Advanced Zinc–Magnesium Alloys Prepared by Mechanical Alloying and Spark Plasma Sintering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Processing
2.2. Microstructure
2.3. Mechanical Properties
3. Results
3.1. Mechanical Alloying
3.2. Compaction by SPS
3.3. Mechanical Properties
4. Discussion
4.1. Microstructure
4.2. Mechanical Properties
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Andani, M.T.; Shayesteh Moghaddam, N.; Haberland, C.; Dean, D.; Miller, M.J.; Elahinia, M. Metals for bone implants. Part 1. Powder metallurgy and implant rendering. Acta Biomater. 2014, 10, 4058–4070. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Thompson, I. Twenty-first century challenges for biomaterials. J. R. Soc. Interface 2010, 7 (Suppl. S4), S379–S391. [Google Scholar] [CrossRef] [Green Version]
- Venezuela, J.; Dargusch, M.S. The influence of alloying and fabrication techniques on the mechanical properties, biodegradability and biocompatibility of zinc: A comprehensive review. Acta Biomater. 2019, 87, 1–40. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zheng, Y.; Qin, L. Progress of biodegradable metals. Prog. Nat. Sci. Mater. Int. 2014, 24, 414–422. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Escobar, D.; Champagne, S.; Yilmazer, H.; Dikici, B.; Boehlert, C.J.; Hermawan, H. Current status and perspectives of zinc-based absorbable alloys for biomedical applications. Acta Biomater. 2019, 97, 1–22. [Google Scholar] [CrossRef]
- Mostaed, E.; Sikora-Jasinska, M.; Drelich, J.W.; Vedani, M. Zinc-based alloys for degradable vascular stent applications. Acta Biomater. 2018, 71, 1–23. [Google Scholar] [CrossRef]
- Kabir, H.; Munir, K.; Wen, C.; Li, Y. Recent research and progress of biodegradable zinc alloys and composites for biomedical applications: Biomechanical and biocorrosion perspectives. Bioact Mater. 2021, 6, 836–879. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Low, H.T.; Kasiri-Asgarani, M.; Farahany, S.; Akbari, E. Fabrication of biodegradable Zn-Al-Mg alloy: Mechanical properties, corrosion behavior, cytotoxicity and antibacterial activities. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 73, 215–219. [Google Scholar] [CrossRef]
