Spectroscopic Characterization and Antioxidant Properties of Mandelic Acid and Its Derivatives in a Theoretical and Experimental Approach
Abstract
:1. Introduction
1.1. Mandelic Acid and Its Derivatives—Properties and Applications of Studied Compounds
1.2. The Antioxidant Reaction Mechanisms Description
2. Materials and Methods
2.1. Materials
2.2. FTIR and Raman Spectra
2.3. NMR Spectra
2.4. Evaluation of Antioxidant Activity
2.5. Computational Details
- Ropt is the optimal value of a bond length. For C-C type of bonds in a benzene ring, the Ropt value is equal to 1.334;
- Ri is the length of the ith bond;
- n is the number of bond lengths in the ring;
- Rar is the average bond length;
- α is the normalization factor necessary to obtain a HOMA value equal to 1 for ideally aromatic benzene or 0 for an ideally alternating cyclohexatriene Kekulé ring.
3. Results
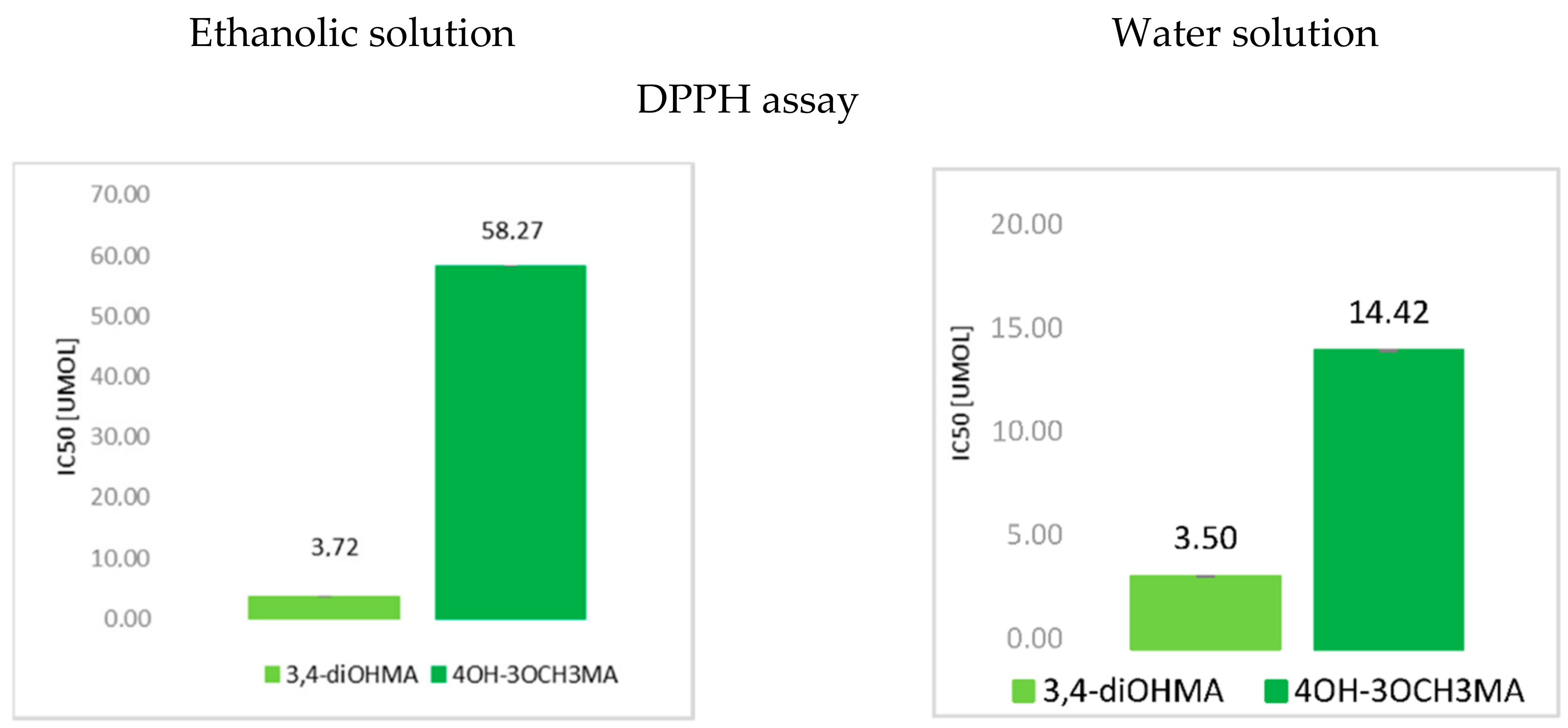
3.1. The Antioxidant Activity of Mandelic Acid and Its Derivatives
3.2. Computational Results
3.2.1. Structure of Mandelic Acid and Their Derivatives
3.2.2. Bond Dissociation Enthalpy, Ionization Potentials, Proton Dissociation Enthalpies, Proton Affinities and Electron Transfer Enthalpies for Mandelic Acid and Its Derivatives
3.2.3. Aromaticity
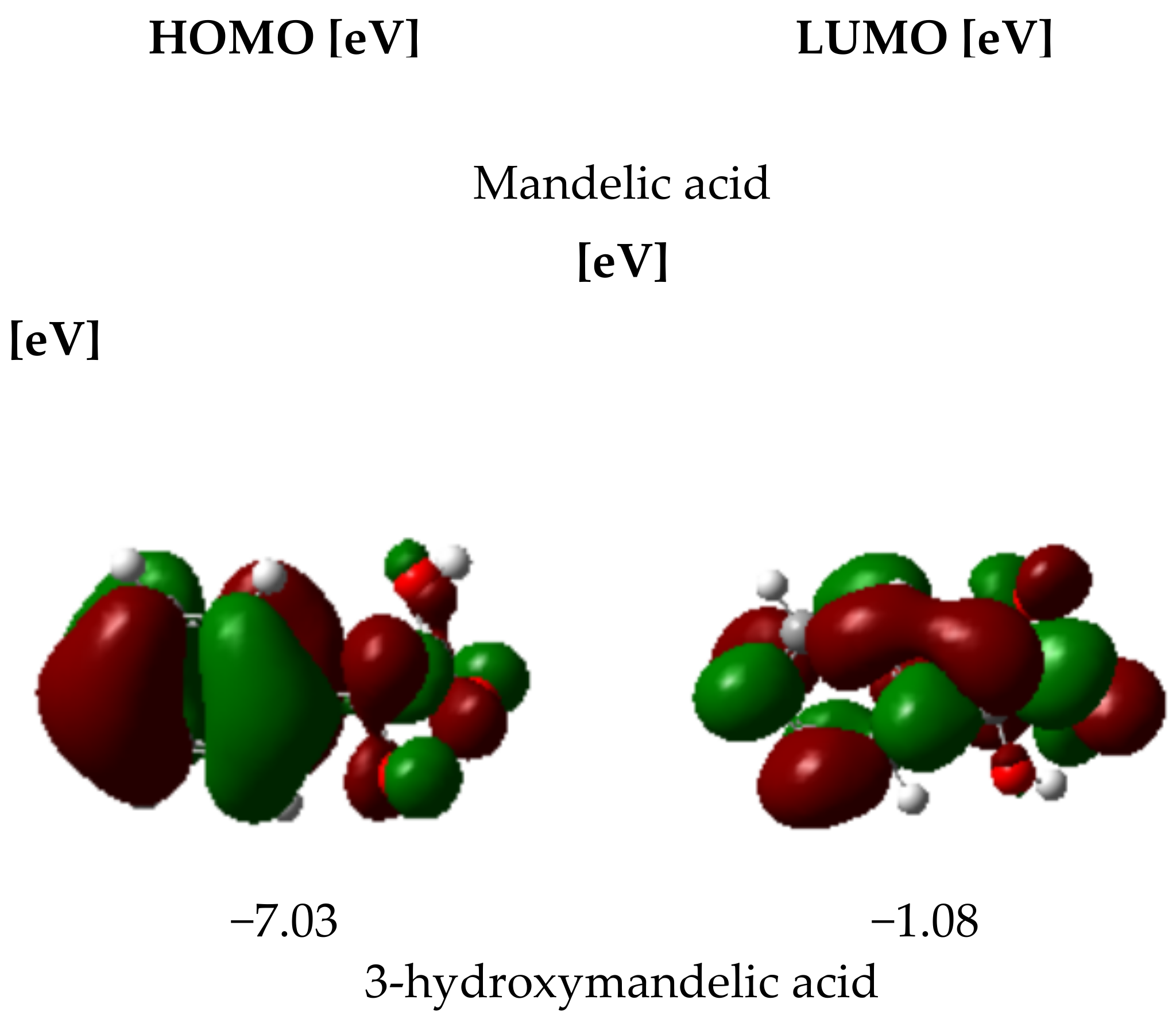
3.2.4. HOMO and LUMO Parameters
3.2.5. Electron Charge Distribution and EPS Distribution
3.3. FT-IR and Raman Spectroscopy
NMR Study
- 13C NMR
- 1H NMR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl radical |
| BDE | bond dissociation energy |
| CUPRAC | Cupric ion reducing antioxidant capacity |
| DFT | density functional theory |
| ETE | electron transfer enthalpy |
| FRAP | ferric reducing ability of plasma |
| HAT | hydrogen atom transfer |
| IP | ionization potential |
| MA | Mandelic acid |
| PA | proton affinity |
| PCET | proton-coupled electron transfer |
| SAMMA | mandelic acid condensation polymer |
| SPLET | sequential proton loss electron transfer |
| 3OH-MA | 3-hydroxymandelic acid |
| 3,4-diOH-MA | 3,4-dihydroxymandelic acid |
| 4OH-3OCH3-MA | 4-hydroxy-3-methoxymandelic acid |
| PDE | proton dissociation enthalpy |
| HOMA | harmonic oscillator model of aromaticity |
| HOMO | highest Occupied Molecular Orbital |
| LUMO | lowest Occupied Molecular Orbital |
References
- Kiris, B.; Asç, Y.S. Parametric Analysis of Mandelic Acid Separation from Aqueous Solutions by Using Secondary Amine Mixture (Amberlite LA-2) in Various Diluents. J. Chem. Eng. Data 2019, 64, 3331–3336. [Google Scholar] [CrossRef]
- Tang, L.; Cheng, H.; Cui, S.; Wang, X.; Song, L.; Zhou, W.; Li, S. DL-mandelic acid intercalated Zn-Al layered double hydroxide: A novel antimicrobial layered material. Colloids Surf. B 2018, 165, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, M.; Shafiq, S.; Ullah, H.; Sadiq, A.; Ullah, F. Isolation of quercetin and mandelic acid from Aesculus indica fruit and their biological activities. BMC Biochem. 2018, 19, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brittain, H.G. Mandelic Acid. Anal. Profiles Drug Subst. Excip. 2002, 29, 197–211. [Google Scholar]
- Ni, K.; Wang, H.; Zhao, L.; Zhang, M.; Zhang, S.; Ren, Y.; Wei, D. Efficient production of (R)-(−)-mandelic acid in biphasic system by immobilized recombinant E. coli. J. Biotechnol. 2013, 167, 433–440. [Google Scholar] [CrossRef]
