Tracing the Status of Silica Fume in Cementitious Materials Subjected to Deterioration Mechanisms with Raman Microscope
Abstract
:1. Introduction
- Carbonation, a deterioration mechanism of concrete, is a chemical reaction between atmospheric carbon dioxide (CO2) and cement hydration products. Whilst virtually almost all of the Ca-bearing hydration products [i.e., calcium silicate hydrate (C–S–H), calcium hydroxide (Ca(OH)2, CH), and various calcium aluminate or ferro-aluminate hydrates] can react with the CO2 to produce calcium carbonate (CaCO3), silica gel, hydrated aluminum, and iron oxides, the dominant reaction is the reaction between calcium hydroxide (CH) and CO2, which will convert CH into calcium carbonate [17,18]. Therefore, carbonation can reduce the pH of the concrete pore solution, which can subsequently trigger the corrosion of reinforcing bars. Additionally, owing to the increased volume from the formation of calcium carbonate, the microstructure of the cement matrix could be densified [19]. On the other hand, due to the carbonation shrinkage, it could also be possible that some microcracks could be formed [20]. All these could potentially affect the stability of the SF agglomerates.
- Sulfate attack occurs when sulfate salts (i.e., SO42−) ingress into the cementitious materials and subsequently react with the hydrates/phases of the cement (e.g., calcium hydroxide, tricalcium aluminate hydrates, monosulfoaluminate, unreacted aluminate, or ferrite phase) to form gypsum or/and ettringite (AFt) [21,22]. The formation of gypsum and ettringite is generally considered to be harmful to the hardened cementitious materials. This is because the formation of ettringite is accompanied by local volume increase and subsequent pressure build-up to the surrounding matrix, leading to the cracking, spalling, and even destruction of cementitious materials [23]. Again, once the cracks are formed [24,25], it could promote further interactions between the cement matrix and surrounding environment. As a result, the stability of the SF agglomerates could be affected.
- Chloride ingress is another severe deterioration mechanism to the steel reinforced concrete, as it could cause the depassivation of the passive film on the steel surface. On the other hand, chloride ions can react with hydrated aluminate phases, yielding the so-called Friedel’s salt (3CaO Al2O3 CaCl2 10H2O) [26,27,28]. As Friedel’s salt occupies more volume than aluminate phases, there is a pore refinement owing to the intrusion of chloride ions. More importantly, the consumption of aluminate hydrates could change the chemistry environment of the cement matrices, which can potentially affect the stability of the SF agglomerates within cementitious materials.
2. Materials and Methods
2.1. Materials
2.2. Manufacture of SF Blended PC Paste Samples
2.3. Deterioration Regimes
- Carbonation: a modified carbonation chamber (LEEC, Nottingham, UK) was employed in the carbonation test. The chamber was set to maintain a constant temperature of 20 (±1) °C, a carbon dioxide (CO2) concentration of 5 (±0.5)%, and relative humidity (RH) of 60 (±5)%, based on a laboratory established regime [31].
- Chloride attack: the 165 g/L sodium chloride (NaCl) solution as specified in the NT BUILD 443 [29] was used as the aggressive solution for the chloride ingress test. The 63 µm powders were placed at the bottom of the tank containing NaCl solution. The tank was then closed tightly and placed in the curing room at a constant temperature of 20 (±1) °C.
- Sulfate attack: the sodium sulfate (Na2SO4) solution, with a concentration of 50 g/L as specified in the ASTM C1012 [30], was used. Similar to the chloride attack, the 63 µm powders were immersed in the Na2SO4 solution for 3 months and then removed from the tank.
