Degradation of CdS Yellow and Orange Pigments: A Preventive Characterization of the Process through Pump–Probe, Reflectance, X-ray Diffraction, and Raman Spectroscopy
Abstract
1. Introduction
1.1. Yellow and Orange Cadmium Pigments
1.2. CdS Degradation Phenomena
2. Materials and Methods
2.1. Mock-Up Samples
2.2. Reflectance Measurements
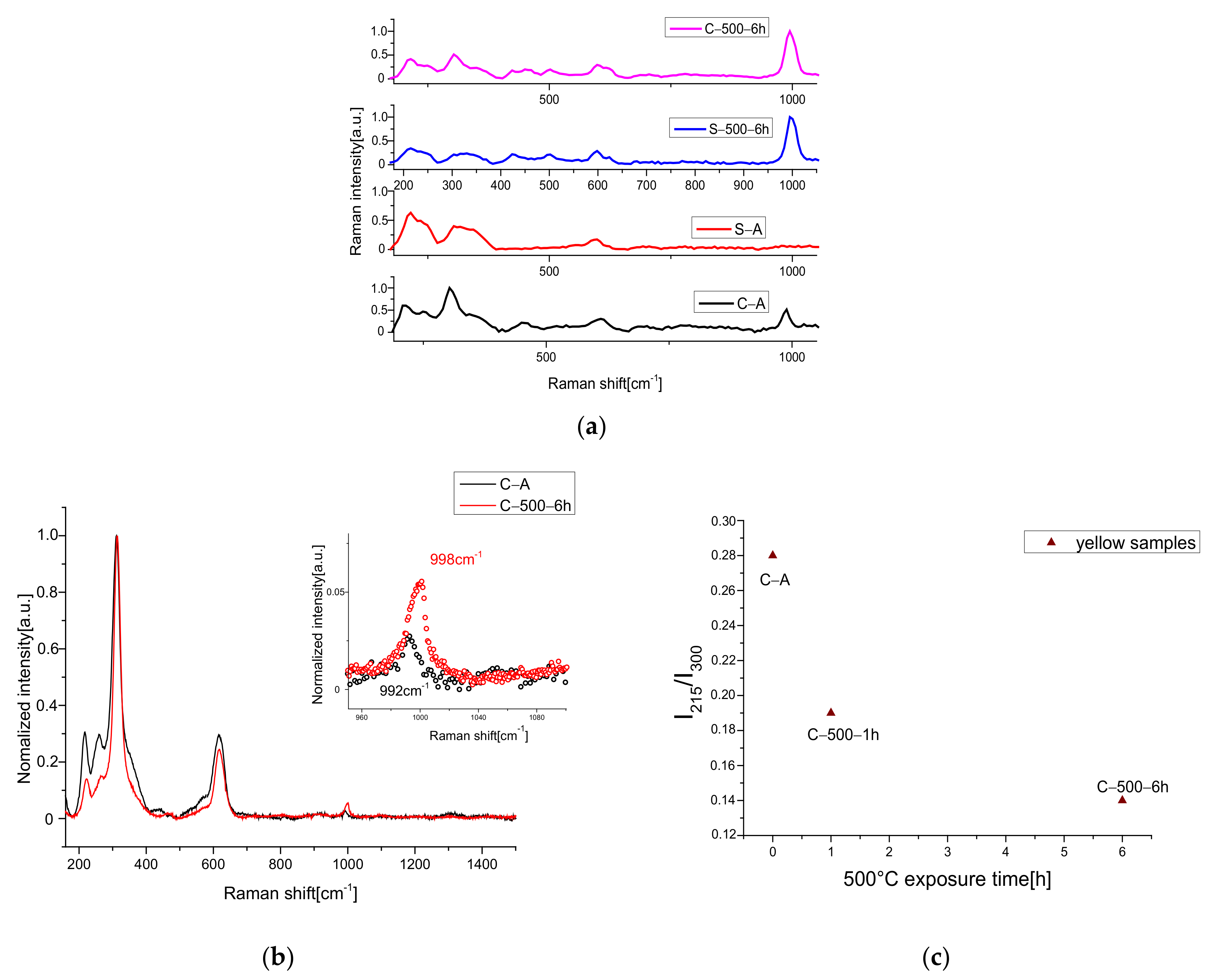
2.3. Raman Measurements
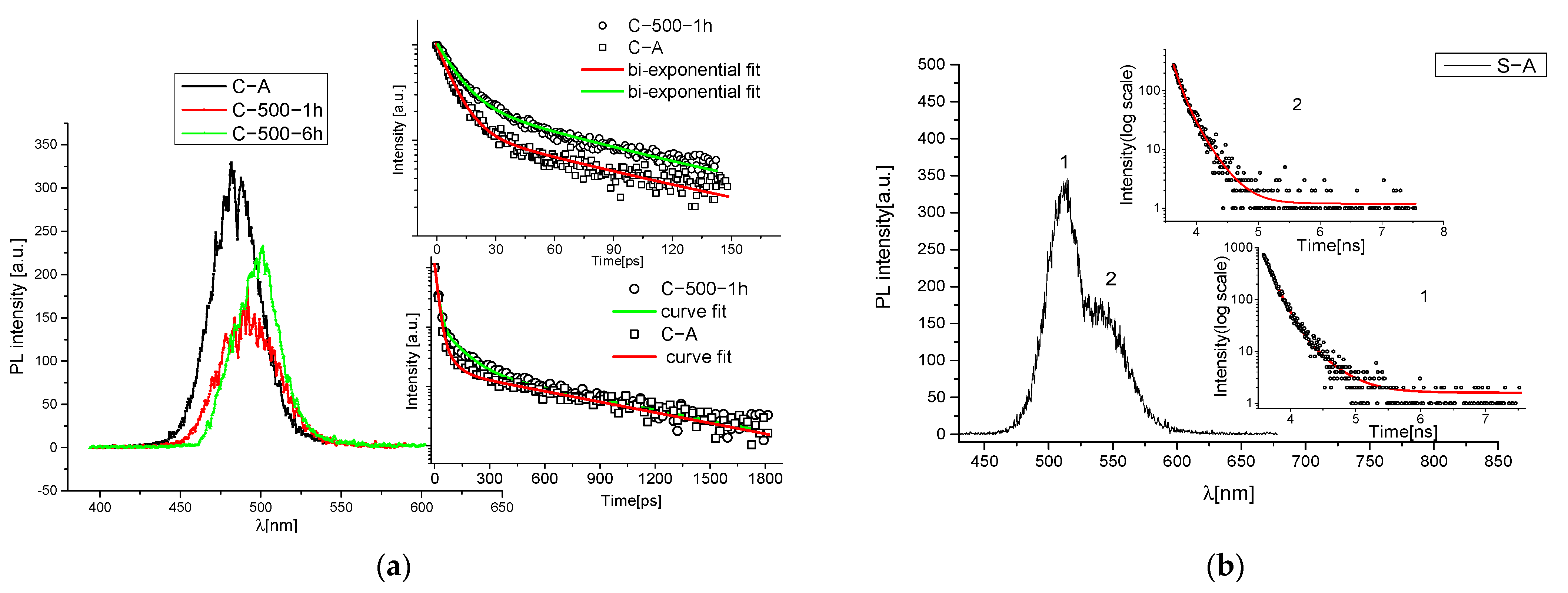
