Silica- Iron Oxide Nanocomposite Enhanced with Porogen Agent Used for Arsenic Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization Material SiO2/Fe(acac)3/NaF
2.2. Arsenic Adsorption Studies
2.3. Arsenic Desorption Studies
2.4. Arsenic Removal from Real Ground Water
3. Results and Discussion
3.1. Synthesis and Characterization Material SiO2/Fe(acac)3/NaF
X-ray Diffractogram
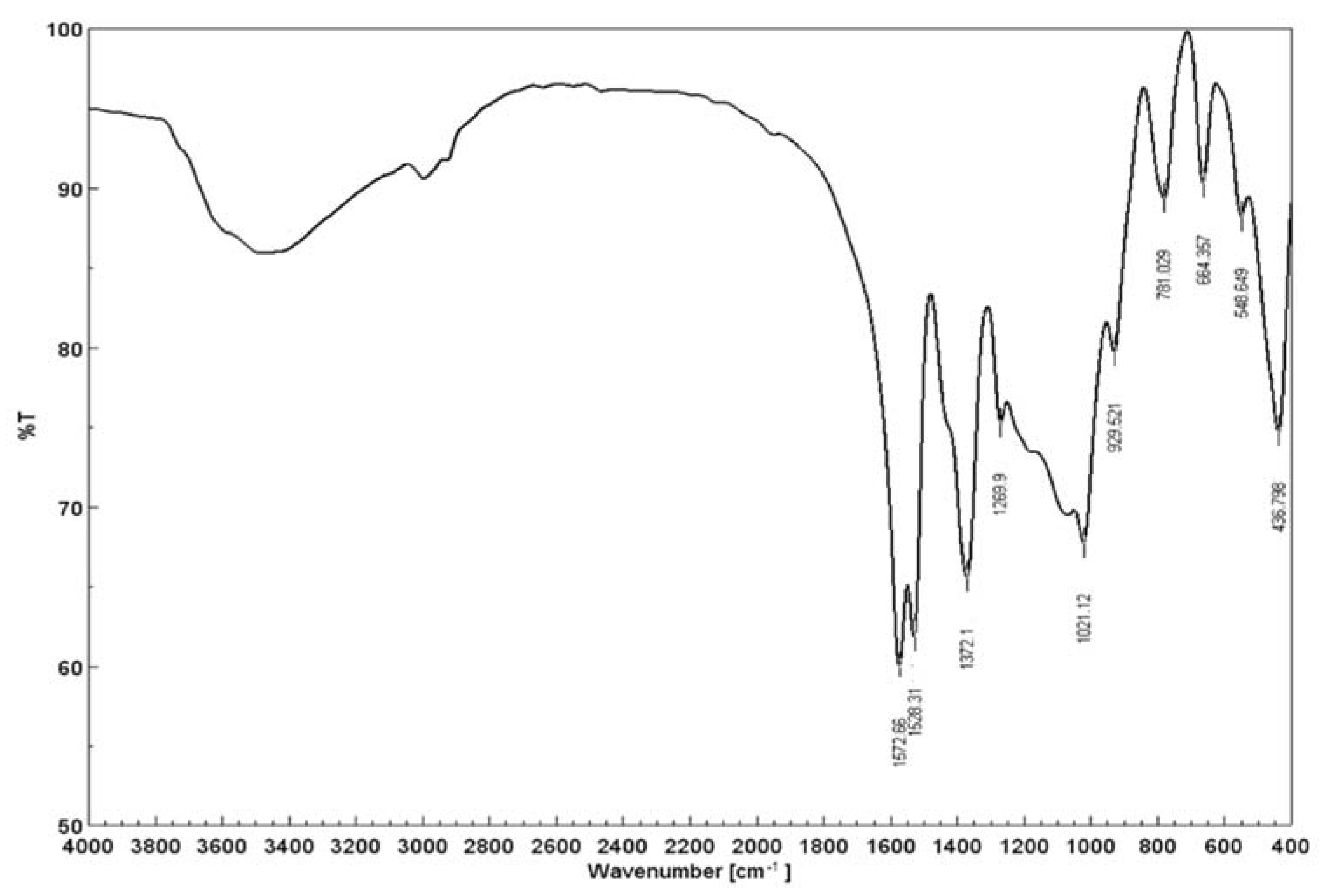
3.2. Raman and Infrared Spectroscopy
3.3. FT-IR Spectra
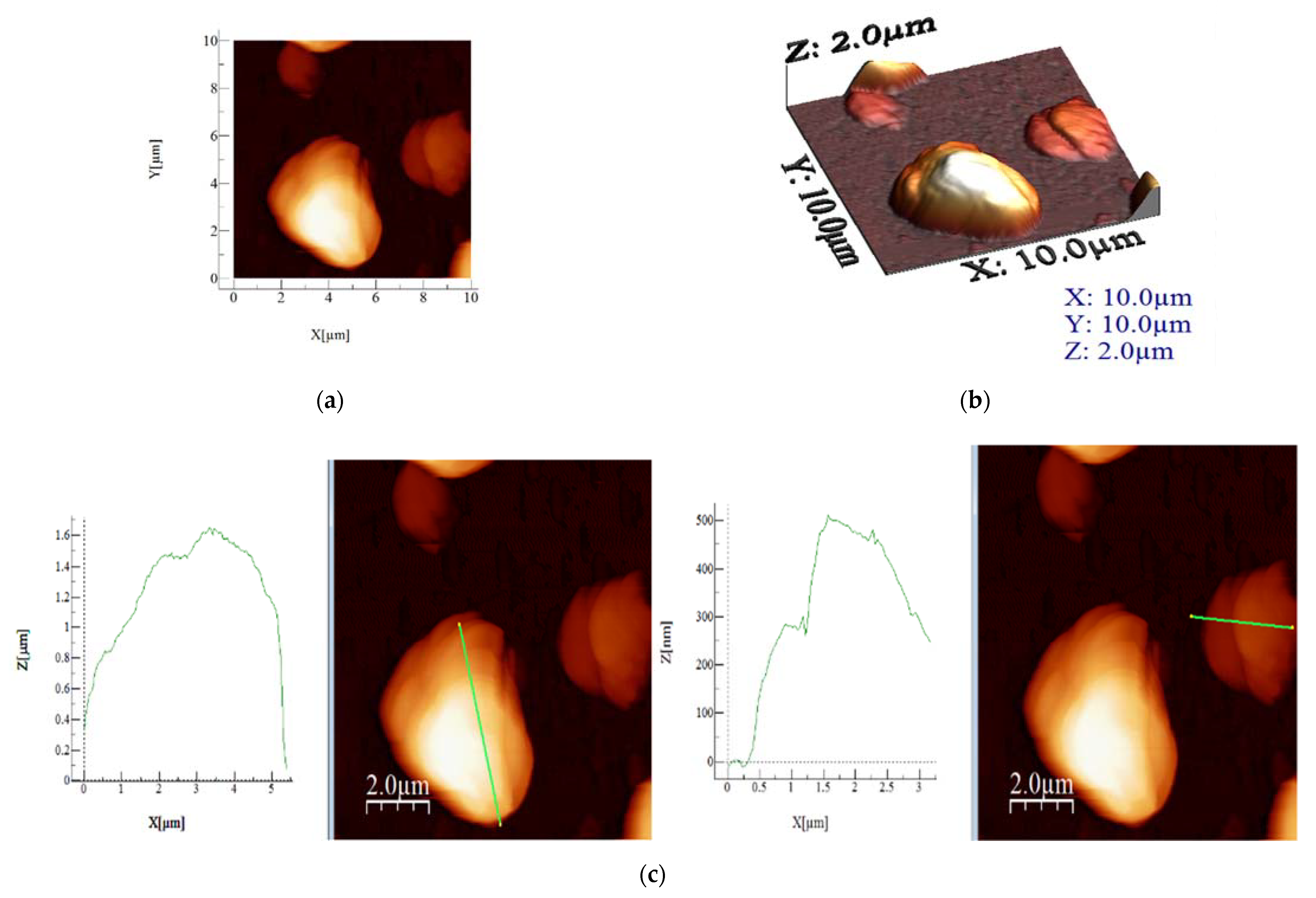
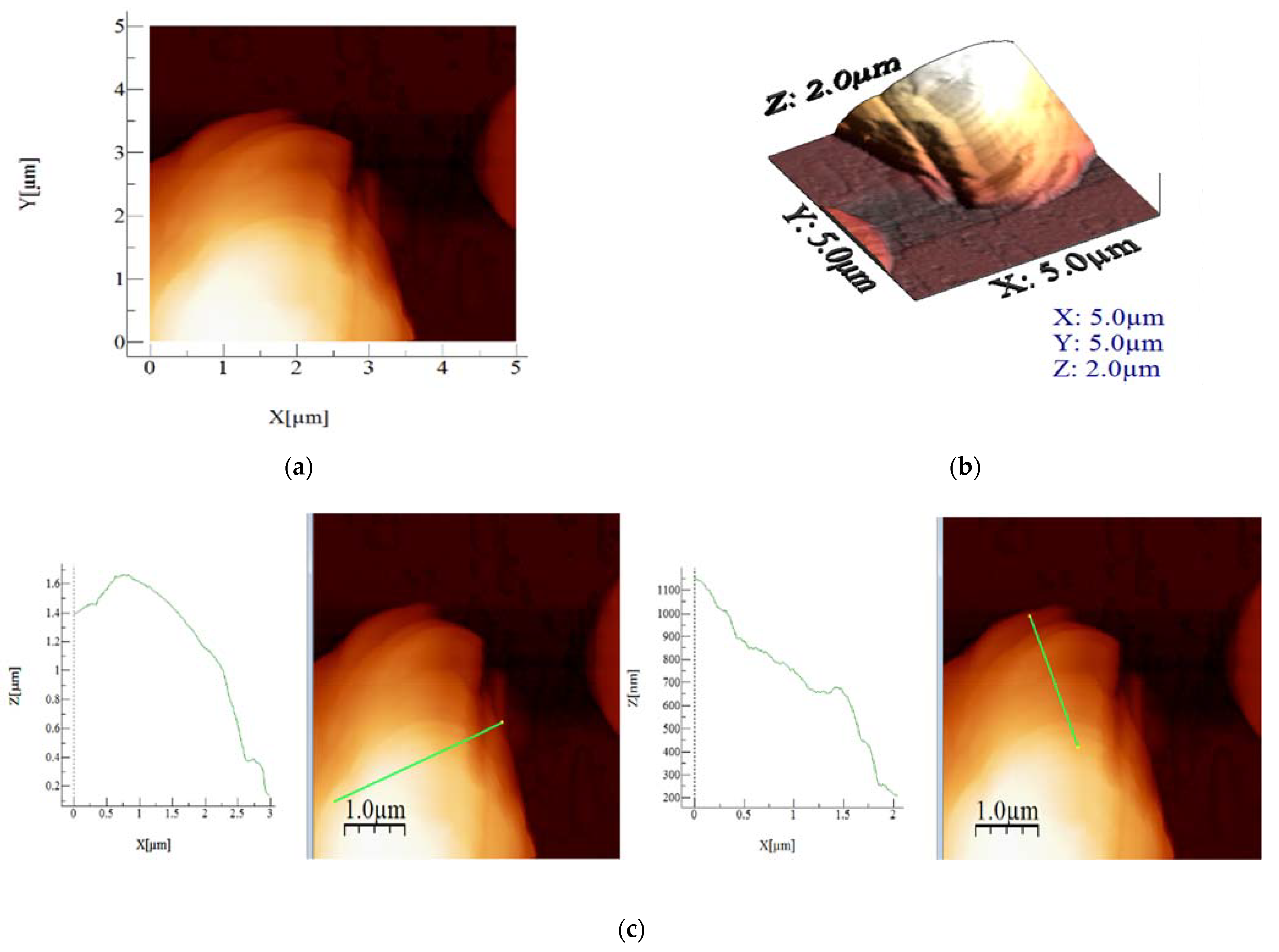
3.4. Morphological Study with Atomic Force Microscopy
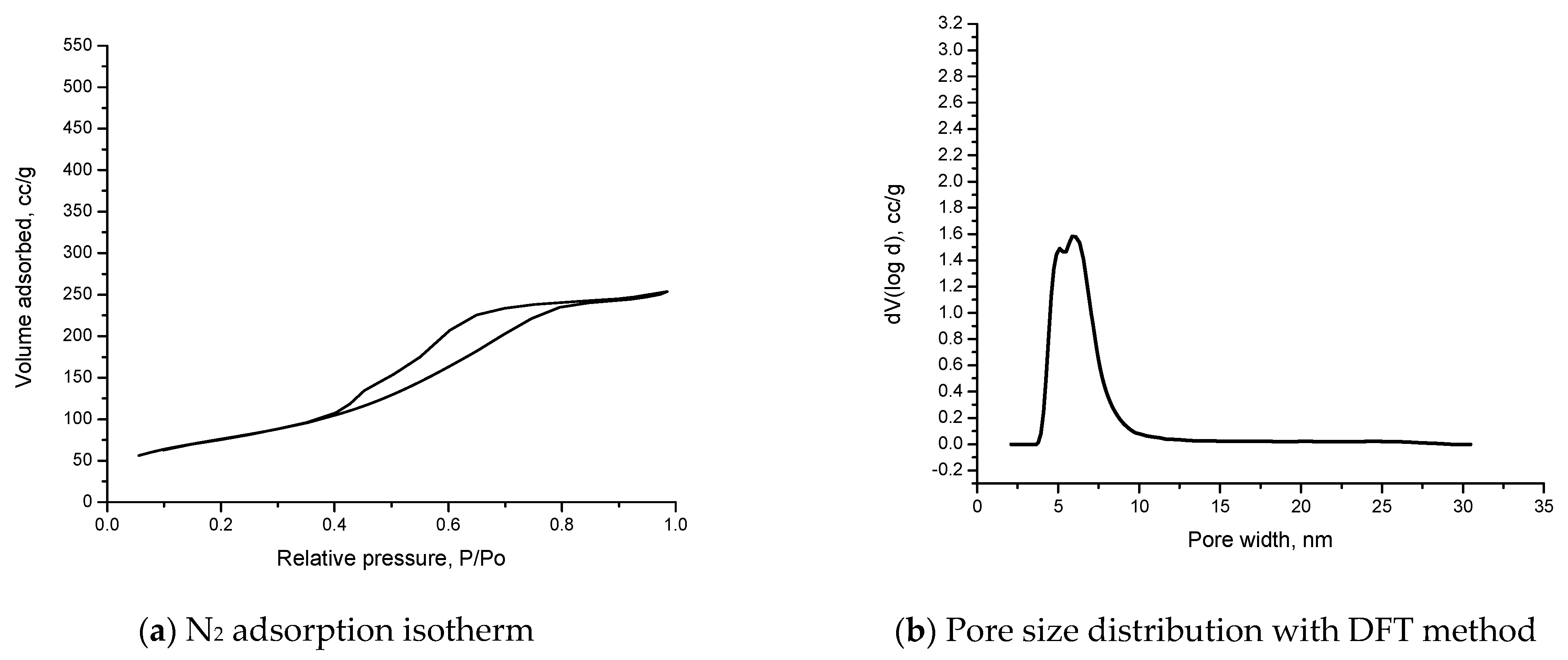
3.5. Nitrogen Adsorption–Desorption Isotherms
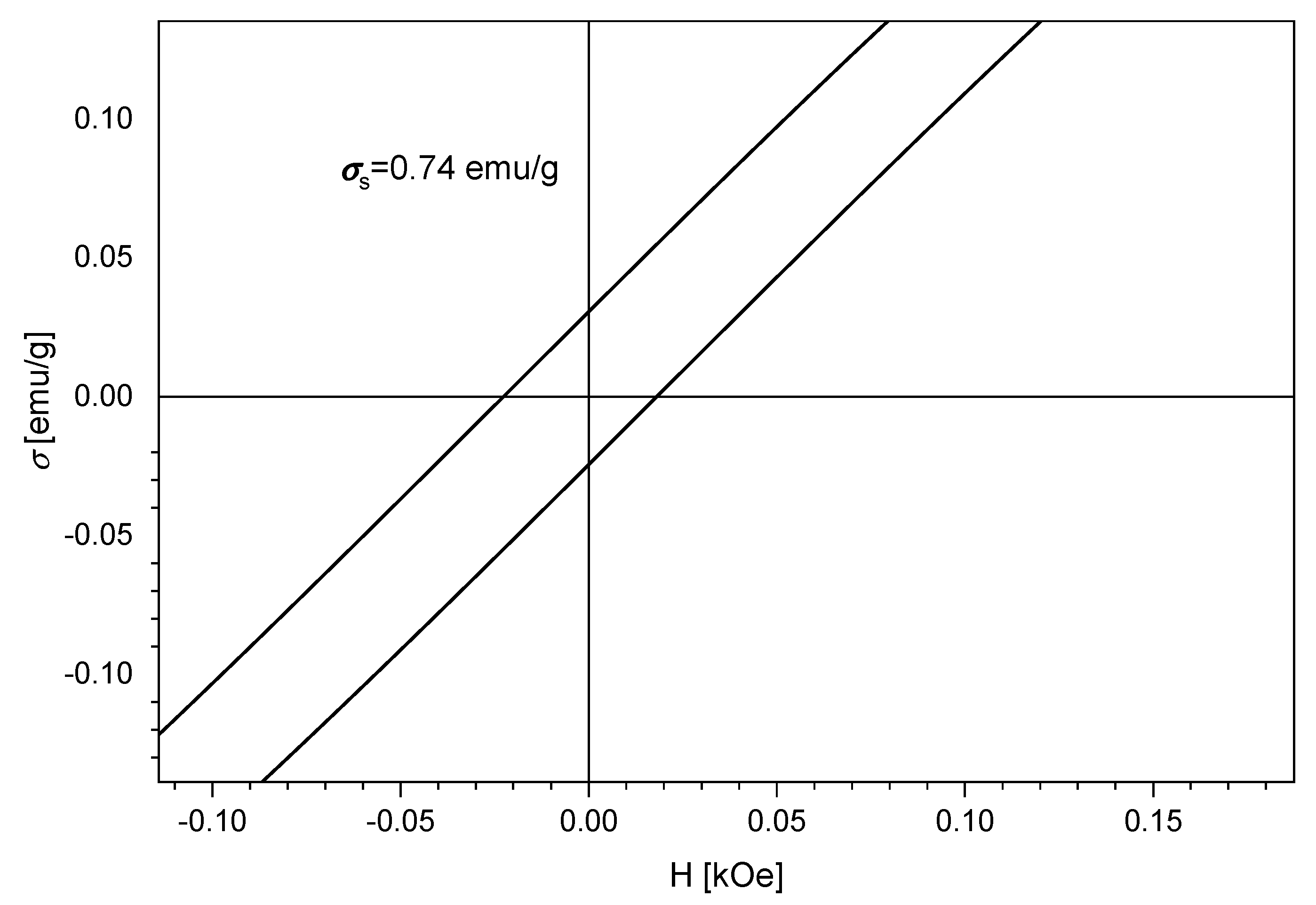
3.6. Magnetic Measurements
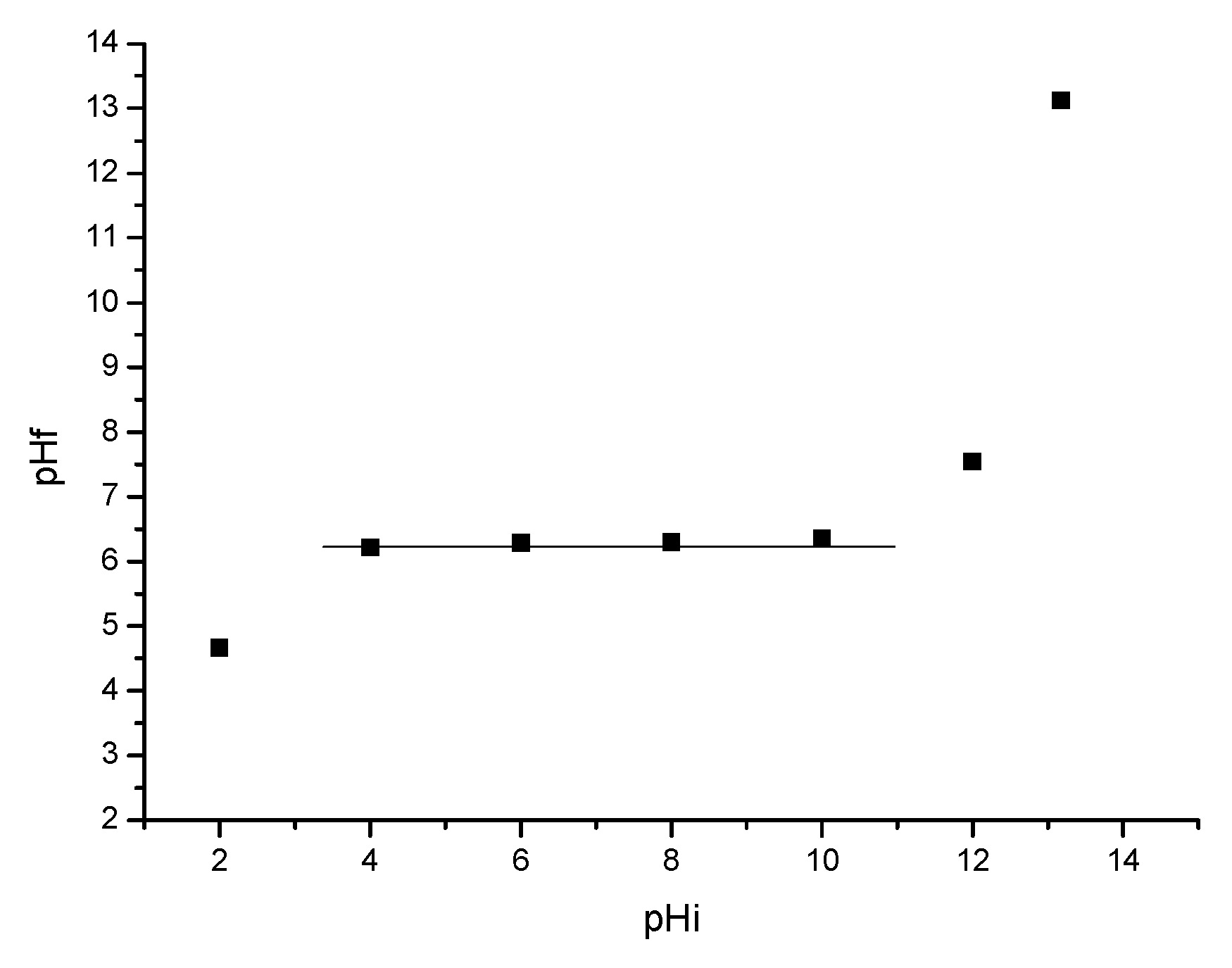
3.7. Point of Zero Charge Determination (pHpZc)
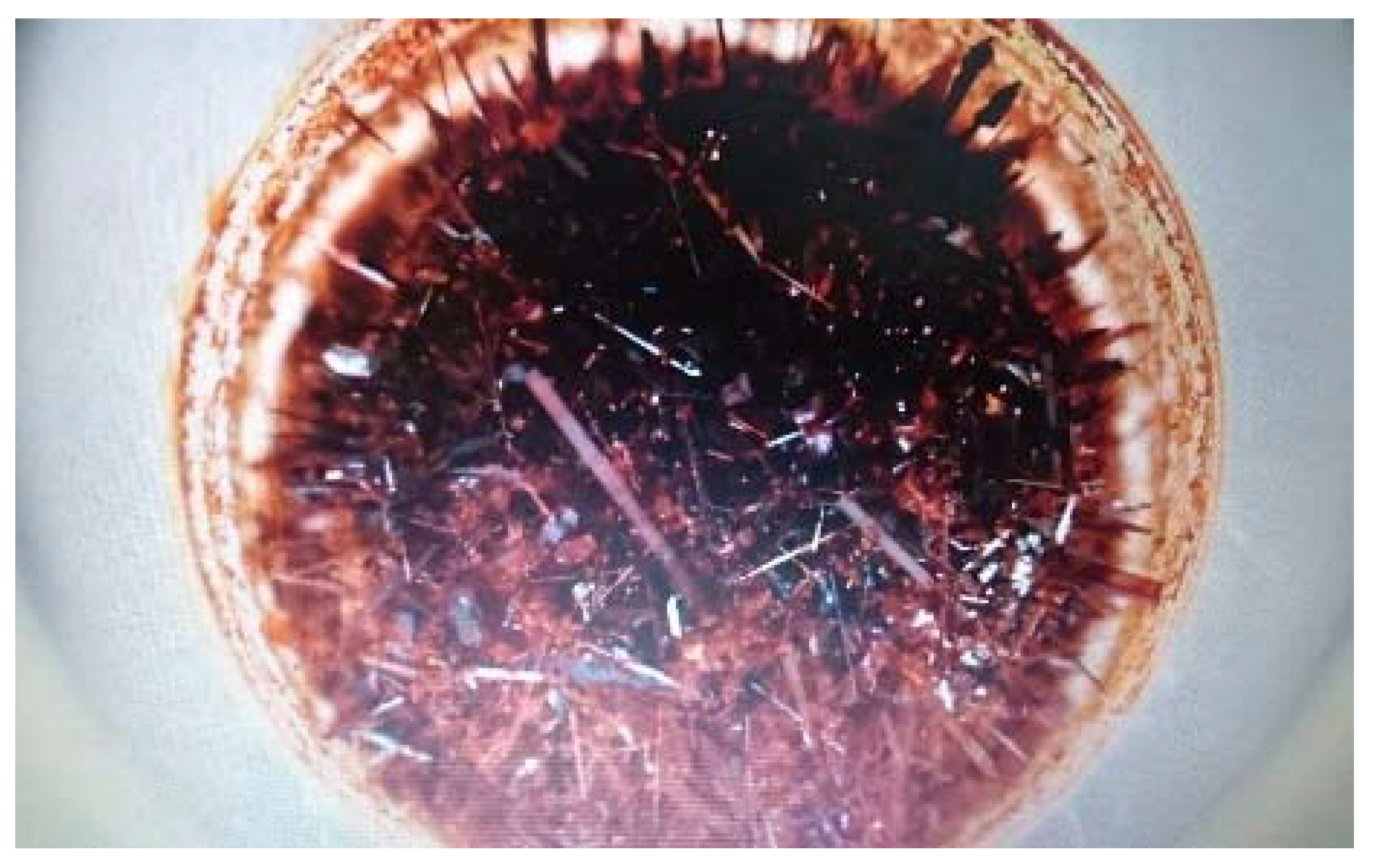
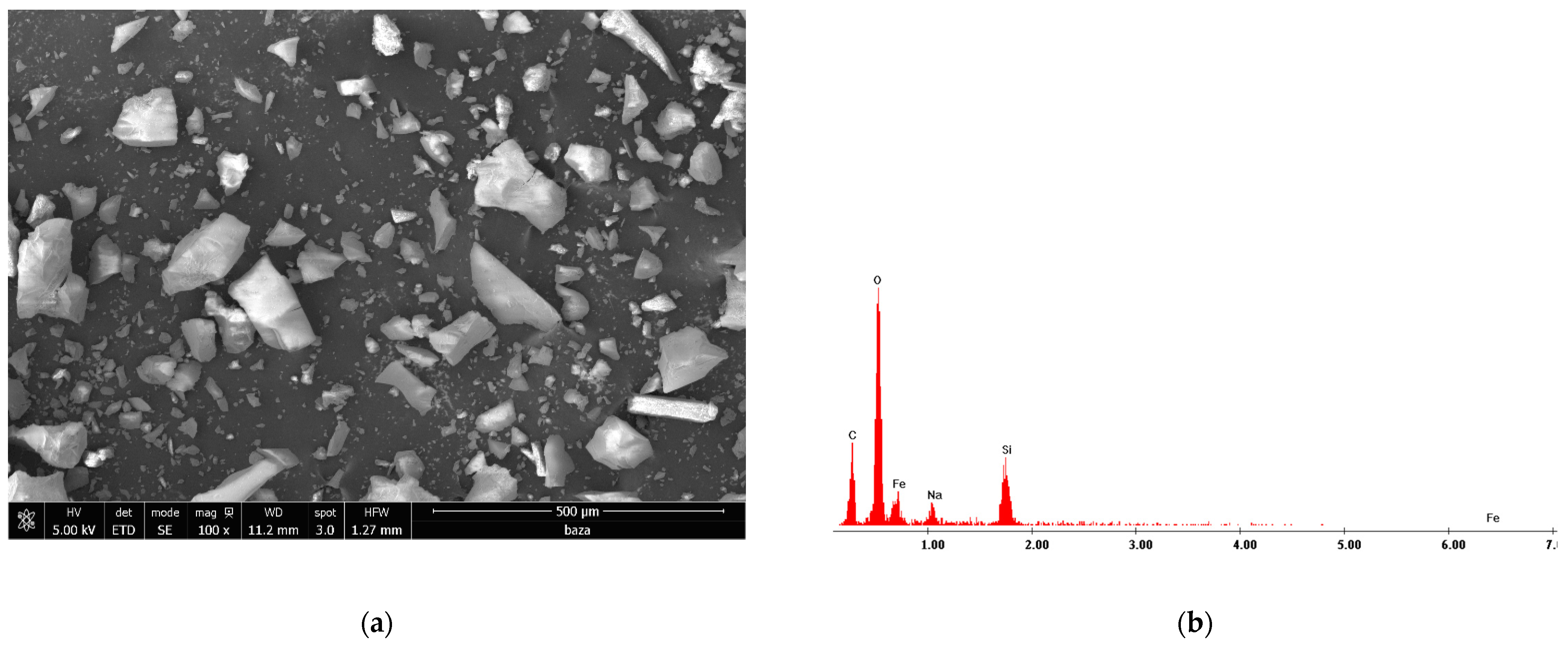
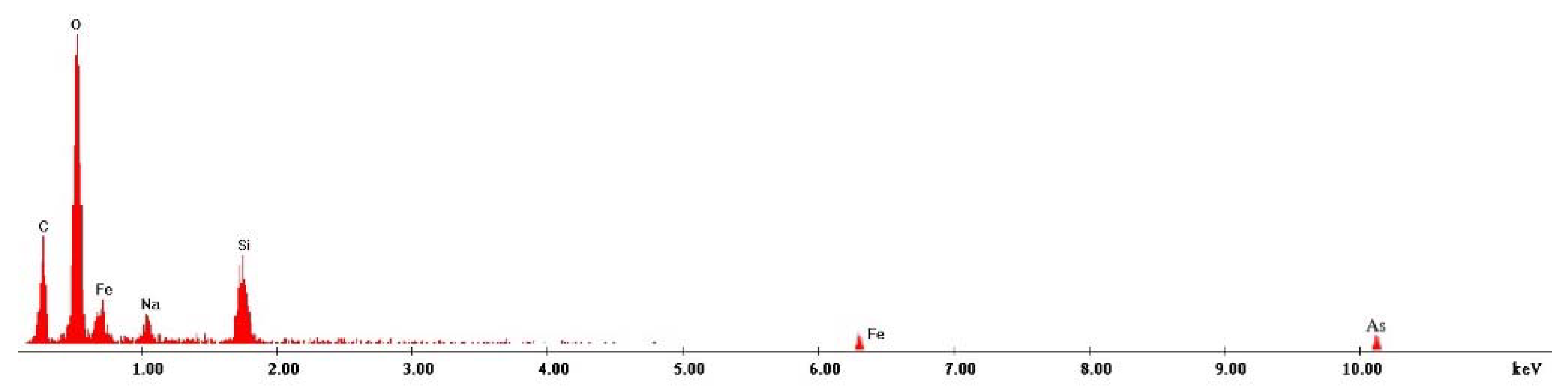
3.8. Scanning Electron Microscopy Characterization
3.9. Arsenic Adsorption Studies
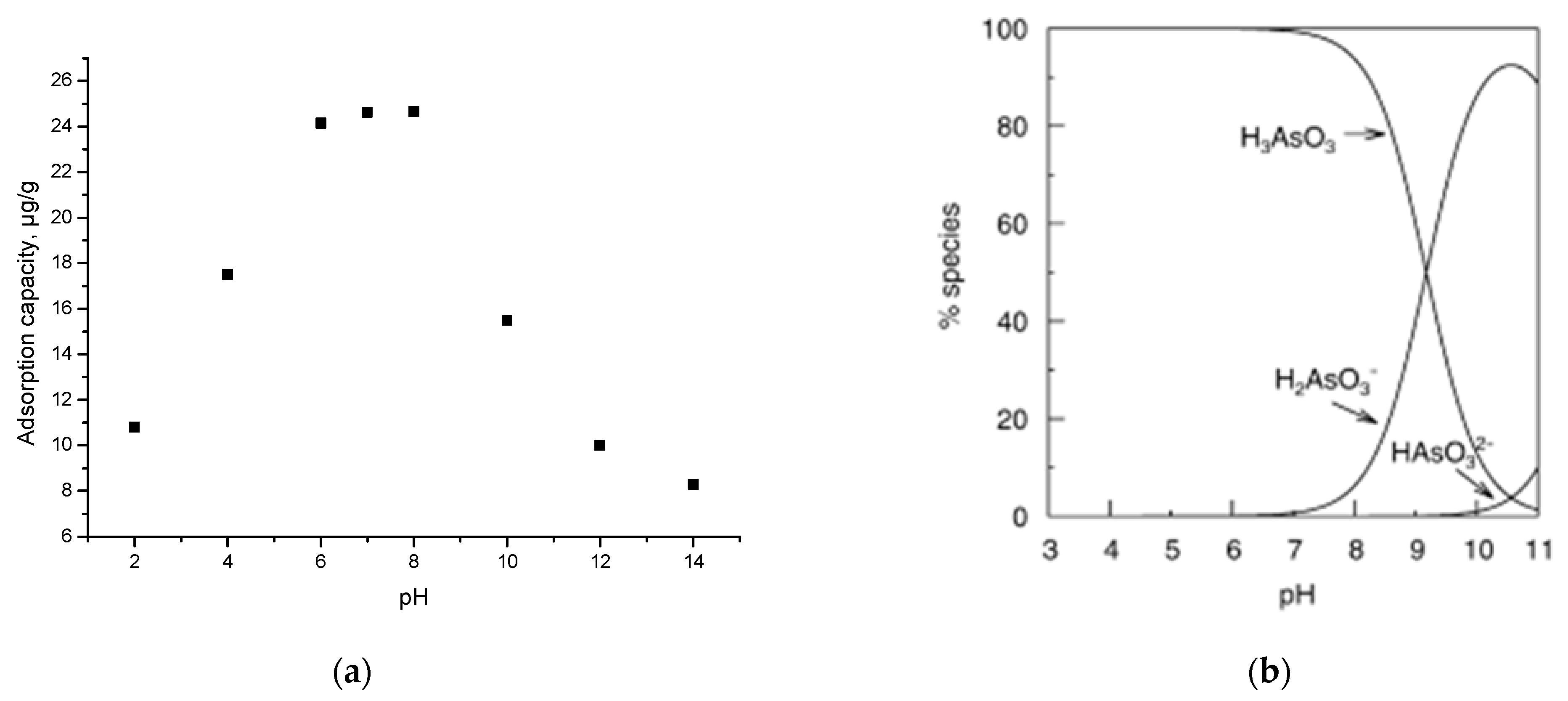
3.9.1. pH Effect
3.9.2. Influence of the Adsorbent Dose
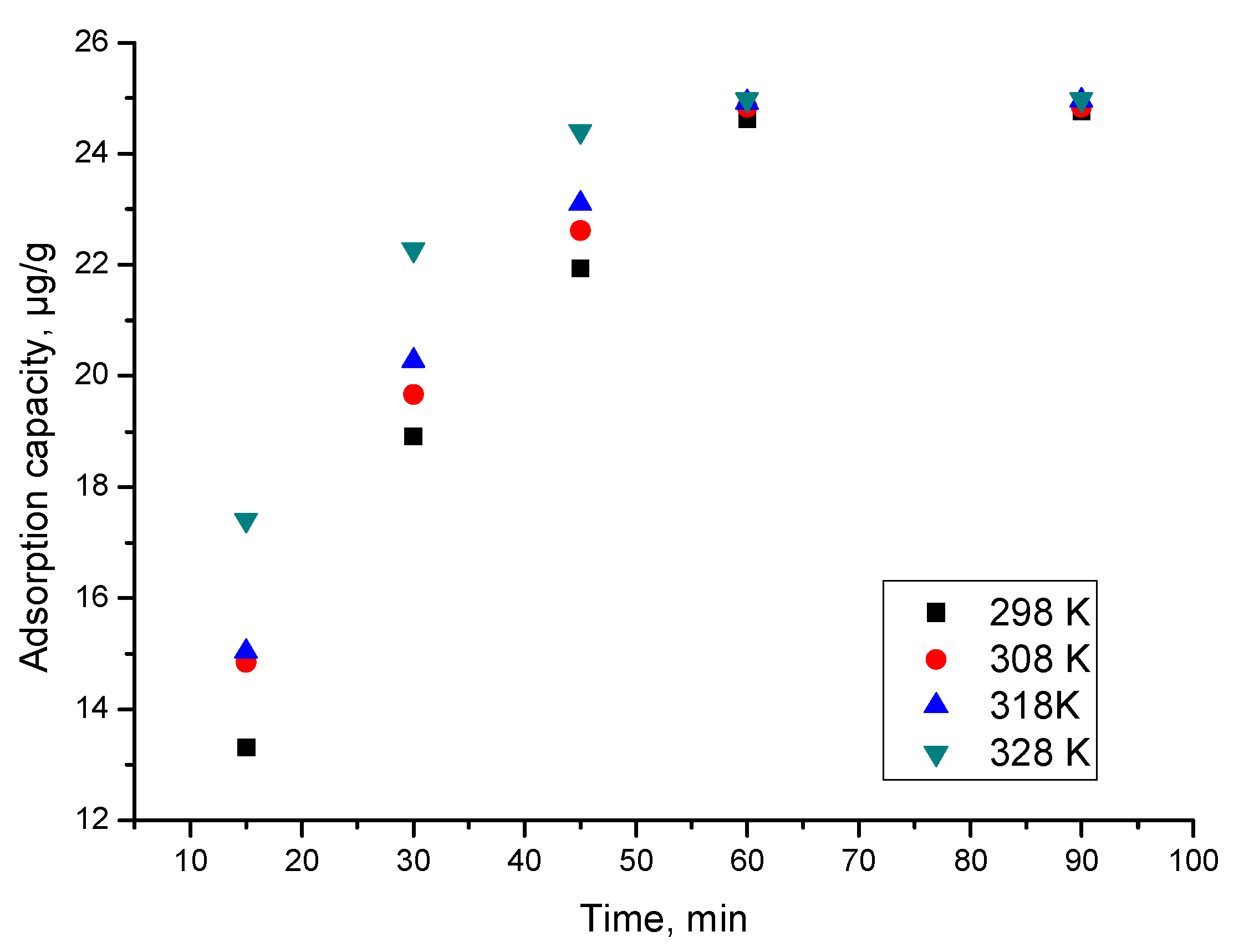
3.9.3. Contact Time Effect
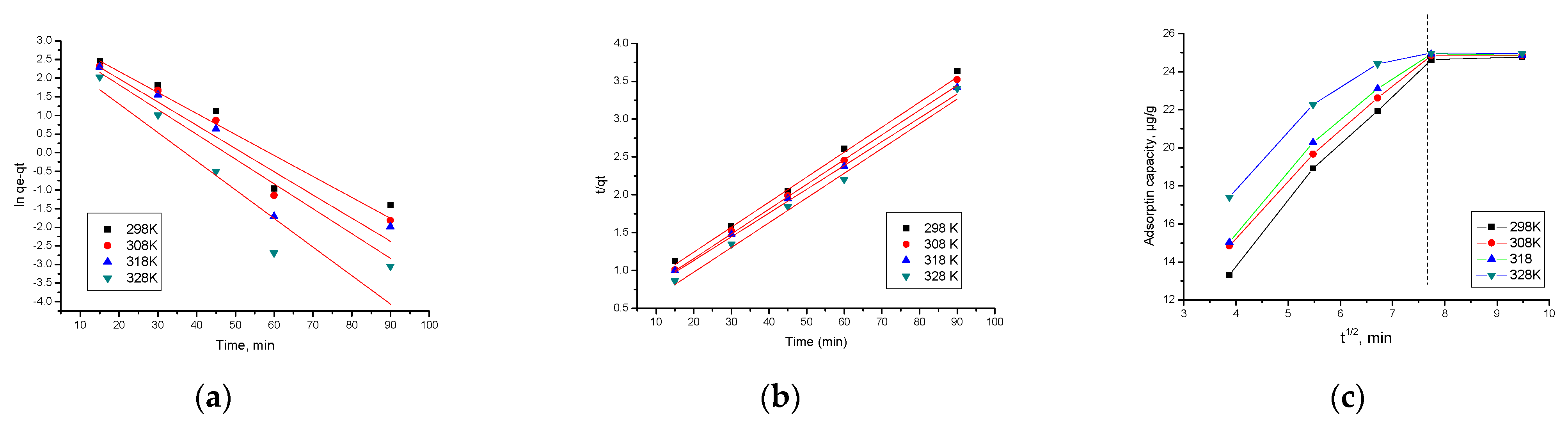
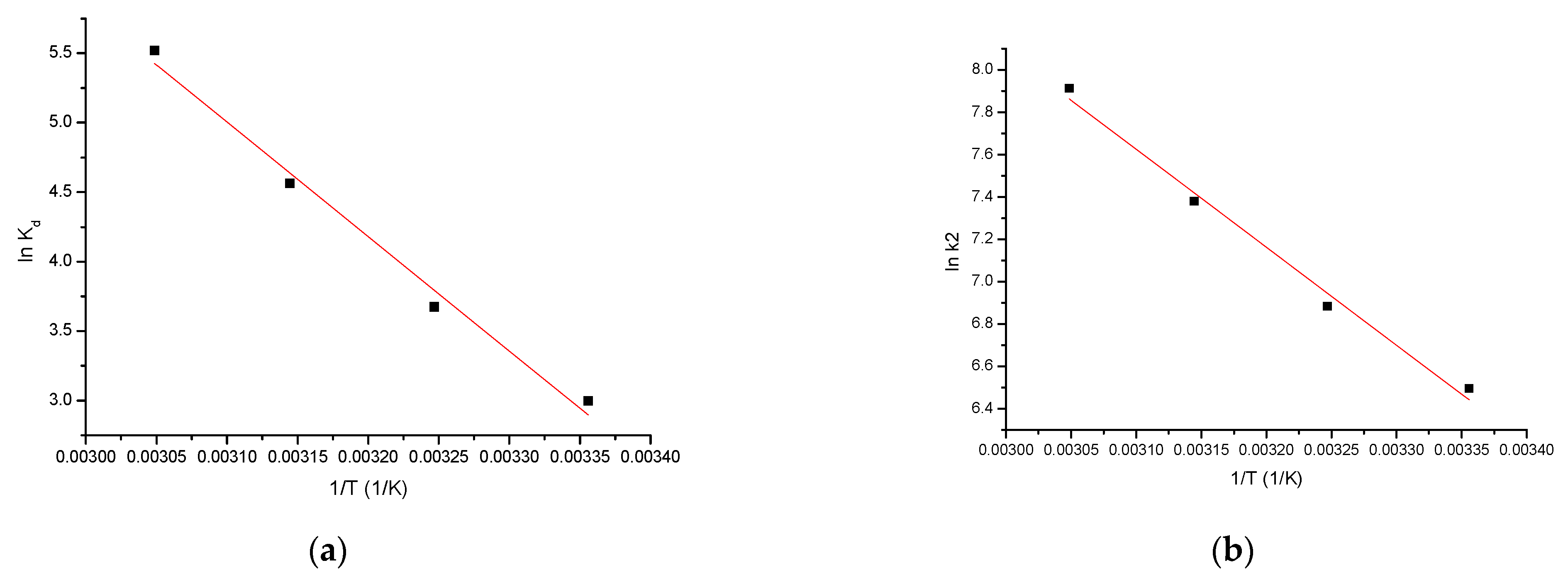
3.10. Kinetic Studies
3.11. Theromdinamic Studies
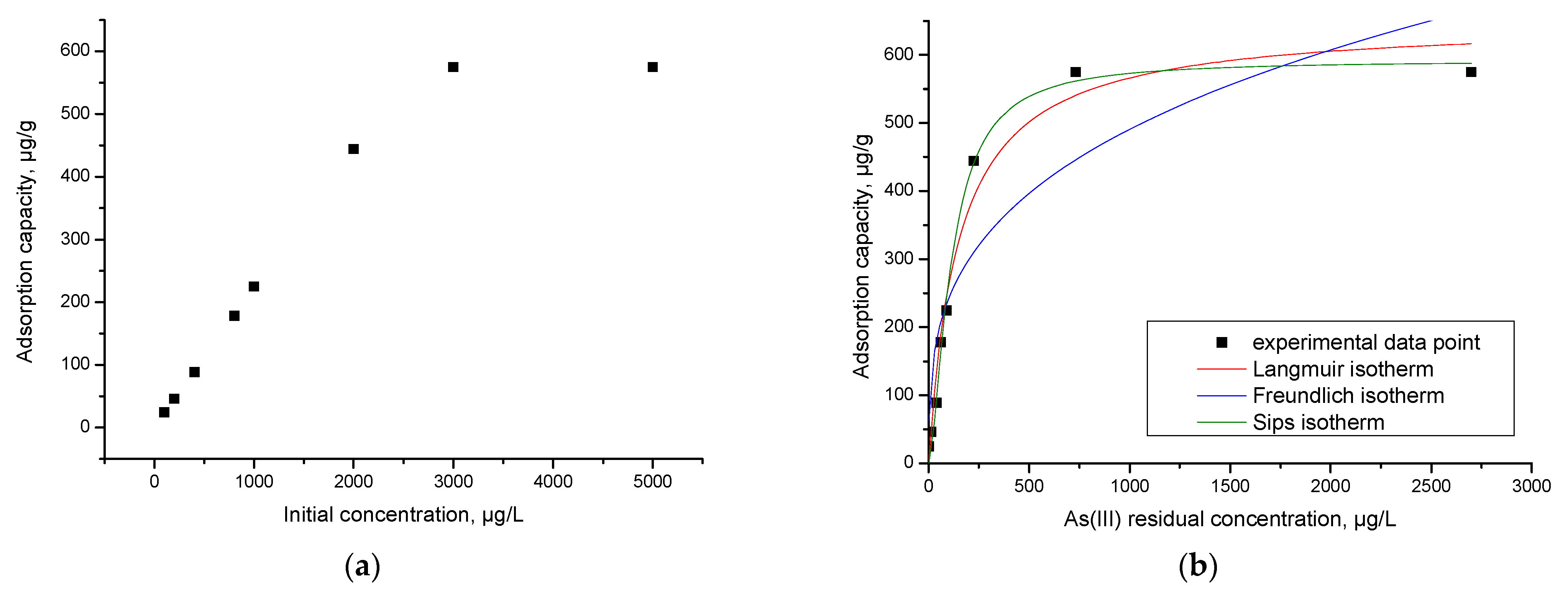
3.12. Initial Concentration Effect. Equilibrium Studies
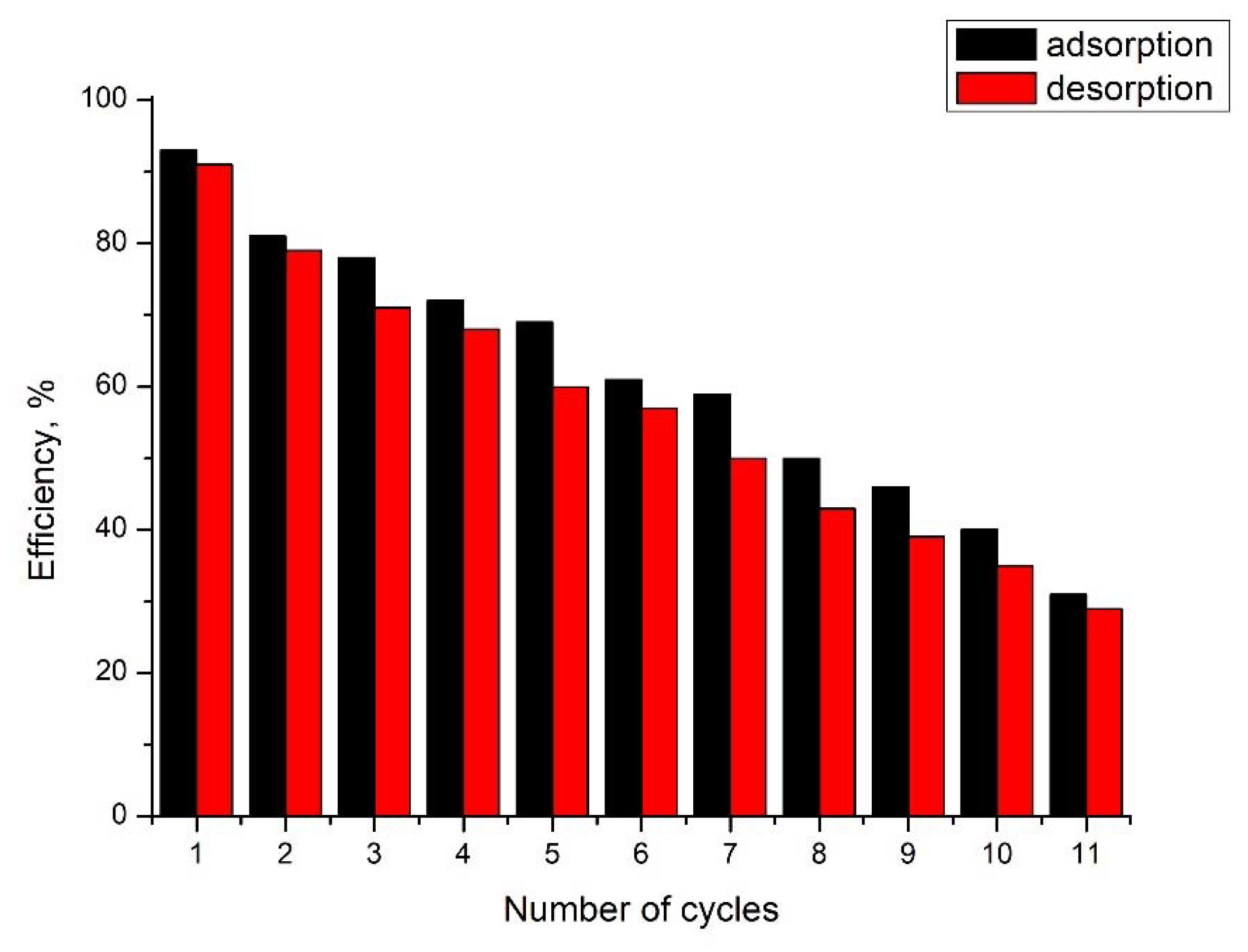
3.13. Arsenic Adsorption/Desorption Studies
Arsenic Removal from Real Ground Water, Competing Ions Influence
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rathi, B.S.; Kumar, P.S. A review on sources, identification and treatment strategies for the removal of toxic Arsenic from water system. J. Hazard. Mater. 2021, 418, 126299. [Google Scholar] [CrossRef]
- Abhinaya, M.; Parthiban, R.; Kumar, P.S.; Vo, D.-V.N. A review on cleaner strategies for extraction of chitosan and its application in toxic pollutant removal. Environ. Res. 2021, 196, 110996. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Goswami, R.; Patel, A.K.; Srivastava, M.; Das, N. Scenario, perspectives and mechanism of arsenic and fluoride Co-occurrence in the groundwater: A review. Chemosphere 2020, 249, 126126. [Google Scholar] [CrossRef]