- Dambatta, M.S.; Izman, S.; Kurniawan, D.; Farahany, S.; Yahaya, B.; Hermawan, H. Influence of thermal treatment on microstructure, mechanical and degradation properties of Zn–3Mg alloy as potential biodegradable implant material. Mater. Des. 2015, 85, 431–437. [Google Scholar] [CrossRef]
- Ren, T.; Gao, X.; Xu, C.; Yang, L.; Guo, P.; Liu, H. Evaluation of as-extruded ternary Zn–Mg–Zr alloys for biomedical implantation material: In vitro and in vivo behavior. Mater. Corros. 2019, 70, 1056–1070. [Google Scholar] [CrossRef]
- Vojtěch, D.; Kubásek, J.; Šerák, J.; Novák, P. Mechanical and corrosion properties of newly developed biodegradable Zn-based alloys for bone fixation. Acta Biomater. 2011, 7, 3515–3522. [Google Scholar] [CrossRef]
- Jarzębska, A.; Bieda, M.; Kawałko, J.; Rogal, Ł.; Koprowski, P.; Sztwiertnia, K. A new approach to plastic deformation of biodegradable zinc alloy with magnesium and its effect on microstructure and mechanical properties. Mater. Lett. 2018, 211, 58–61. [Google Scholar] [CrossRef]
- Jarzębska, A.; Bieda, M.; Maj, Ł.; Chulist, R.; Wojtas, D.; Strąg, M. Controlled Grain Refinement of Biodegradable Zn-Mg Alloy: The Effect of Magnesium Alloying and Multi-Pass Hydrostatic Extrusion Preceded by Hot Extrusion. Metall. Mater. Trans. A 2020, 51, 6784–6796. [Google Scholar] [CrossRef]
- Kubasek, J.; Vojtěch, D. Zn-based alloys as an alternative biodegradable materials. Proc. Metal. 2012, 5, 23–25. [Google Scholar]
- Wang, L.-Q.; Ren, Y.-P.; Sun, S.-N.; Zhao, H.; Li, S.; Qin, G.-W. Microstructure, Mechanical Properties and Fracture Behavior of As-Extruded Zn–Mg Binary Alloys. Acta Metall. Sin. 2017, 30, 931–940. [Google Scholar] [CrossRef]
- Kubásek, J.; Vojtěch, D.; Pospíšilová, I.; Michalcová, A.; Maixner, J. Microstructure and mechanical properties of the micrograined hypoeutectic Zn–Mg alloy. Int. J. Miner. Metall. Mater. 2016, 23, 1167–1176. [Google Scholar] [CrossRef]
- Li, H.F.; Xie, X.H.; Cong, Y.; Zhou, F.Y.; Qiu, K.J. Development of biodegradable Zn-1X binary alloys with nutrient alloying elements Mg, Ca and Sr. Sci. Rep. 2015, 5, 10719. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Qiu, K.; Yang, Y.; Pu, Z.; Li, L. Effects of alloying elements (Ca and Sr) on microstructure, mechanical property and in vitro corrosion behavior of biodegradable Zn–1.5Mg alloy. J. Alloy. Compd. 2016, 664, 444–452. [Google Scholar] [CrossRef]