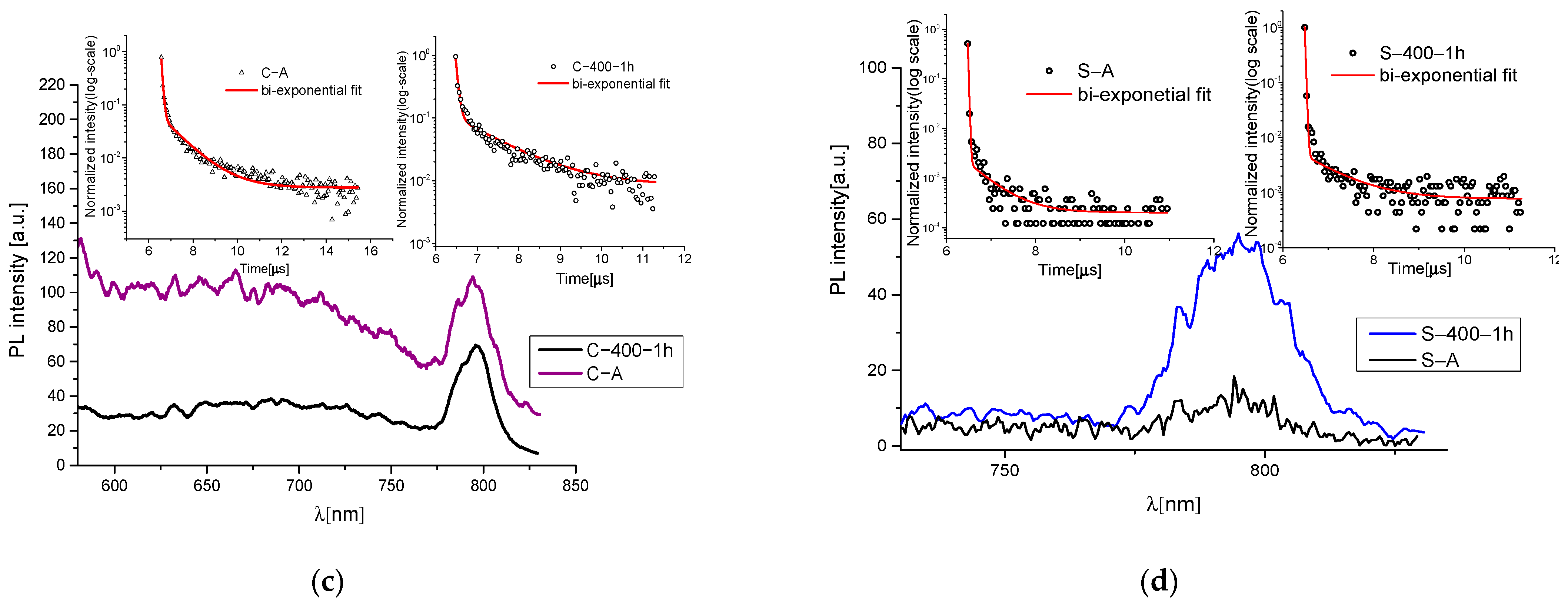
2.4. Time-Resolved Photoluminescence (TR-PL)
- Excitation with 100 fs long pulses delivered by an optical parametric amplifier (Light Conversion TOPAS-800-fs-UV-1) pumped by a regenerative Ti:sapphire amplifier (Coherent Libra-F-1K-HE230). The repetition frequency was 1 kHz.
- Excitation with 100 fs long pulses from Ti:sapphire oscillator Coherent Chameleon Ultra II having a repetition rate of 80 MHz.