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Alka, S.; Shahir, S.; Ibrahim, N.; Ndejiko, M.J.; Vo, D.-V.N.; Manan, F.A. Arsenic removal technologies and future trends: A mini review. J. Clean. Prod. 2021, 278, 123805. [Google Scholar] [CrossRef]
- Garelick, H.; Jones, H.; Dybowska, A.; Valsami-Jones, E. Arsenic Pollution Sources. In Reviews of Environmental Contamination Volume 197: International Perspectives on Arsenic Pollution and Remediation; Springer: New York, NY, USA, 2008; pp. 17–60. [Google Scholar]
- Ratnaike, R.N. Acute and chronic arsenic toxicity. Postgrad. Med. J. 2003, 79, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Szymańska-Chabowska, A.; Antonowicz-Juchniewicz, J.; Andrzejak, R. Some aspects of arsenic toxicity and carcinogenicity in living organism with special regard to its influence on cardiovascular system, blood and bone marrow. Int. J. Occup. Med. Environ. Health 2002, 15, 101–116. [Google Scholar] [PubMed]
- Duker, A.A.; Carranza, E.J.M.; Hale, M. Arsenic geochemistry and health. Environ. Int. 2005, 31, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Kapaj, S.; Peterson, H.; Liber, K.; Bhattacharya, P. Human Health Effects From Chronic Arsenic Poisoning–A Review. J. Environ. Sci. Health Part A 2006, 41, 2399–2428. [Google Scholar] [CrossRef]
- Berg, M.; Tran, H.C.; Nguyen, T.C.; Pham, H.V.; Schertenleib, R.; Giger, W. Arsenic Contamination of Groundwater and Drinking Water in Vietnam: A Human Health Threat. Environ. Sci. Technol. 2001, 35, 2621–2626. [Google Scholar] [CrossRef] [Green Version]
- Chung, J.-Y.; Yu, S.-D.; Hong, Y.-S. Environmental Source of Arsenic Exposure. J. Prev. Med. Public Health 2014, 47, 253–257. [Google Scholar] [CrossRef] [Green Version]
- Choong, T.S.Y.; Chuah, T.G.; Robiah, Y.; Gregory Koay, F.L.; Azni, I. Arsenic toxicity, health hazards and removal techniques from water: An overview. Desalination 2007, 217, 139–166. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef] [Green Version]
- Vaclavikova, M.; Gallios, G.P.; Hredzak, S.; Jakabsky, S. Removal of arsenic from water streams: An overview of available techniques. Clean Technol. Environ. Policy 2008, 10, 89–95. [Google Scholar] [CrossRef]
- Ungureanu, G.; Santos, S.; Boaventura, R.; Botelho, C. Arsenic and antimony in water and wastewater: Overview of removal techniques with special reference to latest advances in adsorption. J. Environ. Manag. 2015, 151, 326–342. [Google Scholar] [CrossRef] [PubMed]
