WSSe Nanocomposites for Enhanced Photocatalytic Hydrogen Evolution and Methylene Blue Removal under Visible-Light Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. Characterization
2.4. Photocatalytic Hydrogen Production
2.5. Photocatalytic MB Degradation Activity
3. Results
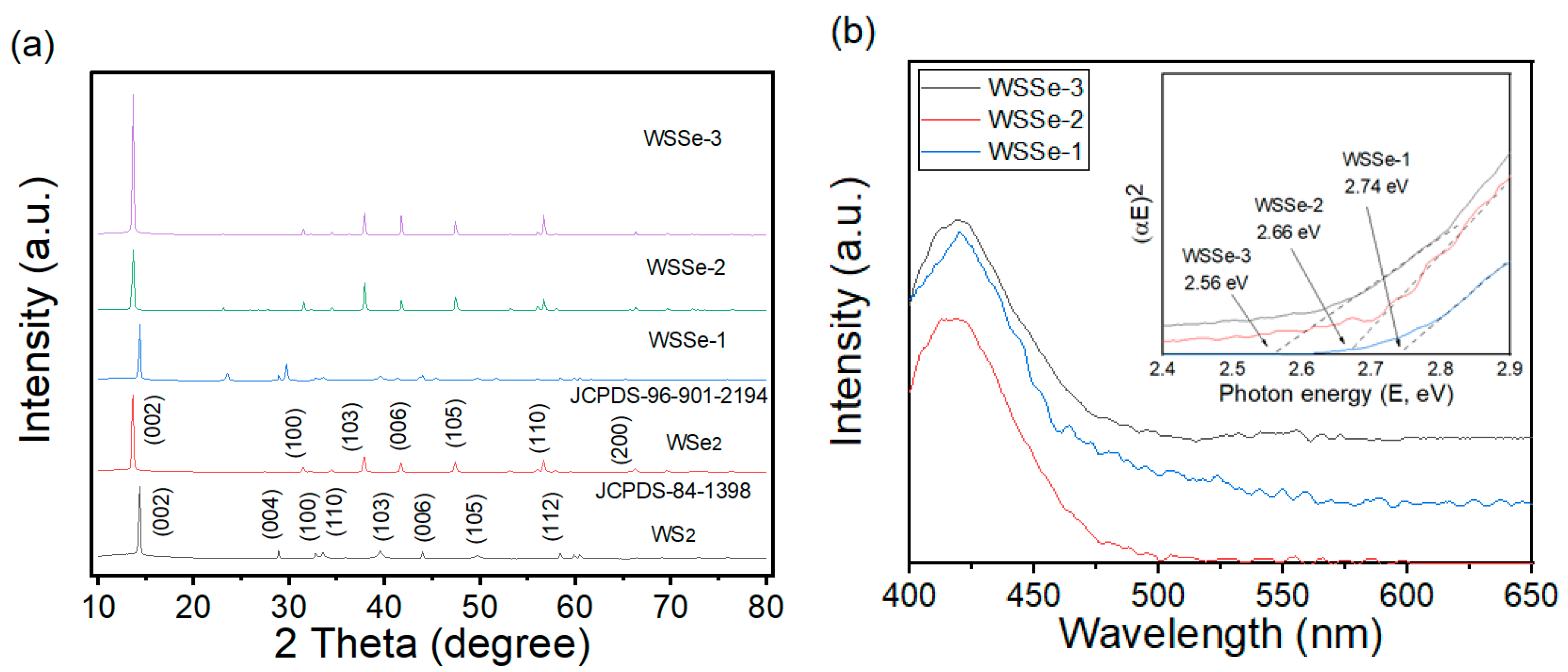
3.1. XRD and UV-Vis Analysis
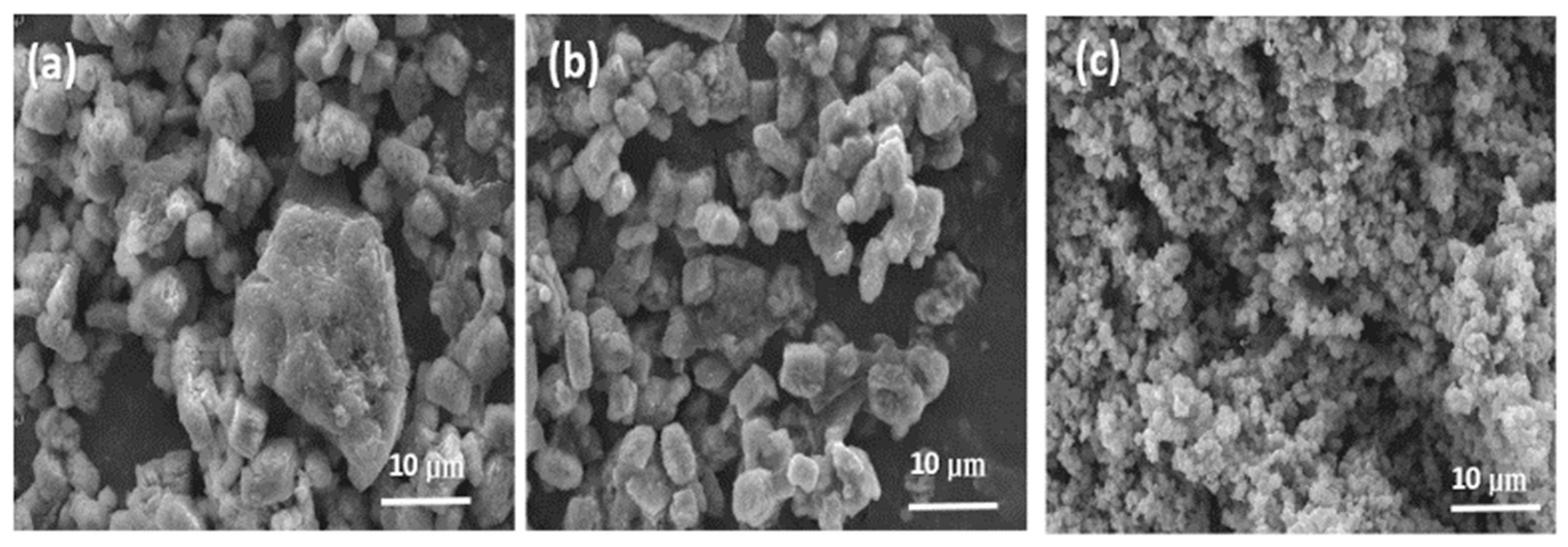
3.2. SEM and TEM Analysis
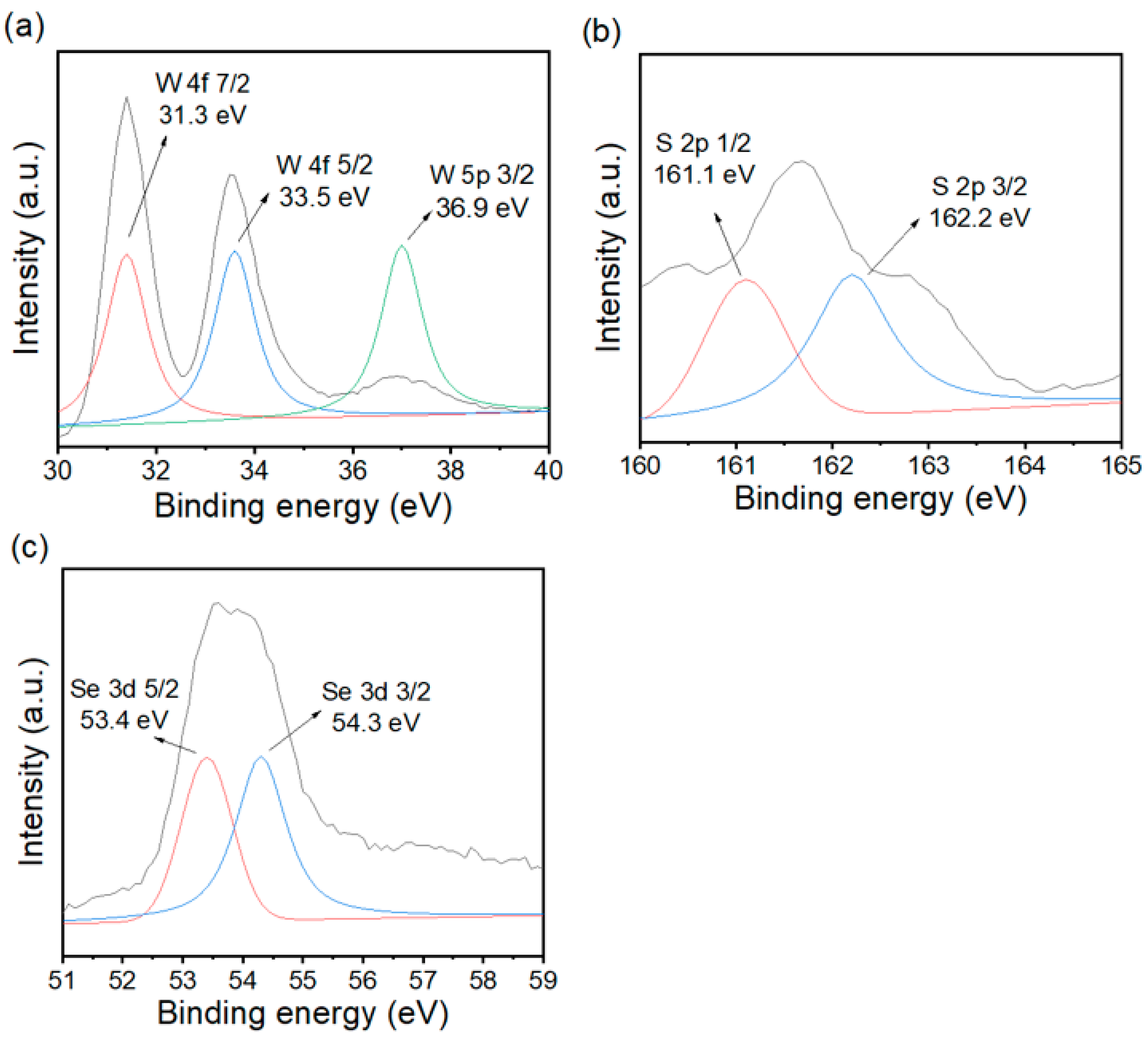
3.3. XPS and N2 Adsorption–Desorption Analysis
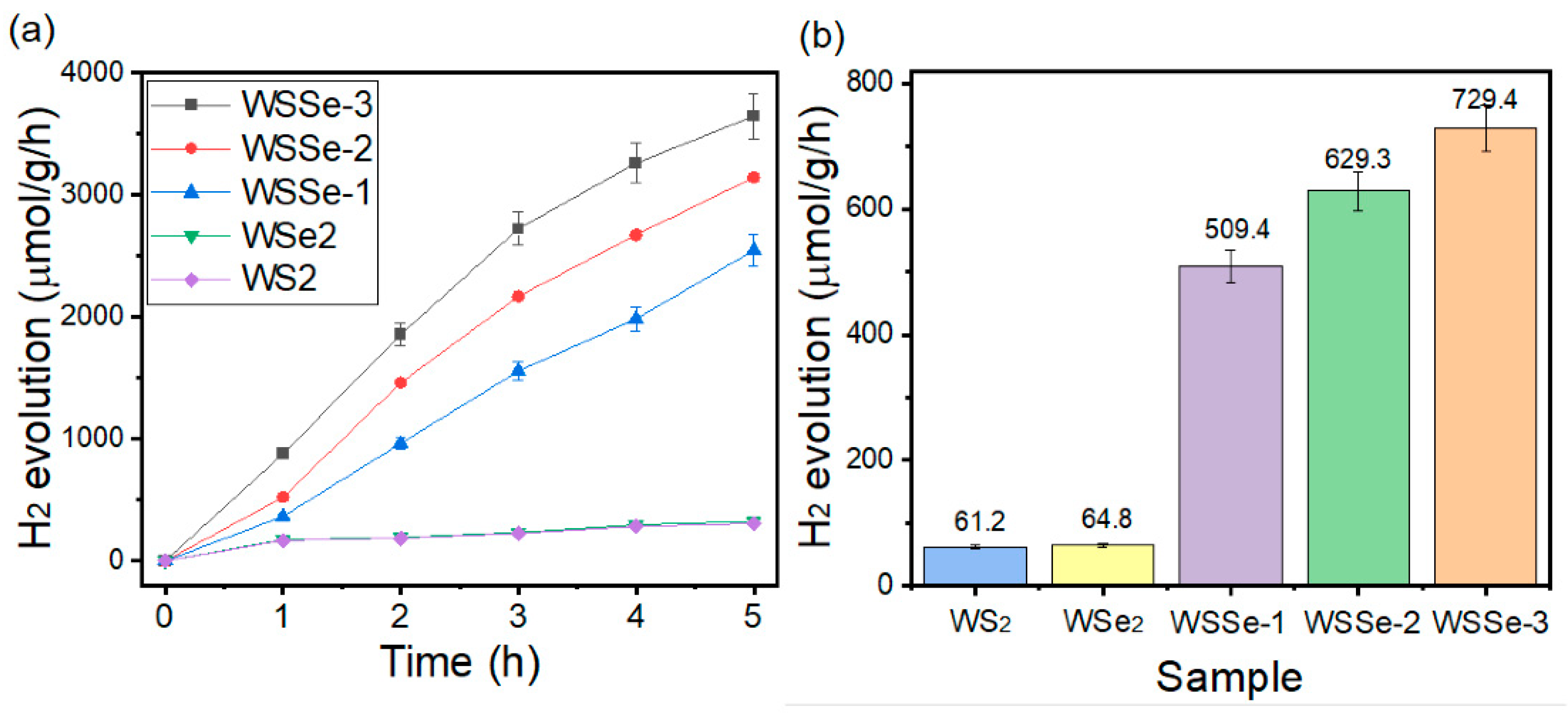
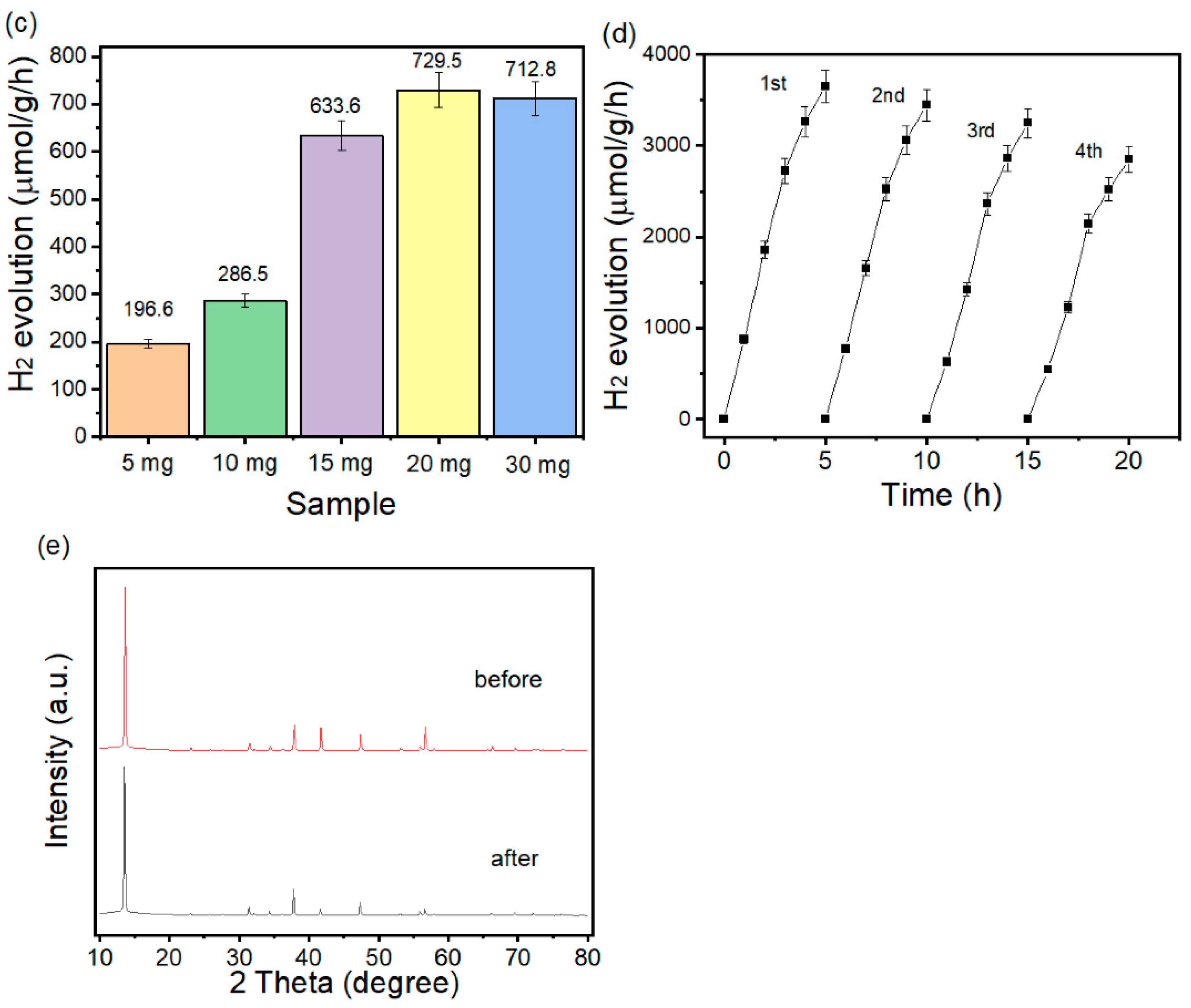
3.4. Photocatalytic Hydrogen Production Studies
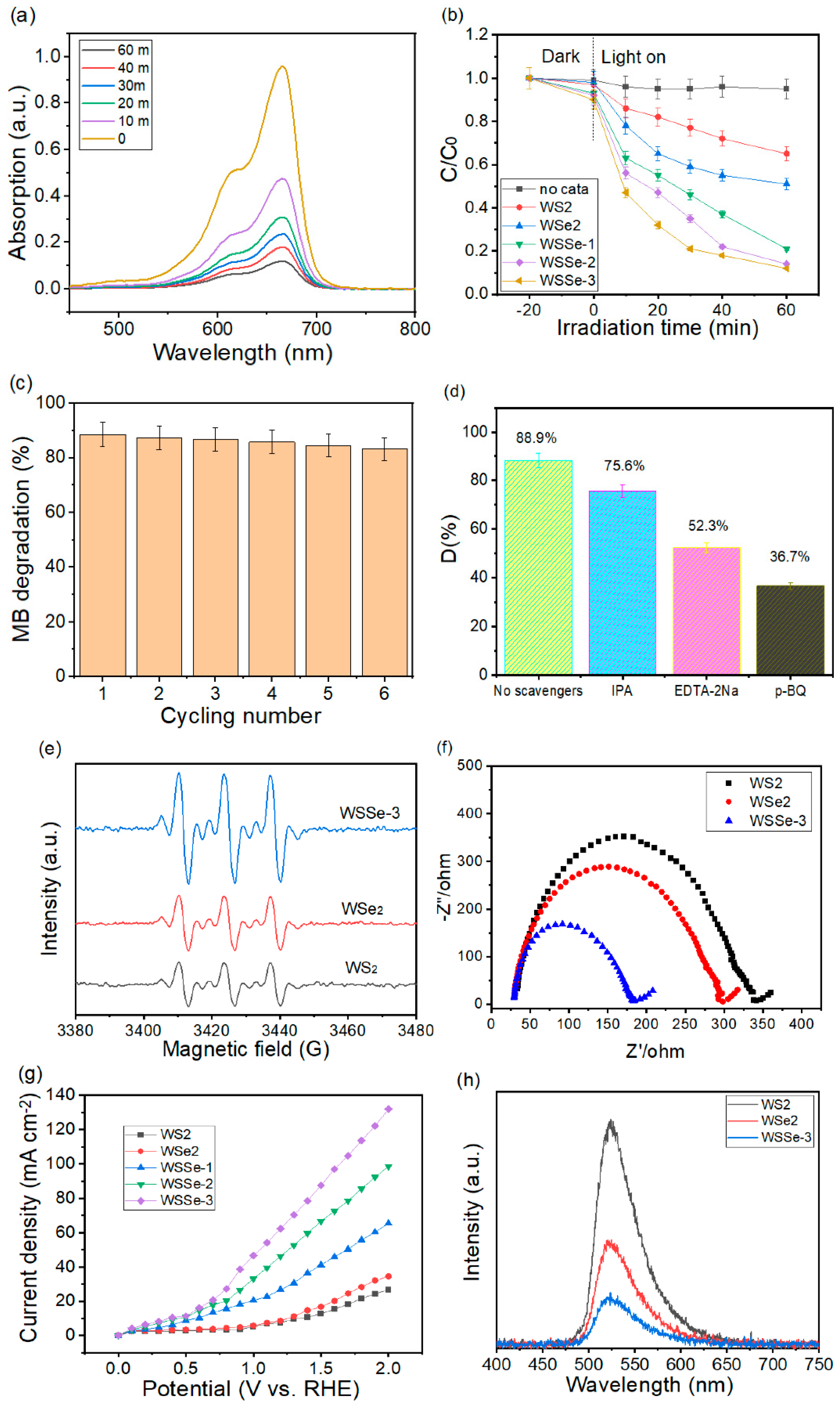
3.5. Photocatalytic MB Degradation Studies
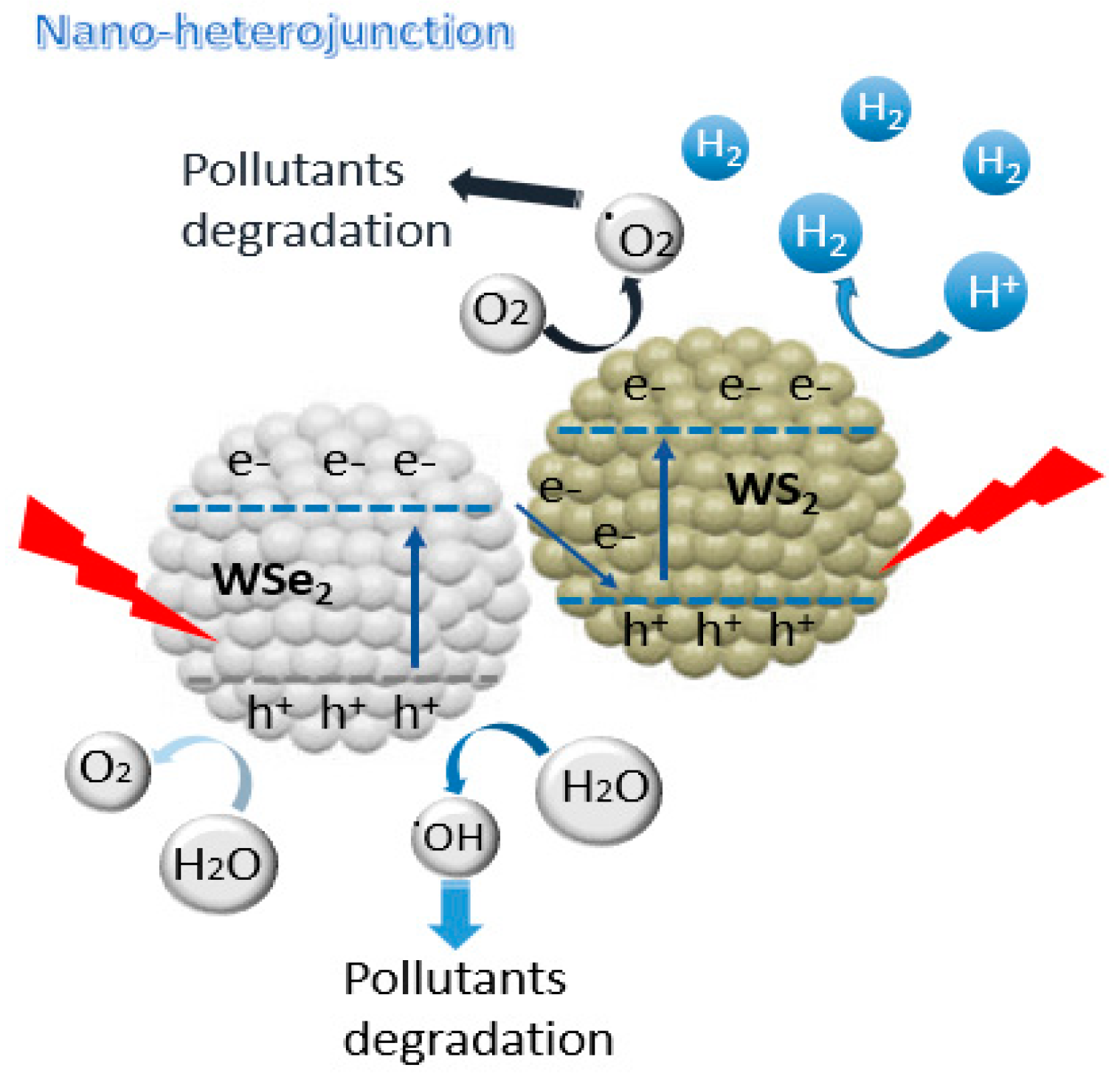
3.6. Proposed Photocatalytic H2 and MB Degradation Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hong, W.; Li, C.; Tang, T.; Xu, H.; Yu, Y.; Liu, G.; Wang, F.; Leia, C.; Zhu, H. The photocatalytic activity of the SnO2/TiO2/PVDF composite membrane in rhodamine B degradation. New J. Chem. 2021, 45, 2631. [Google Scholar] [CrossRef]
- Hao, X.; Guo, Q.; Li, M.; Jin, Z.; Wang, Y. TiO2 as an interfacial-charge-transfer-bridge to construct Eosin Y-mediated direct Z-scheme electron transfer over Co9S8 quantum dots/TiO2 photocatalyst. Catal. Sci. Technol. 2020, 10, 5267–5280. [Google Scholar] [CrossRef]
- Mannaa, M.A.; Qasim, K.F.; Alshorifi, F.T.; El-Bahy, S.M.; Salama, R.S. Role of NiO Nanoparticles in Enhancing Structure Properties of TiO2 and Its Applications in Photodegradation and Hydrogen Evolution. ACS Omega 2021, 6, 30386. [Google Scholar] [CrossRef] [PubMed]