2.5. Pump and Probe Transient Absorption Spectroscopy (TAS)
2.6. XRD Measurements
2.7. SEM-EDS
3. Results and Discussion
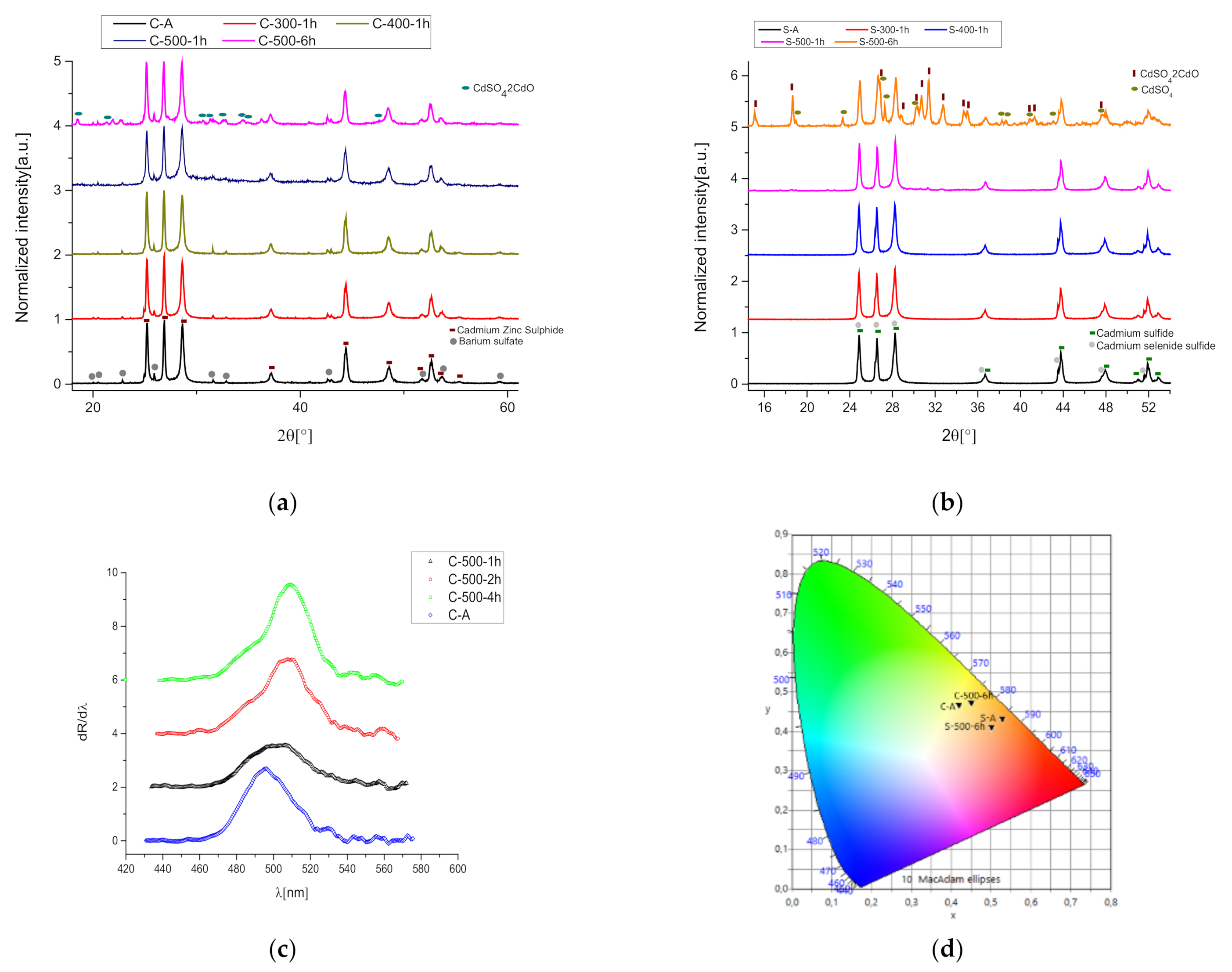
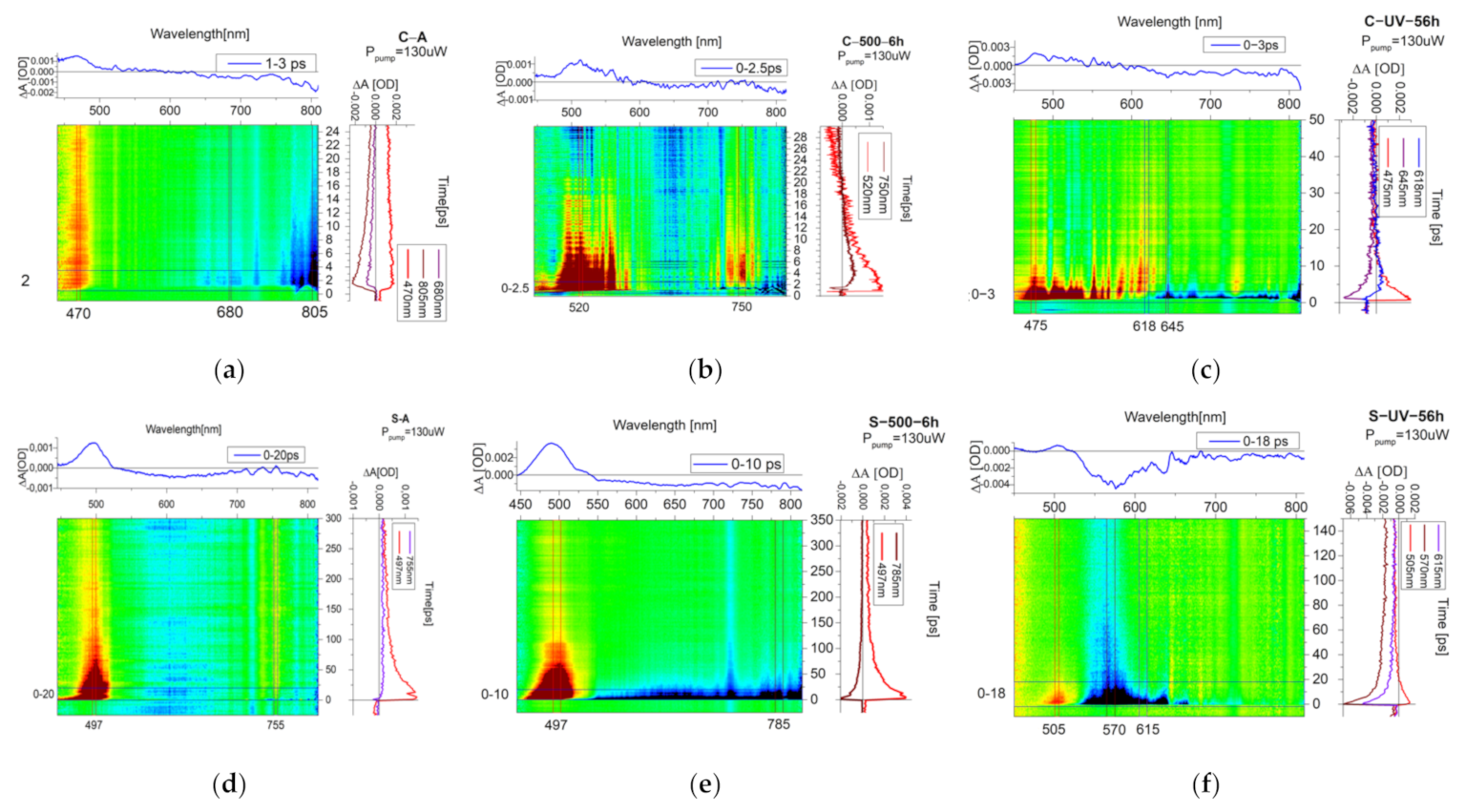
3.1. Heating Process
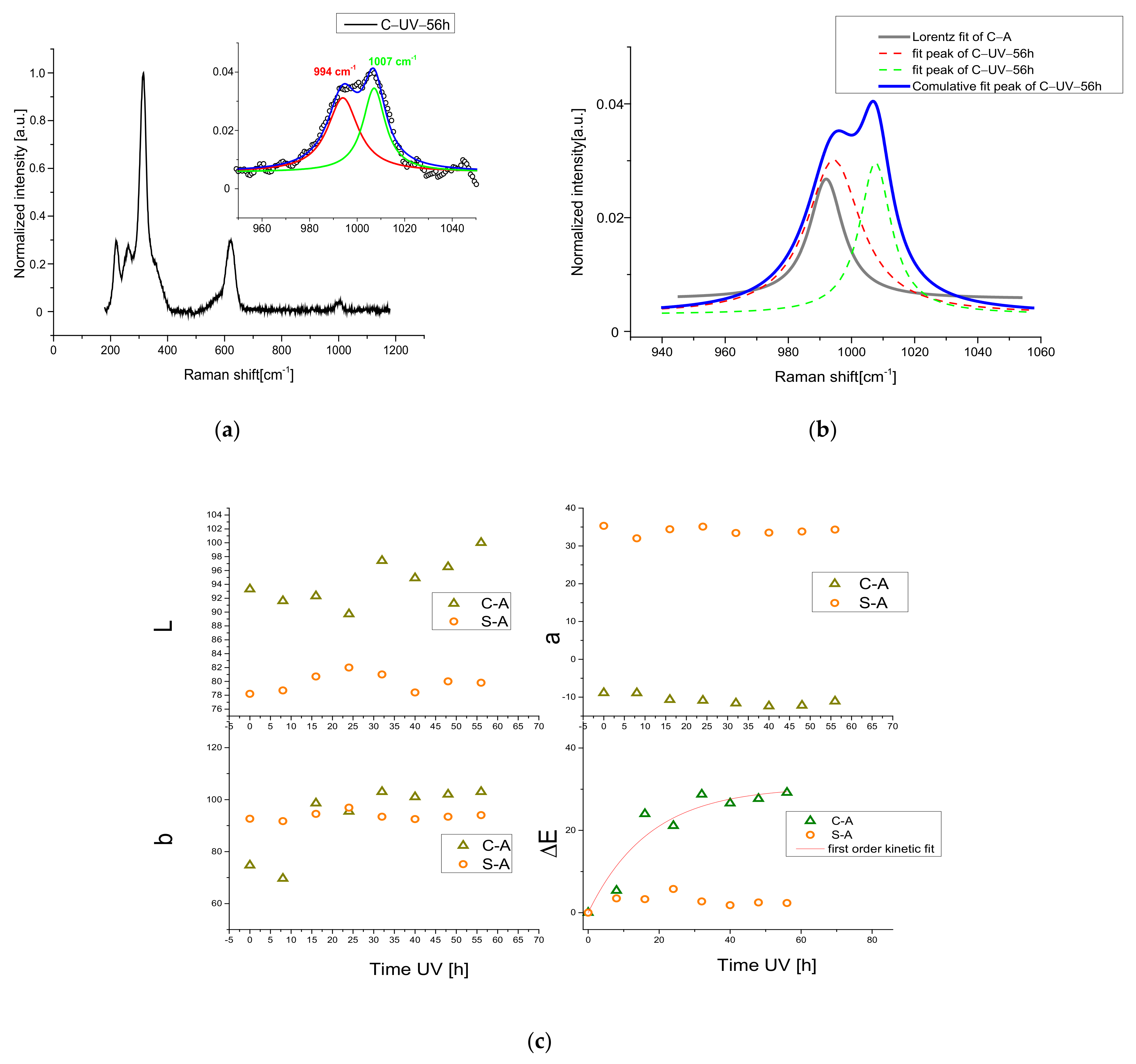
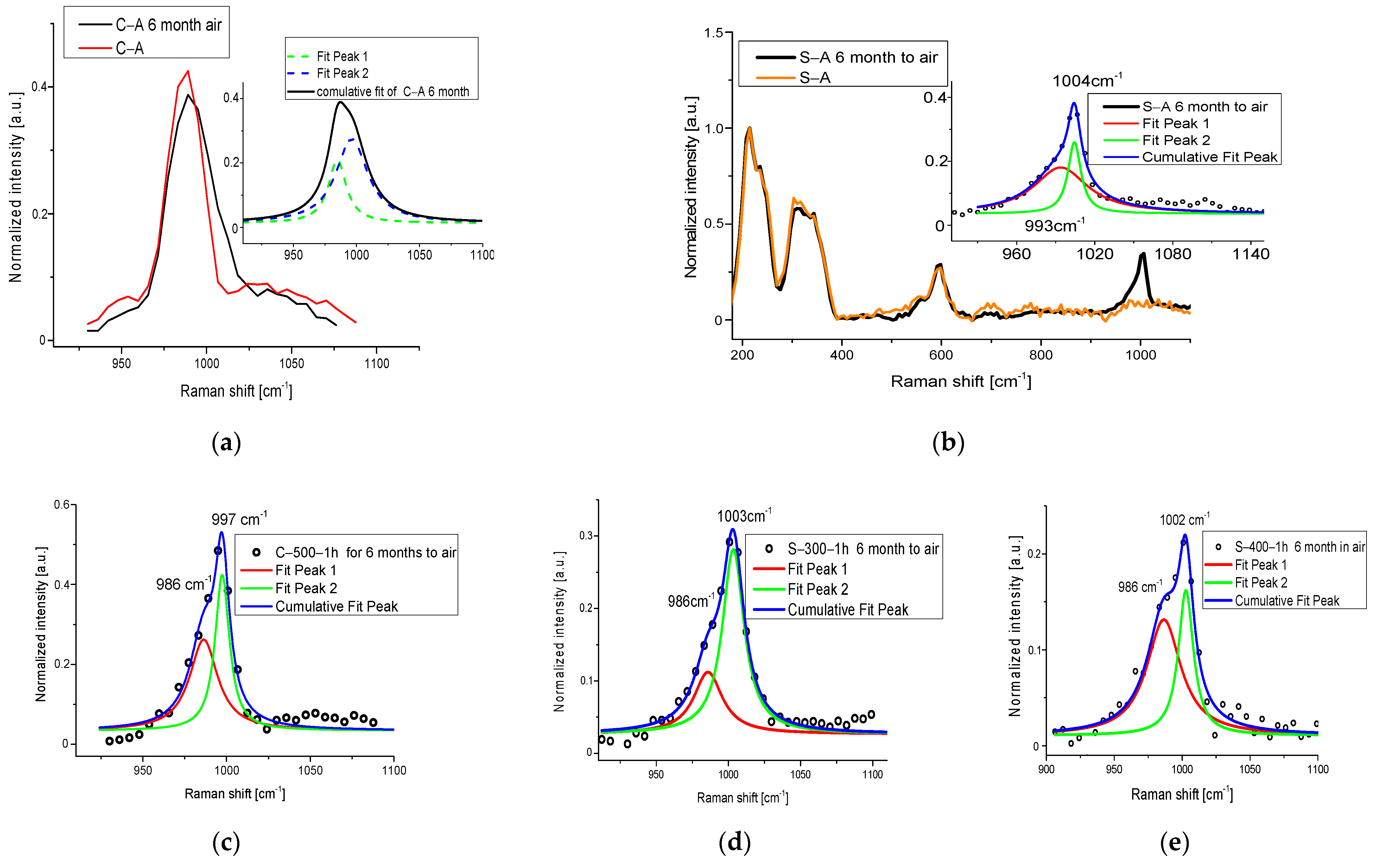
3.2. UV Exposure Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shank, J.W.; Feller, R.L. Artists’ Pigments. A Handbook of Their History and Characteristics, Vol. 1. Leonardo 1989, 22, 267–268. [Google Scholar] [CrossRef]
- Barnett, J.R.; Miller, S.; Pearce, E. Colour and art: A brief history of pigments. Opt. Laser Technol. 2006, 38, 445–453. [Google Scholar] [CrossRef]
- Anaf, W.; Schalm, O.; Janssens, K.; De Wael, K. Understanding the (in) stability of semiconductor pigments by a thermodynamic approach. Dye. Pigment. 2015, 113, 409–415. [Google Scholar] [CrossRef]
- Anaf, W.; Trashin, S.; Schalm, O.; Van Dorp, D.; Janssens, K.; De Wael, K. Electrochemical photodegradation study of semiconductor pigments: Influence of environmental parameters. Anal. Chem. 2014, 86, 9742–9748. [Google Scholar] [CrossRef] [PubMed]
- Chiriu, D.; Pisu, F.A.; Ricci, P.C.; Carbonaro, C.M. Application of raman spectroscopy to ancient materials: Models and results from archaeometric analyses. Materials 2020, 13, 2456. [Google Scholar] [CrossRef] [PubMed]
- Chiriu, D.; Pala, M.; Pisu, F.A.; Cappellini, G.; Ricci, P.C.; Carbonaro, C.M. Time through colors: A kinetic model of red vermilion darkening from Raman spectra. Dye. Pigment. 2021, 184, 108866. [Google Scholar] [CrossRef]
- Chiriu, D.; Ricci, P.C.; Carbonaro, C.M.; Nadali, D.; Polcaro, A.; Collins, P. Raman identification of cuneiform tablet pigments: Emphasis and colour technology in ancient Mesopotamian mid-third millennium. Heliyon 2017, 3, e00272. [Google Scholar] [CrossRef][Green Version]
- Pisu, F.A.; Carbonaro, C.M.; Corpino, R.; Ricci, P.C.; Chiriu, D. Fresco paintings: Development of an aging model from 1064 nm excited raman spectra. Crystals 2021, 11, 257. [Google Scholar] [CrossRef]
- Yu, J.; Warren, W.S.; Fischer, M.C. Visualization of vermilion degradation using pump-probe microscopy. Sci. Adv. 2019, 5, eaaw3136. [Google Scholar] [CrossRef]
