Archaeometric Characterization of the Industrial Production of Porcelains in the Vieillard & Co. Manufactory (Bordeaux, France, 19th Century)
Abstract
:1. Introduction
2. Materials: Archaeological Sherds
3. Analytical Techniques
3.1. Archaeological Samples Preparation
3.2. Scanning Electron Microscopy-Energy Dispersive X-ray Spectroscopy (SEM-EDS)
3.3. X-ray Diffraction (XRD)
3.4. Particle Induced X-ray and Gamma Emission (PIXE-PIGE)
4. Results and Discussion
4.1. Body Characterization
4.1.1. Biscuits of Porcelain
4.1.2. Porcelains
4.2. Glaze Examination
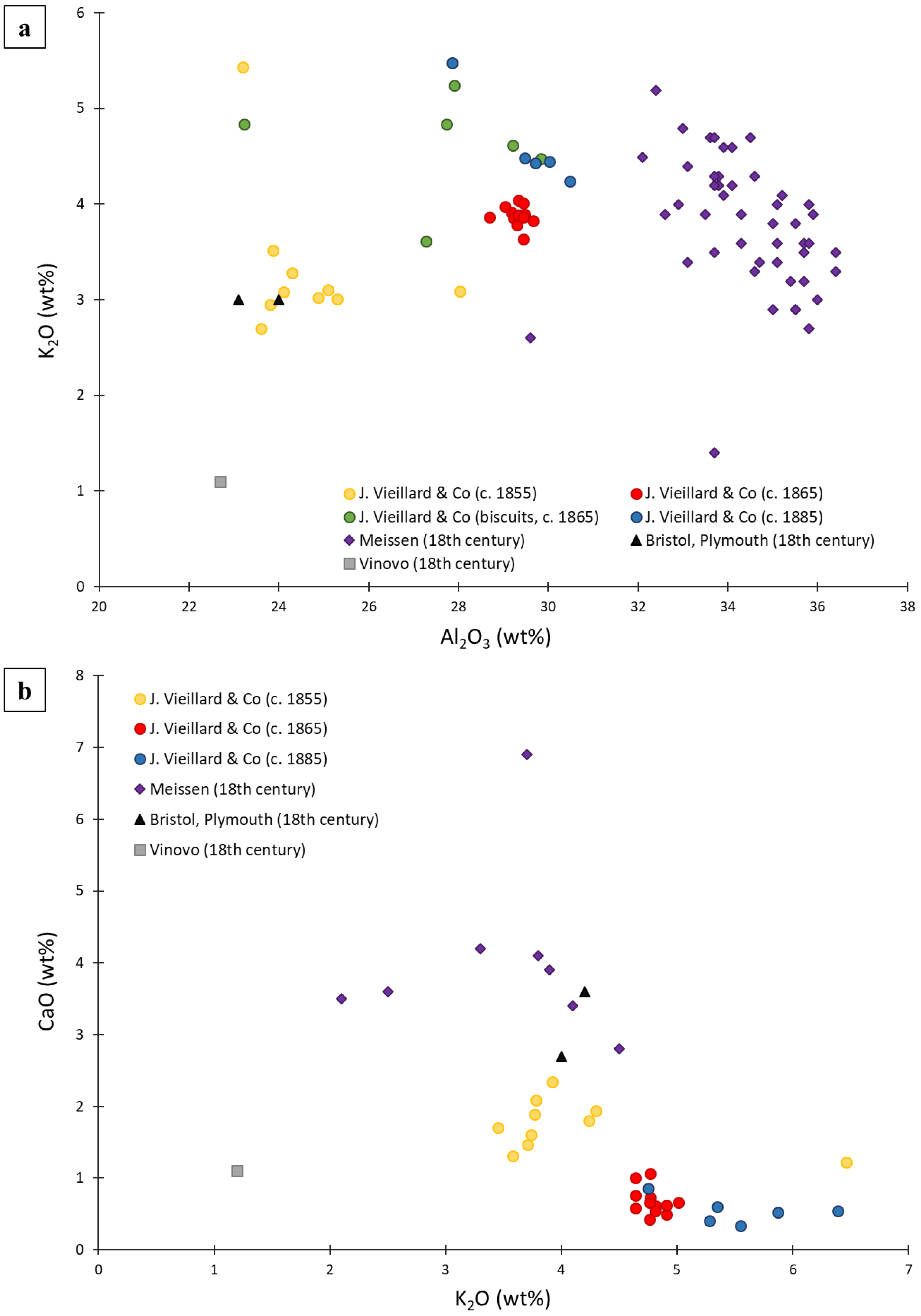
4.3. Comparison with Other European Hard-Paste Porcelain Productions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marache, V.; Sireix, C.; Briceno-Boucey, L.; Valleix-Wattiaux, M.-P. Aquitaine—Gironde, Bordeaux, Rue Lucien Faure, Sondages (Mai et Décembre 2015); Bordeaux Métropole: Bordeaux, France, 2018; p. 105. [Google Scholar]
- du Pasquier, J.J. Vieillard & Cie. Histoire de la Faïence Fine à Bordeaux. De L’anglomanie au Rêve Orientaliste; Mollat: Bordeaux, France, 2002; ISBN 978-2-909351-73-5. [Google Scholar]
- Edwards, H.G.M. 18th and 19th Century Porcelain Analysis: A Forensic Provenancing Assessment; Springer: Cham, Germany, 2020. [Google Scholar] [CrossRef]
- Edwards, H.G.M. Porcelain Analysis and Its Role in the Forensic Attribution of Ceramic Specimens; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Pichon, L.; Moignard, B.; Lemasson, Q.; Pacheco, C.; Walter, P. Development of a Multi-Detector and a Systematic Imaging System on the AGLAE External Beam. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2014, 318, 27–31. [Google Scholar] [CrossRef]
