Aluminum Perlite Syntactic Foams
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
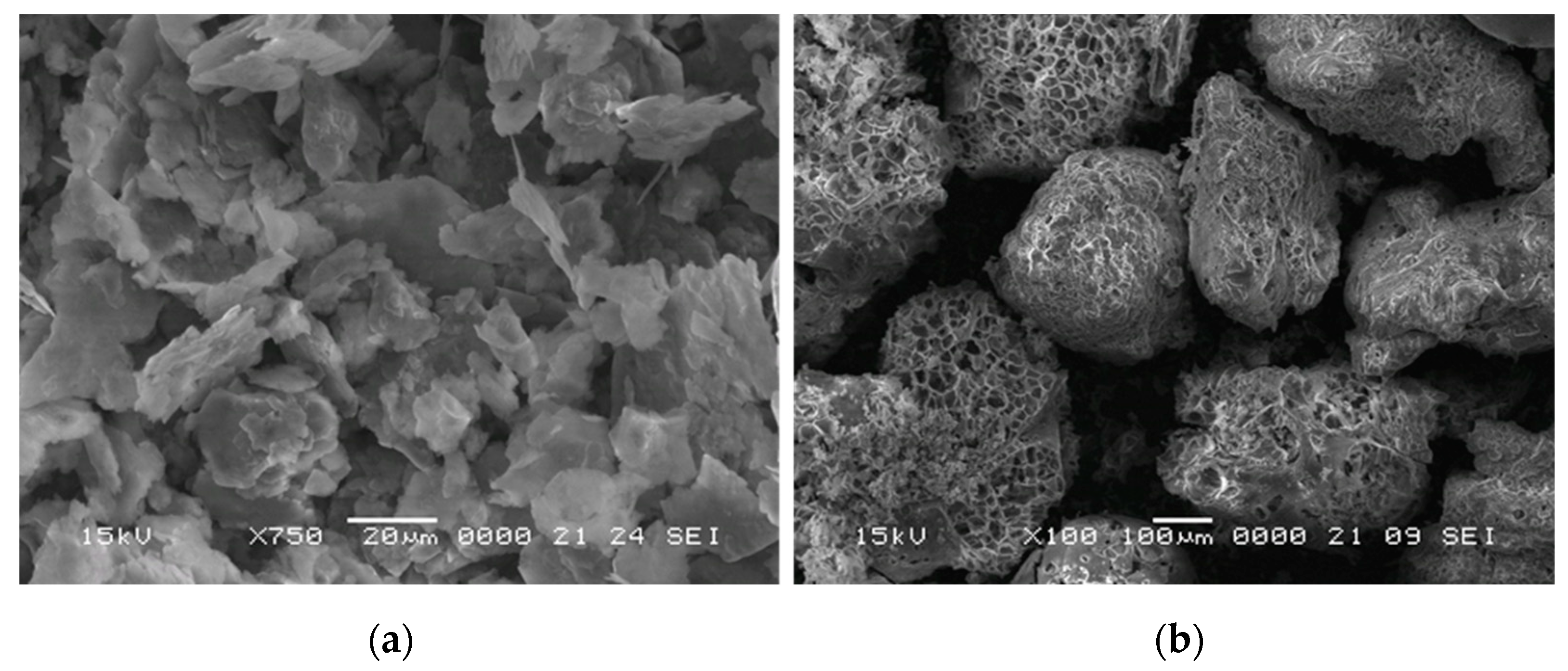
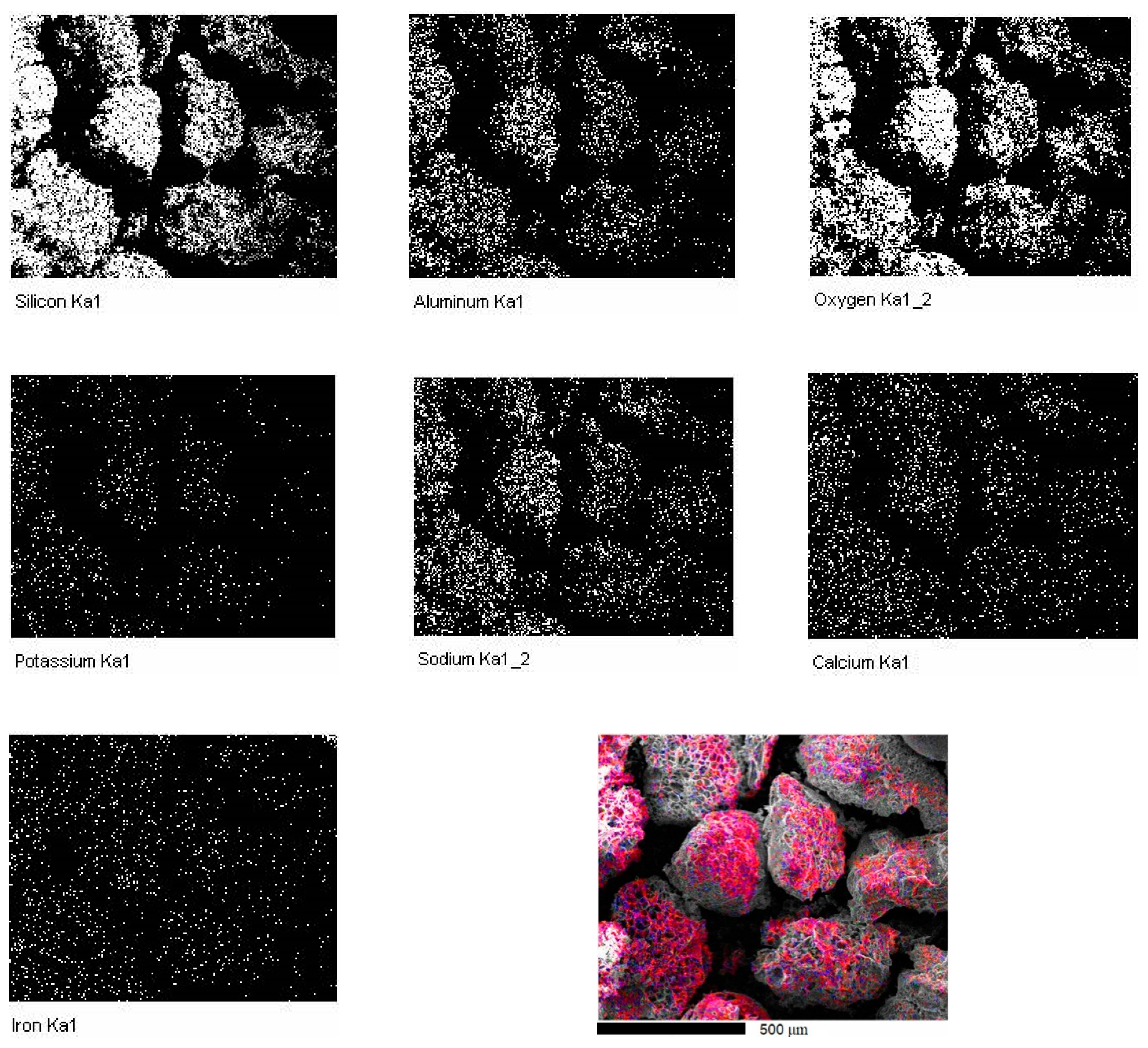
3.1. The Sintered Samples
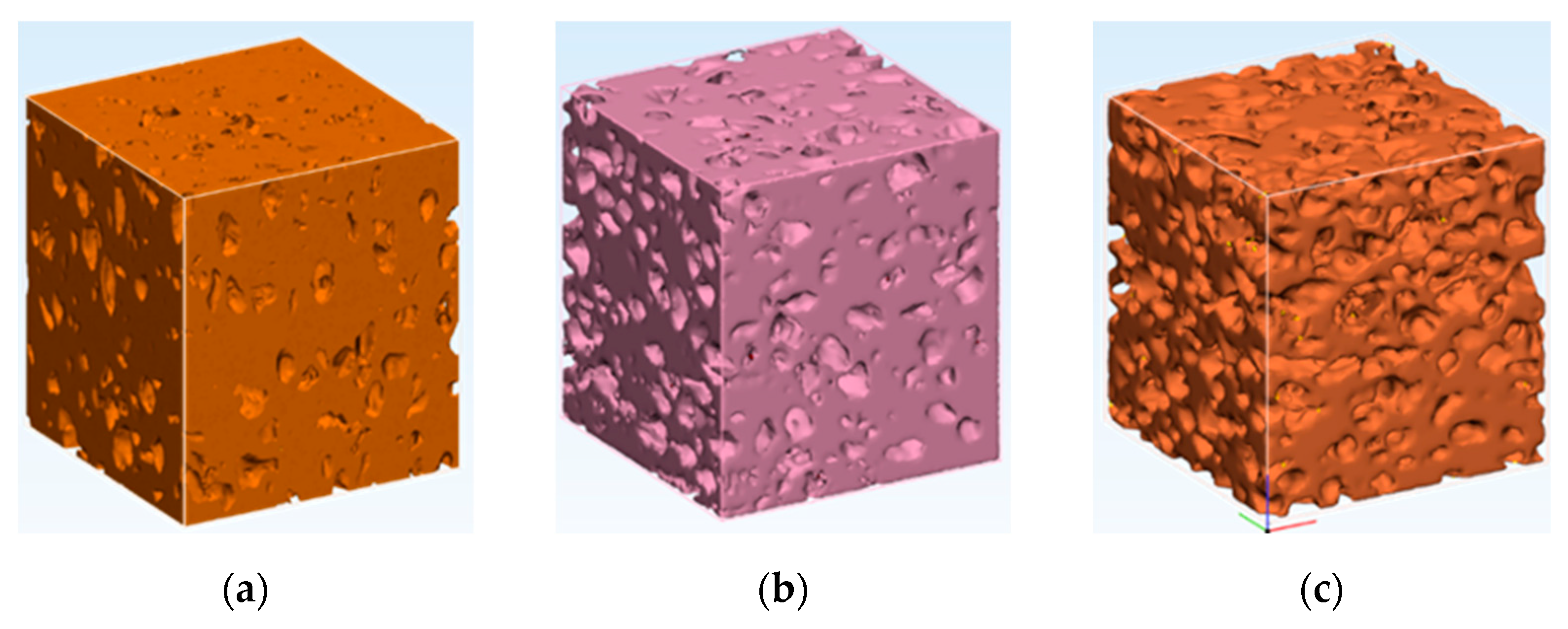
3.2. Samples 3D Structure
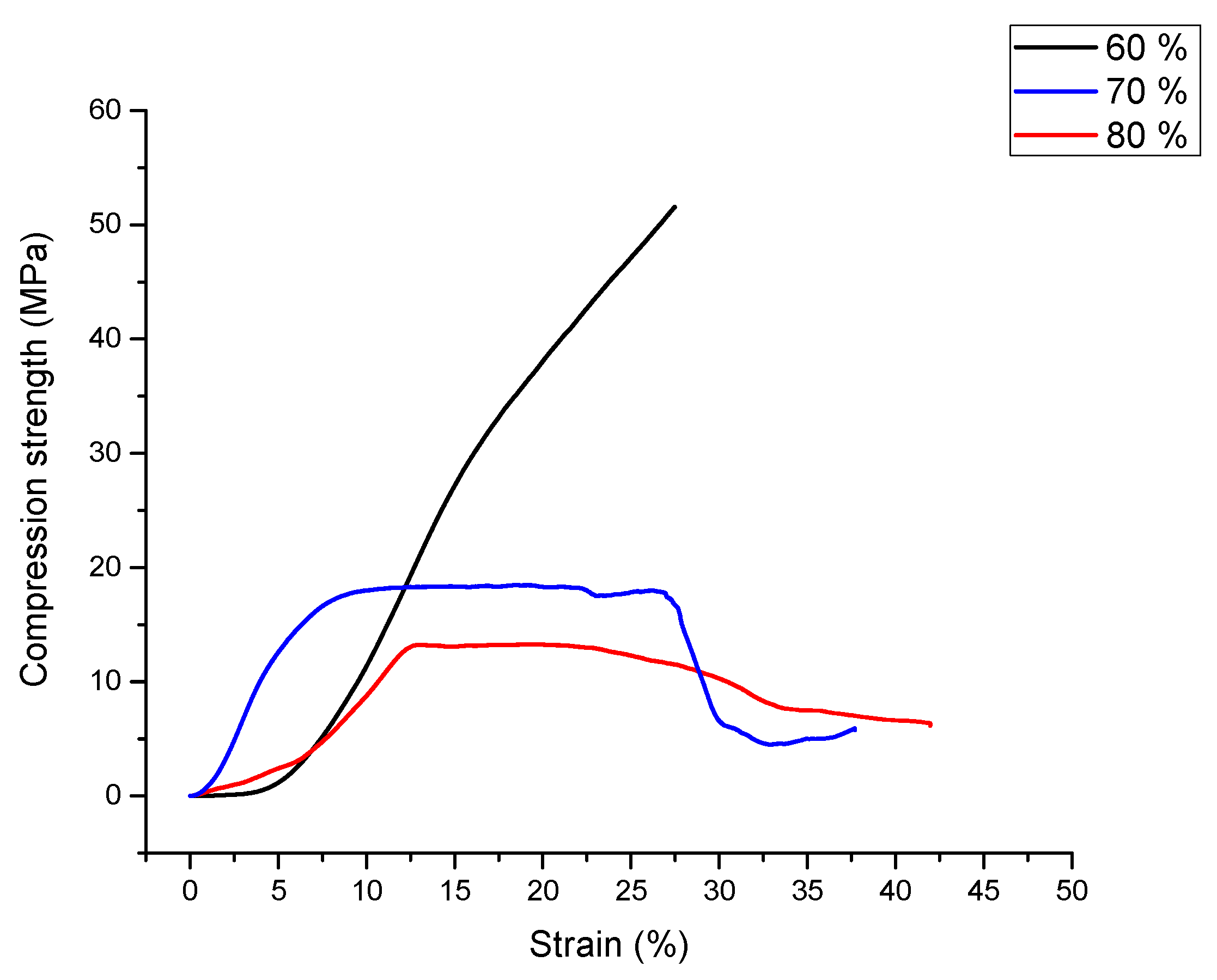
3.3. Compression Test Results
4. Conclusions
- Metallic foams are attracting increasing interest due to the unique combination of physical and mechanical properties such as low density and high specific strength.
- Syntactic metallic foams were successfully produced by Spark Plasma Sintering using aluminum and expanded perlite.
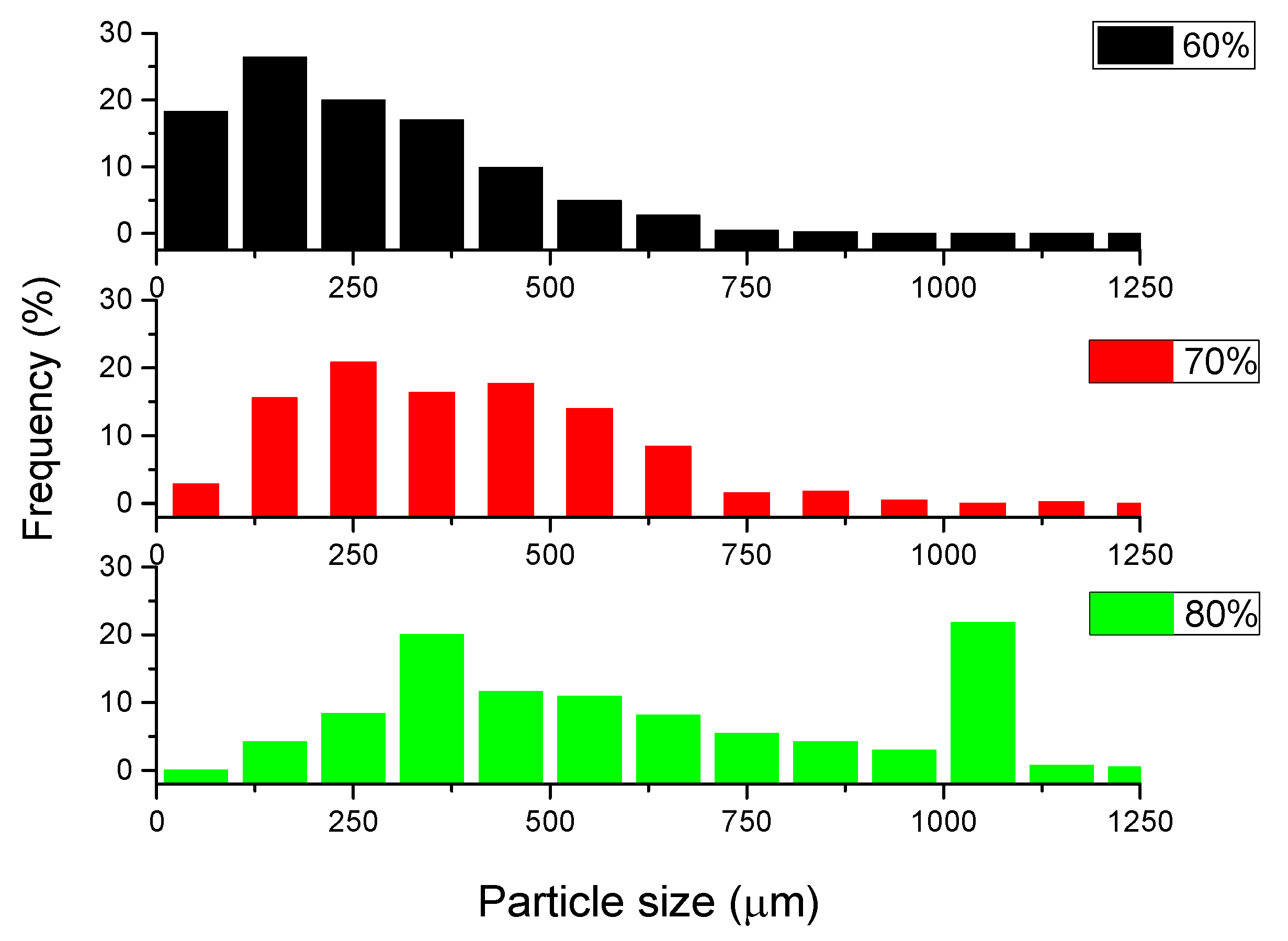
- The particle size of expanded perlite in the sintered samples measured by SEM is in accordance with those resulted by image reconstruction of structures based on μCT. The results confirm that the pressure applied during the Spark Plasma Sintering was enough for good sinterability, preserving at the same time the filler particles.
- Samples with a content of 70, 80 vol% show the specific behavior of syntactic foams to compressive loadings, while those having 60 vol% behave similarly to the bulk aluminum.
- Compression and plateau strengths are in the range reported in literature for this type of materials.