- Villafaña, T.E.; Samineni, P.; Warren, W.S.; Fischer, M.C. Historical pigments revealed by pump-probe microscopy. In Proceedings of the Laser Science, Rochester, NY, USA, 14–18 October 2012; LS2012. [Google Scholar]
- Traill, R.J.; Boyle, R.W. Hawelite, Isometric Cadmium Sulphide, A New Mineral. Am. Mineral. J. Earth Planet. Mater. 1955, 40, 555–559. [Google Scholar]
- Leone, B.; Burnstock, A.; Jones, C.; Hallebeek, P.; Boon, J.J.; Keune, K. The deterioration of cadmium sulphide yellow artists’ pigments. In Proceedings of the 14th Triennial Meeting, ICOM Committee for Conservation, The Hague, The Netherlands, 12–16 September 2005. [Google Scholar]
- Van Der Snickt, G.; Dik, J.; Cotte, M.; Janssens, K.; Jaroszewicz, J.; De Nolf, W.; Groenewegen, J.; Van Der Loeff, L. Characterization of a degraded cadmium yellow (CdS) pigment in an oil painting by means of synchrotron radiation based X-ray techniques. Anal. Chem. 2009, 81, 2600–2610. [Google Scholar] [CrossRef]
- Kulkarni, V.G.; Garn, P.D. Study of the formation of cadmium sulfoselenide. Thermochim. Acta 1986, 99, 33–36. [Google Scholar] [CrossRef]
- Mass, J.; Sedlmair, J.; Patterson, C.S.; Carson, D.; Buckley, B.; Hirschmugl, C. SR-FTIR imaging of the altered cadmium sulfide yellow paints in Henri Matisse’s le Bonheur de vivre (1905-6)-examination of visually distinct degradation regions. Analyst 2013, 138, 6032–6043. [Google Scholar] [CrossRef]
- Pouyet, E.; Cotte, M.; Fayard, B.; Salomé, M.; Meirer, F.; Mehta, A.; Uffelman, E.S.; Hull, A.; Vanmeert, F.; Kieffer, J.; et al. 2D X-ray and FTIR micro-analysis of the degradation of cadmium yellow pigment in paintings of Henri Matisse. Appl. Phys. A Mater. Sci. Process. 2015, 121, 967–980. [Google Scholar] [CrossRef]
- Van Der Snickt, G.; Janssens, K.; Dik, J.; De Nolf, W.; Vanmeert, F.; Jaroszewicz, J.; Cotte, M.; Falkenberg, G.; Van Der Loeff, L. Combined use of Synchrotron Radiation Based Micro-X-ray Fluorescence, Micro-X-ray Diffraction, Micro-X-ray Absorption Near-Edge, and Micro-Fourier Transform Infrared Spectroscopies for Revealing an Alternative Degradation Pathway of the Pigment Cadmium Ye. Anal. Chem. 2012, 84, 10221–10228. [Google Scholar] [CrossRef]