- Miranda-Hernández, J.G.; Herrera-Hernández, H.; González-Morán, C.O.; Rivera Olvera, J.N.; Estrada-Guel, I.; Botello Villa, F. Synthesis and Characterization of Zn-Ni Advanced Alloys Prepared by Mechanical Milling and Sintering at Solid-State Process. Adv. Mater. Sci. Eng. 2017, 2017, 7967848. [Google Scholar] [CrossRef] [Green Version]
- Salleh, E.M.; Ramakrishnan, S.; Hussain, Z. Synthesis of Biodegradable Mg-Zn Alloy by Mechanical Alloying: Effect of Milling Time. Procedia Chem. 2016, 19, 525–530. [Google Scholar] [CrossRef] [Green Version]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Ivanov, E.; Boldyrev, V.V. The science and technology of mechanical alloying. Mater. Sci. Eng. A 2001, 304–306, 151–158. [Google Scholar] [CrossRef]
- Sikora-Jasinska, M.; Mostaed, E.; Mostaed, A.; Beanland, R.; Mantovani, D.; Vedani, M. Fabrication, mechanical properties and in vitro degradation behavior of newly developed ZnAg alloys for degradable implant applications. Mater. Sci. Eng. C 2017, 77, 1170–1181. [Google Scholar] [CrossRef]
- Bowen, P.; Seitz, J.-M.; Guillory, R.J.; Braykovich, J.; Zhao, S.; Goldman, J. Evaluation of wrought Zn-Al alloys (1, 3, and 5 wt.% Al) through mechanical and in vivo testing for stent applications. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2017, 106, 245–258. [Google Scholar] [CrossRef]
- Krystýnová, M.; Doležal, P.; Fintová, S.; Březina, M.; Zapletal, J.; Wasserbauer, J. Preparation and Characterization of Zinc Materials Prepared by Powder Metallurgy. Metals 2017, 7, 396. [Google Scholar] [CrossRef]
- Ali, A.; Kolawole, M.; Aweda, J.O.; Abdulkareem, S. Mechanical Properties of Powder Metallurgy Processed Biodegradable Zn-Based Alloy for Biomedical Application. J. Mater. Metall. Eng. 2019, 13, 558–563. [Google Scholar]
- Choi, H.J.; Lee, S.W.; Park, J.S.; Bae, D.H. Tensile behavior of bulk nanocrystalline aluminum synthesized by hot extrusion of ball-milled powders. Scr. Mater. 2008, 59, 1123–1126. [Google Scholar] [CrossRef]
