Powder 3D Printing of Bone Scaffolds with Uniform and Gradient Pore Sizes Using Cuttlebone-Derived Calcium Phosphate and Glass-Ceramic
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Scaffold Fabrication
2.3. Materials Characterization
2.4. Scaffold Characterization
2.5. Preliminary Biological Evaluations
2.5.1. Cell Harvest and Cell Seeding
2.5.2. Cytotoxicity Test (LDH Assay)
2.5.3. Cell Metabolic Activity Evaluation (AlamarBlue Assay)
2.5.4. Cell Proliferation Assay (DNA Quantification)
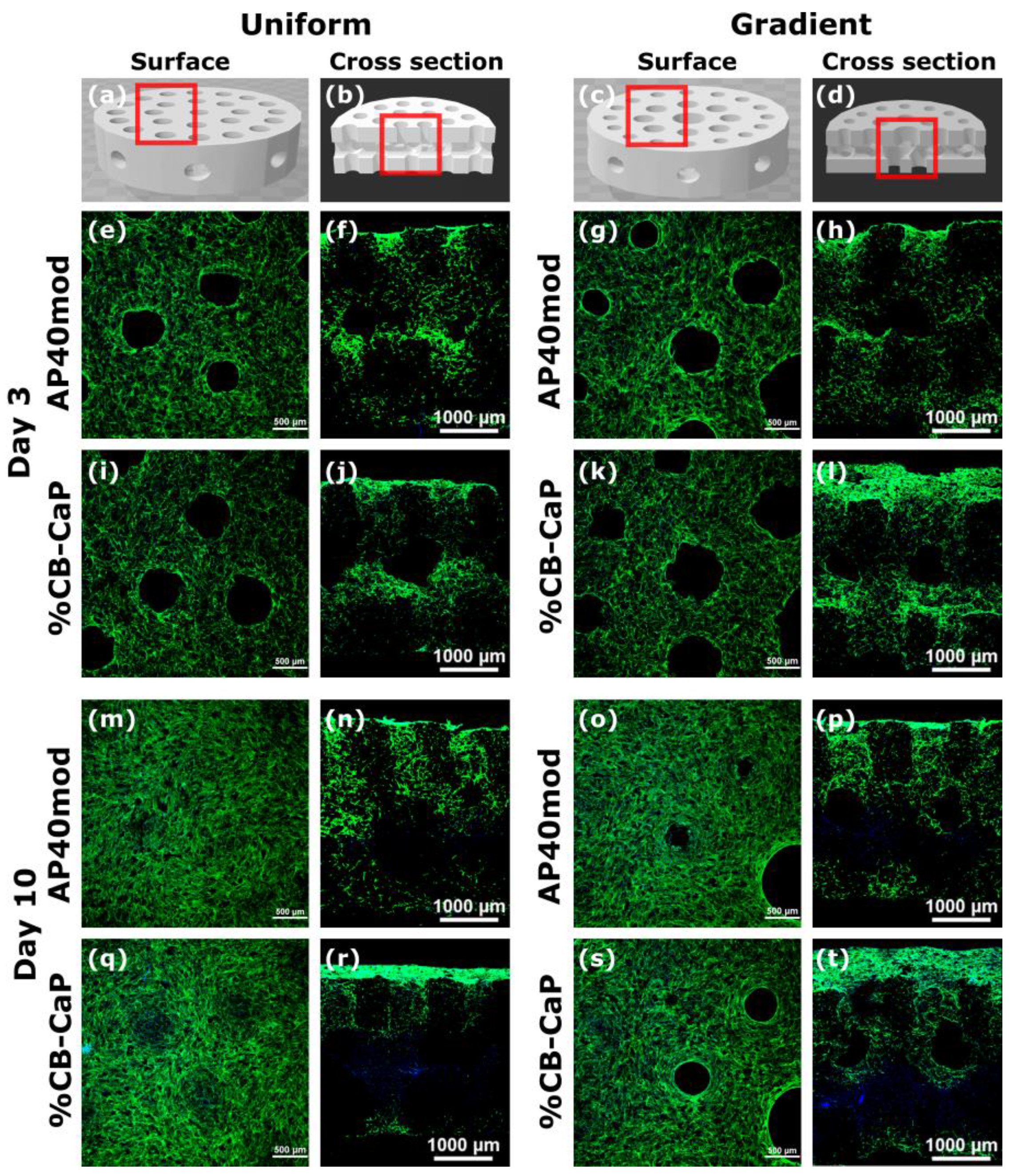
2.5.5. Cell Distribution and Migration (Confocal Microscopy Analysis)
2.5.6. Statistical Analysis
3. Results and Discussion
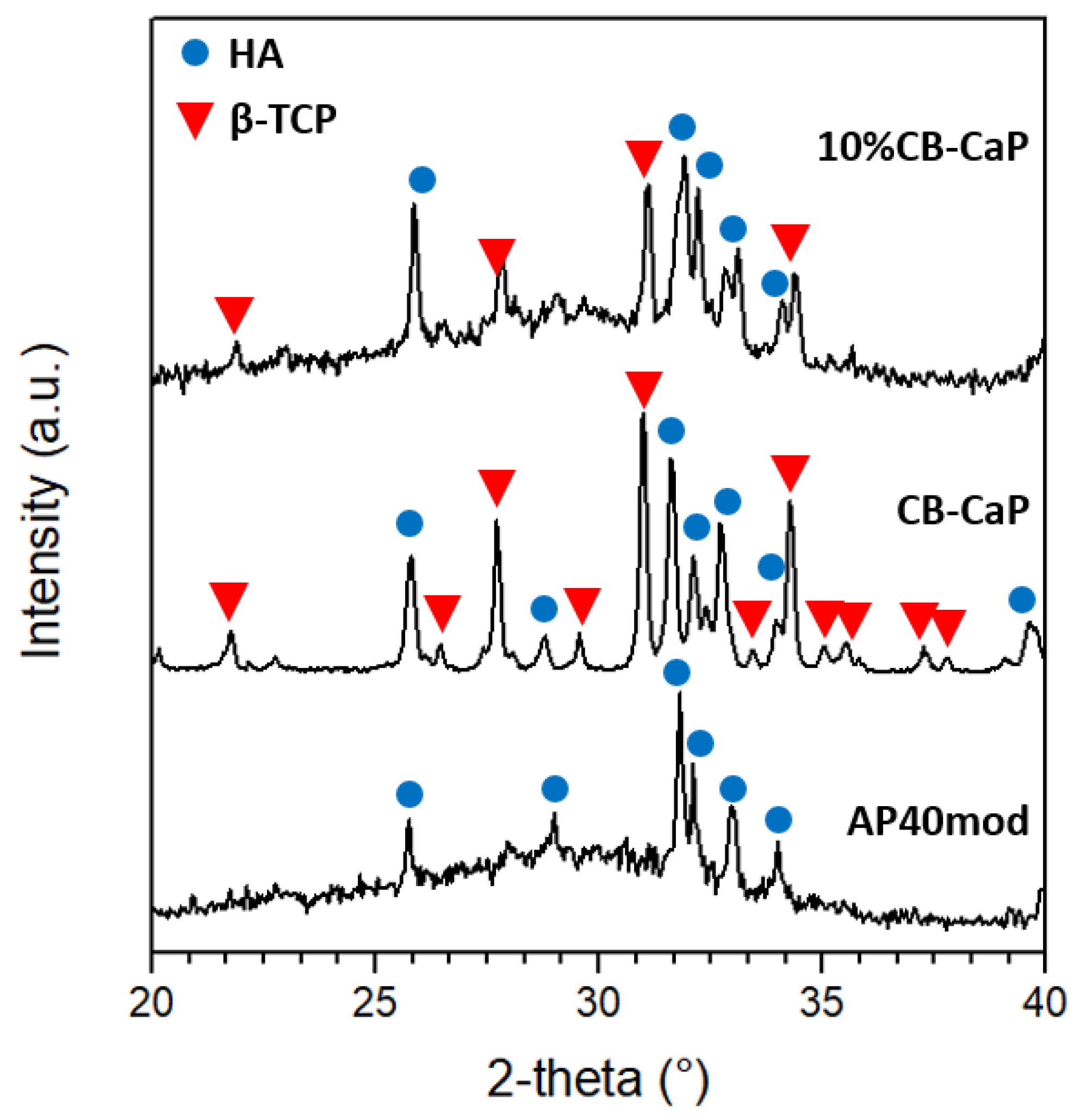
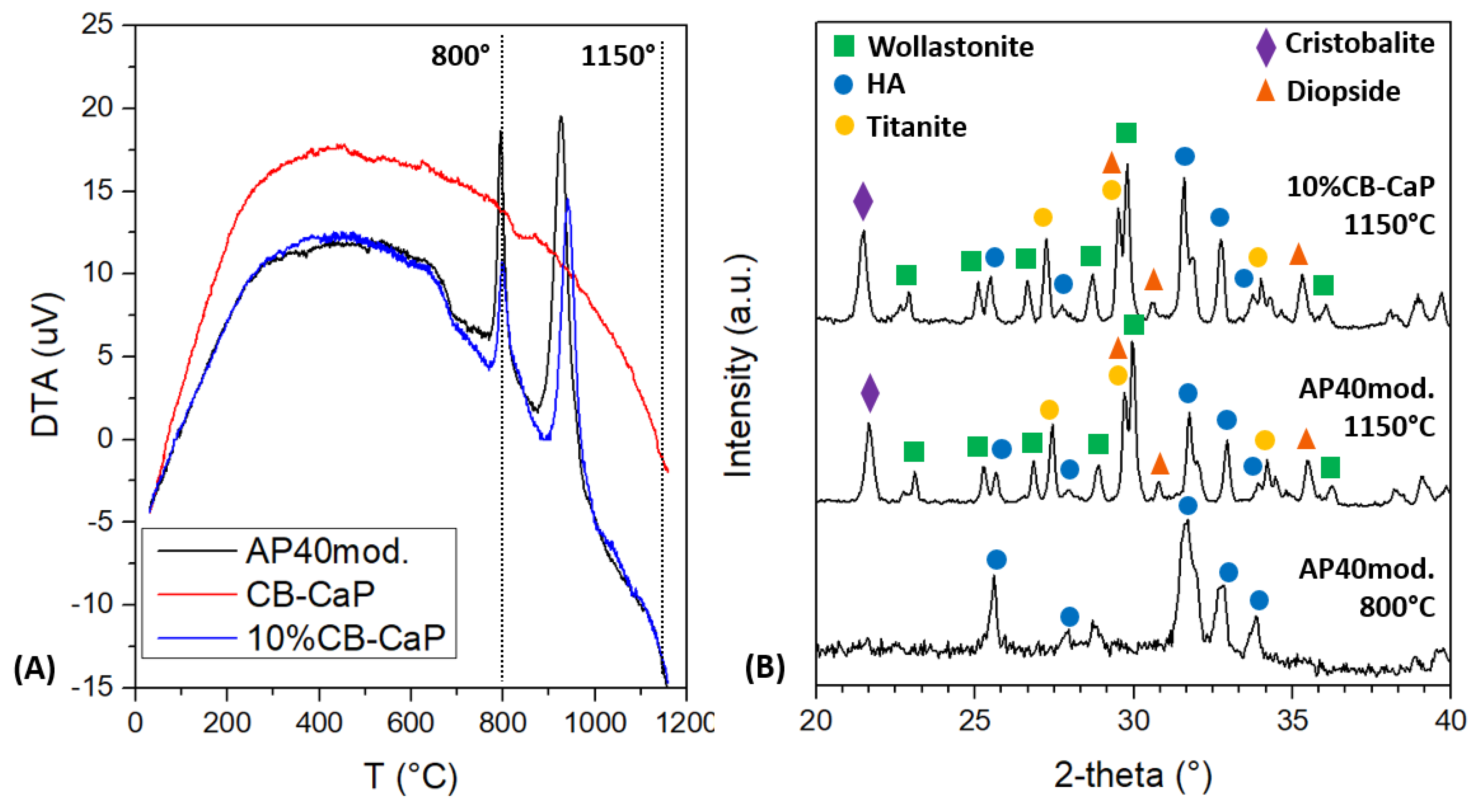
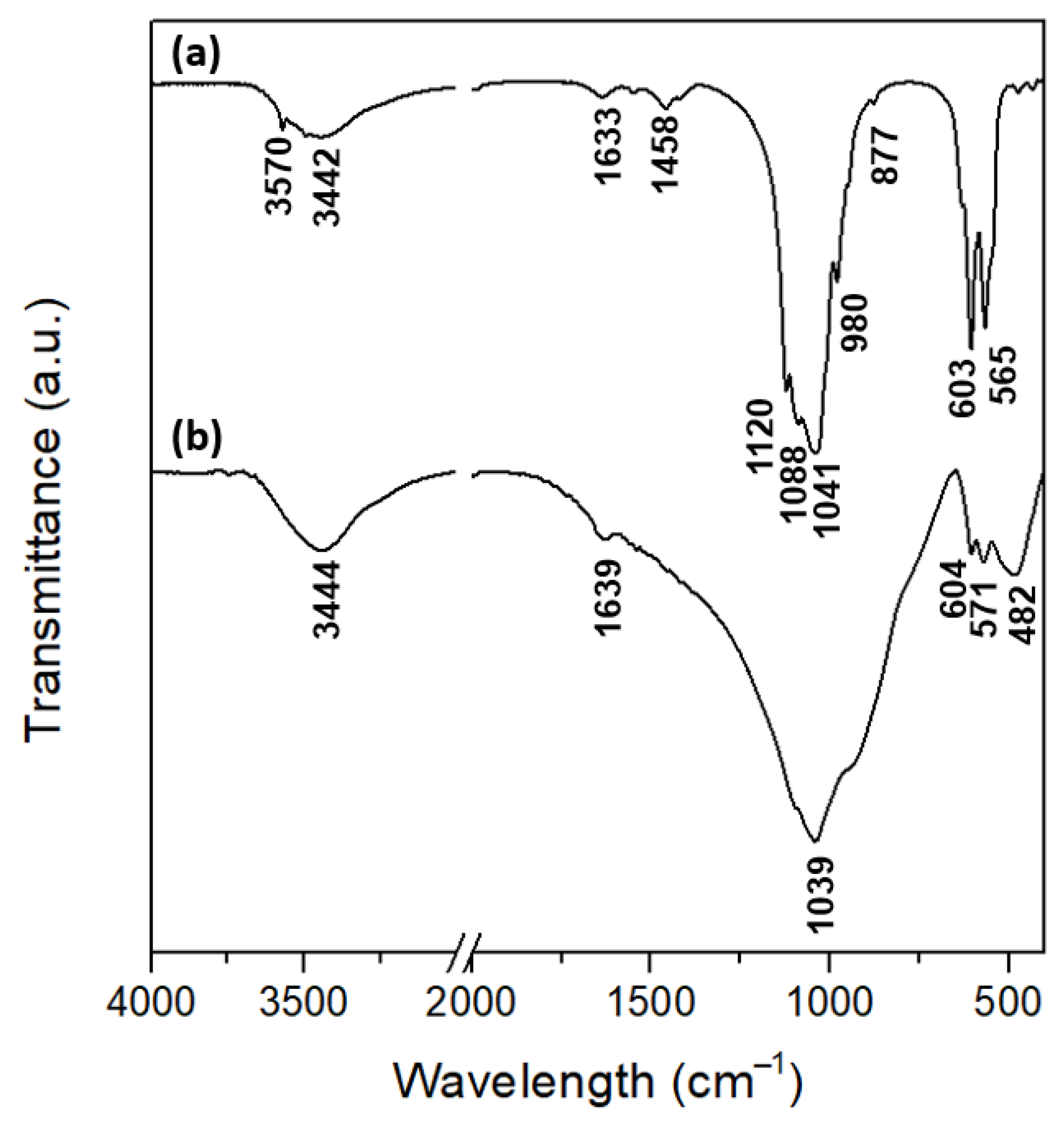
3.1. Materials Characterization
3.2. Scaffold Characterization
3.3. Preliminary Biological In Vitro Tests: Cytotoxicity and Cytocompatibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hing, K. Biomimetic bone regeneration. Biomim. Biomater. Struct. Appl. 2013, 207–237. [Google Scholar] [CrossRef]
- Fratzl, P.; Weinkamer, R. Nature’s hierarchical materials. Prog. Mater. Sci. 2007, 52, 1263–1334. [Google Scholar] [CrossRef] [Green Version]
- Rho, J.Y.; Kuhn-Spearing, L.; Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.; He, Z.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B.; et al. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Bone tissue regeneration: The role of scaffold geometry. Biomater. Sci. 2015, 3, 231–245. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Madrid, A.P.M.; Vrech, S.M.; Sanchez, M.A.; Rodriguez, A.P. Advances in additive manufacturing for bone tissue engineering scaffolds. Mater. Sci. Eng. C 2019, 100, 631–644. [Google Scholar] [CrossRef]
