Effect of Ethylene Glycol: Citric Acid Molar Ratio and pH on the Morphology, Vibrational, Optical and Electronic Properties of TiO2 and CuO Powders Synthesized by Pechini Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the CuO and TiO2
2.2. Characterization Techniques
3. Results
3.1. Structural and Morphological Analysis
3.1.1. X-ray Diffraction
3.1.2. Scanning and Transmission Electron Microscopy
3.2. Characterization of Vibrational Properties
FTIR Spectroscopy
3.3. Characterization of Photophysical Properties
3.3.1. UV-Vis Spectroscopy
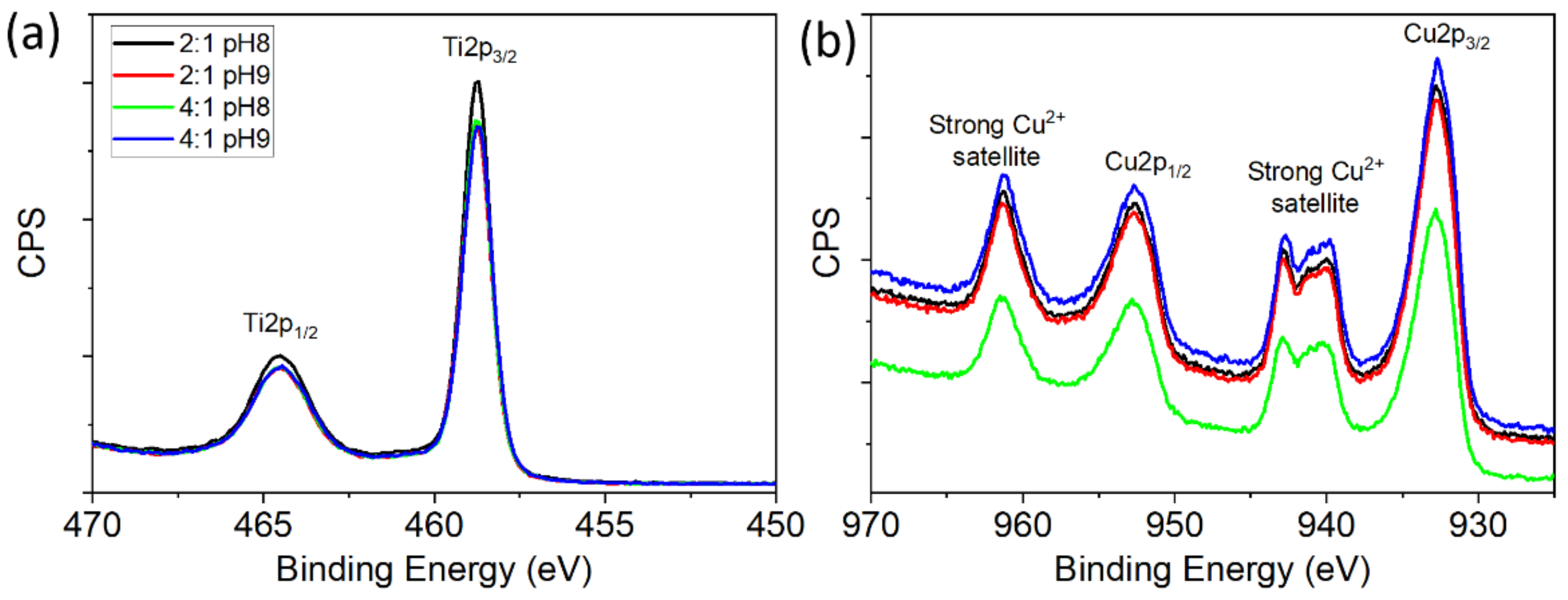
3.3.2. XPS Measurements
| Sample | Eg (eV) | EVB (eV) | ECB (eV) |
|---|---|---|---|
| 2:1 pH8—TiO2 | 3.17 ± 0.04 | 2.6 ± 0.1 | −0.57 ± 0.07 |
| 2:1 pH9—TiO2 | 3.17 ± 0.03 | 2.6 ± 0.1 | −0.57 ± 0.08 |
| 4:1 pH8—TiO2 | 3.18 ± 0.03 | 2.6 ± 0.2 | −0.6 ± 0.1 |
| 4:1 pH9—TiO2 | 3.18 ± 0.03 | 2.6 ± 0.1 | −0.58 ± 0.07 |
| 2:1 pH8—CuO | 1.494 ± 0.003 | 0.99 ± 0.06 * −0.504 ± 0.005 ** | −0.50 ± 0.03 |
| 2:1 pH9—CuO | 1.489 ± 0.004 | 0.99 ± 0.05 * −0.51 ± 0.01 ** | −0.50 ± 0.03 |
| 4:1 pH8—CuO | 1.502 ± 0.004 | 0.97 ± 0.03 * −0.55 ± 0.01 ** | −0.53 ± 0.02 |
| 4:1 pH9—CuO | 1.51 ± 0.01 | 0.89 ± 0.07 * −0.616 ± 0.005 ** | −0.62 ± 0.04 |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, M.; He, J. Fine Tuning of the Morphology of Copper Oxide Nanostructures and Their Application in Ambient Degradation of Methylene Blue. J. Colloid Interface Sci. 2011, 355, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, K.; Xu, D.; Yang, G.; Huang, H.; Nie, F.; Liu, C.; Yang, S. CuO Nanostructures: Synthesis, Characterization, Growth Mechanisms, Fundamental Properties, and Applications. Prog. Mater. Sci. 2014, 60, 208–337. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, X.; Rao, R.; Qian, N.X.; Ma, Y.Q. Study on Vapor-Thermal Synthesis and Sulfur-Doping of TiO2/Graphene Composites. Appl. Surf. Sci. 2020, 507, 144856. [Google Scholar] [CrossRef]
- Vargas, M.A.; Rodríguez-Páez, J.E. Amorphous TiO2 Nanoparticles: Synthesis and Antibacterial Capacity. J. Non. Cryst. Solids 2017, 459, 192–205. [Google Scholar] [CrossRef]
- Crespo-Monteiro, N.; Hamandi, M.; Higuita, M.A.U.; Guillard, C.; Dappozze, F.; Jamon, D.; Vocanson, F.; Jourlin, Y. Influence of the Micro-Nanostructuring of Titanium Dioxide Films on the Photocatalytic Degradation of Formic Acid under UV Illumination. Nanomaterials 2022, 12, 1008. [Google Scholar] [CrossRef]
- Dar, M.A.; Ahsanulhaq, Q.; Kim, Y.S.; Sohn, J.M.; Kim, W.B.; Shin, H.S. Versatile Synthesis of Rectangular Shaped Nanobat-like CuO Nanostructures by Hydrothermal Method; Structural Properties and Growth Mechanism. Appl. Surf. Sci. 2009, 255, 6279–6284. [Google Scholar] [CrossRef]