- Comelli, D.; Maclennan, D.; Ghirardello, M.; Phenix, A.; Schmidt Patterson, C.; Khanjian, H.; Gross, M.; Valentini, G.; Trentelman, K.; Nevin, A. Degradation of Cadmium Yellow Paint: New Evidence from Photoluminescence Studies of Trap States in Picasso’s Femme (époque des “demoiselles d’Avignon”). Anal. Chem. 2019, 91, 3421–3428. [Google Scholar] [CrossRef]
- Armani, N.; Salviati, G.; Nasi, L.; Bosio, A.; Mazzamuto, S.; Romeo, N. Role of thermal treatment on the luminescence properties of CdTe thin films for photovoltaic applications. Thin Solid Film. 2007, 515, 6184–6187. [Google Scholar] [CrossRef]
- Fan, Y.; Deng, M.; Chen, G.; Zhang, Q.; Luo, Y.; Li, D.; Meng, Q. Effect of calcination on the photocatalytic performance of CdS under visible light irradiation. J. Alloys Compd. 2011, 509, 1477–1481. [Google Scholar] [CrossRef]
- Monico, L.; Chieli, A.; De Meyer, S.; Cotte, M.; de Nolf, W.; Falkenberg, G.; Janssens, K.; Romani, A.; Miliani, C. Frontispiece: Role of the Relative Humidity and the Cd/Zn Stoichiometry in the Photooxidation Process of Cadmium Yellows (CdS/Cd1−xZnxS) in Oil Paintings. Chem.–A Eur. J. 2018, 24, 11584–11593. [Google Scholar] [CrossRef]
- Giacopetti, L.; Nevin, A.; Comelli, D.; Valentini, G.; Nardelli, M.B.; Satta, A. First principles study of the optical emission of cadmium yellow: Role of cadmium vacancies. AIP Adv. 2018, 8, 065202. [Google Scholar] [CrossRef]
- Giacopetti, L.; Satta, A. Degradation of Cd-yellow paints: Ab initio study of native defects in {10.0} surface CdS. Microchem. J. 2016, 126, 214–219. [Google Scholar] [CrossRef]
- Giacopetti, L.; Satta, A. Reactivity of Cd-yellow pigments: Role of surface defects. Microchem. J. 2018, 137, 502–508. [Google Scholar] [CrossRef]
- Paulus, J.; Knuutinen, U. Cadmium colours: Composition and properties. Appl. Phys. A Mater. Sci. Process. 2004, 79, 397–400. [Google Scholar] [CrossRef]
- Mikhailov, M.M.; Yuryev, S.A.; Lapin, A.N.; Lovitskiy, A.A. The effects of heating on BaSO4 powders’ diffuse reflectance spectra and radiation stability. Dye. Pigment. 2019, 163, 420–424. [Google Scholar] [CrossRef]
