Properties of Titanium Zirconium Molybdenum Alloy after Exposure to Indium at Elevated Temperatures
Abstract
:1. Introduction
2. Materials and Experimental Procedures
2.1. Chemical Reactivity and Compound Formation
2.2. TZM Sample Preparation
2.3. Metallographic Samples
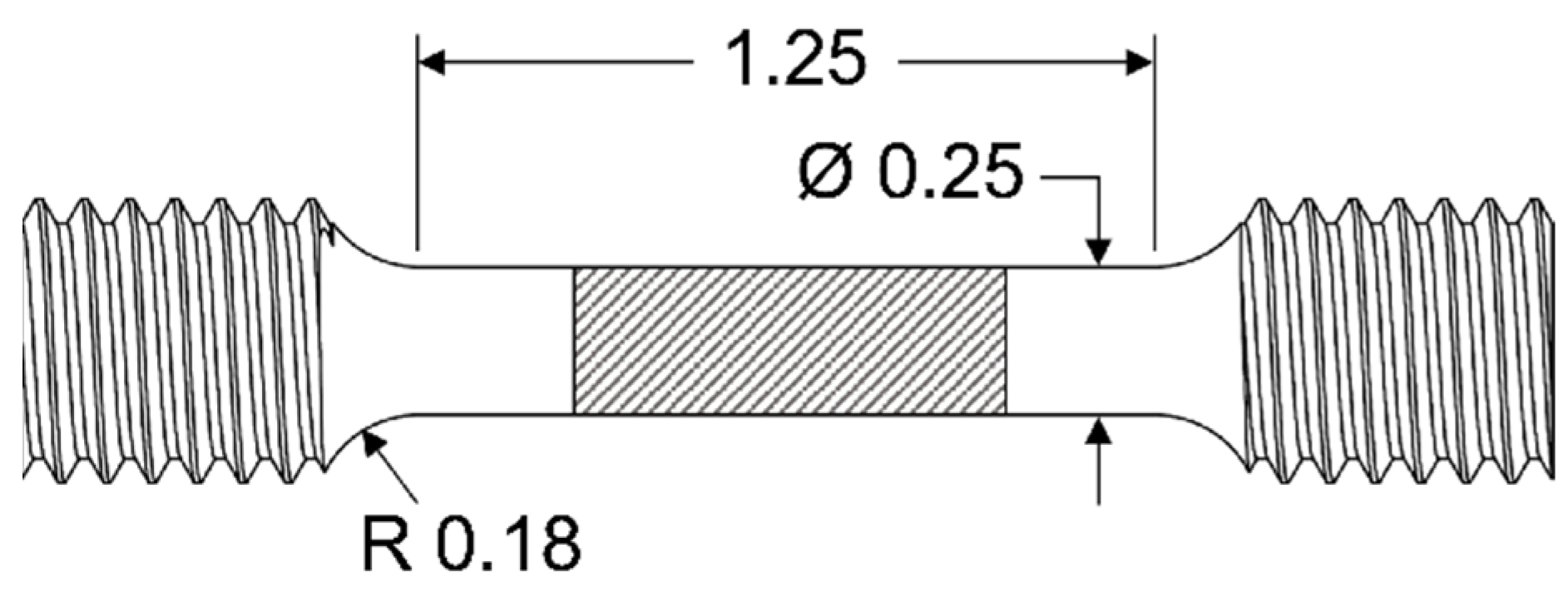
2.4. Tensile Samples
2.5. Fracture Toughness Testing
3. Results and Discussion
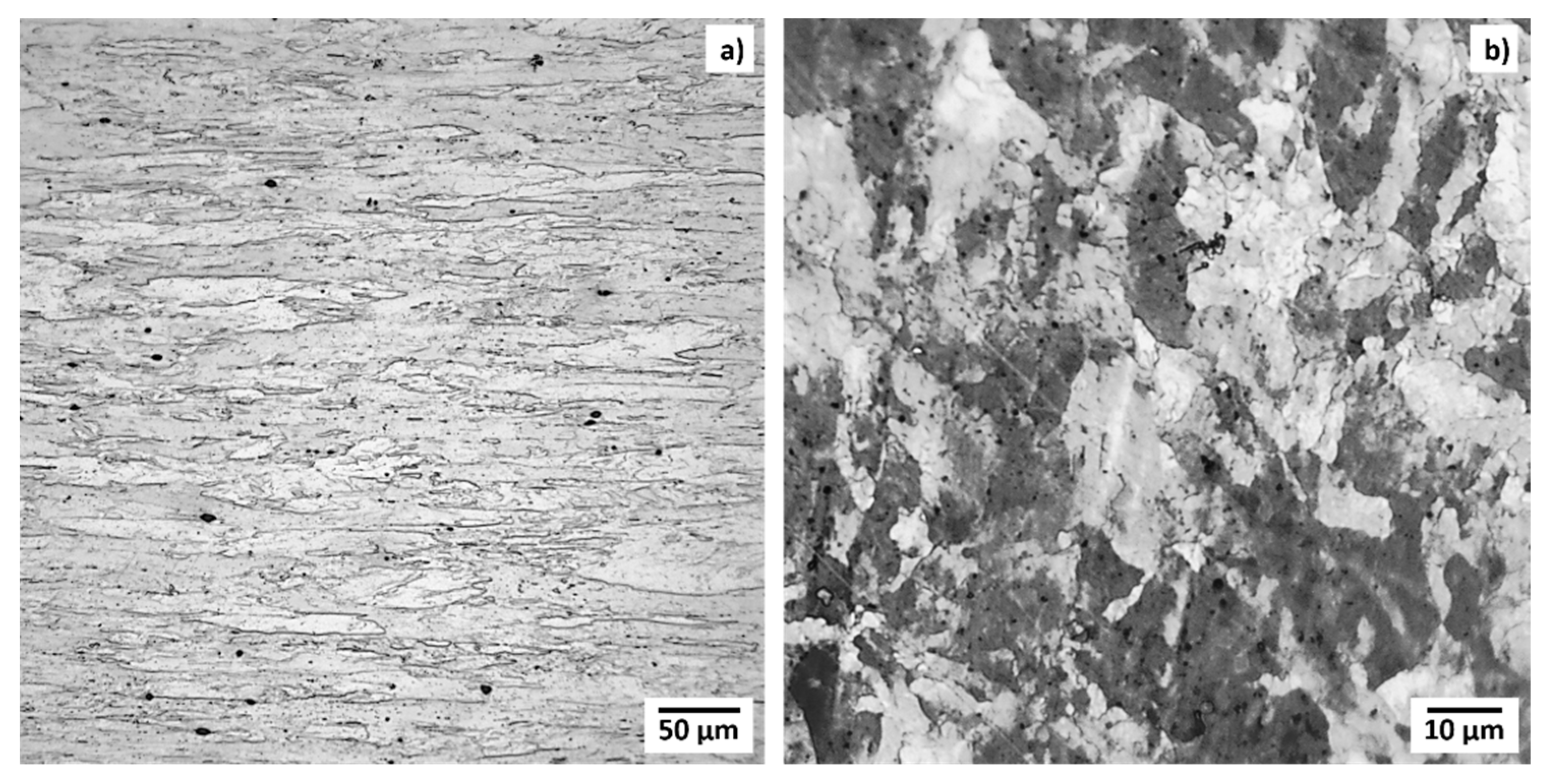
3.1. Metallographic Characterization
