Analysis of Electromagnetic Shielding Properties of a Material Developed Based on Silver-Coated Copper Core-Shell Spraying
Abstract
:1. Introduction
2. Experimental
2.1. Materials Specimen Preparation
2.2. Measurement of Shielding Effectiveness (SE)
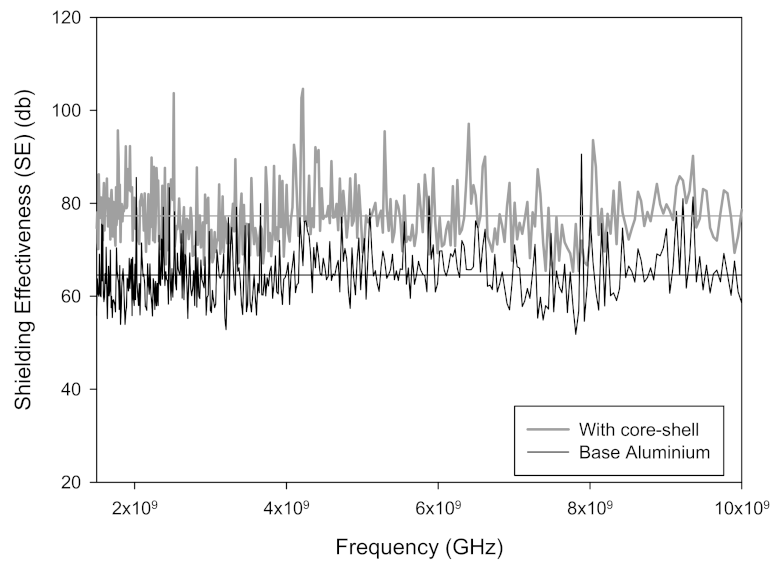
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Voronin, A.S.; Fadeev, Y.V.; Makeev, M.O.; Mikhalev, P.A.; Osipkov, A.S.; Provatorov, A.S.; Ryzhenko, D.S.; Yurkov, G.Y.; Simunin, M.M.; Karpova, D.V.; et al. Low Cost Embedded Copper Mesh Based on Cracked Template for Highly Durability Transparent EMI Shielding Films. Materials 2022, 15, 1449. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Tai, N.H. Carbon materials and their composites for electromagnetic interference shielding effectiveness in X-band. Carbon 2019, 152, 159–187. [Google Scholar] [CrossRef]
- Song, P.; Liu, B.; Qiu, H.; Shi, X.; Cao, D.; Gu, J. MXenes for polymer matrix electromagnetic interference shielding composites: A review. Compos. Commun. 2021, 24, 100653. [Google Scholar] [CrossRef]
- Jing, H.; Miao, Z.; Zeng, Z.; Liu, H.; Zhou, S.; Zou, H.; Liang, M. Carbonization of Graphene-Doped Isocyanate-Based Polyimide Foams to Achieve Carbon Foams with Excellent Electromagnetic Interference Shielding Performance. Materials 2021, 14, 7551. [Google Scholar] [CrossRef] [PubMed]
- Zeranska-Chudek, K.; Wróblewska, A.; Kowalczyk, S.; Plichta, A.; Zdrojek, M. Graphene Infused Ecological Polymer Composites for Electromagnetic Interference Shielding and Heat Management Applications. Materials 2021, 14, 2856. [Google Scholar] [CrossRef] [PubMed]
- Yim, Y.-J.; Lee, J.; Tugirumubano, A.; Go, S.; Kim, H.; Kwac, L. Electromagnetic Interference Shielding Behavior of Magnetic Carbon Fibers Prepared by Electroless FeCoNi-Plating. Materials 2021, 14, 3774. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, S.; Deshmukh, K.; Ahamed, M.B.; Pasha, S.K. Recent advances in electromagnetic interference shielding properties of metal and carbon filler reinforced flexible polymer composites: A review. Compos. Part A: Appl. Sci. Manuf. 2018, 114, 49–71. [Google Scholar] [CrossRef]
- Joshi, A.; Datar, S. Carbon nanostructure composite for electromagnetic interference shielding. Pramana 2015, 84, 1099–1116. [Google Scholar] [CrossRef]