- Mabrouk, M.; Beherei, H.H.; Das, D.B. Recent progress in the fabrication techniques of 3D scaffolds for tissue engineering. Mater. Sci. Eng. C 2020, 110, 110716. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Brunello, G.; Sivolella, S.; Meneghello, R.; Ferroni, L.; Gardin, C.; Piattelli, A.; Zavan, B.; Bressan, E. Powder-based 3D printing for bone tissue engineering. Biotechnol. Adv. 2016, 34, 740–753. [Google Scholar] [CrossRef]
- Zocca, A.; Colombo, P.; Gomes, C.M.; Günster, J. Additive Manufacturing of Ceramics: Issues, Potentialities, and Opportunities. J. Am. Ceram. Soc. 2015, 98, 1983–2001. [Google Scholar] [CrossRef]
- Jang, S.; Park, S. Development of ceramic additive manufacturing: Process and materials technology. Biomed. Eng. Lett. 2020, 10, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Ben-Nissan, B. Natural bioceramics: From coral to bone and beyond. Curr. Opin. Solid State Mater. Sci. 2003, 7, 283–288. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, R.; Inzana, J.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D Printing of Calcium Phosphate Ceramics for Bone Tissue Engineering and Drug Delivery. Ann. Biomed. Eng. 2017, 45, 23–44. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Hou, Y.; Li, Z.; Wang, Z.; Yan, X. Powder-Based 3D Printed Porous Structure and Its Application as Bone Scaffold. Front. Mater. 2020, 7, 1–5. [Google Scholar] [CrossRef]
- Gayer, C.; Ritter, J.; Bullemer, M.; Grom, S.; Jauer, L.; Meiners, W.; Pfister, A.; Reinauer, F.; Vučak, M.; Wissenbach, K.; et al. Development of a solvent-free polylactide/calcium carbonate composite for selective laser sintering of bone tissue engineering scaffolds. Mater. Sci. Eng. C. 2019, 101, 660–673. [Google Scholar] [CrossRef]