- Radepont, M.; Lemasson, Q.; Pichon, L.; Moignard, B.; Pacheco, C. Towards a Sharpest Interpretation of Analytical Results by Assessing the Uncertainty of PIXE/RBS Data at the AGLAE Facility. Measurement 2018, 114, 501–507. [Google Scholar] [CrossRef]
- Brongniart, A. Traité des Arts Céramiques ou des Poteries Considérées Dans Leur Histoire, Leur Pratique et Leur Théorie; Béchet Jeune: Paris, France, 1854; Volume 2. [Google Scholar]
- de Milly, N.-C.T. L’Art de la Porcelaine; Saillant et Nyon: Paris, France, 1772. [Google Scholar]
- Bastenaire-Daudenart, F. L’Art de Fabriquer la Porcelaine; Malher et Cie: Paris, France, 1827. [Google Scholar]
- Blondel, N. Céramique: Vocabulaire Technique; Editions du Patrimoine-Centre des Monuments Nationaux: Paris, France, 2001; ISBN 978-2-7577-0399-1. [Google Scholar]
- Meite, N.; Konan, L.K.; Bamba, D.; Goure-Doubi, B.I.H.; Oyetola, S. Structural and Thermomechanical Study of Plastic Films Made from Cassava-Starch Reinforced with Kaolin and Metakaolin. Mater. Sci. Appl. 2018, 9, 41–54. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Kim, Y.J.; Moon, H.-S. Phase Transformation Sequence from Kaolinite to Mullite Investigated by an Energy-Filtering Transmission Electron Microscope. J. Am. Ceram. Soc. 1999, 82, 2841–2848. [Google Scholar] [CrossRef]
- Roisine, G. Céramiques Glaçurées de Bernard Palissy: À la Recherche des Secrets D’un Maître de la Renaissance. Ph.D. Thesis, Université Paris Sciences & Lettres, Paris, France, 2018. [Google Scholar]