- The best results were obtained for a fraction of 70 vol% expanded perlite, with a value of absorbed energy of 7.49 MJ/m3 and an efficiency of 90%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, C.E.; Mabuchi, M.; Yamada, Y.; Shimojima, K.; Chino, Y.; Hosokawa, H.; Asahina, T. Processing of fine-grained aluminum foam by spark plasma sintering. J. Mater. Sci. Lett. 2003, 22, 1407–1409. [Google Scholar] [CrossRef]
- Liu, P.S.; Chen, G.F. Porous Materials—Processing and Applications, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; ISBN 978-0-12-407788-1. [Google Scholar] [CrossRef]
- Fiedler, T.; Movahedi, N.; York, L.; Broxtermann, S. Functionally-Graded Metallic Syntactic Foams Produced via Particle Pre-Compaction. Metals 2020, 10, 314. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, K.; Bock, J.; Jacobi, A.M. Thermal-hydraulic performance of metal foam heat exchangers under dry operating conditions. Appl. Therm. Eng. 2017, 119, 222–232. [Google Scholar] [CrossRef]
- Bolat, C.; Goksenli, A. Fabrication Optimization of Al 7075/Expanded Glass Syntactic Foam by Cold Chamber Die Casting. Arch. Found. Eng. 2020, 3, 112–118. [Google Scholar] [CrossRef]
- Anbuchezhiyan, G.; Muthuramalingam, T.; Mohan, B. Effect of process parameters on mechanical properties of hollow glass microsphere reinforced magnesium alloy syntactic foams under vacuum die casting. Arch. Civ. Mech. Eng. 2018, 18, 1645–1650. [Google Scholar] [CrossRef]
- Banhart, J. Light-metal foams-history of innovation and technological challenges. Adv. Eng. Mater. 2013, 15, 82–111. [Google Scholar] [CrossRef]
