Indium Recovery by Adsorption on MgFe2O4 Adsorbents
Abstract
1. Introduction
- Mg2+, Fe3+—divalent and trivalent cations
- X—degree of inversion
2. Materials and Methods
2.1. Material Synthesis and Characterization
2.1.1. MgFe2O4 Composite Synthesis
2.1.2. MgFe2O4 Composite Physico-Chemical Characterization
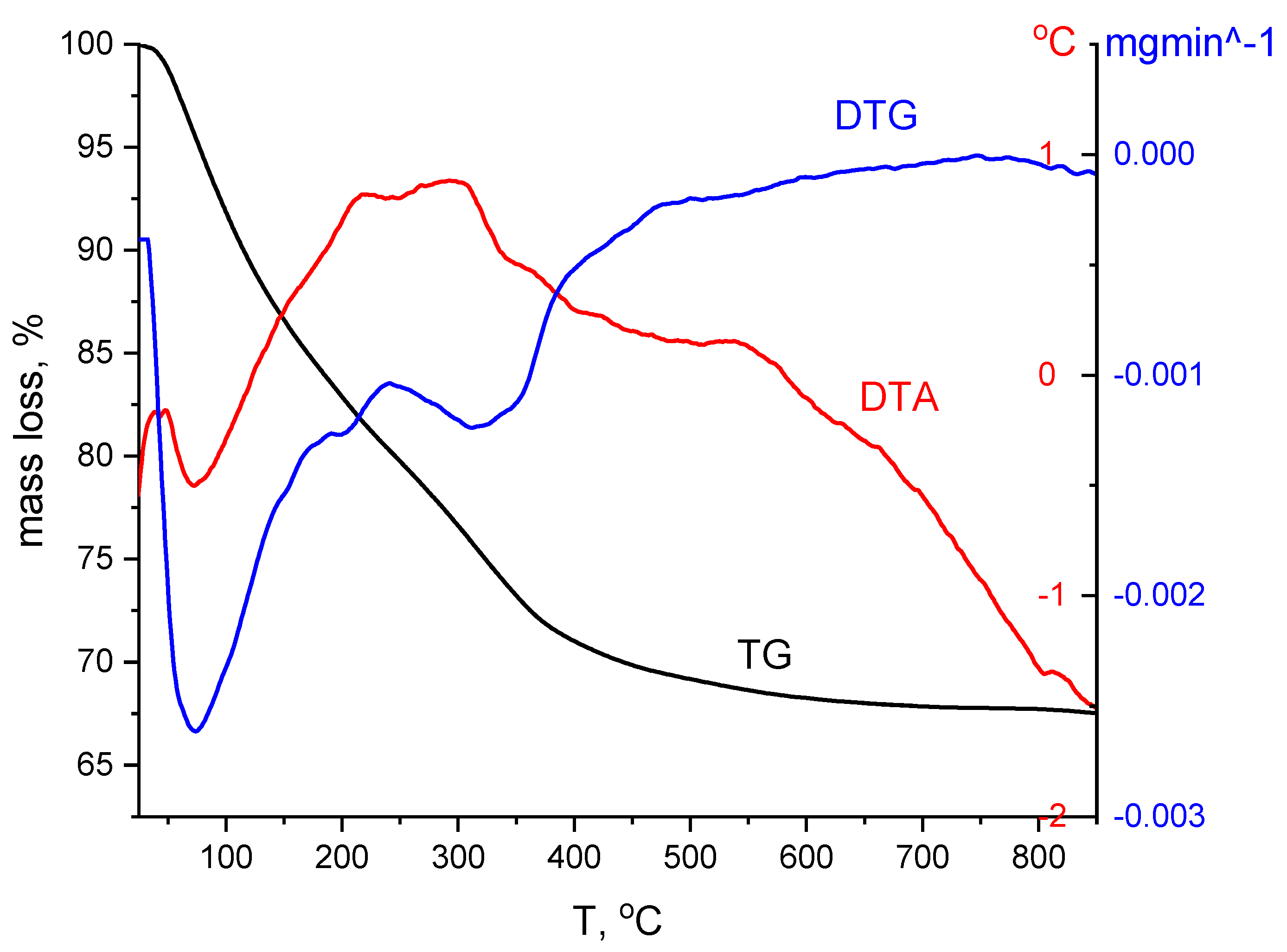
Thermogravimetric Analysis, DTG
Fourier Transform Infra-Red Spectroscopy, FT-IR
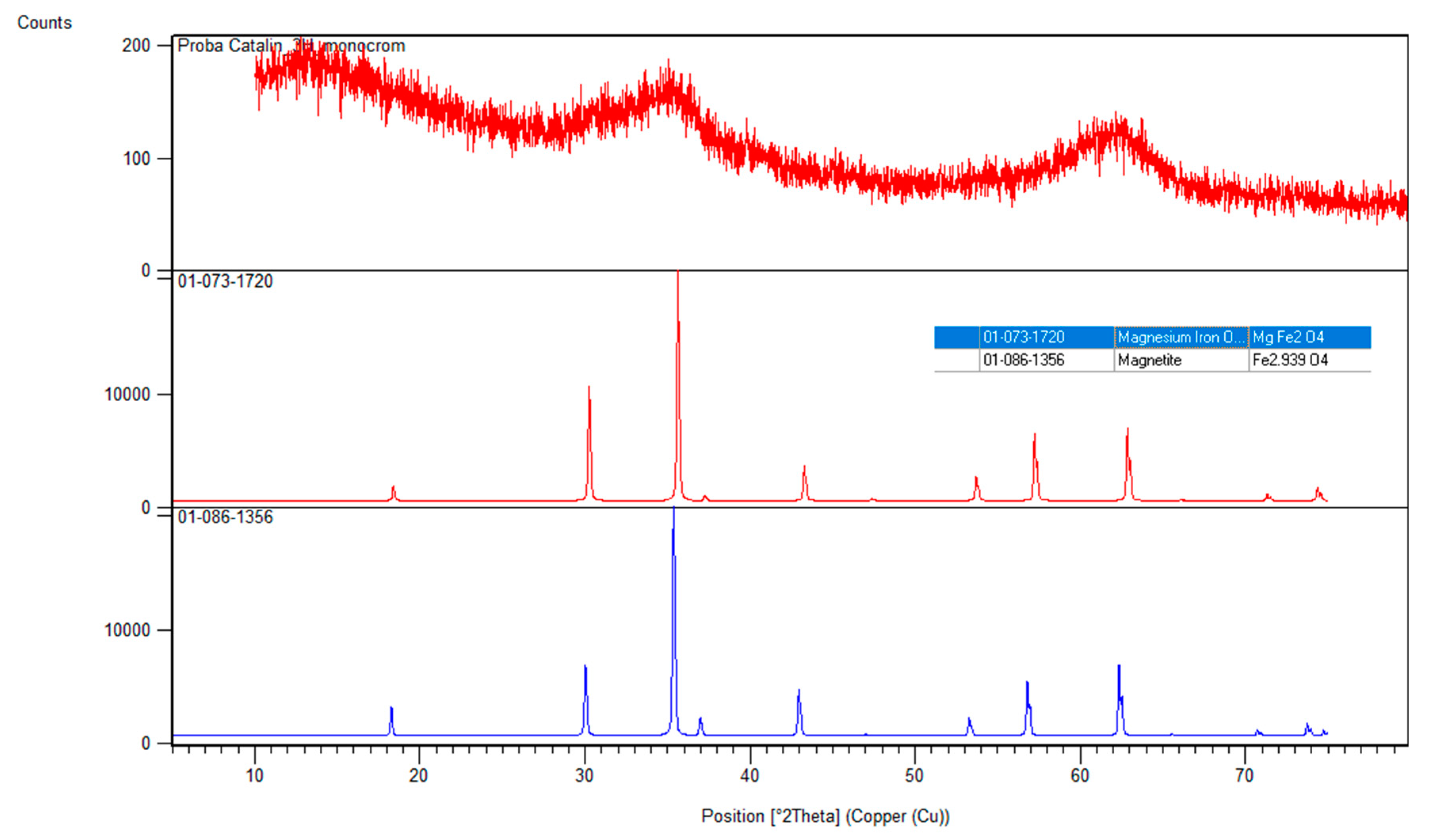