- El-Hakam, S.A.; ALShorifi, F.T.; Salama, R.S.; Gamal, S.; El-Yazeed, W.S.A.; Ibrahim, A.A.; Ahmed, A.I. Application of nanostructured mesoporous silica/ bismuth vanadate composite catalysts for the degradation of methylene blue and brilliant green. J. Mater. Res. Technol. 2022, 18, 1963. [Google Scholar] [CrossRef]
- Alshorifi, F.T.; Alswat, A.A.; Mannaa, M.A.; Alotaibi, M.T.; El-Bahy, S.M.; Salama, R.S. Facile and Green Synthesis of Silver Quantum Dots Immobilized onto a Polymeric CTS−PEO Blend for the Photocatalytic Degradation of p-Nitrophenol. ACS Omega 2021, 6, 30432. [Google Scholar] [CrossRef]
- Chen, Z.; Li, S.; Peng, Y.; Hu, C. Tailoring aromatic ring-terminated edges of g-C3N4 nanosheets for efficient photocatalytic hydrogen evolution with simultaneous antibiotic removal. Catal. Sci. Technol. 2020, 10, 5470–5479. [Google Scholar] [CrossRef]
- Shen, Q.; Gómez-García, C.J.; Sun, W.; Lai, X.; Pang, H.; Ma, H. Improving the photocatalytic H2 evolution activity of Keggin polyoxometalates anchoring copperazole complexes. Green Chem. 2021, 23, 3104. [Google Scholar] [CrossRef]
- Irfan, M.; Shukrullah, S.; Naz, M.Y.; Ahmad, I.; Shoukat, B.; Legutko, S.; Petrů, J.; Rahman, S.; Alsaiari, M.A. Si/SiO2/Al2O3 Supported Growth of CNT Forest for the Production of La/ZnO/CNT Photocatalyst for Hydrogen Production. Materials 2022, 15, 3226. [Google Scholar] [CrossRef]
- Lettieri, S.; Pavone, M.; Fioravanti, A.; Amato, L.S.; Maddalena, P. Charge Carrier Processes and Optical Properties in TiO2 and TiO2-Based Heterojunction Photocatalysts: A Review. Materials 2021, 14, 1645. [Google Scholar] [CrossRef]