- Qiu, C.L.; Attallah, M.M.; Wu, X.H.; Andrews, P. Influence of hot isostatic pressing temperature on microstructure and tensile properties of a nickel-based superalloy powder. Mater. Sci. Eng. A 2013, 564, 176–185. [Google Scholar] [CrossRef]
- Tang, F.; Anderson, I.E.; Gnaupel-Herold, T.; Prask, H. Pure Al matrix composites produced by vacuum hot pressing: Tensile properties and strengthening mechanisms. Mater. Sci. Eng. A 2004, 383, 362–373. [Google Scholar] [CrossRef]
- Cui, Z.; Luo, M.; Zhang, Y.; Gong, D.; Wang, W.; Wang, J. Fabrication of high strength and plasticity of Zn-Mg composites with core–shell structure by spark plasma sintering. Mater. Lett. 2020, 279, 128525. [Google Scholar] [CrossRef]
- Cheng, Y.; Cui, Z.; Cheng, L.; Gong, D.; Wang, W. Effect of particle size on densification of pure magnesium during spark plasma sintering. Adv. Powder Technol. 2017, 28, 1129–1135. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Yang, Y.; Yang, M.; Zheng, H.; Xie, D. Laser Additive Manufacturing of Zinc Targeting for Biomedical Application. Int. J. Bioprinting 2022, 8, 501. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Jauer, L.; Voshage, M.; Chen, Y.; Poprawe, R.; Schleifenbaum, J.H. Densification behavior of pure Zn metal parts produced by selective laser melting for manufacturing biodegradable implants. J. Mater. Processing Technol. 2018, 258, 128–137. [Google Scholar] [CrossRef]
- Chua, K.; Khan, I.; Malhotra, R.; Zhu, D. Additive manufacturing and 3D printing of metallic biomaterials. Eng. Regen. 2021, 2, 288–299. [Google Scholar] [CrossRef]
- Pinc, J.; Školáková, A.; Veřtát, P.; Čapek, J.; Žofková, Z.; Rieszová, L. Microstructural characterization and optimization of the ZnMg0.8(CaO)0.26 alloy processed by ball milling and subsequent extrusion. Manuf. Technol. 2020, 20, 484–491. [Google Scholar] [CrossRef]
- Rocha, C.; Leal Neto, R.; Gonçalves, V.; Carvalho, L.L.; Ambrozio, F. An Investigation of the Use of Stearic Acid as a Process Control Agent in High Energy Ball Milling of Nb-Al and Ni-Al Powder Mixtures; Transtec Publications: Bäch, Switzerland, 2003; Volume 416, pp. 144–149. [Google Scholar]