- Chen, Y.-F.; Wang, M.-C.; Hon, M.-H. Phase Transformation and Growth of Mullite in Kaolin Ceramics. J. Eur. Ceram. Soc. 2004, 24, 2389–2397. [Google Scholar] [CrossRef]
- d’Albis, A. Traité de la Porcelaine de Sèvres; Editions Faton: Dijon, France, 2003; ISBN 978-2-87844-055-3. [Google Scholar]
- Martín-Márquez, J.; Rincón, J.M.; Romero, M. Mullite Development on Firing in Porcelain Stoneware Bodies. J. Eur. Ceram. Soc. 2010, 30, 1599–1607. [Google Scholar] [CrossRef] [Green Version]
- Tite, M.; Freestone, I.; Wood, N. An Investigation into the Relationship Between the Raw Materials Used in the Production of Chinese Porcelain and Stoneware Bodies and the Resulting Microstructures. Archaeometry 2012, 54, 37–55. [Google Scholar] [CrossRef]
- Fabbri, B.; Gualtieri, S.; Amato, F. Capodimonte Porcelain: A Unique Manufacture. In UCL Qatar Series in Archaeology and Cultural Heritage; Martinon-Torres, M., Ed.; Bloomsbury Qatar Foundation: Doha, Qatar, 2014; Volume 1, ISBN 978-9927-101-75-5. [Google Scholar]
- Gültekin, E.; Topateş, G.; Kurama, S. The Effects of Sintering Temperature on Phase and Pore Evolution in Porcelain Tiles. Ceram. Int. 2017, 43, 11511–11515. [Google Scholar] [CrossRef]
- Turco, F.; Davit, P.; Cossio, R.; Agostino, A.; Operti, L. Accuracy Improvement by Means of Porosity Assessment and Standards Optimization in SEM-EDS and XRF Elemental Analyses on Archaeological and Historical Pottery and Porcelain. J. Archaeol. Sci. Rep. 2017, 12, 54–65. [Google Scholar] [CrossRef]