- Yang, X.; Liu, X.; Shen, K.; Fu, Y.; Zhang, M.; Zhu, C.; Cheng, Y. Enantioselective fluorescent recognition of mandelic acid by unsymmetrical salalen and salan sensors. Org. Biomol. Chem. 2011, 9, 6011–6021. [Google Scholar] [CrossRef]
- Xiaa, M.; Yanga, M.; Wanga, Y.; Tiana, F.; Hua, J.; Yanga, W.; Taoa, S.; Lub, L.; Dinga, X.; Jianga, S.; et al. DL-Mandelic acid exhibits high sperm-immobilizing activity and low vaginal irritation: A potential non-surfactant spermicide for contraception. Biomed. Pharmacother. 2020, 126, 110114–110121. [Google Scholar] [CrossRef]
- Emel’yanenkoa, V.N.; Turovtsevc, V.V.; Fedina, Y.A. Experimental and theoretical thermodynamic properties of RS-(±)- and S-(+)-mandelic acids. Thermochim. Acta 2018, 665, 37–42. [Google Scholar] [CrossRef]
- Rahimpoor, R.; Bahrami, A.; Nematollahi, D.; Shahna, F.G.; Farhadian, M. Facile and sensitive determination of urinary mandelic acid by combination of metal organic frameworks with microextraction by packed sorbents. J. Chromatogr. B Biomed. Appl. 2019, 1114, 45–54. [Google Scholar] [CrossRef]
- Nath, M.; Roy, P.; Mishra, R.; Thakur, M. Structure-cytotoxicity relationship for apoptotic inducers organotin(IV) derivatives of mandelic acid and L-proline and their mixed ligand complexes having enhanced cytotoxicity. Appl. Organomet. Chem. 2018, 33, e4663. [Google Scholar] [CrossRef]
- Gumbhirt, K.; Mason, W.D. Determination of m-hydroxymandelic acid, mhydroxyphenylglycol and their conjugates in human plasma using liquid chromatography with electrochemical detection. J. Pharm. Biomed. Anal. 1994, 12, 943–949. [Google Scholar] [CrossRef]
- Davis, B.A.; Boulton, A.A. Excretion of m-hydroxymandehc acid in human urine. J. Chromatogr. 1981, 222, 271–275. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Z.; Wang, J. A Thermophilic Biofunctional Multienzyme Cascade Reaction for Cell-Free Synthesis of Salvianic Acid A and 3,4-Dihydroxymandelic Acid. ACS Sustain. Chem. Eng. 2019, 22, 18247–18253. [Google Scholar] [CrossRef]
- Ley, J.P.; Engelhart, K.; Bernhardt, J.; Bertram, H.J. 3,4-Dihydroxymandelic Acid, a Noradrenalin Metabolite with Powerful Antioxidative Potential. J. Agric. Food Chem. 2002, 50, 5897–5902. [Google Scholar] [CrossRef]
- Niu, D.; Li, H.; Zhang, X. Improved synthesis of 3-methoxy-4-hydroxymandelic acid by glyoxalic acid method. Tetrahedron 2013, 69, 8174–8177. [Google Scholar] [CrossRef]
- Soler Arias, E.A.; Trigo, R.H.; Miceli, D.D.; Vidal, P.N.; Hernandez Blanco, M.F.; Castillo, V.A. Urinary vanillylmandelic acid: Creatinine ratio in dogs with pheochromocytoma. Domest. Anim. Endocrinol. 2021, 74, 106559–106566. [Google Scholar] [CrossRef]
- Hrdlicka, V.; Barek, J.; Navratil, T. Differential pulse voltammetric determination of homovanillic acid as a tumor biomarker in human urine after hollow fiber-based liquid-phase microextraction. Talanta 2021, 221, 121594–121601. [Google Scholar] [CrossRef]
- Xie, J.; Schaich, K.M. Re-evaluation of the 2,2-Diphenyl-1-picrylhydrazyl Free Radical (DPPH) Assay for Antioxidant Activity. J. Agric. Food Chem. 2014, 62, 4251–4260. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Reprint of Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods 2015, 18, 782–796. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Z. Free-Radical-Scavenging Effect of Carbazole Derivatives on DPPH and ABTS Radicals. J. Am. Oil Chem. Soc. 2007, 84, 1095–1100. [Google Scholar] [CrossRef]