- Rathi, B.S.; Kumar, P.S.; Show, P.-L. A review on effective removal of emerging contaminants from aquatic systems: Current trends and scope for further research. J. Hazard. Mater. 2021, 409, 124413. [Google Scholar] [CrossRef] [PubMed]
- Rathi, B.S.; Kumar, P.S.; Ponprasath, R.; Rohan, K.; Jahnavi, N. An effective separation of toxic arsenic from aquatic environment using electrochemical ion exchange process. J. Hazard. Mater. 2021, 412, 125240. [Google Scholar] [CrossRef]
- Saravanan, A.; Karishma, S.; Kumar, P.S.; Varjani, S.; Yaashikaa, P.R.; Jeevanantham, S.; Ramamurthy, R.; Reshma, B. Simultaneous removal of Cu(II) and reactive green 6 dye from wastewater using immobilized mixed fungal biomass and its recovery. Chemosphere 2021, 271, 129519. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Jeevanantham, S.; Karishma, S.; Yaashikaa, P.R. Modeling analysis on the effective elimination of toxic pollutant from aquatic environment using pyrolysis assisted palmyra palm male inflorescence. Environ. Res. 2021, 197, 111146. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Kumar, P.S.; Yaashikaa, P.R.; Karishma, S.; Jeevanantham, S.; Swetha, S. Mixed biosorbent of agro waste and bacterial biomass for the separation of Pb(II) ions from water system. Chemosphere 2021, 277, 130236. [Google Scholar] [CrossRef]
- Saravanan, A.; Senthil Kumar, P.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P.R.; Reshma, B. Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development. Chemosphere 2021, 280, 130595. [Google Scholar] [CrossRef] [PubMed]
- Gulledge, J.H.; O’Connor, J.T. Removal of Arsenic (V) From Water by Adsorption on Aluminum and Ferric Hydroxides. J. AWWA 1973, 65, 548–552. [Google Scholar] [CrossRef]
- Amen, R.; Bashir, H.; Bibi, I.; Shaheen, S.M.; Niazi, N.K.; Shahid, M.; Hussain, M.M.; Antoniadis, V.; Shakoor, M.B.; Al-Solaimani, S.G.; et al. A critical review on arsenic removal from water using biochar-based sorbents: The significance of modification and redox reactions. Chem. Eng. J. 2020, 396, 125195. [Google Scholar] [CrossRef]
- Gayathri, R.; Gopinath, K.P.; Kumar, P.S. Adsorptive separation of toxic metals from aquatic environment using agro waste biochar: Application in electroplating industrial wastewater. Chemosphere 2021, 262, 128031. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.; Saravanan, A. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef]
- Lata, S.; Samadder, S.R. Removal of arsenic from water using nano adsorbents and challenges: A review. J. Environ. Manag. 2016, 166, 387–406. [Google Scholar] [CrossRef]