- Rahnama, A.; Gharagozlou, M. Preparation and Properties of Semiconductor CuO Nanoparticles via a Simple Precipitation Method at Different Reaction Temperatures. Opt. Quantum Electron. 2012, 44, 313–322. [Google Scholar] [CrossRef]
- Vargas, M.A.; Rivera-Muñoz, E.M.; Diosa, J.E.; Mosquera, E.E.; Rodríguez-Páez, J.E. Nanoparticles of ZnO and Mg-Doped ZnO: Synthesis, Characterization and Efficient Removal of Methyl Orange (MO) from Aqueous Solution. Ceram. Int. 2021, 47, 15668–15681. [Google Scholar] [CrossRef]
- Vollath, D. Nanomaterials: An Introduction to Synthesis, Properties and Applications, 2nd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; ISBN 9783527333790. [Google Scholar]
- Ribeiro, P.C.; De Melo Da Costa, A.C.F.; Kiminami, R.H.G.A.; Sasaki, J.M.; Lira, H.L. Synthesis of TiO2 by the Pechini Method and Photocatalytic Degradation of Methyl Red. Mater. Res. 2013, 16, 468–472. [Google Scholar] [CrossRef]
- Pechini, M.P. Method of Preparing Lead and Alkaline Earth Titanates and Nobates and Coating method using the same to form a capacitor. U.S. Patent 3,330,697, 11 July 1967. [Google Scholar]
- Xu, Y.; Yuan, X.; Huang, G.; Long, H. Polymeric Precursor Synthesis of Ba2Ti9O20. Mater. Chem. Phys. 2005, 90, 333–338. [Google Scholar] [CrossRef]
- Mashreghi, A.; Davoudi, F. The Effect of Ethylene Glycol/Citric Acid Molar Ratio in the Initial Precursor of TiO2 Nanoparticle Paste Synthesized by a Polymerizable Complex Method on the Photovoltaic Properties of Dye-Sensitized Solar Cells. Mater. Sci. Semicond. Process. 2015, 30, 618–624. [Google Scholar] [CrossRef]
- Hossein, M.; Morteza, A. Effect of Diverse Pechini Sol-Gel Parameters on the Size, Morphology, Structural and Optical Properties of the Tenorite (CuO) NPs: A Facile Approach for Desired Properties. Ceram. Int. 2020, 46, 17197–17208. [Google Scholar] [CrossRef]
- Shoja Razavi, R.; Reza Loghman-Estarki, M.; Farhadi-Khouzani, M.; Barekat, M.; Jamali, H. Large Scale Synthesis of Zinc Oxide Nano- and Submicro-Structures by Pechinis Method: Effect of Ethylene Glycol/Citric Acid Mole Ratio on Structural and Optical Properties. Curr. Nanosci. 2011, 7, 807–812. [Google Scholar] [CrossRef]
- Mai, Y.J.; Wang, X.L.; Xiang, J.Y.; Qiao, Y.Q.; Zhang, D.; Gu, C.D.; Tu, J.P. CuO/Graphene Composite as Anode Materials for Lithium-Ion Batteries. Electrochim. Acta 2011, 56, 2306–2311. [Google Scholar] [CrossRef]
- Narayanan, M.; Vigneshwari, P.; Natarajan, D.; Kandasamy, S.; Alsehli, M.; Elfasakhany, A.; Pugazhendhi, A. Synthesis and Characterization of TiO2 NPs by Aqueous Leaf Extract of Coleus Aromaticus and Assess Their Antibacterial, Larvicidal, and Anticancer Potential. Environ. Res. 2021, 200, 111335. [Google Scholar] [CrossRef]
