, H.; Song, K.; Tang, A. Microstructure, mechanical properties, bio-corrosion properties and cytotoxicity of as-extruded Mg-Sr alloys. Mater. Sci. Eng. C 2017, 70, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wan, P.; Ge, Y.; Fan, X.; Tan, L.; Li, J.; Yang, K. Tailoring the degradation and biological response of a magnesium–strontium alloy for potential bone substitute application. Mater. Sci. Eng. C 2015, 58, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tie, D.; Guan, R.; Wang, N.; Shang, Y.; Cui, T.; Li, J. Microstructures, mechanical properties, and degradation behaviors of heat-treated Mg-Sr alloys as potential biodegradable implant materials. J. Mech. Behav. Biomed. Mater. 2017, 77, 47–57. [Google Scholar] [CrossRef]
- Cho, D.H.; Lee, B.W.; Park, J.Y.; Cho, K.M.; Park, I.M. Effect of Mn addition on corrosion properties of biodegradable Mg-4Zn-0.5Ca-xMn alloys. J. Alloys Compd. 2017, 695, 1166–1174. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, B.; Baek, S.-M.; Sohn, S.-D.; Shin, H.-J.; Jeong, H.Y.; Yim, C.D.; You, B.S.; Ha, H.-Y.; Park, S.S. Influence of alloyed Al on the microstructure and corrosion properties of extruded Mg–8Sn–1Zn alloys. Corros. Sci. 2015, 95, 133–142. [Google Scholar] [CrossRef]
- Remennik, S.; Bartsch, I.; Willbold, E.; Witte, F.; Shechtman, D. New, fast corroding high ductility Mg–Bi–Ca and Mg–Bi–Si alloys, with no clinically observable gas formation in bone implants. Mater. Sci. Eng. B 2011, 176, 1653–1659. [Google Scholar] [CrossRef]
- Tok, H.Y.; Hamzah, E.; Bakhsheshi-Rad, H.R. The role of bismuth on the microstructure and corrosion behavior of ternary Mg-1.2Ca-xBi alloys for biomedical applications. J. Alloys Compd. 2015, 640, 335–346. [Google Scholar] [CrossRef]
- Li, T.; Zhang, H.; He, Y.; Wen, N.; Wang, X. Microstructure, Mechanical Properties and InVitro Degradation Behavior of a Novel Biodegradable Mg-1.5Zn-0.6Zr-0.2Sc Alloy. J. Mater. Sci. Technol. 2015, 31, 744–750. [Google Scholar] [CrossRef]
- Cai, S.; Lei, T.; Li, N.; Feng, F. Effects of Zn on microstructure, mechanical properties and corrosion behavior of Mg–Zn alloys. Mater. Sci. Eng. C 2012, 32, 2570–2577. [Google Scholar] [CrossRef]
- Jiang, P.; Blawert, C.; Zheludkevich, M.L. The Corrosion Performance and Mechanical Properties of Mg-Zn Based Alloys—A Review. Corros. Mater. Degrad. 2020, 1, 92–158. [Google Scholar] [CrossRef]
- Romzi, M.; Alias, J.; Ramli, M. Effect of zinc (Zn) on the microstructure and corrosion behaviour of magnesium (Mg). Mater. Today Proc. 2021, 48, 1873–1879. [Google Scholar] [CrossRef]
- Jingyuan, Y.; Jianzhong, W.; Qiang, L.; Jian, S.; Jianming, C.; Xudong, S. Effect of Zn on Microstructures and Properties of Mg-Zn Alloys Prepared by Powder Metallurgy Method. Rare Met. Mater. Eng. 2016, 45, 2757–2762. [Google Scholar] [CrossRef]
- El Mahallawy, N.; Diaa, A.A.; Akdesir, M.; Palkowski, H. Effect of Zn addition on the microstructure and mechanical properties of cast, rolled and extruded Mg-6Sn-xZn alloys. Mater. Sci. Eng. A 2017, 680, 47–53. [Google Scholar] [CrossRef]
- Lee, Y.C.; Dahle, A.K.; StJohn, D.H. The role of solute in grain refinement of magnesium. Met. Mater. Trans. A 2000, 31, 2895–2906. [Google Scholar] [CrossRef]
- Wang, W.; Han, J.; Yang, X.; Li, M.; Wan, P.; Tan, L.; Zhang, Y.; Yang, K. Novel biocompatible magnesium alloys design with nutrient alloying elements Si, Ca and Sr: Structure and properties characterization. Mater. Sci. Eng. B 2016, 214, 26–36. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Wan, P.; Zhai, Z.; Mao, Z.; Ouyang, Z.; Yu, D.; Sun, Q.; Tan, L.; Ren, L.; et al. Biodegradable Mg-Cu alloy implants with antibacterial activity for the treatment of osteomyelitis: In vitro and in vivo evaluations. Biomaterials 2016, 106, 250–263. [Google Scholar] [CrossRef]
- Lotfpour, M.; Emamy, M.; Dehghanian, C.; Tavighi, K. Influence of Cu Addition on the Structure, Mechanical and Corrosion Properties of Cast Mg-2 % Zn Alloy. J. Mater. Eng. Perform. 2017, 26, 2136–2150. [Google Scholar] [CrossRef]