- Li, N.; He, Y.; Lian, J.; Liua, Q.; Zhang, X.; Zhang, X. Facile fabrication of NiO/Ag3PO4 Z-scheme photocatalyst with enhanced visible-light-driven photocatalytic activity. New J. Chem. 2020, 44, 12806–12814. [Google Scholar] [CrossRef]
- Dong, H.; Li, J.; Chen, M.; Wang, H.; Jiang, X.; Xiao, Y.; Tian, B.; Zhang, X. High-throughput Production of ZnO-MoS2-Graphene Heterostructures for Highly Efficient Photocatalytic Hydrogen Evolution. Materials 2019, 12, 2233. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zheng, J.; Li, W.; Wang, R.; Li, D.; Guo, X.; Rodriguez, R.D.; Jia, X. Engineering Z-scheme TiO2-OV-BiOCl via oxygen vacancy for enhanced photocatalytic degradation of imidacloprid. Dalton Trans. 2020, 49, 11010–11018. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Patnaik, S.; Parida, K. Dynamic charge transfer through Fermi level equilibration in the p-CuFe2O4/n-NiAl LDH interface towards photocatalytic application. Catal. Sci. Technol. 2020, 10, 6285–6298. [Google Scholar] [CrossRef]
- Zhen, Y.; Yang, C.; Shen, H.; Xue, W.; Gu, C.; Feng, J.; Zhang, Y.; Fu, F.; Liang, Y. Photocatalytic performance and mechanism insights of S-scheme g-C3N4/Bi2MoO6 heterostructure in phenol degradation and hydrogen evolution reaction under visible light. Phys. Chem. Chem. Phys. 2020, 22, 26278–26288. [Google Scholar] [CrossRef] [PubMed]
- Velpandian, M.; Pulipaka, S.; Tikoo, A.; Meduri, P. Improved charge carrier dynamics of WS2 nanostructures by the way of CdS@WS2 heterostructures for use in water splitting and water purification. Sustain. Energy Fuels 2020, 4, 4096–4107. [Google Scholar] [CrossRef]