X-ray Diffraction Analysis, XRD
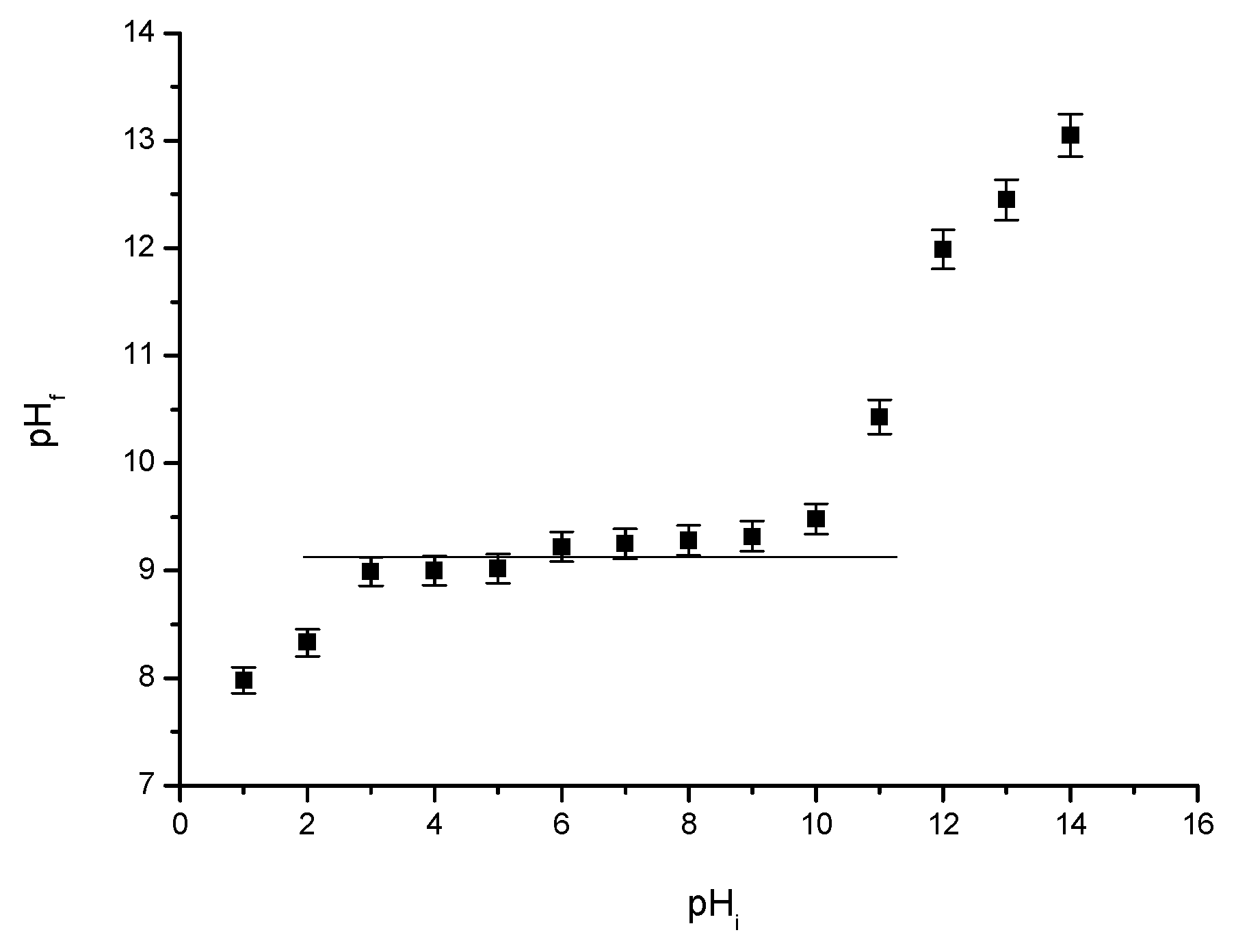
pHpZc
2.1.3. Adsorption Studies
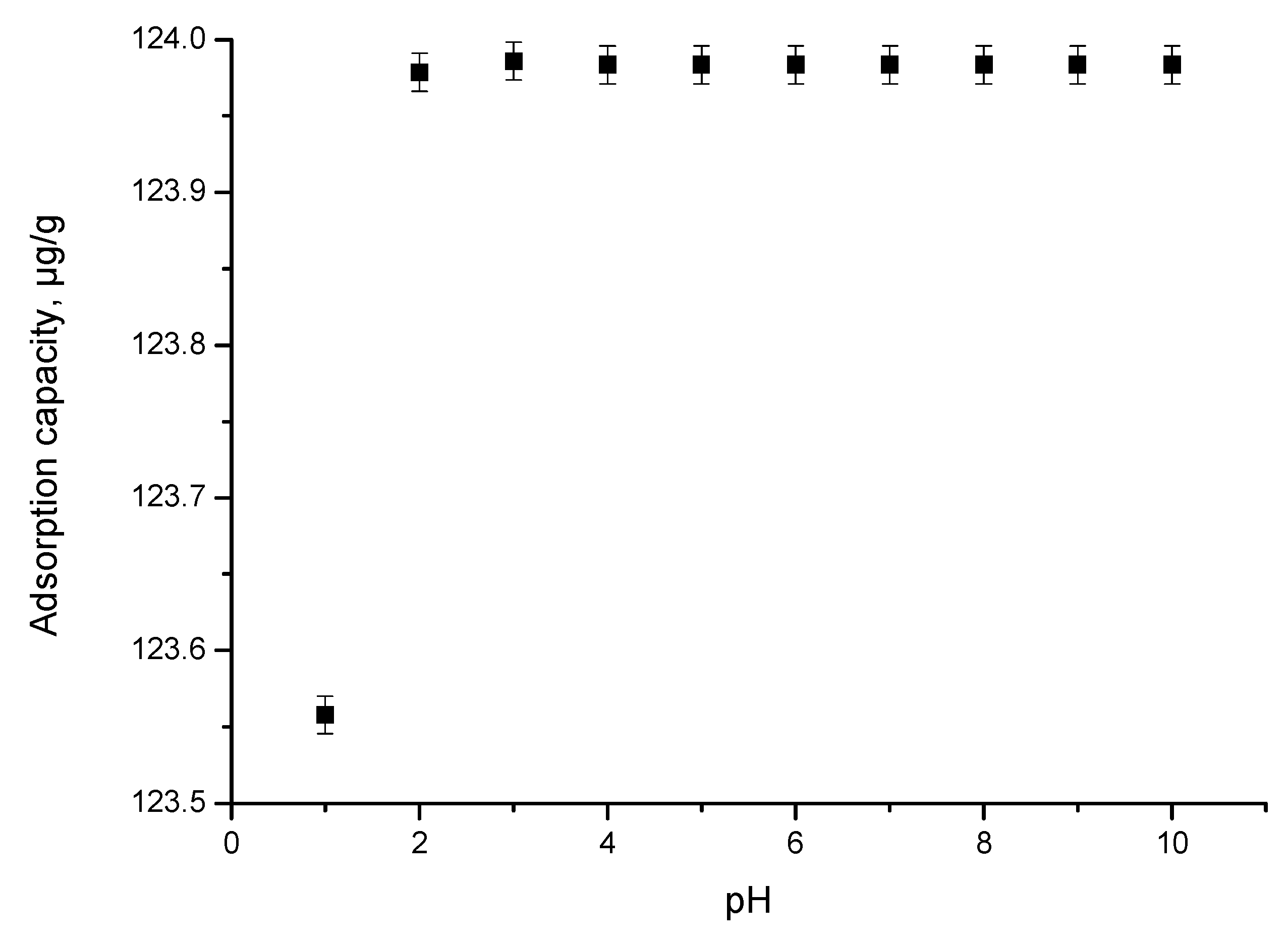
pH Effect
Contact Time and Temperature Effect
Initial Concentration Effect
Kinetics Adsorption
Adsorption Isotherm Models
Thermodynamic Studies
- k2—speed constant (g min−1 mg−1)
- A—Arrhenius constant (g min mg−1)
- Ea—activation energy (kJ mol−1)
- T—absolute temperature (K)
- R—ideal gas constant (8.314 J mol−1 k−1).