- Hanus, A.; Pawlica, L. Structure and mechanical properties of Mg-Si alloys at elevated temperatures. J. Achiev. Mater. Manuf. 2009, 35, 37–46. [Google Scholar]
- Okamoto, H. Mg-Mn (Magnesium-Manganese). J. Phase Equilibria Diffus. 2012, 33, 496. [Google Scholar] [CrossRef][Green Version]
- Martienssen, W.; Warlimont, H. Springer Handbook of Condensed Matter and Materials Data, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Liu, H.; Chen, Y.; Tang, Y.; Wei, S.; Niu, G. The microstructure, tensile properties, and creep behavior of as-cast Mg-(1-10)%Sn alloys. J. Alloys Compd. 2007, 440, 122–126. [Google Scholar] [CrossRef]
- Dahle, A.K.; Lee, Y.C.; Nave, M.D.; Schaffer, P.L.; StJohn, D. Development of the as-cast microstructure in magnesium–aluminium alloys. J. Light Met. 2001, 1, 61–72. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Zhao, H.; Wei, S.; Gao, W. Effects of strontium on microstructure and mechanical properties of as-cast Mg-5 wt.%Sn alloy. J. Alloys Compd. 2010, 504, 345–350. [Google Scholar] [CrossRef]
- Tingting, G.; Xuerong, L.; Kumar, V.R.; Cheng, Z.; Jun, W.; Jianwei, Y.; Jian, C. Influence of Coarse Mg3Bi2 Particles on Deformation Behaviors of Mg-Bi Alloys. Front. Mater. 2021, 8, 633789. [Google Scholar]
- Hakimi, O.; Aghion, E.; Goldman, J. Improved stress corrosion cracking resistance of a novel biodegradable EW62 magnesium alloy by rapid solidification, in simulated electrolytes. Mater. Sci. Eng. C 2015, 51, 226–232. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, P.M. Development of Mg based biomaterial with improved mechanical and degradation properties using powder metallurgy. J. Magnes. Alloys 2020, 8, 883–898. [Google Scholar] [CrossRef]
- Kubásek, J.; Dvorský, D.; Čavojský, M.; Vojtěch, D.; Beronská, N.; Fousová, M. Superior Properties of Mg–4Y–3RE–Zr Alloy Prepared by Powder Metallurgy. J. Mater. Sci. Technol. 2017, 33, 652–660. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Asif, M. Room temperature mechanical properties of Mg–Cu–Al alloys synthesized using powder metallurgy method. Mater. Sci. Eng. A 2015, 644, 129–136. [Google Scholar] [CrossRef]
- Galindez, Y.; Correa, E.; Zuleta, A.A.; Escobar, A.V.; Calderon, D.; Toro, L.; Chacón, P.; Echeverría, F.E. Improved Mg–Al–Zn Magnesium Alloys Produced by High Energy Milling and Hot Sintering. Met. Mater. Int. 2021, 27, 1113–1130. [Google Scholar] [CrossRef]
- Zhou, T.; Yang, M.; Zhou, Z.; Hu, J.; Chen, Z. Microstructure and mechanical properties of rapidly solidified/powder metallurgy Mg–6Zn and Mg–6Zn–5Ca at room and elevated temperatures. J. Alloys Compd. 2013, 560, 161–166. [Google Scholar] [CrossRef]
- Hu, Y.; Dong, D.; Wang, X.; Chen, H.; Qiao, Y. Synthesis and Properties of Mg-Mn-Zn Alloys for Medical Applications. Materials 2021, 14, 1855. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, S.K.; Mishra, R.S.; Brennan, R.C.; Cho, K. Achieving extraordinary structural efficiency in a wrought magnesium rare earth alloy. Mater. Res. Lett. 2020, 8, 151–157. [Google Scholar] [CrossRef]
- Su, J.; Sanjari, M.; Kabir, A.; Jung, I.-H.; Jonas, J.; Yue, S.; Utsunomiya, H. Characteristics of magnesium AZ31 alloys subjected to high speed rolling. Mater. Sci. Eng. A 2015, 636, 582–592. [Google Scholar] [CrossRef]
- She, J.; Pan, F.; Zhang, J.; Tang, A.; Luo, S.; Yu, Z.; Song, K.; Rashad, M. Microstructure and mechanical properties of Mg-Al-Sn extruded alloys. J. Alloys Compd. 2016, 657, 893–905. [Google Scholar] [CrossRef]
- Hu, G.S.; Zhang, D.F.; Zhao, D.Z.; Shen, X.; Jiang, L.Y.; Pan, F.S. Microstructures and mechanical properties of extruded and aged Mg-Zn-Mn-Sn-Y alloys. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2014, 24, 3070–3075. [Google Scholar] [CrossRef]