- Abbasi, H.; Antunes, M.; Velasco, J.I. Recent advances in carbon-based polymer nanocomposites for electromagnetic interference shielding. Prog. Mater. Sci. 2019, 103, 319–373. [Google Scholar] [CrossRef]
- Lakin, I.I.; Abbas, Z.; Azis, R.S.; Alhaji, I.A. Complex Permittivity and Electromagnetic Interference Shielding Effectiveness of OPEFB Fiber-Polylactic Acid Filled with Reduced Graphene Oxide. Materials 2020, 13, 4602. [Google Scholar] [CrossRef]
- Hong, J.; Xu, P. Electromagnetic Interference Shielding Anisotropy of Unidirectional CFRP Composites. Materials 2021, 14, 1907. [Google Scholar] [CrossRef] [PubMed]
- Geetha, S.; Kumar, K.K.S.; Rao, C.R.K.; Vijayan, M.; Trivedi, D.C.K. EMI shielding: Methods and materials—A review. J. Appl. Polym. Sci. 2009, 112, 2073–2086. [Google Scholar] [CrossRef]
- Bagotia, N.; Choudhary, V.; Sharma, D. Studies on toughened polycarbonate/multiwalled carbon nanotubes nanocomposites. Compos. Part B: Eng. 2017, 124, 101–110. [Google Scholar] [CrossRef]
- Luo, X.; Chung, D. Electromagnetic interference shielding using continuous carbon-fiber carbon-matrix and polymer-matrix composites. Compos. Part B Eng. 1999, 30, 227–231. [Google Scholar] [CrossRef]
- Singh, A.K.; Shishkin, A.; Koppel, T.; Gupta, N. A review of porous lightweight composite materials for electromagnetic interference shielding. Compos. Part B Eng. 2018, 149, 188–197. [Google Scholar] [CrossRef]
- Huang, J.-C. EMI shielding plastics: A review. Adv. Polym. Technol. 1995, 14, 137–150. [Google Scholar] [CrossRef]
- Xin, W.; Xi, G.-Q.; Cao, W.-T.; Ma, C.; Liu, T.; Ma, M.-G.; Bian, J. Lightweight and flexible MXene/CNF/silver composite membranes with a brick-like structure and high-performance electromagnetic-interference shielding. RSC Adv. 2019, 9, 29636–29644. [Google Scholar] [CrossRef] [Green Version]
- Liao, S.-Y.; Wang, X.-Y.; Li, X.-M.; Wan, Y.-J.; Zhao, T.; Hu, Y.-G.; Zhu, P.-L.; Sun, R.; Wong, C.-P. Flexible liquid metal/cellulose nanofiber composites film with excellent thermal reliability for highly efficient and broadband EMI shielding. Chem. Eng. J. 2021, 422, 129962. [Google Scholar] [CrossRef]
- Al-Saleh, M.H.; Sundararaj, U. Electromagnetic interference shielding mechanisms of CNT/polymer composites. Carbon 2009, 47, 1738–1746. [Google Scholar] [CrossRef]