- Lan, Y.; Lu, Y.; Ren, Z. Mini Review on Photocatalysis of Titanium Dioxide Nanoparticles and Their Solar Applications. Nano Energy 2013, 2, 1031–1045. [Google Scholar] [CrossRef]
- Saebnoori, E.; Koupaei, N.; Hassanzadeh Tabrizi, S.A. The solution plasma synthesis, characterisation, and antibacterial activities of dispersed CuO nanoparticles. Mater.Technol. 2021, 36, 1–10. [Google Scholar] [CrossRef]
- Kohtani, S.; Yoshioka, E.; Miyabe, H. Photocatalytic hydrogenation on semiconductor particles. In Hydrogenation; Karamé, I., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 291–308. ISBN 0000957720. [Google Scholar]
- Diamanti, M. Special Issue: Novel Photoactive Materials. Materials. 2018, 11, 2553. [Google Scholar] [CrossRef] [Green Version]
- Valencia, S.; Marín, J.M.; Restrepo, G. Study of the Bandgap of Synthesized Titanium Dioxide Nanoparticules Using the Sol-Gel Method and a Hydrothermal Treatment. Open Mater. Sci. J. 2010, 4, 9–14. [Google Scholar] [CrossRef]
- Reddy, K.M.; Manorama, S.V.; Reddy, A.R. Bandgap Studies on Anatase Titanium Dioxide Nanoparticles. Mater. Chem. Phys. 2003, 78, 239–245. [Google Scholar] [CrossRef]
- Kuznetsov, V.N.; Serpone, N. Visible Light Absorption by Various Titanium Dioxide Specimens. J. Phys. Chem. B 2006, 110, 25203–25209. [Google Scholar] [CrossRef] [PubMed]
- Hossain, F.M.; Sheppard, L.; Nowotny, J.; Murch, G.E. Optical Properties of Anatase and Rutile Titanium Dioxide: Ab Initio Calculations for Pure and Anion-Doped Material. J. Phys. Chem. Solids 2008, 69, 1820–1828. [Google Scholar] [CrossRef]
- Wang, Y.; Lany, S.; Ghanbaja, J.; Fagot-Revurat, Y.; Chen, Y.P.; Soldera, F.; Horwat, D.; Mücklich, F.; Pierson, J.F. Electronic Structures of Cu2O, Cu4O3, and CuO: A Joint Experimental and Theoretical Study. Phys. Rev. B 2016, 94, 245418. [Google Scholar] [CrossRef] [Green Version]
- Heinemann, M.; Eifert, B.; Heiliger, C. Band Structure and Phase Stability of the Copper Oxides Cu2O, CuO, and Cu4O3. Phys. Rev. B-Condens. Matter Mater. Phys. 2013, 87, 3–7. [Google Scholar] [CrossRef]
- Tamayo, K.; Marín, L.; Gross, K.; Amaya, C.; Reyes, D.; Lopera, W.; Gómez, M. Influence of thermal annealing on the structural and optical properties of nanostructures based on copper oxides. Ing. Compet. 2022, 24, 1–16. [Google Scholar]
- Vargas, M.A.; Franco, Y.; Ochoa, Y.; Ortegón, Y.; Rodriguez Paez, J.E. TiO2 sintetizado por el método de Precursor Polimérico (Pechini): Estructura de la resina intermedia. Bol. Soc. Esp. Ceram. Vidr. 2011, 50, 267–272. [Google Scholar] [CrossRef]
- Joni, I.M.; Nulhakim, L.; Panatarani, C. Characteristics of TiO2 particles prepared by simple solution method using TiCl3 precursor. J. Phys. Conf. Ser. 2018, 1080, 012042. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.Y.; Lee, H.K.; Moon, J.T.; Joo, J.B. Synthesis of Spherical TiO2 Particles with Disordered Rutile Surface for Photocatalytic Hydrogen Production. Catalysts 2019, 9, 491. [Google Scholar] [CrossRef] [Green Version]