- Chen, D.; Ren, Y.-P.; Guo, Y.; Pei, W.-L.; Zhao, H.-D.; Qin, G.-W. Microstructures and tensile properties of as-extruded Mg-Sn binary alloys. Trans. Nonferrous Met. Soc. China 2010, 20, 1321–1325. [Google Scholar] [CrossRef]
- Cheng, W.; Park, S.; You, B.; Koo, B. Microstructure and mechanical properties of binary Mg–Sn alloys subjected to indirect extrusion. Mater. Sci. Eng. A 2010, 527, 4650–4653. [Google Scholar] [CrossRef]
- Mg-ga, T.; Mgsga, M.; Mgga, G.M. Ga-Mg (Gallium-Magnesium) H-Hf (Hydrogen-Hafnium). J. Phase Equilibria 1991, 12, 119–120. [Google Scholar]
- Minárik, P.; Král, R.; Pešička, J.; Daniš, S.; Janeček, M. Microstructure characterization of LAE442 magnesium alloy processed by extrusion and ECAP. Mater. Charact. 2016, 112, 1–10. [Google Scholar] [CrossRef]
- Minárik, P.; Jablonska, E.; Král, R.; Lipov, J.; Ruml, T.; Blawert, C.; Hadzima, B.; Chmelík, F. Effect of equal channel angular pressing on in vitro degradation of LAE442 magnesium alloy. Mater. Sci. Eng. C 2017, 73, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Li, W.; Li, A.; Shi, X. Microstructure and properties evolution of Mg–2Y–0.6Nd–0.6Zr alloy rolled at room and liquid nitrogen temperature. Sci. Rep. 2021, 11, 20893. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.; Klarner, A.D.; Poorganji, B.; Dean, D.; Luo, A.A.; Elahinia, M. Microstructural, mechanical and corrosion characteristics of heat-treated Mg-1.2Zn-0.5Ca (wt.%) alloy for use as resorbable bone fixation material. J. Mech. Behav. Biomed. Mater. 2017, 69, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Henderson, H.; Ramaswamy, V.; Wilson-Heid, A.E.; Kesler, M.; Allen, J.B.; Manuel, M.V. Mechanical and degradation property improvement in a biocompatible Mg-Ca-Sr alloy by thermomechanical processing. J. Mech. Behav. Biomed. Mater. 2018, 80, 285–292. [Google Scholar] [CrossRef]
- Lin, D.-J.; Hung, F.-Y.; Lui, T.-S.; Yeh, M.-L. Heat treatment mechanism and biodegradable characteristics of ZAX1330 Mg alloy. Mater. Sci. Eng. C 2015, 51, 300–308. [Google Scholar] [CrossRef]
- Lu, F.; Ma, A.; Jiang, J.; Guo, Y.; Yang, D.; Song, D.; Chen, J. Significantly improved corrosion resistance of heat-treated Mg–Al–Gd alloy containing profuse needle-like precipitates within grains. Corros. Sci. 2015, 94, 171–178. [Google Scholar] [CrossRef]
- Tang, H.; Wu, T.; Wang, H.; Jian, X.; Wu, Y. Corrosion behavior of HA containing ceramic coated magnesium alloy in Hank’s solution. J. Alloys Compd. 2017, 698, 643–653. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, W.; Ngai, T. Polymer coatings on magnesium—based implants for orthopedic applications. J. Appl. Polym. Sci. 2021, 60, 32–51. [Google Scholar] [CrossRef]
- Echeverry-Rendon, M.; Allain, J.P.; Robledo, S.M.; Echeverria, F.; Harmsen, M.C. Coatings for biodegradable magnesium-based supports for therapy of vascular disease: A general view. Mater. Sci. Eng. C 2019, 102, 150–163. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Hopkins, C.; Chow, D.H.; Qin, L. Biodegradable Magnesium Based Implants in Orthopedics—A General Review and Perspectives. Adv. Sci. 2020, 7, 1902443. [Google Scholar] [CrossRef]
- Shen, S.; Cai, S.; Li, Y.; Ling, R.; Zhang, F.; Xu, G.; Wang, F. Microwave aqueous synthesis of hydroxyapatite bilayer coating on magnesium alloy for orthopedic application. Chem. Eng. J. 2017, 309, 278–287. [Google Scholar] [CrossRef]
- Yu, N.; Cai, S.; Wang, F.; Zhang, F.; Ling, R.; Li, Y.; Jiang, Y.; Xu, G. Microwave assisted deposition of strontium doped hydroxyapatite coating on AZ31 magnesium alloy with enhanced mineralization ability and corrosion resistance. Ceram. Int. 2017, 43, 2495–2503. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.; Hamzah, E.; Ismail, A.; Sharer, Z.; Abdul-Kadir, M.; Daroonparvar, M.; Saud, S.; Medraj, M. Synthesis and corrosion behavior of a hybrid bioceramic-biopolymer coating on biodegradable Mg alloy for orthopaedic implants. J. Alloys Compd. 2015, 648, 1067–1071. [Google Scholar] [CrossRef]