- Arora, R. Nano adsorbents for removing the arsenic from waste/ground water for energy and environment management—A review. Mater. Today Proc. 2021, 45, 4437–4440. [Google Scholar] [CrossRef]
- Thekkudan, V.N.; Vaidyanathan, V.K.; Ponnusamy, S.K.; Charles, C.; Sundar, S.; Vishnu, D.; Anbalagan, S.; Vaithyanathan, V.K.; Subramanian, S. Review on nanoadsorbents: A solution for heavy metal removal from wastewater. IET Nanobiotechnol. 2017, 11, 213–224. [Google Scholar] [CrossRef]
- Gallegos-Garcia, M.; Ramírez-Muñiz, K.; Song, S. Arsenic Removal from Water by Adsorption Using Iron Oxide Minerals as Adsorbents: A Review. Miner. Process. Extr. Metall. Rev. 2012, 33, 301–315. [Google Scholar] [CrossRef]
- Habuda-Stanić, M.; Nujić, M. Arsenic removal by nanoparticles: A review. Environ. Sci. Pollut. Res. 2015, 22, 8094–8123. [Google Scholar] [CrossRef]
- Liu, B.; Kim, K.-H.; Kumar, V.; Kim, S. A review of functional sorbents for adsorptive removal of arsenic ions in aqueous systems. J. Hazard. Mater. 2020, 388, 121815. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, H.; Wang, L.; Qu, Z.; Yan, N. Surface nano-traps of Fe0/COFs for arsenic(III) depth removal from wastewater in non-ferrous smelting industry. Chem. Eng. J. 2020, 381, 122559. [Google Scholar] [CrossRef]
- Dias, A.C.; Fontes, M.P.F. Arsenic (V) removal from water using hydrotalcites as adsorbents: A critical review. Appl. Clay Sci. 2020, 191, 105615. [Google Scholar] [CrossRef]
- Toyos-Rodríguez, C.; Calleja-García, J.; Torres-Sánchez, L.; López, A.; Abu-Dief, A.M.; Costa, A.; Elbaile, L.; Crespo, R.D.; Garitaonandia, J.S.; Lastra, E.; et al. A Simple and Reliable Synthesis of Superparamagnetic Magnetite Nanoparticles by Thermal Decomposition of Fe(acac)3. J. Nanomater. 2019, 2019, 2464010. [Google Scholar] [CrossRef] [Green Version]
- Khanna, S.; Utsav; Marathey, P.; Paneliya, S.; Vanpariya, A.; Ray, A.; Banerjee, R.; Mukhopadhyay, I. Fabrication of silicon nanohorns via soft lithography technique for photoelectrochemical application. Int. J. Hydrogen Energy 2021, 46, 16404–16413. [Google Scholar] [CrossRef]
- Nandanwar, R.; Bamne, J.; Singh, N.; Sharma, P.K.; Singh, P.; Umar, A.; Haque, F.Z. Synthesis of Titania/Silica Nanocomposite for Enhanced Photodegradation of Methylene Blue and Methyl Orange Dyes under UV and Mercury Lights. ES Mater. Manuf. 2022, 16, 78–88. [Google Scholar] [CrossRef]