- Lin, K.; Liu, J.; Wu, J.M.; Sun, Y.; Li, F.; Zhou, Y.; Shi, Y. Selective laser sintered nano-HA/PDLLA composite microspheres for bone scaffolds applications. Rapid Prototyp. J. 2020, 26, 1131–1143. [Google Scholar] [CrossRef]
- Cheng, L.; Suresh, K.S.; He, H.; Rajput, R.S.; Feng, Q.; Ramesh, S.; Wang, Y.; Krishnan, S.; Ostrovidov, S.; Camci-Unal, G.; et al. 3d printing of micro-and nanoscale bone substitutes: A review on technical and translational perspectives. Int. J. NanoMed. 2021, 16, 4289–4319. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Li, J.; Liu, C.; Lao, C.; Fu, Y.; Liu, C.; Li, Y.; Wang, P.; He, Y. 3D printing of ceramics: A review. J. Eur. Ceram. Soc. 2018, 39, 661–687. [Google Scholar] [CrossRef]
- Ma, H.; Feng, C.; Chang, J.; Wu, C. 3D-printed bioceramic scaffolds: From bone tissue engineering to tumor therapy. Acta Biomater. 2018, 79, 37–59. [Google Scholar] [CrossRef] [PubMed]
- Ressler, A.; Žužić, A.; Ivanišević, I.; Kamboj, N.; Ivanković, H. Ionic substituted hydroxyapatite for bone regeneration applications: A review. Open Ceram. 2021, 6, 100122. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, Y.; Yuan, Z.; Ding, H.; Hu, Y.; Yang, W.; Cai, K. Construction of Zn-incorporated multilayer films to promote osteoblasts growth and reduce bacterial adhesion. Mater. Sci. Eng. C 2017, 75, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Akram, M.; Ahmed, R.; Shakir, I.; Ibrahim, W.A.W.; Hussain, R. Extracting hydroxyapatite and its precursors from natural resources. J. Mater. Sci. 2014, 49, 1461–1475. [Google Scholar] [CrossRef]