- Lewandowski, W.; Lewandowska, H.; Golonko, A.; Świderski, G.; Świsłocka, R.; Kalinowska, M. Correlations between molecular structure and biological activity in “logical series” of dietary chromone derivatives. PLoS ONE 2020, 15, e0229477. [Google Scholar] [CrossRef] [PubMed]
- Esin Celik, S.; Özyürek, M.; Güclü, K.; Apak, R. Solvent effects on the antioxidant capacity of lipophilic and hydrophilic antioxidants measured by CUPRAC, ABTS/persulphate and FRAP methods. Talanta 2010, 81, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Özyürek, M.; Guclu, K.; Capanoglu, E. Antioxidant activity/capacity measurement: I. Classification, physicochemical principles, mechanisms and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, D.R.; Gagliardi, C.J.; Hull, J.F.; Fecenko Murphy, C.; Kent, C.A.; Westlake, B.C.; Paul, A.; Ess, D.H.; Granville McCafferty, D.; Meyer, T.J. Proton-Coupled Electron Transfer. Chem. Rev. 2012, 112, 4016–4093. [Google Scholar] [CrossRef]
- Hammes-Schiffer, S.; Stuchebrukhov, A.A. Theory of Coupled Electron and Proton Transfer Reactions. Chem. Rev. 2010, 110, 6939–6960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Xiao, H.; Zheng, J.; Liang, G. Structure-Thermodynamics-Antioxidant Activity Relationships of Selected Natural Phenolic Acids and Derivatives: An Experimental and Theoretical Evaluation. PLoS ONE 2015, 10, e0121276. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.S.; Johnson, E.R.; DiLabio, G.A. Predicting the Activity of Phenolic Antioxidants: Theoretical Method, Analysis of Substituent Effects, and Application to Major Families of Antioxidants. J. Am. Chem. Soc. 2001, 123, 1173–1183. [Google Scholar] [CrossRef]
- Saqiba, M.; Iqbala, S.; Mahmooda, A.; Akrama, R. Theoretical investigation for exploring the antioxidant potential of chlorogenic acid: A DFT study. Int. J. Food Prop. 2015, 19, 745–751. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Liu, Y.; Zhang, L.; An, L.; Chen, R.; Liu, Y.; Luo, Q.; Li, Y.; Wang, H.; Xue, Y. Computational study on the antioxidant property of coumarin-fused coumarins. Food Chem. 2020, 304, 125446. [Google Scholar] [CrossRef]
- Amić, A.; Marković, Z.; Marković, J.M.D.; Stepanić, V.; Lučić, B.; Amic, D. Towards an improved prediction of the free radical scavenging potency of flavonoids: The significance of double PCET mechanisms. Food Chem. 2014, 152, 578–585. [Google Scholar] [CrossRef]
- Chen, X.; Liang, L.; Han, C. Borate suppresses the scavenging activity of gallic acid and plant polyphenol extracts on DPPH radical: A potential interference to DPPH assay. Lwt-Food Sci. Technol. 2020, 131, 109769–109802. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Wianowska, D.; Olszowy, M. On practical problems in estimation of antioxidant activity of compounds by DPPH method (Problems in estimation of antioxidant activity). Food Chem. 2012, 131, 1037–1043. [Google Scholar] [CrossRef]
- Leopoldini, M.; Marino, T.; Russo, N.; Toscano, M. Antioxidant Properties of Phenolic Compounds: H-Atom versus Electron Transfer Mechanism. J. Phys. Chem. A 2004, 108, 4916–4922. [Google Scholar] [CrossRef]
- Magalhaes, L.M.; Barreiros Salette, L.; Marcela, R.; Segundo, A. Kinetic matching approach applied to ABTS assay for high-throughput determination of total antioxidant capacity of food products. J. Food Compos. Anal. 2014, 33, 187–194. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved Abts Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Sridhar, K.; Linton Charles, A. In vitro antioxidant activity of Kyoho grape extracts in DPPH• and ABTS• assays: Estimation methods for EC50 using advanced statistical programs. Food Chem. 2018, 275, 41–49. [Google Scholar] [CrossRef]