- Hernández-Escobar, D.; Champagne, S.; Yilmazer, H.; Dikici, B.; Boehlert, J.; Hermawan, H. Microstructural evolution and intermetallic formation in Zn-Mg hybrids processed by High-Pressure Torsion. Philos. Mag. 2019, 99, 557–584. [Google Scholar] [CrossRef]
- Jin, H.; Zhao, S.; Guillory, R.; Bowen, P.K.; Yin, Z.; Griebel, A. Novel high-strength, low-alloys Zn-Mg (<0.1 wt.% Mg) and their arterial biodegradation. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 84, 67–79. [Google Scholar] [CrossRef]
- Pinc, J.; Školáková, A.; Veřtát, P.; Duchoň, J.; Kubásek, J.; Lejček, P. Microstructure evolution and mechanical performance of ternary Zn-0.8Mg-0.2Sr (wt.%) alloy processed by equal-channel angular pressing. Mater. Sci. Eng. A 2021, 824, 141809. [Google Scholar] [CrossRef]
- Dambatta, M.S.; Izman, S.; Kurniawan, D.; Hermawan, H. Processing of Zn-3Mg alloy by equal channel angular pressing for biodegradable metal implants. J. King Saud Univ. Sci. 2017, 29, 455–461. [Google Scholar] [CrossRef]
- Kubásek, J.; Pinc, J.; Hosová, K.; Straková, M.; Molnárová, O.; Duchoň, J. The evolution of microstructure and mechanical properties of Zn-0.8Mg-0.2Sr alloy prepared by casting and extrusion. J. Alloys Compd. 2022, 906, 164308. [Google Scholar] [CrossRef]
- Gong, H.; Wang, K.; Strich, R.; Zhou, J.G. In vitro biodegradation behavior, mechanical properties, and cytotoxicity of biodegradable Zn–Mg alloy. J. Biomed. Mater. Res. Part. Appl. Biomater. 2015, 103, 1632–1640. [Google Scholar] [CrossRef] [Green Version]
- Guleryuz, L.F.; Wang, K.; Strich, R.; Zhou, J.G. Microstructure and mechanical properties of Zn-Mg alloys as implant materials manufactured by powder metallurgy method. AIP Conf. Proc. 2017, 1809, 020020. [Google Scholar]
- Bi, G.; Han, Y.; Jiang, J.; Zhang, D.; Qiu, D. Microstructure and mechanical properties of an extruded Mg-Dy-Ni alloy. Mater. Sci. Eng. A 2019, 760, 246–257. [Google Scholar] [CrossRef]
- Cáceres, C.H.; Davidson, C.J.; Griffiths, J.R.; Newton, C.J. Effects of solidification rate and ageing on the microstructure and mechanical properties of AZ91 alloy. Mater. Sci. Eng. A 2002, 325, 344–355. [Google Scholar] [CrossRef]