- Guo, Y.; Su, Y.; Gu, R.; Zhang, Z.; Li, G.; Lian, J.; Ren, L. Enhanced corrosion resistance and biocompatibility of biodegradable magnesium alloy modified by calcium phosphate/collagen coating. Surf. Coat. Technol. 2020, 401, 126318. [Google Scholar] [CrossRef]
- Li, Y.; Gao, J.; Yang, L.; Shen, J.; Jiang, Q.; Wu, C.; Zhu, D.; Zhang, Y. Biodegradable and Bioactive Orthopedic Magnesium Implants with Multilayered Protective Coating. ACS Appl. Bio Mater. 2019, 2, 3290–3299. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xue, J.; Luo, D.; Wang, H.; Gong, X.; Han, Z.; Ren, L. One-step fabrication of biomimetic superhydrophobic surface by electrodeposition on magnesium alloy and its corrosion inhibition. J. Colloid Interface Sci. 2017, 491, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Mousa, H.M.; Park, C.H.; Kim, C.S. Enhanced corrosion resistance and biocompatibility of AZ31 Mg alloy using PCL/ZnO NPs via electrospinning. Appl. Surf. Sci. 2017, 396, 249–258. [Google Scholar] [CrossRef]
- Tian, P.; Liu, X.; Ding, C. In vitro degradation behavior and cytocompatibility of biodegradable AZ31 alloy with PEO/HT composite coating. Colloids Surfaces B Biointerfaces 2015, 128, 44–54. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J.; Xie, X.; Zhang, P.; Lai, Y.; Li, Y.; Qin, L. Surface coating reduces degradation rate of magnesium alloy developed for orthopaedic applications. J. Orthop. Transl. 2013, 1, 41–48. [Google Scholar] [CrossRef]
- Pan, Y.; He, S.; Wang, D.; Huang, D.; Zheng, T.; Wang, S.; Dong, P.; Chen, C. In vitro degradation and electrochemical corrosion evaluations of microarc oxidized pure Mg, Mg-Ca and Mg-Ca-Zn alloys for biomedical applications. Mater. Sci. Eng. C 2015, 47, 85–96. [Google Scholar] [CrossRef]
- Razavi, M.; Fathi, M.; Savabi, O.; Tayebi, L.; Vashaee, D. Biodegradable Magnesium Bone Implants Coated with a Novel Bioceramic Nanocomposite. Materials 2020, 13, 1315. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jiang, B.; Yang, H.; Yang, Q.; Xia, X.; Pan, F. High temperature oxidation behavior of Mg-Y-Sn, Mg-Y, Mg-Sn alloys and its effect on corrosion property. Appl. Surf. Sci. 2015, 353, 1013–1022. [Google Scholar] [CrossRef]
- Lin, Z.; Zhao, Y.; Chu, P.K.; Wang, L.; Pan, H.; Zheng, Y.; Wu, S.; Liu, X.; Cheung, K.M.C.; Wong, T.; et al. A functionalized TiO2/Mg2TiO4 nano-layer on biodegradable magnesium implant enables superior bone-implant integration and bacterial dis-infection. Biomaterials 2019, 219, 119372. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Jia, S.; Qiao, L.; Su, Y.; Gu, R.; Li, G.; Lian, J. A multifunctional polypyrrole/zinc oxide composite coating on biodegradable magnesium alloys for orthopedic implants. Colloids Surfaces B Biointerfaces 2020, 194, 111186. [Google Scholar] [CrossRef] [PubMed]
- Caralapatti, V.K.; Narayanswamy, S. Effect of high repetition laser shock peening on biocompatibility and corrosion resistance of magnesium. Opt. Laser Technol. 2017, 88, 75–84. [Google Scholar] [CrossRef]
- Jin, W.; Wu, G.; Feng, H.; Wang, W.; Zhang, X.; Chu, P.K. Improvement of corrosion resistance and biocompatibility of rare-earth WE43 magnesium alloy by neodymium self-ion implantation. Corros. Sci. 2015, 94, 142–155. [Google Scholar] [CrossRef]
- Zhao, Y.; Jamesh, M.I.; Li, W.K.; Wu, G.; Wang, C.; Zheng, Y.; Yeung, K.W.; Chu, P.K. Enhanced antimicrobial properties, cytocompatibility, and corrosion resistance of plasma-modified biodegradable magnesium alloys. Acta Biomater. 2013, 10, 544–556. [Google Scholar] [CrossRef]
- Yazdimamaghani, M.; Razavi, M.; Vashaee, D.; Moharamzadeh, K.; Boccaccini, A.R.; Tayebi, L. Porous magne-sium-based scaffolds for tissue engineering. Mater. Sci. Eng. C 2017, 71, 1253–1266. [Google Scholar] [CrossRef]
- Li, M.; Yang, X.; Wang, W.; Zhang, Y.; Wan, P.; Yang, K.; Han, Y. Evaluation of the osteo-inductive potential of hollow three-dimensional magnesium-strontium substitutes for the bone grafting application. Mater. Sci. Eng. C 2017, 73, 347–356. [Google Scholar] [CrossRef]