- Pawlowsky-Glahn, V.; Buccianti, A. (Eds.) Compositional Data Analysis: Theory and Applications; Wiley: Chichester, UK, 2011; ISBN 978-0-470-71135-4. [Google Scholar]
- Bourry, É. Traité des Industries Céramiques; Gauthier-Villars: Paris, France, 1897. [Google Scholar]
- Salvétat, A. Leçons de Céramique Professées à l’École Centrale des Arts et Manufactures; Mallet-Bachelier: Paris, France, 1857. [Google Scholar]
- Martinón-Torres, M.; Freestone, I.C.; Hunt, A.; Rehren, T. Mass-Produced Mullite Crucibles in Medieval Europe: Manufacture and Material Properties. J. Am. Ceram. Soc. 2008, 91, 2071–2074. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Wang, M.-C.; Hon, M.-H. Transformation Kinetics for Mullite in Kaolin–Al2O3 Ceramics. J. Mater. Res. 2003, 18, 1355–1362. [Google Scholar] [CrossRef]
- Jouannet, F. Statistique du Département de la Gironde, Sous les Auspices de M. le Préfet et du Conseil Général—Tome 2, Part 2; P. Dupont & Cie: Paris, France, 1843. [Google Scholar]
- Schulle, W.; Ullrich, B. Ergebisse gefügeanalytischer Untersuchungen an Böttgerporzellan. Silikattechnik 1982, 33, 44–47. [Google Scholar]
- Ullrich, B.; Lange, P.; Friedmar, K. Thüringer und Meissener Porzellan des späten 18. und frühen 19. Jahrhunderts—ein werkstoffhistorischer Vergleich. Keram. Z. 2010, 62, 185–190. [Google Scholar]
- Domoney, K.; Shortland, A.; Kuhn, S. Characterization of 18th-Century Meissen Porcelain using SEM-EDS. Archaeometry 2012, 54, 454–474. [Google Scholar] [CrossRef]