- Zhou, Y.; Lou, Q.; Jiang, B.; Li, Q.; Pan, F. Strength-ductility synergy in Mg98.3Y1.3Ni0.4 alloy processed by high temperature homogenization and rolling. Scr. Mater. 2022, 208, 114345. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, D.; Wang, F.; Taylor, J.A.; Zhang, M. Effect of Grain Refinement on Tensile Properties of Cast Zinc Alloys. Metall. Mater. Trans. A 2016, 47, 830–841. [Google Scholar] [CrossRef]
- Chen, C.; Fan, S.; Niu, J.; Huang, H.; Jin, Z.; Kong, L. Alloying design strategy for biodegradable zinc alloys based on first-principles study of solid solution strengthening. Mater. Des. 2021, 204, 109676. [Google Scholar] [CrossRef]
- Cahoon, J.R.; Broughton, W.H.; Kutzak, A.R. The determination of yield strength from hardness measurements. Metall. Trans. 1971, 2, 1979–1983. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, C.; Li, G.; Zheng, Y.; Nie, J.-F. Microstructure, mechanical properties and creep behavior of extruded Zn-xLi (x = 0.1, 0.3 and 0.4) alloys for biodegradable vascular stent applications. Mater. Sci. Eng. A 2020, 777, 139082. [Google Scholar] [CrossRef]
- Kubásek, J.; Dvorský, D.; Čapek, J.; Pinc, J.; Vojtěch, D. Zn-Mg Biodegradable Composite: Novel Material with Tailored Mechanical and Corrosion Properties. Materials 2019, 12, 3930. [Google Scholar] [CrossRef] [Green Version]
- Dvorsky, D.; Kubasek, J.; Vojtech, D. A new approach in the preparation of biodegradable Mg-MgF2 composites with tailored corrosion and mechanical properties by powder metallurgy. Mater. Lett. 2018, 227, 78–81. [Google Scholar] [CrossRef]
- Dvorský, D.; Kubásek, J.; Kristianová, E.; Vojtěch, D. Improved corrosion resistance of WE43 magnesium alloy with continuous network of MgF2 prepared by powder metallurgy. IOP Conf. Series: Mater. Sci. Eng. 2018, 461, 012016. [Google Scholar] [CrossRef]
- Dvorský, D.; Kubásek, J.; Roudnická, M.; Průša, F.; Nečas, D.; Minárik, P. The effect of powder size on the mechanical and corrosion properties and the ignition temperature of WE43 alloy prepared by spark plasma sintering. J. Magnes. Alloy. 2021, 9, 1349–1362. [Google Scholar] [CrossRef]