- Lefebvre, L.-P.; Banhart, J.; Dunand, D.C. Porous Metals and Metallic Foams: Current Status and Recent Developments. Adv. Eng. Mater. 2008, 10, 775–787. [Google Scholar] [CrossRef] [Green Version]
- Atwater, M.A.; Guevara, L.N.; Darling, K.A.; Tschopp, M.A. Solid State Porous Metal Production: A Review of the Capabilities, Characteristics, and Challenges. Adv. Eng. Mater. 2018, 20, 1700766. [Google Scholar] [CrossRef] [Green Version]
- Neikov, O.D. Handbook of Non-Ferrous Metal Powders, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; ISBN 9780081005439. [Google Scholar] [CrossRef]
- Ashby, M.; Evans, T.; Fleck, N.A.; Hutchinson, J.W.; Wadley, H.N.G.; Gibson, L.J. Metal Foams: A Design Guide, 1st ed.; Butterworth-Heinemann: Oxford, UK, 2000; ISBN 9780080511467. [Google Scholar]
- Banhart, J. Manufacture, characterisation and application of cellular metals and metal foams. Prog. Mater. Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- Vida-Simiti, I.; Nemeş, D.; Jumate, N.; Thalmaier, G.; Sechel, N. Self-Ordered Nanoporous Alumina Templates Formed by Anodization of Aluminum in Oxalic Acid. JOM 2012, 64, 1143–1147. [Google Scholar] [CrossRef]
- Salleh, Z.; Islam, M.; Ku, H. Study on Compressive Properties of Syntactic Foams for Marine Applications. J. Multifunct. Compos. 2014, 2, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Orbulov, I.N.; Dobranszky, J. Producing metal matrix syntactic foams by pressure infiltration. Period. Polytech. Mech. Eng. 2008, 52, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Orbulov, I.N.; Dobranszky, J.; Nemeth, A. Microstructural characterisation of syntactic foams. J. Mater. Sci. 2009, 44, 4013–4019. [Google Scholar] [CrossRef]