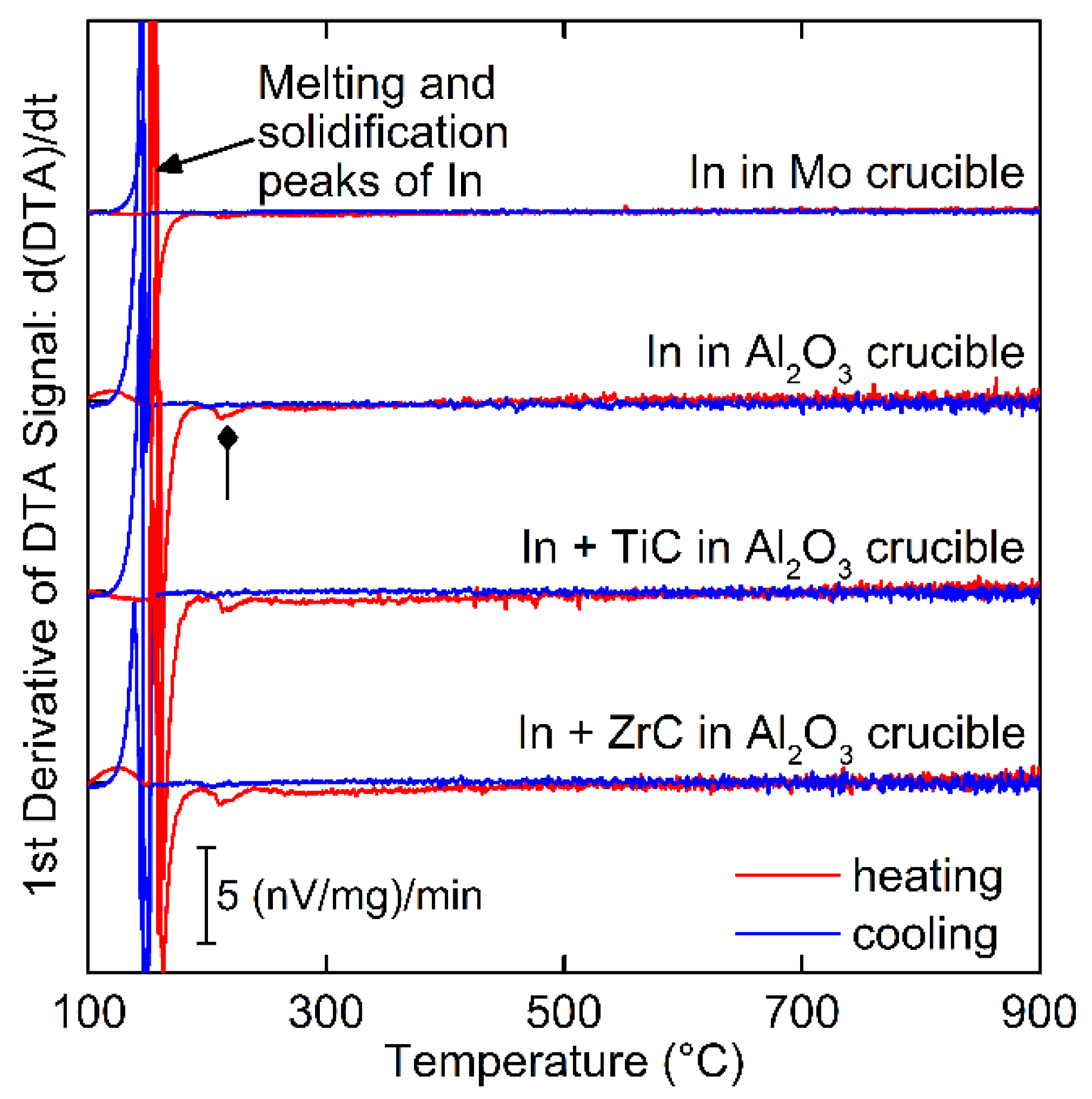
3.2. Chemical Reactivity and Compound Formation
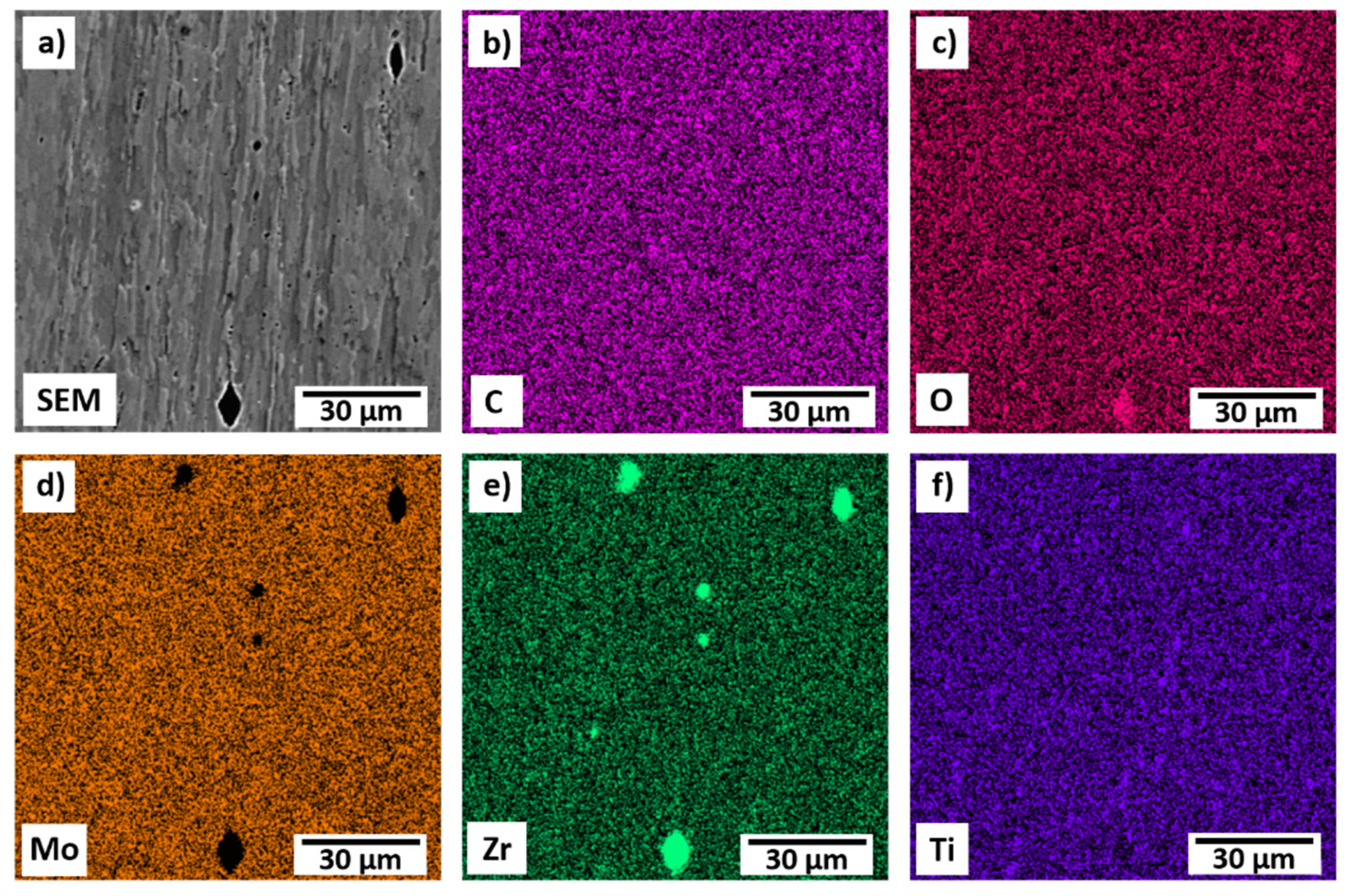
3.3. TZM-Indium Interface
3.4. Mechanical Properties
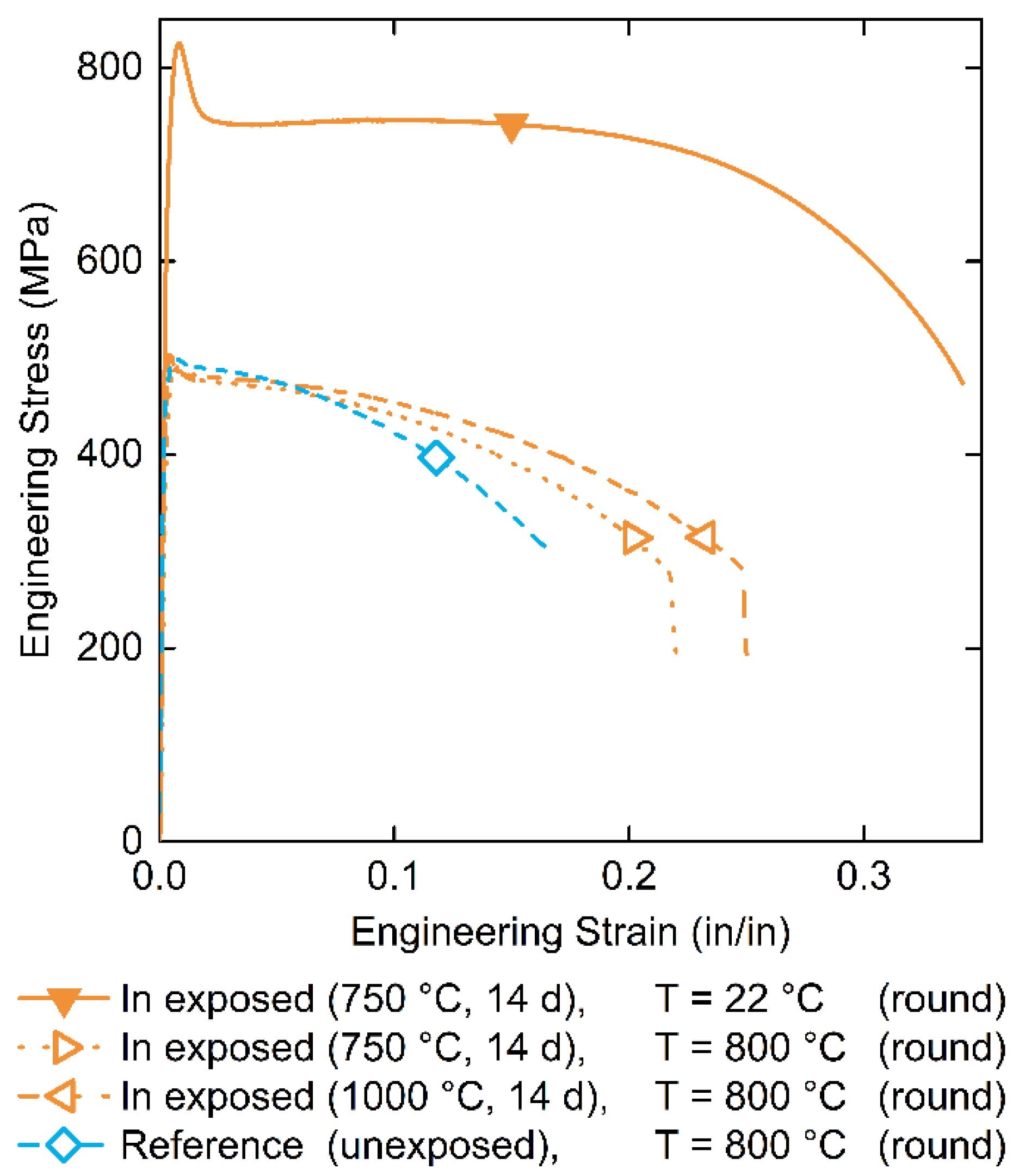