- ΔG°—free Gibbs energy standard variation (J mol−1)
- ΔH°—enthalpy standard variation (J mol−1)
- ΔS°—entropy standard variation (J mol−1 k−1)
- T—absolute temperature (K)
3. Results and Discussion
3.1. Material Synthesis and Characterization
3.1.1. Thermogravimetric Analysis, DTG
3.1.2. X-ray Diffraction Analysis, XRD
3.1.3. FT-IR Spectra
3.1.4. Point of Zero Charge (pHpZc) Determination
3.2. Adsorption Studies
3.2.1. pH Effect
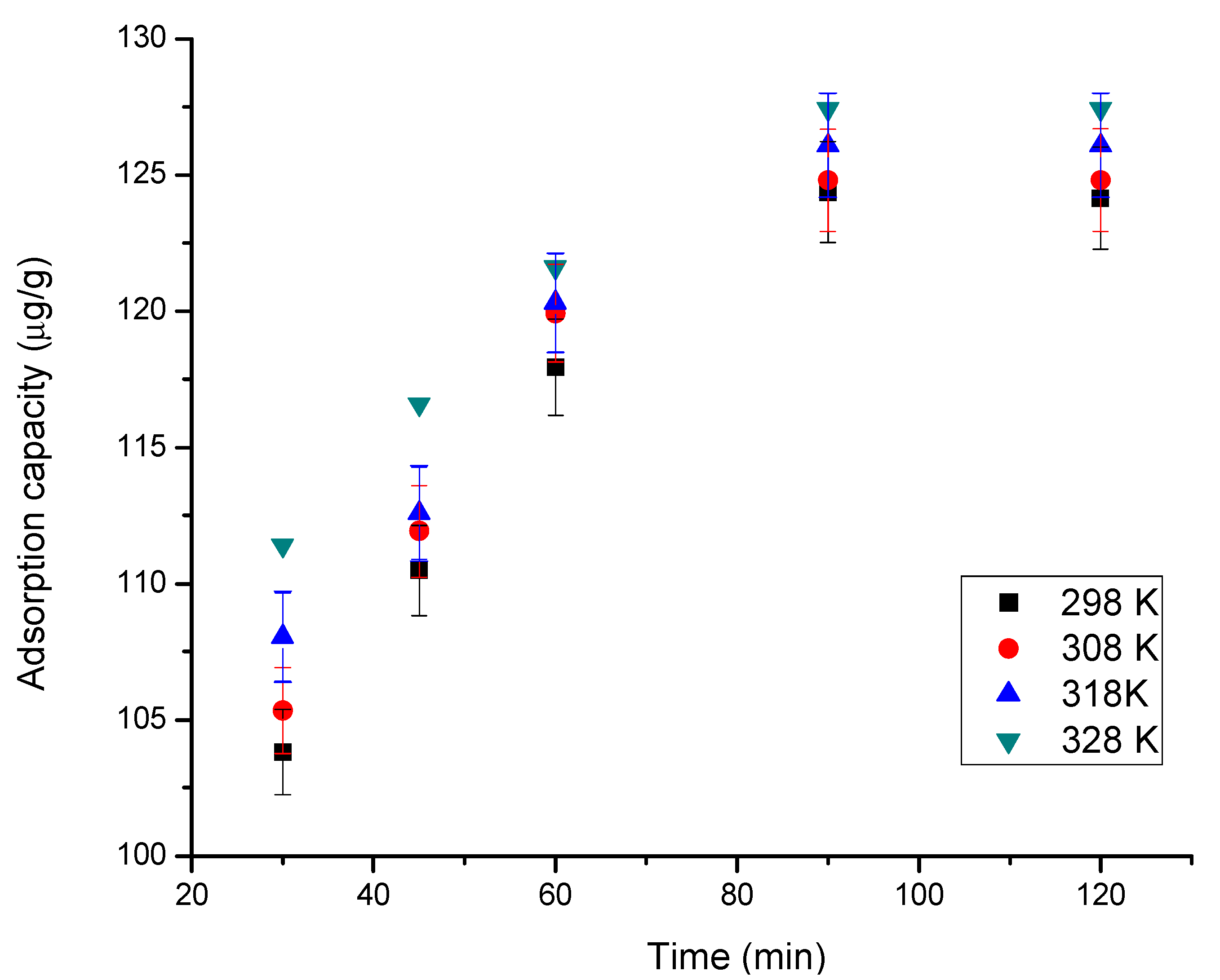
3.2.2. Contact Time and Temperature Effect
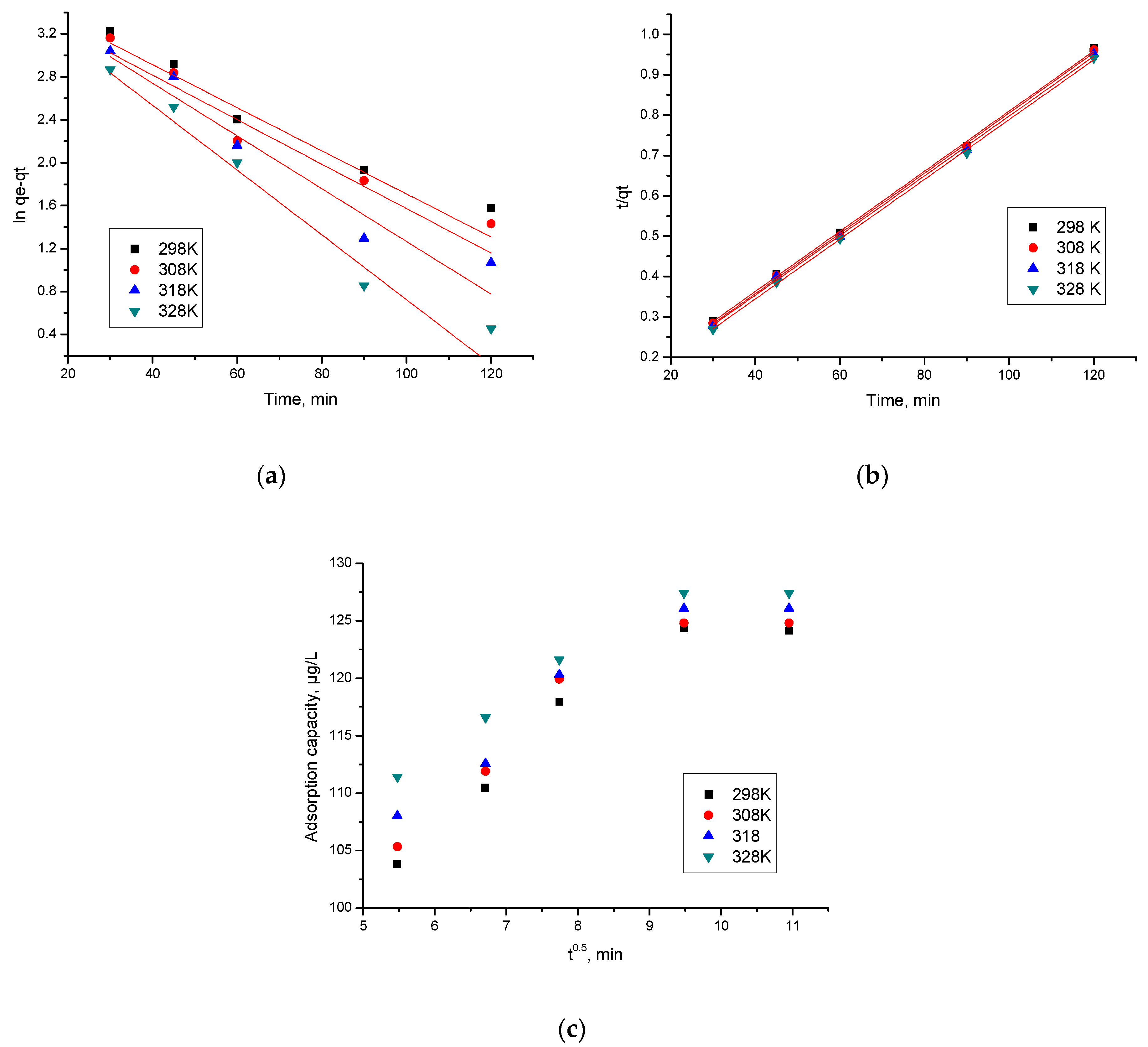
3.2.3. Kinetic Studies
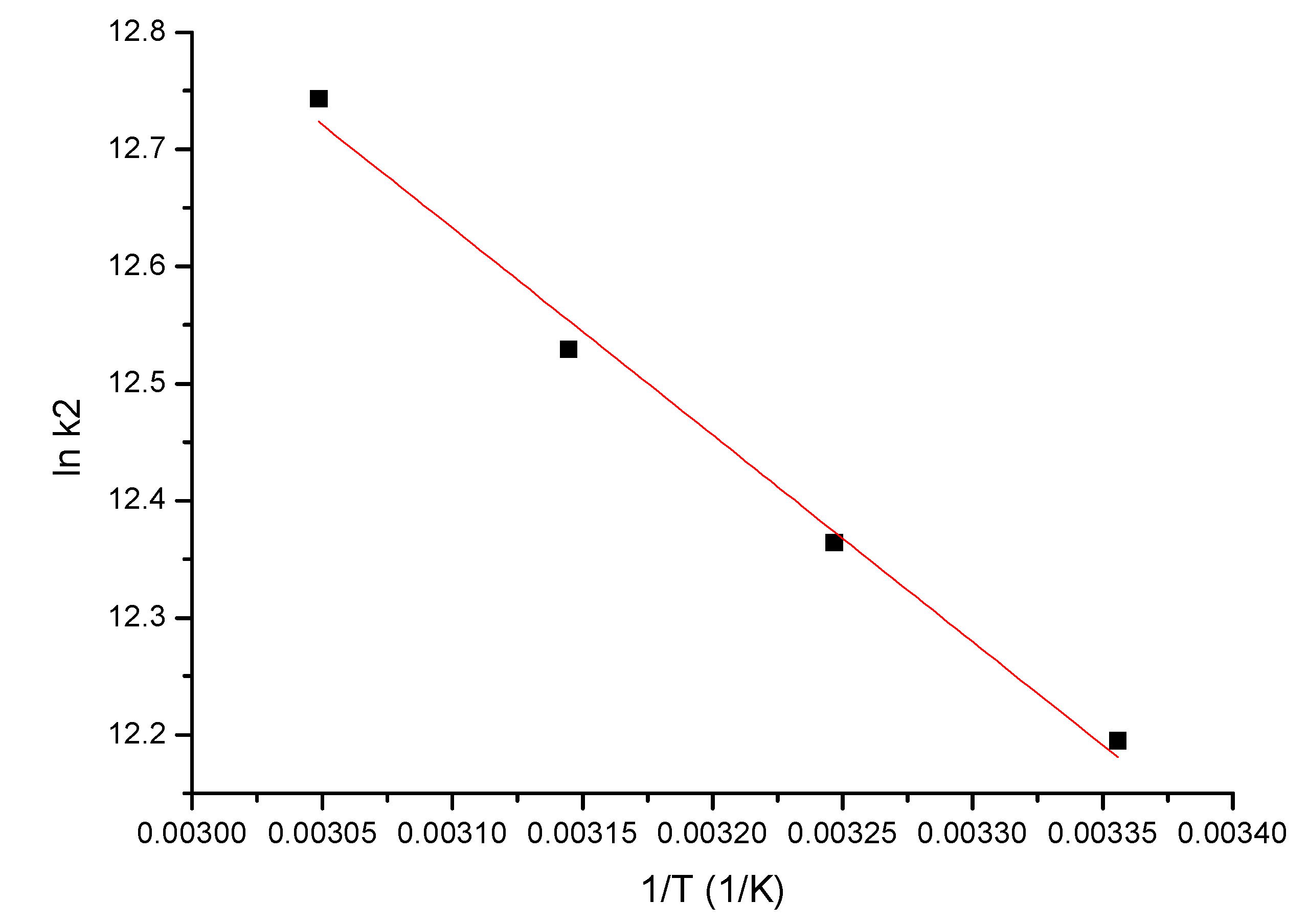
3.2.4. Activation Energy
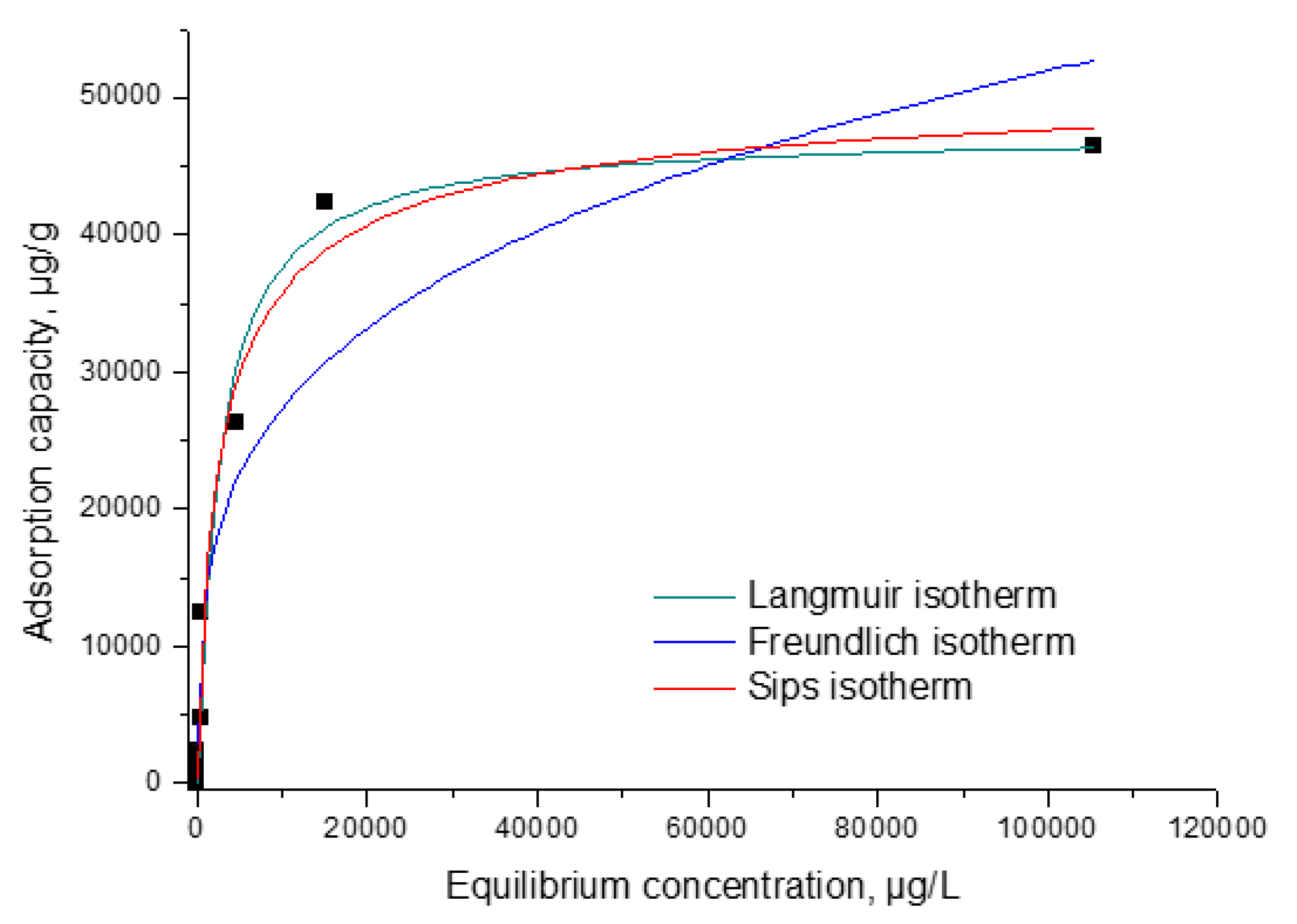
3.2.5. Equilibrium Studies
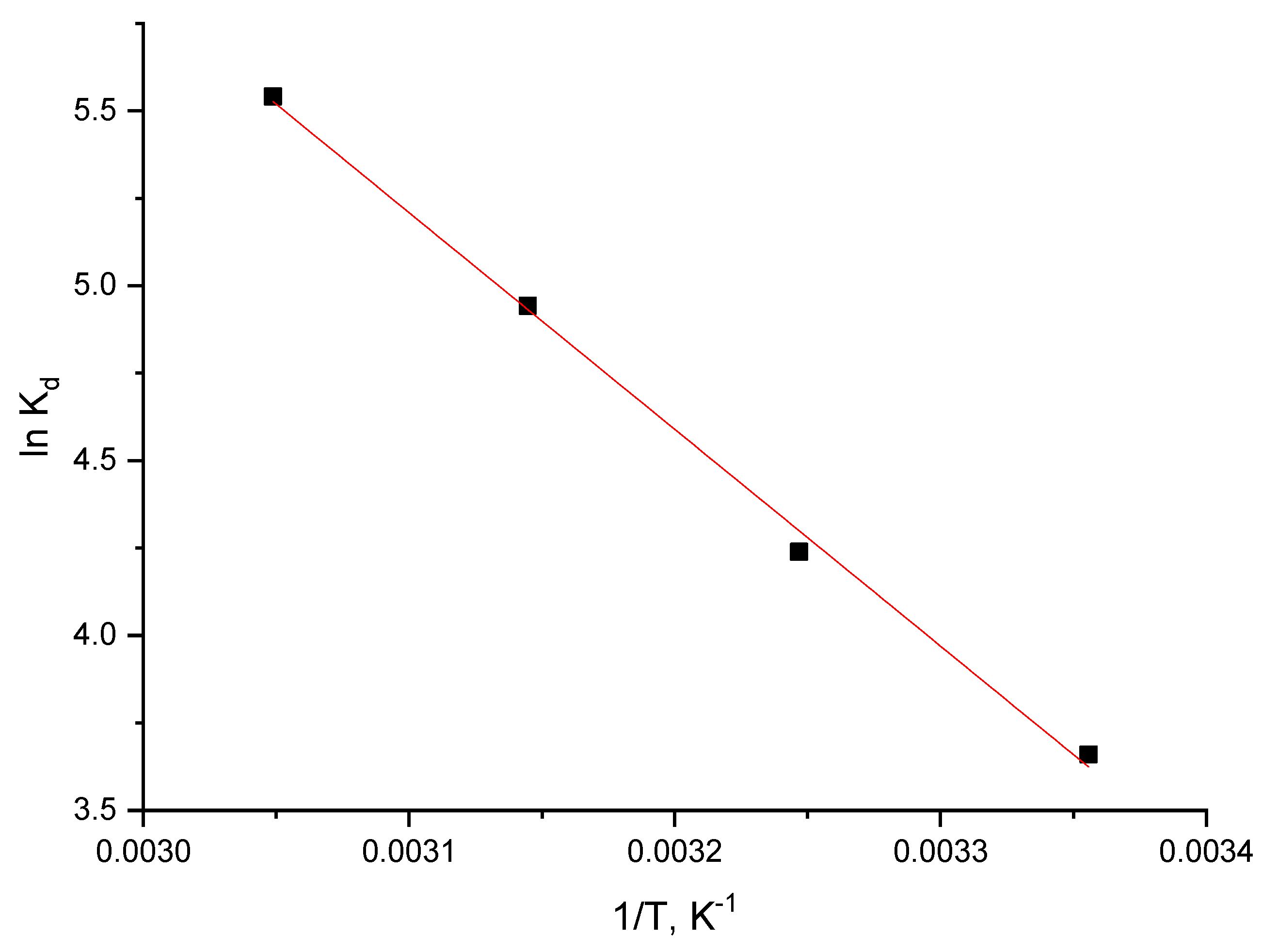
3.2.6. Thermodynamic Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Adhikari, B.B.; Gurung, M.; Kawakita, H.; Ohto, K. Solid phase extraction, preconcentration and separation of indium with methylene crosslinked calix[4]- and calix[6]arene carboxylic acid resins. Chem. Eng. Sci. 2012, 78, 144–154. [Google Scholar] [CrossRef]
- Li, M.; Feng, C.; Li, M.; Zeng, Q.; Gan, Q. Synthesis and application of a surface-grafted In (III) ion-imprinted polymer for selective separation and pre-concentration of indium (III) ion from aqueous solution. Hydrometallurgy 2015, 154, 63–71. [Google Scholar] [CrossRef]
- Li, G.; Zhang, B.; Ma, Z.; Wang, Z. Facile synthesis of hydroxyl- and amine-riched porous polymer for indium recovery in water. Microporous Mesoporous Mater. 2021, 323, 111162. [Google Scholar] [CrossRef]
- Duan, H.; Wang, J.; Liu, L.; Huang, Q.; Li, J. Rethinking China’s strategic mineral policy on indium: Implication for the flat screens and photovoltaic industries. Prog. Photovolt. Res. Appl. 2016, 24, 83–93. [Google Scholar] [CrossRef]
- Díez, E.; Gómez, J.M.; Rodríguez, A.; Bernabé, I.; Sáez, P.; Galán, J. A new mesoporous activated carbon as potential adsorbent for effective indium removal from aqueous solutions. Microporous Mesoporous Mater. 2020, 295, 109984. [Google Scholar] [CrossRef]
- Zimmermann, Y.-S.; Niewersch, C.; Lenz, M.; Kül, Z.Z.; Corvini, P.F.-X.; Schäffer, A.; Wintgens, T. Recycling of Indium From CIGS Photovoltaic Cells: Potential of Combining Acid-Resistant Nanofiltration with Liquid–Liquid Extraction. Environ. Sci. Technol. 2014, 48, 13412–13418. [Google Scholar] [CrossRef]
- Assefi, M.; Maroufi, S.; Nekouei, R.K.; Sahajwalla, V. Selective recovery of indium from scrap LCD panels using macroporous resins. J. Clean. Prod. 2018, 180, 814–822. [Google Scholar] [CrossRef]
- Alfantazi, A.; Moskalyk, R. Processing of indium: A review. Miner. Eng. 2003, 16, 687–694. [Google Scholar] [CrossRef]
- Lee, S.-K.; Lee, U.-H. Adsorption and desorption property of iminodiacetate resin (Lewatit® TP207) for indium recovery. J. Ind. Eng. Chem. 2016, 40, 23–25. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.; Gao, X.; Liu, C.; Guo, L.; Zhang, S.; Liu, X.; Liu, C. Adsorption behavior of indium(III) on modified solvent impregnated resins (MSIRs) containing sec-octylphenoxy acetic acid. Hydrometallurgy 2012, 121–124, 60–67. [Google Scholar] [CrossRef]
- Li, J.; Gao, S.; Duan, H.; Liu, L. Recovery of valuable materials from waste liquid crystal display panel. Waste Manag. 2009, 29, 2033–2039. [Google Scholar] [CrossRef]