- Gao, D.; Zhong, W.; Wang, X.; Chen, F.; Yu, H. Increasing unsaturated Se number and facilitating atomic hydrogen adsorption of WSe2+x nanodots for improving photocatalytic H2 production of TiO2. J. Mater. Chem. A 2022, 10, 7989–7998. [Google Scholar] [CrossRef]
- Wang, W.; Gu, W.; Li, G.; Xie, H.; Wong, P.K.; An, T. Few-layered Tungsten Selenide as Cocatalyst for Visible-lightdriven Photocatalytic Production of Hydrogen Peroxide for Bacterial Inactivation. Environ. Sci. Nano 2020, 7, 3877–3887. [Google Scholar] [CrossRef]
- Bano, K.; Mittal, S.K.; Singh, P.P.; Kaushal, S. Sunlight driven photocatalytic degradation of organic pollutants using a MnV2O6/BiVO4 heterojunction: Mechanistic perception and degradation pathways. Nanoscale Adv. 2021, 3, 6446. [Google Scholar] [CrossRef]
- Afroz, K.; Moniruddin, M.; Bakranov, N.; Nuraje, N. Heterojunction strategy to improve visible light sensitive water splitting performance of photocatalytic materials. J. Mater. Chem. A 2018, 6, 21696–21718. [Google Scholar] [CrossRef]
- Hu, Q.; Niu, J.; Zhang, K.Q.; Yao, M. Fabrication of Mn-Doped SrTiO3/Carbon Fiber with Oxygen Vacancy for Enhanced Photocatalytic Hydrogen Evolution. Materials 2022, 15, 4723. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Q. Photo(electro)catalyst of Flower-Like Cobalt Oxide Co-Doped g-C3N4: Degradation of Methylene Blue under Visible Light Illumination. Materials 2022, 15, 4104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, Z.; Gong, T.; Pan, R. Recent Advances of Emerging Janus Two-dimensional Materials: From Fundamental Physics to Device Applications. J. Mater. Chem. A 2020, 8, 8813–8830. [Google Scholar] [CrossRef]
- Fan, Z.; Meng, F.; Zhang, M.; Wu, Z.; Sun, Z.; Li, A. Solvothermal synthesis of hierarchical TiO2 nanostructures with tunable morphology and enhanced photocatalytic activity. Appl. Surf. Sci. 2016, 360, 298. [Google Scholar] [CrossRef]