3.4.1. Tensile Properties
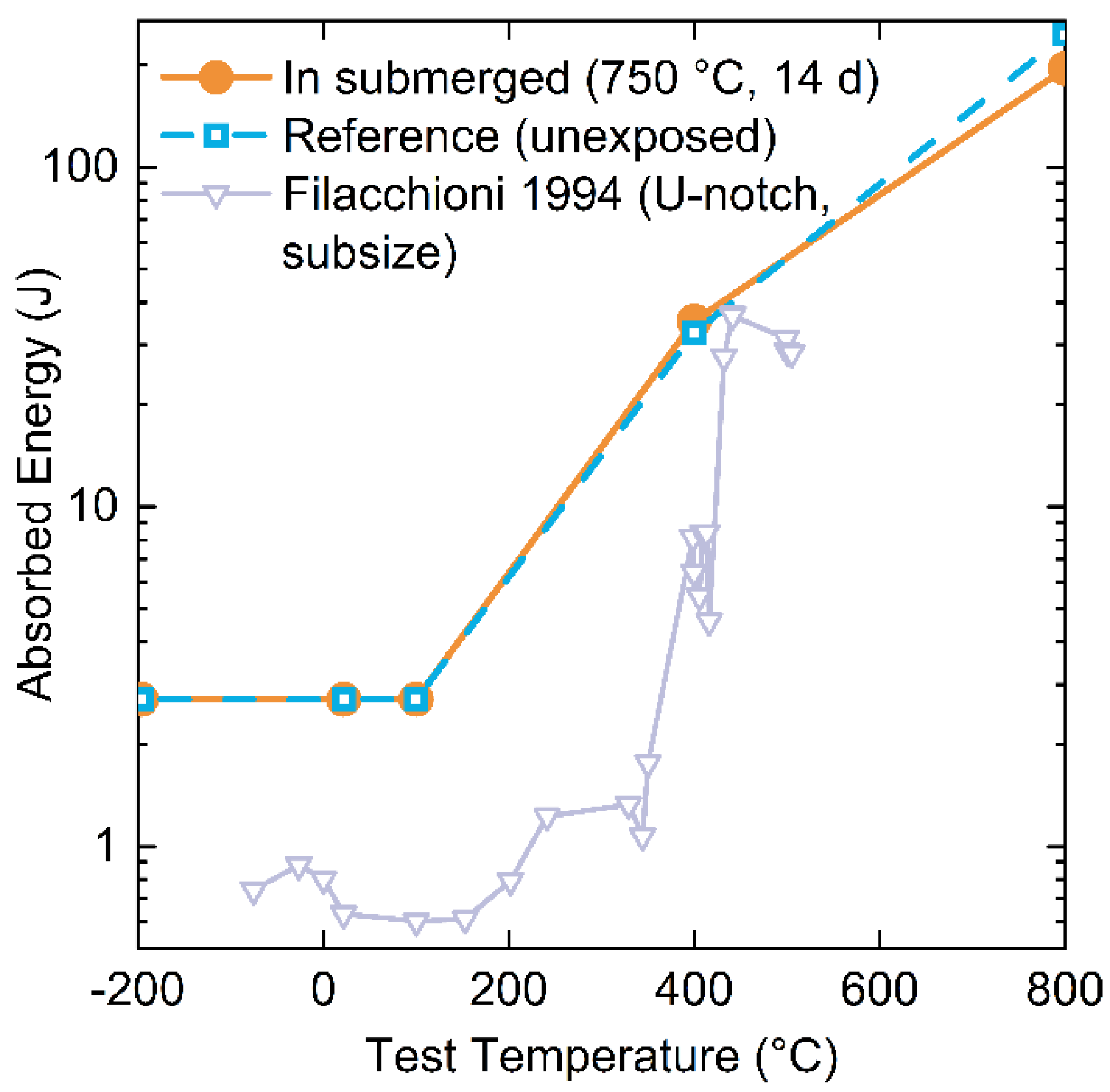
3.4.2. Fracture Toughness
4. Summary
- The TZM microstructure, as analyzed by LOM and SEM, did not reveal any changes or detrimental corrosive interactions with the constituents of TZM. No surface corrosion or other interactions with In were observed in the samples.
- No compounds were found to form between In and pure Mo, TiC, or ZrC at temperatures up to 900 °C using DTA and XRD.