- Kim, H.M.; Kim, K.; Lee, C.Y.; Joo, J.; Cho, S.J.; Yoon, H.S.; Pejaković, D.A.; Yoo, J.W.; Epstein, A.J. Electrical conductivity and electromagnetic interference shielding of multiwalled carbon nanotube composites containing Fe catalyst. Appl. Phys. Lett. 2004, 84, 589–591. [Google Scholar] [CrossRef] [Green Version]
- Qi, Q.; Ma, L.; Zhao, B.; Wang, S.; Liu, X.; Lei, Y.; Park, C.B. An Effective Design Strategy for the Sandwich Structure of PVDF/GNP-Ni-CNT Composites with Remarkable Electromagnetic Interference Shielding Effectiveness. ACS Appl. Mater. Interfaces 2020, 12, 36568–36577. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gupta, M.C.; Dudley, K.L.; Lawrence, R.W. Novel Carbon Nanotube−Polystyrene Foam Composites for Electromagnetic Interference Shielding. Nano Lett. 2005, 5, 2131–2134. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wang, Y.; Huang, Y.; Ma, Y.; Liu, Z.; Cai, J.; Zhang, C.; Gao, H.; Chen, Y. Electromagnetic interference shielding of graphene/epoxy composites. Carbon 2009, 47, 922–925. [Google Scholar] [CrossRef]
- Yan, D.-X.; Pang, H.; Li, B.; Vajtai, R.; Xu, L.; Ren, P.-G.; Wang, J.-H.; Li, Z.-M. Structured Reduced Graphene Oxide/Polymer Composites for Ultra-Efficient Electromagnetic Interference Shielding. Adv. Funct. Mater. 2014, 25, 559–566. [Google Scholar] [CrossRef]
- Cao, M.-S.; Wang, X.-X.; Cao, W.-Q.; Yuan, J. Ultrathin graphene: Electrical properties and highly efficient electromagnetic interference shielding. J. Mater. Chem. C 2015, 3, 6589–6599. [Google Scholar] [CrossRef]
- Yan, D.-X.; Ren, P.-G.; Pang, H.; Fu, Q.; Yang, M.-B.; Li, Z.-M. Efficient electromagnetic interference shielding of lightweight graphene/polystyrene composite. J. Mater. Chem. 2012, 22, 18772–18774. [Google Scholar] [CrossRef]
- Roh, J.-S.; Chi, Y.-S.; Kang, T.J.; Nam, S.-W. Electromagnetic Shielding Effectiveness of Multifunctional Metal Composite Fabrics. Text. Res. J. 2008, 78, 825–835. [Google Scholar] [CrossRef]
- Han, G.Y.; Kim, J.S.; Ahn, D.G. A study on electromagnetic interference shielding effectiveness of the metal powders and nano carbon black/fiber reinforced epoxy composites. J. Korean Soc. Precis. Eng. 2006, 23, 100–107. [Google Scholar]