- Průša, F.; Vojtěch, D.; Kučera, V. Příprava ultrajemnozrných a nanokrystalických materiálů mechanických legováním a slinováním v plazmatu. Chem. Listy 2017, 111, 314–321. [Google Scholar]
- Yang, Y.; Yuan, F.; Gao, C.; Feng, P.; Xue, L.; He, S. A combined strategy to enhance the properties of Zn by laser rapid solidification and laser alloying. J. Mech. Behav. Biomed. Mater. 2018, 82, 51–60. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, H.; Fang, H.; Yu, K.; Zhang, Z.; Xu, X. Effects of the Intermetallic Phases on Microstructure and Properties of Biodegradable Magnesium Matrix and Zinc Matrix Prepared by Powder Metallurgy. Mater. Trans. 2018, 59, 1837–1844. [Google Scholar] [CrossRef] [Green Version]

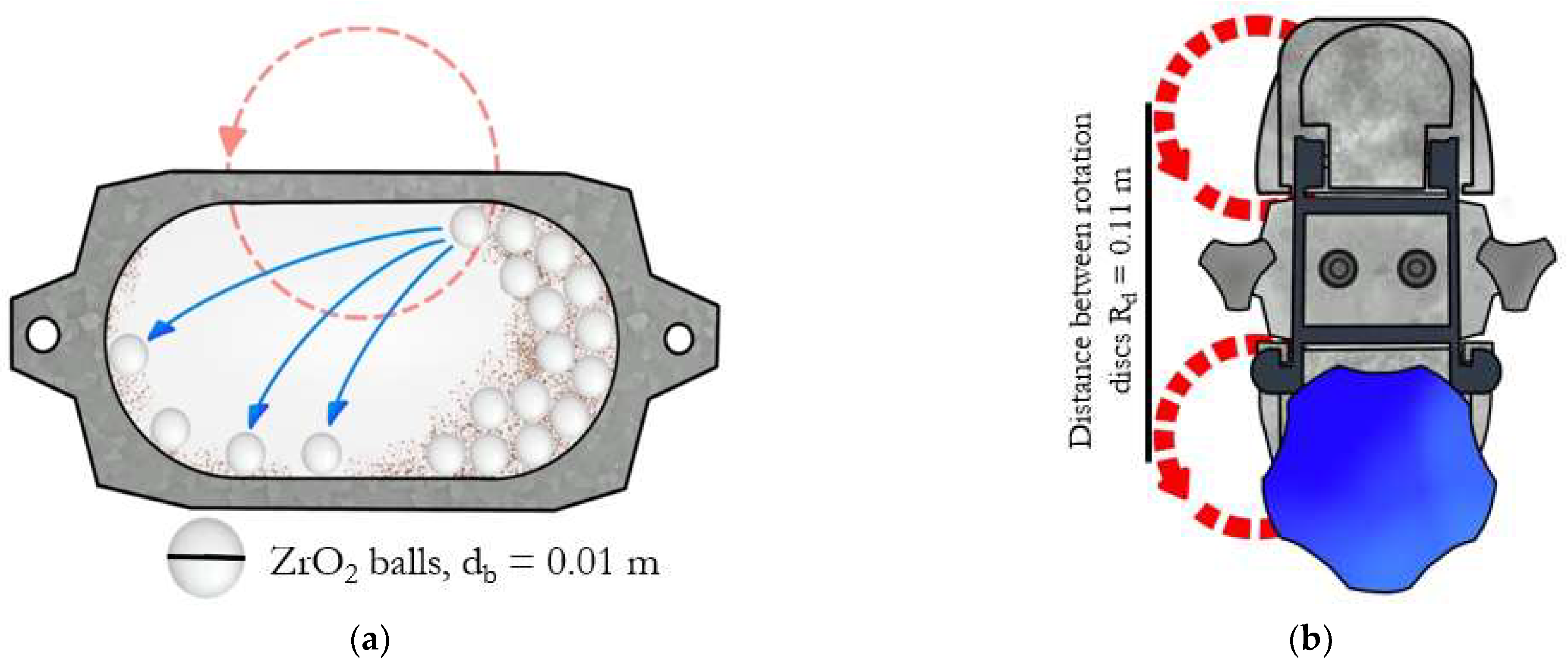
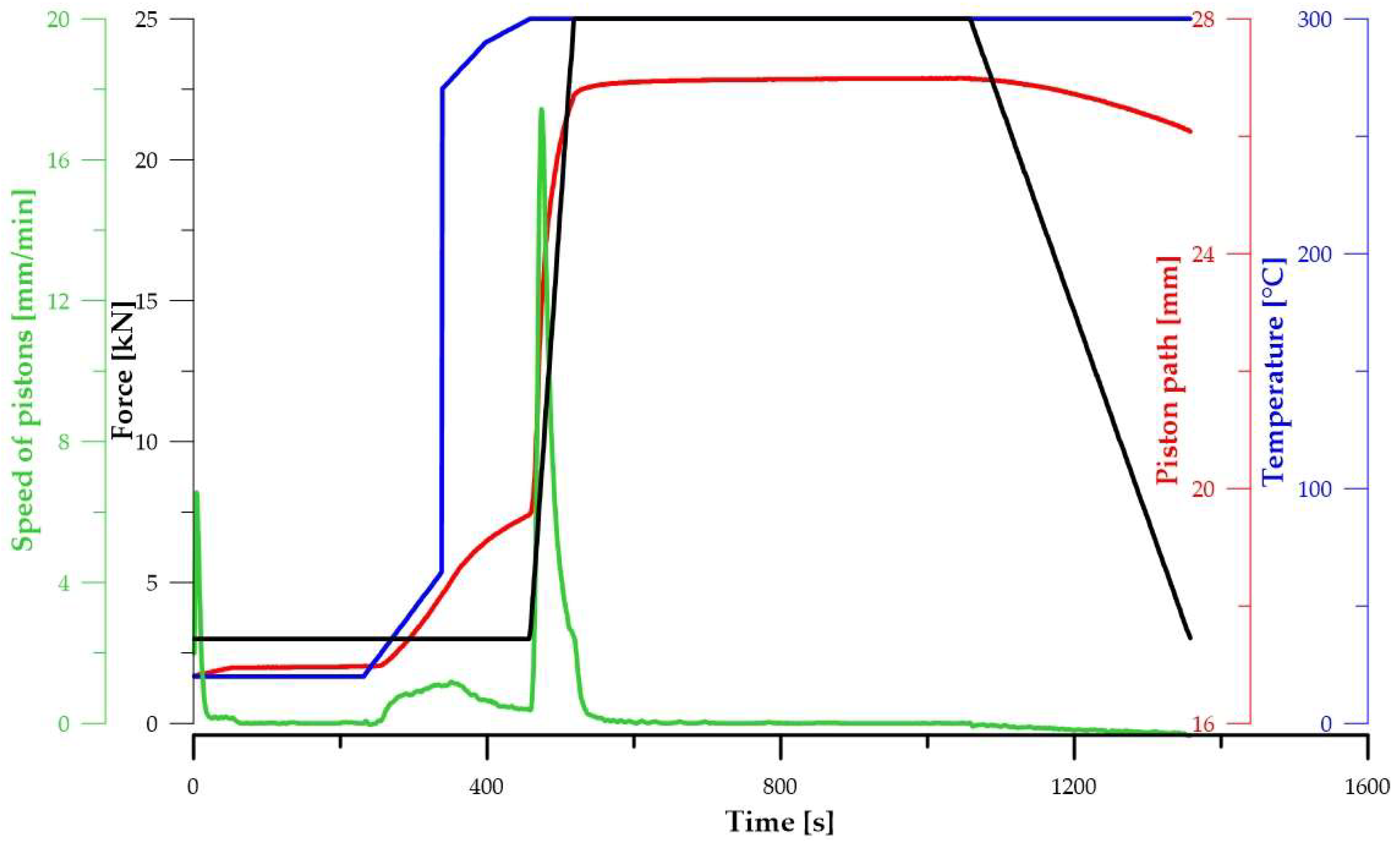
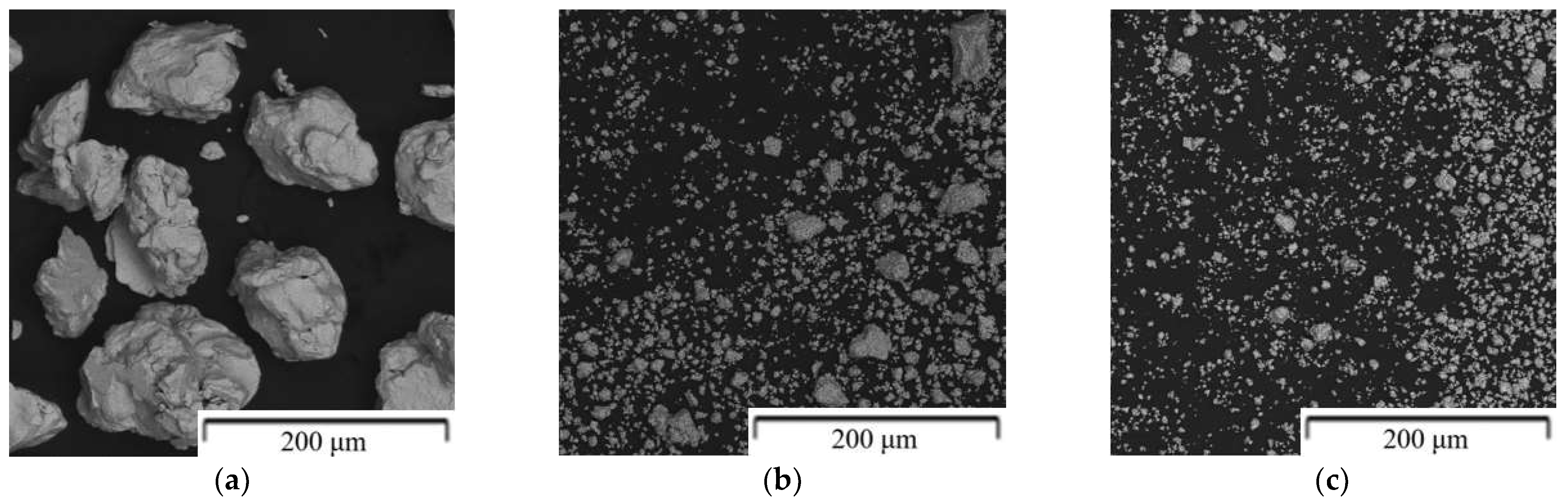
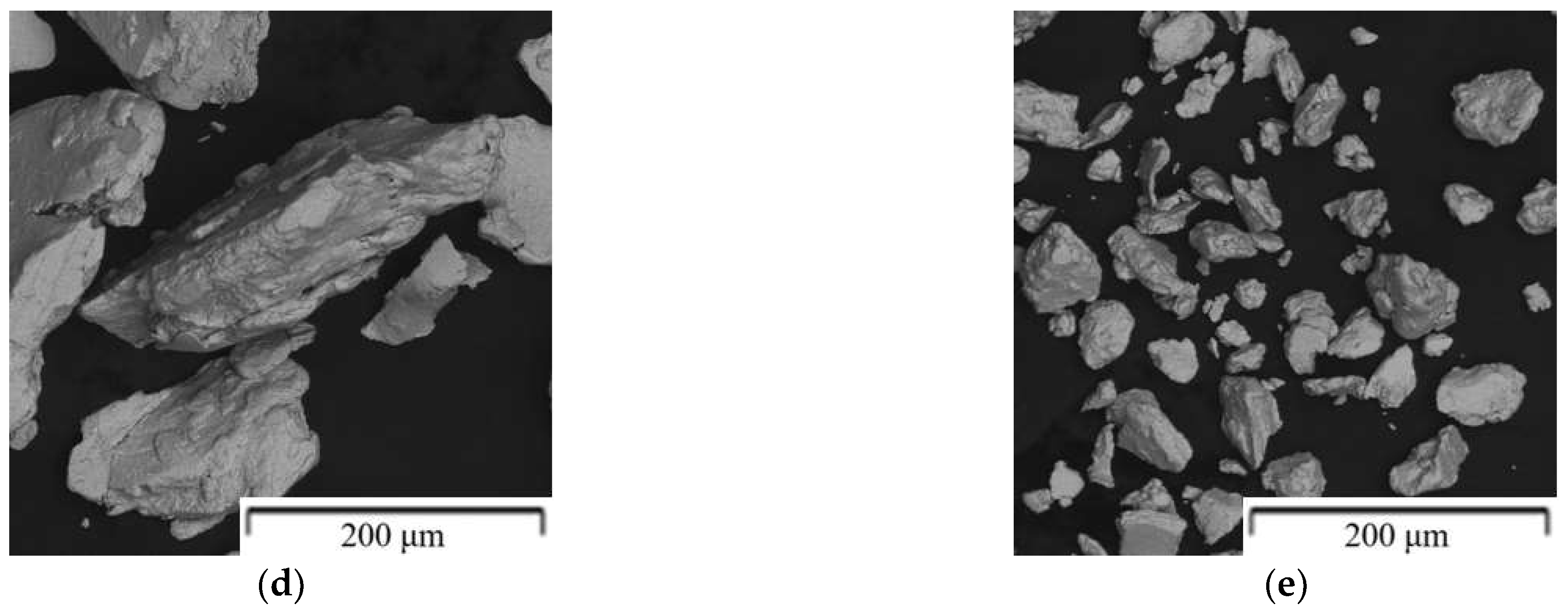

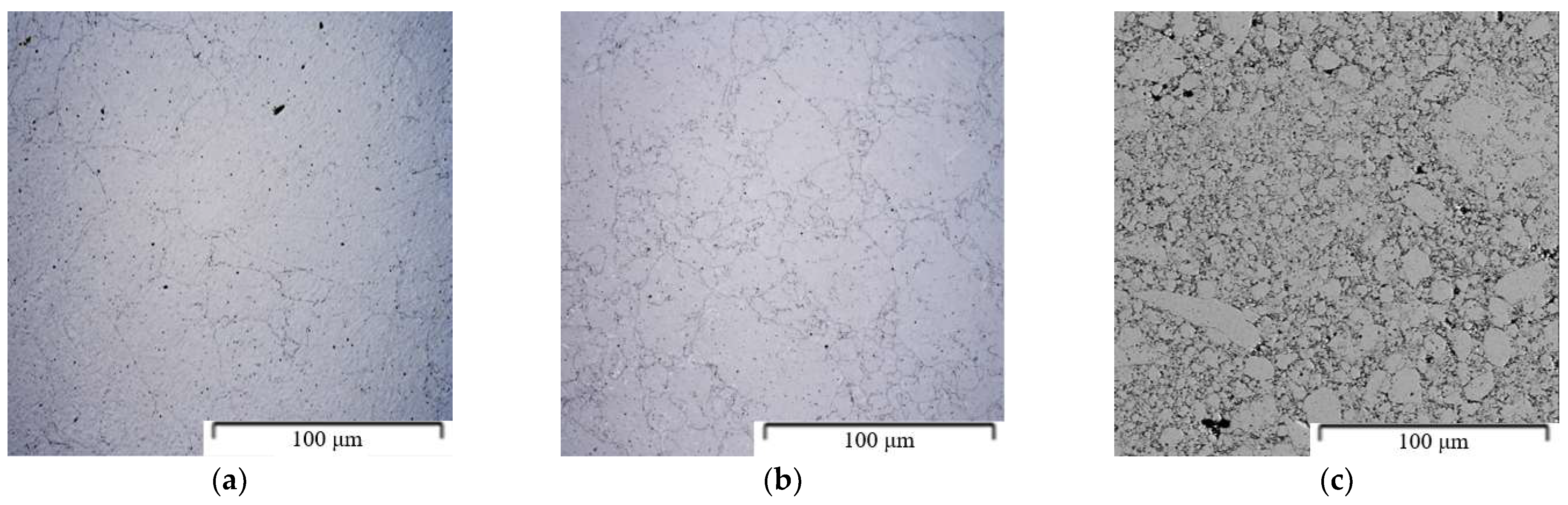
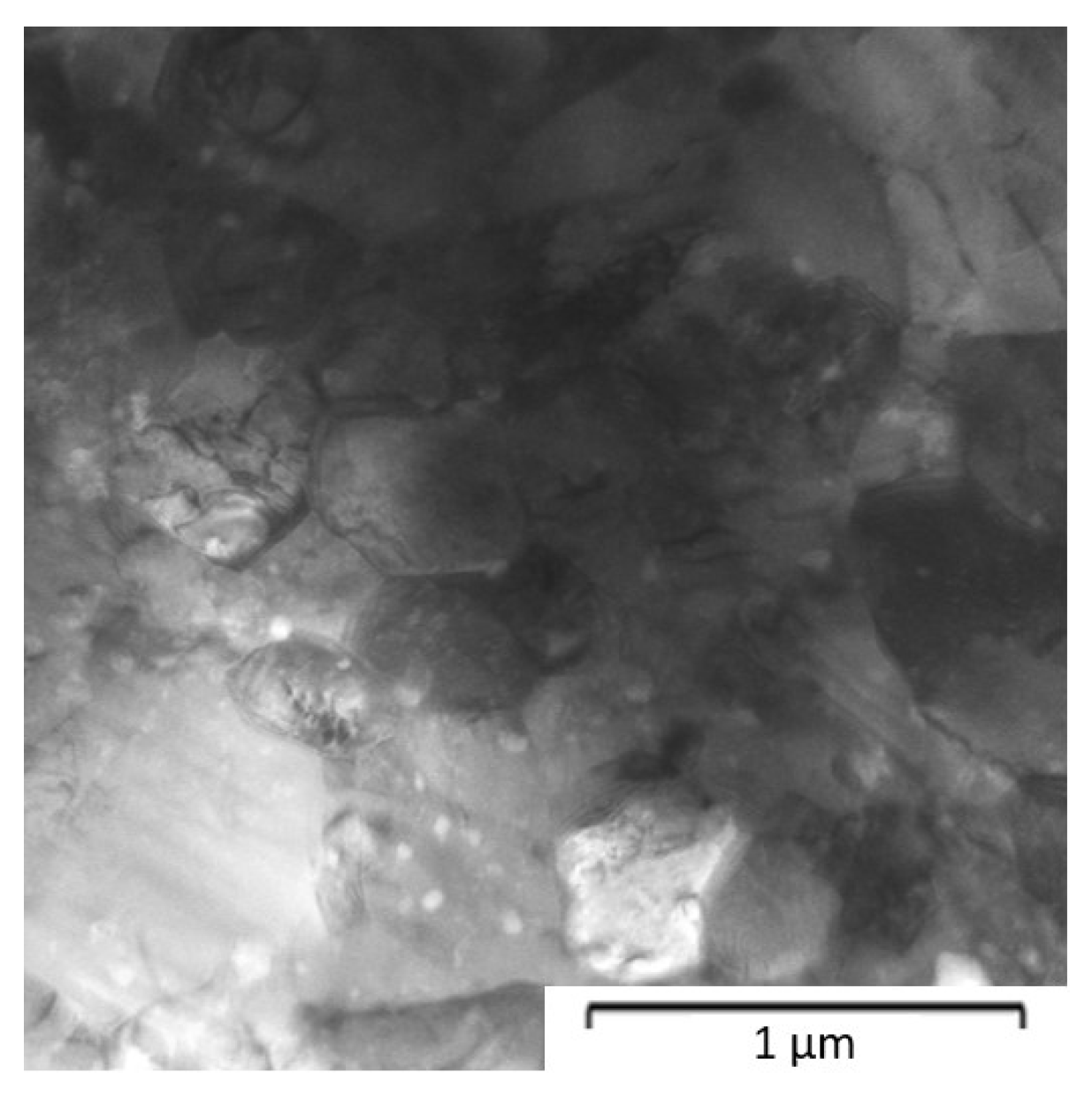
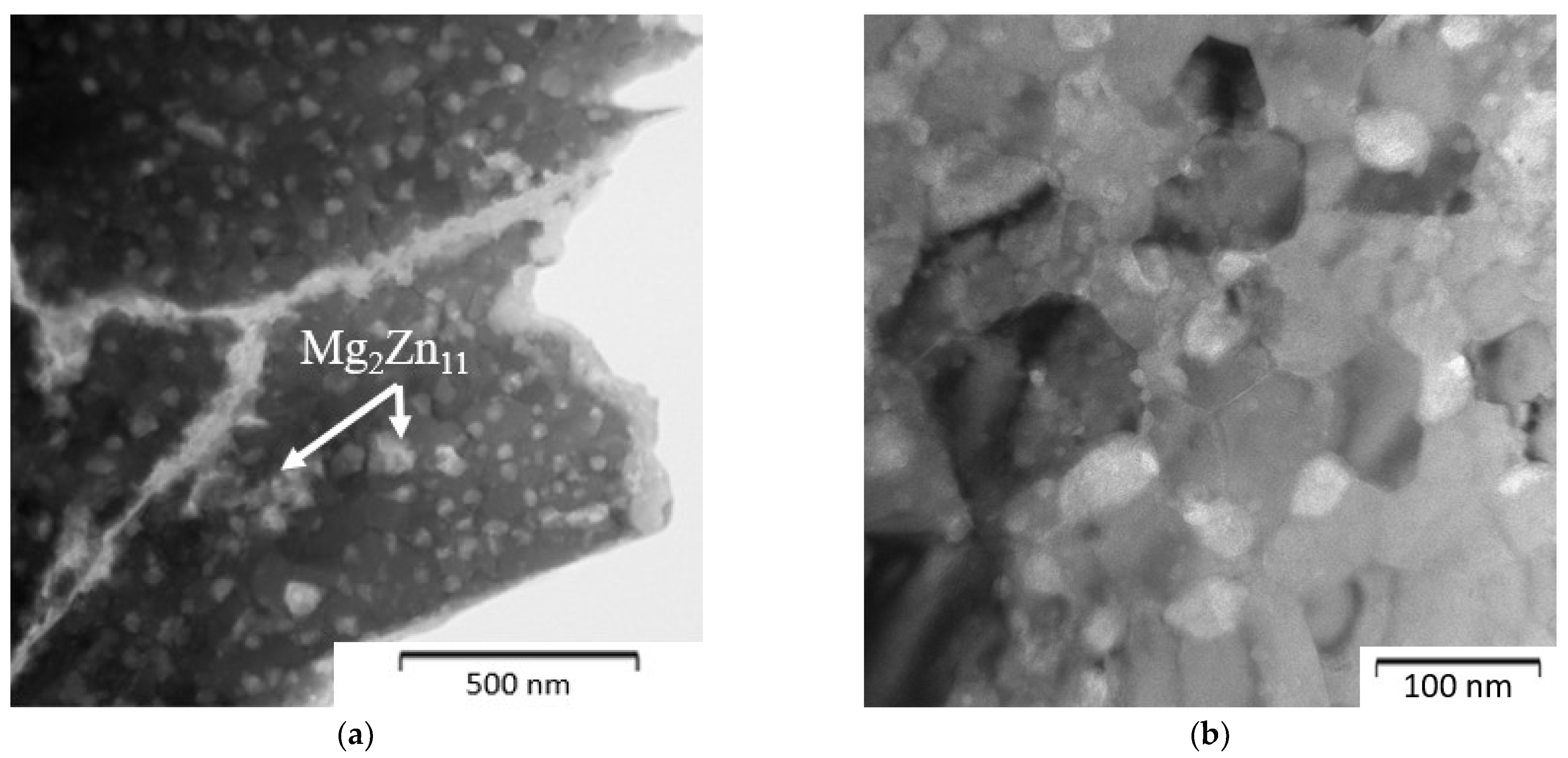
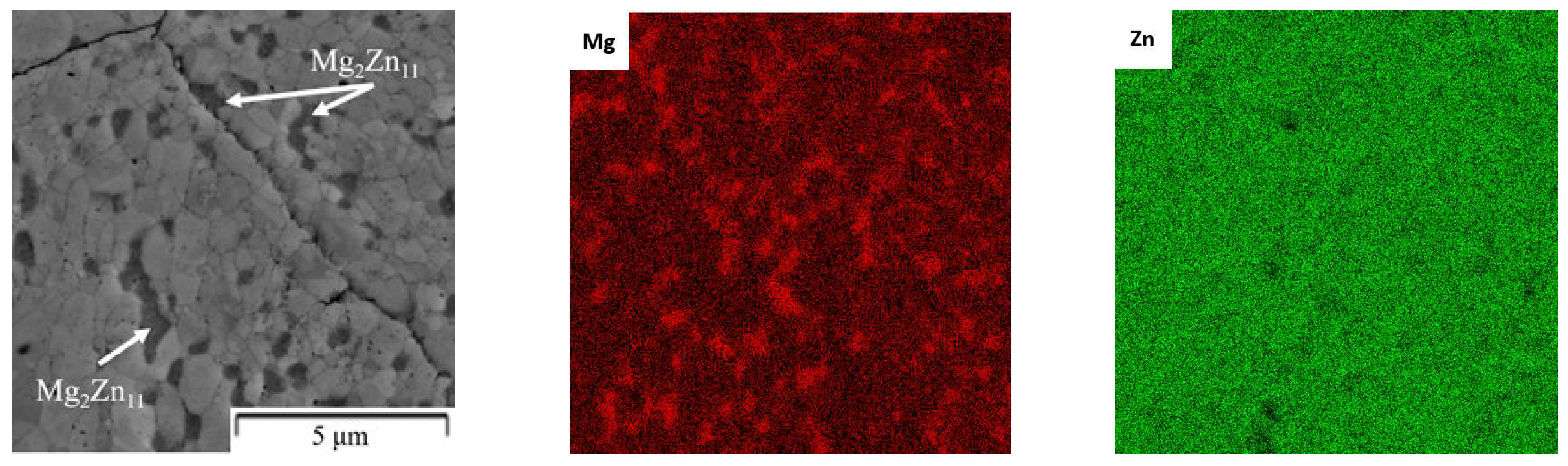

| Composition of the Alloy | Amount of Stearic Acid [g] | RPM [Rotation/min] | Time [min] | Balls/Powder Weight Ratio | Selected for Compaction |
|---|---|---|---|---|---|
| Zn | - | - | - | - | ✔ |
| Zn-1Mg | - | 400 | 10 | 10:1 | - |
| Zn-1Mg | - | 800 | 10 | 10:1 | - |
| Zn-1Mg | - | 1200 | 10 | 10:1 | - |
| Zn-1Mg | - | 1200 | 30 | 10:1 | - |
| Zn-1Mg | 0.08 | 1200 | 30 | 10:1 | - |
| Zn-1Mg | 0.08 | 1200 | 60 | 5:1 | ✔ |
| Zn-1Mg | 0.08 | 800 | 120 | 5:1 | - |
| Zn-6Mg | 0.08 | 1200 | 60 | 5:1 | ✔ |
| Zn-16Mg | 0.08 | 1200 | 60 | 5:1 | ✔ |
| RPM [Rot./min] | Angular Speed ωd [Rad/s] | Deformation Energy EC [kJ] |
|---|---|---|
| 400 | 42 | 271 |
| 800 | 84 | 622 |
| 1200 | 126 | 1012 |
| Composition (Weight of Milling Balls: Powder) | RPM [Rot./min] | Milling Time [min] | Zn [wt.%] | Mg2Zn11 [wt.%] | MgZn2 [wt.%] | Mg [wt.%] |
|---|---|---|---|---|---|---|
| Zn | - | - | 100 | - | - | - |
| Mg | - | - | - | - | - | 100 |
| Zn-1Mg (10:1) | 400 | 10 | 99 | 1 | ||
| Zn-1Mg (10:1) | 800 | 10 | 94 | 5 | 1 | - |
| Zn-1Mg (10:1) | 1200 | 10 | 94 | 6 | - | |
| Zn-1Mg (5:1) | 1200 | 30 | 100 | - | ||
| Zn-1Mg (5:1) * | 1200 | 30 | 99 | 2 | <1 | |
| Zn-1Mg (5:1) * | 800 | 60 | 97 | 3 | - | <1 |