- Terzioğlu, P.; Öğüt, H.; Kalemtaş, A. Natural calcium phosphates from fish bones and their potential biomedical applications. Mater. Sci. Eng. C. 2018, 91, 899–911. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, Y.J. Fabrication of strontium-substituted hydroxyapatite scaffolds using 3D printing for enhanced bone regeneration. J. Mater. Sci. 2021, 56, 1673–1684. [Google Scholar] [CrossRef]
- Li, K.; Li, S.; Ai, F.; Yan, J.; Zhou, K. Fabrication and Characterization of Sr-doped Hydroxyapatite Porous Scaffold. JOM 2021, 73, 1745–1753. [Google Scholar] [CrossRef]
- Mocioiu, A.M.; Tutuianu, R.; Cursaru, L.M.; Piticescu, R.M.; Stanciu, P.; Vasile, B.S.; Trusca, R.; Sereanu, V.; Meghea, A. 3D structures of hydroxyapatite obtained from Rapana venosa shells using hydrothermal synthesis followed by 3D printing. J. Mater. Sci. 2019, 54, 13901–13913. [Google Scholar] [CrossRef]
- Shi, Y.; Pan, T.; Zhu, W.; Yan, C.; Xia, Z. Artificial bone scaffolds of coral imitation prepared by selective laser sintering. J. Mech. Behav. Biomed. Mater. 2020, 104, 103664. [Google Scholar] [CrossRef]
- Cestari, F.; Petretta, M.; Yang, Y.; Motta, A.; Grigolo, B.; Sglavo, V.M. 3D printing of PCL/nano-hydroxyapatite scaffolds derived from biogenic sources for bone tissue engineering. Sustain. Mater. Technol. 2021, 29, e00318. [Google Scholar] [CrossRef]
- Mostafaei, A.; Elliott, A.M.; Barnes, J.E.; Li, F.; Tan, W.; Cramer, C.L.; Nandwana, P.; Chmielus, M. Binder jet 3D printing–Process parameters, materials, properties, and challenges. Prog. Mater. Sci. 2021, 119, 100707. [Google Scholar] [CrossRef]