- Thalmaier, G.; Sechel, N.A.; Vida-Simiti, I. Syntactic Aluminum Foam from Recycled Sawing Chips. JOM 2020, 72, 3377–3382. [Google Scholar] [CrossRef]
- Dudina, D.V.; Bokhonov, B.B.; Olevsky, E.A. Fabrication of Porous Materials by Spark Plasma Sintering: A Review. Materials 2019, 12, 541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohatgi, P.K.; Gupta, N.; Schultz, B.F.; Luong, D.D. The Synthesis, Compressive Properties, and Applications of Metal Matrix Syntactic Foams. JOM 2011, 63, 36–42. [Google Scholar] [CrossRef]
- Altenaiji, M.; Guan, Z.; Cantwell, W.; Zhao, Y.; Schleyer, G. Characterisation of aluminium matrix syntactic foams under drop weight impact. Mater. Des. 2014, 59, 296–302. [Google Scholar] [CrossRef]
- Abdullah, Z.; Ahmad, S.; Rafter, M.F.M.; Manaf, N.S.A. Fabrication of 316L Stainless Steel Foams via Powder Metallurgy Technique. J. Eng. Appl. Sci. 2016, 11, 7583–7587. [Google Scholar]
- Duarte, I.; Ferreira, J.M.F. Composite and Nanocomposite Metal Foams. Materials 2016, 9, 79. [Google Scholar] [CrossRef] [Green Version]
- Matli, P.; Sheng, J.; Parande, G.; Manakari, V.; Chua, B.; Wong, S.; Gupta, M. A Novel Method of Light Weighting Aluminium Using Magnesium Syntactic Composite Core. Crystals 2020, 10, 917. [Google Scholar] [CrossRef]