- Rocchetti, L.; Amato, A.; Fonti, V.; Ubaldini, S.; de Michelis, I.; Kopacek, B.; Vegliò, F.; Beolchini, F. Cross-current leaching of indium from end-of-life LCD panels. Waste Manag. 2015, 42, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Bakry, A.M.; Alamier, W.M.; Salama, R.S.; El-Shall, M.S.; Awad, F.S. Remediation of water containing phosphate using ceria nanoparticles decorated partially reduced graphene oxide (CeO2-PRGO) composite. Surfaces Interfaces 2022, 31, 102006. [Google Scholar] [CrossRef]
- Calagui, M.J.C.; Senoro, D.B.; Kan, C.-C.; Salvacion, J.W.; Futalan, C.M.; Wan, M.-W. Adsorption of indium(III) ions from aqueous solution using chitosan-coated bentonite beads. J. Hazard. Mater. 2014, 277, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Limin, Y.; Wenquan, J.; Zhongzhen, F. Purification of indium electrolyte by coprecipitation. Mater. Rev. 2011, 25, 72–74. [Google Scholar]
- Swain, B.; Mishra, C.; Hong, H.S.; Cho, S.-S.; Lee, S.k. Commercial process for the recovery of metals from ITO etching industry wastewater by liquid–liquid extraction: Simulation, analysis of mechanism, and mathematical model to predict optimum operational conditions. Green Chem. 2015, 17, 3979–3991. [Google Scholar] [CrossRef]
- Gupta, B.; Mudhar, N.; Singh, I. Separations and recovery of indium and gallium using bis(2,4,4-trimethylpentyl)phosphinic acid (Cyanex 272). Sep. Purif. Technol. 2007, 57, 294–303. [Google Scholar] [CrossRef]
- Lee, M.; Ahn, J.; Lee, E. Solvent extraction separation of indium and gallium from sulphate solutions using D2EHPA. Hydrometallurgy 2002, 63, 269–276. [Google Scholar] [CrossRef]
- Trochimczuk, A.W.; Czerwińska, S. In(III) and Ga(III) sorption by polymeric resins with substituted phenylphosphinic acid ligands. React. Funct. Polym. 2005, 63, 215–220. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, G.; Hu, Z. Extraction and separation of gallium, indium and thallium with several carboxylic acids from chloride media. Talanta 2003, 59, 905–912. [Google Scholar] [CrossRef]
- Yang, J.; Retegan, T.; Ekberg, C. Indium recovery from discarded LCD panel glass by solvent extraction. Hydrometallurgy 2013, 137, 68–77. [Google Scholar] [CrossRef]
- Wu, M.; Sun, D.D.; Tay, J.H. Effect of operating variables on rejection of indium using nanofiltration membranes. J. Membr. Sci. 2004, 240, 105–111. [Google Scholar] [CrossRef]
- He, Y.; Ma, E.; Xu, Z. Recycling indium from waste liquid crystal display panel by vacuum carbon-reduction. J. Hazard. Mater. 2014, 268, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Medvecky, L.; Briančin, J. Possibilities of simultaneous determination of indium and gallium in binary InGa alloys by anodic stripping voltammetry in acetate buffer. Chem. Pap. 2004, 58, 93–100. [Google Scholar]
- Ferreira, E.D.M.M.; Morelli, T.; Moreira, I.M.N.S.; De Carvalho, M.S. Studies on indium sorption from iodide medium by polyurethane foam. J. Braz. Chem. Soc. 2004, 15, 563–569. [Google Scholar] [CrossRef]
- Liu, J.S.; He, Z.G.; Cai, J.; Cai, C.G.; Zhou, B.X.; Cai, W.M. Separation of indium (III), gallium (III), and zinc (II) with Levextrel resin containing di(2-ethylhexyl) phosphoric acid (CL-P204): Part I. Selection of separation conditions. Rare Met. 2003, 22, 235–240. [Google Scholar]
- Liu, J.; Chen, H.; Chen, X.; Guo, Z.; Hu, Y.; Liu, C.; Sun, Y. Extraction and separation of In(III), Ga(III) and Zn(II) from sulfate solution using extraction resin. Hydrometallurgy 2006, 82, 137–143. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, J.; Zhou, B.; Yao, S.; Li, H.; Xu, W. Synthesis of coated solvent impregnated resin for the adsorption of indium (III). Hydrometallurgy 2010, 101, 148–155. [Google Scholar] [CrossRef]