- Apak, R.; Guclu, K.; Ozyurek, M.; Esin Celik, S. Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim. Acta. 2008, 160, 413–419. [Google Scholar] [CrossRef]
- Ozyurek, M.; Guclu, K.; Apak, R. The main and modified CUPRAC methods of antioxidant measurement. Trends Anal. Chem. 2011, 30, 652–664. [Google Scholar] [CrossRef]
- Elsa Madhu, S.; Sreeja, H.; Sudendara Priya, J. A preliminary study on phytochemical, antioxidant and cytotoxic activity of leaves of Naregamia alata Wight & Arn. Mater. Today 2020, 25, 343–348. [Google Scholar]
- Marković, Z.; Tošović, J.; Milenković, D.; Marković, S. Revisiting the solvation enthalpies and free energies of the proton and electron in various solvents. Comput. Theor. Chem. 2015, 1077, 11–17. [Google Scholar] [CrossRef]
- Szeląg, M.; Urbaniak, A.; Bluyssen, H.A.R. A theoretical antioxidant pharmacophore for natural hydroxycinnamic acids. De Gruyter 2015, 13, 17–31. [Google Scholar] [CrossRef]
- Świderski, G.; Łyszczek, R.; Wojtulewski, S.; Kalinowska, M.; Świsłocka, R.; Lewandowski, W. Comparison of structural, spectroscopic, theoretical and thermal properties of metal complexes (Zn(II), Mn(II), Cu(II), Ni(II) and Co(II)) of pyridazine-3- carboxylic acid and pyridazine-4-carboxylic acids. Inorg. Chim. Acta 2020, 512, 119865. [Google Scholar] [CrossRef]
- Bird, C.W. A New Aromaticity Index and its Application to Fivemembered Ring Heterocycles. Tetrahedron 1985, 41, 1409. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Barczynski, P.; Musumarra, G.; Pisano, D.; Szafran, M. Aromaticity as a Quantitative Concept. 1. A Statistical Demonstration of the Orthogonality of “Classical” and “Magnetic” Aromaticity in Five- and Six-Membered Heterocycles. J. Am. Chem. Soc. 1989, 111, 7–15. [Google Scholar] [CrossRef]
- Varsanyi, G. Vibrational Spectra of 700 Benzene Derivatives, Vol. I–II.; Academic Kiado: Budapest, Hungary, 1973. [Google Scholar]
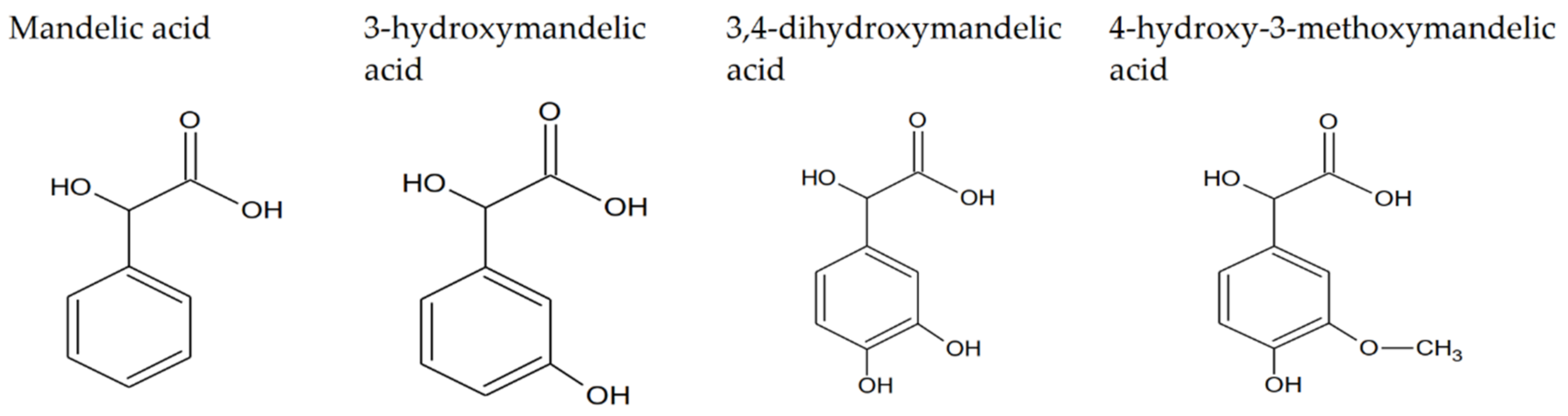

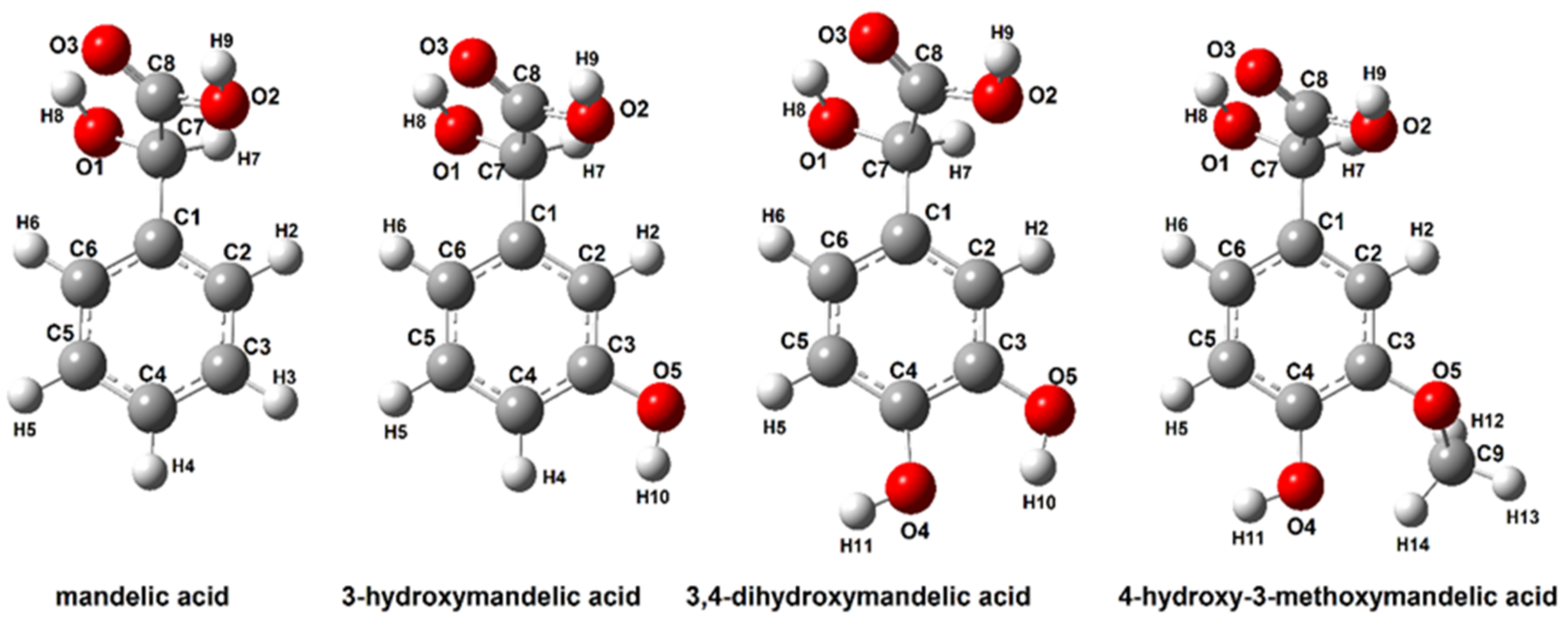
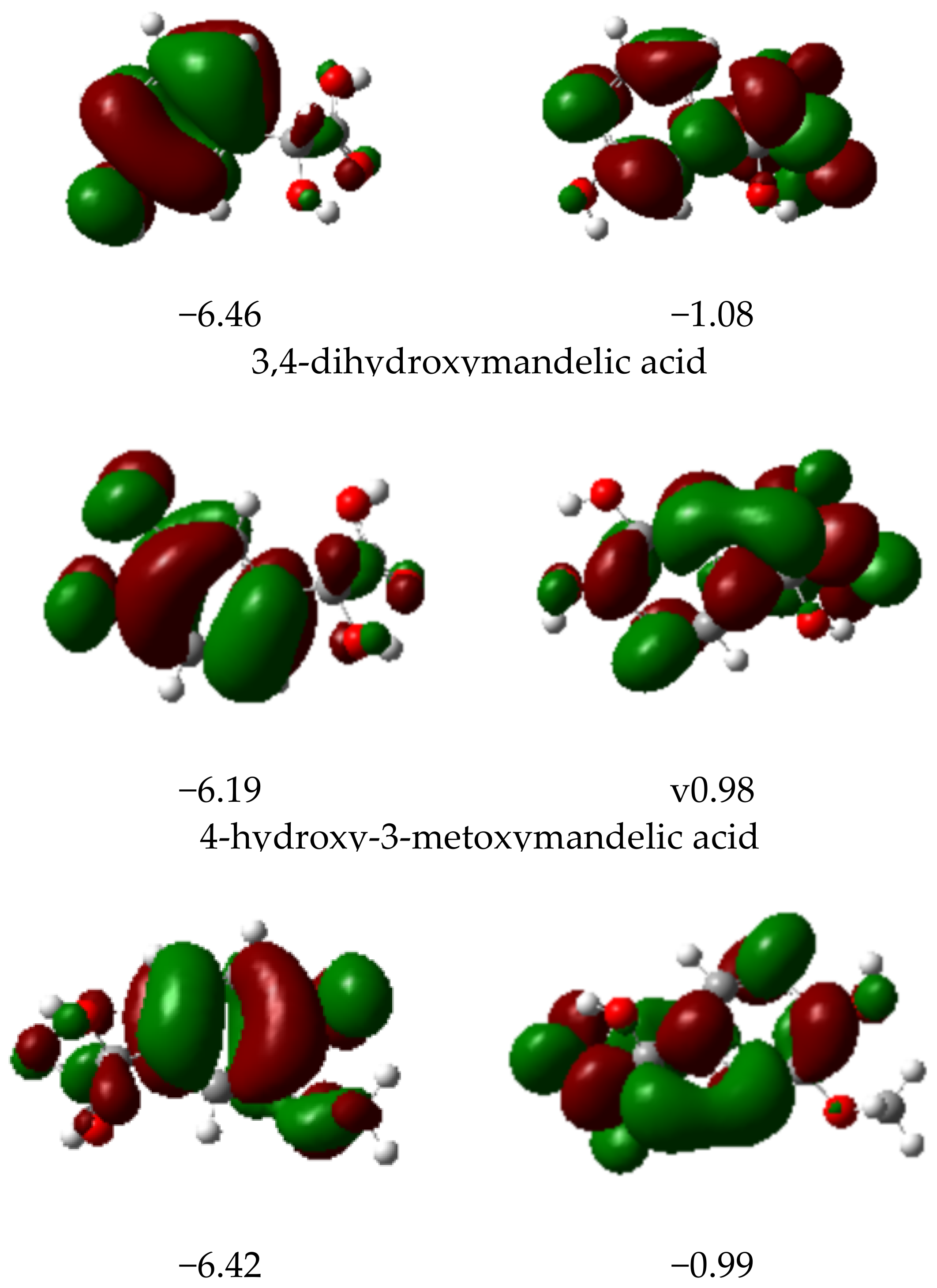
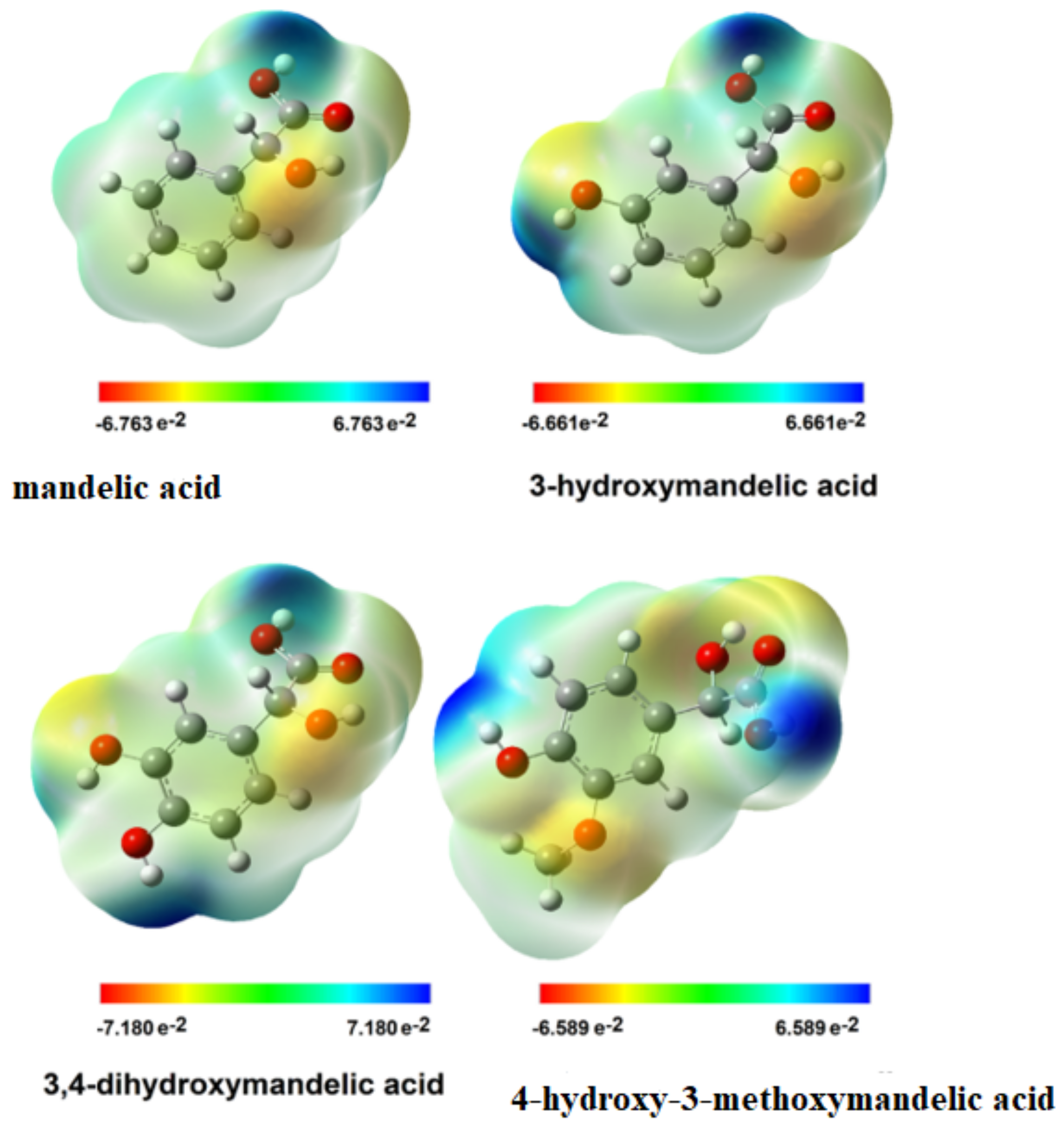
| Mandelic Acid | 3-Hydroxymanndelic Acid | 3,4-Dihydroxymandelic Acid | 4-Hydroxy-3-Metoxymandelic Acid | |
|---|---|---|---|---|
| Energy [hartree] | −535.51 | −610.76 | −685.99 | −725.31 |
| Energy [kJ/mol] | −1,406,909.87 | −1,604,604.26 | −1,802,266.09 | −1,905,551.83 |
| Dipole moment [De] | 2.41 | 1.92 | 0.27 | 1.47 |
| BDE [kcal/mol] | ||||
|---|---|---|---|---|
| Compound | Vacuum | Water | Ethanol | |
| Solvent | ||||
| MA | 97.65 | 407.23 | 409.11 | |
| 3OH-MA | 77.06 | 396.1 | 389.21 | |
| 3,4-diOH-MA | ||||
| 3-OH radical | 70.74 | 382.56 | 384.45 | |
| 4-OH radical | 62.21 | 382.45 | 384.33 | |
| 4OH-3OCH3-MA | 69.9 | 382.16 | 383.9 | |
| IP [kcal/mol] | ||||
| MA | 199.33 | 131.92 | 139.63 | |
| 3OH-MA | 188.75 | 136.01 | 142.10 | |
| 3,4-diOH-MA | 178.05 | 113.81 | 121.47 | |
| 4OH-3OCH3-MA | 176.92 | 112.78 | 120.36 | |
| PDE [kcal/mol] | ||||
| MA | 212.48 | 0.06 | −4.18 | |
| 3OH-MA | 202.49 | −15.16 | −13.31 | |
| 3,4-diOH-MA | ||||
| 3-OH radical | 206.85 | −6.49 | −10.67 | |
| 4-OH radical | 198.32 | −6.60 | −10.78 | |