- Bolat, Ç.; Bilge, G.; Gökşenli, A. An Investigation on the Effect of Heat Treatment on the Compression Behavior of Aluminum Matrix Syntactic Foam Fabricated by Sandwich Infiltration Casting. Mater. Res. 2021, 24. [Google Scholar] [CrossRef]
- Jain, H.; Mondal, D.; Gupta, G.; Kumar, R.; Singh, S. Synthesis and characterization of 316L stainless steel foam made through two different removal process of space holder method. Manuf. Lett. 2020, 26, 33–36. [Google Scholar] [CrossRef]
- Castro, G.; Nutt, S. Synthesis of syntactic steel foam using gravity-fed infiltration. Mater. Sci. Eng. A 2012, 553, 89–95. [Google Scholar] [CrossRef]
- Kemény, A.; Leveles, B.; Bubonyi, T.; Orbulov, I.N. Effect of particle size and volume ratio of ceramic hollow spheres on the mechanical properties of bimodal composite metal foams. Compos. Part A Appl. Sci. Manuf. 2021, 140, 106152. [Google Scholar] [CrossRef]
- Wright, A.; Kennedy, A. The Processing and Properties of Syntactic Al Foams Containing Low Cost Expanded Glass Particles. Adv. Eng. Mater. 2017, 19, 1600467. [Google Scholar] [CrossRef] [Green Version]
- Broxtermann, S.; Taherishargh, M.; Belova, I.; Murch, G.; Fiedler, T. On the compressive behaviour of high porosity expanded Perlite-Metal Syntactic Foam (P-MSF). J. Alloys Compd. 2017, 691, 690–697. [Google Scholar] [CrossRef]
- Taherishargh, M.; Belova, I.; Murch, G.; Fiedler, T. Low-density expanded perlite–aluminium syntactic foam. Mater. Sci. Eng. A Struct. 2014, 604, 127–134. [Google Scholar] [CrossRef]