- Fang, G.Z.; Wang, Q.C.; Zhou, J.; Lei, Y.P.; Chen, Z.X.; Wang, Z.Q. Metal organic framework-templated synthesis of bimetallic selenides with rich phase boundaries for sodium-ion storage and oxygen evolution reaction. ACS Nano 2019, 13, 5635. [Google Scholar] [CrossRef] [PubMed]
- Pataniya, P.M.; Sumesh, C.K. Enhanced electrocatalytic hydrogen evolution reaction by injection of photogenerated electrons in Ag/WS2 nanohybrids. Appl. Surf. Sci. 2021, 563, 150323. [Google Scholar] [CrossRef]
- Keerthana, B.G.T.; Murugakoothan, P. Synthesis and characterization of CdS/TiO2 nanocomposite: Methylene blue adsorption and enhanced photocatalytic activities. Vacuum 2019, 159, 476. [Google Scholar] [CrossRef]
- Mansha, M.S.; Iqbal, T. Experimental and theoretical study of novel rGO/AgVO3 nano-hetrostructures for their application as efficient photocatalyst. Opt. Mater. 2022, 131, 112591. [Google Scholar] [CrossRef]
- Alkanad, K.; Hezam, A.; Shekar, G.C.S.; Drmosh, Q.A.; Kala, A.L.A.; AL-Gunaid, M.Q.A.; Lokanath, N.K. Magnetic recyclable α-Fe2O3–Fe3O4/Co3O4–CoO nanocomposite with a dual Z-scheme charge transfer pathway for quick photo-Fenton degradation of organic pollutants. Catal. Sci. Technol. 2021, 11, 3084. [Google Scholar] [CrossRef]
- Majhi, D.; Das, K.; Bariki, R.; Padhan, S.; Mishra, A.; Dhiman, R.; Dash, P.; Nayakc, B.; Mishra, B.G. A facile reflux method for in situ fabrication of a non-cytotoxic Bi2S3/b-Bi2O3/ZnIn2S4 ternary photocatalyst: A novel dual Z-scheme system with enhanced multifunctional photocatalytic activity. J. Mater. Chem. A 2020, 8, 21729–21743. [Google Scholar] [CrossRef]