- Song, Q.; Liu, Y.; Zhang, L.; Xu, Z. Facile indium recovery from waste liquid crystal displays: Chloride-facilitated indium electroreduction and stepwise Cu/MoO2 and indium electrodeposition. J. Hazard. Mater. 2021, 415, 125599. [Google Scholar] [CrossRef]
- Armstrong, R.D.; Todd, M.; Atkinson, J.W.; Scott, K. Selective electrodeposition of metals from simulated waste solutions. J. Appl. Electrochem. 1996, 26, 379–384. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Salama, R.S.; El-Hakam, S.A.; Khder, A.S.; Ahmed, A.I. Synthesis of 12-tungestophosphoric acid supported on Zr/MCM-41 composite with excellent heterogeneous catalyst and promising adsorbent of methylene blue. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 631, 127753. [Google Scholar] [CrossRef]
- Salama, R.S.; El-Sayed, E.-S.M.; El-Bahy, S.M.; Awad, F.S. Silver nanoparticles supported on UiO-66 (Zr): As an efficient and recyclable heterogeneous catalyst and efficient adsorbent for removal of Indigo Carmine. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 626, 127089. [Google Scholar] [CrossRef]
- Atkins, P.; de Paula, J. Atkins’ Physical Chemistry; Oxford University Press: Oxford, UK, 2005; p. 1008. [Google Scholar]
- de Freitas, F.; Battirola, L.D.; de Andrade, R.L.T. Adsorption of Cu2+ and Pb2+ Ions by Pontederia rotundifolia (L.f.) (Pontederiaceae) and Salvinia biloba Raddi (Salviniaceae) Biomass. Water Air Soil Pollut. 2018, 229, 349. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Tuzen, M.; Soylak, M. Chromium speciation in environmental samples by solid phase extraction on Chromosorb 108. J. Hazard. Mater. 2006, 129, 266–273. [Google Scholar] [CrossRef]
- Fortes, M.; Martins, A.; Benedetto, J. Indium adsorption onto ion exchange polymeric resins. Miner. Eng. 2003, 16, 659–663. [Google Scholar] [CrossRef]
- Tokuyama, H.; Iwama, T. Solid-phase extraction of indium(III) ions onto thermosensitive poly(N-isopropylacrylamide). Sep. Purif. Technol. 2009, 68, 417–421. [Google Scholar] [CrossRef]
- Wang, S.; Sun, H.; Ang, H.M.; Tadé, M.O. Adsorptive remediation of environmental pollutants using novel graphene-based nanomaterials. Chem. Eng. J. 2013, 226, 336–347. [Google Scholar] [CrossRef]
- Tuzen, M.; Soylak, M. A solid phase extraction procedure for Indium prior to its graphite furnace atomic absorption spectrometric determination. J. Hazard. Mater. 2006, 129, 179–185. [Google Scholar] [CrossRef]
- Hoogerstraete, T.V.; Onghena, B.; Binnemans, K. Homogeneous Liquid–Liquid Extraction of Metal Ions with a Functionalized Ionic Liquid. J. Phys. Chem. Lett. 2013, 4, 1659–1663. [Google Scholar] [CrossRef]
- Liu, H.-M.; Wu, C.-C.; Lin, Y.-H.; Chiang, C.-K. Recovery of indium from etching wastewater using supercritical carbon dioxide extraction. J. Hazard. Mater. 2009, 172, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Bossche, A.V.D.; Vereycken, W.; Hoogerstraete, T.V.; Dehaen, W.; Binnemans, K. Recovery of Gallium, Indium, and Arsenic from Semiconductors Using Tribromide Ionic Liquids. ACS Sustain. Chem. Eng. 2019, 7, 14451–14459. [Google Scholar] [CrossRef]
- Ozkantar, N.; Yilmaz, E.; Soylak, M.; Tuzen, M. Solid-phase extraction of iridium from soil and water samples by using activated carbon cloth prior to its spectrophotometric determination. Environ. Monit. Assess. 2015, 187, 501. [Google Scholar] [CrossRef]
- Memon, F.N.; Ayyilidiz, H.F.; Kara, H.; Memon, S.; Kenar, A.; Leghari, M.K.; Topkafa, M.; Sherazi, S.T.H.; Memon, N.A.; Durmaz, F.; et al. Application of central composite design for the optimization of on-line solid phase extraction of Cu2+ by calix[4]arene bonded silica resin. Chemom. Intell. Lab. Syst. 2015, 146, 158–168. [Google Scholar] [CrossRef]