- Cai, W.; Yang, H.; Guo, X. A Facile Synthesis of Nanocrystalline Spherical TiO2 Particles and its Photoluminescent Properties. Procedia Eng. 2014, 94, 71–75. [Google Scholar] [CrossRef] [Green Version]
- Pal, M.; García-Serrano, J.; Santiago, P.; Pal, U. Size-Controlled Synthesis of Spherical TiO2 Nanoparticles: Morphology, Crystallization, and Phase Transition. J. Phys. Chem. C 2007, 111, 96–102. [Google Scholar] [CrossRef]
- Fazio, G.; Ferrighi, L.; Di Valentin, C. Spherical vs Faceted Anatase TiO2 Nanoparticles: A Model Study of Structural and Electronic Properties. J. Phys. Chem. C 2015, 119, 20735–20746. [Google Scholar] [CrossRef]
- Tellez, L.; Rubio, F.; Peña, R.; Rubio, J. Seguimiento por espectroscopia infrarroja (FT-IR) de la copolimerizacion de TEOS (tetraetilortosilicato) y PDMS (polidimetilsiloxano) en presencia de TBT (tetrabutiltitanio). Bol. Soc. Esp. Ceram. 2004, 43, 883–890. [Google Scholar] [CrossRef]
- Guzmán, A.; Fernandez, A.; Rodriguez, J. Estudio de las reacciones de hidrólisis y condensación del titanio en presencia de acetilacetona. Rev. Acad. Colomb. Cienc. 2007, 31, 529–536. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; John Wiley and Sons: New York, NY, USA, 1997. [Google Scholar]
- Praveen, P.; Viruthagiri, G.; Mugundan, S.; Shanmugam, N. Structural, optical and morphological analyses of pristine titanium di-oxide nanoparticles-Synthesized via sol-gel route. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 117, 622–629. [Google Scholar] [CrossRef]
- Rashidzadeh, M. Synthesis of High-Thermal Stable Titanium Dioxide Nanoparticles. Int. J. Photoenergy 2008, 2008, 4. [Google Scholar] [CrossRef] [Green Version]
- Ba-Abbad, M.M.; Kadhum, A.A.; Mohamad, A.B.; Takriff, M.S.; Sopian, K. Synthesis and Catalytic Activity of TiO2 Nanoparticles for Photochemical Oxidation of Concentrated Chlorophenols under Direct Solar Radiation. Int. J. Electrochem. Sci. 2012, 7, 4871–4888. [Google Scholar]
- Gohari, G.; Mohammadi, A.; Akbari, A.; Panahirad, S.; Reza Dadpour, M.; Fotopoulos, V.; Kimura, S. Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci. Rep. 2020, 10, 912. [Google Scholar] [CrossRef]
- Bagheri, S.; Shameli, K.; Abd Hamid, S.B. Synthesis and Characterization of Anatase Titanium Dioxide Nanoparticles Using Egg White Solution via Sol-Gel Method. Compos. Nanoparticles 2013, 2013, 848205. [Google Scholar] [CrossRef]
- Yu, J.; Su, Y.; Cheng, B.; Zhou, M. Effects of pH on the microstructures and photocatalytic activity of mesoporous nanocrystalline titania powders prepared via hydrothermal method. J. Mol. Catal. A Chem. 2006, 258, 104–112. [Google Scholar] [CrossRef]