- Nakayama, M.; Takeda, A.; Maruyama, H.; Kumbhar, V.; Crosnier, O. Cobalt-substituted iron-based wolframite synthesized via polyol route for efficient oxygen evolution reaction. Electrochem. Commun. 2020, 120, 106834. [Google Scholar] [CrossRef]
- Wang, C.; Wang, R.; Peng, Y.; Chen, J.; Li, J. Iron tungsten mixed composite as a robust oxygen evolution electrocatalyst. Chem. Commun. 2019, 55, 10944. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; He, Q.; Hu, Y.; TaylorIsimjan, T.; Hou, R.; Yang, X. Interface engineering of porous Fe2P-WO2.92 catalyst with oxygen vacancies for highly active and stable large-current oxygen evolution and overall water splitting. J. Energy Chem. 2022, 65, 574. [Google Scholar] [CrossRef]
- He, J.; Zhou, X.; Xu, P.; Sun, J. Promoting electrocatalytic water oxidation through tungsten-modulated oxygen vacancies on hierarchical FeNi-layered double hydroxide. Nano Energy 2021, 80, 105540. [Google Scholar] [CrossRef]
- Palanisamy, G.; Bhuvaneswari, K.; Bharathi, G.; Pazhanivel, T.; Dhanalakshmi, M. Improved photocatalytic performance of magnetically recoverable Bi2Te3/ CdS/CuFe2O4 nanocomposite for MB dye under visible light exposure. Solid State Sci. 2021, 115, 106584. [Google Scholar] [CrossRef]
- Joorabi, F.T.; Kamali, M.; Sheibani, S. Effect of aqueous inorganic anions on the photocatalytic activity of CuO–Cu2O nanocomposite on MB and MO dyes degradation. Mater. Sci. Semicond. Process. 2022, 139, 106335. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, R.D.; Purohit, L.P. Novel ZnO tetrapod-reduced graphene oxide nanocomposites for enhanced photocatalytic degradation of phenolic compounds and MB dye. J. Mol. Liq. 2021, 327, 114814. [Google Scholar] [CrossRef]