- Kamrani, S.; Fleck, C. Biodegradable magnesium alloys as temporary orthopaedic implants: A review. BioMetals 2019, 32, 185–193. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, P.; Wang, N.; Peng, L.; Kang, B.; Zeng, H.; Yuan, G.; Ding, W. Challenges and Solutions for the Additive Manufacturing of Biodegradable Magnesium Implants. Engineering 2020, 6, 1267–1275. [Google Scholar] [CrossRef]
- Sezer, N.; Evis, Z.; Koç, M. Additive manufacturing of biodegradable magnesium implants and scaffolds: Review of the recent advances and research trends. J. Magnes. Alloy. 2020, 9, 392–415. [Google Scholar] [CrossRef]
- Xie, K.; Wang, N.; Guo, Y.; Zhao, S.; Tan, J.; Wang, L.; Li, G.; Wu, J.; Yang, Y.; Xu, W.; et al. Additively manufactured biodegradable porous magnesium implants for elimination of implant-related infections: An in vitro and in vivo study. Bioact. Mater. 2021, 8, 140–152. [Google Scholar] [CrossRef]
- Ying-liang, C.; Ting-wei, Q.I.N.; Hui-min, W.; Zhao, Z. Comparison of corrosion behaviors of AZ31, AZ91, AM60 and ZK60 magnesium alloys. Trans. Nonferrous Met. Soc. China 2008, 19, 517–524. [Google Scholar]
- Singh, I.B.; Singh, M.; Das, S. A comparative corrosion behavior of Mg, AZ31 and AZ91 alloys in 3. 5 % NaCl solution. J. Magnes. Alloy. 2015, 3, 142–148. [Google Scholar] [CrossRef]
- Fu, J.; Du, W.; Liu, K.; Du, X.; Zhao, C.; Liang, H.; Mansoor, A.; Li, S.; Wang, Z. Effect of the Ca2Mg6Zn3 Phase on the Corrosion Behavior of Biodegradable Mg-4.0Zn-0.2Mn-xCa Alloys in Hank’s Solution. Materials 2022, 15, 2079. [Google Scholar] [CrossRef]



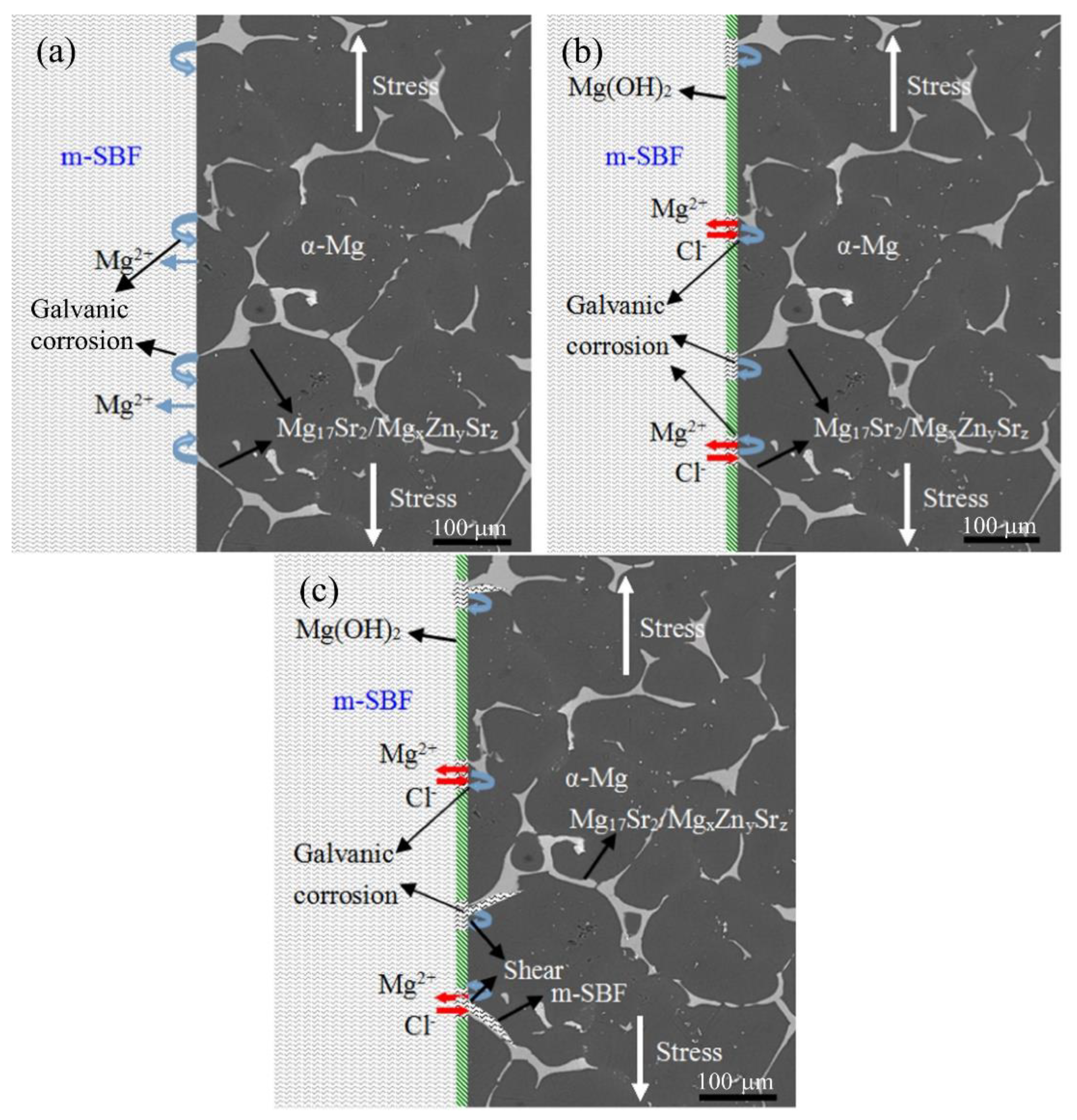



| ASTM Code | Chemical Symbol | Whole Blood Level (Mean) | Blood Serum Levels (Mean) | General Daily Allowance for Adults (mg) | Toxicology and Pathophysiology | ||||
|---|---|---|---|---|---|---|---|---|---|
| French, μg/L [48] * | Elderly Swedish, (Mean ± SD) μM/L [49] *** | Benin, μg/L (Male) [50] ** | French, μg/L [48] * | Elderly Swedish, (Mean ± SD) μM/L [49] *** | Male | Female | |||
| Mg | - | - | 27858 | - | - | 260 [31]/400–420 [51] | 220 [31]/310–320 [51] | Non-toxic except at high levels [31]. However, the upper limit is dependent on various factors such as gender/age/diet. | |
| X | Ca | - | - | - | - | - | 1000 [31,51] | 1000–1300 [31,51] | An essential element of the body [31]. Makes up the human skeletal system. |
| C | Cu | 1523 | 12.9 ± 1.91 | 874.925 | 1642 | 15.1 ± 2.92 | 0.9 [51] | 0.9 [51] | It is an essential trace element of the body [34]. Above 3 mg/L of whole blood concentration of Cu leads to gastrointestinal symptoms of toxicity [34]. |
| F | Fe | - | - | 472457 | - | - | 8 [51] | 18 [51] | Essential for normal metabolism of cells [33]. Have been reported to be toxic to cells under certain conditions [36]. |
| M | Mn | 33.8 | 0.144 ± 0.043 | 19.936 | 14.2 | 0.0284 ± 0.021 | 2.3 [51] | 1.8 [51] | It is a trace element, Mn2+ is the predominant form in human body [52]. Leads to neurotoxic effects (manganism) only when exposed to inhalation [52]. Almost entirely excreted in the feces [52]. |
| Z | Zn | 6663 | 95.9 ± 12.7 | 4937.58 | 1529 | 11.2 ± 1.7 | 11 [51] | 8 [51] | Zn is an essential [31] trace element [36]. The plasma zinc is regulated via homeostatic control [31]. |
| J | Sr | 9.6 | - | 31.792 | 23.8 | - | Sr is not an essential element. Strontium ranelate is used for treatment of osteoporosis [53]. | ||
| L | Li | 0.268 | - | 0.474 | 5749 | - | - | - | Non-Essential trace element without which the human body can lead a healthy life [46]. Long-term dosage and high dosage can be toxic. Excreted almost completely via kidneys with low tissue accumulation [46]. |
| W | Y | - | - | - | - | - | - | Water-insoluble Y compounds are non-toxic but water-soluble compounds are mildly toxic [54]. Y and its compounds have been reported to have caused liver and lung damage in animals [54]. | |