- Alguacil, F.; Lopez, F.; Rodriguez, O.; Martinez-Ramirez, S.; Garcia-Diaz, I. Sorption of indium (III) onto carbon nanotubes. Ecotoxicol. Environ. Saf. 2016, 130, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Naaz, F.; Dubey, H.K.; Kumari, C.; Lahiri, P. Structural and magnetic properties of MgFe2O4 nanopowder synthesized via co-precipitation route. SN Appl. Sci. 2020, 2, 808. [Google Scholar] [CrossRef]
- Bloesser, A.; Kurz, H.; Timm, J.; Wittkamp, F.; Simon, C.; Hayama, S.; Weber, B.; Apfel, U.-P.; Marschall, R. Tailoring the Size, Inversion Parameter, and Absorption of Phase-Pure Magnetic MgFe2O4 Nanoparticles for Photocatalytic Degradations. ACS Appl. Nano Mater. 2020, 3, 11587–11599. [Google Scholar] [CrossRef]
- Hammache, Z.; Soukeur, A.; Omeiri, S.; Bellal, B.; Trari, M. Physical and photo-electrochemical properties of MgFe2O4 prepared by sol gel route: Application to the photodegradation of methylene blue. J. Mater. Sci. Mater. Electron. 2019, 30, 5375–5382. [Google Scholar] [CrossRef]
- Becker, A.; Kirchberg, K.; Marschall, R. Magnesium Ferrite (MgFe2O4) Nanoparticles for Photocatalytic Antibiotics Degradation. Z. Für Phys. Chem. 2020, 234, 645–654. [Google Scholar] [CrossRef]
- Sun, J.R.; Wang, Z.G.; Wang, Y.Y.; Wei, K.F.; Li, F.S. Structure and Magnetic Properties of MgFe2O4 Nanoparticles Prepared by the Low-Temperature Solid-State Reaction Method. Mater. Sci. Forum 2011, 686, 316–318. [Google Scholar] [CrossRef]
- Reddy, S.; Swamy, B.E.K.; Chandra, U.; Mahathesha, K.R.; Sathisha, T.V.; Jayadevappa, H. Synthesis of MgFe2O4 nanoparticles and MgFe2O4 nanoparticles/CPE for electrochemical investigation of dopamine. Anal. Methods 2011, 3, 2792–2796. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substabces. Kungl. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.-S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. A Comparison of Chemisorption Kinetic Models Applied to Pollutant Removal on Various Sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Equilibria and Capacities for Adsorption on Carbon. J. Sanit. Eng. Div. 1964, 90, 79–108. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Maensiri, S.; Sangmanee, M.; Wiengmoon, A. Magnesium Ferrite (MgFe2O4) Nanostructures Fabricated by Electrospinning. Nanoscale Res. Lett. 2008, 4, 221–228. [Google Scholar] [CrossRef]
- Upadhyay, S.; Sreenivas, K. Comparative Studies of MgFe2O4 Nanoparticles Synthesized using Different Precursors by Sol Gel Auto Combustion Method. J. At. Mol. Condens. Nano Phys. 2015, 2, 101–108. [Google Scholar] [CrossRef]
- Ilhan, S.; Izotova, S.G.; Komlev, A.A. Synthesis and characterization of MgFe2O4 nanoparticles prepared by hydrothermal decomposition of co-precipitated magnesium and iron hydroxides. Ceram. Int. 2015, 41, 577–585. [Google Scholar] [CrossRef]
- Nicola, R.; Costişor, O.; Ciopec, M.; Negrea, A.; Lazău, R.; Ianăşi, C.; Picioruş, E.-M.; Len, A.; Almásy, L.; Szerb, E.I.; et al. Silica-Coated Magnetic Nanocomposites for Pb2+ Removal from Aqueous Solution. Appl. Sci. 2020, 10, 2726. [Google Scholar] [CrossRef]
- Sepahvand, R. Synthesis and Characterization of Carbon Nanotubes Decorated with Magnesium Ferrite (MgFe2O4) Nanoparticles by Citrate-Gel Method. J. Sci. Islam. Repub. Iran 2011, 22, 177–182. [Google Scholar] [CrossRef]