- Riveros, G.; Gómez, H.; Henríquez, R.; Schrebler, R.; Córdova, R.; Marotti, R.E.; Dalchiele, E.A. Electrodeposition and characterization of ZnX (X=Se, Te) semiconductor thin films. J. Chil. Chem. Soc. 2002, 47, 411–429. [Google Scholar] [CrossRef]
- Marotti, R.E.; Guerra, D.N.; Bello, C.; Machado, G.; Dalchiele, E.A. Bandgap energy tuning of electrochemically grown ZnO thin films by thickness and electrodeposition potential. Sol. Energy Mater. Sol. Cells 2004, 82, 85–103. [Google Scholar] [CrossRef]
- Henríquez, R.; Grez, P.; Muñoz, E.; Gómez, H.; Badán, J.A.; Marotti, R.E.; Dalchiele, E.A. Optical properties of CdSe and CdO thin films electrochemically prepared. Thin Solid Film. 2010, 518, 1774–1778. [Google Scholar] [CrossRef]
- Nair, P.K.; Daza, O.G.; Reádigos, A.A.C.; Campos, J.; Nair, M.T.S. Formation of conductive CdO layer on CdS thin films during air heating. Semicond. Sci. Technol. 2001, 16, 651. [Google Scholar] [CrossRef]
- Gueli, A.M.; Gallo, S.; Pasquale, S. Optical and colorimetric characterization on binary mixtures prepared with coloured and white historical pigments. Dye. Pigment. 2018, 157, 342–350. [Google Scholar] [CrossRef]
- Cesaratto, A.; D’Andrea, C.; Nevin, A.; Valentini, G.; Tassone, F.; Alberti, R.; Frizzi, T.; Comelli, D. Analysis of cadmium-based pigments with time-resolved photoluminescence. Anal. Methods 2014, 6, 130–138. [Google Scholar] [CrossRef]
- Zou, B.S.; Volkov, V.V.; Wang, Z.L. Optical properties of amorphous ZnO, CdO, and PbO nanoclusters in solution. Chem. Mater. 1999, 11, 3037–3043. [Google Scholar] [CrossRef]
- Nevin, A.; Cesaratto, A.; Bellei, S.; D’Andrea, C.; Toniolo, L.; Valentini, G.; Comelli, D. Time-resolved photoluminescence spectroscopy and imaging: New approaches to the analysis of cultural heritage and its degradation. Sensors 2014, 14, 6338–6355. [Google Scholar] [CrossRef] [PubMed]
- Srihari, V.; Sridharan, V.; Ravindran, T.R.; Chandra, S.; Arora, A.K.; Sastry, V.S.; Sundar, C.S. Raman scattering of cadmium oxide: In B1 phase. In Proceedings of the AIP Conference, Manipal, India, 26–30 December 2010. [Google Scholar]