| V | Gd | - | - | - | - | - | - | - | Highly toxic as a free ion [54]. It is used after chelation as MRI contrast agents. The strength of the chelation determines the toxicity [54]. |
| A | Al | - | 0.709 ± 0.539 | 3.726 | 0.424 ± 0.752 | - | - | Aluminum is rated as Generally Regarded As Safe (GRAS) by US FDA [32]. However, concerns of its role in Alzheimer’s due to its accumulation in the brain exist, although the consensus for this is disputed [45]. | |
| N | Ni | 18.8 | 0.144 ± 0.175 | - | 5.94 | 0.0446 ± 0.0527 | - | - | Circumstantial evidence as an essential element [37]. Potentially leads to Cancer in forms administered other than orally [37]. |
| B | Bi | 4.72 | - | <0.010 | 0.01 | - | - | - | Found to be toxic in high doses [55]. |
| Q | Ag | 0.127 | - | - | 0.234 | - | - | - | Ag is reported to be extremely toxic and is potentially fatal in the case of ingestion in the form of silver salts [56]. |
| T | Sn | 5.59 | - | 0.257 | 0.443 | - | - | - | Not an essential element [57]. Found in cans and some vitamin supplements. Relatively non-toxic (barring respiratory forms), but in chronic doses, tends to accumulate in bones, kidney, and liver and may cause liver and kidney problems [57]. |
| K | Zr | - | - | - | - | - | - | - | Zr dental implants have been found to be biocompatible with good osseointegration with good soft tissue response [58]. Majority is excreted via urine while absorption is dependent on the species of Zr [59]. |
| S | S | - | - | - | - | - | - | - | Circumstantial evidence as an essential element has been reported [37]. Previously considered as biologically inert (breast implants), but exposure to severe and long-term doses could lead to inflammation of liver and spleen [42]. |
| E | Nd | - | - | - | - | - | - | - | Low to moderate toxicity has been observed [54]. |
| E | La | - | - | - | - | - | - | - | Animal tests involving injection of La in solution form has been reported to cause low blood pressure, hyperglycemia, hepatic alterations, and degeneration of the spleen [54]. |
| E | Ce | - | - | <0.010 | - | - | - | - | Experiments involving high dosage of Cerium injection in animals have led to fatal cardiovascular collapse [54]. |
| Astm Code | Element | In Vitro Test Results | Reported Concentration | Cell Line(S)/Cell Description | Duration (Day) | Medium | Ref. |
|---|---|---|---|---|---|---|---|
| - | Mg | Cells were fully viable. | 160 × 103 ng/mL | U-2OS/(human osteosarcoma) | [63] | ||
| Increased cell viability of cells. Viability was 171.1 ± 11.6% after day 5. | 7 × 104 cells/μL | hBMMSCs/(human bone marrow mesenchymal stem cells) | 1, 3, 5 | α-MEM | [4] | ||
| A | Al | Hemolysis and adhered platelets decreased for Mg-Al alloy as compared with Mg element. | 20 ± 7 μM/L | Platelets | 7 | DMEM | [62] |
| Al showed no decrease in cell viability | L929/NIH3T3/(fibroblasts) MC3T3-E1/(osteoblasts) | ||||||
| Al alloyed Mg showed no observed negative effects on cell viabilities. | ECV304/VSMC/blood vessel related cell | ||||||
| X | Ca | Not toxic when Mg-1Ca was tested on cells. It also showed good viability. | - | L929 | 7 | DMEM | [64] |
| C | Cu | Low Cu concentration, i.e., Cu wt.% of 0.03 and 0.19 stimulates growth of tested cells and promotes initial cell adhesion and spreading. | 0.03–0.19 wt.% | HUVEC/MC3T3-E1 | 1 | α-MEM and Endothelial cell medium, respectively | [65] |
| High Cu concentration such as Cu wt.% above 0.57% is slightly toxic for cell proliferation. | >0.57% | HUVEC/(Human Umbilical Vein Endothelial cells) and MC3T3-E1 | 1–5 | ||||
| F | Fe | Low iron concentrations are favorable for metabolism of cells. | <10 μg/mL | HUVEC/(Human Umbilical Vein Endothelial cells) | 3 | (Cell proliferation agent) WST-8 | [66] |
| No difference in metabolism of cells compared to zero Fe concentration. | 50 μg/mL | 1 | |||||
| High concentrations are cytotoxic to cells. | >50 μg/mL | 1, 3 | |||||