- Sun, C.; Tian, X.; Wang, L.; Liu, Y.; Wirth, C.M.; Günster, J.; Li, D.; Jin, Z. Effect of particle size gradation on the performance of glass-ceramic 3D printing process. Ceram. Int. 2017, 43, 578–584. [Google Scholar] [CrossRef]
- Hausner, H.H. Powder Characteristics and their Effect on Powder Processing. Powder Technol. 1981, 30, 3–8. [Google Scholar] [CrossRef]
- Bortolotti, M.; Lutterotti, L.; Pepponi, G. Combining XRD and XRF analysis in one Rietveld-like fitting. Powder Diffr. 2017, 32, S225–S230. [Google Scholar] [CrossRef]
- Graulis, S.; Chateigner, D.; Downs, R.T.; Yokochi, A.F.T.; Quirós, M.; Lutterotti, L.; Manakova, E.; Butkus, J.; Moeck, P.; le Bail, A. Crystallography Open Database-An open-access collection of crystal structures. J. Appl. Crystallogr. 2009, 42, 726–729. [Google Scholar] [CrossRef]
- Ardanova, L.I.; Get’man, E.I.; Loboda, S.N.; Prisedsky, V.V.; Tkachenko, T.V.; Marchenko, V.I.; Antonovich, V.P.; Chivireva, N.A.; Chebishev, K.A.; Lyashenko, A.S. Isomorphous Substitutions of Rare Earth Elements for Calcium in Synthetic Hydroxyapatites. Inorg. Chem. 2010, 49, 10687–10693. [Google Scholar] [CrossRef]
- Yashima, M.; Sakai, A.; Kamiyama, T.; Hoshikawa, A. Crystal structure analysis of β-tricalcium phosphate Ca3(PO4)2 by neutron powder diffraction. J. Solid State Chem. 2003, 175, 272–277. [Google Scholar] [CrossRef]
- Ohashi, Y. Polysynthetically-twinned structures of enstatite and wollastonite. Phys. Chem. Miner. 1984, 10, 217–229. [Google Scholar] [CrossRef]
- Speer, J.A.; Gibbs, G.V. The crystal structure of synthetic titanite, CaTiOSiO4, and the domain textures of natural titanites. Am. Miner. 1976, 61, 238–247. [Google Scholar]