| Zn-1Mg (5:1) * | 1200 | 60 | 94 | 6 | - | |
| Zn-1Mg (5:1) * | 800 | 120 | 93 | 7 | - | |
| Zn-6Mg (5:1) * | 1200 | 60 | 33 | 65 | 2 | - |
| Zn-16Mg (5:1) * | 1200 | 60 | 6 | - | 94 | - |
| Composition | RPM/Milling Time [min] | HV1 | Compression Test | 3-Point Bend Test | |||
|---|---|---|---|---|---|---|---|
| σCYS [MPa] | σUCS [MPa] | σYS [MPa] | σUS [MPa] | Strain [%] | |||
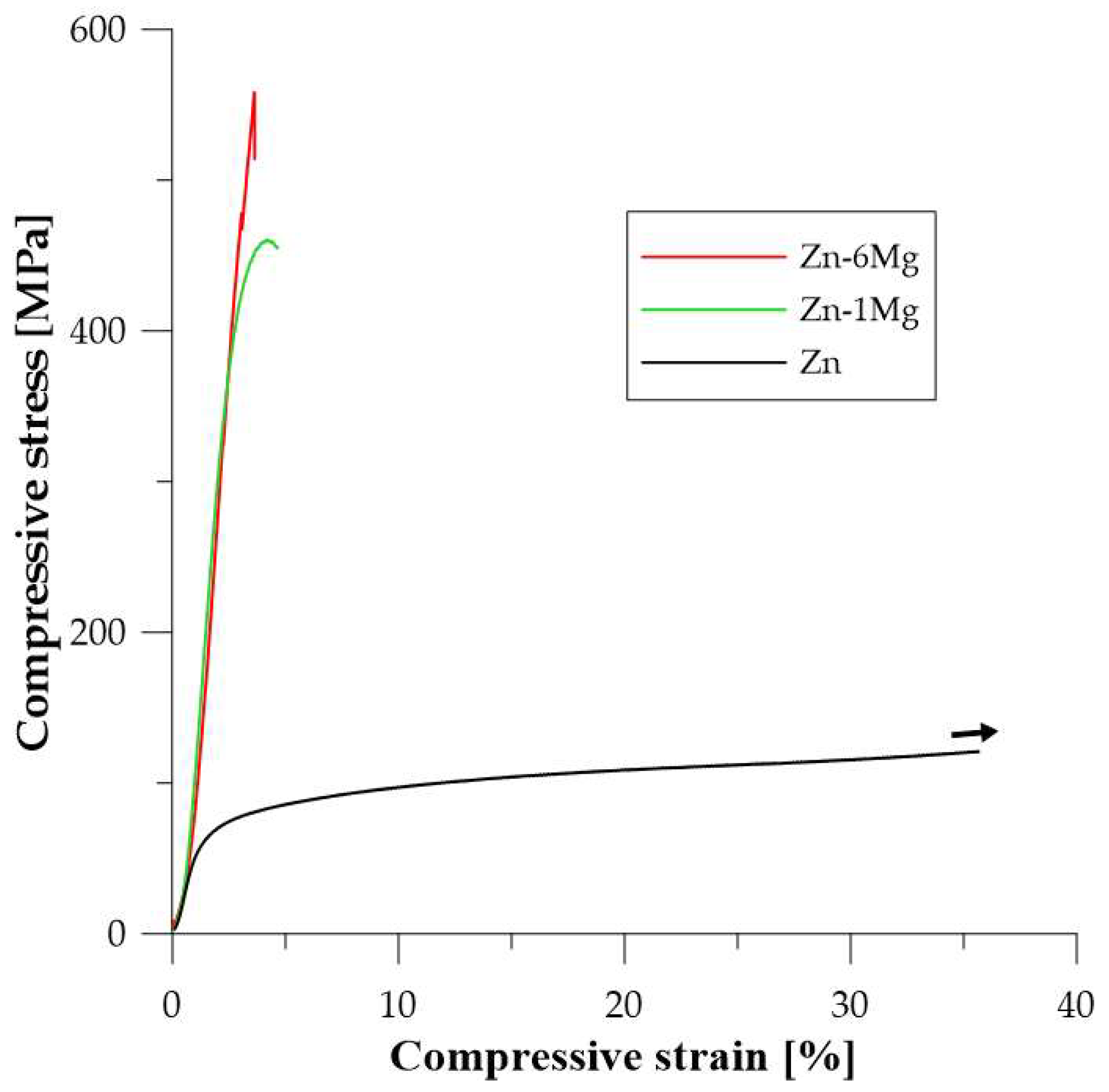
| Zn | - | 38 ± 2 | 86 ± 2 | - | 104 ± 13 | 136 ± 12 | 0.73 ± 0.11 |
| Zn-1 Mg * | 1200/60 min | 123 ± 2 | 361 ± 3 | 436 ± 6 | - | 59 ± 5 | 0.07 ± 0.01 |
| Zn-6 Mg * | 1200/60 min | 271 ± 12 | - | 574 ± 29 | - | 74 ± 15 | 0.02 ± 0.01 |
| Zn-16 Mg * | 1200/60 min | 222 ± 12 | - | 241 | - | - | - |
| Composition | Preparation | Hardness | σCYS [MPa] | σUCS [MPa] | E [%] | Ref. |
|---|---|---|---|---|---|---|
| Zn | MA & SPS | 38 ± 2 | 86 ± 2 | - | - | This work |
| Zn-1Mg | 123 ± 2 | 361 ± 3 | 436 ± 6 | - | ||
| Zn-6Mg | 271 ± 12 | - | 574 ± 29 | - | ||
| Zn-1Mg | MA, Cold press, sintered, forged | 81 ± 5 | - | 245 ± 12 | 5.6 ± 1.4 | [58] |
| Zn | MA, Hydrostatic pressing & sintering | 40 ± 1 | - | 100 ± 35 | - | [28] |
| Zn-1Mg | 60 ± 23 | - | 178 ± 13 | - | ||
| Zn-6Mg | 41 ± 1 | - | 122 ± 10 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nečas, D.; Marek, I.; Pinc, J.; Vojtěch, D.; Kubásek, J. Advanced Zinc–Magnesium Alloys Prepared by Mechanical Alloying and Spark Plasma Sintering. Materials 2022, 15, 5272. https://doi.org/10.3390/ma15155272
Nečas D, Marek I, Pinc J, Vojtěch D, Kubásek J. Advanced Zinc–Magnesium Alloys Prepared by Mechanical Alloying and Spark Plasma Sintering. Materials. 2022; 15(15):5272. https://doi.org/10.3390/ma15155272
Chicago/Turabian StyleNečas, David, Ivo Marek, Jan Pinc, Dalibor Vojtěch, and Jiří Kubásek. 2022. "Advanced Zinc–Magnesium Alloys Prepared by Mechanical Alloying and Spark Plasma Sintering" Materials 15, no. 15: 5272. https://doi.org/10.3390/ma15155272
APA StyleNečas, D., Marek, I., Pinc, J., Vojtěch, D., & Kubásek, J. (2022). Advanced Zinc–Magnesium Alloys Prepared by Mechanical Alloying and Spark Plasma Sintering. Materials, 15(15), 5272. https://doi.org/10.3390/ma15155272