- Al-Amin, M.; Chandra Dey, S.; Ur Rashid, T.; Ashaduzzaman, M.; Shamsuddin, S.M. Solar Assisted Photocatalytic Degradation of Reactive Azo Dyes in Presence of Anatase Titanium Dioxide. Int. J. Latest Res. Eng. Technol. 2016, 2, 14–21. [Google Scholar]
- Adnan Ouda, A.; Mohamad Alosfur, F.K.; Ridha, N.J.; Abud, S.H.; Umran, N.M.; Al-aaraji, H.H.; Madlool, R.A. Facile method to synthesis of anatase TiO2 nanorods. J. Phys. Conf. Ser. 2018, 1032, 012038. [Google Scholar] [CrossRef]
- Sethi, D.; Sakthivel, R. ZnO/TiO2 composites for photocatalytic inactivation of Escherichia coli. J. Photochem. Photobiol. B Biol. 2017, 168, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.; Memic, A. Size-dependent antimicrobial properties of CuO nanoparticles against Gram-positive and-negative bacterial strains. Int. J. Nanomed. 2012, 7, 3527–3535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elango, M.; Deepa, M.; Subramanian, R.; Mohamed Musthafa, A. Synthesis, Characterization, and Antibacterial Activity of Polyindole/Ag–Cuo Nanocomposites by Reflux Condensation Method. Polym. Plast. Technol. Eng. 2018, 57, 1440–1451. [Google Scholar] [CrossRef]
- Stefan, M.; Pana, O.; Leostean, C.; Bele, C.; Silipas, D.; Senila, M.; Gautron, E. Synthesis and characterization of Fe3O4–TiO2 core-shell nanoparticles. J. Appl. Phys. 2014, 116, 114312. [Google Scholar] [CrossRef]
- Le, Y.L.; Weng, X.; Zhou, M.; Zhang, Q.; Deng, L. Structural and Visible-Near Infrared Optical Properties of Cr-Doped TiO2 for Colored Cool Pigments. Nanoscale Res. Lett. 2017, 12, 597. [Google Scholar] [CrossRef] [Green Version]
- Escobedo Morales, A.; Sanchez Mora, E.; Pal, U. Use of diffuse reflectance spectroscopy for optical characterization of un-supported nanostructures. Rev. Mex. Fís. 2007, 53, 18–22. [Google Scholar]
- Weizhong, J.; Ying, W.; L, G. Influence of TiO2 film on photo-catalytic property of enamels. J. Non. Cryst. Solids 2007, 353, 4191–4194. [Google Scholar] [CrossRef]
- Puzenat, E. Photocatalytic self-cleaning materials: Principles and impact on atmosphere. EPJ Web Conf. 2009, 1, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Fernandes Machado, N.R.C.; Santana, V. Influence of thermal treatment on the structure and photocatalytic activity of TiO2 P25. Catalysis Today 2005, 107, 595–601. [Google Scholar] [CrossRef]
- Sawicka-Chudy, P.; Sibiński, M.; Wisz, G.; Rybak-Wilusz, E.; Cholewa, M. Numerical analysis and optimization of Cu2O/TiO2, CuO/TiO2, heterojunction solar cells using SCAPS. J. Phys. Conf. Ser. 2018, 1033, 012002. [Google Scholar] [CrossRef] [Green Version]
- X-ray Photoelectron Spectroscopy (XPS) Reference Pages. Available online: www.xpsfitting.com (accessed on 7 March 2022).