- Pradeep, A.; Priyadharsini, P.; Chandrasekaran, G. Sol–gel route of synthesis of nanoparticles of MgFe2O4 and XRD, FTIR and VSM study. J. Magn. Magn. Mater. 2008, 320, 2774–2779. [Google Scholar] [CrossRef]
- Robles, J. The Engineering of Pt/Carbon Catalyst Preparation-For Application on Proton Exchange Fuel Cell Membrane; Univrsity of Illinois at Chicago: Chicago, IL, USA, 2004. [Google Scholar]
- Vidal, C.; do Nascimento, R.; Raulino, G.; Clecius, A.; Melo, D. Adsorção: Aspectos Teóricos e Aplicações Ambientais; Imprensa Universitária: Fortaleza, Brazil, 2014. [Google Scholar]
- Werner, A.; Rieger, A.; Mosch, M.; Haseneder, R.; Repke, J.-U. Nanofiltration of indium and germanium ions in aqueous solutions: Influence of pH and charge on retention and membrane flux. Sep. Purif. Technol. 2017, 194, 319–328. [Google Scholar] [CrossRef]
- Baes, C.F.; Mesmer, R.S. The Hydrolysis of Cations. Ber. Der Bunsenges. Für Phys. Chem. 1977, 81, 245–246. [Google Scholar]
- Akama, Y.; Suzuki, S.; Monobe, Y. Study on the adsorption and selective separation of indium from zinc with chelating cellulose. Cellul. Chem. Technol. 2016, 50, 147–152. [Google Scholar]
- Zhang, S.; Ning, S.; Liu, H.; Zhou, J.; Wang, S.; Zhang, W.; Wang, X.; Wei, Y. Highly-efficient separation and recovery of ruthenium from electroplating wastewater by a mesoporous silica-polymer based adsorbent. Microporous Mesoporous Mater. 2020, 303, 110293. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, F.; Cheng, W.; Wang, J.; Ma, J. Adsorption Equilibrium and Kinetics of the Removal of Ammoniacal Nitrogen by Zeolite X/Activated Carbon Composite Synthesized from Elutrilithe. J. Chem. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Marinho, R.S.; da Silva, C.N.; Afonso, J.C.; da Cunha, J.W.S.D. Recovery of platinum, tin and indium from spent catalysts in chloride medium using strong basic anion exchange resins. J. Hazard. Mater. 2011, 192, 1155–1160. [Google Scholar] [CrossRef]
- Kwak, N.-S.; Baek, Y.; Hwang, T.S. The synthesis of poly(vinylphosphonic acid-co-methacrylic acid) microbeads by suspension polymerization and the characterization of their indium adsorption properties. J. Hazard. Mater. 2012, 203–204, 213–220. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Equation | References |
|---|---|---|
| Pseudo-first order kinetic model (Lagergren) | (1) where: qe—equilibrium adsorption capacity (mg g−1) qt—adsorption capacity at a specific time—t (mg g−1) k1—pseudo-first order speed constant (min−1) t—contact time (min) | [53] |
| Pseudo-second order kinetic model (Ho and McKay) | (2) qe—equilibrium absorption capacity (mg g−1) qt—adsorption capacity at a specific time—t (mg g−1) k2—pseudo-second order speed constant (g mg−1 min−1) t—contact time (min) | [54,55] |
| Intraparticle diffusion (Weber and Morris model) | Qt = kdiff • t1/2 + C (3) where: Qt—adsorption capacity at t time, µg/g kdiff—speed constant for intraparticle diffusion, µg/g·min1/2 C—constant correlated with the thickness of the liquid film surrounding the adsorbent particles. | [56,57] |
| Parameters | Equation | References |
|---|---|---|
| The adsorption capacity of the material | (4) where: qe—the maximum adsorption capacity (µg g−1) C0—initial concentration of metallic ion in solution (µg L−1) Ce—the equilibrium concentration of metallic ion in solution (µg L−1) V—volume of the aqueous solution with metallic ion content (L) m—mass of the adsorbent (g) | |
| Langmuir isotherm nonlinear expression | (5) where: qe—the maximum adsorption capacity (µg g−1) Ce the equilibrium concentration of metallic ion in solution (µg L−1) qL—Langmuir maximum adsorption capacity (µg g−1) KL—Langmuir constant | [58] |