- Bezur, A.; Casadio, F. Du Paquier’s Porcelain: Artistic Expression and Technological Mastery. In Fired by Passion: Vienna Baroque Porcelain of Claudius Innocentius Du Paquier; Arnoldsche Art Publishers: Stuttgart, Germany, 2009; pp. 1163–1211. [Google Scholar]
- Neelmeijer, C.; Pietsch, U.; Ulbricht, H. Eighteenth-century Meissen porcelain reference data obtained by proton-beam analysis (PIXE–PIGE). Archaeometry 2014, 56, 527–540. [Google Scholar] [CrossRef]
- Tite, M.S.; Bimson, M. A technological study of English porcelains. Archaeometry 1991, 33, 3–27. [Google Scholar] [CrossRef]
- Turco, F.; Davit, P.; Maritano, C.; Operti, L.; Fenoglio, G.; Agostino, A. 18th Century Piedmontese Porcelains from Palazzo Madama Museum). Archaeometry 2016, 58, 765–778. [Google Scholar] [CrossRef]
- Aza, A.H.; Torre, Á.G.; Aranda, M.A.G.; Valle, F.J.; Aza, S. Rietveld Quantitative Analysis of Buen Retiro Porcelains. J. Am. Ceram. Soc. 2004, 87, 449–454. [Google Scholar] [CrossRef]
- Bezur, A.; Casadio, F. Chapter 8—The Analysis of Porcelain Using Hand-Held and Portable X ray Fluorescence Spectrometers. In Handheld XRF for Art and Archaeology; Shugar, A.N., Mass, J.L., Eds.; Leuven University Press: Leuven, Belgium, 2012; pp. 249–312. [Google Scholar]
- Casadio, F.; Bezur, A.; Domoney, K.; Eremin, K.; Lee, L.; Mass, J.L.; Shortland, A.; Zumbulyadis, N. X-ray fluorescence applied to overglaze enamel decoration on 18th and 19th Century porcelain from Central Europe. Stud. Conserv. 2012, 57, 61–72. [Google Scholar] [CrossRef]


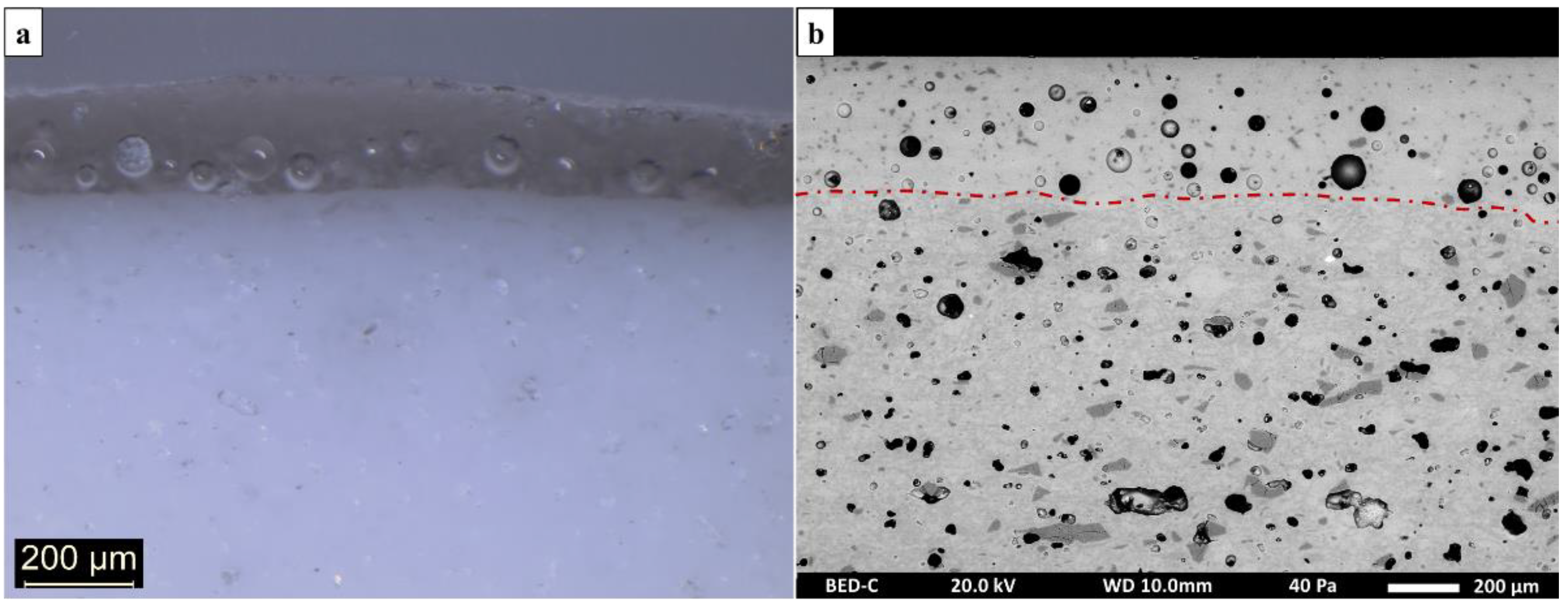
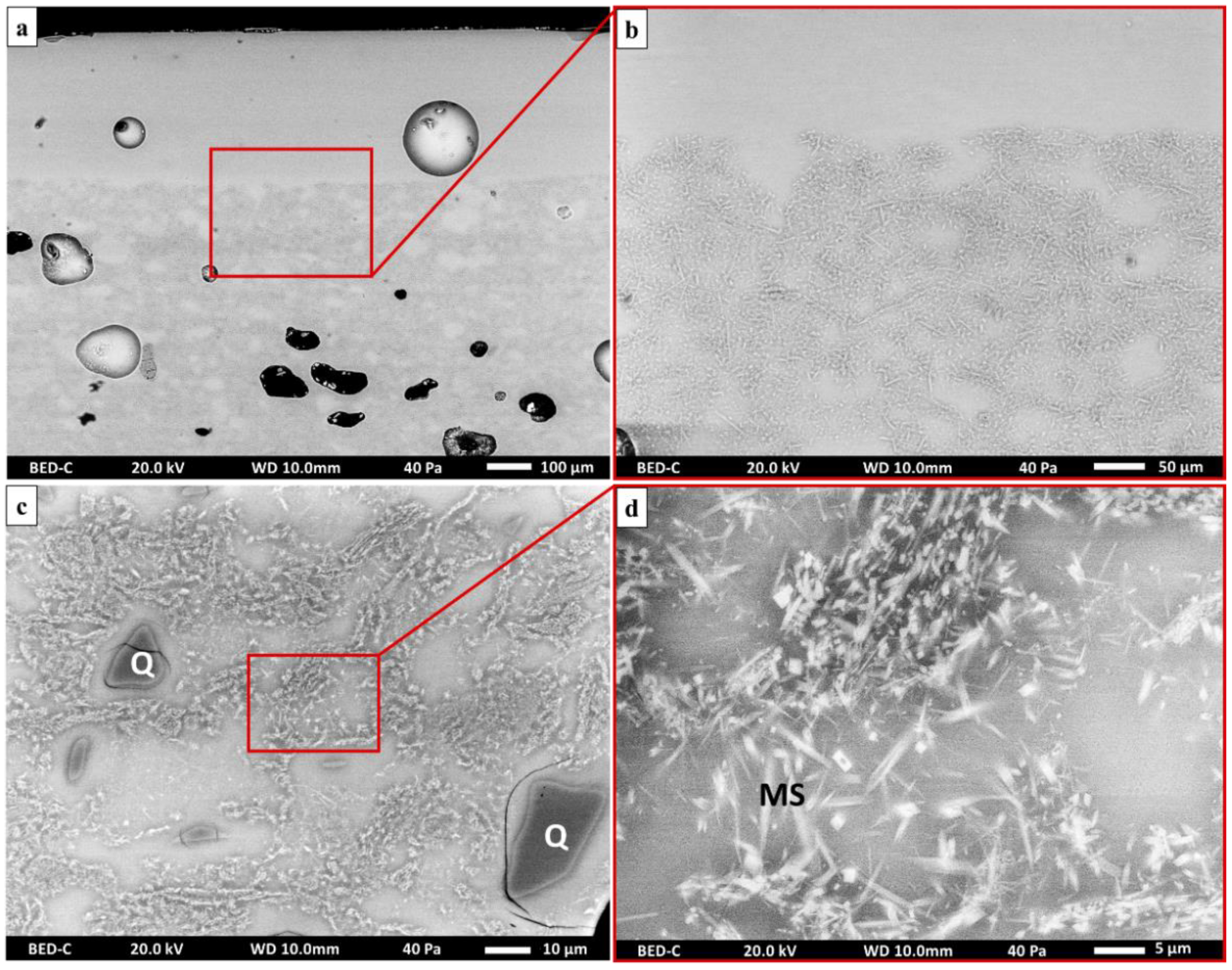