- Kumar, S.; Ojha, A.K. Synthesis, characterizations and antimicrobial activities of well dispersed ultra-long CdO nanowires. AIP Adv. 2013, 3, 052109. [Google Scholar] [CrossRef]
- Ganesh, V.; Manthrammel, M.A.; Shkir, M.; AlFaify, S. Investigation on physical properties of CdO thin films affected by Tb doping for optoelectronics. Appl. Phys. A Mater. Sci. Process. 2019, 125, 249. [Google Scholar] [CrossRef]
- Popović, Z.V.; Stanišić, G.; Stojanović, D.; Kostić, R. Infrared and Raman Spectra of CdO. Phys. Status Solidi B 1991, 165, K109–K112. [Google Scholar] [CrossRef]
- Chi, T.T.K.; Gouadec, G.; Colomban, P.; Wang, G.; Mazerolles, L.; Liem, N.Q. Off-resonance Raman analysis of wurtzite CdS ground to the nanoscale: Structural and size-related effects. J. Raman Spectrosc. 2011, 42, 1007–1015. [Google Scholar] [CrossRef]
- Rosi, F.; Grazia, C.; Gabrieli, F.; Romani, A.; Paolantoni, M.; Vivani, R.; Brunetti, B.G.; Colomban, P.; Miliani, C. UV–Vis-NIR and micro Raman spectroscopies for the non destructive identification of Cd1−xZnxS solid solutions in cadmium yellow pigments. Microchem. J. 2016, 124, 856–867. [Google Scholar] [CrossRef]
- Falgayrac, G.; Sobanska, S.; Brémard, C. Heterogeneous microchemistry between CdSO4 and CaCO3 particles under humidity and liquid water. J. Hazard. Mater. 2013, 248, 415–423. [Google Scholar] [CrossRef]
| Sample | L | a | b | X0 [nm] | Simulated Color | Sample | L | a | b | X0 [nm] | Simulated Color |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C-A | 94.1 | −11.1 | 101 | 496.9 |  | C-A | 94.1 | −11.1 | 101 | 496.9 |  |
| C-300 °C-1 h | 103 | −17.3 | 101 | 494.2 |  | C-500 °C-2 h | 100 | −5.91 | 84 | 506.4 |  |
| C-400 °C-1 h | 93.7 | −8.89 | 101 | 497.7 |  | C-500 °C-4 h | 104 | −5.66 | 99.3 | 508 |  |
| C-500 °C-1 h | 85.8 | −5.08 | 75.6 | 502.3 |  | C-500 °C-6 h | 91 | −0.81 | 86.9 | 511 |  |
| S-A | 78.2 | 35.3 | 92.6 | 514.5, 556.0 |  | S-A | 78.2 | 35.3 | 92.6 | 514.5, 556.0 |  |
| S-300 °C-1 h | 92.2 | 42.4 | 107 | 512.5 |  | S-500 °C-2 h | 89,7 | 37.1 | 95 | 514.0, 553.0 |  |
| S-400 °C-1 h | 76.8 | 28.4 | 85.3 | 514.0, 552.8 |  | S-500 °C-4 h | 91,5 | 43.2 | 105 | 513.0, 555.0 |  |