- Sahu, S.; Ansari, M.Z.; Mondal, D.P. Microstructure and compressive deformation behavior of 2014 aluminium cenosphere syntactic foam made through stircasting technique. Mater. Today Proc. 2020, 25, 785–788. [Google Scholar] [CrossRef]
- Movahedi, N.; Murch, G.E.; Belova, I.V.; Fiedler, T. Effect of Heat Treatment on the Compressive Behavior of Zinc Alloy ZA27 Syntactic Foam. Materials 2019, 12, 792. [Google Scholar] [CrossRef] [Green Version]
- Movahedi, N.; Taherishargh, M.; Belova, I.V.; Murch, G.E.; Fiedler, T. Mechanical and Microstructural Characterization of an AZ91–Activated Carbon Syntactic Foam. Materials 2019, 12, 3. [Google Scholar] [CrossRef] [Green Version]
- Braszczyńska-Malik, K.; Kamieniak, J. Analysis of interface between components in AZ91 magnesium alloy foam composite with Ni-P coated fly ash cenospheres. J. Alloys Compd. 2017, 720, 352–359. [Google Scholar] [CrossRef]
- Jha, N.; Mondal, D.; Goel, M.; Majumdar, J.; DAS, S.; Modi, O. Titanium cenosphere syntactic foam with coarser cenosphere fabricated by powder metallurgy at lower compaction load. Trans. Nonferrous Met. Soc. China 2014, 24, 89–99. [Google Scholar] [CrossRef]
- Xue, X.; Zhao, Y. Ti Matrix Syntactic Foam Fabricated by Powder Metallurgy: Particle Breakage and Elastic Modulus. JOM 2011, 63, 43–47. [Google Scholar] [CrossRef]