| Freundlich isotherm nonlinear expression | (6) where: qe—the maximum adsorption capacity (µg g−1) Ce—the equilibrium concentration of metallic ion in solution (µg g−1) KF and nF—the characteristic constants that can be related to the relative adsorption capacity of the adsorbent and the intensity of adsorption | [35] |
| Sips isotherm nonlinear expression | (7) where: qS—the maximum adsorption capacity (µg g−1) KS—constant related to the adsorption capacity of the adsorbent nS—the heterogeneity factor | [59] |
| Pseudo-First-Order | ||||
| Temperature (K) | qe,exp (µg g−1) | k1 (min−1) | qe,calc (µg g−1) | R2 |
| 298 | 124.3 | 0.020 | 39.61 | 0.8930 |
| 308 | 125.0 | 0.022 | 41.15 | 0.8883 |
| 318 | 126.0 | 0.023 | 41.63 | 0.9063 |
| 328 | 127.4 | 0.030 | 42.17 | 0.9114 |
| Pseudo-second-order | ||||
| Temperature (K) | qe,exp (µg g−1) | k2(g µg−1∙min−1) | qe,calc (µg g−1) | R2 |
| 298 | 124.3 | 197.7 | 113.6 | 0.9991 |
| 308 | 125.0 | 234.2 | 119.0 | 0.9992 |
| 318 | 126.0 | 276.2 | 126.5 | 0.9992 |
| 328 | 127.4 | 342.4 | 128.2 | 0.9996 |
| Intraparticle diffusion model (IPD) | ||||
| Temperature (K) | Kdiff (µg·g−1 min−1/2) | C | R2 | |
| 298 | 3.06 | 84.7 | 0.8646 | |
| 308 | 3.54 | 87.7 | 0.8370 | |
| 318 | 3.67 | 90.0 | 0.8721 | |
| 328 | 3.88 | 96.1 | 0.8646 | |
| Langmuir Isotherm | |||
| qm,exp(µg g−1) | KL(L µg−1) | qL(µg g−1) | R2 |
| 46,396 | 3.84 × 10−4 | 51,616 | 0.9717 |
| Freundlich isotherm | |||
| KF(µg g−1) | 1/nF | R2 | |
| 2107.4 | 0.28 | 0.9049 | |
| Sips isotherm | |||
| KS | qS(µg/g) | 1/nS | R2 |
| 0.28 | 47,475 | 0.03 | 0.9877 |
| Materials | q, mg/g | References |
|---|---|---|
| Cellulose with iminodiacetic acid | 0.172 | [70] |
| CCB | 7.89 | [14] |
| Amberlite IRA-400AR | 14.93 | [73] |
| Coated solvent impregnated resins | 23.8 | [74] |
| Modified solvent impregnated resins | 26.25 | [10] |
| Dowex 1 | 31.00 | [73] |
| Modified solvent impregnated resin (MSIR—impregnation of sec-octylphenoxy acetic acid (CA-12) on styrene–divinylbenzene copolymer support (HZ818) after nitration of the benzene rings) | 26.0 | [10] |
| Non-imprinted polymers In(III)-NIP | 29.75 | [2] |
| LewatitTP 260 | 1.437 | [7] |
| Lewatit TP 208 | 1.488 | [7] |
| Amberlite IRA 743 | 0.84 | [7] |
| MgFe2O4 | ~46.4 | This paper |
| ΔH° (J mol−1) | ΔS° (J mol−1 K−1) | ΔG° (kJ mol−1) | R2 | |||
|---|---|---|---|---|---|---|
| 51.52 | 203.0 | 298 K | 308 K | 318 K | 328 K | 0.9975 |
| −60.44 | −62.47 | −64.50 | −66.53 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciocărlie, L.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Ianasi, P.; Ianasi, C.; Nemes, N.S. Indium Recovery by Adsorption on MgFe2O4 Adsorbents. Materials 2022, 15, 7054. https://doi.org/10.3390/ma15207054
Ciocărlie L, Negrea A, Ciopec M, Duteanu N, Negrea P, Ianasi P, Ianasi C, Nemes NS. Indium Recovery by Adsorption on MgFe2O4 Adsorbents. Materials. 2022; 15(20):7054. https://doi.org/10.3390/ma15207054
Chicago/Turabian StyleCiocărlie, Loredana, Adina Negrea, Mihaela Ciopec, Narcis Duteanu, Petru Negrea, Paula Ianasi, Catalin Ianasi, and Nicoleta Sorina Nemes. 2022. "Indium Recovery by Adsorption on MgFe2O4 Adsorbents" Materials 15, no. 20: 7054. https://doi.org/10.3390/ma15207054
APA StyleCiocărlie, L., Negrea, A., Ciopec, M., Duteanu, N., Negrea, P., Ianasi, P., Ianasi, C., & Nemes, N. S. (2022). Indium Recovery by Adsorption on MgFe2O4 Adsorbents. Materials, 15(20), 7054. https://doi.org/10.3390/ma15207054