| 4OH-3OCH3-MA | 207.14 | −5.86 | −10.10 | |
| PA [kcal/mol] | ||||
| MA | 318.21 | 42.12 | 40.92 | |
| 3OH-MA | 331.68 | 30.38 | 28.85 | |
| 3,4-diOH-MA | ||||
| 3-OH radical | 329.34 | 29.84 | 28.28 | |
| 4-OH radical | 315.39 | 23.25 | 21.30 | |
| 4OH-3OCH3-MA | 326.11 | 28.64 | 26.99 | |
| ETE [kcal/mol] | ||||
| MA | 93.60 | 89.86 | 94.55 | |
| 3OH-MA | 59.55 | 90.47 | 86.71 | |
| 3,4-diOH-MA | ||||
| 3-OH radical | 55.56 | 77.47 | 82.53 | |
| 4-OH radical | 61.01 | 83.96 | 89.39 | |
| Aromaticity Indice | Solution/Gas Phase | Mandelic Acid | 3-Hydroxy- Mandelic Acid | 3,4-Dihydroxy- Mandelic Acid | 4-Hydroxy-3-Methoxy Mandelic Acid |
|---|---|---|---|---|---|
| HOMA | Water | 0.989 | 0.988 | 0.980 | 0.986 |
| Gas phase | 0.989 | 0.988 | 0.988 | 0.984 | |
| Ethanol | 0.984 | 0.989 | 0.980 | 0.980 | |
| I6 | Water | 98.90 | 97.74 | 96.57 | 95.84 |
| Gas phase | 98.90 | 97.74 | 97.75 | 95.81 | |
| Ethanol | 98.53 | 98.65 | 96.53 | 95.84 | |
| BAC | Water | 0.984 | 0.959 | 0.940 | 0.930 |
| Gas phase | 0.984 | 0.959 | 0.959 | 0.918 | |
| Ethanol | 0.975 | 0.980 | 0.939 | 0.930 |
| Mandelic Acid | |||||
|---|---|---|---|---|---|
| Solvent | ΔE [eV] | Hardness [eV] | Softness [eV] | Electrophilicity [eV] | Electronegativity [eV] |
| Ethanol | 0.223 | 0.112 | 8.965 | 0.102 | 0.151 |
| Water | 0.223 | 0.112 | 8.954 | 0.102 | 0.151 |
| Vacuum | 0.218 | 0.109 | 9.154 | 0.050 | 0.149 |
| 3-hydroxymandelic acid | |||||
| Ethanol | 0.203 | 0.102 | 9.830 | 0.097 | 0.140 |
| Water | 0.204 | 0.102 | 9.814 | 0.097 | 0.140 |
| Vacuum | 0.198 | 0.099 | 10.120 | 0.097 | 0.139 |
| 3,4-dihydroxymandelic acid | |||||
| Ethanol | 0.190 | 0.100 | 10.406 | 0.090 | 0.133 |
| Water | 0.193 | 0.100 | 10.388 | 0.090 | 0.133 |
| Vacuum | 0.191 | 0.100 | 10.449 | 0.090 | 1.132 |
| 4-hydroxy-3-methoxymandelic acid | |||||
| Ethanol | 0.200 | 0.100 | 10.004 | 0.090 | 0.137 |
| Water | 0.201 | 0.100 | 9.970 | 0.137 | 0.137 |
| Vacuum | 0.200 | 0.100 | 10.016 | 0.090 | 0.136 |
| NBO Atom Charge Distribution | |||
|---|---|---|---|
| Mandelic Acid | |||
| Atom * | Ethanol | Water | Vacuum |
| C1 | −0.063 | −0.064 | −0.060 |
| C2 | −0.196 | −0.198 | −0.192 |
| C3 | −0.202 | −0.201 | −0.197 |
| C4 | −0.205 | −0.205 | −0.200 |
| C5 | 0.201 | −0.202 | −0.194 |
| C6 | −0.108 | −0.197 | 0.190 |
| C7 | 0.030 | 0.029 | 0.036 |
| C8 | 0.810 | 0.810 | 0.799 |
| H2 | 0.215 | 0.219 | 0.207 |
| H3 | 0.215 | 0.215 | 0.205 |
| H4 | 0.214 | 0.215 | 0.205 |
| H5 | 0.214 | 0.215 | 0.206 |
| H6 | 0.218 | 0.215 | 0.220 |
| H7 | 0.212 | 0.212 | 0.201 |
| H8 | 0.487 | 0.487 | 0.481 |
| H9 | 0.585 | 0.506 | 0.488 |
| O1 | −0.750 | −0.751 | −0.729 |
| O2 | −0.670 | −0.670 | −0.671 |
| O3 | −0.636 | −0.637 | −0.613 |
| 3-hydroxymandelic acid | |||
| C1 | −0.042 | −0.043 | −0.04 |
| C2 | −0.273 | −0.273 | −0.273 |
| C3 | 0.319 | 0.318 | −0.323 |
| C4 | −0.257 | −0.258 | −0.250 |
| C5 | −0.181 | −0.182 | −0.176 |
| C6 | −0.229 | −0.230 | −0.226 |
| C7 | 0.031 | 0.030 | 0.037 |
| C8 | 0.810 | 0.810 | 0.800 |
| H2 | 0.223 | 0.223 | 0.218 |
| H4 | 0.223 | 0.223 | 0.218 |
| H5 | 0.216 | 0.216 | 0.206 |
| H6 | 0.216 | 0.217 | 0.209 |
| H7 | 0.213 | 0.213 | 0.203 |
| H8 | 0.487 | 0.488 | 0.481 |
| H9 | 0.506 | 0.506 | 0.488 |
| H10 | 0.487 | 0.488 | 0.468 |
| O1 | −0.758 | −0.751 | −0.732 |
| O2 | −0.669 | −0.669 | −0.669 |
| O3 | −0.636 | −0.637 | −0.613 |
| O5 | −0.692 | −0.692 | −0.672 |
| 3,4-dihydroxymandelic acid | |||