- Utsav; Khanna, S.; Makani, N.H.; Paneliya, S.; Mukhopadhyay, I.; Banerjee, R. Thermal crowning mechanism in gold–silica nanocomposites: Plasmonic-photonic pairing in archetypal two-dimensional structures. Phys. Chem. Chem. Phys. 2021, 23, 17197–17207. [Google Scholar] [CrossRef]
- Ianăşi, C.; Picioruş, M.; Nicola, R.; Ciopec, M.; Negrea, A.; Nižňanský, D.; Len, A.; Almásy, L.; Putz, A.-M. Removal of cadmium from aqueous solutions using inorganic porous nanocomposites. Korean J. Chem. Eng. 2019, 36, 688–700. [Google Scholar] [CrossRef]
- Abdel-Khalek, H.; Abd-El Salam, M.; El-Mahalawy, A.M. The Electrical Conductivity and Dielectric Response of Cupric Acetylacetonate Thin Films. J. Electron. Mater. 2019, 48, 3736–3752. [Google Scholar] [CrossRef]
- Jović Orsini, N.; Babić-Stojić, B.; Spasojević, V.; Calatayud, M.P.; Cvjetićanin, N.; Goya, G.F. Magnetic and power absorption measurements on iron oxide nanoparticles synthesized by thermal decomposition of Fe(acac)3. J. Magn. Magn. Mater. 2018, 449, 286–296. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Wiley: Hoboken, NJ, USA, 2004; 368p. [Google Scholar]
- Bauer, M.; Kauf, T.; Christoffers, J.; Bertagnolli, H. Investigations into the metal species of the homogeneous iron(iii) catalyzed Michael addition reactions. Phys. Chem. Chem. Phys. 2005, 7, 2664–2670. [Google Scholar] [CrossRef] [PubMed]
- Hester, R.E.; Plane, R.A. Metal-Oxygen Bonds in Complexes: Raman Spectra of Trisacetylacetonato and Trisoxalato Complexes of Aluminum, Gallium, and Indium. Inorg. Chem. 1964, 3, 513–517. [Google Scholar] [CrossRef]
- Krishnan, V.; Gross, S.; Müller, S.; Armelao, L.; Tondello, E.; Bertagnolli, H. Structural Investigations on the Hydrolysis and Condensation Behavior of Pure and Chemically Modified Alkoxides. 2. Germanium Alkoxides. J. Phys. Chem. B 2007, 111, 7519–7528. [Google Scholar] [CrossRef]
- Loridant, S. Raman spectroscopy as a powerful tool to characterize ceria-based catalysts. Catal. Today 2021, 373, 98–111. [Google Scholar] [CrossRef]
- Ianasi, C.; Costisor, O.; Putz, A.-M.; Lazau, R.; Adina, N.; Niznansky, D.; Sacarescu, L.; Savii, C. Low temperature superparamagnetic nanocomposites obtained by Fe(acac)3-SiO2-PVA hybrid xerogel thermolysis. Process. Appl. Ceram. 2016, 10, 265. [Google Scholar] [CrossRef]
- Ercuta, A. Sensitive AC Hysteresigraph of Extended Driving Field Capability. IEEE Trans. Instrum. Meas. 2020, 69, 1643–1651. [Google Scholar] [CrossRef]