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Ghijsen, J.; Tjeng, L.H.; Van Elp, J.; Eskes, H.; Westerink, J.; Sawatzky, G.A.; Czyzyk, M.T. Electronic structure of Cu2O and CuO. Phys. Rev. B 1988, 38, 11322. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Controllable Growth, Microstructure and Electronic Structure of Copper Oxide Thin Films. Other. Ph.D. Thesis, University of Lorrain, Nancy, France, 2015. [Google Scholar]
- Hussain, M.; Ibupoto, Z.H.; Abbassi, M.A.; Khan, A.; Pozina, G.; Nur, O.; Willander, M. Synthesis of CuO/ZnO composite nanostructures, their optical characterization and valence band offset determination by x-ray photoelectron spectroscopy. J. Nanoelectron. Optoelectron 2014, 9, 348–356. [Google Scholar] [CrossRef]
- Galakhov, V.R.; Finkelstein, L.D.; Zatsepin, D.A.; Kurmaev, E.Z.; Samokhvalov, A.A.; Naumov, S.V.; Tatarinova, G.K. Interaction of Cu 3d and O 2p states in Mg1-xCuxO solid solutions with NaCl structure: X-rayphotoelectron and x-ray emission study. Phys. Rev. B 2000, 62, 4922. [Google Scholar] [CrossRef]
- Ansari, S.A.; Cho, M.H. Highly Visible Light Responsive, Narrow Band gap TiO2 Nanoparticles Modified by Elemental Red Phosphorus for Photocatalysis and Photoelectrochemical Applications. Sci. Rep. 2016, 6, 25405. [Google Scholar] [CrossRef]
- Arif Khan, M.; Nayan, N.; Shadiullah; Ahmad, M.K.; Fhong, S.C. Surface Study of CuO Nanopetals by Advanced Nanocharacterization Techniques with Enhanced Optical and Catalytic Properties. Nanomaterials 2020, 10, 1298. [Google Scholar] [CrossRef]
- Feng, N.; Liu, F.; Huang, M.; Zheng, A.; Wang, Q.; Chen, T.; Cao, G.; Xu, J.; Fan, J.; Deng, F. Unravelling the Efficient Photocatalytic Activity of Boron-induced Ti3+ Species in the Surface Layer of TiO2. Sci. Rep. 2016, 6, 34765. [Google Scholar] [CrossRef] [Green Version]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Urbano, M.A.; Marín, L.; Castillo, W.M.; Rodríguez, L.A.; Magén, C.; Manotas-Albor, M.; Diosa, J.E.; Gross, K. Effect of Ethylene Glycol: Citric Acid Molar Ratio and pH on the Morphology, Vibrational, Optical and Electronic Properties of TiO2 and CuO Powders Synthesized by Pechini Method. Materials 2022, 15, 5266. https://doi.org/10.3390/ma15155266
Vargas-Urbano MA, Marín L, Castillo WM, Rodríguez LA, Magén C, Manotas-Albor M, Diosa JE, Gross K. Effect of Ethylene Glycol: Citric Acid Molar Ratio and pH on the Morphology, Vibrational, Optical and Electronic Properties of TiO2 and CuO Powders Synthesized by Pechini Method. Materials. 2022; 15(15):5266. https://doi.org/10.3390/ma15155266
Chicago/Turabian StyleVargas-Urbano, Mónica A., Lorena Marín, Winny Mónica Castillo, Luis Alfredo Rodríguez, César Magén, Milton Manotas-Albor, Jesús Evelio Diosa, and Katherine Gross. 2022. "Effect of Ethylene Glycol: Citric Acid Molar Ratio and pH on the Morphology, Vibrational, Optical and Electronic Properties of TiO2 and CuO Powders Synthesized by Pechini Method" Materials 15, no. 15: 5266. https://doi.org/10.3390/ma15155266
APA StyleVargas-Urbano, M. A., Marín, L., Castillo, W. M., Rodríguez, L. A., Magén, C., Manotas-Albor, M., Diosa, J. E., & Gross, K. (2022). Effect of Ethylene Glycol: Citric Acid Molar Ratio and pH on the Morphology, Vibrational, Optical and Electronic Properties of TiO2 and CuO Powders Synthesized by Pechini Method. Materials, 15(15), 5266. https://doi.org/10.3390/ma15155266