- Wyckoff, R.W.G. IX. Die Kristallstruktur von β-Cristobalit SiO2 (bei hohen Temperaturen stabile Form). Z. Für Krist. Mater. 1925, 62, 189–200. [Google Scholar] [CrossRef]
- Thompson, R.M.; Downs, R.T. The crystal structure of diopside at pressure to 10 GPa. Am. Miner. 2008, 93, 177–186. [Google Scholar] [CrossRef]
- Dorozhkina, E.I.; Dorozhkin, S.V. Mechanism of the solid-state transformation of a calcium-deficient hydroxyapatite (CDHA) into biphasic calcium phosphate (BCP) at elevated temperatures. Chem. Mater. 2002, 14, 4267–4272. [Google Scholar] [CrossRef]
- Kokubo, T.; Shigematsu, M.; Nagashima, Y.; Tashiro, M.; Nakamura, T.; Yamamuro, T.; Higashi, S. Apatite-and wollastonite-containg glass-ceramics for prosthetic application. Bull. Inst. Chem. Res. Kyoto Univ. 1982, 60, 260–268. [Google Scholar]
- Dorozhkin, S.V. Calcium orthophosphates: Occurrence, properties, biomineralization, pathological calcification and biomimetic applications. Biomatter 2011, 1, 121–164. [Google Scholar] [CrossRef]
- Rehman, I.; Bonfield, W. Characterization of hydroxyapatite and carbonated apatite by photo acoustic FTIR spectroscopy. J. Mater. Sci. Mater. Med. 1997, 8, 1–4. [Google Scholar] [CrossRef]
- Obadia, L.; Julien, M.; Quillard, S.; Rouillon, T.; Pilet, P.; Guicheux, J.; Bujoli, B.; Bouler, J.M. Na-doped β-tricalcium phosphate: Physico-chemical and in vitro biological properties. J. Mater. Sci. Mater. Med. 2011, 22, 593–600. [Google Scholar] [CrossRef]
- Panda, R.N.; Hsieh, M.F.; Chung, R.J.; Chin, T.S. FTIR, XRD, SEM and solid state NMR investigations of carbonate-containing hydroxyapatite nano-particles synthesized by hydroxide-gel technique. J. Phys. Chem. Solids. 2003, 64, 193–199. [Google Scholar] [CrossRef]