- Padmini, M.; Balaganapathi, T.; Thilakan, P. Rutile-TiO2: Post heat treatment and its influence on the photocatalytic degradation of MB dye. Ceram. Int. 2022, 48, 16685. [Google Scholar] [CrossRef]
- Arif, N.; Wang, Z.X.; Wang, Y.T.; Dou, Y.C.; Li, K.; Liu, S.Q.; Liu, F.T. Design of earth-abundant Z-scheme g-C3N4/rGO/ FeOOH ternary heterojunctions with excellent photocatalytic activity. CrystEngComm 2021, 23, 1991. [Google Scholar] [CrossRef]
- Shelke, H.D.; Machale, A.R.; Survase, A.A.; Pathan, H.M.; Lokhande, C.D.; Lokhande, A.C.; Shaikh, S.F.; Rana, A.H.S.; Palaniswami, M. Multifunctional Cu2SnS3 Nanoparticles with Enhanced Photocatalytic Dye Degradation and Antibacterial Activity. Materials 2022, 15, 3126. [Google Scholar] [CrossRef]
- Li, B.; Wang, W.; Zhao, J.; Wang, Z.; Su, B.; Hou, Y.; Ding, Z.; Ong, W.J.; Wang, S. All-solid-state direct Z-scheme NiTiO3/Cd0.5Zn0.5S heterostructures for photocatalytic hydrogen evolution with visible light. J. Mater. Chem. A 2021, 9, 10270. [Google Scholar] [CrossRef]

| Samples | Specific Surface Area (m2/g, BET) a | Total Pore Volume (cm3/g, BET) b | Average Pore Diameter (nm, BJH) b |
|---|---|---|---|
| WSe2 | 33.6 ± 5 | 0.182 ± 0.02 | 38.4 ± 5 |
| WS2 | 23.3 ± 5 | 0.113 ± 0.02 | 48.5 ± 5 |
| WSSe-3 | 67.4 ± 5 | 0.325 ± 0.02 | 8.7 ± 3 |
| Photocatalyst | Tafel Slope (mV/dec) | Overpotential at 10 mA/cm2 | Exchange Current Density (mA/cm2) | Electrolytes | Reference |
|---|---|---|---|---|---|
| Co0.5Fe0.5WO4 | 36.3 | 360 mV | 10 | 1.0 M KOH | [30] |
| Fe-WOx | 51.7 | 0.38 V | 10 | 1.0 M KOH | [31] |