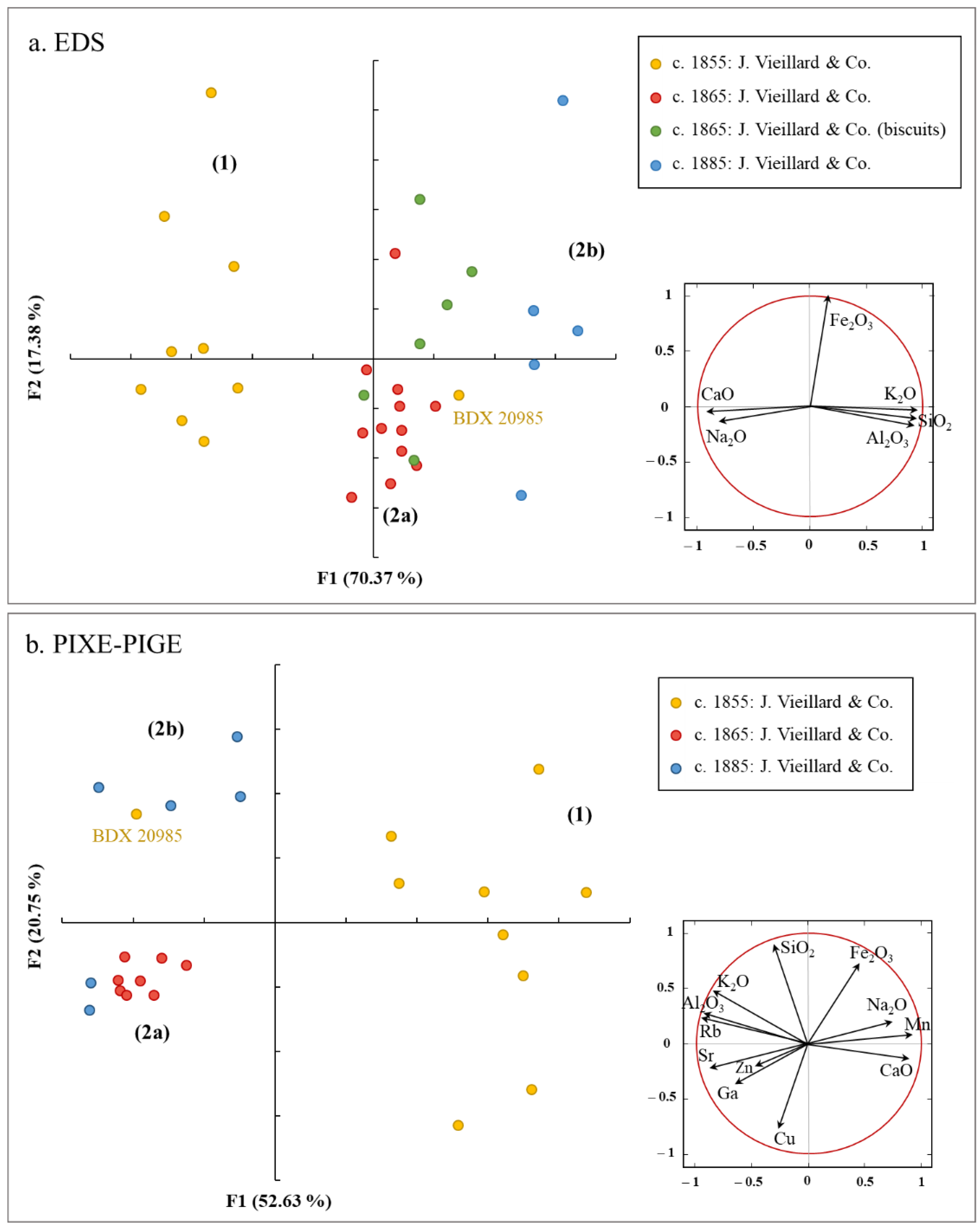
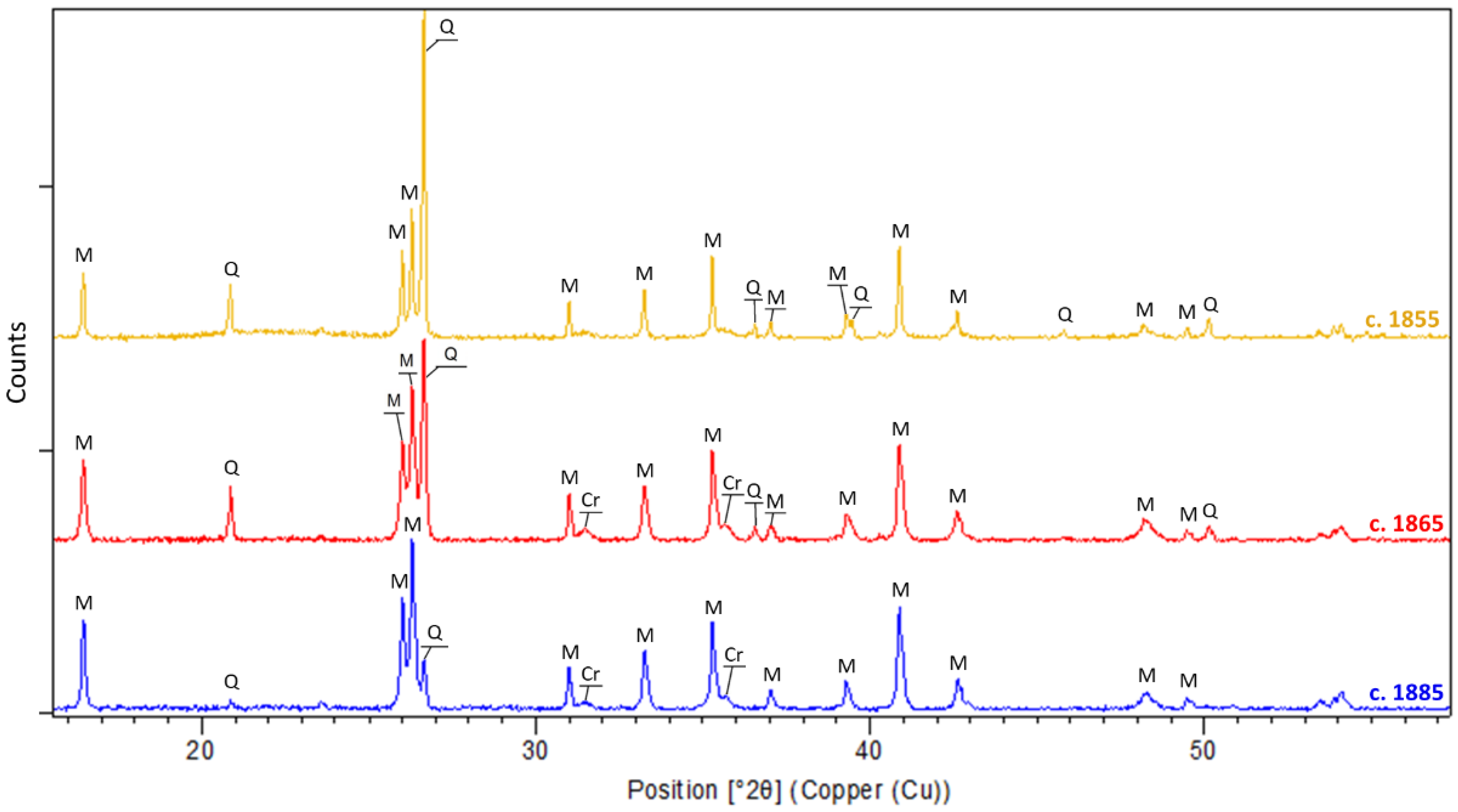
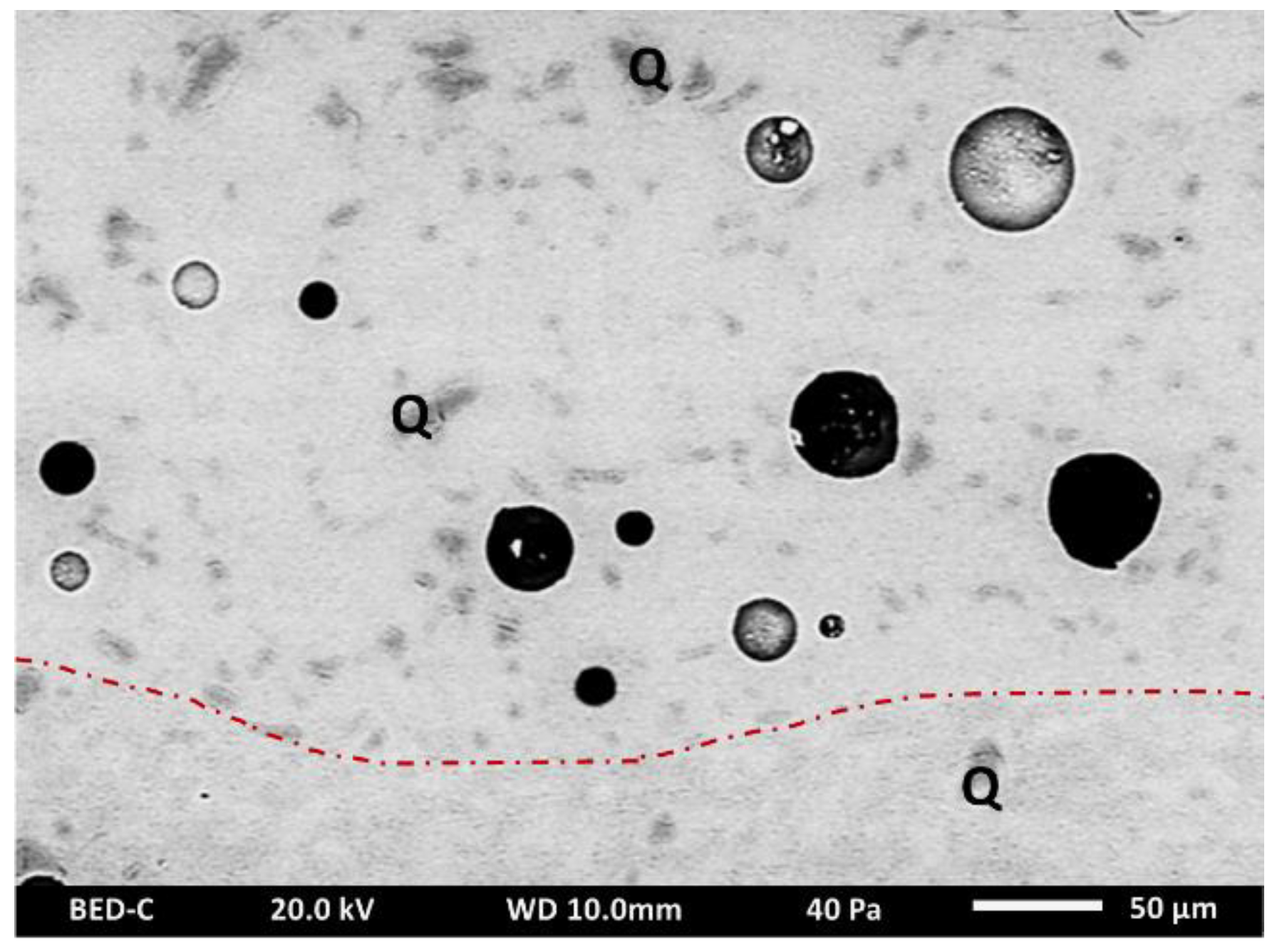

| Sample Number | Chronology | Manufactory | Type | Shape |
|---|---|---|---|---|
| BDX 20979 | c. 1855 | J. Vieillard & Co. (father’s production) | porcelain | bucket |
| BDX 20980 | - | |||
| BDX 20981 | plate | |||
| BDX 20982 | - | |||
| BDX 20983 | - | |||
| BDX 20984 | - | |||
| BDX 20985 | plate | |||
| BDX 20986 | - | |||
| BDX 20987 | - | |||
| BDX 20988 | tureen lid | |||
| BDX 20989 | c. 1865 | J. Vieillard & Co. (sons’ production) | plate | |
| BDX 20990 | plate | |||
| BDX 20991 | - | |||
| BDX 20992 | - | |||
| BDX 20993 | - | |||
| BDX 20994 | - | |||
| BDX 20995 | - | |||
| BDX 20996 | - | |||
| BDX 20997 | - | |||
| BDX 20998 | - | |||
| BDX 20999 | vase | |||
| BDX 21000 | pyrometric cone | |||
| BDX 21862 | biscuit of porcelain | - | ||
| BDX 21863 | - | |||
| BDX 21864 | - | |||
| BDX 21865 | - | |||
| BDX 21866 | - | |||
| BDX 21867 | - | |||
| BDX 21001 | c. 1885 | porcelain | goblet | |
| BDX 21002 | saucer | |||
| BDX 21003 | teapot | |||
| BDX 21004 | insulator | |||
| BDX 21005 | - | |||
| BDX 21006 | plate |
| Na2O | Al2O3 | SiO2 | K2O | CaO | Fe2O3 | |
|---|---|---|---|---|---|---|
| c. 1865—J. Vieillard & Co (biscuits) | ||||||
| Mean (n = 6) | 0.8 | 29.2 | 64.6 | 4.8 | 0.2 | 0.3 |
| SD | 0.1 | 1.1 | 0.6 | 0.6 | 0.1 | 0.1 |
| Na2O | MgO | Al2O3 | SiO2 | P2O5 | K2O | CaO | TiO2 | Fe2O3 | |
|---|---|---|---|---|---|---|---|---|---|
| c. 1855—J. Vieillard & Co | |||||||||