- Sposito, G. On Points of Zero Charge. Environ. Sci. Technol. 1998, 32, 2815–2819. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substabces. Kungl. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Weber, W.J.; Morris, J.C. Equilibria and Capacities for Adsorption on Carbon. J. Sanit. Eng. Div. 1964, 90, 79–108. [Google Scholar] [CrossRef]
- Huang, Y.; Lee, X.; Grattieri, M.; Macazo, F.C.; Cai, R.; Minteer, S.D. A sustainable adsorbent for phosphate removal: Modifying multi-walled carbon nanotubes with chitosan. J. Mater. Sci. 2018, 53, 12641–12649. [Google Scholar] [CrossRef]
- Ofomaja, A.E. Intraparticle diffusion process for lead(II) biosorption onto mansonia wood sawdust. Bioresour. Technol. 2010, 101, 5868–5876. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Bhaumik, M.; Agarwal, S.; Gupta, V.K.; Maity, A. Enhanced removal of Cr(VI) from aqueous solutions using polypyrrole wrapped oxidized MWCNTs nanocomposites adsorbent. J. Colloid Interface Sci. 2016, 470, 257–267. [Google Scholar] [CrossRef]
- Zhang, S.; Ning, S.; Liu, H.; Zhou, J.; Wang, S.; Zhang, W.; Wang, X.; Wei, Y. Highly-efficient separation and recovery of ruthenium from electroplating wastewater by a mesoporous silica-polymer based adsorbent. Microporous Mesoporous Mater. 2020, 303, 110293. [Google Scholar] [CrossRef]
- Atkins, P.; de Paula, J. Atkins’ Physical Chemistry; Oxford University Press: Oxford, UK, 2005; 1008p. [Google Scholar]
- Zhang, Y.; Yu, F.; Cheng, W.; Wang, J.; Ma, J. Adsorption Equilibrium and Kinetics of the Removal of Ammoniacal Nitrogen by Zeolite X/Activated Carbon Composite Synthesized from Elutrilithe. J. Chem. 2017, 2017, 1936829. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Kucuker, M.A.; Wieczorek, N.; Kuchta, K.; Copty, N.K. Biosorption of neodymium on Chlorella vulgaris in aqueous solution obtained from hard disk drive magnets. PLoS ONE 2017, 12, e0175255. [Google Scholar] [CrossRef]
- Khosa, M.A.; Ullah, A. In-situ modification, regeneration, and application of keratin biopolymer for arsenic removal. J. Hazard. Mater. 2014, 278, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, R.; Samadder, S.R. Low cost and easy synthesis of aluminium oxide nanoparticles for arsenite removal from groundwater: A complete batch study. J. Mol. Liq. 2018, 250, 192–201. [Google Scholar] [CrossRef]
- Gupta, V.K.; Saini, V.K.; Jain, N. Adsorption of As(III) from aqueous solutions by iron oxide-coated sand. J. Colloid Interface Sci. 2005, 288, 55–60. [Google Scholar] [CrossRef]