- Chemical analysis of the TZM-In interface using EDS and XRD indicated no In diffused into the TZM sample. An upper bound for the diffusion coefficient was estimated to be 4.13 × 10−19 m2 s−1 at 1000 °C.
- Mechanical properties measured using tensile testing and Charpy V-notch bars indicated no improvement or degradation in yield strength, ultimate tensile strength, elongation to failure, and fracture toughness. Anticipated temperature-dependent trends for mechanical properties based on literature observations were followed from samples from this study.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cockeram, B.; Smith, R.; Snead, L. The influence of fast neutron irradiation and irradiation temperature on the tensile properties of wrought LCAC and TZM molybdenum. J. Nucl. Mater. 2005, 346, 145–164. [Google Scholar] [CrossRef]
- Chakraborty, S.; Banerjee, S.; Singh, K.; Sharma, I.; Grover, A.; Suri, A. Studies on the development of protective coating on TZM alloy and its subsequent characterization. J. Mater. Process. Technol. 2008, 207, 240–247. [Google Scholar] [CrossRef]
- Calderon, H.; Kostorz, G.; Ullrich, G. Microstructure and plasticity of two molybdenum-base alloys (TZM). Mater. Sci. Eng. A 1993, 160, 189–199. [Google Scholar] [CrossRef]
- Majumdar, S.; Kapoor, R.; Raveendra, S.; Sinha, H.; Samajdar, I.; Bhargava, P.; Chakravartty, J.; Sharma, I.; Suri, A. A study of hot deformation behavior and microstructural characterization of Mo–TZM alloy. J. Nucl. Mater. 2009, 385, 545–551. [Google Scholar] [CrossRef]
- Byun, T.; Li, M.; Cockeram, B.; Snead, L. Deformation and fracture properties in neutron irradiated pure Mo and Mo alloys. J. Nucl. Mater. 2008, 376, 240–246. [Google Scholar] [CrossRef]
- ASTM B387-18; Standard Specification for Molybdenum and Molybdenum Alloy Bar, Rod, and Wire. ASTM International: West Conshohocken, PA, USA, 2018; pp. 1–4. [CrossRef]
- Mrotzek, T.; Hoffmann, A.; Martin, U. Hardening mechanisms and recrystallization behaviour of several molybdenum alloys. Int. J. Refract. Met. Hard Mater. 2006, 24, 298–305. [Google Scholar] [CrossRef]
- Ryan, N.; Martin, J. The formation and stability of group IVA carbides and nitrides in molybdenum. J. Less Common Met. 1969, 17, 363–376. [Google Scholar] [CrossRef]
- Meng, X.; Li, L.; Li, C.; Andruczyk, D.; Tritz, K.; Maingi, R.; Huang, M.; Zhang, D.; Xu, W.; Sun, Z.; et al. Corrosion characteristics of Mo and TZM alloy for plasma facing components in molten lithium at 623 K. Corros. Sci. 2022, 200, 110202. [Google Scholar] [CrossRef]
- Tuncay, B.; Ozyurek, D. The Role of Zirconium on the Microstructure and Electrochemical Corrosion in TZM Alloys. Prot. Met. Phys. Chem. Surf. 2022, 58, 356–364. [Google Scholar] [CrossRef]
- Davis, J.R. ASM Specialty Handbook: Heat-Resistant Materials; ASM International: Almere, The Netherlands, 1997. [Google Scholar]
- DiStefano, J.; Pint, B.; DeVan, J. Oxidation of refractory metals in air and low pressure oxygen gas. Int. J. Refract. Met. Hard Mater. 2000, 18, 237–243. [Google Scholar] [CrossRef]
- Smolik, G.; Petti, D.; Schuetz, S. Oxidation and volatilization of TZM alloy in air. J. Nucl. Mater. 2000, 283–287, 1458–1462. [Google Scholar] [CrossRef] [Green Version]
- Fu, T.; Cui, K.; Zhang, Y.; Wang, J.; Zhang, X.; Shen, F.; Yu, L.; Mao, H. Microstructure and Oxidation Behavior of Anti-Oxidation Coatings on Mo-Based Alloys through HAPC Process: A Review. Coatings 2021, 11, 883. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, K.; Fu, T.; Wang, J.; Shen, F.; Zhang, X.; Yu, L. Formation of MoSi2 and Si/MoSi2 coatings on TZM (Mo–0.5Ti–0.1Zr–0.02C) alloy by hot dip silicon-plating method. Ceram. Int. 2021, 47, 23053–23065. [Google Scholar] [CrossRef]