| M | Mn | Serious toxic effect to the tested cell lines. | 1.8 μM/L | L929/(fibroblasts) | 7 | DMEM | [62] |
| NIH3T3/(fibroblasts) | |||||||
| MC3T3-E1/(osteoblasts) | |||||||
| ECV304/blood vessel related cell | |||||||
| VSMC/blood vessel related cell | |||||||
| S | Si | Increased cell viability of cells. | 71 ± 27 μM/L | MC3T3-E1/(osteoblasts) | |||
| Toxic for the cells tested. | ECV304/VSMC/blood vessel related cell | ||||||
| Z | Zn | Hemolysis and adhered platelets decreased for Mg-Zn as compared with Mg element. | 2.6 ± 1 μM/L | Platelets | |||
| Mg-Zn showed no decrease in cell viability | L929/NIH3T3/(fibroblasts) | ||||||
| MC3T3-E1/(osteoblasts) | |||||||
| ECV304/blood vessel related cell | |||||||
| Zn alloyed Mg showed no observed negative effects on cell viabilities | VSMC/blood vessel related cell | ||||||
| K | Zr | Serious toxic effect to the tested cell line | 6.9 ± 1 μM/L | NIH3T3/L929/(fibroblasts) | |||
| ECV304/VSMC/blood vessel related cell | |||||||
| Platelets | |||||||
| T | Sn | Hemolysis and adhered platelets decreased for Mg-Sn as compared with Mg element. | 15.8 ± 7.8 μM/L | L929/NIH3T3/(fibroblasts) | |||
| Sn showed no decrease in cell viability. | |||||||
| MC3T3-E1/(osteoblasts) | |||||||
| VSMC/ECV304/blood vessel related cell | |||||||
| Showed negative effects on blood-vessel-related cell viabilities. Especially toxic from Mg-1Sn alloy extract. | |||||||
| Showed negative effects on blood-vessel-related cell viabilities. | ECV304 cells | ||||||
| Toxic at the tested concentration | MG63 | ||||||
| Mg-1Sn, Mg-3Sn are harmless to tested cells. | 1–3 wt.% | ATDC5 | 6 | [67] | |||
| V | Gd | Good cell viability results when tested with ATDC5 cells [9]. Mg10Gd promoted cell maturation and hypertrophy in vitro indicating enhanced healing possibilities in vivo [9]. | - | L929/(fibroblasts) | 7 | α-MEM + DMEM/F12-HAM | [68] |
| Q | Ag | Serious toxic effect to the tested cell lines. | 0.9 ± 0.5 μM/L | NIH3T3/(fibroblasts) | 7 | DMEM | [62] |
| MC3T3-E1/(osteoblasts) | |||||||
| ECV304/blood vessel related cell | |||||||
| ATDC5 | |||||||
| Good cell viability when tested with ATDC5 cells. | - | L929/(fibroblasts) | α-MEM + DMEM/F12-HAM | [68] | |||
| In | No significant viability changes. | 5 ± 1.8 μM/L | NIH3T3/(fibroblasts) | 7 | DMEM | [62] | |
| MC3T3-E1/(osteoblasts) | |||||||
| Toxic for tested cells. | ECV304/VSMC/blood vessel related cell | ||||||
| MC3T3-E1/(Murine calvarial preosteoblasts) | |||||||
| E | Nd and La | Cytotoxic for tested cells at high concentrations. Cytotoxicity decreased with lower concentrations. | - | MC3T3-E1/(Murine calvarial preosteoblasts) | 5 | [69] | |
| Ce | Severely cytotoxic for MC3T3-E1 cells even in low concentrations. | - |
| Electrochemical Test | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Composition (wt.%) | Work History | Duration | Corrosion Potential | Corrosion Current Density | Corrosion Rate | Immersion Test, CR (Mass Loss) | Ref | ||||
| SBF | Hank’s | SBF | Hank’s | SBF | Hank’s | Hank’s | (DMEM + 10%FBS) | ||||
| HRS | Ecorr (V) | Icorr (μA/cm2) | mm/yr | ||||||||
| Pure Mg | As-Cast | 168 | - | - | - | - | 9.55 ± 1.19 P | - | - | 0.66 ± 0.36 | [85] |
| 720 | - | - | - | - | - | 2.08 ± 0.2 | - | [84] | |||
| 72 | - | - | - | - | - | 1.318681 | - | [65] | |||
| 168 | - | - | - | - | - | 1.507064 | - | ||||
| 500 | 1.886 | 1.533 | 86.06 | 15.98 | 1.94 | 0.36 | - | - | [62] | ||
| As-Rolled | 500 | 1.796 | 1.544 | 37.24 | 9.58 | 0.84 | 0.22 | - | - | ||
| Mg–1Al | As-Cast | 500 | 1.777 | 1.522 | 91.81 | 17.58 | 2.07 | 0.4 | - | - | |
| 1.764 | 1.5 | 360.2 | 51.39 | 8.12 | 1.34 | - | - | ||||
| As-Rolled | 1.685 | 1.391 | 136.8 | 172.9 | 3.09 | 3.9 | - | - | |||
| 1.708 | 1.514 | 53.95 | 26 | 1.22 | 0.59 | - | - | ||||
| Mg–1In | As-Cast | 1.905 | 1.561 | 103 | 19.48 | 2.32 | 0.44 | - | - | ||
| As-Rolled | 1.863 | 1.472 | 42.6 | 16 | 0.96 | 0.36 | - | - | |||