| C1 | −0.073 | −0.074 | −0.081 |
| C2 | −0.253 | −0.253 | −0.213 |
| C3 | 0.274 | 0.273 | 0.254 |
| C4 | 0.271 | 0.271 | 0.283 |
| C5 | −0.257 | −0.257 | −0.265 |
| C6 | −0.208 | −0.208 | −0.178 |
| C7 | 0.032 | 0.031 | 0.039 |
| C8 | 0.809 | 0.818 | 0.799 |
| H2 | 0.218 | 0.219 | 0.217 |
| H5 | 0.219 | 0.220 | 0.284 |
| H6 | 0.220 | 0.220 | 0.221 |
| H7 | 0.211 | 0.212 | 0.200 |
| H8 | 0.486 | 0.497 | 0.481 |
| H9 | 0.505 | 0.505 | 0.488 |
| H10 | 0.488 | 0.489 | 0.470 |
| H11 | 0.488 | 0.489 | 0.466 |
| O1 | −0.751 | −0.752 | −0.730 |
| O2 | −0.671 | −0.671 | −0.671 |
| O3 | −0.638 | −0.639 | −0.613 |
| O4 | −0.686 | −0.687 | −0.659 |
| O5 | −0.686 | −0.687 | −0.711 |
| 4-hydroxy-3-methoxymandelic acid | |||
| C1 | −0.082 | −0.083 | −0.077 |
| C2 | −0.212 | −0.213 | −0.207 |
| C3 | 0.264 | 0.263 | 0.273 |
| C4 | 0.282 | 0.282 | 0.277 |
| C5 | −0.262 | −0.262 | −0.265 |
| C6 | −0.190 | −0.190 | −0.187 |
| C7 | 0.032 | 0.032 | 0.039 |
| C8 | 0.809 | 0.810 | 0.799 |
| H2 | 0.223 | 0.224 | 0.219 |
| H5 | 0.219 | 0.220 | 0.201 |
| H6 | 0.221 | 0.221 | 0.222 |
| H7 | 0.211 | 0.212 | 0.200 |
| H8 | 0.486 | 0.487 | 0.480 |
| H9 | 0.505 | 0.506 | 0.487 |
| H10 | 0.188 | 0.188 | 0.183 |
| H12 | 0.490 | 0.491 | 0.470 |
| H13 | 0.171 | 0.171 | 0.162 |
| H14 | 0.180 | 0.179 | 0.182 |
| O1 | −0.751 | −0.752 | −0.731 |
| O2 | −0.670 | −0.671 | −0.670 |
| O3 | −0.638 | −0.639 | −0.515 |
| O4 | −0.687 | −0.687 | −0.676 |
| O5 | −0.589 | −0.591 | −0.569 |
| Mandelic Acid | 3-Hydroxymandelic Acid | 3,4-Dihydroxymandelic Acid | 4-Hydroxy-3-Methoxymandelic acid | Assignment | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IRKBr | IRATR | Raman | Theor. (1) | IRKBr | IRATR | Raman | Theor. | IRKBr | IRATR | Raman | Theor. | IRKBr | IRATR | Raman | Theor. | ||||||
| cm−1 (int.) | cm−1 (int.) | cm−1 (int.) | cm−1 | Int. | cm−1 (int.) | cm−1 (int.) | cm−1 (int.) | cm−1 | Int. | cm−1 (int.) | cm−1 (int.) | cm−1 (int.) | cm−1 | Int. | cm−1 (int.) | cm−1 (int.) | cm−1 (int.) | cm−1 | Int. | [45] | |
| 3420 s | 3408 m | 3427 w | 3850 | 93.8 | 3402 s | 3850 | 93.8 | νOHar | |||||||||||||
| 3338 s | 3327 m | 3834 | 62.7 | 3335 s | 3330 m | 3792 | 113.0 | νOHar | |||||||||||||
| 3400 s | 3401 m | 3755 | 86.1 | 3755 | 88.0 | 3756 | 86.1 | 3353 vs | 3329 m | 3756 | 89.4 | νOH | |||||||||
| 3734 | 109.5 | 3730 | 112.2 | 3732 | 112.3 | 3728 | 78.9 | νOH | |||||||||||||
| 3070 m | 3074 w | 3064 vs | 3197 | 5.4 | 3062 vw | 3066 vw | 3070 s | 3198 | 5.8 | 3198 w | 3175 w | 3209 | 1.1 | 3087 vw | 3069 vs | 3203 | 1.7 | ν(CH) | 2 | ||
| 3031 m | 3038 w | 3049 m | 3188 | 16.96 | 3035 w | 3032 vw | 3185 | 9.4 | 3032 w | 3031 s | 3186 | 1.6 | 3034 vw | 3034 s | 3182 | 2.9 | ν(CH) | 20a | |||
| 2967 m | 2972 m | 3177 | 19.9 | 3173 | 6.3 | 2974 w | 2973 w | 2974 m | ν(CH) | 20b | |||||||||||
| 2927 m | 2936 w | 3167 | 2.1 | 3170 | 5.2 | 2945 w | 2911 w | 2916 m | 3154 | 14.4 | 2935 w | 2932 w | 2935 s | 3146 | 18.6 | ν(CH) | 7b | ||||
| 2716 m | 2722 m | 3015 | 17.4 | 2628 m | 2622 w | 3016 | 16.5 | 3017 | 18.1 | 3014 | 38.5 | νCH | |||||||||
| 1716 vs | 1711 vs | 1719 m | 1797 | 335.6 | 1715 vs | 1713 vs | 1796 | 333.4 | 1708 vs 1695 vs | 1692 vs | 1648 m | 1795 | 336.2 | 1743 vs 1718 s | 1743 s 1715 s | 1716 m | 1796 | 317.3 | νC=O | ||
| 1603 m | 1642 | 4.2 | 1605 s | 1603 s | 1609 m | 1644 | 31.1 | 1622 m | 1620 w | 1618 s | 1659 | 8.0 | 1611 m | 1610 m | 1609 s | 1647 | 22.7 | ν(CC) | 8a | ||