2.4. Raman Microscope Test
3. Results and Discussion
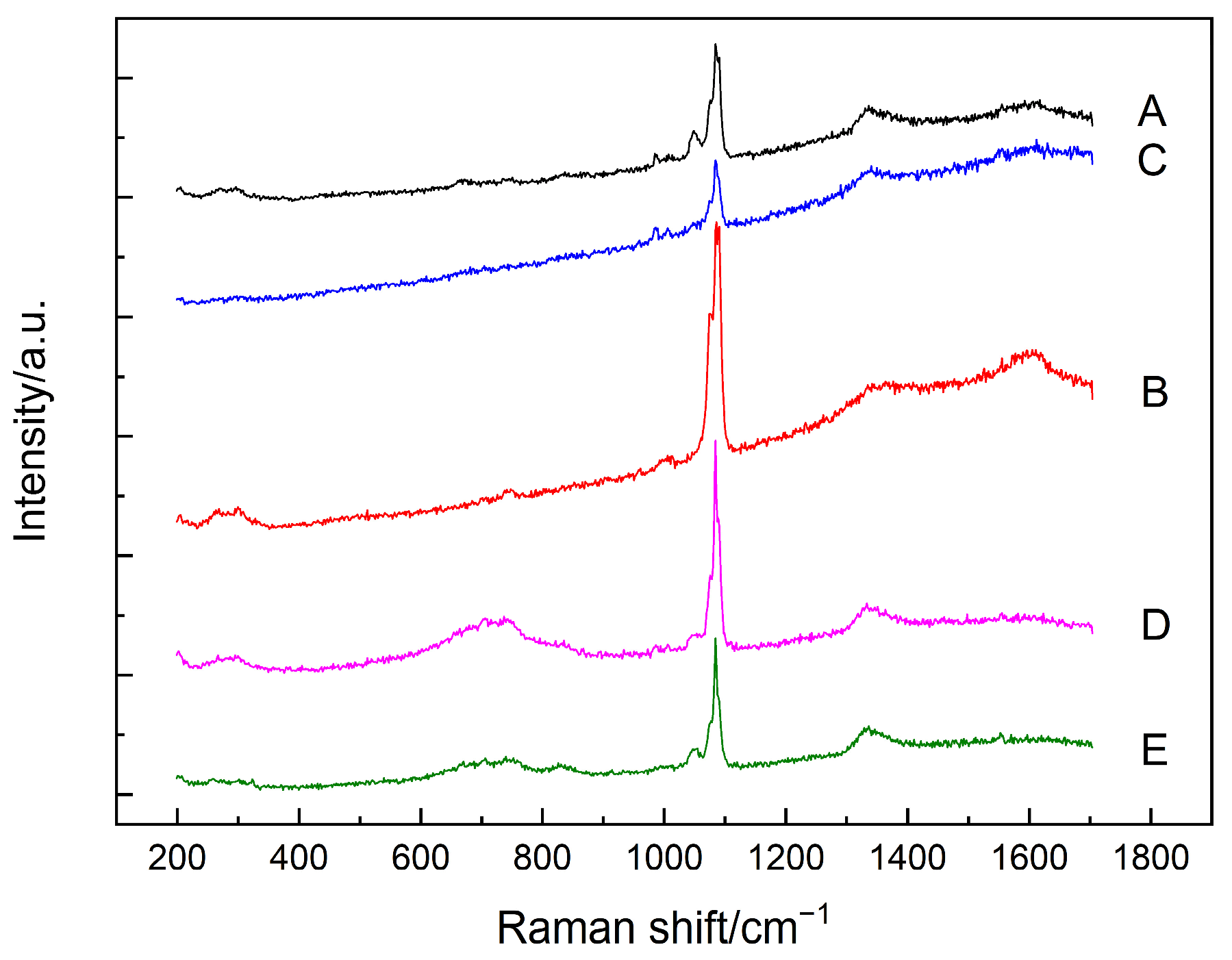
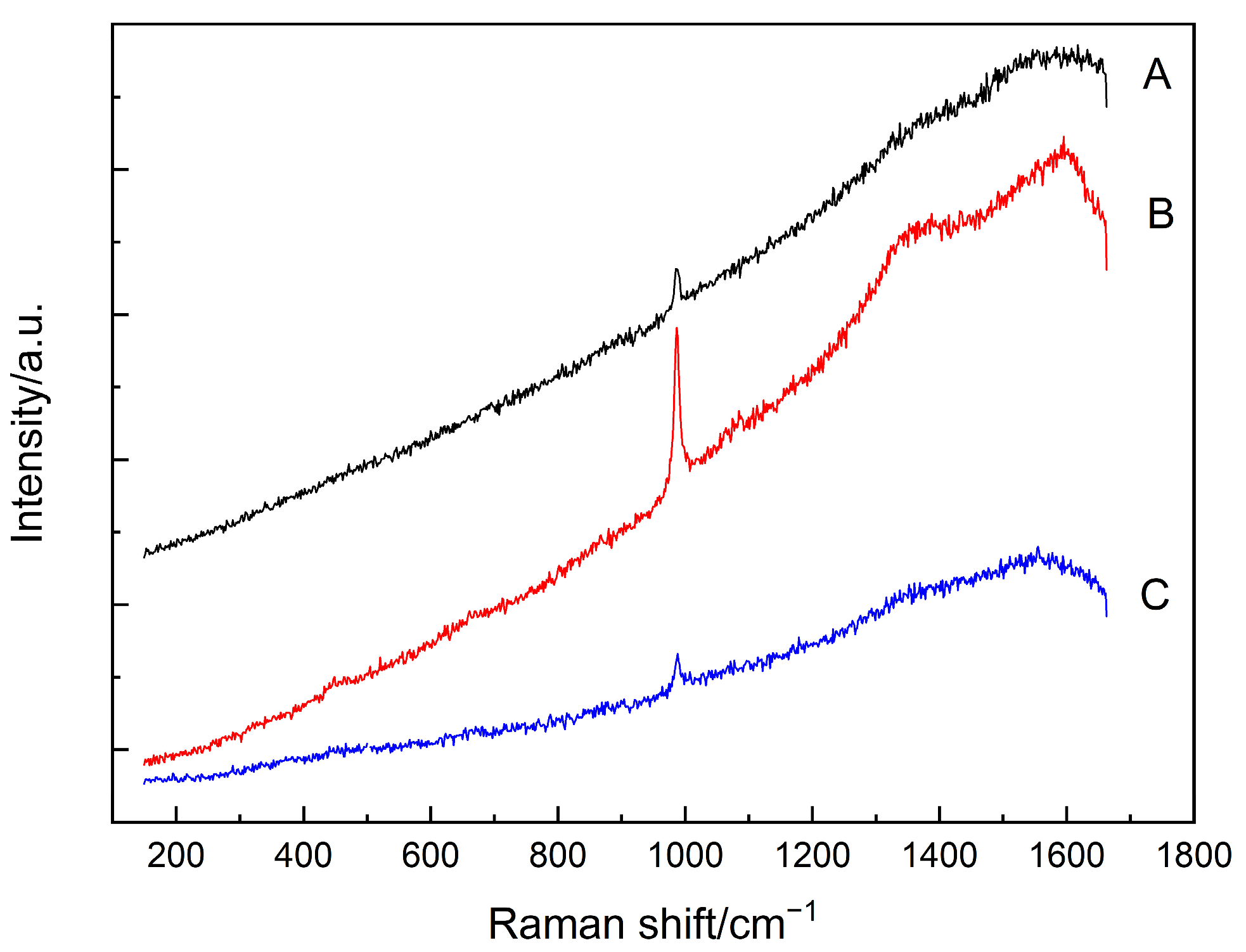
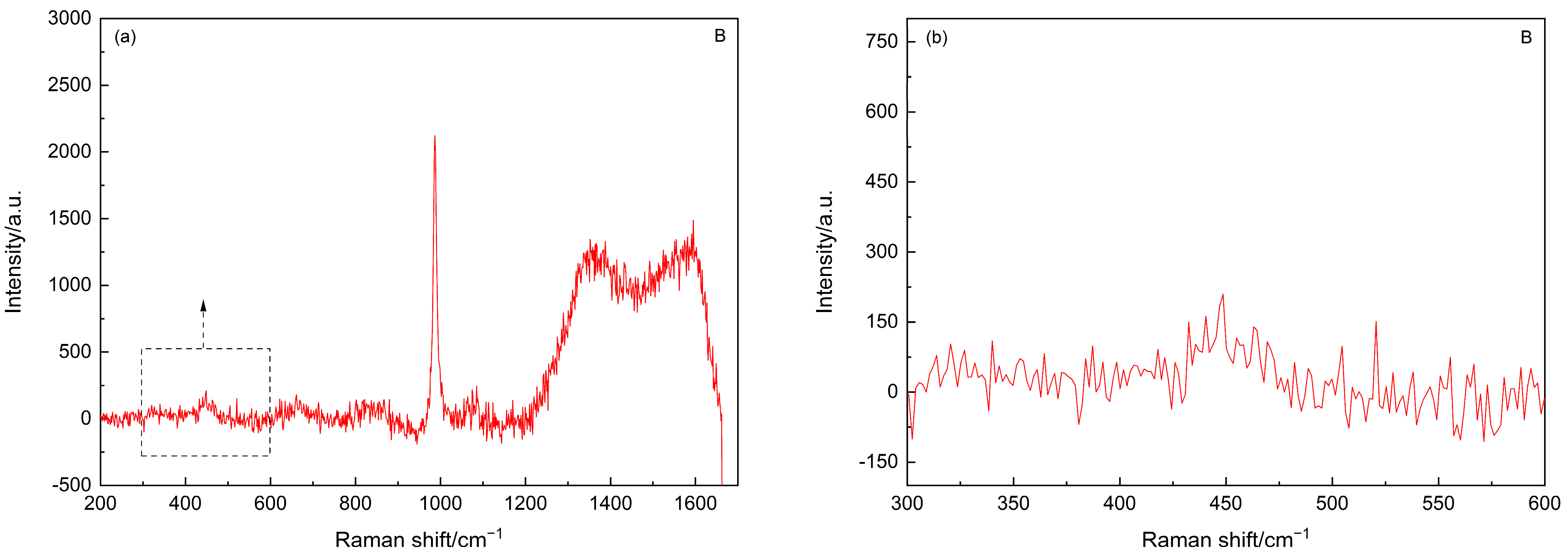
3.1. Carbonation
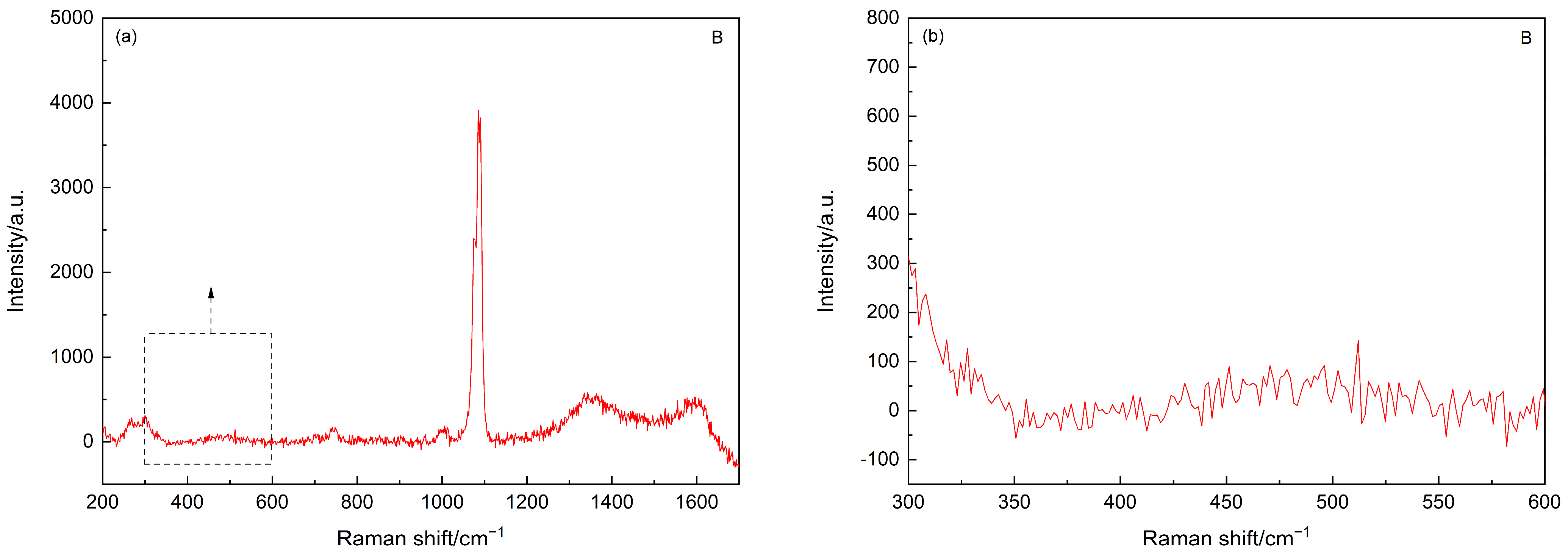
3.2. Chloride Attack
- Cl− ions, compared to OH−, have a relatively smaller ionic size and a greater tendency to diffuse inside the ‘membrane’ (e.g., adsorption/coating of Ca2+ on hydrated C3S), which could facilitate the build-up of internal pressure. This hence causes an early rupture of the ‘membrane’, leading to the unlocking of the C3S phase, which comes in contact with water and promotes the hydration reaction [41].
- The NaCl (used during deterioration) could react with CH in the pore solution and increase the amount of CaCl2 in the cement matrix. The CaCl2 is a well-established inorganic chloride-based accelerator and can flocculate hydrophilic colloids (e.g., C–S–H), facilitating the diffusion of ions and water through the initial C–S–H layer and thus allowing a higher rate of hydration during the early diffusion-controlled period [42].
- The CaCl2 could enhance the C–S–H nucleation by a homogeneous precipitation, and this accelerates the hydration [43].
3.3. Sulfate Attack
3.4. Discussion
4. Conclusions
- (1)
- The amorphous silica was identified in the SF–PC blends exposed to the carbonation and sulfate attack, as evidenced by the Raman bands at about 350–540 cm−1. No silica phases were identified in the chloride attacked SF–PC blends, which could be attributed to the enhanced hydration of cement and hence continued hydration of SF. These results indicate that there is a potential hazard to the living system, especially the long-term servicing structures exposed to a contiguous deterioration environment.