- Mukherjee, D.P.; Das, S.K. SiO2-Al2O3-CaO glass-ceramics: Effects of CaF2 on crystallization, microstructure and properties. Ceram. Int. 2013, 39, 571–578. [Google Scholar] [CrossRef]
- Nyquist, R.A.; Kagel, R.O. Infrared Spectra of Inorganic Compounds. In Handbook of Infrared and Raman Spectra of Inorganic Compounds and Organic Salts; Academic Press: Cambridge, UK, 1971; pp. 1–18. [Google Scholar] [CrossRef]
- Agathopoulos, S.; Tulyaganov, D.U.; Ventura, J.M.G.; Kannan, S.; Saranti, A.; Karakassides, M.A.; Ferreira, J.M.F. Structural analysis and devitrification of glasses based on the CaO-MgO-SiO2 system with B2O3, Na2O, CaF2 and P2O5 additives. J. Non. Cryst. Solids 2006, 352, 322–328. [Google Scholar] [CrossRef]
- Rojaee, R.; Fathi, M.; Raeissi, K. Electrophoretic deposition of nanostructured hydroxyapatite coating on AZ91 magnesium alloy implants with different surface treatments. Appl. Surf. Sci. 2013, 285, 664–673. [Google Scholar] [CrossRef]
- Burgess, R.J.; Agathocleous, M.; Morrison, S.J. Metabolic regulation of stem cell function. J. Intern. Med. 2014, 276, 12–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caliari, S.R.; Harley, B.A.C. Structural and biochemical modification of a collagen scaffold to selectively enhance MSC tenogenic, chondrogenic, and osteogenic differentiation. Adv. Healthc. Mater. 2014, 3, 1086–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]


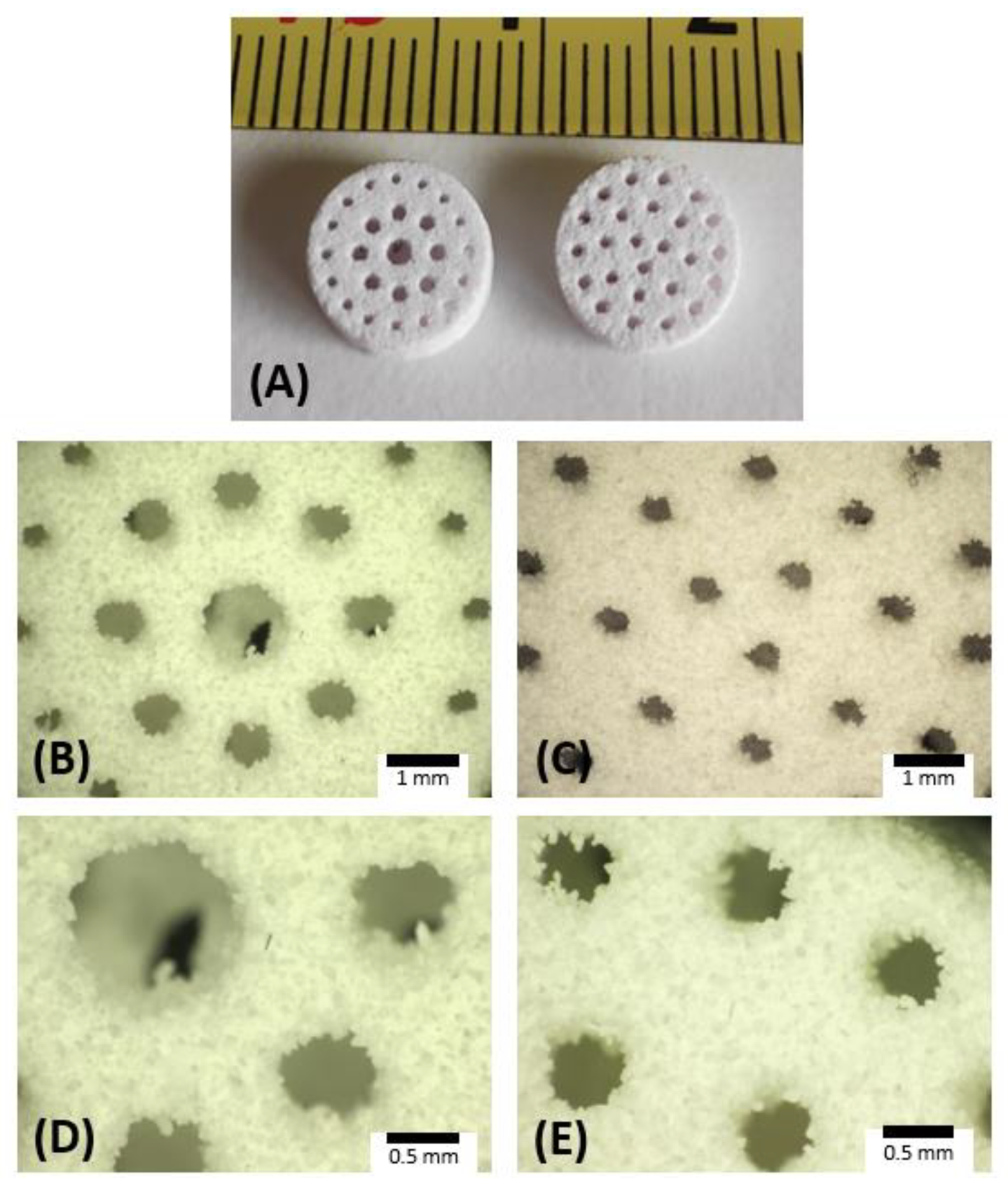


| Material | CAD Model | AP40mod | 10%CB-CaP | |||
|---|---|---|---|---|---|---|
| Pores Geometry | Grad. | Unif. | Gradient | Uniform | Gradient | Uniform |
| Diameter (mm) | 9.0 | 9.0 | 8.41 ± 0.05 | 8.45 ± 0.08 | 8.46 ± 0.09 | 8.48 ± 0.09 |