- Katona, B.; Szlancsik, A.; Tábi, T.; Orbulov, I.N. Compressive characteristics and low frequency damping of aluminium matrix syntactic foams. Mater. Sci. Eng. A Struct. 2019, 739, 140–148. [Google Scholar] [CrossRef]



| Perlite Amount (%vol.) | Density (g/cm3) | Porosity (%) |
|---|---|---|
| 60 | 1.73 | 35.9 |
| 70 | 1.23 | 54.4 |
| 80 | 1.16 | 57.0 |
| Perlite Amount (%vol.) | Compression Strength (MPa) | Plateau Strength (MPa) | Absorbed Energy (MJ/m3) | Energy Absorption Efficiency (%) |
|---|---|---|---|---|
| 70 | 18 | 18 | 7.49 | 90 |
| 80 | 12 | 12.5 | 3.22 | 70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thalmaier, G.; Sechel, N.A.; Csapai, A.; Popa, C.O.; Batin, G.; Gábora, A.; Mankovits, T.; Vida-Simiti, I. Aluminum Perlite Syntactic Foams. Materials 2022, 15, 5446. https://doi.org/10.3390/ma15155446
Thalmaier G, Sechel NA, Csapai A, Popa CO, Batin G, Gábora A, Mankovits T, Vida-Simiti I. Aluminum Perlite Syntactic Foams. Materials. 2022; 15(15):5446. https://doi.org/10.3390/ma15155446
Chicago/Turabian StyleThalmaier, György, Niculina Argentina Sechel, Alexandra Csapai, Catalin Ovidiu Popa, Gabriel Batin, Andras Gábora, Tamas Mankovits, and Ioan Vida-Simiti. 2022. "Aluminum Perlite Syntactic Foams" Materials 15, no. 15: 5446. https://doi.org/10.3390/ma15155446
APA StyleThalmaier, G., Sechel, N. A., Csapai, A., Popa, C. O., Batin, G., Gábora, A., Mankovits, T., & Vida-Simiti, I. (2022). Aluminum Perlite Syntactic Foams. Materials, 15(15), 5446. https://doi.org/10.3390/ma15155446