- Oh, K.; Hong, S.M.; Seo, Y. Effect of crosslinking reaction on the electromagnetic interference shielding of a Fe-Si-Al alloy (Sendust)/polymer composite at high frequency. Polym. Adv. Technol. 2014, 25, 1366–1370. [Google Scholar] [CrossRef]
- Jung, D.; Lee, H.; Kang, Y.; Park, S. Air-stable silver-coated copper particles of sub-micrometer size. J. Colloid Interface Sci. 2011, 364, 574–581. [Google Scholar] [CrossRef]
- López-Lorente, A.I.; Simonet, B.M.; Valcárcel, M. Analytical potential of hybrid nanoparticles. Anal. Bioanal. Chem. 2011, 399, 43–54. [Google Scholar] [CrossRef]
- Khatami, M.; Alijani, H.Q.; Nejad, M.S.; Varma, R.S. Core@ shell Nanoparticles: Greener Synthesis Using Natural Plant Products. Appl. Sci. 2018, 8, 411. [Google Scholar] [CrossRef] [Green Version]
- Xie, S.; Deng, Y.; Mei, J.; Yang, Z.; Lau, W.M.; Liu, H. Carbon coated CoS2 thermal battery electrode material with enhanced discharge performances and air stability. Electrochim. Acta. 2017, 231, 287–293. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.-H. Effects of Pretreatment and Ag Coating Processes Conditions on the Properties of Ag-Coated Cu Flakes. Korean J. Mater. Res. 2014, 24, 617–624. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Lee, J.-H. A Method for Application of Ammonium-based Pretreatment Solution in Preparation of Copper Flakes Coated by Electroless Ag Plating. J. Microelectron. Packag. Soc. 2015, 22, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.J.; Kim, J.H.; Lee, J.-H. Effects of Different Pretreatment Methods and Amounts of Reductant on Preparation of Silver-coated Copper Flakes Using Electroless Plating. J. Microelectron. Packag. Soc. 2016, 23, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Oh, T.S. Polymeric composite material for shielding of electromagnetic interference. Polym. Sci. Technol. 1991, 2, 179–190. [Google Scholar]
- Lim, J.; Kim, Y.; Kim, Y.; Song, P.; Kwon, A. Effects of Composition on the Electromagnetic Wave Shielding/Absorption and Corrosion in Zn-Ni Alloy Thin Film. Korean J. Met. Mater. 2022, 60, 62–67. [Google Scholar] [CrossRef]
- Lyu, X.; Jia, Y.; Mao, X.; Li, D.; Li, G.; Zhuang, L.; Wang, X.; Yang, D.; Wang, Q.; Du, A.; et al. Gradient-Concentration Design of Stable Core–Shell Nanostructure for Acidic Oxygen Reduction Electrocatalysis. Adv. Mater. 2020, 32, 2003493. [Google Scholar] [CrossRef] [PubMed]