| Thickness (mm) | 3.0 | 3.0 | 3.11 ± 0.06 | 3.09 ± 0.06 | 2.96 ± 0.07 | 2.94 ± 0.89 |
| Mass (mg) | 197 ± 2 | 195 ± 6 | 150 ± 5 | 148 ± 6 | ||
| Pores size (mm) | 1.5 1.0 0.7 | 0.85 | 1.17 ± 0.03 0.62 ± 0.05 0.34 ± 0.05 | 0.47 ± 0.04 | 1.26 ± 0.03 0.74 ± 0.07 0.44 ± 0.07 | 0.50 ± 0.06 |
| Total porosity a (%) | 61 | 62 | 70 | 70 | ||
| Bulk density b (g/cm3) | 1.57 ± 0.02 | 1.40 ± 0.09 | ||||
| Apparent porosity b (%) | 47 ± 4 | 53 ± 3 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cestari, F.; Yang, Y.; Wilbig, J.; Günster, J.; Motta, A.; Sglavo, V.M. Powder 3D Printing of Bone Scaffolds with Uniform and Gradient Pore Sizes Using Cuttlebone-Derived Calcium Phosphate and Glass-Ceramic. Materials 2022, 15, 5139. https://doi.org/10.3390/ma15155139
Cestari F, Yang Y, Wilbig J, Günster J, Motta A, Sglavo VM. Powder 3D Printing of Bone Scaffolds with Uniform and Gradient Pore Sizes Using Cuttlebone-Derived Calcium Phosphate and Glass-Ceramic. Materials. 2022; 15(15):5139. https://doi.org/10.3390/ma15155139
Chicago/Turabian StyleCestari, Francesca, Yuejiao Yang, Janka Wilbig, Jens Günster, Antonella Motta, and Vincenzo M. Sglavo. 2022. "Powder 3D Printing of Bone Scaffolds with Uniform and Gradient Pore Sizes Using Cuttlebone-Derived Calcium Phosphate and Glass-Ceramic" Materials 15, no. 15: 5139. https://doi.org/10.3390/ma15155139
APA StyleCestari, F., Yang, Y., Wilbig, J., Günster, J., Motta, A., & Sglavo, V. M. (2022). Powder 3D Printing of Bone Scaffolds with Uniform and Gradient Pore Sizes Using Cuttlebone-Derived Calcium Phosphate and Glass-Ceramic. Materials, 15(15), 5139. https://doi.org/10.3390/ma15155139