| Mean (n = 10) | 2.4 | 0.2 | 24.2 | 68.6 | 0.2 | 3.3 | 0.7 | nd | 0.4 |
| SD | 0.3 | 0.1 | 0.7 | 0.7 | 0.1 | 0.8 | 0.2 | 0.1 | |
| c. 1865—J. Vieillard & Co | |||||||||
| Mean (n = 12) | 1.5 | 0.1 | 29.3 | 64.4 | 0.2 | 3.9 | 0.4 | 0.1 | 0.3 |
| SD | 0.1 | 0.1 | 0.3 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| c. 1885—J. Vieillard & Co | |||||||||
| Mean (n = 6) | 1.3 | 0.2 | 27.5 | 65.6 | 0.2 | 4.6 | 0.3 | 0.1 | 0.3 |
| SD | 0.3 | 0.1 | 2.3 | 2.3 | 0.1 | 0.5 | 0.1 | 0.1 | 0.1 |
| Na2O | MgO | Al2O3 | SiO2 | K2O | CaO | TiO2 | Fe2O3 | Mn | Cu | Zn | Ga | Rb | Sr | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt% | wt% | wt% | wt% | wt% | wt% | wt% | wt% | ppm | ppm | ppm | ppm | ppm | ppm | |
| c. 1855— J. Vieillard & Co | ||||||||||||||
| Mean (n = 10) | 1.7 | 0.3 | 24.3 | 68.7 | 3.3 | 0.8 | 0.1 | 0.4 | 78 | 38 | 69 | 36 | 197 | 104 |
| SD | 0.3 | 0.1 | 1.4 | 1.6 | 0.8 | 0.5 | 0.1 | 0.1 | 27 | 27 | 13 | 5 | 35 | 14 |
| c. 1865— J. Vieillard & Co | ||||||||||||||
| Mean (n = 8) | 1.1 | nd | 28.8 | 64.8 | 4.0 | 0.3 | 0.1 | 0.3 | 41 | 39 | 74 | 48 | 281 | 122 |
| SD | 0.1 | 0.3 | 0.4 | 0.1 | 0.1 | 0.1 | 0.1 | 3 | 5 | 11 | 3 | 13 | 7 | |
| c. 1885— J. Vieillard & Co | ||||||||||||||
| Mean (n = 6) | 0.9 | 0.3 | 27.0 | 66.0 | 4.7 | 0.3 | 0.1 | 0.4 | 38 | 27 | 71 | 37 | 277 | 110 |
| SD | 0.2 | 0.1 | 2.3 | 2.3 | 0.6 | 0.1 | 0.1 | 0.1 | 10 | 11 | 19 | 10 | 42 | 19 |
| Na2O | MgO | Al2O3 | SiO2 | P2O5 | K2O | CaO | Fe2O3 | |
|---|---|---|---|---|---|---|---|---|
| c. 1855—J. Vieillard & Co | ||||||||
| Mean (n = 10) | 3.3 | 0.2 | 16.1 | 74.5 | 0.2 | 4.1 | 1.7 | 0.2 |