- (2)
- This study clearly demonstrated the potential of employing the Raman microscope for tracing the status of silica fume in cementitious materials subjected to deterioration mechanisms, indicating that the use of Raman microscopes could be an effective approach to monitoring the status of nanomaterials, such as SF, in concrete structures.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jabir, H.A.; Abid, S.R.; Murali, G.; Ali, S.H.; Klyuev, S.; Fediuk, R.; Vatin, N.; Promakhov, V.; Vasilev, Y. Experimental tests and reliability analysis of the cracking impact resistance of UHPFRC. Fibers 2020, 8, 74. [Google Scholar] [CrossRef]
- Diab, A.M.; Elyamany, H.E.; Abd Elmoaty, M.; Sreh, M.M. Effect of nanomaterials additives on performance of concrete resistance against magnesium sulfate and acids. Constr. Build. Mater. 2019, 210, 210–231. [Google Scholar] [CrossRef]
- Wu, Z.M.; Shi, C.J.; Khayat, K.H.; Wan, S. Effects of different nanomaterials on hardening and performance of ultra-high strength concrete (UHSC). Cem. Concr. Compos. 2016, 70, 24–34. [Google Scholar] [CrossRef] [Green Version]
- Mahdikhani, M.; Bamshad, O.; Shirvani, M.F. Mechanical properties and durability of concrete specimens containing nano silica in sulfuric acid rain condition. Constr. Build. Mater. 2018, 167, 929–935. [Google Scholar] [CrossRef]
- Langaroudi, M.A.M.; Mohammadi, Y. Effect of nano-clay on workability, mechanical, and durability properties of self-consolidating concrete containing mineral admixtures. Constr. Build. Mater. 2018, 191, 619–634. [Google Scholar] [CrossRef]
- Hawreen, A.; Bogas, J.A. Creep, shrinkage and mechanical properties of concrete reinforced with different types of carbon nanotubes. Constr. Build. Mater. 2019, 198, 70–81. [Google Scholar] [CrossRef]
- Pedro, D.; De Brito, J.; Evangelista, L. Durability performance of high-performance concrete made with recycled aggregates, fly ash and densified silica fume. Cem. Concr. Compos. 2018, 93, 63–74. [Google Scholar] [CrossRef]
- Wetzel, A.; Middendorf, B. Influence of silica fume on properties of fresh and hardened ultra-high performance concrete based on alkali-activated slag. Cem. Concr. Compos. 2019, 100, 53–59. [Google Scholar] [CrossRef]
- Holsapple, M.P.; Farland, W.H.; Landry, T.D.; Monteiro-Riviere, N.A.; Carter, J.M.; Walker, N.J. Research strategies for safety evaluation of nanomaterials, part II: Toxicological and safety evaluation of nanomaterials, current challenges and data needs. Toxicol. Sci. 2005, 88, 12–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoet, P.H.M.; Brüske-Hohlfeld, I.; Salata, O.V. Nanoparticles—Known and unknown health risks. J. Nanobiotechnol. 2004, 2, 12. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.J.; Cahyadi, J.H. Effects of densified silica fume on microstructure and compressive strength of blended cement pastes. Cem. Concr. Res. 2003, 33, 1543–1548. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, B.; Yan, P. Comparative study of effect of raw and densified silica fume in the paste, mortar and concrete. Constr. Build. Mater. 2016, 105, 82–93. [Google Scholar] [CrossRef]
- Yue, Y.F.; Wang, J.J.; Bai, Y. Tracing the status of silica fume in cementitious materials with Raman microscope. Constr. Build. Mater. 2018, 159, 610–616. [Google Scholar] [CrossRef]
- Chen, T.; Gao, X. Effect of carbonation curing regime on strength and microstructure of Portland cement paste. J. CO2 Util. 2019, 34, 74–86. [Google Scholar] [CrossRef]
- Irbe, L.; Beddoe, R.E.; Heinz, D. The role of aluminium in C-A-S-H during sulfate attack on concrete. Cem. Concr. Res. 2019, 116, 71–80. [Google Scholar] [CrossRef]