| S-500 °C-1 h | 76.4 | 29.9 | 89.7 | 514.3, 552.9 |  | S-500 °C-6 h | 70.4 | 32.1 | 62.6 | 515.0, 556.0 |  |
| Sample | E (eV) | τ1 [ps] | τ2 [ps] | τ3 [ps] |
|---|---|---|---|---|
| C-A | 2.56 | 8–13 | 50 | 730 |
| C-300-1 h | 2.55 | 8–13 | 150 | 727 |
| C-400-1 h | 2.56 | 8–13 | 200 | 730 |
| C-500-1 h | 2.51 | 8–13 | 100 | 728 |
| C-500-6 h | 2.48 | 8–13 | 50 | 707 |
| Calculation of Cadmium Saturation for S-500-6 h Sample | ||
|---|---|---|
| Point 1 [%] | Point 5 [%] | |
| Cd (Se,S) | 26.3 | 27.6 |
| CdSO4(8/3 H2O) | 24.4 | 26.3 |
| CdO | 49.3 | 46.1 |
| Point 2 [%] | Point 3 [%] | |
| CdZnS | 32.7 | 38.7 |
| CdSO4(8/3 H2O) | 32.2 | 23.7 |
| CdO | 35.1 | 37.6 |
| C-UV-56 h | Point 1 [%] | Point 2 [%] |
|---|---|---|
| CdZnS | 13.3 | 64 |
| CdSO4 (8/3 H2O) | 86.7 | 36 |
| CdO | - | - |
| -S2− in excess | 1.3 | 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assunta Pisu, F.; Ricci, P.C.; Porcu, S.; Carbonaro, C.M.; Chiriu, D. Degradation of CdS Yellow and Orange Pigments: A Preventive Characterization of the Process through Pump–Probe, Reflectance, X-ray Diffraction, and Raman Spectroscopy. Materials 2022, 15, 5533. https://doi.org/10.3390/ma15165533
Assunta Pisu F, Ricci PC, Porcu S, Carbonaro CM, Chiriu D. Degradation of CdS Yellow and Orange Pigments: A Preventive Characterization of the Process through Pump–Probe, Reflectance, X-ray Diffraction, and Raman Spectroscopy. Materials. 2022; 15(16):5533. https://doi.org/10.3390/ma15165533
Chicago/Turabian StyleAssunta Pisu, Francesca, Pier Carlo Ricci, Stefania Porcu, Carlo Maria Carbonaro, and Daniele Chiriu. 2022. "Degradation of CdS Yellow and Orange Pigments: A Preventive Characterization of the Process through Pump–Probe, Reflectance, X-ray Diffraction, and Raman Spectroscopy" Materials 15, no. 16: 5533. https://doi.org/10.3390/ma15165533
APA StyleAssunta Pisu, F., Ricci, P. C., Porcu, S., Carbonaro, C. M., & Chiriu, D. (2022). Degradation of CdS Yellow and Orange Pigments: A Preventive Characterization of the Process through Pump–Probe, Reflectance, X-ray Diffraction, and Raman Spectroscopy. Materials, 15(16), 5533. https://doi.org/10.3390/ma15165533