| SD | 0.5 | 0.1 | 0.6 | 1.0 | 0.1 | 0.9 | 0.3 | 0.1 |
| c. 1865—J. Vieillard & Co | ||||||||
| Mean (n = 12) | 1.9 | nd | 13.1 | 79.4 | nd | 4.8 | 0.7 | 0.2 |
| SD | 0.2 | 0.3 | 0.4 | 0.1 | 0.2 | 0.1 | ||
| c. 1885—J. Vieillard & Co | ||||||||
| Mean (n = 6) | 1.6 | 0.2 | 14.2 | 77.8 | nd | 5.5 | 0.5 | 0.3 |
| SD | 0.2 | 0.1 | 1.0 | 1.6 | 0.6 | 0.2 | 0.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beauvoit, E.; Cantin, N.; Lemasson, Q.; Chapoulie, R.; Ben Amara, A. Archaeometric Characterization of the Industrial Production of Porcelains in the Vieillard & Co. Manufactory (Bordeaux, France, 19th Century). Materials 2022, 15, 5311. https://doi.org/10.3390/ma15155311
Beauvoit E, Cantin N, Lemasson Q, Chapoulie R, Ben Amara A. Archaeometric Characterization of the Industrial Production of Porcelains in the Vieillard & Co. Manufactory (Bordeaux, France, 19th Century). Materials. 2022; 15(15):5311. https://doi.org/10.3390/ma15155311
Chicago/Turabian StyleBeauvoit, Emmie, Nadia Cantin, Quentin Lemasson, Rémy Chapoulie, and Ayed Ben Amara. 2022. "Archaeometric Characterization of the Industrial Production of Porcelains in the Vieillard & Co. Manufactory (Bordeaux, France, 19th Century)" Materials 15, no. 15: 5311. https://doi.org/10.3390/ma15155311
APA StyleBeauvoit, E., Cantin, N., Lemasson, Q., Chapoulie, R., & Ben Amara, A. (2022). Archaeometric Characterization of the Industrial Production of Porcelains in the Vieillard & Co. Manufactory (Bordeaux, France, 19th Century). Materials, 15(15), 5311. https://doi.org/10.3390/ma15155311