- Zhu, Q.; Jiang, L.; Chen, Y.; Xu, J.; Mo, L. Effect of chloride salt type on chloride binding behavior of concrete. Constr. Build. Mater. 2012, 37, 512–517. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford: London, UK, 1997. [Google Scholar]
- Auroy, M.; Poyet, S.; Le Bescop, P.; Torrenti, J.-M.; Charpentier, T.; Moskura, M. Comparison between natural and accelerated carbonation (3% CO2): Impact on mineralogy, microstructure, water retention and cracking. Cem. Concr. Res. 2018, 109, 64–80. [Google Scholar] [CrossRef]
- Arandigoyen, M.; Bicer-Simsir, B.; Alvarez, J.I.; Lange, D.A. Variation of microstructure with carbonation in lime and blended pastes. Appl. Surf. Sci. 2006, 252, 7562–7571. [Google Scholar] [CrossRef] [Green Version]
- Lesti, M.; Tiemeyer, C.; Plank, J. CO2 stability of Portland cement based well cementing systems for use on carbon capture & storage (CCS) wells. Cem. Concr. Res. 2013, 45, 45–54. [Google Scholar] [CrossRef]
- Neville, A.M. Properties of Concrete, 5th ed.; Prentice Hall: San Francisco, CA, USA, 1995. [Google Scholar]
- Neville, A.M. The confused world of sulfate attack on concrete. Cem. Concr. Res. 2004, 34, 1275–1296. [Google Scholar] [CrossRef]
- Cohen, M.D. Theories of expansion in sulfoaluminate-type expansive cements: Schools of thought. Cem. Concr. Res. 1983, 13, 809–818. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olek, J. Mechanism of sulfate attack: A fresh look: Part 2. Proposed mechanisms. Cem. Concr. Res. 2003, 33, 341–346. [Google Scholar] [CrossRef]
- Irassar, E.F.; Bonavetti, V.L.; González, M. Microstructural study of sulfate attack on ordinary and limestone Portland cements at ambient temperature. Cem. Concr. Res. 2003, 33, 31–41. [Google Scholar] [CrossRef]
- Talero, R.; Trusilewicz, L.; Delgado, A.; Pedrajas, C.; Lannegrand, R.; Rahhal, V. Comparative and semi-quantitative XRD analysis of Friedel’s salt originating from pozzolan and Portland cement. Constr. Build. Mater. 2011, 25, 2370–2380. [Google Scholar] [CrossRef]
- Yue, Y.F.; Wang, J.J.; Basheer, P.M.; Bai, Y. Raman spectroscopic investigation of Friedel’s salt. Cem. Concr. Compos. 2018, 86, 306–314. [Google Scholar] [CrossRef]
- Shi, X.M.; Xie, N.; Fortune, K.; Gong, J. Durability of steel reinforced concrete in chloride environments: An overview. Constr. Build. Mater. 2012, 30, 125–138. [Google Scholar] [CrossRef]
- NT BUILD 443; Concrete, Hardened: Accelerated Chloride Penetration. Nordtest Register: Espoo, Finland, 1996.
- ASTM C1012; Standard Test Method for Length Change of Hydraulic-Cement Mortars Exposed to a Sulfate Solution. ASTM International: West Conshohocken, PA, USA, 2019.
- Russell, D.; Basheer, P.M.; Rankin, G.I.B.; Long, A.E. Effect of relative humidity and air permeability on prediction of the rate of carbonation of concrete. Proc. Inst. Civ. Eng. Struct. Build. 2001, 3, 319–326. [Google Scholar] [CrossRef]
- Yue, Y.F.; Bai, Y.; Basheer, P.M.; Boland, J.J.; Wang, J.J. Monitoring the cementitious materials subjected to sulfate attack with optical fiber excitation Raman spectroscopy. Opt. Eng. 2013, 52, 104107. [Google Scholar] [CrossRef]
- Martinez-Ramirez, S.; Sanchez-Cortes, S.; Garcia-Ramos, J.; Domingo, C.; Fortes, C.; Blanco-Varela, M. Micro-Raman spectroscopy applied to depth profiles of carbonates formed in lime mortar. Cem. Concr. Res. 2003, 33, 2063–2068. [Google Scholar] [CrossRef]