| Mg–1Mn | As-Cast | 1.811 | 1.511 | 109.1 | 24.27 | 2.46 | 0.55 | - | - | ||
| As-Rolled | 1.825 | 1.486 | 20.15 | 5.71 | 0.45 | 0.13 | - | - | |||
| Mg–1Si | As-Cast | 1.568 | 1.513 | 296 | 47.95 | 6.68 | 1.08 | - | - | ||
| As-Rolled | 1.634 | 1.452 | 28.36 | 21.17 | 0.64 | 0.48 | - | - | |||
| Mg–1Sn | As-Cast | 1.893 | 1.621 | 108.8 | 16.3 | 2.45 | 0.37 | - | - | ||
| As-Rolled | 1.787 | 1.471 | 54.84 | 13.76 | 1.24 | 0.31 | - | - | |||
| Mg–1Y | As-Cast | 1.703 | 1.49 | 140 | 27.67 | 3.16 | 0.62 | - | - | ||
| As-Rolled | 1.848 | 1.502 | 73.06 | 16.63 | 1.65 | 0.38 | - | - | |||
| Mg–1Zn | As-Cast | 1.822 | 1.609 | 67.3 | 10.47 | 1.52 | 0.24 | - | - | ||
| As-Rolled | 1.805 | 1.549 | 40.78 | 7.55 | 0.92 | 0.17 | - | - | |||
| Mg–1Zr | As-Cast | 1.886 | 1.55 | 97.69 | 21.73 | 2.2 | 0.49 | - | - | ||
| As-Rolled | 1.633 | 1.522 | 40.2 | 12.15 | 0.91 | 0.27 | - | ||||
| Mg0.03Cu | As-Cast | 72 | - | - | - | - | - | 10.54945 | - | [65] | |
| As-Cast | 168 | - | - | - | - | - | 9.230769 | - | |||
| Mg0.5Sr | As-cast | 372 * | - | - | - | - | - | - | 1.157076 H | - | [110] |
| Cast (Homogenized at 450 °C + Quenched) | 372 * | - | - | - | - | - | - | 0.777605 H | - | ||
| Cast (Aged 150 °C, 360 h + Quenched) | 372 * | - | - | - | - | - | - | 0.827372 H | - | ||
| Mg–0.5Ca | As-Cast | 84 | −1.986 K | 186 K | 1.52 K | - | [86] | ||||
| Mg-Fe (Mg30Fe70) | Ball-milled, SPS (500 °C, 600 MPa, 10 min) | 240 | - | - | - | - | - | - | 0.00292 d | - | |
| Mg-0.69La | As-Cast | 250 | - | - | - | - | 14.7 ± 0.92 ** | - | - | - | [69] |
| Mg-1.27Ce | - | - | - | - | 9.6 ± 0.78 ** | - | - | - | |||
| Mg-2.13Nd | - | - | - | - | 4.1 ± 0.29 ** | - | - | - | |||
| Materials | YS, MPa | UTS, MPa | UCS, MPa | Elongation, % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| As-Cast | As-Rolled | As-Extruded | As-Cast | As-Rolled | As-Extruded | As-Cast | As-Rolled | As-Extruded | As-Cast | As-Rolled | As-Extruded | Ref | |
| Mg | 20.83 | 113.2 | - | 86.69 | 169.6 | - | - | - | - | 13.06 | 12.26 | - | [62] |
| Mg-1Al | 42.34 | 168.8 | - | 159.94 | 230.1 | - | - | - | - | 16.58 | 6.09 | - | |
| Mg-1Ag | 23.86 | 126.9 | - | 116.26 | 196.6 | - | - | - | - | 13.34 | 6.687 | - | |
| Mg-1In | 35.62 | 133.5 | - | 145.82 | 191.6 | - | - | - | - | 14.96 | 9.473 | - | |
| Mg-1Mn | 28.9 | 116.5 | - | 82.99 | 172.1 | - | - | - | - | 7.536 | 3.741 | - | |
| Mg-1Si | 80.3 | 120.7 | - | 194.21 | 196.1 | - | - | - | - | 14.85 | 3.582 | - | |
| Mg-1Sn | 35.28 | 146 | - | 149.18 | 203.2 | - | - | - | - | 20.04 | 6.647 | - | |
| Mg-1Y | 25.54 | 146.8 | - | 74.59 | 199.9 | - | - | - | - | 9.992 | 9.154 | - | |
| Mg-1Zn | 25.54 | 160.5 | - | 133.39 | 239.7 | - | - | - | - | 18.25 | 7.124 | - | |
| Mg-1Zr | 67.2 | 131 | - | 172.03 | 182.9 | - | - | - | - | 27.02 | 17.27 | - | |
| Mg-1Ca | 40.26 | 123.7 | 136.2 | 71.54 | 166.8 | 240.13 | - | - | - | 1.911 | 3.196 | 10.81 | |
| Mg-0.57Cu | - | - | - | 104.14 | - | - | 167.48 | - | - | - | - | - | [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, S.F.; Islam, M.T.; Saheb, N.; Baig, M.M.A. Magnesium for Implants: A Review on the Effect of Alloying Elements on Biocompatibility and Properties. Materials 2022, 15, 5669. https://doi.org/10.3390/ma15165669
Hassan SF, Islam MT, Saheb N, Baig MMA. Magnesium for Implants: A Review on the Effect of Alloying Elements on Biocompatibility and Properties. Materials. 2022; 15(16):5669. https://doi.org/10.3390/ma15165669
Chicago/Turabian StyleHassan, S. Fida, M. T. Islam, N. Saheb, and M. M. A. Baig. 2022. "Magnesium for Implants: A Review on the Effect of Alloying Elements on Biocompatibility and Properties" Materials 15, no. 16: 5669. https://doi.org/10.3390/ma15165669
APA StyleHassan, S. F., Islam, M. T., Saheb, N., & Baig, M. M. A. (2022). Magnesium for Implants: A Review on the Effect of Alloying Elements on Biocompatibility and Properties. Materials, 15(16), 5669. https://doi.org/10.3390/ma15165669