- Singh, T.S.; Pant, K.K. Equilibrium, kinetics and thermodynamic studies for adsorption of As(III) on activated alumina. Sep. Purif. Technol. 2004, 36, 139–147. [Google Scholar] [CrossRef]
- Khosa, M.; Ullah, A. A Sustainable Role of Keratin Biopolymer in Green Chemistry: A Review. J. Food Process. Beverages 2013, 1, 8. [Google Scholar]
- Ranjan, D.; Talat, M.; Hasan, S.H. Biosorption of arsenic from aqueous solution using agricultural residue ‘rice polish’. J. Hazard. Mater. 2009, 166, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Water Day 2022: Groundwater, Invisible but Vital to Health. Available online: https://www.who.int/news-room/feature-stories/detail/world-water-day-2022-groundwater-invisible-but-vital-to-health (accessed on 24 May 2022).



| Parameters | Content |
|---|---|
| Ca2+ (mg/L) | 89 ± 8.9 |
| Mg2+ (mg/L) | 44 ± 4.4 |
| Na+ (mg/L) | 120 ± 12 |
| K+ (mg/L) | 1.75 ± 0.17 |
| Fen+ (mg/L) | 0.6 ± 0.06 |
| Mnn+ (mg/L) | 0.4 ± 0.04 |
| Ptotal, (mg/L) | 5.6 ± 0.56 |
| , (mg/L) | 4.4 ± 0.44 |
| , (mg/L) | 12.3 ± 1.23 |
| , (mg/L) | 0.74 ± 0.07 |
| , (mg/L) | 8.2 ± 0.82 |
| As(III) (μg/L) | 104 ± 10.4 |
| Sample Name | Ironed Area (µm2) | Sa (µm) | Sq (µm) | Sp (µm) | Sv (µm) | Sy (µm) | Average Height (µm) |
|---|---|---|---|---|---|---|---|
| Material (10 µm × 10 µm) | 141.851 | 0.3358 | 0.4618 | 1.9228 | −0.0648 | 1.9876 | 0.2449 |
| Material (5 µm × 5 µm) | 40.807 | 0.5047 | 0.5878 | 1.8672 | −0.1181 | 1.9853 | 0.4144 |
| Sample | Surface Area, m2/g | Pore Size Distribution, DFT Method, nm | Total Pore Volume | Fhh Value |
|---|---|---|---|---|
| Material | 276 | 5.564 | 3.940 e−1 cc/g for pores smaller than 130.1 nm | 2.6644 |
| Pseudo-First Order Kinetic Model | ||||
| Temperature (K) | qe,exp (µg/g) | k1 (min−1) | qe,calc (µg/g) | R2 |
| 298 | 24.62 | 0.0564 | 10.05 | 0.9076 |
| 308 | 24.84 | 0.0624 | 11.39 | 0.8437 |
| 318 | 24.93 | 0.0645 | 13.07 | 0.7943 |
| 328 | 24.98 | 0.0768 | 19.00 | 0.8333 |
| Pseudo-second order kinetic model | ||||
| Temperature (K) | qe,exp (µg g−1) | k2 (g µg−1∙min−1) | qe,calc (µg g−1) | R2 |
| 298 | 24.62 | 661.5 | 19.60 | 0.9930 |
| 308 | 24.84 | 975.8 | 21.27 | 0.9952 |
| 318 | 24.93 | 1601.7 | 26.11 | 0.9951 |
| 328 | 24.98 | 2727.2 | 26.25 | 0.9973 |
| Intraparticle diffusion model | ||||
| Temperature (K) | kdiff (mg·g−1 min−1/2) | C | R2 | |