| 1588 w | 1588 w | 1627 | 0.4 | 1641 | 86.1 | 1606 s | 1603 m | 1605 s | 1646 | 46.7 | 1634 | 22.9 | ν(CC) | 8b | |||||||
| 1497 w | 1497 w | 1524 | 10.5 | 1466 vs | 1465 s | 1529 | 12.0 | 1537 s | 1534 m | 1530 vw | 1544 | 183.7 | 1517 vs | 1515 s | 1548 | 225.6 | ν(CC) | 19a | |||
| 1460 sh | 1460 sh | 1461 m | 1510 | 8.9 | δas(CH3) | ||||||||||||||||
| 1451 m | 1488 | 6.7 | δas(CH3) | ||||||||||||||||||
| 1452 m | 1453 m | 1483 | 9.2 | 1485 | 96.8 | 1452 m | 1450 m | 1449 vw | 1491 | 2.2 | 1439 s | 1437 s | 1447 m | 1481 | 6.7 | ν(CC) | 19b | ||||
| 1378 m | 1377 m | 1423 | 18.3 | 1420 m | 1420 m | 1423 | 19.5 | 1431 s | 1428 s | 1414 vw | 1426 | 18.9 | βOH; δCHOH | ||||||||
| 1366 | 0.1 | 1368 | 18.8 | 1377 sh | 1389 | 14.8 | 1380 m | 1377 m | 1376 w | ν(CC) | 3 | ||||||||||
| 1348 | 4.0 | 1359 w | 1348 | 33.1 | 1351 | 85.4 | ν(CC); βCH | 14 | |||||||||||||
| 1299 s | 1296 s | 1295 w | 1340 | 92.0 | 1268 vs | 1265 s | 1265 w | 1342 | 77.8 | 1350 s | 1347 s | 1355 m | 1341 | 88.8 | 1365 sh | βOH; νC–OH | |||||
| 1253 w | 1253 vw | 1256 vw | 1307 | 2.5 | 1249 vs | 1245 vs | 1324 | 7.1 | 1283 s | 1280 s | 1293 m | 1319 | 25.0 | 1270 vs | 1267 s | 1265 m | τCHOH(CH2); β(CH) | ||||
| 1303 | 237.0 | 1305 | 203.3 | νC–OH; α(CCC); νC–CH3 | |||||||||||||||||
| 1229 m | 1228 m | 1222 vw | 1253 | 27.5 | 1232 sh | 1254 | 37.4 | 1259 s | 1256 s | 1261 w | 1251 | 54.2 | 1237 vs | 1234 s | 1251 | 55.7 | ωCHOH(CH2) | ||||
| 1192 m | 1192 m | 1192 m | 1207 | 6.4 | 1214 vs | 1208 vs | 1220 s | 1219 vs | 1193 m | β(CH); βOHar | 9a | ||||||||||
| 1156 vw | 1154 vw | 1155 w | 1195 | 8.7 | 1168 s | 1167 m | 1167 w | 1196 | 47.2 | 1151 s | 1148 s | 1155 m | 1208 | 43.3 | 1150 vs | 1148 vs | 1147 w | 1207 | 27.5 | β(CH); ρ(CH3) | 9b |
| 1170 | 166.8 | 1179 | 39.5 | 1180 | 75.2 | 1185 | 48.4 | βOH | |||||||||||||
| 1132 m | 1131 s | 1171 | 5.0 | ρ(CH3) | |||||||||||||||||
| 1062 s | 1062 s | 1058 vw | 1089 | 79.7 | 1083 s | 1079 vs | 1082 m | 1085 | 66.0 | 1119 s | 1116 s | 1111 m | 1102 | 86.3 | 1061 s | 1057 s | 1061 m | 1101 | 116.1 | β(CH) | 18a |
| 1028 w | 1030 w | 1030 m | 1048 | 7.1 | 1089 vs | 1084 vs | 1085 w | 1032 s | 1031 s | 1032 m | β(CH) | 18b | |||||||||
| 1018 | 0.6 | 1013 | 4.8 | 960 | 18.2 | α(CCC) | 13 | ||||||||||||||
| 954 w | 954 w | 953 w | 1040 | 89.4 | νO–(CH3) | ||||||||||||||||
| 1004 w | 1004 w | 1004 s | 1118 | 104.8 | 1001 m | 1001 w | 1000 vs | 1118 | 83.1 | 982 m | 980 m | 1124 | 208.4 | α(CCC); νC–OH | 12 | ||||||
| 940 m | 940 m | 985 | 0.1 | 869 s | 982 | 0.1 | 920 sh | 923 sh | 937 | 1.9 | 924 w | 913 m | 947 | 0.3 | γ(CH) | 17a | |||||
| 889 m | 887 m | 892 | 22.1 | 932 m | 967 w | 919 | 16.2 | 881 s | 880 s | 908 m | 911 | 23.4 | 880 m | 936 | 12.6 | νC–COOH; α(CCC); γOH | |||||
| 855 w | 854 w | 858 w | 869 | 2.0 | 826 m | 826 m | 821 w | 860 | 6.6 | 868 m | 867 m | 869 | 8.3 | 863 m | 861 s | 878 | 7.8 | βC=O | |||
| 816 | 1.7 | 836 | 18.2 | 835 | 20.1 | γC=O; α(CCC) | |||||||||||||||
| 768 w | 768 w | 768 vw | 763 | 16.7 | 732 s | 731 vs | 725 m | 788 | 28.5 | 835 m | 833 m | 792 | 36.6 | 825 m | 822 s | 824 w | 811 | 28.1 | γ(CH) | 11 | |
| 733 s | 731 s | 732 w | 727 | 50.9 | 697 s | 695 s | 720 | 54.8 | 807 s | 801 s | 784 vs | 725 | 1.8 | 775 m | 773 s | 777 m | 746 | 1.4 | φ(CC) | 4 | |
| 697 s | 697 s | 709 | 33.2 | 674 m | 671 s | 695 | 26.7 | 732 m | 731 m | 718 m | 706 | 36.1 | 732 m | 731 m | 707 | 31.8 | α(CCC) | 1 | |||