- Gabrielli, C.; Jaouhari, R.; Joiret, S.; Maurin, G. In situ Raman spectroscopy applied to electrochemical scaling. Determination of the structure of vaterite. J. Raman Spectrosc. 2000, 31, 497–501. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.Q.; Tang, L.P.; Dong, Z.J. XRD and 29Si MAS NMR study on carbonated cement paste under accelerated carbonation using different concentration of CO2. Mater. Today Commun. 2019, 19, 464–470. [Google Scholar] [CrossRef]
- Ferrari, A. Resonant Raman Spectroscopy of Disordered, Amorphous, and Diamondlike Carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef] [Green Version]
- Garbev, K.; Stemmermann, P.; Black, L.; Breen, C.; Yarwood, J.; Gasharova, B. Structural features of C–S–H (I) and its carbonation in air—a Raman spectroscopic study. Part I: Fresh phases. J. Am. Ceram. Soc. 2007, 90, 900–907. [Google Scholar] [CrossRef]
- Kamitsos, E.I.; Kapoutsis, J.A.; Jain, H.; Hsieh, C.H. Vibrational study of the role of trivalent ions in sodium trisilicate glass. J. Non-Cryst. Solids 1994, 171, 31–45. [Google Scholar] [CrossRef]
- Iqbal, Z.; Vepřek, S.; Webb, A.P.; Capezzuto, P. Raman scattering from small particle size polycrystalline silicon. Solid State Commun. 1981, 37, 993–996. [Google Scholar] [CrossRef]
- Rodgers, K.A.; Hampton, W.A. Laser Raman identification of silica phases comprising microtextural components of sinters. Mineral. Mag. 2003, 67, 1–13. [Google Scholar] [CrossRef]
- Singh, N.B.; Ojha, P.N. Effect of CaCl2 on the hydration of tricalcium silicate. J. Mater. Sci. 1981, 16, 2675–2681. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Monteiro, P.J.M.; Gartner, E.M.; Denbeaux, G.P. A soft X-ray microscope investigation into the effects of calcium chloride on tricalcium silicate hydration. Cem. Concr. Res. 2005, 35, 19–25. [Google Scholar] [CrossRef]
- Nicoleau, L. Accelerated growth of calcium silicate hydrates: Experiments and simulations. Cem. Concr. Res. 2011, 41, 1339–1348. [Google Scholar] [CrossRef]
- Black, L.; Breen, C.; Yarwood, J.; Deng, C.S.; Phipps, J.; Maitland, G. Hydration of tricalcium aluminate (C3A) in the presence and absence of gypsum—studied by Raman spectroscopy and X-ray diffraction. J. Mater. Chem. 2006, 16, 1263–1272. [Google Scholar] [CrossRef]




| Oxides/% | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | SO3 |
|---|---|---|---|---|---|---|---|---|
| PC | 23.00 | 6.15 | 2.95 | 61.30 | 1.80 | 0.68 | 0.22 | 2.50 |
| SF | 93.00 | 0.70 | 1.20 | 0.30 | 1.20 | 1.80 | 1.50 | 0.30 |
| Deterioration Mechanisms | Raman Bands (cm−1) | Assignments |
|---|---|---|
| Carbonation | 350–560 | amorphous silica |
| 1360/1605 | carbon | |
| 517 | crystal silicon | |
| 600–800 | C–S–H | |
| 1085 | calcite/aragonite | |
| 1074/1090 | vaterite | |
| Chloride attack | 210/250, 356/395, 513/530, 778 | Friedel’s salt |
| 630–730 | C–S–H | |
| 1084 | calcite/aragonite | |
| Sulfate attack | 400–540 | amorphous silica |
| 1356/1603 | carbon | |
| 420–480 | C–S–H | |
| 989 | ettringite |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, Y.; Wang, J.; Bai, Y. Tracing the Status of Silica Fume in Cementitious Materials Subjected to Deterioration Mechanisms with Raman Microscope. Materials 2022, 15, 5195. https://doi.org/10.3390/ma15155195
Yue Y, Wang J, Bai Y. Tracing the Status of Silica Fume in Cementitious Materials Subjected to Deterioration Mechanisms with Raman Microscope. Materials. 2022; 15(15):5195. https://doi.org/10.3390/ma15155195
Chicago/Turabian StyleYue, Yanfei, Jingjing Wang, and Yun Bai. 2022. "Tracing the Status of Silica Fume in Cementitious Materials Subjected to Deterioration Mechanisms with Raman Microscope" Materials 15, no. 15: 5195. https://doi.org/10.3390/ma15155195