| 298 | 1.32 | 6.71 | 0.8492 | |
| 308 | 1.78 | 9.05 | 0.8373 | |
| 318 | 1.84 | 9.58 | 0.8019 | |
| 328 | 2.10 | 14.01 | 0.8345 | |
| ΔH° (kJ/mol) | ΔS° (J/mol∙K) | ΔG° (kJ/mol) | R2 | |||
|---|---|---|---|---|---|---|
| 68.56 | 254.6 | 298 K | 308 K | 318 K | 328 K | 0.9906 |
| −75.67 | −78.21 | −80.75 | −83.29 | |||
| Langmuir Isotherm | |||
| qm,exp (µg/g) | KL (L/µg) | qL (µg/g) | R2 |
| 574.9 | 0.067 | 650.28 | 0.9711 |
| Freundlich isotherm | |||
| KF (µg/g) | 1/nF | R2 | |
| 58.61 | 0.31 | 0.8065 | |
| Sips isotherm | |||
| KS | qS (µg/g) | 1/nS | R2 |
| 6.21 | 591.9 | 0.56 | 0.9916 |
| Materials | q, µg/g | References |
|---|---|---|
| Allyl alcohol treated chicken feathers | 0.115 | [64] |
| TrisilanolCyclohexyl treated chicken feathers | 0.110 | [64] |
| Aluminum oxide NPs | 0.500 | [65] |
| Iron oxide-coated sand | 0.029 | [66] |
| Activated alumina | 0.180 | [67] |
| Modified chicken feathers | 0.130 | [64,68]. |
| Rice polish | 0.140 | [69] |
| SiO2/Fe(acac)3/NaF | 575.100 | This paper |
| Parameters | Content |
|---|---|
| Ca2+ (mg/L) | 8.90 ± 0.89 |
| Mg2+ (mg/L) | 41.20 ± 4.12 |
| Na+ (mg/L) | 119.00 ± 11.9 |
| K+ (mg/L) | 0.85 ± 0.08 |
| Fen+ (mg/L) | 0.20 ± 0.02 |
| Mnn+ (mg/L) | 0.03 ± 0.003 |
| Ptotal, (mg/L) | 2.60 ± 0.26 |
| , (mg/L) | 2.40 ± 0.24 |
| , (mg/L) | 11.30 ± 1.13 |
| , (mg/L) | 0.34 ± 0.03 |
| , (mg/L) | 4.20 ± 0.42 |
| As(III) (μg/L) | 9.30 ± 0.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mladin, G.; Ciopec, M.; Negrea, A.; Duteanu, N.; Negrea, P.; Ianasi, P.; Ianași, C. Silica- Iron Oxide Nanocomposite Enhanced with Porogen Agent Used for Arsenic Removal. Materials 2022, 15, 5366. https://doi.org/10.3390/ma15155366
Mladin G, Ciopec M, Negrea A, Duteanu N, Negrea P, Ianasi P, Ianași C. Silica- Iron Oxide Nanocomposite Enhanced with Porogen Agent Used for Arsenic Removal. Materials. 2022; 15(15):5366. https://doi.org/10.3390/ma15155366
Chicago/Turabian StyleMladin, Georgiana, Mihaela Ciopec, Adina Negrea, Narcis Duteanu, Petru Negrea, Paula Ianasi, and Cătălin Ianași. 2022. "Silica- Iron Oxide Nanocomposite Enhanced with Porogen Agent Used for Arsenic Removal" Materials 15, no. 15: 5366. https://doi.org/10.3390/ma15155366
APA StyleMladin, G., Ciopec, M., Negrea, A., Duteanu, N., Negrea, P., Ianasi, P., & Ianași, C. (2022). Silica- Iron Oxide Nanocomposite Enhanced with Porogen Agent Used for Arsenic Removal. Materials, 15(15), 5366. https://doi.org/10.3390/ma15155366