- Sharma, I.; Chakraborty, S.; Suri, A. Preparation of TZM alloy by aluminothermic smelting and its characterization. J. Alloys Compd. 2005, 393, 122–127. [Google Scholar] [CrossRef]
- Danisman, C.B.; Yavas, B.; Yucel, O.; Sahin, F.; Goller, G. Processing and characterization of spark plasma sintered TZM alloy. J. Alloys Compd. 2016, 685, 860–868. [Google Scholar] [CrossRef]
- Malkowski, T.F.; Speck, J.S.; Denbaars, S.; Nakamura, S. An exploratory study of acidic ammonothermal growth in a TZM autoclave at high temperatures. J. Cryst. Growth 2018, 499, 85–89. [Google Scholar] [CrossRef]
- Malkowski, T.F.; Pimputkar, S.; Speck, J.S.; Denbaars, S.; Nakamura, S. Acidic ammonothermal growth of gallium nitride in a liner-free molybdenum alloy autoclave. J. Cryst. Growth 2016, 456, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Pimputkar, S.; Speck, J.; Nakamura, S. Basic ammonothermal GaN growth in molybdenum capsules. J. Cryst. Growth 2016, 456, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Häusler, J.; Schnick, W. Ammonothermal Synthesis of Nitrides: Recent Developments and Future Perspectives. Chem. Eur. J. 2018, 24, 11864–11879. [Google Scholar] [CrossRef] [PubMed]
- Pimputkar, S.; Speck, J.S.; Denbaars, S.; Nakamura, S. Prospects for LED lighting. Nat. Photon. 2009, 3, 180–182. [Google Scholar] [CrossRef]
- Amano, H.; Baines, Y.; Beam, E.; Borga, M.; Bouchet, T.; Chalker, P.R.; Charles, M.; Chen, K.J.; Chowdhury, N.; Chu, R.; et al. The 2018 GaN power electronics roadmap. J. Phys. D Appl. Phys. 2018, 51, 163001. [Google Scholar] [CrossRef]
- Bhuiyan, A.G.; Hashimoto, A.; Yamamoto, A.; Bhuiyan, A.G.; Hashimoto, A.; Yamamoto, A. Indium nitride (InN): A review on growth, characterization, and properties. J. Appl. Phys. 2003, 94, 2779–2808. [Google Scholar] [CrossRef]
- Hertrampf, J.; Becker, P.; Widenmeyer, M.; Weidenkaff, A.; Schlücker, E.; Niewa, R. Ammonothermal Crystal Growth of Indium Nitride. Cryst. Growth Des. 2018, 18, 2365–2369. [Google Scholar] [CrossRef]
- Johnson, G.D. Corrosion Studies of Liquid Metal Heat Pipe Systems at 1000° to 1800 °C; Springer: Boston, MA, USA, 1970; pp. 321–337. [Google Scholar] [CrossRef]
- White, C.E.T.; Okamoto, H. Phase Diagrams of Indium Alloys and Their Engineering Applications; ASM International: Materials Park, OH, USA, 1992; Volume 8. [Google Scholar]
- Kononnako, V.I.; Shveikin, G.P.; Sukhman, A.L.; Lomovtsev, V.I.; Mitrofanov, B.V. Chemical compatibility of titanium carbide with aluminum, gallium, and indium melts. Sov. Powder Met. Met. Ceram. 1976, 15, 699–702. [Google Scholar] [CrossRef]
- Walker, P.; Tarn, W.H. CRC Handbook of Metal Etchants; CRC Press LLC: Boca Raton, FL, USA, 1990. [Google Scholar]
- Kelly, A. Metallography and Microstructures; ASM International: Materials Park, OH, USA, 2004; Volume 9. [Google Scholar] [CrossRef]
- ASTM E1823-20b; Standard Terminology Relating to Fatigue and Fracture Testing. ASTM International: West Conshohocken, PA, USA, 2020; pp. 1–25. [CrossRef]
- Shabalin, I.L. Ultra-High Temperature Materials I; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Filacchioni, G.; Casagrande, E.; De Angelis, U.; De Santis, G.; Ferrara, D. Effects of strain rate on tensile properties of TZM and Mo–5%Re. J. Nucl. Mater. 2002, 307–311, 705–709. [Google Scholar] [CrossRef]
- Allmann, R.; Pies, W.; Weiss, A. d8269, XIV.3.1 Simple Oxo-Compounds of Indium (Oxoindates); Springer: Berlin/Heidelberg, Germany, 1980; Volume 7d2, pp. 303–318. [Google Scholar] [CrossRef]