| 609 m | 608 m | 617 w | 660 | 35.1 | 637 w | 660 | 39.8 | 646 m | 662 m | 661 | 43.8 | 707 m | 693 s | 701 m | 662 | 27.0 | γC=O; βOH | ||||
| 528 m | 570 | 52.2 | 505 m | 545 | 20.4 | 603 w | 585 w | 549 | 28.8 | 634 w | 546 | 19.8 | γOH | ||||||||
| 494 m | 501 w | 498 | 4.1 | 462 m | 475 | 14.4 | 519 m | 475 | 20.3 | 533 w | 471 | 21.8 | φ(CC); γOH | 16b | |||||||
| 467 w | 413 | 4.4 | 413 | 53.2 | 468 m | 403 | 48.3 | 465 w | 402 | 35.2 | φ(CC); βOH | 16a | |||||||||
| Compound | ||||||||
|---|---|---|---|---|---|---|---|---|
| Mandelic Acid | 3-OH-Mandelic Acid | 3,4-Dihydoxymandelic Acid | 4-Hydroxy-3-Methoxymandelic Acid | |||||
| 13C NMR | ||||||||
| assignment | Calc. | Exp. | Calc. | Exp. | Calc. | Exp. | Calc. | Exp. |
| C1 * | 146.02 | 140.39 | 147.49 | 141.62 | 138.31 | 131.10 | 137.91 | 131.13 |
| C2 | 129.71 | 126.85 | 113.89 | 113.47 | 118.61 | 114.14 | 128.73 | 110.80 |
| C3 | 133.36 | 128.34 | 163.51 | 157.13 | 150.89 | 144.90 | 153.63 | 146.12 |
| C4 | 133.39 | 127.87 | 118.26 | 114.51 | 149.05 | 144.90 | 156.53 | 147.26 |
| C5 | 133.15 | 128.34 | 134.76 | 129.00 | 117.19 | 115.06 | 119.51 | 115.01 |
| C6 | 133.58 | 126.85 | 124.10 | 117.31 | 120.68 | 117.83 | 125.42 | 119.33 |
| C7 | 75.56 | 72.63 | 75.08 | 72.33 | 74.74 | 72.13 | 74.81 | 72.21 |
| C8 | 182.50 | 174.36 | 182.60 | 174.04 | 182.56 | 174.48 | 182.73 | 174.39 |
| C9 | - | - | - | - | - | - | 60.07 | 55.56 |
| 1H NMR | ||||||||
| H2 | 7.89 | 7.45 | 7.17 | 6.82 | 7.25 | 6.80 | 7.35 | 6.96 |
| H3 | 7.69 | 7.35 | -- | - | - | - | - | - |
| H4 | 7.62 | 7.34 | 7.23 | 7.11 | - | - | - | - |
| H5 | 7.61 | 7.35 | 6.98 | 7.11 | 6.95 | 6.64 | 6.90 | 6.72 |
| H6 | 7.75 | 7.45 | 7.47 | 7.13 | 7.25 | 6.65 | 7.45 | 6.95 |
| H7 | 3.34 | 5.08 | 3.17 | 4.89 | 3.14 | 4.80 | 3.15 | 4.88 |
| H8 | 5.47 | 5.08 | 5.46 | 6.81 | 5.33 | 5.55 | 5.33 | 5.69 |
| H9 | 6.60 | 12.69 | 6.05 | 12.41 | 6.55 | 12.38 | 6.51 | 12.44 |
| H10 | - | - | 4.57 | 9.36 | 4.54 | 8.90 | - | - |
| H11 | - | - | - | - | 5.33 | 8.82 | 4.53 | 8.93 |
| H12 | - | - | - | - | - | - | 3.92 | 3.74 |
| H13 | - | - | - | - | - | - | 3.44 | 3.74 |
| H14 | 3.15 | 3.74 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parcheta, M.; Świsłocka, R.; Świderski, G.; Matejczyk, M.; Lewandowski, W. Spectroscopic Characterization and Antioxidant Properties of Mandelic Acid and Its Derivatives in a Theoretical and Experimental Approach. Materials 2022, 15, 5413. https://doi.org/10.3390/ma15155413
Parcheta M, Świsłocka R, Świderski G, Matejczyk M, Lewandowski W. Spectroscopic Characterization and Antioxidant Properties of Mandelic Acid and Its Derivatives in a Theoretical and Experimental Approach. Materials. 2022; 15(15):5413. https://doi.org/10.3390/ma15155413
Chicago/Turabian StyleParcheta, Monika, Renata Świsłocka, Grzegorz Świderski, Marzena Matejczyk, and Włodzimierz Lewandowski. 2022. "Spectroscopic Characterization and Antioxidant Properties of Mandelic Acid and Its Derivatives in a Theoretical and Experimental Approach" Materials 15, no. 15: 5413. https://doi.org/10.3390/ma15155413
APA StyleParcheta, M., Świsłocka, R., Świderski, G., Matejczyk, M., & Lewandowski, W. (2022). Spectroscopic Characterization and Antioxidant Properties of Mandelic Acid and Its Derivatives in a Theoretical and Experimental Approach. Materials, 15(15), 5413. https://doi.org/10.3390/ma15155413