- Beitollai, H.; Garkani Nejad, F.; Tajik, S.; Jahani, S.; Biparva, P. Voltammetric determination of amitriptyline based on graphite screen printed electrode modified with a copper oxide nanoparticles. Int. J. Nano Dimens. 2017, 8, 197–205. [Google Scholar]
- Jahani, S.; Beitollahi, H. Selective Detection of Dopamine in the Presence of Uric Acid Using NiO Nanoparticles Decorated on Graphene Nanosheets Modified Screen-printed Electrodes. Electroanalysis 2016, 28, 2022–2028. [Google Scholar] [CrossRef]
- Khorasani-Motlagh, M.; Noroozifar, M.; Jahani, S. Preparation and Characterization of Nano-Sized Magnetic Particles LaCoO3 by Ultrasonic-Assisted Coprecipitation Method. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2015, 45, 1591–1595. [Google Scholar] [CrossRef]
- Niroomand, S.; Khorasani-Motlagh, M.; Noroozifar, M.; Jahani, S.; Moodi, A. Photochemical and DFT studies on DNA-binding ability and antibacterial activity of lanthanum(III)-phenanthroline complex. J. Mol. Struct. 2017, 1130, 940–950. [Google Scholar] [CrossRef]
- Beitollahi, H.; Tajik, S.; Jahani, S. Electrocatalytic Determination of Hydrazine and Phenol Using a Carbon Paste Electrode Modified with Ionic Liquids and Magnetic Core-shell Fe3O4@SiO2/MWCNT Nanocomposite. Electroanalysis 2015, 28, 1093–1099. [Google Scholar] [CrossRef]
- Jahani, S.; Khorasani-Motlagh, M.; Noroozifar, M. DNA interaction of europium(III) complex containing 2,2′-bipyridine and its antimicrobial activity. J. Biomol. Struct. Dyn. 2015, 34, 612–624. [Google Scholar] [CrossRef] [PubMed]
- Jahani, S.; Beitollahi, H. Carbon paste electrode modified with TiO2/Fe3O4/MWCNT nanocomposite and ionic liquids as a voltammetric sensor for sensitive ascorbic acid and tryptophan detection. Anal. Bioanal. Electrochem. 2016, 8, 158–168. [Google Scholar]
- Singh, P.; Singh, H.; Ahn, S.; Castro-Aceituno, V.; Jiménez, Z.; Simu, S.Y.; Kim, Y.J.; Yang, D.C. Pharmacological importance, characterization and applications of gold and silver nanoparticles synthesized by Panax ginseng fresh leaves. Artif. Cells Nanomed. Biotechnol. 2016, 45, 1415–1424. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Srivastava, O.N. One-Step Green Synthesis of Gold Nanoparticles Using Black Cardamom and Effect of pH on Its Synthesis. Nanoscale Res. Lett. 2015, 10, 1055. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Singh, H.; Castro-Aceituno, V.; Ahn, S.; Kim, Y.J.; Farh, M.E.-A.; Yang, D.C. Engineering of mesoporous silica nanoparticles for release of ginsenoside CK and Rh2 to enhance their anticancer and anti-inflammatory efficacy: In Vitro studies. J. Nanopart. Res. 2017, 19, 257. [Google Scholar] [CrossRef]
- Singh, P.; Ahn, S.; Kang, J.-P.; Veronika, S.; Huo, Y.; Singh, H.; Chokkaligam, M.; Farh, M.E.-A.; Aceituno, V.C.; Kim, Y.J.; et al. In vitro anti-inflammatory activity of spherical silver nanoparticles and monodisperse hexagonal gold nanoparticles by fruit extract of Prunus serrulata: A green synthetic approach. Artif. Cells Nanomed. Biotechnol. 2017, 46, 2022–2032. [Google Scholar] [CrossRef] [Green Version]
- Seddighi, N.S.; Salari, S.; Izadi, A.R. Evaluation of antifungal effect of iron-oxide nanoparticles against different Candida species. IET Nanobiotechnol. 2017, 11, 883–888. [Google Scholar] [CrossRef]
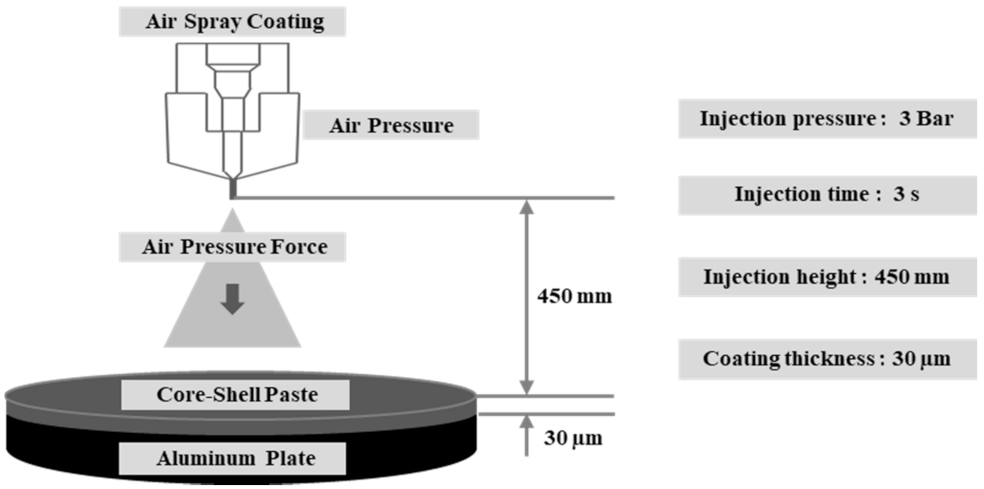
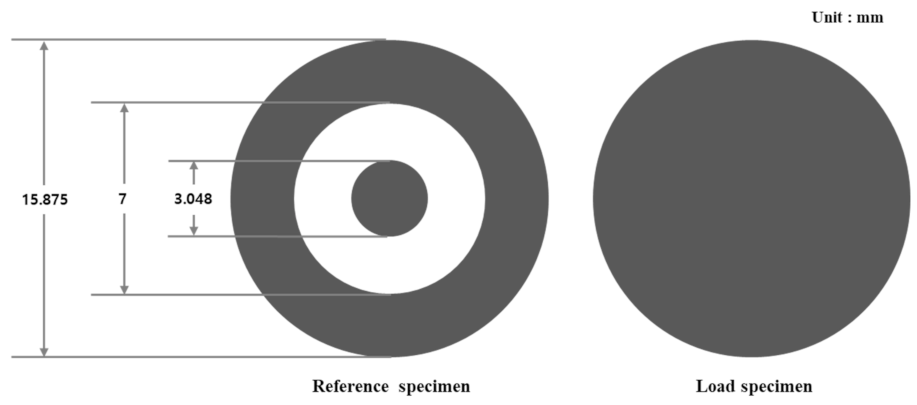
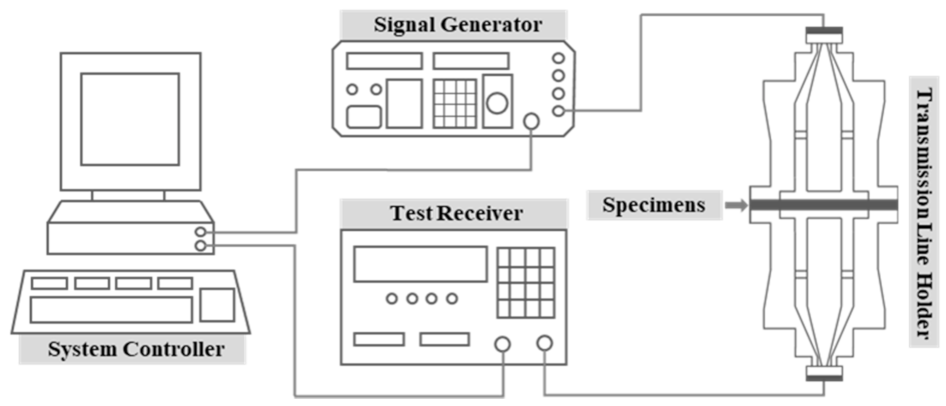
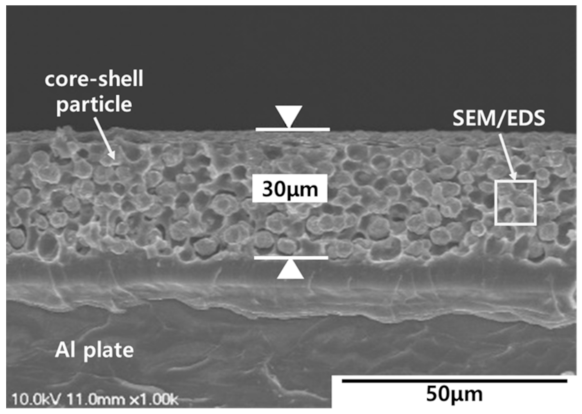

| Features | Contents |
|---|---|
| State | Solid |
| Appearance | Powder |
| Particle size | 2.0 µm, 4.0–4.4 µm, 6.0 µm |
| Particle shape | Spherical |
| Specific surface area | 0.40 m2/g |
| Bulk density | 4.2 g/m3 |
| Purity | 99.9% |
| Alternate Name | Silver-coated copper powder |
| Location | Sheet Resistance (Ω/□) |
|---|---|
| 1 | 0.1309 |
| 2 | 0.1410 |
| 3 | 0.1254 |
| 4 | 0.1321 |
| 5 | 0.1387 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, Y.-J.; Yun, J.-H.; Kang, M.-S. Analysis of Electromagnetic Shielding Properties of a Material Developed Based on Silver-Coated Copper Core-Shell Spraying. Materials 2022, 15, 5448. https://doi.org/10.3390/ma15155448
Jeon Y-J, Yun J-H, Kang M-S. Analysis of Electromagnetic Shielding Properties of a Material Developed Based on Silver-Coated Copper Core-Shell Spraying. Materials. 2022; 15(15):5448. https://doi.org/10.3390/ma15155448
Chicago/Turabian StyleJeon, Yu-Jae, Jong-Hwan Yun, and Min-Soo Kang. 2022. "Analysis of Electromagnetic Shielding Properties of a Material Developed Based on Silver-Coated Copper Core-Shell Spraying" Materials 15, no. 15: 5448. https://doi.org/10.3390/ma15155448