- Waseem, O.A.; Woller, K.B. Evolution in microstructure and hardness of Titanium–Zirconium–Molybdenum (TZM) alloy after depth marker implantation for erosion diagnostic in fusion devices. Mater. Chem. Phys. 2021, 258, 123883. [Google Scholar] [CrossRef]
- Okamoto, H. In-O (Indium-Oxygen). J. Phase Equilibria Diffus. 2007, 28, 591–592. [Google Scholar] [CrossRef]
- Filipek, E.; Rychlowska-Himmel, I.; Paczesna, A. Thermal stability of In2(MoO4)3 and phase equilibria in the MoO3–In2O3 system. J. Therm. Anal. Calorim. 2012, 109, 711–716. [Google Scholar] [CrossRef] [Green Version]
- Nicholas, M.G.; Old, C.F. Liquid metal embrittlement. J. Mater. Sci. 1979, 14, 1–18. [Google Scholar] [CrossRef]
- Cockeram, B. The mechanical properties and fracture mechanisms of wrought low carbon arc cast (LCAC), molybdenum–0.5pct titanium–0.1pct zirconium (TZM), and oxide dispersion strengthened (ODS) molybdenum flat products. Mater. Sci. Eng. A 2006, 418, 120–136. [Google Scholar] [CrossRef]
- Filacchioni, G.; Casagrande, E.; De Angelis, U.; De Santis, G.; Ferrara, D. Tensile and impact properties of TZM and Mo-5% Re. J. Nucl. Mater. 1994, 212–215, 1288–1291. [Google Scholar] [CrossRef]
- Davis, J.R. Tensile Testing; ASM International: Materials Park, OH, USA, 2004. [Google Scholar]
- Steichen, J. Tensile properties of neutron irradiated TZM and tungsten. J. Nucl. Mater. 1976, 60, 13–19. [Google Scholar] [CrossRef]
- Kuhn, H.; Medlin, D. (Eds.) Fracture Toughness and Fracture Mechanics; ASM International: Materials Park, OH, USA, 2000; Volume 8. [Google Scholar] [CrossRef]
- Hu, P.; Yang, F.; Wang, K.-S.; Yu, Z.-T.; Tan, J.-F.; Song, R.; Hu, B.-L.; Wang, H.; He, H.-C.; Volinsky, A. Preparation and ductile-to-brittle transition temperature of the La-TZM alloy plates. Int. J. Refract. Met. Hard Mater. 2015, 52, 131–136. [Google Scholar] [CrossRef]


| Sample | Mo | Fe | Ni | Si | C | O | N | Ti | Zr |
|---|---|---|---|---|---|---|---|---|---|
| TZM 1 | Balance | <0.001 | 0.002 | <0.001 | 0.025 | 0.0191 | 0.002 | 0.47 | 0.085 |
| TZM 2 | Balance | 0.0008 | 0.0008 | 0.0007 | 0.027 | 0.003 | 0.002 | 0.53 | 0.08 |
| Pulling Temperature (°C) | Indium Exposure | Ultimate Tensile Strength (MPa) | Yield Strength (MPa) | Total Elongation (%) | Reduction of Area (%) |
|---|---|---|---|---|---|
| 22 | 750 °C, 14 d | 825 ± 8 | 763 ± 8 | 35.0 ± 0.4 | 70.0 ± 0.7 |
| 800 | 750 °C, 14 d | 491 ± 5 | 470 ± 5 | 22.0 ± 0.2 | 71.5 ± 0.7 |
| 800 | 1000 °C, 14 d | 510 ± 5 | 502 ± 5 | 25.0 ± 0.3 | 73.5 ± 0.7 |
| 800 | unexposed | 479 ± 5 | 446 ± 4 | 32.0 ± 0.3 | 75.5 ± 0.8 |
| 800 | unexposed | 499 ± 5 | 485 ± 5 | 17.2 ± 0.2 | 71.9 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metzger, F.; Rienzi, V.; Mascetti, C.; Nguyen, T.; Pimputkar, S. Properties of Titanium Zirconium Molybdenum Alloy after Exposure to Indium at Elevated Temperatures. Materials 2022, 15, 5270. https://doi.org/10.3390/ma15155270
Metzger F, Rienzi V, Mascetti C, Nguyen T, Pimputkar S. Properties of Titanium Zirconium Molybdenum Alloy after Exposure to Indium at Elevated Temperatures. Materials. 2022; 15(15):5270. https://doi.org/10.3390/ma15155270
Chicago/Turabian StyleMetzger, Florian, Vincent Rienzi, Christopher Mascetti, Tri Nguyen, and Siddha Pimputkar. 2022. "Properties of Titanium Zirconium Molybdenum Alloy after Exposure to Indium at Elevated Temperatures" Materials 15, no. 15: 5270. https://doi.org/10.3390/ma15155270
APA StyleMetzger, F., Rienzi, V., Mascetti, C., Nguyen, T., & Pimputkar, S. (2022). Properties of Titanium Zirconium Molybdenum Alloy after Exposure to Indium at Elevated Temperatures. Materials, 15(15), 5270. https://doi.org/10.3390/ma15155270