| Fe2P-WO2.92/NF | 46.3 | 267 mV | 100 | 1.0 M KOH | [32] |
| FeNiW-LDH | 55.7 | 202 mV | 10 | 1.0 M KOH | [33] |
| WSSe | 65.2 | 252 mV | 10 | 0.1 M KOH | This work |
| Photocatalyst | Catalyst Loading | Methylene Blue | Time | Degradation Efficiency | Reference |
|---|---|---|---|---|---|
| rGO/AgVO3 | 20 mg | 25 ppm | 2.5 h | 88% | [27] |
| Bi2Te3/CdS/CuFe2O4 | 50 mg | 40 ppm | 2 h | 97% | [34] |
| CuO–Cu2O | 40 mg | 5 ppm | 4 h | 90% | [35] |
| ZnO | 30 mg | 20 ppm | 1.5 h | 98% | [36] |
| R–TiO2 | 50 mg | 10 ppm | 6 h | 90% | [37] |
| WSSe | 20 mg | 10 ppm | 1 h | 89% | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tien, T.-M.; Chung, Y.-J.; Huang, C.-T.; Chen, E.L. WSSe Nanocomposites for Enhanced Photocatalytic Hydrogen Evolution and Methylene Blue Removal under Visible-Light Irradiation. Materials 2022, 15, 5616. https://doi.org/10.3390/ma15165616
Tien T-M, Chung Y-J, Huang C-T, Chen EL. WSSe Nanocomposites for Enhanced Photocatalytic Hydrogen Evolution and Methylene Blue Removal under Visible-Light Irradiation. Materials. 2022; 15(16):5616. https://doi.org/10.3390/ma15165616
Chicago/Turabian StyleTien, Tsung-Mo, Yu-Jen Chung, Chen-Tang Huang, and Edward L. Chen. 2022. "WSSe Nanocomposites for Enhanced Photocatalytic Hydrogen Evolution and Methylene Blue Removal under Visible-Light Irradiation" Materials 15, no. 16: 5616. https://doi.org/10.3390/ma15165616
APA StyleTien, T.-M., Chung, Y.-J., Huang, C.-T., & Chen, E. L. (2022). WSSe Nanocomposites for Enhanced Photocatalytic Hydrogen Evolution and Methylene Blue Removal under Visible-Light Irradiation. Materials, 15(16), 5616. https://doi.org/10.3390/ma15165616






